Abstract
Although effective in controlling malaria, indoor residual spraying results in elevated exposure to insecticides such as dichlorodiphenyltrichloroethane (DDT) and pyrethroids. These chemicals cross the placenta, but no studies have examined their associations with birth outcomes in populations residing in indoor residual spraying areas. We investigated this question in the Venda Health Examination of Mothers, Babies and Their Environment (VHEMBE), a birth cohort study of 751 South African children born between 2012 and 2013. We measured maternal peripartum serum DDT and urine pyrethroid metabolite concentrations and collected data on birth weight, length, head circumference, and duration of gestation. We analyzed the data using marginal structural models with inverse-probability-of-treatment weights, generalized propensity scores, and standard conditional linear regression. Using all 3 analytical methods, p,p′-DDT, o,p′-DDT, and to a lesser extent p,p′-dichlorodiphenyldichloroethylene were related to elevated birth weight, birth length, and head circumference among girls. Changes in gestational duration did not mediate this relationship, suggesting that these exposures accelerate fetal growth, which is consistent with the known estrogenic properties of o,p′-DDT and p,p′-DDT. No associations with pyrethroid metabolites were found. Results suggest that prenatal exposure to DDT is related to elevated birth size. Further studies are needed to elucidate the implications of these findings.
Keywords: birth outcomes, birth weight, DDT, indoor residual spraying, insecticides, marginal structural models, pyrethroids, South Africa
Indoor residual spraying (IRS), the use of insecticides on the interior walls of residences, is conducted by 83 countries to control malaria (1). The procedure protects 106 million people but potentially subjects them to elevated exposure to insecticides, with poorly understood health consequences (2, 3). Dichlorodiphenyltrichloroethane (DDT) and/or pyrethroid insecticides are commonly used for IRS in malaria-endemic areas.
DDT is a broad-spectrum organochlorine insecticide that was heavily used in agriculture and for public health purposes between the 1940s and the 1970s, when it was banned in most Western countries because of concerns about adverse health effects in humans and wildlife. In 2004, the Stockholm Convention on Persistent Organic Pollutants imposed an international ban on DDT, but an exemption was included for the control of insectborne diseases such as malaria. Pyrethroids are synthetic derivatives of pyrethrins, which are naturally produced by chrysanthemum flowers. These insecticides are commonly used in agriculture and commercial products; their use has increased substantially since the implementation of organophosphate pesticide bans in the early 2000s, making them the most commonly used retail insecticides in the United States (4–6).
DDT and pyrethroids readily cross the human placenta, which has led to hypotheses suggesting that exposure to these insecticides may be associated with adverse birth outcomes; but results from epidemiologic studies have been mixed. For instance, maternal and umbilical cord blood concentrations of p,p′-DDT have been found to be associated with decreased birth weight, birth length, and head circumference in Bolivia (n = 200) (7), decreased birth weight and birth length in California (n = 385) (8), and reduced birth weight but not birth length and head circumference in Valencia, Spain (n = 494) (9). In addition, analysis of 3 different subsamples of participants from the California-based Child Health and Development Study, conducted when DDT was still in use, produced conflicting results, with Jusko et al. (10) (n = 399) finding a positive association between exposure to p,p′-DDT and gestational age and no association with birth weight, Kezios et al. (11) (n = 600) reporting positive relationships with birth weight and null associations with gestational age, and Farhang et al. (12) (n = 420) finding no association with birth weight or gestational age. Studies of the association between p,p′-DDT’s breakdown product, p,p′-dichlorodiphenyldichloroethylene (DDE), and birth weight or gestational duration have yielded similarly inconsistent results (7–40).
Few studies have examined associations between exposure to pyrethroids and birth outcomes, and, like studies of DDT/DDE, results have been mixed. Birth weight was not associated with third-trimester urinary concentrations of the pyrethroid metabolite 3-phenoxybenzoic acid (3-PBA) in studies conducted in New York, New York (41) and Laizhou Bay, China (42), but a positive association was found with maternal first-trimester serum 3-PBA levels in Tokyo, Japan (43). In addition, no association was found between maternal urinary concentrations of the pyrethroid metabolites cis- and trans-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane-1-carboxylic acid (DCCA) and birth weight in the Chinese study (42), but the researchers found positive associations between 3-PBA, cis-DCCA, and trans-DCCA and birth length and between trans-DCCA and gestational age at birth.
However, prior research has been almost exclusively conducted in high-income countries. It is unclear whether results may be applicable to populations residing in malaria-endemic areas, which have different genetic backgrounds and lifestyles, experience high exposure to insecticides due to IRS (2, 3), and may be uniquely susceptible to adverse effects due to poverty, malnutrition, and poor health. Our objective was thus to determine whether exposure to IRS insecticides was associated with adverse birth outcomes among children living in Limpopo Province, South Africa, a malaria-endemic area where spraying with DDT or the pyrethroid deltamethrin occurs annually.
METHODS
Participants
We used data from the Venda Health Examination of Mothers, Babies and Their Environment (VHEMBE), a birth cohort study taking place in the rural Vhembe district of Limpopo Province. Mothers were recruited between August 2012 and December 2013 when they presented to Tshilidzini Hospital in the town of Thohoyandou to give birth. Eligible women were at least 18 years of age, had contractions more than 5 minutes apart, spoke Tshivenda (the most commonly spoken language in Vhembe) at home, lived within 20 km of the hospital, planned to remain in the area for at least 2 years, and had not been diagnosed with malaria during pregnancy (no otherwise-eligible woman was diagnosed with malaria). Of the 920 eligible women, 152 refused enrollment, 3 did not provide a sufficient blood sample for DDT analysis, and 14 did not complete a baseline questionnaire. Data on birth weight (n = 1), birth length (n = 6), and head circumference (n = 6) were missing for a few participants, leaving sample sizes ranging between 745 and 751, depending on the outcome, for DDT/DDE analyses. Thirteen participants did not provide urine samples and one 3-PBA measurement did not meet quality control standards, leaving sample sizes between 732 and 738 for pyrethroid analyses.
Written informed consent was obtained from all participants. Ethics approval was obtained from the University of California, Berkeley (Berkeley, California), the University of Pretoria (Pretoria, Gauteng, South Africa), the Limpopo Department of Health and Social Development (Polokwane, Limpopo, South Africa), and McGill University (Montreal, Quebec, Canada).
Data collection
Trained, bilingual (Tshivenda and English) staff originating from the study area conducted structured questionnaire-based interviews shortly after delivery. Staff collected data on demographic factors, household assets (based on the Demographic and Health Surveys) (44), nutrition (using a locally validated food frequency questionnaire) (45), food insecurity (based on the US National Center for Health Statistics’ Household Food Security Survey) (46), stress (based on a scale developed for the Soweto-based Birth to Twenty Study) (47), lifestyle, and health and pregnancy history, including the date of the last menstrual period. We defined poverty as an income below 386 rands per person per month (about US$30) based on Statistics South Africa guidelines (48), and low energy intake (<11,000 kJ/day) was defined on the basis of US Institute of Medicine guidelines (49). Energy and fat intakes were estimated by a South African expert nutritionist using Food Finder 3 software (South Africa Medical Research Council/WAMTechnology, Stellenbosch, South Africa). In addition, because income may not fully represent socioeconomic status in our study area, we constructed a household asset index via principal components analysis. The questionnaire was developed in English, translated into Tshivenda, and back-translated into English by native speakers in the translated language.
