Abstract
The association between a single interpregnancy interval (IPI) and birth outcomes has not yet been explored using matched methods. We modeled the odds of preterm birth, being small for gestational age, and having low birth weight in a second, live-born infant in a cohort of 192,041 sibling pairs born in Western Australia between 1980 and 2010. The association between IPI and birth outcomes was estimated from the interaction between birth order and IPI (with 18–23 months as the reference category), using conditional logistic regression. Matched analysis showed the odds of preterm birth were higher for siblings born following an IPI of <6 months (adjusted interaction odds ratio = 1.22, 95% confidence interval: 1.06, 1.38) compared with those born after an IPI of 18–23 months. There were no significant differences for IPIs of <6 months for other outcomes (small for gestational age or low birth weight). This is the first study to use matched analyses to investigate the association between a single IPI on birth outcomes. IPIs of <6 months were associated with increased odds of preterm birth in second-born infants, although the association is likely smaller than previously estimated by unmatched studies.
Keywords: birth intervals, family planning, pregnancy outcome, preterm birth, siblings
Editor’s note: An invited commentary on this article appears on page 000, and the authors’ response appears on page 22.
Adverse birth outcomes remain a leading cause of infant death, neonatal morbidity, and childhood illness in high-income countries (1). Epidemiologic studies have shown that the interval between pregnancies could be linked to length of gestation, fetal growth, and birth weight (2). Notably, intervals of <6 months have been associated with increased odds of preterm birth (3, 4), low birth weight (2), infants being small for gestational age (5), congenital anomalies (5), and perinatal death (4, 6). Considering this, the World Health Organization recommends at least 2 years between pregnancies in order to minimize the risk of adverse perinatal outcomes (7).
While several hypotheses have been proposed, including nutritional depletion and anemia after giving birth (7, 8), a causal mechanism for the association between birth spacing and adverse birth outcomes has not yet been confirmed. It is also unclear how much of the association between interpregnancy interval (IPI) and perinatal outcomes is causal and how much is due to other factors associated with interpregnancy interval resulting in confounding. IPI is correlated with many potential confounders, including socioeconomic status, age, obstetrical history, and race/ethnicity (9, 10), all of which relate to maternal characteristics. For example, in the United States, short IPIs are more common among mothers with higher education and non-Hispanic black mothers (10).
Previous research into the association between IPI and birth outcomes has relied largely on traditional retrospective cohort studies (2, 4–6). This raises the possibility that, despite efforts to adjust for confounders, the association between IPI and birth outcomes could be induced by unmeasured or poorly measured maternal characteristics rather than being caused by the interval itself. Several recent studies have addressed the potential for unmeasured confounding by applying a sibling-matched (also known as “maternally matched”) design (11, 12). These studies have controlled for maternal factors that remain constant between pregnancies, such as genetic factors and some aspects of lifestyle, by comparing 2 IPIs per mother. For all 3 studies that applied a matched design, effect estimates were attenuated in comparison with results from an unmatched design, implying that there could have been consistent, unmeasured confounding occurring (3, 11, 12).
A key limitation of the sibling-matched designs applied previously is that they use information on 2 or more consecutive pregnancies per mother in order to provide the 2 IPIs per mother required for matching. Considering that 35%–42% of multiparous women in Australia and other high-income countries have only 2 children (13, 14), and maternal characteristics are known to vary by parity (15), the results of previous matched studies might not be representative of the entire population of multiparous women. This study aimed to investigate the association between the first IPI and birth outcomes using a sibling-matched design and to compare these results with a traditional unmatched cohort analysis.
METHODS
We created a retrospective cohort of first- and second-born singleton births in Western Australia between 1980 and 2010. We aimed to measure the association between adverse birth outcomes and the interval between first and second live-born pregnancies, accounting for individual predisposition to these outcomes.
Data source and definitions
Maternal and infant information was derived from the Midwives Notification System, a statutory data collection of all births in Western Australia of ≥20 weeks’ gestation. This data collection covers >99% of births in Western Australia (16). For consistency with previously published studies (3, 11, 12), we restricted the data set to include consecutive live-born singleton infants. Date of birth, infant weight, gestation, and sex are variables included in this data collection. The accuracy of these variables is estimated to exceed 98% (16). IPI was defined as the time between birth of the first infant and estimated conception of the second infant. Consistent with previous studies (3, 11, 12), intervals were grouped into 7 categories: <6 months, 6–11 months, 12–17 months, 18–23 months, 24–59 months, and ≥60 months, with 18–23 months as the reference interval. Birth outcomes included preterm birth (gestation of <37 weeks), low birth weight (<2,500 grams), and being small for gestational age (<10th percentile for birth weight according to sex and gestational age, based on the birth-weight distribution in 5-calendar-year blocks). We performed additional supplemental analyses that accounted for categories of preterm birth, including spontaneous and iatrogenic, moderate (gestational age of 33–36 weeks), very (gestational age of 28–32 weeks), and extreme (gestational age of <28 weeks) preterm birth. Information on potential confounding factors—including maternal age, race/ethnicity (white or nonwhite), and residence (Perth metropolitan or nonmetropolitan), as recorded by the medical professional attending the birth—was also ascertained. Socioeconomic status was derived from the mother’s Statistical Local Area of residence based on the Index of Relative Socioeconomic Advantage and Disadvantage provided by Australian Bureau of Statistics. These scores were grouped by quintiles relative to the population in Western Australia (17).
