Abstract
Because early puberty has been linked to diseases later in life, identification of modifiable causes of early puberty is of interest. We explored the possible associations between maternal smoking during pregnancy and pubertal development in sons and daughters. Between 2012 and 2017, 15,819 children from the Danish National Birth Cohort, born during 2000–2003, provided half-yearly information on puberty from the age of 11 years. We estimated adjusted age differences (in months) at attaining various pubertal milestones, including Tanner stages, per 10 daily cigarettes smoked in the first trimester of gestation. In sons, exposure to smoking in utero was associated with earlier genital development (Tanner 2, −1.3 months, 95% confidence interval (CI): −2.5, 0.0; Tanner 5, −3.7 months, 95% CI: −5.3, −2.0), pubic hair development (Tanner 2, −1.8 months, 95% CI: −2.9, −0.6; Tanner 5, −2.9 months, 95% CI: −4.2, −1.7), and voice break (−2.4 months, 95% CI: −3.6, −1.3). In daughters, maternal smoking was associated with earlier breast development (Tanner 2, −3.4 months, 95% CI: −5.3, −1.5; Tanner 5, −4.7 months, 95% CI: −6.5, −2.9), pubic hair development stages 3–5 (Tanner 5, −2.5 months, 95% CI: −4.1, −1.0), and menarche (−3.1 months, 95% CI: −4.0, −2.3). Fetal exposure to tobacco smoke might advance timing of puberty in boys and girls.
Keywords: maternal exposure, menarche, prenatal exposure delayed effects, puberty, sexual development, sexual maturation, smoking, tobacco smoking
The timing of puberty has become earlier during the last century in girls, but it remains unsettled whether this also happened to boys (1, 2). A decline is a potential source of concern, given that early puberty is related to several adult diseases, such as obesity, diabetes mellitus, cardiovascular disease, breast cancer, and testicular cancer (3–6). Modifiable causes of early puberty might, therefore, provide another avenue for prevention of some chronic diseases.
Maternal smoking during pregnancy might be such a modifiable cause of early puberty. Maternal smoking during pregnancy has been related to other markers of reproductive health in sons and daughters, such as poor semen quality and reduced fecundability (7). Likewise, maternal smoking during pregnancy could advance timing of puberty through one or more mechanisms. First, tobacco smoke contains several toxic compounds (8), which might result in androgenization of the fetal hormonal milieu (9–11), leading to altered timing of puberty in rodents (12, 13), but results from observational studies have been conflicting (14–16). Second, these toxic tobacco compounds might also alter expression of genes involved in neuronal development due to changes in DNA methylation observed in fetuses of women who smoke during pregnancy (17). Because genes involved in timing of puberty are expressed mainly in the neural tissue, this provides a second potential mechanism for advanced timing of puberty (18). Third, maternal smoking during pregnancy might also advance timing of puberty through low birth weight and childhood obesity, which are both associated with advanced pubertal development (19–22).
A widely used marker of pubertal development in girls, age at menarche, has been reported to occur earlier in daughters of smoking mothers than in daughters of nonsmoking mothers in some studies (16, 23–31), whereas other studies reported no association (29, 32, 33) or even later age at menarche (34–36). Only a few studies in sons have been published, and some have indicated younger age at voice break, regular shaving, first ejaculation, and acne, although these studies were either low in power or prone to recall bias of the pubertal milestones (37–39). Other important markers of puberty are less well studied. These markers include breast and pubic hair development in daughters (26, 37, 40) and genital and pubic hair development in sons (39, 41).
In this cohort study, we used detailed information on various markers of puberty, collected half-yearly during puberty, and detailed information on smoking collected during pregnancy. The aim was to investigate whether maternal smoking during pregnancy is associated with earlier timing of puberty in their sons and daughters.
METHODS
Study population
This population-based cohort study is based on the Puberty Cohort, a subcohort of the Danish National Birth Cohort (DNBC). The DNBC holds information on approximately 92,000 mothers and their children born during 1996–2003 (42). Mothers were interviewed twice during pregnancy and at 6 and 18 months postpartum. Additionally, they completed questionnaires when the children were 7 years and 11 years of age.
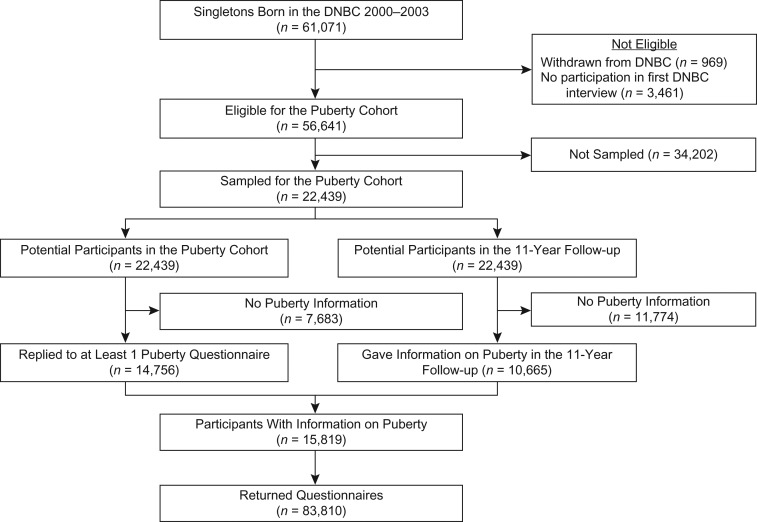
Children eligible for participation in the Puberty Cohort were live-born singletons from the DNBC, born during 2000–2003, whose mothers had participated in the first pregnancy interview and had not withdrawn from the DNBC before May 2012 (n = 56,641). We sampled 22,439 children and invited them to give half-yearly information on puberty through web-based questionnaires from the age of 11.5 years to full maturity (defined as Tanner stage 5 for both pubic hair development and breast or genital development) or 18 years of age, whichever came first. From August 2012 to March 2017, 14,756 children returned at least 1 questionnaire. Furthermore, 10,665 of the 22,439 invited children gave information on puberty in the DNBC’s 11-year follow-up, which had similar questions on puberty to the Puberty Cohort. When this information was added, a total of 15,819 children (7,696 sons and 8,123 daughters) participated in the Puberty Cohort (participation rate 70%) and returned on average 5.3 (range, 1–11) questionnaires (Figure 1). In total, 83,810 questionnaires were returned. The participants were by March 2017 between 14 and 17 years of age.
Figure 1.
Flow diagram of participants in the Puberty Cohort, Danish National Birth Cohort (DNBC), Denmark, 2000–2017.
