Abstract
To investigate the association of alcohol intake with colorectal cancer risk according to race/ethnicity as well as sex, lifestyle-related factors, alcoholic beverage type, and anatomical subsite, we analyzed data from 190,698 black, Native Hawaiian, Japanese-American, Latino, and white persons in Hawaii and California in the Multiethnic Cohort Study, with 4,923 incident cases during a 16.7-year follow-up period (1993–2013). In multivariate Cox regression models, the hazard ratio was 1.16 (95% confidence interval (CI): 1.01, 1.34) for 15.0–29.9 g/day of alcohol and 1.28 (95% CI: 1.12, 1.45) for ≥30.0 g/day among men, and 1.06 (95% CI: 0.85, 1.32) and 1.15 (95% CI: 0.92, 1.43), respectively, among women, compared with nondrinkers (P for heterogeneity according to sex = 0.74). An increased risk was apparent among Native Hawaiians, Japanese Americans, Latinos, and white persons and among individuals with body mass index <25.0 (calculated as weight (kg)/height (m)2), never-users of nonsteroidal antiinflammatory drugs, and those with lower intake of dietary fiber and folate. Beer and wine, but not liquor, consumption was positively related to colorectal cancer risk. The association was stronger for rectum and left-colon tumors than for right-colon tumors. Our findings suggest that the positive association between alcohol and colorectal cancer varies according to race/ethnicity, lifestyle factors, alcoholic beverage type, and anatomical subsite of tumors.
Keywords: Alcohol, cohort, colorectal cancer, multiethnic population
The latest literature review by the World Cancer Research Fund (WCRF)/American Institute for Cancer Research (AICR) concluded that the evidence for alcoholic drinks being a risk factor for colorectal cancer was convincing based on evidence for intakes above approximately 30 g per day (1). In meta-analysis, a 10-g increase in daily alcohol consumption increased colorectal cancer risk by 7% overall, 8% for men and 4% for women (1). Increased risk with alcohol consumption was also observed across geographical locations (Europe, North America, and Asia), anatomical subsites of tumor (colon and rectum), and types of alcoholic beverage (wine, beer, and spirits) (1). These subgroup analyses were, however, based on limited data. Particularly, racial/ethnic-specific associations have rarely been examined due to the homogeneity of the populations in previous studies. In addition, it is still uncertain whether the association between alcohol intake and colorectal cancer varies by age, obesity status, and folate intake (2–5).
Using data from the Multiethnic Cohort Study (MEC), a large population-based prospective cohort, we examined possible heterogeneity in the alcohol-colorectal cancer association according to race/ethnicity as well as sex, age, lifestyle-related factors, type of alcoholic beverage, and anatomical subsite of tumor.
METHODS
Study population
The MEC consists of more than 215,000 men and women in Hawaii and California, aged 45–75 years, the majority of whom are black, Native Hawaiian, Japanese American, Latino, or white (6). They entered the cohort by completing a 26-page mailed questionnaire in 1993–1996. Participants were asked to check any of the 9 racial/ethnic backgrounds: black or African American, Chinese, Filipino, Hawaiian, Japanese, Korean, Mexican or other Latino (originally “Hispanic”), white or Caucasian, and other (specify). Cohort members were grouped into 5 races/ethnicities for analysis. To be more inclusive, persons of mixed ancestry were assigned according to the following priority ranking: black, Native Hawaiian, Latino, Japanese American, and white. In the current analysis, we excluded participants who were not assigned to one of the 5 racial/ethnic groups (n = 13,987), had prior colorectal cancer reported on the baseline questionnaire (n = 2,251) or from tumor registries (n = 301), and reported implausible diets based on total energy intake (distance from the mean greater than 3 times a robust standard deviation based on a truncated normal distribution) or its components (distance from mean greater than 3.5 times robust standard deviations) (n = 8,403), leaving a total of 190,698 cohort members for analysis.
Data collection
Dietary intake was assessed at baseline using a self-administered quantitative food frequency questionnaire (QFFQ) with over 180 food items, including alcoholic beverages (6). The QFFQ queried average consumption during the previous year of regular/draft beer, light beer, white/pink wine, red wine, and hard liquor with 9 categories of frequency, ranging from “never or hardly ever” to “4 or more times a day,” and 4 portion sizes (≤1, 2, 3, and ≥4 cans/bottles/glasses/drinks). Daily intakes of nutrients and ethanol from the QFFQ were calculated using a food composition table that has been developed and maintained by the University of Hawaii Cancer Center for the MEC. The following conversion factors were used: 1 can of regular/draft beer, 13.0 g of ethanol; 1 can of light beer, 11.5 g; 1 glass of white/pink wine, 11.3 g; 1 glass of red wine, 11.2 g; and 1 drink of hard liquor, 15.4 g. A calibration study was conducted and showed satisfactory correlations between the QFFQ and three 24-hour recalls for all sex/ethnic groups being studied (7), with correlations for alcohol intake in the range of 0.56–0.88 across groups.
Case ascertainment
Incident colorectal cancer cases were identified by linkage to the Surveillance, Epidemiology, and End Results Program statewide tumor registries in Hawaii and California. Deaths were identified by linkage to death certificate files in both states and the National Death Index. Case and death ascertainment were complete through December 31, 2013. Invasive adenocarcinoma of the large bowel was the event of interest for this analysis.