Statistical analysis
Characteristics of women were compared according to first IPI, using χ2 tests for independence. We then used a sibling-matched design that requires only a single IPI per mother, based on a method previously applied by Cheslack-Postava et al. (18),which measured the association between IPI and the incidence of autism. In traditional cohort studies, the first birth would not be included in the model and is therefore noninformative. However, in a sibling-matched analysis, information from the first birth is included in the model, and a conditional logistic regression model can be used to estimate the association between IPI and outcomes in the second birth accounting for covariate information within clusters (19). In a sibling-matched analysis, more information on the mother is included, which can allow for more comprehensive adjustment of maternal factors.
In our traditional cohort analysis, which does not account for sibling pairs, we estimated the unmatched odds of adverse birth outcomes in the second birth as a function of IPI category, using logistic regression. As part of this unmatched model, we adjusted for age, race/ethnicity, socioeconomic status, and birth year. The statistical model is given as where
In this model, only second births are of interest; the first birth does not contribute any information. is equal to 1 if the second birth from birth pair i results in the adverse outcome of interest; n is the total number of birth pairs in the study; is the probability that the second birth from pair i results in the adverse outcome of interest; is a vector of covariates specific to the second birth of pair i including an intercept term, maternal age at delivery, maternal race/ethnicity, maternal socioeconomic status, and year of birth; and β is a vector of unknown regression coefficients describing the association between the covariates and the probability of an adverse outcome.
For the sibling-matched design, we applied a conditional logistic regression model that estimated the odds of adverse birth outcomes in the second birth with an interaction term for IPI and birth order. We adjusted for maternal age, birth year, and socioeconomic status, as these factors can potentially vary over time. This statistical model is given as , where
is equal to 1 if birth j (j = 1, first birth; j = 2, second birth) from birth pair i results in the adverse outcome of interest; nd is the total number of discordant birth pairs in the study (i.e., exactly 1 birth in the pair resulted in an adverse outcome); is the birth-pair-specific intercept; is the probability that birth j from pair i results in the adverse outcome of interest; is a vector of covariates specific to birth j of pair i including maternal age at delivery, maternal race/ethnicity, maternal socioeconomic status, and year of birth; β is a vector of unknown regression coefficients describing the association between the covariates and the probability of an adverse outcome; and λ is an unknown regression parameter describing the association between second births and the adverse outcome of interest.
In both models, the association between IPI for birth pair i (; measured in months) and the probability of an adverse outcome for the second birth is described by the unknown parameters, . IPI is modeled as a categorical variable where and months; where months represents the reference category; and is the indicator function taking a value of 1 if the input statement is true and the value of zero if the input statement is false. In the sibling-matched design model, we ensure that only second births are used to estimate the IPI associations through use of the indicator function in the IPI formula, .
Analyses were performed using SAS, version 9.4 (SAS Institute, Inc., Cary, North Carolina). For more information, see Web Appendix 1 (available at https://academic.oup.com/aje).
Supplemental analysis
To explore the potential influence of random measurement error, which would have occurred more commonly during the earlier years of the cohort (e.g., when there was a lower likelihood that births had ultrasound-confirmed gestational age), we performed supplementary analyses, in which our matched analysis of sibling pairs was restricted to those born after 1995. To allow further comparison with matched analyses, an unmatched analysis restricted to discordant sibling pairs was also performed.
Ethical approval
This study was approved by the Department of Health Western Australia Human Research Ethics Committee (RA 2011/64) and the Curtin University Human Research Ethics Committee (RA RDHS-30-16).