Maternal smoking during pregnancy
The main exposure was smoking during the mother’s first trimester of pregnancy. In the first 3 interviews in the DNBC, the women were asked: “Did you smoke during pregnancy?”, “Do you smoke now?”, “Did you have periods during your pregnancy where you did not smoke (for at least one week)?”, “In which weeks of gestation did you not smoke?” (yes/no for each of week 1 through 42), and “How much did you smoke on average?” (number of daily cigarettes). From this information, we derived the variables that described average daily smoking in the first trimester (gestational week 1–12) and throughout pregnancy: continuous and categorical (nonsmoker, light-smoker (1–10 daily cigarettes), heavy-smoker (>10 daily cigarettes)).
Timing of puberty
The outcome was age at attaining various pubertal milestones. We used a translated version of the questionnaire used in the British Avon Longitudinal Study of Parents and Children (41). The questionnaire included the following items: menarche (yes/no; if yes, then year and month), first ejaculation of semen (yes/no; if yes, then year and month), voice break (yes—sometimes, yes—definitive changes, no), axillary hair (yes/no), and acne (yes/no). The children also provided information on their current pubertal stage in terms of Tanner stages 1–5 for each of the following: pubic hair and genital development for boys and pubic hair and breast development for girls (43, 44). To collect information on Tanner stages, we used the Sexual Maturation Scale, which uses illustrations of each of the 5 Tanner stages assisted by a short description of each stage (45). The children were asked to indicate which of the 5 illustrated Tanner stages best represented their current pubertal stage. The questionnaire is available in Danish (46).
Covariates
Identification of potential confounders was guided by directed acyclic graphs (47). The potential confounders considered were prepregnancy body mass index (BMI), alcohol consumption in the first trimester, time to pregnancy (including assisted reproductive technology), parity, maternal age at delivery, maternal age at menarche, highest social class of parents, and cohabitation of parents during pregnancy. Confounders were categorized as shown in Table 1. We retrieved parity and maternal age at delivery from the Danish Medical Birth Registry and highest social class of parents from Statistics Denmark; the latter was based on the International Standard Class of Occupation and Education codes (ISCO-88 and ISCED). Information on all other potential confounders were retrieved from the DNBC provided by the women during pregnancy. Paternal smoking in the first trimester, duration of exclusive breastfeeding, exposure to postnatal smoking (defined as maternal smoking in the first 6 postnatal months), and childhood BMI at 7 years were retrieved from the DNBC, and birth weight was retrieved from the Danish Medical Birth Registry.
Table 1.
Background Characteristics According to Maternal Smoking in the First Trimester of Pregnancy for 15,766a Children in the Puberty Cohort, Danish National Birth Cohort, Denmark, 2000– 2017
| Background Characteristic | Smoking in the First Trimester | |||||||
|---|---|---|---|---|---|---|---|---|
| Nonsmoker (n = 11,347) | 1–10 Daily Cigarettes (n = 3,512) | >10 Daily Cigarettes (n = 907) | Missing | |||||
| No. | % | No. | % | No. | % | No. | % | |
| Maternal characteristics | ||||||||
| Prepregnancy BMIb | 217 | 1.4 | ||||||
| <18.5 | 683 | 6.1 | 303 | 8.8 | 69 | 7.8 | ||
| 18.5–24.9 | 6,973 | 62.2 | 2,139 | 62.0 | 504 | 56.8 | ||
| 25.0–29.9 | 2,414 | 21.5 | 679 | 19.7 | 205 | 23.1 | ||
| ≥30.0 | 1,139 | 10.2 | 331 | 9.6 | 110 | 12.4 | ||
| Alcohol in the first trimester, units/weekc | 4 | 0.0 | ||||||
| 0 | 5,780 | 51.0 | 1,864 | 53.1 | 502 | 55.3 | ||
| 0.1–1.0 | 3,724 | 32.8 | 988 | 28.1 | 204 | 22.5 | ||
| 1.1–3.0 | 1,339 | 11.8 | 441 | 12.6 | 116 | 12.8 | ||
| >3.0 | 500 | 4.4 | 219 | 6.2 | 85 | 9.4 | ||
| Paternal smoking in the first trimester | 9 | 0.1 | ||||||
| No | 8,927 | 78.7 | 1,674 | 47.7 | 349 | 38.5 | ||
| Yes | 2,413 | 21.3 | 1,837 | 52.3 | 557 | 61.5 | ||
| Time to pregnancy (including ART) | 44 | 0.3 | ||||||
| 0 month | 2,312 | 20.4 | 574 | 16.4 | 137 | 15.1 | ||
| 1–2 months | 2,232 | 19.7 | 586 | 16.8 | 121 | 13.4 | ||
| 3–5 months | 1,870 | 16.5 | 565 | 16.2 | 109 | 12.0 | ||
| 6–12 months | 1,460 | 12.9 | 440 | 12.6 | 132 | 14.6 | ||
| >12 months | 825 | 7.3 | 312 | 8.9 | 102 | 11.3 | ||
| ART | 1,186 | 10.5 | 240 | 6.9 | 37 | 4.1 | ||
| Not planned | 1,434 | 12.7 | 781 | 22.3 | 267 | 29.5 | ||
| Parity | 0 | 0.0 | ||||||
| First child | 5,571 | 49.1 | 1,989 | 56.6 | 372 | 41.0 | ||
| Second child or later | 5,776 | 50.9 | 1,523 | 43.4 | 535 | 59.0 | ||
| Maternal age at delivery, yearsd | 30.8 (4.2) | 29.9 (4.7) | 30.7 (5.0) | 6 | 0.0 | |||
| Maternal age at menarche | 123 | 0.8 | ||||||
| Earlier than peers | 2,826 | 25.1 | 934 | 26.8 | 241 | 26.7 | ||
| Same time as peers | 6,413 | 56.9 | 2,019 | 58.0 | 524 | 58.2 | ||
| Later than peers | 2,022 | 18.0 | 528 | 15.2 | 136 | 15.1 | ||
| Highest social class of parents | 31 | 0.2 | ||||||
| High-grade professional | 2,953 | 26.1 | 632 | 18.0 | 98 | 10.8 | ||
| Low-grade professional | 3,992 | 35.3 | 978 | 27.9 | 198 | 21.9 | ||
| Skilled worker | 2,925 | 25.8 | 1,110 | 31.6 | 307 | 33.9 | ||
| Unskilled worker | 1,199 | 10.6 | 680 | 19.4 | 261 | 28.8 | ||
| Student | 205 | 1.8 | 90 | 2.6 | 16 | 1.8 | ||
| Economically inactive | 48 | 0.4 | 18 | 0.5 | 25 | 2.8 | ||
| Cohabitation of parents | 9 | 0.1 | ||||||
| Did not live together | 118 | 1.0 | 137 | 3.9 | 71 | 7.8 | ||
| Lived together | 11,224 | 99.0 | 3,373 | 96.1 | 834 | 92.2 | ||
| Child’s characteristics | ||||||||
| Birthweight, gramsd | 3,571 (586) | 3,448 (596) | 3,319 (597) | 57 | 0.4 | |||
| Duration of exclusive breastfeeding, months | 2,298 | 14.6 | ||||||
| 0 | 486 | 5.0 | 189 | 6.4 | 72 | 9.4 | ||
| <4 | 2,193 | 22.5 | 1,026 | 34.8 | 351 | 45.8 | ||
| ≥4 | 7,072 | 72.5 | 1,736 | 58.8 | 343 | 44.8 | ||
| Exposure to postnatal smokinge | 2,346 | 14.9 | ||||||
| No | 9,507 | 97.8 | 1,071 | 36.4 | 95 | 12.5 | ||
| Yes | 214 | 2.2 | 1,868 | 63.6 | 665 | 87.5 | ||
| Child’s BMI at age 7 yearsd | 15.6 (1.7) | 15.9 (1.8) | 16.1 (2.1) | 4,755 | 30.2 | |||
Abbreviations: ART, assisted reproductive technology; BMI, body mass index.