Two registered nurses blinded to participants’ exposure status abstracted information from maternal and child medical records in order to collect data on gestational age at birth, birth weight, and maternal human immunodeficiency virus (HIV) status. Birth weight was measured by hospital staff using a Tanita BD-815U neonatal scale (Tanita Corporation of America, Inc., Arlington Heights, Illinois) that we provided. For this study, gestational age at birth was based on the date of the last menstrual period as reported during the delivery interview. Some gestational age values were implausible, possibly because of incorrect recollection of the date of the last menstrual period. Unlikely values (above the first or below the 99th birth-weight-for-gestational-age percentile) were replaced with gestational ages indicated in medical records if the latter values were situated between the first and 99th birth-weight-for-gestational-age percentiles. We also generated a secondary variable based on gestational age values from medical records as the reference value, with unlikely values being replaced with those based on delivery interviews (correlation between primary and secondary variables = 0.78; P < 0.001). Study nurses also measured birth length using a Seca 417 portable infantometer (Seca Corporation, Chino, California) and head circumference using measuring tapes, following protocols developed by the US Centers for Disease Control and Prevention for the National Health and Nutrition Examination Survey (50). Length and head circumference measures were taken in triplicate and averaged. Small (<10th percentile) and large (>10th percentile) size-for-gestational-age values were estimated on the basis of a revised version of the World Health Organization growth standards (51). Quality control data showed good agreement between nurses for birth length (intraclass correlation coefficient (ICC) = 0.98, P < 0.001; mean difference = 0.27 (standard deviation (SD), 0.41) cm) and head circumference (ICC = 0.98, P < 0.001; mean difference = 0.15 (SD, 0.16) cm).
Measurement of IRS insecticides
Urine and blood samples were collected from women either before (nblood = 590; nurine = 455) or shortly after (nblood = 161; nurine = 283) delivery, and they were immediately processed and stored at −80°C until shipment to analytical laboratories. The Emory University Environmental Health Laboratory (Atlanta, Georgia) measured concentrations of p,p′-DDT, p,p′-DDE, and o,p′-DDT in serum as well as levels of polychlorinated biphenyls 118, 138, 153, and 180 using high-resolution gas chromatography–isotope dilution mass spectrometry (52). The limits of detection for p,p′-DDT, p,p′-DDE, and o,p′-DDT were 0.01, 0.03, and 0.01 ng/mL serum, and the limits of quantification were 0.03, 0.09, and 0.03 ng/mL serum, respectively. DDT/DDE and polychlorinated biphenyl concentrations were lipid-adjusted and expressed in ng/g lipid. Total lipid levels were estimated on the basis of triglyceride and total cholesterol measurements using standard enzymatic methods (Roche Chemicals, Indianapolis, Indiana). The Institut National de Santé Publique du Québec measured pyrethroid metabolites in urine using gas chromatography–mass spectrometry. Limits of detection were 0.0025 μg/L for cis-(2,2-dibromovinyl)-2,2-dimethylcyclopropane-1-carboxylic acid (cis-DBCA), 0.0045 μg/L for cis-DCCA, 0.0038 μg/L for trans-DCCA, 0.0047 μg/L for 3-PBA, and 0.005 μg/L for 4-fluoro-3-phenoxybenzoic acid. Metabolite concentrations (expressed in μg/L) were corrected for dilution by dividing values by urine specific gravity, which was measured using a portable refractometer (Atago PAL-10S; Atago Company Ltd., Tokyo, Japan) at the time of sample collection.
Statistical analysis
Data on biomarkers of exposure were log10-transformed to reduce the influence of outliers. We used analysis of variance and Pearson’s correlation coefficients to examine bivariate associations. We used marginal structural models with inverse-probability-of-treatment weights to estimate the causal effect of exposure to IRS insecticides on birth outcomes. We used stabilized weights which were estimated by means of the Super Learner algorithm, a loss-based supervised learning method that uses a weighted combination of prediction algorithms to return a function that minimizes cross-validated risk (53). Covariates considered included maternal age, education, marital status, postdelivery weight, postdelivery body mass index (weight (kg)/height (m)2), parity, daily energy intake, fat intake, fruit and vegetable consumption, food security, HIV status, smoking, exposure to environmental tobacco smoke, total serum polychlorinated biphenyl levels, alcohol consumption, stress, household income, asset index, child’s sex, season of birth, and mode of delivery (see Web Table 1, available at https://academic.oup.com/aje). We obtained percentile-based 95% confidence intervals and P values by bootstrapping the procedure 1,000 times. Based on observation of the propensity scores and of a reasonable range of weights (0.4–3.1), we concluded that the positivity assumption was not violated. We also examined effect modification by poverty, malnutrition, maternal HIV status, and child sex by including cross-product terms.
In order to evaluate the robustness of our results to different analysis strategies, we also reran all analyses applying 1) a generalized propensity score method for continuous exposure (54) and 2) standard conditional regression methods (i.e., multiple linear and logistic regression models). The generalized propensity score, which is equal to the conditional density of the exposure given the covariates, was estimated using the Super Learner algorithm including the above covariates and was included in models with a cubic spline to limit residual confounding; robust estimators were used to compute the variance. Variables included in standard regression models included those that were moderately (P < 0.20) associated with biomarkers of exposure and outcomes. We conducted generalized additive analyses with a cubic spline to evaluate the linearity assumption.
We also evaluated whether gestational age at birth mediated relationships between exposures and birth size using standard and counterfactual framework approaches. We first used the Baron and Kenny (55) method to estimate the indirect (mediated by gestational age) and direct (not mediated by gestational age) effects of exposures on birth size. As opposed to the Baron and Kenny method, the counterfactual approach (56) allows for exposure-mediator interactions and produces total effects that decompose into direct and indirect effects, thereby always generating mediation proportions between 0 and 1 (57). We thus used the latter approach to estimate the controlled direct effect by computing the average change in birth size due to a 10-fold increase in exposure when gestational age was set at the population average (39 weeks), the natural direct effect by estimating the change in birth size due to a 10-fold increase in exposure with gestational age values taking on their natural values when exposure was equal to the median, and the natural indirect effect by estimating the change in birth size due a change in gestational age in response to a 10-fold increase in exposure.
We also conducted sensitivity analysis to evaluate the robustness of our results. We repeated analyses by expressing DDT/DDE on a serum basis and included triglyceride and total cholesterol concentrations in the models. Similarly, we expressed concentrations of pyrethroid metabolites on a urine volume basis and conducted separate analyses by including urine specific gravity or creatinine concentration in models. We also fitted separate gestational age models using values based primarily on questionnaire (primary variable) or medical record (secondary variable) data, as described above. In addition, we reran the analyses using a variable representing pyrethroid exposure intensity (μg/hour), computed by multiplying metabolite concentrations by the volume of urine produced (participants were asked to empty their bladders) and dividing by time since the last void (58–60). Finally, we conducted analyses excluding potential outliers identified using the generalized extreme studentized deviate many-outlier procedure (61). Results were not substantially affected by these different specifications, and no substantial departure from linearity was observed. We present results with log10-transformed DDT/DDE and pyrethroid metabolites expressed linearly and corrected for lipid levels and specific gravity, respectively, and using the primary variable for gestational age.
Missing data for covariates (<2.1%) were imputed at random on the basis of observed univariate probability distributions. Machine-read values were used for DDT/DDE or pyrethroid concentrations between the limit of detection and the limit of quantification. Values below the limit of detection were imputed on the basis of a log-normal probability distribution whose parameters were estimated by maximum likelihood estimation (62). All analyses were conducted using STATA, version 13.1 (StataCorp LLC, College Station, Texas) or R, version 3.1.2 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Population characteristics
All participants were black/African and were born in South Africa. Women were aged 26.4 (SD, 6.3) years, on average, and most had a low level of education, with 54.9% not having completed high school and only 14.6% having pursued studies beyond high school (Table 1). Poverty and malnutrition are common in this population. Most of the women (61.3%) lived in households with incomes at or below the South African food poverty level, 43.9% were food-insecure, and 60.3% had a low energy intake during pregnancy. Most women (56.7%) were parous, and 13.8% were HIV-positive.
Table 1.