Statistical analysis
Cox proportional hazards models of colorectal cancer with age as the time metric were used to calculate hazard ratios and 95% confidence intervals. Follow-up started at cohort entry and ended at the earliest of the following events: invasive colorectal cancer diagnosis, death, or study closure (December 2013). Colorectal cancer cases other than adenocarcinoma (n = 328) were censored at the date of diagnosis. Alcohol intake was categorized according to grams of ethanol per day (0, 0.1–4.9, 5.0–14.9, 15.0–29.9, and ≥30.0), and indicator variables denoting category membership were included in the models. To test for linear trend, the sex- and ethnicity-specific median value within the category was included as a continuous variable in separate models. The proportional hazards assumption was tested by Schoenfeld residuals and found to be met. Models adjusted for sex and race/ethnicity as a stratum variable and age at cohort entry as a covariate. The magnitude of the association between alcohol intake and colorectal cancer risk did not differ significantly between men and women (P = 0.74). Thus, subgroup analyses were performed for men and women combined, with adjustment for sex as a stratum variable. Multivariate models were further adjusted for the following baseline covariates: family history of colorectal cancer (yes/no), history of colorectal polyps (yes/no), body mass index (BMI, calculated as weight (kg)/height (m)2: <25.0, 25.0–29.9 and ≥30.0), pack-years of cigarette smoking (continuous), multivitamin use (at least once a week during the previous year: yes/no), nonsteroidal antiinflammatory drug (NSAID) use (at least 2 times per week for 1 month or longer: yes/no), physical activity (hours spent in vigorous work or sports per day), menopausal hormone therapy use (use of estrogen: never, past, current), and daily intakes of total energy (excluding energy from alcohol; log-transformed kcal), red meat (g/1,000 kcal), dietary fiber (g/1,000 kcal), calcium (mg), folate (dietary folate equivalents), and vitamin D (International Units). Participants with missing data on covariates (n = 22,629) were excluded from the multivariate models, resulting in 168,069 participants. Participants with missing covariates tended to be older (63.1 vs. 59.5 years), female (64.9% vs. 53.7%), and black (26.1% vs. 16.0%) or Latino (32.7% vs. 21.4%) compared with those with complete data. A sensitivity analysis using multiple imputation for missing data found that the results were similar to the complete-case approach; therefore, the latter is presented. Tests for heterogeneity were based on the Wald statistics for cross-product terms of trend variables and subgroup membership. Tests for heterogeneity according to anatomical subsite were based on the Wald statistics using competing risk methodology (8), where each subsite was a different event. A possible nonlinear relationship between alcohol intake and colorectal cancer risk was examined nonparametrically using restricted cubic splines (9). In supplemental analyses, alcohol intake was updated as a time-dependent variable using data from a 10-year follow-up survey (2003–2007) that were available for 85,495 (45%) of the 190,698 participants. All statistical tests were 2-sided. All analyses were performed using SAS, version 9.4 (SAS Institute, Inc., Cary, North Carolina).
RESULTS
During a mean follow-up of 16.7 years, 4,923 incident cases of colorectal adenocarcinoma (2,545 in men and 2,378 in women) were identified. At baseline, 38.4% of men and 62.1% of women did not consume alcohol, while 15.1% of men and 3.9% of women consumed 30.0 g or more per day (Table 1). White persons tended to drink more alcohol compared with the other racial/ethnic groups. Men and women who had higher alcohol consumption were more likely to be younger at baseline, less likely to be obese, and more likely to be smokers with higher pack-years. Also, they tended to have lower dietary fiber intakes. Among women, higher alcohol consumers tended to be users of menopausal hormone therapy and NSAIDs and to have lower energy intake, while men with higher alcohol consumption tended to be physically active and to have higher energy intake.
Table 1.
Baseline Characteristics of Participants According to Alcohol Consumption in the Multiethnic Cohort Study (n = 85,785 Men and 104,913 Women), Hawaii and California, 1993–1996
| Characteristic | Alcohol Intake, g/day | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.1–4.9 | 5.0–14.9 | 15.0–29.9 | ≥30.0 | ||||||
| % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | |
| Men | ||||||||||
| All men | 38.4 | 21.1 | 14.5 | 10.9 | 15.1 | |||||
| Age at cohort entry, years | 61.33 (8.85) | 59.90 (8.84) | 59.17 (8.74) | 58.90 (8.82) | 59.43 (8.66) | |||||
| Ethnicity | ||||||||||
| Black | 16.2 | 13.9 | 12.0 | 11.3 | 11.9 | |||||
| Native Hawaiian | 7.5 | 6.0 | 6.6 | 6.5 | 7.4 | |||||
| Japanese American | 34.7 | 27.9 | 25.4 | 27.7 | 24.0 | |||||
| Latino | 23.0 | 28.5 | 28.2 | 22.1 | 20.0 | |||||
| White | 18.6 | 23.7 | 27.8 | 32.5 | 36.6 | |||||
| Family history of colorectal cancer | 7.2 | 6.9 | 7.2 | 7.4 | 7.4 | |||||
| History of intestinal polyps | 6.8 | 6.4 | 6.5 | 7.2 | 7.7 | |||||
| Body mass indexa | ||||||||||
| <25.0 | 36.2 | 34.0 | 34.9 | 36.8 | 38.5 | |||||
| 25.0–29.9 | 44.9 | 48.5 | 48.5 | 48.8 | 46.3 | |||||
| ≥30.0 | 18.8 | 17.5 | 16.6 | 14.4 | 15.2 | |||||