a 15,766 of 15,819 children with nonmissing information on maternal smoking in the first trimester (53 missing).
b BMI calculated as weight (kg)/height (m)2.
c 1 unit = 12 g of pure alcohol.
d Values are expressed as mean (standard deviations).
e Exposure to postnatal smoking defined as maternal smoking during the first 6 months after birth.
Statistical analysis
Analyses were performed using Stata MP, version 13.1 (StataCorp LLC, College Station, Texas). Because we had half-yearly information on puberty, the outcomes were either left, interval, or right censored; the outcome was left censored when the milestone was already attained by the first questionnaire, interval censored when the pubertal milestone was attained between 2 questionnaires, and right censored when the milestone was not attainted by the last questionnaire (Web Table 1, available at https://academic.oup.com/aje). Therefore, we fitted a multivariable regression model for interval censored data, assuming normally distributed residuals, using Stata’s intreg package. The exposure, maternal smoking in the first trimester, was first included in categories, with nonsmoking as the referent, and plotted on a graph to visually inspect a potentially dose-dependent pattern. Then, maternal smoking in the first trimester was included as a continuous variable in units of 10 daily cigarettes to estimate the difference in age at attaining each pubertal milestone (in months) per 10 daily cigarettes smoked by the mother in the first trimester as a test for trend. Maternal age was introduced as a second-order polynomial variable to allow for departure of linearity.
We performed 6 subanalyses. First, a maternal-paternal comparison was performed, where paternal smoking was intended to be a negative control (48). This was performed by using an exposure variable consisting of 4 groups of parental smoking during first trimester: “No parent smokes,” “only mother smokes,” “only father smokes,” and “both parents smoke.” Second, we conducted a multidimensional bias analysis for unmeasured confounding under different scenarios for age at voice break in sons and age at menarche in girls (for a detailed description see Web Appendix 1) (49). Third, we analyzed smoking throughout the entire pregnancy (continuous), rather than solely in the first trimester, as the exposure of interest. Fourth, fifth, and sixth, we further adjusted for childhood BMI, exposure to postnatal smoking, and duration of exclusive breastfeeding. To evaluate the risk of bias due to missing information on these 3 variables, we also restricted the main analysis to having nonmissing information on any of these 3 variables and compared the results with those from the main analysis.
Normality of the interval-censored residuals was checked in R x64, version 3.3.1 (R Foundation for Statistical Computing, Vienna, Austria), by fitting a stepwise cumulative incidence function, using the nonparametric distribution estimator in the icenReg package. The nonparametric distribution was compared visually with the cumulative incidence function based on the normal distribution. Further, we stratified the plots by levels of covariates to check that the mean and standard deviation of the residuals were independent of the covariates.
We used inverse probability weights to account for both the sampling approach applied in the Puberty Cohort (see Web Appendix 2 and Web Table 2 for detailed description) and potential selection bias due to nonparticipation. In short, we sampled participants for the Puberty Cohort from 12 prenatal exposures hypothesized to be important for the timing of puberty, including maternal smoking during pregnancy, supplemented with a random sample of 8,000 children. From the sampling fractions for each exposure group, we derived sampling weights corresponding to the inverse probability of being sampled. To account for selection bias due to nonparticipation, we created selection weights (50), which estimate the inverse probability of participation and were estimated using a multivariable logistic regression model. The variables used to estimate the selection weights were a priori believed to be important for participation: maternal smoking and alcohol consumption in the first trimester, prepregnancy BMI, paternal smoking in the first trimester, parity, maternal age at delivery, maternal age at menarche, highest social class of parents, and cohabitation of parents during pregnancy. Finally, the selection weights were multiplied by the sampling weights and included in the analyses. All models were fitted using robust standard errors to take into account the weighting approach and clustering of siblings. Estimates were computed with 95% confidence intervals.
Ethical approval
The Committee for Biomedical Research Ethics in Denmark approved the collection of data in the DNBC ((KF)01-471/94). A written informed consent was obtained from mothers upon recruitment covering both mother’s and offspring’s participation until the children turned 18 years of age. The present study was approved by the Danish Data Protection Agency (2012-41-0379 and 2015-57-0002) and the Steering Committee of the DNBC (2012-04 and 2015-47).
RESULTS
The prevalence of maternal smoking in the first trimester was 28%. Heavily smoking mothers (>10 daily cigarettes, 6%) were slightly more likely to consume alcohol, to have a smoking partner, to have had an unplanned pregnancy, to have had a longer time to pregnancy, to be parous, to have lower social class, to be living without the father during pregnancy, to have children with lower birth weight, to breastfeed less, and to smoke after pregnancy than nonsmoking mothers (Table 1).