Characteristics of Participants in the Venda Health Examination of Mothers, Babies and Their Environment (n = 751), Limpopo Province, South Africa, 2012–2013
| Characteristic | No. of Persons | % |
|---|---|---|
| Age, years | ||
| <25 | 377 | 50.2 |
| 25–35 | 283 | 37.7 |
| >35 | 91 | 12.1 |
| Education | ||
| <12th grade | 412 | 54.9 |
| 12th grade | 229 | 30.5 |
| >12th grade | 110 | 14.6 |
| Household incomea | ||
| Above poverty level | 291 | 38.7 |
| At or below poverty level | 460 | 61.3 |
| Food securityb,c | ||
| High | 421 | 56.1 |
| Low | 242 | 32.3 |
| Very low | 87 | 11.6 |
| Energy intake,d kJ/day | ||
| <11,000 | 453 | 60.3 |
| ≥11,000 | 298 | 39.7 |
| Prestudy paritye | ||
| 0 | 325 | 43.3 |
| 1 | 201 | 26.8 |
| ≥2 | 225 | 30.0 |
| HIV statusc | ||
| Negative | 645 | 86.2 |
| Positive | 103 | 13.8 |
| Smoking | ||
| No | 748 | 99.6 |
| Yes | 3 | 0.4 |
| Alcohol consumption | ||
| No | 710 | 94.5 |
| Yes | 41 | 5.5 |
| Child sex | ||
| Male | 387 | 51.5 |
| Female | 364 | 48.5 |
| Birth weight, gc | ||
| ≥2,500 | 687 | 91.6 |
| <2,500 | 63 | 8.4 |
| Gestational age, weeks | ||
| ≥37 | 648 | 86.3 |
| <37 | 103 | 13.7 |
| Size for gestational age | ||
| >10th percentile | 41 | 5.5 |
| 10th–90th percentiles | 528 | 70.3 |
| <10th percentile | 182 | 24.2 |
| Mode of deliveryc | ||
| Vaginal | 569 | 77.3 |
| Cesarean | 167 | 22.7 |
Abbreviation: HIV, human immunodeficiency virus.
a Poverty was defined as a household income below 386 rands per person per month (about US$30) based on Statistics South Africa guidelines for mid-2013 (48).
b Food insecurity was defined on the basis of the US National Center for Health Statistics’ Household Food Security Survey (46).
c Numbers do not add up to 751 because of missing data.
d Low energy intake (<11,000 kJ/day) was defined on the basis of US Institute of Medicine guidelines (49).
e Percentages do not add up to 100 because of rounding.
Birth outcomes
The average duration of gestation was 39.3 (SD, 2.3) weeks, with 13.7% of children being born preterm (<37 weeks). The mean birth weight was 3,126 (SD, 452) g, and the prevalence of low birth weight (<2,500 g) was 8.4%. Almost one-quarter (24.2%) of the children were born small for gestational age (<10th percentile of weight for gestational age), and 5.5% were large for gestational age (>10th percentile of weight for gestational age). Mean birth length and head circumference were 48.9 (SD, 2.2) cm and 34.4 (SD, 1.5) cm, respectively.
Exposure to IRS insecticides
Table 2 shows the distribution of biomarkers of exposure to IRS insecticides, as well as their detection and quantification frequencies. Except for p,p′-DDT (98% detection) and o,p′-DDT (91% detection), the concentrations of all biomarkers of exposure were above the limit of detection. DDT/DDE (r = 0.69–0.85; P < 0.001) and pyrethroid (r = 0.46–0.90; P < 0.001) levels were intercorrelated within insecticide classes but not between insecticide classes (r = 0.00–0.05; P = 0.19–0.85) (Web Table 2).
Table 2.
Maternal Peripartum Serum Concentrations of Dichlorodiphenyltrichloroethane (ng/g Lipids) and Urinary Pyrethroid Metabolites (μg/L; Specific-Gravity–Corrected) Among Participants in the Venda Health Examination of Mothers, Babies and Their Environment, Limpopo Province, South Africa, 2012–2013
| Compound | No. of Persons | Detection Frequency, % | Quantification Frequency, % | Geometric Mean (GSD) | Minimum | Percentile | Maximum | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 25th | 50th | 75th | 90th | |||||||
| p,p′-DDT | 751 | 98.0 | 90.7 | 69.6 (6.7) | <LOD | 18.9 | 55.3 | 261.0 | 946.2 | 15,027.6 |
| p,p′-DDE | 751 | 100 | 97.2 | 287.9 (4.8) | 4.0 | 91.8 | 242.2 | 878.9 | 2,577.7 | 26,301.3 |
| o,p′-DDT | 751 | 90.6 | 43.3 | 8.9 (4.6) | <LOD | 3.4 | 7.1 | 22.7 | 72.0 | 2,029.3 |
| cis-DBCA | 738 | 100 | 99.6 | 0.23 (3.41) | <0.01 | 0.10 | 0.23 | 0.47 | 1.12 | 17.83 |
| 3-PBA | 737 | 100 | 100 | 0.72 (2.80) | 0.02 | 0.38 | 0.71 | 1.36 | 2.39 | 58.90 |
| cis-DCCA | 738 | 100 | 99.9 | 0.31 (2.94) | 0.01 | 0.15 | 0.30 | 0.59 | 1.03 | 103.50 |
| trans-DCCA | 738 | 100 | 99.6 | 0.36 (3.43) | 0.01 | 0.16 | 0.34 | 0.78 | 1.49 | 132.88 |
Abbreviations: DBCA, (2,2-dibromovinyl)-2,2-dimethylcyclopropane-1-carboxylic acid; DCCA, (2,2-dichlorovinyl)-2,2-dimethylcyclopropane-1-carboxylic acid; DDE, dichlorodiphenyldichloroethylene; DDT, dichlorodiphenyltrichloroethane; F3-PBA, 4-fluoro-3-phenoxybenzoic acid; GSD, geometric standard deviation; LOD, limit of detection; 3-PBA, 3-phenoxybenzoic acid.
Associations between exposure to IRS insecticides and birth outcomes
Marginal structural models, propensity score models, and standard conditional models yielded qualitatively similar results. We report results from marginal structural models here and in Tables 3 and 4; results derived from other models are shown in Web Tables 3–6. Maternal serum concentrations of p,p′-DDT, o,p′-DDT, and, to a lesser extent, p,p′-DDE were related to elevated birth weight (βp,p′-DDT = 70.7 g (95% CI: 11.8, 130.8); βo,p′-DDT = 80.4 g (95% CI: 10.2, 149.9); βp,p′-DDE = 58.4 g (95% CI: 17.0, 129.4)), birth length (βp,p′-DDT = 0.41 cm (95% CI: 0.11, 0.72); βo,p′-DDT = 0.48 cm (95% CI: 0.12, 0.82); βp,p′-DDE = 0.40 cm (95% CI: 0.07, 0.72)), and head circumference (βp,p′-DDT = 0.30 cm (95% CI: 0.12, 0.46); βo,p′-DDT = 0.33 cm (95% CI: 0.13, 0.52); βp,p′-DDE = 0.21 cm (95% CI: 0.01, 0.41)) among girls but not among boys.
Table 3.