| Smoking status | ||||||||||
| Never | 33.2 | 35.0 | 30.3 | 25.5 | 17.7 | |||||
| Former | 51.6 | 49.5 | 52.1 | 55.3 | 53.2 | |||||
| Current | 15.1 | 15.5 | 17.6 | 19.2 | 29.1 | |||||
| No. of pack-years among ever-smokers | 21.24 (16.85) | 18.09 (15.67) | 18.03 (15.34) | 19.75 (15.94) | 24.85 (17.67) | |||||
| Multivitamin useb | 46.6 | 49.5 | 49.7 | 48.0 | 45.5 | |||||
| NSAID usec | 50.7 | 51.3 | 51.5 | 50.7 | 51.3 | |||||
| Physical activity, hours/dayd | 0.53 (1.01) | 0.55 (0.95) | 0.61 (0.98) | 0.65 (1.06) | 0.67 (1.13) | |||||
| Daily intake | ||||||||||
| Energy, kcale | 2,284.63 (1,088.38) | 2,263.95 (1,055.41) | 2,357.01 (1,117.41) | 2,360.48 (1,074.59) | 2,433.38 (1,109.59) | |||||
| Red meat, g/1,000 kcal | 19.51 (13.41) | 20.61 (12.92) | 20.83 (12.67) | 20.64 (12.46) | 19.47 (12.04) | |||||
| Dietary fiber, g/1,000 kcal | 11.51 (4.28) | 11.44 (3.91) | 10.91 (3.62) | 10.12 (3.43) | 8.44 (3.19) | |||||
| Calcium, mgf | 1,003.27 (629.85) | 1,022.01 (619.21) | 1,052.86 (629.87) | 1,038.31 (609.31) | 1,030.10 (588.26) | |||||
| Folate, μg DFEsf | 681.09 (547.04) | 699.15 (553.50) | 722.51 (561.50) | 707.52 (540.58) | 703.64 (522.73) | |||||
| Vitamin D, IUsf | 337.14 (340.82) | 347.16 (348.38) | 358.23 (355.82) | 348.11 (344.44) | 329.49 (339.21) | |||||
| Alcohol by typeg | ||||||||||
| Beer, g | 0 | 1.97 (1.36) | 9.29 (2.78) | 21.05 (4.18) | 70.89 (59.86) | |||||
| Wine, g | 0 | 1.34 (1.13) | 9.26 (2.11) | 21.38 (4.09) | 55.66 (33.79) | |||||
| Liquor, g | 0 | 1.81 (1.22) | 8.41 (2.44) | 18.82 (3.03) | 69.68 (58.30) | |||||
| Women | ||||||||||
| All women | 62.1 | 22.1 | 7.5 | 4.3 | 3.9 | |||||
| Age at cohort entry, years | 60.55 (8.79) | 58.40 (8.70) | 57.99 (8.77) | 58.14 (8.85) | 59.36 (8.84) | |||||
| Ethnicity | ||||||||||
| Black | 20.1 | 20.0 | 19.0 | 16.7 | 18.5 | |||||
| Native Hawaiian | 7.6 | 7.0 | 7.8 | 7.0 | 7.0 | |||||
| Japanese American | 34.1 | 19.7 | 12.5 | 10.9 | 7.2 | |||||
| Latino | 22.5 | 23.6 | 18.5 | 11.7 | 9.3 | |||||
| White | 15.7 | 29.7 | 42.2 | 53.7 | 58.0 | |||||
| Family history of colorectal cancer | 8.6 | 8.4 | 8.2 | 9.3 | 8.7 | |||||
| History of intestinal polyps | 4.5 | 3.8 | 3.8 | 4.4 | 5.0 | |||||
| Body mass indexa | ||||||||||
| <25.0 | 43.2 | 46.9 | 53.4 | 59.1 | 57.8 | |||||
| 25.0–29.9 | 32.1 | 33.2 | 30.7 | 27.3 | 28.9 | |||||
| ≥30.0 | 24.7 | 19.9 | 15.9 | 13.7 | 13.3 | |||||
| Smoking status | ||||||||||
| Never | 62.5 | 52.1 | 40.3 | 33.6 | 22.7 | |||||
| Former | 26.1 | 33.3 | 39.4 | 41.8 | 41.5 | |||||
| Current | 11.4 | 14.7 | 20.3 | 24.6 | 35.7 | |||||
| No. of pack-years among ever-smokers | 15.16 (14.28) | 13.73 (13.49) | 14.86 (13.79) | 17.44 (14.88) | 22.41 (16.81) | |||||
| Multivitamin useb | 52.3 | 57.2 | 57.6 | 55.7 | 54.8 | |||||
| NSAID usec | 52.1 | 56.1 | 57.0 | 57.3 | 59.9 | |||||
| Physical activity, hours/dayd | 0.18 (0.52) | 0.24 (0.55) | 0.30 (0.64) | 0.30 (0.63) | 0.28 (0.65) | |||||
| MHT use | ||||||||||
| Never | 55.3 | 51.2 | 50.1 | 48.5 | 47.6 | |||||
| Past | 18.3 | 17.4 | 17.4 | 17.0 | 20.5 | |||||
| Current | 26.4 | 31.4 | 32.6 | 34.5 | 31.9 | |||||
| Daily intake | ||||||||||
| Energy, kcale | 1,947.31 (966.54) | 1,973.10 (952.48) | 1,974.85 (964.26) | 1,882.32 (899.91) | 1,882.14 (932.10) | |||||
| Red meat, g/1,000 kcal | 16.96 (12.25) | 17.50 (12.01) | 17.32 (12.19) | 17.01 (11.86) | 16.57 (11.41) | |||||
| Dietary fiber, g/1,000 kcal | 12.86 (4.39) | 12.70 (4.05) | 12.20 (3.81) | 11.34 (3.55) | 9.54 (3.43) | |||||
| Calcium, mgf | 1,086.32 (734.64) | 1,151.99 (741.90) | 1,177.95 (756.93) | 1,165.40 (754.76) | 1,116.02 (711.84) | |||||
| Folate, μg DFEsf | 662.29 (525.93) | 708.99 (546.09) | 721.40 (546.59) | 704.70 (532.62) | 690.20 (532.57) | |||||
| Vitamin D, IUsf | 329.60 (337.72) | 363.19 (358.54) | 374.39 (367.05) | 372.22 (367.81) | 358.32 (361.24) | |||||
| Alcohol by typeg | ||||||||||
| Beer, g | 0 | 1.61 (1.28) | 9.24 (2.68) | 20.91 (4.06) | 71.93 (60.11) | |||||
| Wine, g | 0 | 1.24 (1.09) | 9.14 (2.06) | 20.95 (3.86) | 50.89 (29.58) | |||||
| Liquor, g | 0 | 1.63 (1.20) | 8.48 (2.54) | 18.33 (3.00) | 58.61 (45.67) | |||||
Abbreviations; DFE, dietary folate equivalent; IU, International Unit; MHT, menopausal hormone therapy; NSAID, nonsteroidal antiinflammatory drug; SD, standard deviation.
a Weight (kg)/height(m)2.
b At least once a week during the previous year.
c At least 2 times per week for 1 month or longer.
d Hours spent in vigorous work or sports per day.
e Excluding energy from alcohol.
f From foods and supplements.
g Among drinkers who consumed only one type of alcohol.
Alcohol consumption of ≥15.0 g/day was associated with an increased risk of colorectal cancer after adjustment for age, sex, and race/ethnicity; hazard ratios were 1.11 (95% confidence interval (CI): 1.00, 1.24) for 15.0–29.9 g/day and 1.37 (95% CI: 1.24, 1.50) for ≥30.0 g/day, compared with nondrinkers (P for trend < 0.001), and these ratios remained similar after excluding participants with missing covariates (Table 2). After further adjustment for covariates, increased risks were still observed (P for trend < 0.001). The association appeared to be stronger among men than among women, but the test for heterogeneity between men and women was not statistically significant (P = 0.74). Based on nonparametric cubic splines, the relationship between alcohol consumption and colorectal cancer risk was linear in both sexes (Figure 1).
Table 2.