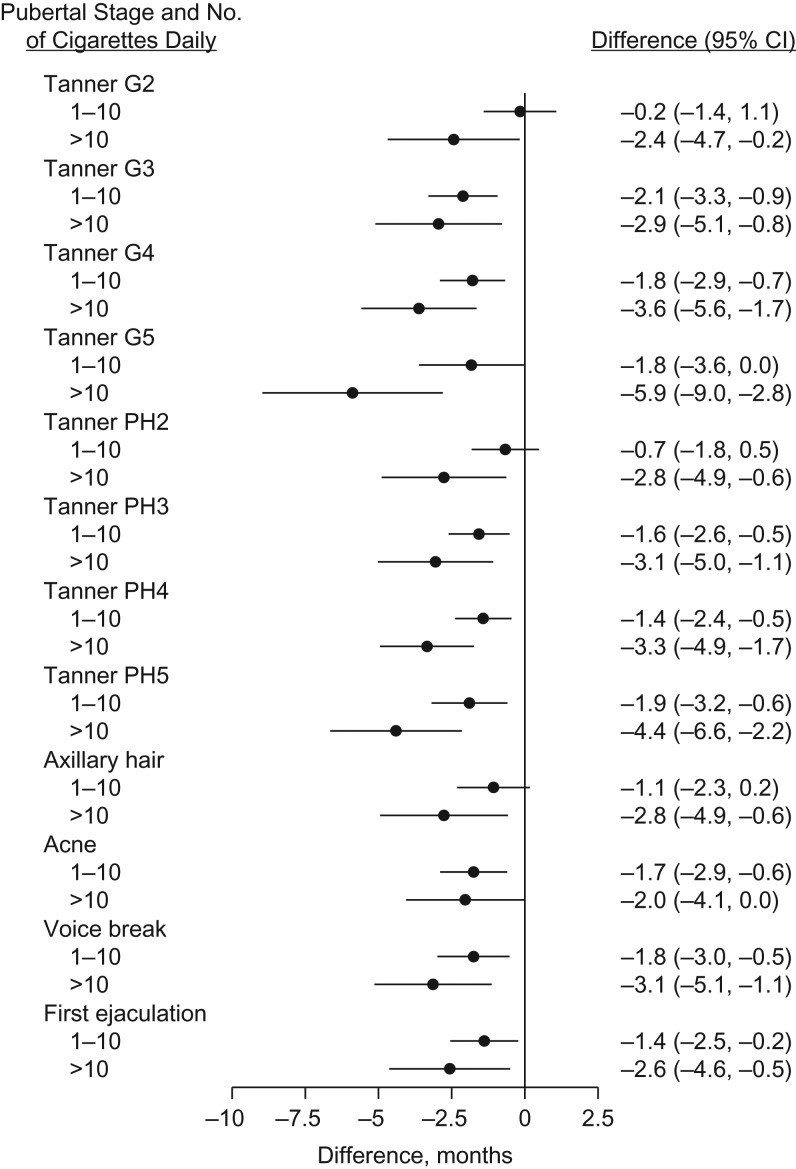
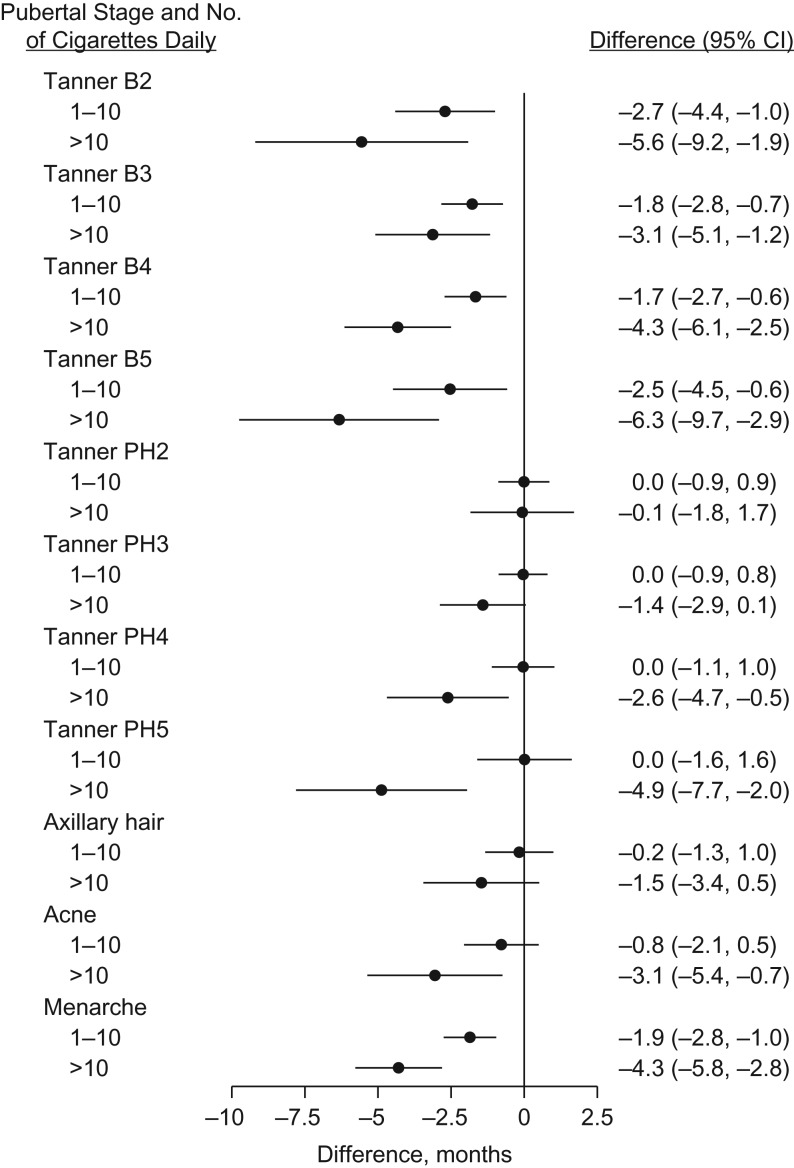
Figures 2 and 3 show the adjusted difference (in months with 95% confidence intervals) in age at attaining the pubertal milestones according to maternal smoking categories (nonsmoker, light smoker, or heavy smoker) in the first trimester. For sons, maternal smoking in the first trimester was consistently associated with earlier age at attaining all pubertal milestones in a dose-dependent manner. For daughters, dose-dependent associations were observed for acne, menarche, and all stages of breast development, but only for Tanner stages 3–5 for pubic hair development.
Figure 2.
Age difference in timing of puberty among sons in relation to maternal smoking in the first trimester of pregnancy, Puberty Cohort, Danish National Birth Cohort, Denmark, 2012–2017. Estimated age differences in timing of puberty with 95% confidence intervals (CIs). The referent was nonsmoking mothers, and the analysis adjusted for prepregnancy body mass index, alcohol units per week in the first trimester, time to pregnancy (including assisted reproductive technology), highest social class of parents, maternal age at menarche, maternal age at delivery, parity, and cohabitation of parents during pregnancy. G2–5, genital stages 2–5; PH2–5, pubic hair stages 2–5.