RESULTS
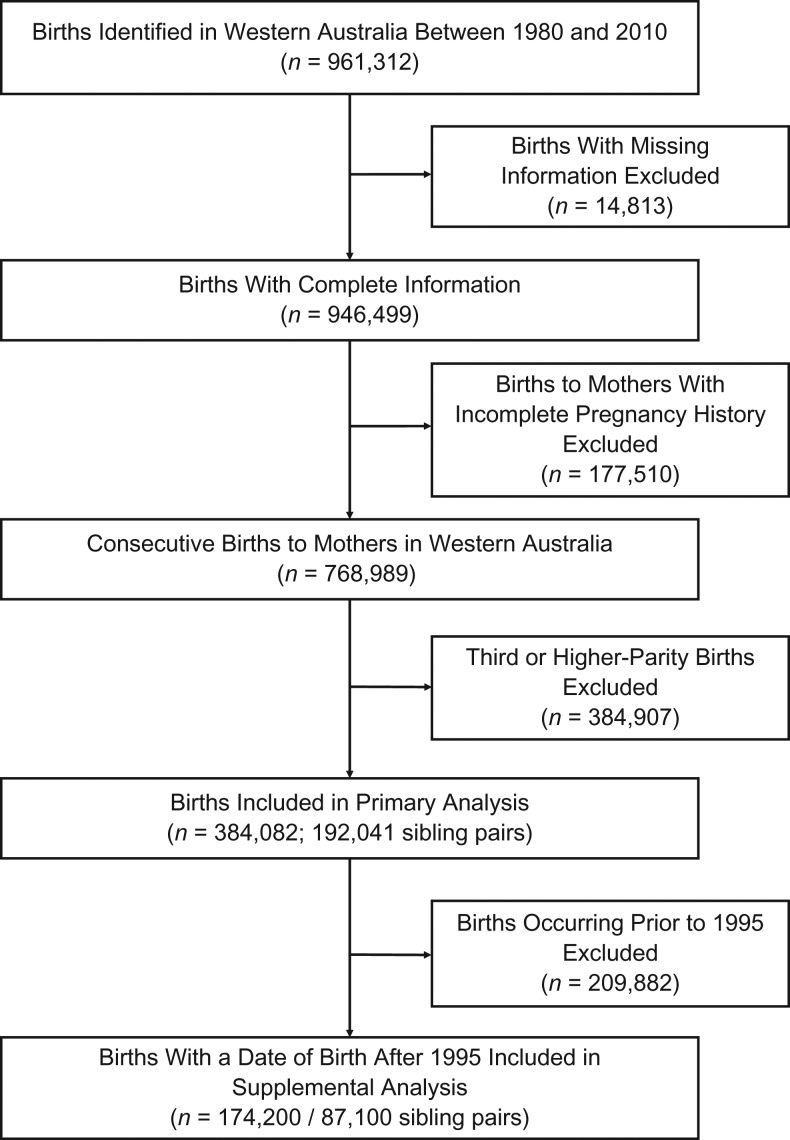
In Western Australia, a total of 961,312 live singleton births were identified with a date of birth between 1980 and 2010. Of these, 946,499 had complete information on maternal and birth characteristics; 177,510 nonconsecutive births were excluded, leaving 768,989 consecutive live births for analysis. Of these, we identified 192,041 first- and second-born sibling pairs (Figure 1).
Figure 1.
Study flow chart for selection of first- and second-born infants from birth records in Western Australia, 1980–2010. Births were excluded if they were missing maternal age; infant’s sex, birth weight, or gestational age; or mother’s socioeconomic status or residence.
IPI between first and second birth
There was considerable variation in maternal characteristics according to IPI between the first and second pregnancy (Table 1). One-third of second-born infants (34%) were born 24–59 months after their sibling. A small percentage of second-born infants were born after either shorter IPIs of <6 months (3%) or longer IPIs of ≥60 months (8%). Short IPIs (<6 months) were more frequently observed for mothers who were younger at first birth (<20 years) (Table 1). IPIs of 18–23 months and 24–59 months were most frequently observed for women with the greatest relative socioeconomic advantage and least frequently for women with the greatest relative socioeconomic disadvantage (P < 0.001). In general, higher parity was associated with shorter IPIs, and lower parity was associated with longer IPIs (P < 0.001).
Table 1.
Characteristics of Women According to the Interval Between First and Second Live, Singleton Births (n = 192,041), Western Australia, 1980–2010
| Characteristic | Interpregnancy Interval | P Value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <6 Months | 6–11 Months | 12–17 Months | 18–23 Months | 24–59 Months | ≥60 Months | ||||||||
| No. | Row % | No. | Row % | No. | Row % | No. | Row % | No. | Row % | No | Row % | ||
| All | 5,871 | 3 | 29,158 | 15 | 42,756 | 22 | 34,405 | 18 | 64,926 | 34 | 14,925 | 8 | |
| Age at first birth, years | <0.001 | ||||||||||||
| 14–19 | 1,354 | 5 | 3,648 | 15 | 4,049 | 16 | 3,224 | 13 | 8,727 | 35 | 4,141 | 16 | |
| 20–24 | 2,115 | 4 | 8,634 | 16 | 11,599 | 21 | 9,205 | 17 | 18,142 | 33 | 5,622 | 10 | |
| 25–29 | 1,499 | 2 | 9,865 | 15 | 15,967 | 24 | 13,084 | 19 | 23,231 | 34 | 3,717 | 6 | |
| 30–34 | 692 | 2 | 5,463 | 15 | 8,997 | 25 | 7,226 | 20 | 12,383 | 34 | 1,291 | 4 | |
| ≥35 | 211 | 3 | 1,548 | 19 | 2,144 | 26 | 1,666 | 20 | 2,443 | 30 | 154 | 2 | |
| Race/ethnicitya | 0.243 | ||||||||||||
| White | 4,716 | 3 | 25,686 | 15 | 38,702 | 23 | 31,324 | 18 | 57,360 | 34 | 12,911 | 7 | |
| Nonwhite | 1,155 | 5 | 3,472 | 16 | 4,054 | 19 | 3,081 | 14 | 7,566 | 36 | 2,014 | 9 | |
| Socioeconomic statusb | <0.001 | ||||||||||||
| Lowest 20% | 522 | 4 | 2,050 | 16 | 2,908 | 23 | 2,170 | 17 | 3,931 | 31 | 939 | 8 | |
| 20%–39% | 538 | 3 | 2,484 | 16 | 3,524 | 23 | 2,700 | 17 | 4,946 | 32 | 1,241 | 8 | |
| 40%–59% | 1,161 | 4 | 5,078 | 16 | 7,018 | 22 | 5,660 | 17 | 10,690 | 33 | 2,820 | 9 | |
| 60%–79% | 2,132 | 3 | 10,209 | 15 | 14,817 | 21 | 12,316 | 18 | 23,959 | 35 | 5,618 | 8 | |
| Highest 20% | 1,518 | 2 | 9,337 | 15 | 14,489 | 23 | 11,559 | 18 | 21,400 | 34 | 4,307 | 7 | |
| Residence | <0.001 | ||||||||||||
| Metropolitan | 4,030 | 3 | 20,394 | 15 | 30,201 | 22 | 24,833 | 18 | 47,925 | 35 | 10,727 | 8 | |
| Nonmetropolitan | 1,841 | 3 | 8,764 | 16 | 12,555 | 23 | 9,572 | 18 | 17,001 | 32 | 4,198 | 8 | |