Alcohol Intake and Colorectal Cancer Risk in the Multiethnic Cohort Study, Hawaii and California, 1993–2013
| Subgroup | No. of Cases | Alcohol Intake, g/day | Per 10-g Increment | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.1–4.9 | 5.0–14.9 | 15.0–29.9 | ≥30.0 | P for Trend | |||||||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |||
| All | ||||||||||||||
| Model 1a | 4,923 | 1.00 | Referent | 0.99 | 0.92, 1.07 | 0.93 | 0.84, 1.03 | 1.11 | 1.00, 1.24 | 1.37 | 1.24, 1.50 | <0.001 | 1.03 | 1.02, 1.04 |
| Model 2a,b | 4,327 | 1.00 | Referent | 0.96 | 0.89, 1.04 | 0.93 | 0.83, 1.03 | 1.13 | 1.01, 1.27 | 1.36 | 1.23, 1.50 | <0.001 | 1.03 | 1.02, 1.04 |
| Model 3b,c | 4,327 | 1.00 | Referent | 0.99 | 0.91, 1.07 | 0.94 | 0.84, 1.05 | 1.12 | 1.00, 1.26 | 1.24 | 1.11, 1.37 | <0.001 | 1.02 | 1.01, 1.03 |
| Sex | ||||||||||||||
| Male | 2,304 | 1.00 | Referent | 1.06 | 0.94, 1.18 | 0.96 | 0.84, 1.10 | 1.16 | 1.01, 1.34 | 1.28 | 1.12, 1.45 | <0.001 | 1.02 | 1.01, 1.03 |
| Female | 2,023 | 1.00 | Referent | 0.93 | 0.83, 1.04 | 0.93 | 0.77, 1.12 | 1.06 | 0.85, 1.32 | 1.15 | 0.92, 1.43 | 0.16 | 1.01 | 0.98, 1.04 |
| P for heterogeneity | 0.74 | |||||||||||||
| Age at cohort entry, years | ||||||||||||||
| 45–54 | 830 | 1.00 | Referent | 0.99 | 0.82, 1.18 | 1.00 | 0.80, 1.25 | 0.81 | 0.61, 1.08 | 1.36 | 1.08, 1.71 | 0.02 | 1.02 | 1.00, 1.04 |
| 55–64 | 1,405 | 1.00 | Referent | 0.99 | 0.86, 1.14 | 0.97 | 0.80, 1.16 | 1.22 | 1.01, 1.49 | 1.17 | 0.97, 1.41 | 0.05 | 1.02 | 1.00, 1.03 |
| 65–75 | 2,092 | 1.00 | Referent | 1.00 | 0.89, 1.12 | 0.89 | 0.76, 1.05 | 1.21 | 1.03, 1.44 | 1.23 | 1.05, 1.44 | 0.002 | 1.02 | 1.00, 1.03 |
| P for heterogeneity | 0.29 | |||||||||||||
| Race/ethnicity | ||||||||||||||
| Black | 800 | 1.00 | Referent | 1.06 | 0.89, 1.26 | 0.85 | 0.65, 1.11 | 1.01 | 0.74, 1.37 | 1.03 | 0.77, 1.38 | 0.95 | 0.99 | 0.97, 1.02 |
| Native Hawaiian | 276 | 1.00 | Referent | 1.29 | 0.94, 1.76 | 1.08 | 0.72, 1.64 | 1.04 | 0.63, 1.70 | 1.64 | 1.12, 2.40 | 0.03 | 1.02 | 0.99, 1.06 |
| Japanese American | 1,554 | 1.00 | Referent | 1.06 | 0.92, 1.21 | 1.02 | 0.84, 1.24 | 1.15 | 0.94, 1.42 | 1.38 | 1.14, 1.68 | 0.001 | 1.04 | 1.02, 1.06 |
| Latino | 808 | 1.00 | Referent | 0.90 | 0.76, 1.08 | 0.91 | 0.72, 1.16 | 1.29 | 0.99, 1.68 | 1.20 | 0.93, 1.55 | 0.03 | 1.01 | 0.98, 1.03 |
| White | 889 | 1.00 | Referent | 0.83 | 0.69, 1.00 | 0.90 | 0.72, 1.12 | 1.06 | 0.85, 1.32 | 1.11 | 0.90, 1.35 | 0.06 | 1.02 | 1.00, 1.04 |
| P for heterogeneity | 0.07 | |||||||||||||
| Family history of colorectal cancer | ||||||||||||||
| No | 3,854 | 1.00 | Referent | 0.97 | 0.89, 1.06 | 0.97 | 0.87, 1.08 | 1.13 | 1.00, 1.28 | 1.25 | 1.12, 1.40 | <0.001 | 1.02 | 1.00, 1.03 |
| Yes | 473 | 1.00 | Referent | 1.13 | 0.90, 1.43 | 0.69 | 0.47, 1.00 | 1.04 | 0.72, 1.50 | 1.06 | 0.75, 1.50 | 0.81 | 1.03 | 0.99, 1.06 |
| P for heterogeneity | 0.23 | |||||||||||||
| Body mass indexd | ||||||||||||||
| <25.0 | 1,711 | 1.00 | Referent | 0.98 | 0.86, 1.12 | 0.99 | 0.83, 1.17 | 1.15 | 0.96, 1.39 | 1.38 | 1.17, 1.63 | <0.001 | 1.03 | 1.02, 1.05 |
| 25.0–29.9 | 1,700 | 1.00 | Referent | 0.96 | 0.85, 1.09 | 0.91 | 0.77, 1.07 | 1.09 | 0.91, 1.30 | 1.10 | 0.93, 1.31 | 0.14 | 1.00 | 0.98, 1.02 |
| ≥30.0 | 916 | 1.00 | Referent | 1.07 | 0.90, 1.26 | 0.92 | 0.71, 1.18 | 1.13 | 0.85, 1.51 | 1.26 | 0.98, 1.62 | 0.07 | 1.01 | 0.99, 1.04 |
| P for heterogeneity | 0.10 | |||||||||||||
| Physical activity, hours/daye | ||||||||||||||
| 0 | 2,114 | 1.00 | Referent | 0.99 | 0.88, 1.11 | 0.91 | 0.77, 1.07 | 1.04 | 0.86, 1.26 | 1.14 | 0.97, 1.35 | 0.12 | 1.01 | 0.99, 1.03 |
| 0.1–0.2 | 965 | 1.00 | Referent | 0.99 | 0.84, 1.17 | 0.90 | 0.72, 1.13 | 1.20 | 0.94, 1.52 | 1.38 | 1.10, 1.72 | 0.002 | 1.03 | 1.00, 1.05 |
| ≥0.3 | 1,248 | 1.00 | Referent | 1.00 | 0.86, 1.17 | 1.02 | 0.85, 1.23 | 1.20 | 0.99, 1.46 | 1.29 | 1.08, 1.55 | 0.002 | 1.02 | 1.00, 1.04 |
| P for heterogeneity | 0.72 | |||||||||||||
| Smoking status | ||||||||||||||
| Never | 1,723 | 1.00 | Referent | 0.95 | 0.84, 1.08 | 0.93 | 0.76, 1.12 | 1.01 | 0.80, 1.29 | 1.35 | 1.07, 1.69 | 0.01 | 1.02 | 0.99, 1.05 |