Associations Between Maternal Peripartum Serum Concentrations of Dichlorodiphenyltrichloroethane and Dichlorodiphenyldichloroethylene and Birth Outcomes (Based on Marginal Structural Models), Venda Health Examination of Mothers, Babies and Their Environment, Limpopo Province, South Africa, 2012–2013a
| Birth Outcome and Sex | No. of Persons | Compound | |||||
|---|---|---|---|---|---|---|---|
| p,p′-DDT | p,p′-DDE | o,p′-DDT | |||||
| βb | 95% CI | β | 95% CI | β | 95% CI | ||
| Birth weight, g | |||||||
| All children | 750 | 12.2 | −24.5, 49.7 | 10.5 | −36.9, 58.1 | 11.4 | −36.0, 60.6 |
| Boys | 387 | −29.9 | −77.0, 17.1 | −28.0 | −88.6, 32.1 | −43.7 | −105.8, 24.7 |
| Girls | 363 | 70.7c | 11.8, 130.8 | 58.4 | −17.0, 129.4 | 80.4c | 10.2, 149.9 |
| P for interaction | 0.012 | 0.074 | 0.012 | ||||
| Birth length, cm | |||||||
| All children | 745 | 0.16 | −0.04, 0.36 | 0.20 | −0.04, 0.44 | 0.20 | −0.05, 0.45 |
| Boys | 383 | 0.01 | −0.23, 0.27 | 0.06 | −0.29, 0.38 | 0.01 | −0.32, 0.36 |
| Girls | 362 | 0.41c | 0.11, 0.72 | 0.40c | 0.07, 0.72 | 0.48c | 0.12, 0.82 |
| P for interaction | 0.036 | 0.138 | 0.072 | ||||
| Head circumference, cm | |||||||
| All children | 745 | 0.07 | −0.05, 0.19 | 0.02 | −0.12, 0.16 | 0.09 | −0.05, 0.24 |
| Boys | 383 | −0.09 | −0.24, 0.06 | −0.13 | −0.31, 0.07 | −0.08 | −0.31, 0.13 |
| Girls | 362 | 0.30c | 0.12, 0.46 | 0.21c | 0.01, 0.41 | 0.33c | 0.13, 0.52 |
| P for interaction | <0.001 | 0.012 | 0.004 | ||||
| Gestational age, weeks | |||||||
| All children | 751 | −0.02 | −0.21, 0.18 | −0.08 | −0.34, 0.15 | −0.04 | −0.29, 0.20 |
| Boys | 387 | −0.19 | −0.46, 0.07 | −0.25 | −0.61, 0.08 | −0.13 | −0.50, 0.22 |
| Girls | 364 | 0.17 | −0.10, 0.45 | 0.09 | −0.25, 0.44 | 0.10 | −0.25, 0.44 |
| P for interaction | 0.064 | 0.160 | 0.336 | ||||
| ORd | 95% CI | OR | 95% CI | OR | 95% CI | ||
| Low birth weight | |||||||
| All children | 750 | 0.81 | 0.60, 1.08 | 0.78 | 0.53, 1.16 | 0.90 | 0.62, 1.32 |
| Boys | 387 | 1.09 | 0.73, 1.64 | 0.95 | 0.52, 1.69 | 1.03 | 0.53, 2.05 |
| Girls | 363 | 0.62c | 0.39, 0.92 | 0.67 | 0.37, 1.13 | 0.74 | 0.47, 1.15 |
| P for interaction | 0.052 | 0.386 | 0.404 | ||||
| Preterm birth | |||||||
| All children | 751 | 0.97 | 0.74, 1.27 | 1.04 | 0.75, 1.41 | 1.06 | 0.77, 1.48 |
| Boys | 387 | 1.18 | 0.83, 1.67 | 1.24 | 0.83, 1.94 | 1.22 | 0.75, 1.99 |
| Girls | 364 | 0.78 | 0.51, 1.16 | 0.86 | 0.54, 1.31 | 0.87 | 0.53, 1.34 |
| P for interaction | 0.138 | 0.220 | 0.266 | ||||
| Small size for gestational age | |||||||
| All children | 750 | 0.92 | 0.73, 1.16 | 0.87 | 0.66, 1.10 | 0.89 | 0.68, 1.16 |
| Boys | 387 | 0.95 | 0.69, 1.29 | 0.93 | 0.66, 1.28 | 0.98 | 0.70, 1.36 |
| Girls | 363 | 0.91 | 0.66, 1.27 | 0.80 | 0.51, 1.18 | 0.85 | 0.54, 1.32 |
| P for interaction | 0.874 | 0.574 | 0.596 | ||||
| Large size for gestational age | |||||||
| All children | 750 | 1.02 | 0.69, 1.51 | 1.13 | 0.72, 1.80 | 0.94 | 0.54, 1.57 |
| Boys | 387 | 0.91 | 0.57, 1.44 | 1.04 | 0.60, 1.75 | 0.59 | 0.26, 1.32 |
| Girls | 363 | 1.28 | 0.66, 2.44 | 1.29 | 0.63, 2.71 | 1.50 | 0.73, 2.83 |
| P for interaction | 0.364 | 0.600 | 0.084 | ||||
Abbreviations: CI, confidence interval; DDE, dichlorodiphenyldichloroethylene; DDT, dichlorodiphenyltrichloroethane; OR, odds ratio.
a Propensity scores and inverse-probability-of-treatment weights were based on conditional probability density functions determined using the Super Learner algorithm (53).
b Change in mean outcome for each 10-fold increase in maternal peripartum serum DDT/DDE concentration.
cP < 0.05.
d OR for each 10-fold increase in maternal peripartum serum DDT/DDE concentration.
Table 4.
Associations Between Maternal Peripartum Urinary Concentrations of Pyrethroid Metabolites and Birth Outcomes (Based on Marginal Structural Models), Venda Health Examination of Mothers, Babies and Their Environment, Limpopo Province, South Africa, 2012–2013a
| Birth Outcome and Sex | No. of Persons | Pyrethroid Metabolite | |||||||
|---|---|---|---|---|---|---|---|---|---|
| cis-DBCA | cis-DCCA | trans-DCCA | 3-PBA | ||||||
| βb | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | ||
| Birth weight, g | |||||||||
| All children | 738 | 4.8 | −65.4, 79.8 | 15.8 | −52.9, 83.4 | 5.2 | −57.0, 65.1 | 7.5 | −83.3, 99.8 |
| Boys | 383 | −21.6 | −106.5, 65.6 | 4.7 | −81.5, 90.2 | −16.7 | −95.4, 58.5 | −25.0 | −127.2, 75.9 |
| Girls | 355 | 30.7 | −84.2, 145.7 | 28.3 | −71.8, 131.1 | 32.5 | −52.3, 132.4 | 51.2 | −108.4, 201.3 |
| P for interaction | 0.518 | 0.714 | 0.382 | 0.428 | |||||
| Birth length, cm | |||||||||
| All children | 733 | 0.03 | −0.26, 0.33 | 0.11 | −0.18, 0.37 | 0.02 | −0.24, 0.26 | 0.12 | −0.20, 0.45 |
| Boys | 379 | −0.08 | −0.48, 0.33 | −0.07 | −0.48, 0.28 | −0.15 | −0.48, 0.14 | −0.03 | −0.40, 0.37 |
| Girls | 354 | 0.12 | −0.25, 0.58 | 0.31 | −0.11, 0.70 | 0.23 | −0.15, 0.60 | 0.31 | −0.16, 0.78 |
| P for interaction | 0.474 | 0.156 | 0.088 | 0.274 | |||||
| Head circumference, cm | |||||||||
| All children | 733 | 0.04 | −0.20, 0.27 | 0.06 | −0.20, 0.28 | −0.02 | −0.25, 0.18 | −0.01 | −0.30, 0.27 |
| Boys | 379 | −0.09 | −0.41, 0.19 | −0.02 | −0.41, 0.28 | −0.03 | −0.35, 0.21 | −0.09 | −0.45, 0.27 |
| Girls | 354 | 0.17 | −0.09, 0.46 | 0.15 | −0.13, 0.45 | −0.01 | −0.29, 0.28 | 0.10 | −0.28, 0.46 |
| P for interaction | 0.182 | 0.440 | 0.938 | 0.540 | |||||
| Gestational age, weeks | |||||||||
| All children | 738 | −0.11 | −0.49, 0.27 | 0.07 | −0.26, 0.39 | 0.01 | −0.27, 0.29 | −0.11 | −0.56, 0.29 |
| Boys | 383 | −0.13 | −0.57, 0.33 | 0.11 | −0.34, 0.56 | 0.01 | −0.38, 0.37 | −0.11 | −0.64, 0.34 |
| Girls | 355 | −0.08 | −0.70, 0.50 | 0.02 | −0.48, 0.54 | 0.02 | −0.40, 0.46 | −0.10 | −0.86, 0.62 |
| P for interaction | 0.820 | 0.784 | 0.910 | 0.962 | |||||
| ORc | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | ||
| Low birth weight | |||||||||
| All children | 738 | 0.97 | 0.45, 2.01 | 0.70 | 0.41, 1.12 | 0.81 | 0.51, 1.22 | 0.73 | 0.36, 1.56 |
| Boys | 383 | 0.75 | 0.32, 1.68 | 0.83 | 0.35, 1.67 | 1.00 | 0.51, 1.77 | 0.98 | 0.41, 2.24 |
| Girls | 355 | 1.09 | 0.37, 2.48 | 0.61 | 0.30, 1.13 | 0.68 | 0.35, 1.17 | 0.57 | 0.19, 1.90 |
| P for interaction | 0.638 | 0.554 | 0.334 | 0.444 | |||||
| Preterm birth | |||||||||
| All children | 738 | 1.11 | 0.69, 1.82 | 0.83 | 0.52, 1.27 | 0.98 | 0.67, 1.44 | 1.12 | 0.66, 1.99 |
| Boys | 383 | 1.22 | 0.72, 2.09 | 0.98 | 0.53, 1.64 | 1.15 | 0.71, 1.75 | 1.31 | 0.71, 2.60 |
| Girls | 355 | 0.99 | 0.43, 2.19 | 0.68 | 0.34, 1.33 | 0.80 | 0.43, 1.47 | 0.90 | 0.33, 2.21 |
| P for interaction | 0.622 | 0.440 | 0.362 | 0.524 | |||||
| Small size for gestational age | |||||||||
| All children | 738 | 0.97 | 0.70, 1.33 | 0.91 | 0.65, 1.32 | 0.99 | 0.72, 1.36 | 1.01 | 0.71, 1.49 |
| Boys | 383 | 1.23 | 0.82, 1.86 | 1.15 | 0.78, 1.84 | 1.16 | 0.81, 1.73 | 1.22 | 0.80, 2.00 |
| Girls | 355 | 0.69 | 0.38, 1.19 | 0.62 | 0.31, 1.19 | 0.73 | 0.41, 1.26 | 0.68 | 0.34, 1.43 |
| P for interaction | 0.088 | 0.082 | 0.140 | 0.186 | |||||
| Large size for gestational age | |||||||||
| All children | 738 | 0.78 | 0.47, 1.34 | 0.91 | 0.45, 1.70 | 0.91 | 0.49, 1.61 | 0.88 | 0.39, 1.84 |
| Boys | 383 | 0.86 | 0.40, 1.92 | 1.25 | 0.44, 2.91 | 1.12 | 0.42, 2.55 | 0.99 | 0.29, 2.99 |
| Girls | 355 | 0.71 | 0.32, 1.58 | 0.60 | 0.21, 1.60 | 0.69 | 0.28, 1.61 | 0.78 | 0.29, 2.02 |
| P for interaction | 0.730 | 0.300 | 0.448 | 0.750 | |||||
Abbreviations: CI, confidence interval; DBCA, (2,2-dibromovinyl)-2,2-dimethylcyclopropane-1-carboxylic acid; DCCA, (2,2-dichlorovinyl)-2,2-dimethylcyclopropane-1-carboxylic acid; OR, odds ratio; 3-PBA, 3-phenoxybenzoic acid.