Figure 3.
Age difference in timing of puberty among daughters in relation to maternal smoking in the first trimester of pregnancy, Puberty Cohort, Danish National Birth Cohort, Denmark, 2012–2017. Estimated age differences in timing of puberty with 95% confidence intervals (CIs). The referent was nonsmoking mothers, and the analysis adjusted for prepregnancy body mass index, alcohol units per week in the first trimester, time to pregnancy (including assisted reproductive technology), highest social class of parents, maternal age at menarche, maternal age at delivery, parity, and cohabitation of parents during pregnancy. B2–5, breast stages 2–5; PH2–5, pubic hair stages 2–5.
Table 2 shows the unadjusted and adjusted difference in age at attaining a given pubertal milestone (in months) per 10 daily cigarettes smoked in the first trimester. In sons, maternal smoking in the first trimester was consistently associated with 1–4 months’ earlier pubertal development for all milestones per 10 daily cigarettes. In daughters, maternal smoking in the first trimester was associated with 1–4.5 months’ earlier age at breast development, pubic hair development (except Tanner stage 2), menarche, and acne per 10 daily cigarettes.
Table 2.
Age Difference in Timing of Puberty in Months per 10 Daily Cigarettes in the First Trimester of Gestation for Children in the Puberty Cohort, Danish National Birth Cohort, Denmark, 2012–2017
| Pubertal Milestone | No.a | Age Differenceb | ||
|---|---|---|---|---|
| Unadjusted | Adjustedc | |||
| Mean | Mean | 95% CI | ||
| Sons | ||||
| Tanner genital stage 2 | 7,446 | −1.3 | −1.3 | −2.5, 0.0 |
| Tanner genital stage 3 | 7,446 | −2.4 | −2.1 | −3.3, −1.0 |
| Tanner genital stage 4 | 7,446 | −2.6 | −2.3 | −3.3, −1.3 |
| Tanner genital stage 5 | 7,446 | −4.0 | −3.7 | −5.3, −2.0 |
| Tanner pubic hair stage 2 | 7,450 | −1.7 | −1.8 | −2.9, −0.6 |
| Tanner pubic hair stage 3 | 7,450 | −2.4 | −2.2 | −3.2, −1.2 |
| Tanner pubic hair stage 4 | 7,450 | −2.3 | −2.0 | −2.9, −1.2 |
| Tanner pubic hair stage 5 | 7,450 | −3.3 | −2.9 | −4.2, −1.7 |
| Axillary hair | 7,455 | −2.5 | −2.0 | −3.2, −0.8 |
| Acne | 7,455 | −2.2 | −1.9 | −3.0, −0.8 |
| Voice break | 7,253 | −3.1 | −2.4 | −3.6, −1.3 |
| First ejaculation | 7,442 | −1.6 | −1.7 | −2.8, −0.6 |
| Daughters | ||||
| Tanner breast stage 2 | 7,866 | −4.5 | −3.4 | −5.3, −1.5 |
| Tanner breast stage 3 | 7,866 | −3.6 | −2.6 | −3.7, −1.6 |
| Tanner breast stage 4 | 7,866 | −3.6 | −2.8 | −3.8, −1.8 |
| Tanner breast stage 5 | 7,866 | −5.9 | −4.7 | −6.5, −2.9 |
| Tanner pubic hair stage 2 | 7,867 | −0.5 | −0.1 | −1.0, 0.8 |
| Tanner pubic hair stage 3 | 7,867 | −1.3 | −0.9 | −1.7, −0.1 |
| Tanner pubic hair stage 4 | 7,867 | −1.8 | −1.4 | −2.5, −0.4 |
| Tanner pubic hair stage 5 | 7,867 | −3.4 | −2.5 | −4.1, −1.0 |
| Axillary hair | 7,872 | −1.7 | −1.0 | −2.1, 0.1 |
| Acne | 7,872 | −2.8 | −2.1 | −3.4, −0.9 |
| Menarche | 7,864 | −4.1 | −3.1 | −4.0, −2.3 |
Abbreviation: CI, confidence interval.
a Some sons and daughters gave information on some but not all pubertal milestones, so different numbers of observations were used for each outcome.
b Change in age (β) in months at attaining pubertal milestones per 10 daily cigarettes in the first trimester with 95% confidence interval.
c Adjusted for prepregnancy body mass index, alcohol units per week in the first trimester, time to pregnancy (including assisted reproductive technology), highest social class of parents, maternal age at menarche, maternal age at delivery, parity, and cohabitation of parents during pregnancy.
The maternal-paternal comparison showed associations between paternal smoking during pregnancy and timing of puberty in the offspring similar in direction and magnitude as the associations between maternal smoking during pregnancy and timing of puberty (Web Figures 1 and 2).
Under realistic scenarios, the multidimensional bias analysis showed that uncontrolled confounding could explain some but not all of the association between maternal smoking during pregnancy and age at voice break and menarche (Web Appendix 1 and Web Tables 3 and 4). We repeated the analysis with maternal smoking exposure throughout pregnancy (continuous), and the results were slightly attenuated (Web Table 5). When adjusting for childhood BMI at age 7 years, the associations were also slightly attenuated (Web Table 6). When adjusting for exposure to postnatal smoking, the results were essentially the same for sons but were attenuated for daughters (Web Table 7). Adjusting for duration of exclusive breastfeeding did not change the estimates (Web Table 8).
DISCUSSION
In this longitudinal study, we found dose-dependent associations between maternal smoking during pregnancy and earlier timing of puberty in both sons and daughters. All pubertal milestones occurred 1–4 months earlier per 10 daily cigarettes in sons, and in daughters, breast development, pubic hair development (except Tanner stage 2 for pubic hair), menarche, and acne occurred 1–4.5 months earlier per 10 daily cigarettes.
Our study is large and used various pubertal milestones including Tanner stages. We accounted for potential sources of selection bias using selection weights (50). Even though we had data on most potential confounders, we cannot rule out confounding from unmeasured factors or residual confounding. Therefore, we performed a maternal-paternal comparison (48), which also showed associations between paternal smoking during pregnancy and timing of puberty in the offspring similar in direction and magnitude as the associations between maternal smoking during pregnancy and timing of puberty. These findings indicate remaining residual confounding or a biological effect of paternal smoking (e.g., through passive smoking or a programming effect on the sperm) (51). Furthermore, if a paternal programming effect exists, we cannot rule out the existence of a maternal programming effect of the ovaries before conception, which might confound the observed associations. To further explore the possibility of residual confounding, we conducted a multidimensional bias analysis for unmeasured confounding for age at voice break and menarche (49). Such an unmeasured confounder might be unhealthy lifestyle in the family that is connected to smoking behavior and could accelerate puberty through diet or exposure to endocrine-disrupting chemicals. Under realistic scenarios, unmeasured confounding explained only part of the observed associations (Web Appendix 1).