| No. of children | <0.001 | ||||||||||||
| 2 | 2,745 | 2 | 15,495 | 13 | 25,221 | 21 | 22,133 | 18 | 45,810 | 38 | 10,404 | 8 | |
| 3 | 1,809 | 4 | 8,843 | 18 | 12,524 | 25 | 9,167 | 18 | 14,334 | 28 | 3,506 | 7 | |
| ≥4 | 1,317 | 7 | 4,820 | 24 | 5,011 | 25 | 3,105 | 15 | 4,782 | 24 | 1,015 | 5 | |
a Race/ethnicity was defined as white or nonwhite: nonwhite included Aboriginal and Torres Strait Islander, Asian, Indian, black, Polynesian, Maori, and other races/ethnicities.
b Socioeconomic status was defined based on the Statistical Local Area of the mother and the Socioeconomic Index for Areas (SEIFA) score for relative advantage and disadvantage produced by the Australian Bureau of Statistics (17).
Unmatched, cohort study results
Of the 192,041 second-born, live singleton births, a total of 24,375 (13%) infants had an outcome of interest: 10,530 (5%) were born preterm, 14,574 (8%) were small for gestational age, and 6,849 (4%) had low birth weight. Of the preterm births, 4,097 (39%) were iatrogenic, and 6,433 (61%) were spontaneous. Compared with infants born 18–23 months after the first sibling, infants born after shorter IPIs (<12 months after the first sibling) had greater odds of preterm birth, with the highest odds of preterm birth associated with the shortest IPIs (<6 months: adjusted odds ratio (aOR) = 1.67, 95% confidence interval (CI): 1.50, 1.85) (Table 2). While we observed similar results for spontaneous preterm births, we did not observe this association for iatrogenic preterm births (Web Table 1). The strongest association between short IPI (<6 months) and preterm birth was observed for very preterm births (aOR = 2.54, 95% CI: 1.95, 3.31).
Table 2.
Odds of Adverse Birth Outcomes in Second-Born Child as Estimated by an Unmatched Cohort Study, According to Interpregnancy Interval Between First and Second Consecutive Live Births (n = 192,041), Western Australia, 1980–2010
| Birth Outcome and Interpregnancy Interval, months | No. of Sibling Pairs | Second Born With Outcome | ORa | 95% CIa | Adjusted ORb | 95% CIb | |
|---|---|---|---|---|---|---|---|
| No. | % | ||||||
| Preterm birth | |||||||
| <6 | 5,871 | 516 | 8.8 | 1.90 | 1.71, 2.10 | 1.67 | 1.50, 1.85 |
| 6–11 | 29,158 | 1,608 | 5.5 | 1.15 | 1.08, 1.22 | 1.10 | 1.03, 1.18 |
| 12–17 | 42,756 | 2,028 | 4.7 | 0.98 | 0.92, 1.05 | 0.97 | 0.91, 1.04 |
| 18–23 | 34,405 | 1,664 | 4.8 | 1.00 | Referent | 1.00 | Referent |
| 24–59 | 64,926 | 3,585 | 5.5 | 1.15 | 1.08, 1.22 | 1.14 | 1.07, 1.21 |
| ≥60 | 14,925 | 1,129 | 7.6 | 1.61 | 1.49, 1.74 | 1.61 | 1.49, 1.74 |
| Small for gestational age | |||||||
| <6 | 5,871 | 484 | 8.2 | 1.20 | 1.09, 1.33 | 0.96 | 0.87, 1.07 |
| 6–11 | 29,158 | 2,093 | 7.2 | 1.03 | 0.97, 1.10 | 0.95 | 0.90, 1.01 |
| 12–17 | 42,756 | 2,924 | 6.8 | 0.98 | 0.93, 1.04 | 0.96 | 0.91, 1.01 |
| 18–23 | 34,405 | 2,395 | 7.0 | 1.00 | Referent | 1.00 | Referent |
| 24–59 | 64,926 | 5,075 | 7.8 | 1.13 | 1.08, 1.19 | 1.14 | 1.08, 1.20 |
| ≥60 | 14,925 | 1,603 | 10.7 | 1.61 | 1.51, 1.72 | 1.72 | 1.61, 1.84 |
| Low birth weight | |||||||
| <6 | 5,871 | 325 | 5.5 | 1.89 | 1.67, 2.15 | 1.51 | 1.32, 1.71 |
| 6–11 | 29,158 | 1,029 | 3.5 | 1.18 | 1.08, 1.29 | 1.09 | 1.00, 1.19 |
| 12–17 | 42,756 | 1,265 | 3.0 | 0.99 | 0.91, 1.07 | 0.96 | 0.89, 1.05 |
| 18–23 | 34,405 | 1,032 | 3.0 | 1.00 | Referent | 1.00 | Referent |
| 24–59 | 64,926 | 2,362 | 3.6 | 1.22 | 1.13, 1.31 | 1.22 | 1.13, 1.31 |
| ≥60 | 14,925 | 836 | 5.6 | 1.92 | 1.75, 2.11 | 2.02 | 1.84, 2.22 |
Abbreviations: CI, confidence interval; OR, odds ratio.