a Propensity scores and inverse-probability-of-treatment weights were based on conditional probability density functions determined using the Super Learner algorithm (53).
b Change in mean outcome for each 10-fold increase in maternal peripartum pyrethroid metabolite concentration.
c OR for each 10-fold increase in maternal peripartum pyrethroid metabolite concentration.
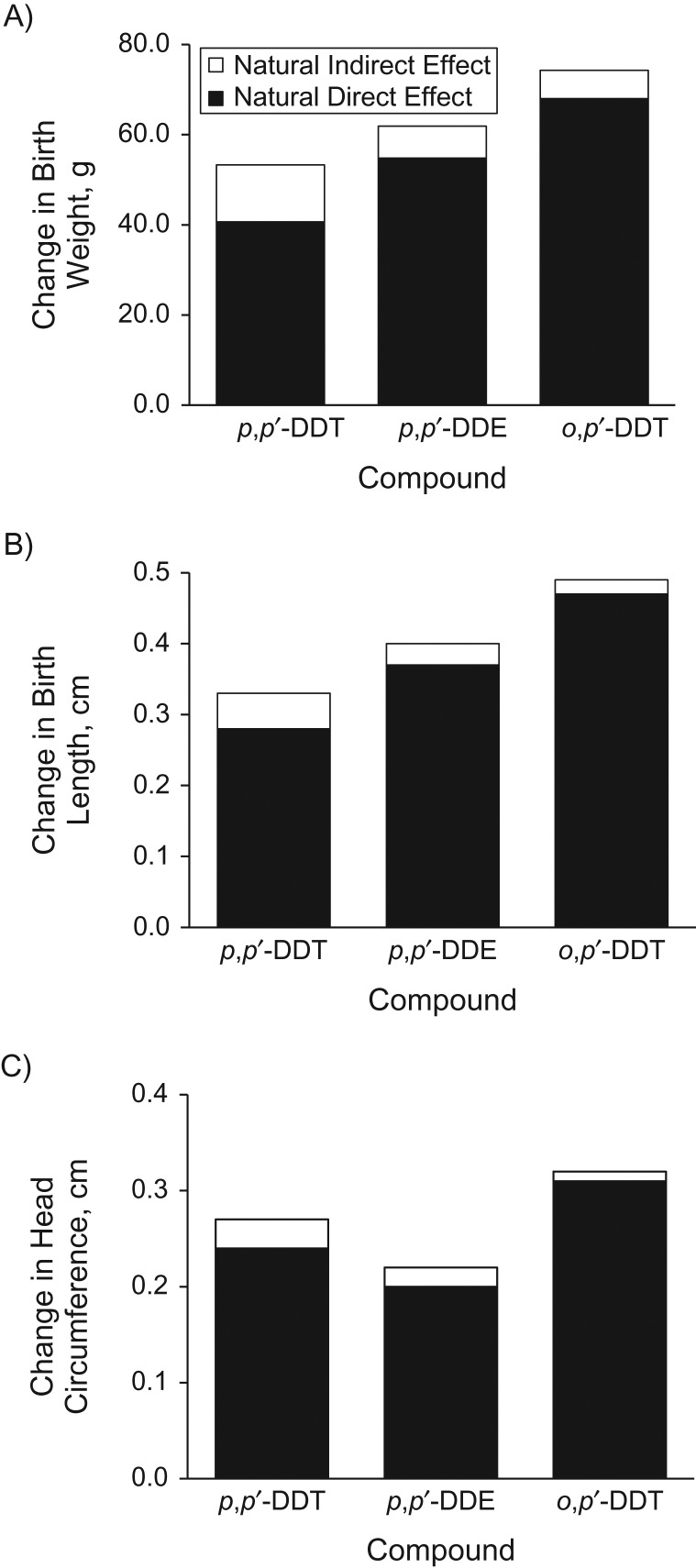
Associations between DDT/DDE and both gestational age and large size for gestational age among girls were positive but imprecise, suggesting that increases in birth size may be due to a combination of increased gestation duration and intrauterine growth. However, mediation analysis using both the Baron and Kenny method (55) and the counterfactual framework showed small and imprecise indirect effects mediated by gestational age which ranged between 6.3 g (95% CI: −14.4, 29.0) and 12.8 g (95% CI: −6.3, 32.0) for associations between DDT/DDE and birth weight and approached zero for associations with birth length and head circumference (Figure 1; Web Tables 7 and 8). In contrast, controlled and natural direct effects (which were similar because exposures and gestational age did not interact) were large, and natural direct effects accounted for large portions (76%–97%) of total effects.
Figure 1.
Direct, indirect (mediated by duration of gestation), and total effects of dichlorodiphenyltrichloroethane (DDT) and dichlorodiphenyldichloroethylene (DDE) on birth weight (A), birth length (B), and head circumference (C), based on the counterfactual framework approach, among girls (n = 364) participating in the Venda Health Examination of Mothers, Babies and Their Environment, Limpopo Province, South Africa, 2012–2013. Models adjusted for maternal age, education, marital status, smoking, alcohol use during pregnancy, postdelivery body mass index (weight (kg)/height (m)2), human immunodeficiency virus status, household income per capita, assets, child’s sex, parity, delivery method, and season of birth.
Associations between birth outcomes and pyrethroid metabolite concentrations were generally positive among girls and negative among boys, but evidence for effect modification by sex was limited, and estimates were imprecise. We found limited evidence of effect modification by household poverty, malnutrition, or maternal HIV status for DDT/DDE and pyrethroids, although associations between DDT/DDE and birth outcomes were generally more positive among children from poor households and children whose mothers were HIV-positive (Web Tables 9–20).
DISCUSSION
We found that maternal serum concentrations of p,p′-DDT, p,p′-DDE, and o,p′-DDT were associated with elevated birth weight, birth length, and head circumference among South African female infants from an area where IRS is used annually to control malaria, but not among males. Estimated associations with birth weight were notable, with a 10-fold elevation in DDT/DDE being related to increases of 58.4–80.4 g. However, we found limited evidence that maternal urinary concentrations of pyrethroid metabolites were associated with birth outcomes.