Pregnant women tend to underreport their smoking behavior (52). In the present study, information on smoking was collected during pregnancy, long before timing of puberty in the children was known, and the resulting misclassification is most likely to be nondifferential, causing bias towards the null. Information on puberty was collected through self-administered questionnaires, which imposes a risk of misclassification but allows for a large sample size and probably less selection bias due to high participation. A high proportion of children had already attained the early pubertal milestones at entry and were, therefore, left censored (Web Table 2). For the estimates related to the early milestones to be unbiased even in the presence of left censoring, the residuals need to be normally distributed. The model check supported this for all later milestones, but for the earliest milestones, this model check is uncertain given that the left part of the distribution was unobserved due to left censoring. In case of skewed residuals, the potential error introduced by left censoring is, however, most likely nondifferential with regard to maternal smoking and cannot possibly explain the associations. This is corroborated by the similar associations for both early and late milestones. The only exception was the null finding for Tanner stage 2 for pubic hair in girls, which is in line with former data on girls of white ethnicity (26, 40). Despite this, we cannot rule out that the null finding for Tanner stage 2 for pubic hair in girls is due to left censoring and a skewed distribution of the residuals for this specific milestone.
Previous studies have indicated either no association or earlier timing of puberty in sons of smoking mothers in terms of voice break (37–39), first ejaculation (38), genital development (39), and pubic hair development (39, 41). However, these studies were limited either by samples being too small (37), lack of confounder adjustment (39), or recalled puberty data in adulthood (38). The first large longitudinal study with detailed confounder adjustment investigated Tanner stages for pubic hair development as the only milestone and did not observe an association between maternal smoking during pregnancy and pubic hair development (41). We overcame the specific limitations mentioned above and found consistent dose-dependent associations between maternal smoking in pregnancy and all pubertal milestones in sons.
In daughters, published results on smoking and pubic hair and breast development have been less consistent (26, 37, 40). One study reported no association with breast development (37), another study reported earlier onset of breast development but not onset of pubic hair development (26), and the third reported earlier onset of pubic hair development but not breast development in daughters of smoking mothers (40). However, the last study found earlier onset of breast development, but not pubic hair development, when only girls of white ethnicity were considered (40), which indicates the presence of heterogeneity of effects by ethnicity. Our results support an association for earlier onset of breast development but not onset of pubic hair development in white girls. Finally, we found earlier age at menarche in daughters of smoking mothers, whereas investigators in other studies have reported conflicting results (16, 23–36). In a recent meta-analysis, maternal smoking during pregnancy was overall associated with slightly earlier age at menarche (53), but the authors also noted heterogeneity of effects between years of birth (53) indicating that important effect modifiers that change over time might be responsible for earlier inconsistent results in the previous literature (16, 23–36). Houghton et al. (54) found heterogeneity according to postnatal growth patterns and suggested that this could be the reason for the inconsistent results in the literature. In an exploratory analysis, we found no evidence of heterogeneity of effect when including a term for interaction between maternal smoking (continuous) and postnatal growth patterns (P = 0.91, data not shown) when defined as change in weight z score (continuous, based on United Kingdom–World Health Organization growth reference (55)) between birth and 7 years of age. It should be noted that maternal smoking during pregnancy is most likely not the main driving factor for the decline in age at menarche observed in girls (1, 2), given that the prevalence of smoking during pregnancy has declined over the last decades (56).
Prepubertal childhood obesity and low birth weight might be mediators for the association between maternal smoking during pregnancy and timing of puberty (19–22). We adjusted for childhood BMI at age 7 years but not birth weight because this has been related to severe collider-stratification bias (57, 58). When adjusting for childhood BMI at age 7 years, our results were only slightly attenuated, indicating that other mechanisms are more important.
We further examined the role of exposure to postnatal smoking and breastfeeding by adjusting for these variables. When adjusting for exposure to postnatal smoking, the results remained unchanged in sons but attenuated in daughters, indicating that exposure to postnatal smoking cannot explain the associations. These results should, however, be interpreted cautiously due to the risk of collinearity between maternal smoking during pregnancy and after birth and due to the failure to establish a link between postnatal smoking and timing of puberty so far (23, 34, 54). Maternal smoking during pregnancy might also affect puberty by way of shorter duration of breastfeeding, which has been associated with delayed age at menarche (28), but when adjusting for duration of exclusive breastfeeding, our results remained unchanged.
Because early puberty might be causally related to later diseases in adulthood (3–6), modifiable causes of early puberty need to be identified. Maternal smoking during pregnancy might be such a modifiable cause. If maternal smoking during pregnancy advances the timing of puberty, maternal smoking could have an impact on the incidence of adult diseases. If the estimated associations are true effects, our results could be generalized to Western populations of white origin given that ours is a population-based study, mainly including white persons. The evidence provided in this study could be used by health professionals as an additional argument in the motivation for smoking cessations before or during pregnancy.
In conclusion, maternal smoking during pregnancy was associated with younger age at all pubertal milestones in sons. In daughters, maternal smoking during pregnancy was associated with younger age at breast development, pubic hair development (except Tanner stage 2), menarche, and acne but not with axillary hair. In utero exposure to tobacco smoke might advance the timing of puberty.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Public Health, Section for Epidemiology, Aarhus University, Aarhus, Denmark (Nis Brix, Andreas Ernst, Lea L. B. Lauridsen, Cecilia H. Ramlau-Hansen); Department of Public Health, Section for Biostatistics, Aarhus University, Aarhus, Denmark (Erik T. Parner); Department of Clinical Epidemiology, Aarhus University Hospital, Aarhus, Denmark (Jørn Olsen); Department of Epidemiology, Fielding School of Public Health, University of California Los Angeles, Los Angeles, California (Jørn Olsen); and Department of Pediatrics, Perinatal Epidemiology Research Unit, Aarhus University Hospital, Aarhus, Denmark (Tine B. Henriksen).