a OR and corresponding 95% CI based on logistic regression.
b Adjusted for age, race/ethnicity, socioeconomic status, and calendar year of birth.
Low birth weight was similarly associated with the shortest IPIs (<6 months: aOR = 1.51, 95% CI: 1.32, 1.71; and 6–11 months: aOR = 1.09, 95% CI: 1.00, 1.19). Infants born after IPIs of ≥24 months also had greater odds of preterm birth (24–59 months: aOR = 1.14, 95% CI: 1.07, 1.21; and ≥60 months: aOR = 1.61, 95% CI: 1.49, 1.74) and low birth weight (24–59 months: aOR = 1.22, 95% CI: 1.13, 1.31; and ≥60 months: aOR = 2.02, 95% CI: 1.84, 2.22) compared with infants born after an IPI of 18–23 months. Infants born after long IPIs (≥60 months) had greater odds of being born small for gestational age compared with infants born after an IPI of 18–23 months (≥60 interval: aOR = 1.72, 95% CI: 1.61, 1.84). Shorter IPIs (<18 months) were not associated with any differences in the odds of being small for gestational age (Table 2).
Matched, sibling-pair study results
In the matched analysis of sibling pairs, the odds of preterm birth among second-born siblings was significantly greater among siblings born after a short IPI (<6 months) or long IPI (≥60 months) in comparison with the odds of preterm birth among siblings born after an IPI of 18–23 months (<6 months: odds ratio for interaction (IOR) = 1.22, 95% CI: 1.06, 1.38; ≥60 months: IOR = 1.73, 95% CI: 1.58, 1.89) (P for interaction < 0.001) (Table 3). We observed no major differences in the odds of preterm birth for other IPIs in comparison with the odds of preterm birth for the reference category of IPI of 18–23 months.
Table 3.
Odds of Adverse Birth Outcomes in Second-Born Child as Estimated by Matched Sibling-Pair Analysis, According to Interpregnancy Interval Between First and Second Consecutive Live Births (n = 192,041), Western Australia, 1980–2010
| Birth Outcome and Interpregnancy Interval, months | No. of Sibling Pairs | No. of Discordant Pairs in Analysis | Crude IOR | Adjusted IORa | ||
|---|---|---|---|---|---|---|
| IOR | 95% CI | IOR | 95% CI | |||
| Preterm birth | ||||||
| <6 | 5,871 | 799 | 1.25 | 1.09, 1.41 | 1.22 | 1.06, 1.38 |
| 6–11 | 29,158 | 2,772 | 1.10 | 0.97, 1.21 | 1.08 | 0.98, 1.19 |
| 12–17 | 42,756 | 3,526 | 1.11 | 1.01, 1.21 | 1.10 | 1.00, 1.20 |
| 18–23 | 34,405 | 2,944 | 1.00 | Referent | 1.00 | Referent |
| 24–59 | 64,926 | 6,118 | 1.07 | 0.98, 1.16 | 1.09 | 1.00, 1.18 |
| ≥60 | 14,925 | 1,681 | 1.59 | 1.47, 1.71 | 1.73 | 1.58, 1.89 |
| Small for gestational age | ||||||
| <6 | 5,871 | 934 | 0.98 | 0.83, 1.14 | 1.01 | 0.86, 1.16 |
| 6–11 | 29,158 | 3,773 | 0.99 | 0.90, 1.08 | 1.01 | 0.92, 1.11 |
| 12–17 | 42,756 | 5,376 | 1.03 | 0.95, 1.12 | 1.05 | 0.96, 1.13 |
| 18–23 | 34,405 | 4,460 | 1.00 | Referent | 1.00 | Referent |
| 24–59 | 64,926 | 9,190 | 1.04 | 0.96, 1.11 | 0.99 | 0.91, 1.07 |
| ≥60 | 14,925 | 2,517 | 1.46 | 1.36, 1.56 | 1.21 | 1.08, 1.34 |
| Low birth weight | ||||||
| <6 | 5,871 | 599 | 1.00 | 0.81, 1.19 | 1.00 | 0.81, 1.29 |
| 6–11 | 29,158 | 1,971 | 1.10 | 0.97, 1.23 | 1.11 | 0.98, 1.24 |
| 12–17 | 42,756 | 2,471 | 1.06 | 0.93, 1.18 | 1.06 | 0.93, 1.18 |
| 18–23 | 34,405 | 2,019 | 1.00 | Referent | 1.00 | Referent |
| 24–59 | 64,926 | 4,653 | 1.02 | 0.91, 1.13 | 1.00 | 0.87, 1.11 |
| ≥60 | 14,925 | 1,334 | 1.62 | 1.47, 1.76 | 1.52 | 1.34, 1.70 |
Abbreviations: CI, confidence interval; IOR, interaction odds ratio.
a Odds of second-born child being born with outcome under analysis compared with odds of first being born with same outcome; IOR and corresponding 95% CI adjusted for maternal age, socioeconomic status, and calendar year of birth, where an interval of 18–23 months was the reference category.