| Former | 1,897 | 1.00 | Referent | 0.99 | 0.88, 1.12 | 0.91 | 0.78, 1.06 | 1.17 | 1.00, 1.36 | 1.14 | 0.98, 1.33 | 0.03 | 1.02 | 1.00, 1.03 |
| Current | 707 | 1.00 | Referent | 1.08 | 0.88, 1.33 | 1.03 | 0.80, 1.33 | 1.12 | 0.85, 1.46 | 1.35 | 1.08, 1.68 | 0.009 | 1.02 | 1.00, 1.04 |
| P for heterogeneity | 0.46 | |||||||||||||
| NSAID use | ||||||||||||||
| Never | 2,215 | 1.00 | Referent | 0.97 | 0.87, 1.09 | 1.02 | 0.87, 1.18 | 1.15 | 0.97, 1.35 | 1.35 | 1.16, 1.56 | <0.001 | 1.03 | 1.01, 1.04 |
| Ever | 2,112 | 1.00 | Referent | 1.00 | 0.90, 1.12 | 0.87 | 0.74, 1.01 | 1.10 | 0.93, 1.29 | 1.12 | 0.96, 1.31 | 0.11 | 1.00 | 0.99, 1.02 |
| P for heterogeneity | 0.02 | |||||||||||||
| MHT use among women | ||||||||||||||
| Never | 1,119 | 1.00 | Referent | 0.90 | 0.77, 1.05 | 0.90 | 0.69, 1.17 | 0.92 | 0.67, 1.28 | 1.09 | 0.79, 1.49 | 0.67 | 1.02 | 0.98, 1.06 |
| Ever | 904 | 1.00 | Referent | 0.95 | 0.81, 1.13 | 0.95 | 0.73, 1.24 | 1.21 | 0.89, 1.64 | 1.23 | 0.90, 1.68 | 0.10 | 1.01 | 0.96, 1.05 |
| P for heterogeneity | 0.56 | |||||||||||||
| Dietary fiber, g/1,000 kcal | ||||||||||||||
| <9.6 | 1,600 | 1.00 | Referent | 1.05 | 0.91, 1.22 | 0.94 | 0.78, 1.12 | 1.22 | 1.02, 1.45 | 1.46 | 1.26, 1.68 | <0.001 | 1.02 | 1.01, 1.04 |
| 9.6–13.2 | 1,430 | 1.00 | Referent | 0.94 | 0.82, 1.08 | 0.92 | 0.77, 1.10 | 0.99 | 0.80, 1.21 | 0.97 | 0.78, 1.22 | 0.92 | 1.00 | 0.96, 1.03 |
| ≥13.3 | 1,297 | 1.00 | Referent | 0.99 | 0.86, 1.14 | 1.02 | 0.83, 1.24 | 1.29 | 1.01, 1.65 | 1.20 | 0.88, 1.65 | 0.09 | 1.06 | 1.01, 1.11 |
| P for heterogeneity | 0.006 | |||||||||||||
| Total folate intake, μg DFEsf | ||||||||||||||
| <350 | 1,520 | 1.00 | Referent | 1.01 | 0.88, 1.16 | 0.93 | 0.77, 1.13 | 1.22 | 1.01, 1.49 | 1.43 | 1.19, 1.71 | <0.001 | 1.03 | 1.01, 1.06 |
| 350–769 | 1,449 | 1.00 | Referent | 0.95 | 0.83, 1.10 | 0.85 | 0.70, 1.03 | 1.07 | 0.88, 1.31 | 1.21 | 1.01, 1.45 | 0.02 | 1.01 | 1.00, 1.03 |
| ≥770 | 1,358 | 1.00 | Referent | 1.00 | 0.87, 1.15 | 1.04 | 0.87, 1.25 | 1.07 | 0.87, 1.32 | 1.08 | 0.89, 1.32 | 0.41 | 1.01 | 1.00, 1.03 |
| P for heterogeneity | 0.03 | |||||||||||||
| Alcohol typeg | ||||||||||||||
| Beer | 639 | 1.00 | Referent | 1.12 | 0.98, 1.28 | 1.06 | 0.87, 1.29 | 1.22 | 0.99, 1.51 | 1.47 | 1.23, 1.76 | <0.001 | 1.04 | 1.02, 1.05 |
| Wine | 344 | 1.00 | Referent | 0.96 | 0.84, 1.09 | 0.94 | 0.68, 1.31 | 1.22 | 0.85, 1.76 | 1.83 | 1.18, 2.83 | 0.02 | 1.08 | 1.01, 1.14 |
| Liquor | 156 | 1.00 | Referent | 1.02 | 0.81, 1.28 | 1.14 | 0.76, 1.71 | 1.40 | 0.90, 2.15 | 1.11 | 0.79, 1.57 | 0.20 | 1.02 | 0.98, 1.06 |
| Anatomical subsite | ||||||||||||||
| Right colon | 2,042 | 1.00 | Referent | 0.94 | 0.84, 1.06 | 0.93 | 0.79, 1.08 | 1.05 | 0.89, 1.26 | 1.06 | 0.90, 1.25 | 0.32 | 1.01 | 0.99, 1.03 |
| Left colon | 1,215 | 1.00 | Referent | 1.13 | 0.98, 1.32 | 1.03 | 0.84, 1.26 | 1.15 | 0.92, 1.43 | 1.35 | 1.11, 1.65 | 0.008 | 1.02 | 1.00, 1.04 |
| Rectum | 988 | 1.00 | Referent | 0.96 | 0.81, 1.14 | 0.88 | 0.70, 1.10 | 1.23 | 0.98, 1.55 | 1.42 | 1.16, 1.75 | <0.001 | 1.02 | 1.00, 1.04 |
| P for heterogeneity | <0.001 | |||||||||||||
Abbreviation: CI, confidence interval; DFE, dietary folate equivalent; HR, hazard ratio; MHT, menopausal hormone therapy; NSAID, nonsteroidal antiinflammatory drug.
a Adjusted for age at cohort entry, sex, and race/ethnicity.
b Excluding participants with missing data on any of the covariates.
c Additionally adjusted for family history of colorectal cancer, history of colorectal polyps, body mass index, pack-years of cigarette smoking, multivitamin use, NSAID use, physical activity, MHT use, and daily intakes of total energy (excluding energy from alcohol), red meat, dietary fiber, calcium, folate, and vitamin D. All subgroup analyses were based on Model 3, excluding covariates corresponding to stratification variables.
d Weight (kg)/height(m)2.
e Hours spent in vigorous work or sports per day.
f From foods and supplements.
g Among exclusive drinkers who drank only one type of alcohol. Nondrinkers of any types of alcohol were the reference group.