This work was supported by the Danish Council for Independent Research (grant DFF 4183-00152 to C.H.R.-H) and the Faculty of Health at Aarhus University. The Danish National Birth Cohort was established with a significant grant from the Danish National Research Foundation. Additional support was obtained from the Danish Regional Committees, the Pharmacy Foundation, the Egmont Foundation, the March of Dimes Birth Defects Foundation, the Health Foundation, and other minor grants. The Danish National Birth Cohort Biobank has been supported by the Novo Nordisk Foundation and the Lundbeck Foundation. Follow-up of mothers and children has been supported by the Danish Medical Research Council (grants SSVF 0646, 271-08-0839/06-066023, O602-01042B, and 0602-02738B), the Lundbeck Foundation (grant 195/04, R100-A9193), the Innovation Fund Denmark (grant 0603-00294B (09-067124)), the Nordea Foundation (grant 02-2013-2014), Aarhus Ideas (grant AU R9-A959-13-S804), University of Copenhagen Strategic Grant (grant IFSV 2012), and the Danish Council for Independent Research (grants DFF–4183-00594 and DFF–4183-00152).
Conflict of interest: none declared.
Abbreviations
- BMI
body mass index
- DNBC
Danish National Birth Cohort
REFERENCES
- 1. Ong KK, Ahmed ML, Dunger DB. Lessons from large population studies on timing and tempo of puberty (secular trends and relation to body size): the European trend. Mol Cell Endocrinol. 2006;254–255:8–12. [DOI] [PubMed] [Google Scholar]
- 2. Euling SY, Herman-Giddens ME, Lee PA, et al. . Examination of US puberty-timing data from 1940 to 1994 for secular trends: panel findings. Pediatrics. 2008;121(suppl 3):S172–S191. [DOI] [PubMed] [Google Scholar]
- 3. Freedman DS, Khan LK, Serdula MK, et al. . The relation of menarcheal age to obesity in childhood and adulthood: the Bogalusa heart study. BMC Pediatr. 2003;3:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Day FR, Elks CE, Murray A, et al. . Puberty timing associated with diabetes, cardiovascular disease and also diverse health outcomes in men and women: the UK Biobank study. Sci Rep. 2015;5:11208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berkey CS, Frazier AL, Gardner JD, et al. . Adolescence and breast carcinoma risk. Cancer. 1999;85(11):2400–2409. [DOI] [PubMed] [Google Scholar]
- 6. Moss AR, Osmond D, Bacchetti P, et al. . Hormonal risk factors in testicular cancer. A case-control study. Am J Epidemiol. 1986;124(1):39–52. [DOI] [PubMed] [Google Scholar]
- 7. Håkonsen LB, Ernst A, Ramlau-Hansen CH. Maternal cigarette smoking during pregnancy and reproductive health in children: a review of epidemiological studies. Asian J Androl. 2014;16(1):39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jauniaux E, Gulbis B, Acharya G, et al. . Maternal tobacco exposure and cotinine levels in fetal fluids in the first half of pregnancy. Obstet Gynecol. 1999;93(1):25–29. [DOI] [PubMed] [Google Scholar]
- 9. Mochizuki M, Maruo T, Masuko K, et al. . Effects of smoking on fetoplacental-maternal system during pregnancy. Am J Obstet Gynecol. 1984;149(4):413–420. [DOI] [PubMed] [Google Scholar]
- 10. Toriola AT, Vääräsmäki M, Lehtinen M, et al. . Determinants of maternal sex steroids during the first half of pregnancy. Obstet Gynecol. 2011;118(5):1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Smith LM, Cloak CC, Poland RE, et al. . Prenatal nicotine increases testosterone levels in the fetus and female offspring. Nicotine Tob Res. 2003;5(3):369–374. [DOI] [PubMed] [Google Scholar]
- 12. Witham EA, Meadows JD, Shojaei S, et al. . Prenatal exposure to low levels of androgen accelerates female puberty onset and reproductive senescence in mice. Endocrinology. 2012;153(9):4522–4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dela Cruz C, Pereira OC. Prenatal testosterone supplementation alters puberty onset, aggressive behavior, and partner preference in adult male rats. J Physiol Sci. 2012;62(2):123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Manning JT, Fink B. Is low digit ratio linked with late menarche? Evidence from the BBC internet study. Am J Hum Biol. 2011;23(4):527–533. [DOI] [PubMed] [Google Scholar]
- 15. Oberg AS, Villamor E. Low digit ratio predicts early age at menarche in Colombian schoolgirls. Paediatr Perinat Epidemiol. 2012;26(5):448–455. [DOI] [PubMed] [Google Scholar]
- 16. D’Aloisio AA, DeRoo LA, Baird DD, et al. . Prenatal and infant exposures and age at menarche. Epidemiology. 2013;24(2):277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Joubert BR, Felix JF, Yousefi P, et al. . DNA methylation in newborns and maternal smoking in pregnancy: genome-wide consortium meta-analysis. Am J Hum Genet. 2016;98(4):680–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Day FR, Thompson DJ, Helgason H, et al. . Genomic analyses identify hundreds of variants associated with age at menarche and support a role for puberty timing in cancer risk. Nat Genet. 2017;49(6):834–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pereira PP, Da Mata FA, Figueiredo AC, et al. . Maternal active smoking during pregnancy and low birth weight in the Americas: a systematic review and meta-analysis. Nicotine Tob Res. 2017;19(5):497–505. [DOI] [PubMed] [Google Scholar]
- 20. Roth CL, DiVall S. Consequences of early life programing by genetic and environmental influences: a synthesis regarding pubertal timing. Endocr Dev. 2016;29:134–152. [DOI] [PubMed] [Google Scholar]
- 21. Wagner IV, Sabin MA, Pfäffle RW, et al. . Effects of obesity on human sexual development. Nat Rev Endocrinol. 2012;8(4):246–254. [DOI] [PubMed] [Google Scholar]
- 22. Møller SE, Ajslev TA, Andersen CS, et al. . Risk of childhood overweight after exposure to tobacco smoking in prenatal and early postnatal life. PLoS One. 2014;9(10):e109184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Windham GC, Bottomley C, Birner C, et al. . Age at menarche in relation to maternal use of tobacco, alcohol, coffee, and tea during pregnancy. Am J Epidemiol. 2004;159(9):862–871. [DOI] [PubMed] [Google Scholar]
- 24. Shrestha A, Nohr EA, Bech BH, et al. . Smoking and alcohol use during pregnancy and age of menarche in daughters. Hum Reprod. 2011;26(1):259–265. [DOI] [PubMed] [Google Scholar]
- 25. Ernst A, Kristensen SL, Toft G, et al. . Maternal smoking during pregnancy and reproductive health of daughters: a follow-up study spanning two decades. Hum Reprod. 2012;27(12):3593–3600. [DOI] [PubMed] [Google Scholar]