When we examined the association between IPI and categories of preterm birth, we found siblings born after a short IPI (<6 months) had higher odds of spontaneous preterm birth (IOR = 1.43, 95% CI: 1.23, 1.62) and preterm birth at a gestational age of 33–36 weeks (IOR = 1.37, 95% CI: 1.19, 1.55); however, there was no association between short IPI (<6 months) and iatrogenic preterm birth or between short IPI and very or extreme preterm birth (Web Table 2). Long IPI (≥60 months) was consistently associated with increased odds of all categories of preterm birth.
Siblings born after an IPI of ≥60 months had greater odds of being small for gestational age (IOR = 1.21, 95% CI: 1.08, 1.34) compared with siblings born after an IPI of 18–23 months (P for interaction = 0.01). We observed no difference in the relative odds of being small for gestational age for shorter IPIs. The odds of low birth weight in second-born siblings was significantly greater among siblings born after an IPI of ≥60 months (IOR = 1.52, 95% CI: 1.34, 1.70) compared with siblings born after an 18–23 months IPI (P for interaction < 0.001). No other difference was observed in the odds of low birth weight for siblings following shorter IPIs (Table 3).
Supplemental analysis
For comparison, results of unmatched analyses restricted to the sample of discordant sibling pairs are presented in Web Table 3.
When we restricted the cohort to siblings with a date of birth after 1995 (n = 87,100), we observed similar results to those of the primary analysis (Web Table 4). However, the confidence intervals for the adjusted interaction odds ratio measuring the association between short IPI (<6 months) and preterm birth crossed the null (IOR = 1.19, 95% CI: 0.96, 1.42; P = 0.13).
DISCUSSION
Our study shows that short IPIs between first and second pregnancies are associated with higher odds of preterm birth, specifically spontaneous preterm birth at 33–36 weeks, but not with infants being small for gestational age or having low birth weight. To our knowledge, this is the first study using matched analysis to examine the association between a single IPI occurring between first and second live-born siblings and birth outcomes. This sibling-matched design offers several benefits in that: 1) Information from the first birth is informative to the model; and 2) mothers who do not go on to have a third birth can be included in the analysis, extending the generalizability of study findings to all multiparous women.
There are several strengths to our study. First, because the epidemiologic method we employed allowed us to include a first birth in the model, we had additional information available in the analysis and were potentially able to restrict some residual confounding present when only the second birth is considered. If uncontrolled, such confounding could artificially inflate risk estimates relating to the IPI (3, 11, 12). This is an important consideration, given that our study, as well as previous studies (20, 21), found that women with shorter IPIs were more likely to be in sociodemographic groups that have an increased likelihood of adverse birth outcomes, even at first birth. Women with prior adverse birth outcomes are at greater risk of a future adverse outcome compared with women with prior healthy birth outcomes (22). While these findings underscore the importance of well-controlled analyses for reliable measurement of the impact of IPI, to our knowledge no previous epidemiologic investigation has used a matched study design to explore the association of the first IPI exclusively. In addition, the coverage and validity of our data source is considered high (16). As a result, our study would have included nearly all births to women with 2 or more live, consecutive singleton births with ≥20 weeks’ gestation in Western Australia between 1980 and 2010.
Our matched analysis, which would have restricted uncontrolled confounding, identified attenuated estimates of the association between IPI and birth outcomes in comparison with unmatched analysis. Using the traditional approach of unmatched models, we observed a 1.5–2-fold increase in the odds of preterm birth and low birth weight associated with an IPI of <12 months in unmatched models compared with an IPI of 18–23 months. In contrast, our results from matched models showed reduced nonsignificant associations between IPIs of <12 months and all these birth outcomes, with exception of a 1.2-fold increase in the odds of preterm birth associated with the shortest IPIs (<6 months) and preterm birth. This attenuated association has been documented in previously published studies. Unmatched analyses elsewhere have reported similar results to those of our unmatched analysis, documenting a 2-fold increase in the risk of preterm birth (3, 11, 12, 21, 23), and a 1.3–1.7-fold increase in the odds of low birth weight (21) and infants being small for gestational age (5) associated with IPI of <6 months (3, 5, 21, 23). They have also shown that intervals ≥60 months are associated with a 1.2–1.5-fold increase in the odds of preterm birth (3, 11, 12) and a 1.3–1.9-fold increase in the odds of being born small for gestational age (11, 12) or with low birth weight (11, 12). Previous studies using matched analyses have consistently shown that the associations between IPI and preterm birth, being small for gestational age, and having low birth weight are attenuated compared with unmatched studies (3, 11, 12), thus suggesting that these measured associations might be due in part to unmeasured confounding. To date, only 3 matched studies have been conducted, 2 of which identified no significant increase in the risk of these adverse birth outcomes for births following intervals of <12 months compared with intervals of 18–23 months (11, 12). The third matched study showed a 1.2-fold increase in the risk of preterm birth when there was less than 6 months between pregnancies, an estimate similar to our own (3).