Positive associations between DDT/DDE and birth size may be regarded as a favorable outcome in study populations such as ours, which has a high prevalence of low birth weight. On the other hand, studies conducted in Western countries and Africa found that elevated birth weight is associated with higher risks of perinatal mortality (63–65), and authors of meta-analyses reported that higher birth weight and, to a lesser extent, birth length were associated with increased risks of breast cancer in women (66, 67). Elevated exposure to estrogens in utero has been proposed as the possible mechanism of action underlying these associations (67), since estrogens stimulate cell proliferation and numerous studies have found positive associations between exposure to estrogens and both birth size and breast cancer risk (68–74). In other words, elevated birth size may in some cases be a marker for high in utero exposure to estrogen, which is related to adverse health effects later in life. In this context, it is noteworthy that we found that associations with birth size were strongest for o,p′-DDT, the most potently estrogenic DDT isomer, followed by p,p′-DDT, which is also estrogenic (p,p′-DDE is antiandrogenic but not estrogenic) (75). Similarly, maternal serum o,p′-DDT concentrations during pregnancy were more strongly associated with breast cancer risk than concentrations of p,p′-DDT or p,p′-DDE among women participating in the Child Health and Development Study, a birth cohort study that enrolled participants when DDT was still being used in the United States (76).
Elevated birth weight may occur through increased duration of gestation or accelerated intrauterine growth. The fact that we found little evidence that associations between DDT/DDE exposure and measures of birth size were mediated by longer gestation thus suggests that DDT/DDE may accelerate intrauterine growth. This is consistent with our finding of elevated, though imprecise, odds of large-for-gestational-age birth.
Our findings conflict with much of the previous literature, which primarily indicated inverse or null associations between birth size and exposure to DDT or pyrethroids. However, none of the prior studies were conducted in populations in which IRS is practiced. Poverty, malnutrition, and poor health, which are prevalent in these populations, may modify susceptibility to the adverse effects of insecticides. We found some evidence supporting this hypothesis within our study population in that associations between DDT/DDE and birth size variables were systematically stronger among children from poor households or who were exposed to HIV. Although statistical evidence for effect modification by these variables was limited within our study population, effects may differ more greatly between Western and IRS populations because of larger differences in income and health status between these populations than within IRS populations. An alternative explanation may be related to the different survival rates for low–birth-weight infants between Western populations and our study area. Because birth weight data are generally not available for stillbirths, prior studies (including ours) had to restrict their analysis to live infants. However, if elevated serum DDT/DDE levels and low birth weight adversely affected survival, restricting analysis to live infants could induce collider stratification (selection) bias by removing infants with this combination of exposure and birth weight from the study sample, which could result in a positive bias. Because, in our study population, people may be more susceptible to the adverse effects of DDT/DDE and low–birth-weight infants are less likely to survive than in Western populations, this bias may have been stronger in our study population.
This study had several strengths. To our knowledge, this was the first study to investigate associations between exposure to IRS insecticides and birth outcomes in a population living in a malaria-endemic area. Results were robust to a variety of sensitivity analyses and different modeling approaches such as marginal structural models, generalized propensity scores, and standard regression. Although residual confounding remains a possibility, we had data on numerous potential confounders and had complete data on virtually all variables.
This study also had a few limitations. Because women do not typically measure their weight in our study population, we did not have data on prepregnancy weight and thus on pregnancy weight gain, an important determinant of infant birth weight. However, it is unlikely that weight gain would have affected pyrethroid exposure. In addition, we previously showed that weight change was inversely associated with the serum concentration of DDT/DDE (77). Weight gain would thus be expected to act as a negative confounder, so its omission from models should result in an underestimation of associations, which could not explain our results. Because of the short elimination half-life of pyrethroids (approximately 5–13 hours) (78, 79), metabolite concentrations in spot urine samples may not reflect long-term exposure. The literature is inconsistent on this topic, however. Although weak (≤0.21) ICCs were reported for pyrethroid metabolite concentrations in spot urine samples among US adults (60), stronger reproducibility was found among Polish adults (ICC = 0.85) (80). Finally, the possibility of chance findings cannot be excluded, given the large number of tests performed.
In summary, we found that prenatal exposure to the estrogenic DDT isomers o,p′-DDT and p,p′-DDT and, to a lesser extent, p,p′-DDE was related to elevated birth size among girls, including birth weight, birth length, and head circumference. Elevated birth size, particularly if due to exposure to estrogenic chemicals, may be associated with adverse health outcomes, including elevated perinatal mortality and breast cancer risk, but additional research is needed to fully elucidate the implications of our findings.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Biostatistics and Occupational Health, Faculty of Medicine, McGill University, Montreal, Quebec, Canada (Jonathan Chevrier); Center for Environmental Research and Children’s Health, School of Public Health, University of California, Berkeley, Berkeley, California (Stephen Rauch, Fraser Gaspar, Brenda Eskenazi); and Centre for Sustainable Malaria Control, School of Health Systems and Public Health, University of Pretoria, Pretoria, Gauteng, South Africa (Madelein Crause, Muvhulawa Obida, Riana Bornman).
This work was supported by the US National Institute of Environmental Health Sciences (grant R01ES020360; Principal Investigator, B.E.) and was undertaken in part thanks to funding from the Canada Research Chairs Program (J.C.).
We thank the staff and participants of the Venda Health Examination of Mothers, Babies and Their Environment for their dedication and important contributions to this work.
Conflict of interest: none declared.
Abbreviations
- DCCA
(2,2-dichlorovinyl)-2,2-dimethylcyclopropane-1-carboxylic acid
- DDE
dichlorodiphenyldichloroethylene
- DDT
dichlorodiphenyltrichloroethane
- HIV
human immunodeficiency virus
- ICC
intraclass correlation coefficient
- IRS
indoor residual spraying
- 3-PBA
3-phenoxybenzoic acid
- SD
standard deviation
REFERENCES
- 1. World Health Organization World Malaria Report 2016 Geneva, Switzerland: World Health Organization; 2016. [Google Scholar]
- 2. Gaspar FW, Chevrier J, Quirós-Alcalá L, et al. . Levels and determinants of DDT and DDE exposure in the VHEMBE cohort. Environ Health Perspect. 2017;125(7):077006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Whitworth KW, Bornman RM, Archer JI, et al. . Predictors of plasma DDT and DDE concentrations among women exposed to indoor residual spraying for malaria control in the South African Study of Women and Babies (SOWB). Environ Health Perspect. 2014;122(6):545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Environmental Protection Agency Chlorpyrifos Revised Risk Assessment and Agreement With Registrants Washington, DC: Environmental Protection Agency; 2000. [Google Scholar]
- 5. Environmental Protection Agency Diazinon Revised Risk Assessment and Agreement With Registrants Washington, DC: Environmental Protection Agency; 2001. [Google Scholar]