Figure 1.
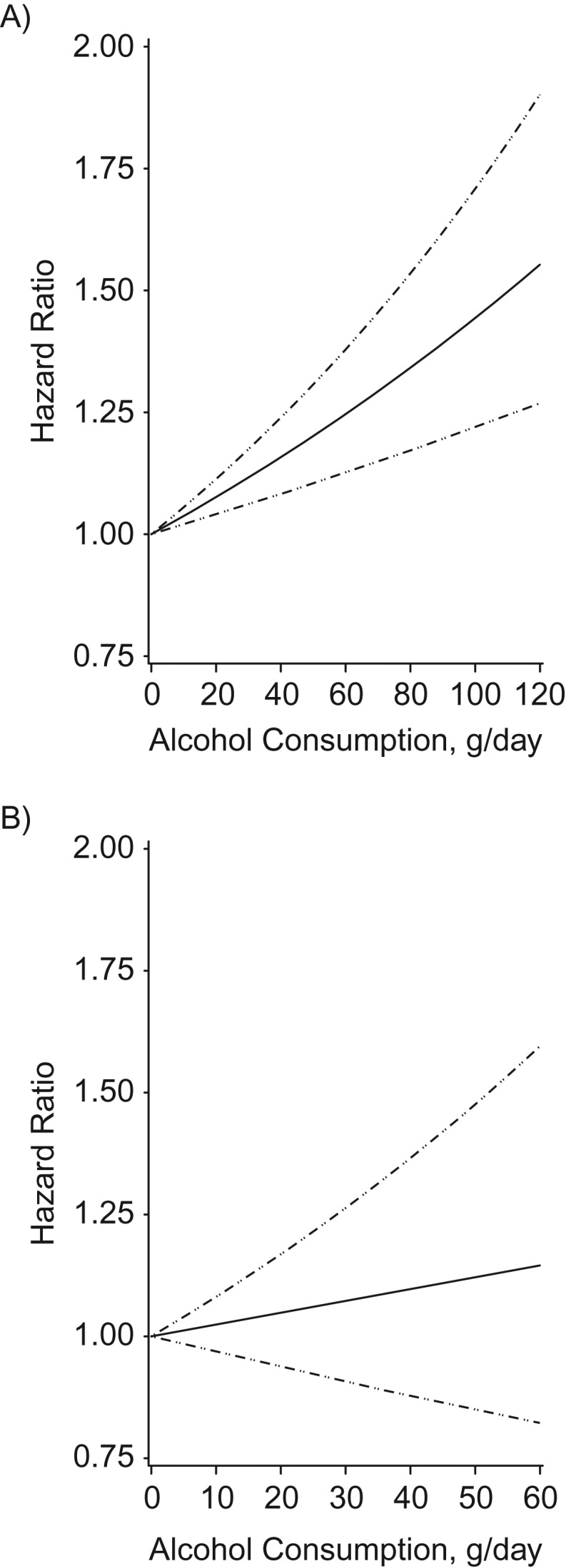
Multivariate association between alcohol intake and colorectal cancer risk, based on restricted cubic splines, among men (A) and women (B) in the Multiethnic Cohort Study, Hawaii and California, 1993–2013. The solid line indicates the hazard ratio, and the dashed lines indicate the 95% confidence intervals for adjusted estimates.
In racial/ethnic-specific analyses combining men and women (Table 2), Native Hawaiian, Japanese-American, Latino, and white persons showed significant or suggestive associations, whereas black persons did not (P for heterogeneity = 0.07). Stronger increased risks of colorectal cancer with alcohol consumption were apparent in participants with lower BMI (<25.0 (P for heterogeneity = 0.02 for <25.0 vs. ≥25.0), NSAID never-users (P for heterogeneity = 0.02), those with lower intake of dietary fiber (<9.6 g/1,000 kcal, P for heterogeneity = 0.006), and those with lower folate intake from foods and supplements (<770 dietary folate equivalents, P for heterogeneity = 0.03). We fitted the models according to folate intake from foods only, limiting to nonusers of multivitamins, which were major sources for supplemental folate at the time of cohort entry (1993–1996). Folate intake measured by the QFFQ at baseline reflected the prefortification era (before 1998). To account for folate fortification, we fitted the models with records split at January 1, 1998, using the prefortification values for the first part and the postfortification values for the latter part. The results were similar regardless of how folate intake was estimated (data not shown). Moreover, alcohol intake assessed in the 10-year follow-up questionnaire was also associated with subsequent diagnosis of colorectal cancer (hazard ratio (HR) = 1.40, 95% CI: 1.08, 1.81 for ≥30.0 g/day vs. nondrinking), suggesting that this association was observed after folic acid fortification of the food supply in the United States.
When examining specific types of alcohol among individuals who drank only one type of alcohol (Table 2), an increased risk was found for beer and wine but not for liquor, compared with nondrinkers of any type of alcohol. In subsite-specific analyses, the association was stronger for the left colon and rectum than for the right colon (P for heterogeneity < 0.001). There was no indication for heterogeneity in the associations according to age group, family history of colorectal cancer, physical activity, smoking status, and menopausal hormone therapy use among women. In particular, an increased risk with higher alcohol consumption in never-smokers as well as in current smokers suggests no confounding effect of smoking on the association between alcohol intake and colorectal cancer risk.
DISCUSSION
In this large, multiethnic, population-based cohort, higher alcohol consumption was associated with an increased risk of colorectal cancer, with risk estimates that were statistically significant for men and of borderline significance for women after adjustment for potential confounders. Among men and women combined, an increased risk was apparent among Native Hawaiian, Japanese-American, Latino, and white persons; NSAID never-users; participants with lower BMI; those with lower dietary fiber intake; and those with lower folate intake. Consumption of alcohol exclusively from beer and wine, but not significantly for liquor, was related to an increased risk of colorectal cancer. The association was stronger for left-colon and rectum than for right-colon tumors.