The potential impact of long IPIs on subsequent birth outcomes has been less commonly explored than that of short IPIs (24). Although the associations between long IPI and birth outcomes were mostly attenuated in matched analyses, we consistently observed higher odds of all adverse birth outcomes for long IPI (≥60 months) in our matched models. These findings would be consistent with some of those from previous matched studies (11, 12). However, the evidence in this area is mixed, with inconsistent results published from matched studies. It is important to note that unlike short IPIs, long IPIs are much more prone to measurement error. Miscarriages and abortions are inherently difficult to capture in population-based studies, and the likelihood that these events occurred between pregnancies and are not accounted for in analyses is greatest for long IPIs. This would introduce some measurement error in the exposure variable for longer IPIs (24). While we were able to account for stillbirths in our cohort and restrict our analyses to consecutive live births, we were not able to account for earlier pregnancy loss. Studies that can comprehensively measure pregnancy outcomes following a previous live birth would be useful for better evaluating the health impacts of long IPIs.
There are several other potential limitations to our study. First, there were a small number of sibling pairs with IPIs of ≥120 months between pregnancies, which made evaluation of the impact of very long intervals on perinatal health impossible due to poorly powered analysis. Second, for consistency with the current World Health Organization recommendations (7) and previously published studies, we restricted our data set to live, singleton consecutive births, and thus these results might not apply to women with a fetal death at any gestational age between births. Given that there is currently no recommendation for the optimal IPI following a stillbirth (7), future research on IPI should aim to include stillbirths. Third, although the sibling-matched design would have restricted time-invariant confounders, such as race/ethnicity and chronic medical conditions, it does not restrict some important time-varying confounders, such as maternal age and changes in risk behavior. We have attempted to control for such confounders as adjustment variables in our models; however, we cannot discount the possibility that some temporal confounders (e.g., smoking cessation, interpregnancy weight gain), which we were unable to measure, could have introduced residual confounding. Finally, an assumption of our approach was that the probability of the outcomes of the first and second birth are exchangeable conditional on the adjustment variables. The presence of unmeasured temporal confounders would violate this assumption. A related limitation is that parity, maternal age, and birth year increase monotonically in time. This collinearity might induce additional uncertainty in the observed effect estimates.
For planning pregnancies, it is important to consider that IPIs of <18 months might be associated with risk of preterm birth. Preterm birth rates are increasing in most countries (25), and strategies for preventing preterm birth are a high priority. Our findings are potentially valuable to clinicians when counseling around family planning and for families considering a second pregnancy. In our study and others (3, 11, 12), the observed optimal IPI for infant health is 18–23 months, suggesting that the current recommendation for at least 2 years between pregnancies for women in a high-income countries (7) might be too long.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: School of Public Health, Curtin University, Bentley, Western Australia, Australia (Annette K. Regan, Eva Malacova, Gavin Pereira); School of Nursing, Midwifery and Paramedicine, Curtin University, Bentley, Western Australia, Australia (Stephen J. Ball); School of Public Health, Yale University, New Haven, Connecticut (Joshua L. Warren); Department of Obstetrics, Gynecology and Reproductive Sciences, University of California San Francisco, San Francisco, California (Amy Padula); Department of Public Health, Environments and Society, London School of Hygiene and Tropical Medicine, London, United Kingdom (Cicely Marston); Sydney School of Public Health, University of Sydney, Camperdown, New South Wales, Australia (Natasha Nassar); and Telethon Kids Institute, University of Western Australia, Nedlands, Western Australia, Australia (Fiona Stanley, Helen Leonard, Nicholas de Klerk).
This work was supported by funding from the National Health and Medical Research Council (grant APP1099655). Funding was received from a Sidney Sax Fellowship (grant 1052236 to G.P.), a Career Development Fellowship (grant 1067066 to N.N.), and a Senior Research Fellowship (grant 1117105 to H.L.) as well as the National Center for Advancing Translational Science (grants UL1 TR001863 and KL2 TR001862 to J.L.W.) and project grants from the National Health and Medical Research Council (grants 1099655 and 1047263 to G.P., S.J.B., N.dK., C.M., and N.N.).
We thank the Linkage and Client Services Teams at the Data Linkage Branch (Department of Health Western Australia) as well as Maureen Hutchinson, the Data Custodian for the Midwives Notification System.
Conflict of interest: none declared.