- 6. Palmquist K, Salatas J, Fairbrother A. Pyrethroid insecticides: use, environmental fate, and ecotoxicology In: Perveen FK, ed. Insecticides: Advances in Integrated Pest Management. London, United Kingdom: IntechOpen Ltd.; 2012:251–278. http://www.intechopen.com/books/insecticides-advances-in-integrated-pest-management/pyrethroid-insecticides-use-environmental-fate-and-ecotoxicology. Accessed November 15, 2017. [Google Scholar]
- 7. Arrebola JP, Cuellar M, Bonde JP, et al. . Associations of maternal o,p′-DDT and p,p′-DDE levels with birth outcomes in a Bolivian cohort. Environ Res. 2016;151:469–477. [DOI] [PubMed] [Google Scholar]
- 8. Fenster L, Eskenazi B, Anderson M, et al. . Association of in utero organochlorine pesticide exposure and fetal growth and length of gestation in an agricultural population. Environ Health Perspect. 2006;114(4):597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lopez-Espinosa MJ, Murcia M, Iñiguez C, et al. . Prenatal exposure to organochlorine compounds and birth size. Pediatrics. 2011;128(1):e127–e134. [DOI] [PubMed] [Google Scholar]
- 10. Jusko TA, Koepsell TD, Baker RJ, et al. . Maternal DDT exposures in relation to fetal and 5-year growth. Epidemiology. 2006;17(6):692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kezios KL, Liu X, Cirillo PM, et al. . Dichlorodiphenyltrichloroethane (DDT), DDT metabolites and pregnancy outcomes. Reprod Toxicol. 2013;35:156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Farhang L, Weintraub JM, Petreas M, et al. . Association of DDT and DDE with birth weight and length of gestation in the Child Health and Development Studies, 1959–1967. Am J Epidemiol. 2005;162(8):717–725. [DOI] [PubMed] [Google Scholar]
- 13. Weisskopf MG, Anderson HA, Hanrahan LP, et al. . Maternal exposure to Great Lakes sport-caught fish and dichlorodiphenyl dichloroethylene, but not polychlorinated biphenyls, is associated with reduced birth weight. Environ Res. 2005;97(2):149–162. [DOI] [PubMed] [Google Scholar]
- 14. Wojtyniak BJ, Rabczenko D, Jönsson BA, et al. . Association of maternal serum concentrations of 2,2′,4,4′5,5′-hexachlorobiphenyl (CB-153) and 1,1-dichloro-2,2-bis(p-chlorophenyl)-ethylene (p,p′-DDE) levels with birth weight, gestational age and preterm births in Inuit and European populations. Environ Health. 2010;9:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Al-Saleh I, Al-Doush I, Alsabbaheen A, et al. . Levels of DDT and its metabolites in placenta, maternal and cord blood and their potential influence on neonatal anthropometric measures. Sci Total Environ. 2012;416:62–74. [DOI] [PubMed] [Google Scholar]
- 16. de Cock M, De Boer MR, Lamoree M, et al. . Prenatal exposure to endocrine disrupting chemicals and birth weight—a prospective cohort study. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2016;51(2):178–185. [DOI] [PubMed] [Google Scholar]
- 17. Dewailly E, Bruneau S, Ayotte P, et al. . Health status at birth of Inuit newborn prenatally exposed to organochlorines. Chemosphere. 1993;27(1–3):359–366. [Google Scholar]
- 18. Dewan P, Jain V, Gupta P, et al. . Organochlorine pesticide residues in maternal blood, cord blood, placenta, and breastmilk and their relation to birth size. Chemosphere. 2013;90(5):1704–1710. [DOI] [PubMed] [Google Scholar]
- 19. Gladen BC, Shkiryak-Nyzhnyk ZA, Chyslovska N, et al. . Persistent organochlorine compounds and birth weight. Ann Epidemiol. 2003;13(3):151–157. [DOI] [PubMed] [Google Scholar]
- 20. Govarts E, Nieuwenhuijsen M, Schoeters G, et al. . Birth weight and prenatal exposure to polychlorinated biphenyls (PCBs) and dichlorodiphenyldichloroethylene (DDE): a meta-analysis within 12 European birth cohorts. Environ Health Perspect. 2012;120(2):162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guo H, Jin Y, Cheng Y, et al. . Prenatal exposure to organochlorine pesticides and infant birth weight in China. Chemosphere. 2014;110:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Karmaus W, Zhu X. Maternal concentration of polychlorinated biphenyls and dichlorodiphenyl dichlorethylene and birth weight in Michigan fish eaters: a cohort study. Environ Health. 2004;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ribas-Fitó N, Sala M, Cardo E, et al. . Association of hexachlorobenzene and other organochlorine compounds with anthropometric measures at birth. Pediatr Res. 2002;52(2):163–167. [DOI] [PubMed] [Google Scholar]
- 24. Rogan WJ, Gladen BC, McKinney JD, et al. . Neonatal effects of transplacental exposure to PCBs and DDE. J Pediatr. 1986;109(2):335–341. [DOI] [PubMed] [Google Scholar]
- 25. Sagiv SK, Tolbert PE, Altshul LM, et al. . Organochlorine exposures during pregnancy and infant size at birth. Epidemiology. 2007;18(1):120–129. [DOI] [PubMed] [Google Scholar]
- 26. Tan J, Loganath A, Chong YS, et al. . Exposure to persistent organic pollutants in utero and related maternal characteristics on birth outcomes: a multivariate data analysis approach. Chemosphere. 2009;74(3):428–433. [DOI] [PubMed] [Google Scholar]
- 27. Tyagi V, Garg N, Mustafa MD, et al. . Organochlorine pesticide levels in maternal blood and placental tissue with reference to preterm birth: a recent trend in North Indian population. Environ Monit Assess. 2015;187(7):471. [DOI] [PubMed] [Google Scholar]
- 28. Vafeiadi M, Vrijheid M, Fthenou E, et al. . Persistent organic pollutants exposure during pregnancy, maternal gestational weight gain, and birth outcomes in the mother-child cohort in Crete, Greece (RHEA Study). Environ Int. 2014;64:116–123. [DOI] [PubMed] [Google Scholar]
- 29. Wolff MS, Engel S, Berkowitz G, et al. . Prenatal pesticide and PCB exposures and birth outcomes. Pediatr Res. 2007;61(2):243–250. [DOI] [PubMed] [Google Scholar]
- 30. Casas M, Nieuwenhuijsen M, Martinez D, et al. . Prenatal exposure to PCB-153, p,p′-DDE and birth outcomes in 9000 mother-child pairs: exposure-response relationship and effect modifiers. Environ Int. 2015;74:23–31. [DOI] [PubMed] [Google Scholar]
- 31. Bjerregaard P, Hansen JC. Organochlorines and heavy metals in pregnant women from the Disko Bay area in Greenland. Sci Total Environ. 2000;245(1–3):195–202. [DOI] [PubMed] [Google Scholar]
- 32. Pathak R, Ahmed RS, Tripathi AK, et al. . Maternal and cord blood levels of organochlorine pesticides: association with preterm labor. Clin Biochem. 2009;42(7-8):746–749. [DOI] [PubMed] [Google Scholar]
- 33. Torres-Arreola L, Berkowitz G, Torres-Sánchez L, et al. . Preterm birth in relation to maternal organochlorine serum levels. Ann Epidemiol. 2003;13(3):158–162. [DOI] [PubMed] [Google Scholar]
- 34. Wood SL, Jarrell JJ, Swaby C, et al. . Endocrine disruptors and spontaneous premature labor: a case control study. Environ Health. 2007;6:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Longnecker MP, Klebanoff MA, Zhou H, et al. . Association between maternal serum concentration of the DDT metabolite DDE and preterm and small-for-gestational-age babies at birth. Lancet. 2001;358(9276):110–114. [DOI] [PubMed] [Google Scholar]
- 36. Mustafa M, Garg N, Banerjee BD, et al. . Inflammatory-mediated pathway in association with organochlorine pesticides levels in the etiology of idiopathic preterm birth. Reprod Toxicol. 2015;57:111–120. [DOI] [PubMed] [Google Scholar]
- 37. Procianoy RS, Schvartsman S. Blood pesticide concentration in mothers and their newborn infants: relation to prematurity. Acta Paediatr Scand. 1981;70(6):925–928. [DOI] [PubMed] [Google Scholar]
- 38. Saxena MC, Siddiqui MK, Bhargava AK, et al. . Role of chlorinated hydrocarbon pesticides in abortions and premature labour. Toxicology. 1980;17(3):323–331. [DOI] [PubMed] [Google Scholar]
- 39. Wassermann M, Ron M, Bercovici B, et al. . Premature delivery and organochlorine compounds: polychlorinated biphenyls and some organochlorine insecticides. Environ Res. 1982;28(1):106–112. [DOI] [PubMed] [Google Scholar]
- 40. Brucker-Davis F, Wagner-Mahler K, Bornebusch L, et al. . Exposure to selected endocrine disruptors and neonatal outcome of 86 healthy boys from Nice area (France). Chemosphere. 2010;81(2):169–176. [DOI] [PubMed] [Google Scholar]