In a meta-analysis conducted as part of a systematic literature review (SLR) by the WCRF/AICR Continuous Update Project in 2017, a summary relative risk of colorectal cancer was 1.07 (95% CI: 1.05, 1.08) per 10-g/day increment of ethanol based on 16 cohort studies (2), which is stronger than in the present study (HR = 1.02, 95% CI: 1.01, 1.03). The association of heavy drinking (≥30.0 g/day) with colorectal cancer risk in the MEC (HR = 1.24, 95% CI: 1.11, 1.37) also appears weaker compared with the Health Professionals Follow-up Study (relative risk (RR) = 1.38, 95% CI: 1.11, 1.72) (10), the Nurses’ Health Study (RR = 1.30, 95% CI: 1.00, 1.69) (10), and the Netherland Cohort Study (HR = 1.32, 95% CI: 1.06, 1.65) (11). We speculate that the upper range of alcohol consumption might be more limited for the MEC data, although proportions of heavy drinkers (≥30.0 g/day) in the MEC (15.1% of men and 3.9% of women at baseline in 1993–1996) were comparable to those in the Health Professionals Follow-up Study (11.9% in 1986) and the Nurses’ Health Study (4.0% in 1986) (12).
In the WCRF/AICR SLR, a nonlinear dose-response relationship was observed with no significant risk increase up to 20 g/day, with a significant linear increase in risk for 30 g/day and above (1). In the MEC, the relationship between alcohol consumption and colorectal cancer risk was linear in both sexes, and the risk appeared elevated at relatively low intake levels for men (e.g., HR = 1.06 for values between 15 and 20 g/day) (Figure 1).
The SLR suggested a possibly greater association among men (RR = 1.08, 95% CI: 1.06, 1.09) than among women (RR = 1.04, 95% CI: 1.00, 1.07) (1), which is consistent with our findings. This greater elevated risk may be because of the generally higher consumption of alcohol among men. Also, men and women may prefer different types of alcoholic drinks, and there may be hormone-related differences in alcohol metabolism or differences in susceptibility to the effect of alcohol (2). Indeed, in the MEC, mean daily alcohol consumption among drinkers was higher at 23.8 (standard deviation, 38.9) g among men than at 11.3 (standard deviation, 22.4) g among women. In addition, proportions of drinkers who only consumed one type of alcohol varied according to sex: for beer, wine, and liquor, respectively, 32.4%, 7.4%, and 4.9% of male drinkers and 13.3%, 32.0%, and 9.3% of female drinkers.
There is limited literature on the alcohol and colorectal cancer association according to race/ethnicity. Our findings in the MEC suggest that the association was strongest among Japanese Americans compared with the other racial/ethnic groups in MEC. In the WCRF/AICR SLR, the summary relative risk of colorectal cancer per 10 g/day of ethanol did not vary much by geographical location: 1.07 (95% CI: 1.06, 1.08) for Asia (based on 7 studies), 1.06 (95% CI: 1.01, 1.12) for North America (based on 4 studies), and 1.05 (95% CI: 1.02, 1.08) for Europe (based on 5 studies) (2). Another meta-analysis reported a stronger association for heavy drinkers (≥4 drinks/day) than for nondrinkers or occasional drinkers in Asian studies (RR = 1.81; 95% CI: 1.33, 2.46) than in European (RR = 1.16; 95% CI: 0.95, 1.43) and North American (RR = 1.59; 95% CI: 1.23, 2.01) studies (3). Potential explanations for these findings include a high prevalence of the slow-metabolizing variant of the aldehyde dehydrogenase gene in Asian populations, which is associated with increased blood levels of acetaldehyde after alcohol ingestion, and, possibly, other nongenetic factors (e.g., body size) (3, 13, 14). However, when we examined alcohol consumption per body weight, Japanese Americans still showed stronger trends than the other racial/ethnic groups.
In the present study, we found that alcohol consumption was not related to an increased risk among NSAID users and individuals with a high dietary fiber intake, while NSAID nonusers and low fiber consumers showed an increased risk with alcohol. In the MEC, NSAID use at baseline was associated with a 21% decrease in risk of colorectal cancer in men (15). Our findings suggest that NSAID use and a high-fiber diet may alleviate the adverse effects of alcohol consumption on incidence of colorectal cancer.
Folate deficiency has been shown to exacerbate the adverse effects of heavy alcohol consumption on incidence of colorectal cancer (4). In the Nurses’ Health Study and Health Professionals Follow-up Study, increased risk of colorectal cancer with alcohol consumption of ≥30 g/day was no longer statistically significant for the postfortification period (10). We also found that an increased risk with alcohol consumption was not found in participants with high folate intake, although folate intake was not related to colorectal cancer risk in our data (for the highest vs. lowest quintiles of folate intake, HR = 1.12, 95% CI: 0.93, 1.36). Thus, our findings offer additional evidence suggesting that adequate intake of folate may protect against the increased risk of colorectal cancer associated with alcohol consumption.
In the MEC, the proportion of current smokers in the highest category of alcohol consumption was high and could have confounded the results. However, we found an increased risk with higher alcohol consumption in never-smokers as well as in former and current smokers, which suggests that smoking did not confound the association between alcohol intake and colorectal cancer risk in our data.
In the WCRF/AICR SLR, significant increase in risk of colorectal cancer was observed for consumption of beer (RR per 10 g/day = 1.08, 95% CI: 1.05, 1.11 based on 5 studies), wine (RR = 1.04, 95% CI: 1.01, 1.08 based on 6 studies), and liquor (RR = 1.08, 95% CI: 1.02, 1.14 based on 4 studies) (2). In the present study, alcohol consumption from beer and wine was associated with an increased risk of colorectal cancer, while liquor consumption was not, among individuals who drank only one type of alcohol. However, liquor-only consumers represented only 6.8% of drinkers in the MEC, while beer-only and wine-only consumers represented 24.2% and 18.0%, respectively.
Previous meta-analyses showed that the association of alcohol drinking with colorectal cancer risk did not differ according to colon and rectal anatomical subsites (2, 3, 14, 16, 17). However, our study showed a stronger association for rectal than colon tumors (P for heterogeneity < 0.001). Another meta-analysis (3) suggested a stronger positive association of moderate and heavy alcohol drinking with cancer in the left colon compared with cancer in the right colon, but the difference was not statistically significant. We also found a stronger association for left- compared with right-colon cancer in the present study (P for heterogeneity < 0.001). The reasons for this disparity in site association remain unclear. Embryological and physiological differences between anatomical subsites might be related to different etiologic pathways for colorectal cancer (18, 19).