Abbreviations
- aOR
adjusted odds ratio
- CI
confidence interval
- IOR
odds ratio for interaction
- IPI
interpregnancy interval
REFERENCES
- 1. Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385(9966):430–440. [DOI] [PubMed] [Google Scholar]
- 2. Merklinger-Gruchala A, Jasienska G, Kapiszewska M. Short interpregnancy interval and low birth weight: a role of parity. Am J Hum Biol. 2015;27(5):660–666. [DOI] [PubMed] [Google Scholar]
- 3. Shachar BZ, Mayo JA, Lyell DJ, et al. Interpregnancy interval after live birth or pregnancy termination and estimated risk of preterm birth: a retrospective cohort study. BJOG. 2016;123(12):2009–2017. [DOI] [PubMed] [Google Scholar]
- 4. Mahande MJ, Obure J. Effect of interpregnancy interval on adverse pregnancy outcomes in northern Tanzania: a registry-based retrospective cohort study. BMC Pregnancy Childbirth. 2016;16(1):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ekin A, Gezer C, Taner CE, et al. Impact of interpregnancy interval on the subsequent risk of adverse perinatal outcomes. J Obstet Gynaecol Res. 2015;41(11):1744–1751. [DOI] [PubMed] [Google Scholar]
- 6. DeFranco EA, Seske LM, Greenberg JM, et al. Influence of interpregnancy interval on neonatal morbidity. Am J Obstet Gynecol. 2015;212(3):386.e1–386.e9. [DOI] [PubMed] [Google Scholar]
- 7. World Health Organization Report of a WHO Technical Consultation on Birth Spacing. Geneva, Switzerland: World Health Organization; 2007. http://www.who.int/reproductivehealth/publications/family_planning/WHO_RHR_07_1/en/. Accessed May 27, 2018.
- 8. Conde-Agudelo A, Belizán JM. Maternal morbidity and mortality associated with interpregnancy interval: cross sectional study. BMJ. 2000;321(7271):1255–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Atreya MR, Muglia LJ, Greenberg JM, et al. Racial differences in the influence of interpregnancy interval on fetal growth. Matern Child Health J. 2017;21(3):562–570. [DOI] [PubMed] [Google Scholar]
- 10. Thoma ME, Copen CE, Kirmeyer SE. Short interpregnancy intervals in 2014: differences by maternal demographic characteristics. NCHS Data Brief. 2016;240:1–8. [PubMed] [Google Scholar]
- 11. Hanley GE, Hutcheon JA, Kinniburgh BA, et al. Interpregnancy interval and adverse pregnancy outcomes: an analysis of successive pregnancies. Obstet Gynecol. 2017;129(3):408–415. [DOI] [PubMed] [Google Scholar]
- 12. Ball SJ, Pereira G, Jacoby P, et al. Re-evaluation of link between interpregnancy interval and adverse birth outcomes: retrospective cohort study matching two intervals per mother. BMJ. 2014;349:g4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smallwood S. New estimates of trends in births by birth order in England and Wales. Popul Trends. 2002;108:32–48. [PubMed] [Google Scholar]
- 14. Hilder L, Zhichao Z, Parker M, et al. Australia’s Mothers and Babies 2012 Perinatal statistics series no. 30. Cat. no. PER 69. Canberra: AIHW National Perinatal Epidemiology and Statistics Unit; 2014.
- 15. Kozuki N, Lee AC, Silveira MF, et al. The associations of parity and maternal age with small-for-gestational-age, preterm, and neonatal and infant mortality: a meta-analysis. BMC Public Health. 2013;13(suppl 3):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Department of Health Western Australia Validation Study of the Western Australian Midwives’ Notification System: 2005 Birth Data. Perth, WA: Department of Health Western Australia; 2007. [Google Scholar]
- 17. Australian Bureau of Statistics Socio-Economic Indexes for Areas. http://www.abs.gov.au/websitedbs/censushome.nsf/home/seifa. Accessed March 1, 2017.
- 18. Cheslack-Postava K, Liu K, Bearman PS. Closely spaced pregnancies are associated with increased odds of autism in California sibling births. Pediatrics. 2011;127(2):246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Neuhaus JM, Kalbfleisch JD. Between- and within-cluster covariate effects in the analysis of clustered data. Biometrics. 1998;54(2):638–645. [PubMed] [Google Scholar]
- 20. Appareddy S, Pryor J, Bailey B. Inter-pregnancy interval and adverse outcomes: evidence for an additional risk in health disparate populations. J Matern Fetal Neonatal Med. 2017;30(21):2640–2644. [DOI] [PubMed] [Google Scholar]
- 21. Cofer FG, Fridman M, Lawton E, et al. Interpregnancy interval and childbirth outcomes in California, 2007–2009. Matern Child Health J. 2016;20(suppl 1):43–51. [DOI] [PubMed] [Google Scholar]
- 22. Lamont K, Scott NW, Jones GT, et al. Risk of recurrent stillbirth: systematic review and meta-analysis. BMJ. 2015;350:h3080. [DOI] [PubMed] [Google Scholar]
- 23. Coo H, Brownell MD, Ruth C, et al. Interpregnancy interval and adverse perinatal outcomes: a record-linkage study using the Manitoba Population Research Data Repository. J Obstet Gynaecol Can. 2017;39(6):420–433. [DOI] [PubMed] [Google Scholar]
- 24. Klebanoff MA. Interpregnancy interval and pregnancy outcomes: causal or not? Obstet Gynecol. 2017;129(3):405–407. [DOI] [PubMed] [Google Scholar]
- 25. Blencowe H, Cousens S, Chou D, et al. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health. 2013;10(suppl 1):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.