- 41. Berkowitz GS, Wetmur JG, Birman-Deych E, et al. . In utero pesticide exposure, maternal paraoxonase activity, and head circumference. Environ Health Perspect. 2004;112(3):388–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ding G, Cui C, Chen L, et al. . Prenatal exposure to pyrethroid insecticides and birth outcomes in rural northern China. J Expo Sci Environ Epidemiol. 2015;25(3):264–270. [DOI] [PubMed] [Google Scholar]
- 43. Zhang J, Yoshinaga J, Hisada A, et al. . Prenatal pyrethroid insecticide exposure and thyroid hormone levels and birth sizes of neonates. Sci Total Environ. 2014;488-489:275–279. [DOI] [PubMed] [Google Scholar]
- 44. DHS Program, US Agency for International Development DHS model questionnaires. Washington, DC: US Agency for International Development; 2012. http://dhsprogram.com/What-We-Do/Survey-Types/DHS-Questionnaires.cfm. Accessed November 15, 2017. [Google Scholar]
- 45. MacIntyre UE, Venter CS, Vorster HH. A culture-sensitive quantitative food frequency questionnaire used in an African population: 2. Relative validation by 7-day weighed records and biomarkers. Public Health Nutr. 2001;4(1):63–71. [DOI] [PubMed] [Google Scholar]
- 46. Blumberg SJ, Bialostosky K, Hamilton WL, et al. . The effectiveness of a short form of the Household Food Security Scale. Am J Public Health. 1999;89(8):1231–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ramchandani PG, Richter LM, Norris SA, et al. . Maternal prenatal stress and later child behavioral problems in an urban South African setting. J Am Acad Child Adolesc Psychiatry. 2010;49(3):239–247. [PubMed] [Google Scholar]
- 48. Statistics South Africa Poverty Trends in South Africa: An Examination of Absolute Poverty Between 2006 and 2011. Pretoria, South Africa: Statistics South Africa; 2014. [Google Scholar]
- 49. Rasmussen KM, Yaktine AL. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: Institute of Medicine and National Research Council; 2009. [PubMed] [Google Scholar]
- 50. Centers for Disease Control and Prevention National Health and Nutrition Examination Survey. Anthropometry Procedures Manual Atlanta, GA: Centers for Disease Control and Prevention;2007. https://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/manual_an.pdf. Accessed November 15, 2017. [Google Scholar]
- 51. Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013;13:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Barr JR, Maggio VL, Barr DB, et al. . New high-resolution mass spectrometric approach for the measurement of polychlorinated biphenyls and organochlorine pesticides in human serum. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;794(1):137–148. [DOI] [PubMed] [Google Scholar]
- 53. Van der Laan MJ, Polley EC, Hubbard AE. Super Learner Berkeley, CA: University of California, Berkeley; 2007. (U.C. Berkeley Division of Biostatistics Working Paper Series, working paper 222). http://biostats.bepress.com/ucbbiostat/paper222. Accessed November 1, 2010. [Google Scholar]
- 54. Hirano K, Imbens G. The propensity score with continuous treatment In: Gelman A, Meng X-L, eds. Applied Bayesian Modelling and Causal Inference From Missing Data. New York, NY: John Wiley & Sons, Inc.; 2004:73–84. [Google Scholar]
- 55. Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. [DOI] [PubMed] [Google Scholar]
- 56. Robins JM, Greenland S. Identifiability and exchangeability for direct and indirect effects. Epidemiology. 1992;3(2):143–155. [DOI] [PubMed] [Google Scholar]
- 57. Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18(2):137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hwang YH, Bornschein RL, Grote J, et al. . Urinary arsenic excretion as a biomarker of arsenic exposure in children. Arch Environ Health. 1997;52(2):139–147. [DOI] [PubMed] [Google Scholar]
- 59. Fortin MC, Bouchard M, Carrier G, et al. . Biological monitoring of exposure to pyrethrins and pyrethroids in a metropolitan population of the province of Quebec, Canada. Environ Res. 2008;107(3):343–350. [DOI] [PubMed] [Google Scholar]
- 60. Morgan MK, Sobus JR, Barr DB, et al. . Temporal variability of pyrethroid metabolite levels in bedtime, morning, and 24-h urine samples for 50 adults in North Carolina. Environ Res. 2016;144(A):81–91. [DOI] [PubMed] [Google Scholar]
- 61. Rosner B. Percentage points for a generalized ESD many-outlier procedure. Technometrics. 1983;25(2):165–172. [Google Scholar]
- 62. Lubin JH, Colt JS, Camann D, et al. . Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect. 2004;112(17):1691–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Basso O, Wilcox AJ, Weinberg CR. Birth weight and mortality: causality or confounding? Am J Epidemiol. 2006;164(4):303–311. [DOI] [PubMed] [Google Scholar]
- 64. Wilcox AJ, Skjaerven R. Birth weight and perinatal mortality: the effect of gestational age. Am J Public Health. 1992;82(3):378–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Abu Habib N, Wilcox AJ, Daltveit AK, et al. . Birthweight, preterm birth and perinatal mortality: a comparison of black babies in Tanzania and the USA. Acta Obstet Gynecol Scand. 2011;90(10):1100–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Xue F, Michels KB. Intrauterine factors and risk of breast cancer: a systematic review and meta-analysis of current evidence. Lancet Oncol. 2007;8(12):1088–1100. [DOI] [PubMed] [Google Scholar]
- 67. Michels KB, Xue F. Role of birthweight in the etiology of breast cancer. Int J Cancer. 2006;119(9):2007–2025. [DOI] [PubMed] [Google Scholar]
- 68. Peck JD, Hulka BS, Savitz DA, et al. . Accuracy of fetal growth indicators as surrogate measures of steroid hormone levels during pregnancy. Am J Epidemiol. 2003;157(3):258–266. [DOI] [PubMed] [Google Scholar]
- 69. Hickey M, Hart R, Keelan JA. The relationship between umbilical cord estrogens and perinatal characteristics. Cancer Epidemiol Biomarkers Prev. 2014;23(6):946–952. [DOI] [PubMed] [Google Scholar]
- 70. Gardner MO, Goldenberg RL, Cliver SP, et al. . Maternal serum concentrations of human placental lactogen, estradiol and pregnancy specific beta 1-glycoprotein and fetal growth retardation. Acta Obstet Gynecol Scand Suppl. 1997;165:56–58. [PubMed] [Google Scholar]
- 71. Gerhard I, Fitzer C, Klinga K, et al. . Estrogen screening in evaluation of fetal outcome and infant’s development. J Perinat Med. 1986;14(5):279–291. [DOI] [PubMed] [Google Scholar]
- 72. Loriaux DL, Ruder HJ, Knab DR, et al. . Estrone sulfate, estrone, estradiol and estriol plasma levels in human pregnancy. J Clin Endocrinol Metab. 1972;35(6):887–891. [DOI] [PubMed] [Google Scholar]
- 73. Markestad T, Bergsjø P, Aakvaag A, et al. . Prediction of fetal growth based on maternal serum concentrations of human chorionic gonadotropin, human placental lactogen and estriol. Acta Obstet Gynecol Scand Suppl. 1997;165:50–55. [PubMed] [Google Scholar]
- 74. Petridou E, Panagiotopoulou K, Katsouyanni K, et al. . Tobacco smoking, pregnancy estrogens, and birth weight. Epidemiology. 1990;1(3):247–250. [DOI] [PubMed] [Google Scholar]
- 75. Agency for Toxic Substances and Disease Registry, US Public Health Service Toxicological Profile for DDT, DDE and DDD. Atlanta, GA: Agency for Toxic Substances and Disease Registry; 2002. [PubMed] [Google Scholar]
- 76. Cohn BA, La Merrill M, Krigbaum NY, et al. . DDT exposure in utero and breast cancer. J Clin Endocrinol Metab. 2015;100(8):2865–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chevrier J, Dewailly E, Ayotte P, et al. . Body weight loss increases plasma and adipose tissue concentrations of potentially toxic pollutants in obese individuals. Int J Obes Relat Metab Disord. 2000;24(10):1272–1278. [DOI] [PubMed] [Google Scholar]
- 78. International Programme on Chemical Safety Deltamethrin (Environmental health criteria 97). Geneva, Switzerland: World Health Organization; 1990. http://www.inchem.org/documents/ehc/ehc/ehc97.htm. Accessed April 4, 2017. [Google Scholar]
- 79. Ratelle M, Coté J, Bouchard M. Time profiles and toxicokinetic parameters of key biomarkers of exposure to cypermethrin in orally exposed volunteers compared with previously available kinetic data following permethrin exposure. J Appl Toxicol. 2015;35(12):1586–1593. [DOI] [PubMed] [Google Scholar]
- 80. Wielgomas B. Variability of urinary excretion of pyrethroid metabolites in seven persons over seven consecutive days—implications for observational studies. Toxicol Lett. 2013;221(1):15–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.