- 26. Maisonet M, Christensen KY, Rubin C, et al. . Role of prenatal characteristics and early growth on pubertal attainment of British girls. Pediatrics. 2010;126(3):e591–e600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rubin C, Maisonet M, Kieszak S, et al. . Timing of maturation and predictors of menarche in girls enrolled in a contemporary British cohort. Paediatr Perinat Epidemiol. 2009;23(5):492–504. [DOI] [PubMed] [Google Scholar]
- 28. Morris DH, Jones ME, Schoemaker MJ, et al. . Determinants of age at menarche in the UK: analyses from the Breakthrough Generations Study. Br J Cancer. 2010;103(11):1760–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fukuda M, Fukuda K, Shimizu T, et al. . Maternal smoking during pregnancy and age at menarche of premenopausal and post-menopausal daughters. Hum Reprod. 2013;28(2):551. [DOI] [PubMed] [Google Scholar]
- 30. Zhang B, Shi H, Wang Q, et al. . Maternal passive smoking during pregnancy and age of menarche in daughters: a study of elementary and middle school students in Shanghai. Asia Pac J Public Health. 2015;27(2 suppl):14S–20S. [DOI] [PubMed] [Google Scholar]
- 31. Behie AM, O’Donnell MH. Prenatal smoking and age at menarche: influence of the prenatal environment on the timing of puberty. Hum Reprod. 2015;30(4):957–962. [DOI] [PubMed] [Google Scholar]
- 32. Dossus L, Kvaskoff M, Bijon A, et al. . Determinants of age at menarche and time to menstrual cycle regularity in the French E3N cohort. Ann Epidemiol. 2012;22(10):723–730. [DOI] [PubMed] [Google Scholar]
- 33. Wang Y, Dinse GE, Rogan WJ. Birth weight, early weight gain and pubertal maturation: a longitudinal study. Pediatr Obes. 2012;7(2):101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ferris JS, Flom JD, Tehranifar P, et al. . Prenatal and childhood environmental tobacco smoke exposure and age at menarche. Paediatr Perinat Epidemiol. 2010;24(6):515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Windham GC, Zhang L, Longnecker MP, et al. . Maternal smoking, demographic and lifestyle factors in relation to daughter’s age at menarche. Paediatr Perinat Epidemiol. 2008;22(6):551–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Terry MB, Ferris JS, Tehranifar P, et al. . Birth weight, postnatal growth, and age at menarche. Am J Epidemiol. 2009;170(1):72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fried PA, James DS, Watkinson B. Growth and pubertal milestones during adolescence in offspring prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol. 2001;23(5):431–436. [DOI] [PubMed] [Google Scholar]
- 38. Håkonsen LB, Olsen J, Støvring H, et al. . Maternal cigarette smoking during pregnancy and pubertal development in sons. A follow-up study of a birth cohort. Andrology. 2013;1(2):348–355. [DOI] [PubMed] [Google Scholar]
- 39. Ravnborg TL, Jensen TK, Andersson AM, et al. . Prenatal and adult exposures to smoking are associated with adverse effects on reproductive hormones, semen quality, final height and body mass index. Hum Reprod. 2011;26(5):1000–1011. [DOI] [PubMed] [Google Scholar]
- 40. Windham GC, Lum R, Voss R, et al. . Age at pubertal onset in girls and tobacco smoke exposure during pre- and postnatal susceptibility windows. Epidemiology. 2017;28(5):719–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Monteilh C, Kieszak S, Flanders WD, et al. . Timing of maturation and predictors of Tanner stage transitions in boys enrolled in a contemporary British cohort. Paediatr Perinat Epidemiol. 2011;25(1):75–87. [DOI] [PubMed] [Google Scholar]
- 42. Olsen J, Melbye M, Olsen SF, et al. . The Danish National Birth Cohort—its background, structure and aim. Scand J Public Health. 2001;29(4):300–307. [DOI] [PubMed] [Google Scholar]
- 43. Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45(239):13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44(235):291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Morris NM, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc. 1980;9(3):271–280. [DOI] [PubMed] [Google Scholar]
- 46.Puberty questionnaire [Danish]. https://www.ssi.dk/~/media/Indhold/DK%20-%20dansk/Forskning/BSMB/Pubertet/Pubertet%20downloads/Pubertetsskema%20PDF-version%202015.ashx. Updated December, 2015. Accessed August 31, 2018.
- 47. Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10(1):37–48. [PubMed] [Google Scholar]
- 48. Smith GD. Assessing intrauterine influences on offspring health outcomes: can epidemiological studies yield robust findings? Basic Clin Pharmacol Toxicol. 2008;102(2):245–256. [DOI] [PubMed] [Google Scholar]
- 49. Vanderweele TJ, Arah OA. Bias formulas for sensitivity analysis of unmeasured confounding for general outcomes, treatments, and confounders. Epidemiology. 2011;22(1):42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hernán MA, Hernández-Díaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15(5):615–625. [DOI] [PubMed] [Google Scholar]
- 51. Hur SS, Cropley JE, Suter CM. Paternal epigenetic programming: evolving metabolic disease risk. J Mol Endocrinol. 2017;58(3):R159–R168. [DOI] [PubMed] [Google Scholar]
- 52. Shipton D, Tappin DM, Vadiveloo T, et al. . Reliability of self reported smoking status by pregnant women for estimating smoking prevalence: a retrospective, cross sectional study. BMJ. 2009;339:b4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yermachenko A, Dvornyk V. A meta-analysis provides evidence that prenatal smoking exposure decreases age at menarche. Reprod Toxicol. 2015;58:222–228. [DOI] [PubMed] [Google Scholar]
- 54. Houghton LC, Goldberg M, Wei Y, et al. . Why do studies show different associations between intrauterine exposure to maternal smoking and age at menarche? Ann Epidemiol. 2018;28(3):197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vidmar SI, Cole TJ, Pan HQ. Standardizing anthropometric measures in children and adolescents with functions for egen: Update. Stata J. 2013;13(2):366–378. [Google Scholar]
- 56. Cnattingius S. The epidemiology of smoking during pregnancy: smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine Tob Res. 2004;6(suppl 2):S125–S140. [DOI] [PubMed] [Google Scholar]
- 57. Hernández-Díaz S, Schisterman EF, Hernán MA. The birth weight “paradox” uncovered? Am J Epidemiol. 2006;164(11):1115–1120. [DOI] [PubMed] [Google Scholar]
- 58. Wilcox AJ. Invited commentary: the perils of birth weight—a lesson from directed acyclic graphs. Am J Epidemiol. 2006;164(11):1121–1123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.