Because of the large sample size and a multiethnic population in the MEC, we were able to examine associations for various subgroups defined by race/ethnicity, as well as sex, age, family history of colorectal cancer, BMI, physical activity, NSAID use, dietary fiber and folate intakes, and, for women, menopausal hormone therapy use. We were also able to control for a wide range of potential confounders due to a validated QFFQ and other comprehensive information. Nevertheless, there are limitations to be considered. Self-reported alcohol consumption is subject to measurement error. Only the alcohol consumption measured at baseline was used for the current analysis. However, using data from a 10-year follow-up survey (2003–2007), which was completed by 45% of the participants included in this analysis, we fitted the models with a time-dependent alcohol intake and found similar results. In addition, we had no information on lifetime consumption and drinking patterns, which may affect the risk of colorectal cancer. In the MEC, race/ethnicity was defined by self-report, which might cause misclassification. However, we found high correspondence between self-reported ethnicity and genetic ancestry (20).
In conclusion, our prospective data confirm that alcohol is a risk factor for colorectal cancer in both men and women, and they suggest that the association varies by race/ethnicity, BMI, NSAID use, dietary fiber and folate consumption, type of alcoholic beverage, and anatomical subsite of tumors. Given the heterogeneity of the associations, multiple underlying mechanisms are likely to be at play.
ACKNOWLEDGMENTS
Author affiliations: Cancer Epidemiology Program, University of Hawaii Cancer Center, Honolulu, Hawaii (Song-Yi Park, Lynne R. Wilkens, Loïc Le Marchand); and Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California (Veronica Wendy Setiawan, Kristine R. Monroe, Christopher A. Haiman).
This work was supported in part by the National Cancer Institute (grant U01CA164973).
Conflict of interest: none declared.
Abbreviations
- AICR
American Institute for Cancer Research
- BMI
body mass index
- CI
confidence interval
- HR
hazard ratio
- MEC
Multiethnic Cohort Study
- NSAID
nonsteroidal antiinflammatory drug
- QFFQ
quantitative food frequency questionnaire
- RR
relative risk
- SLR
systematic literature review
- WCRF
World Cancer Research Fund
REFERENCES
- 1. World Cancer Research Fund/American Institute for Cancer Research Continuous Update Project Report: Diet, Nutrition, Physical Activity and Colorectal Cancer 2017. https://www.wcrf.org/sites/default/files/Colorectal-cancer-report.pdf. Accessed September 6, 2018.
- 2. World Cancer Research Fund/American Institute for Cancer Research World Cancer Research Fund International Systematic Literature Review. The Associations between Food, Nutrition and Physical Activity and the Risk of Colorectal Cancer. 2017. https://www.wcrf.org/sites/default/files/colorectal-cancer-slr.pdf. Accessed September 6, 2018.
- 3. Fedirko V, Tramacere I, Bagnardi V, et al. . Alcohol drinking and colorectal cancer risk: an overall and dose-response meta-analysis of published studies. Ann Oncol. 2011;22(9):1958–1972. [DOI] [PubMed] [Google Scholar]
- 4. Klarich DS, Brasser SM, Hong MY. Moderate alcohol consumption and colorectal cancer risk. Alcohol Clin Exp Res. 2015;39(8):1280–1291. [DOI] [PubMed] [Google Scholar]
- 5. Wang Y, Duan H, Yang H, et al. . A pooled analysis of alcohol intake and colorectal cancer. Int J Clin Exp Med. 2015;8(5):6878–6889. [PMC free article] [PubMed] [Google Scholar]
- 6. Kolonel LN, Henderson BE, Hankin JH, et al. . A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol. 2000;151(4):346–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stram DO, Hankin JH, Wilkens LR, et al. . Calibration of the dietary questionnaire for a multiethnic cohort in Hawaii and Los Angeles. Am J Epidemiol. 2000;151(4):358–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Therneau TM, Grambsh PM. Modeling Survival Data: Extending the Cox Model. New York, NY: Springer; 2000. [Google Scholar]
- 9. Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551–561. [DOI] [PubMed] [Google Scholar]
- 10. Nan H, Lee JE, Rimm EB, et al. . Prospective study of alcohol consumption and the risk of colorectal cancer before and after folic acid fortification in the United States. Ann Epidemiol. 2013;23(9):558–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bongaerts BW, van den Brandt PA, Goldbohm RA, et al. . Alcohol consumption, type of alcoholic beverage and risk of colorectal cancer at specific subsites. Int J Cancer. 2008;123(10):2411–2417. [DOI] [PubMed] [Google Scholar]
- 12. Cho E, Lee JE, Rimm EB, et al. . Alcohol consumption and the risk of colon cancer by family history of colorectal cancer. Am J Clin Nutr. 2012;95(2):413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eriksson CJ. The role of acetaldehyde in the actions of alcohol (update 2000). Alcohol Clin Exp Res. 2001;25(5 suppl ISBRA):15S–32S. [DOI] [PubMed] [Google Scholar]
- 14. Mizoue T, Inoue M, Wakai K, et al. . Alcohol drinking and colorectal cancer in Japanese: a pooled analysis of results from five cohort studies. Am J Epidemiol. 2008;167(12):1397–1406. [DOI] [PubMed] [Google Scholar]
- 15. Park SY, Wilkens LR, Kolonel LN, et al. . Exploring differences in the aspirin-colorectal cancer association by sex and race/ethnicity: the Multiethnic Cohort Study. Cancer Epidemiol Biomarkers Prev. 2017;26(2);162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moskal A, Norat T, Ferrari P, et al. . Alcohol intake and colorectal cancer risk: a dose-response meta-analysis of published cohort studies. Int J Cancer. 2007;120(3):664–671. [DOI] [PubMed] [Google Scholar]
- 17. Cho E, Smith-Warner SA, Ritz J, et al. . Alcohol intake and colorectal cancer: a pooled analysis of 8 cohort studies. Ann Intern Med. 2004;140(8):603–613. [DOI] [PubMed] [Google Scholar]
- 18. Zhang C, Zhong M. Consumption of beer and colorectal cancer incidence: a meta-analysis of observational studies. Cancer Causes Control. 2015;26(4):549–560. [DOI] [PubMed] [Google Scholar]
- 19. Li FY, Lai MD. Colorectal cancer, one entity or three. J Zhejiang Univ Sci B. 2009;10(3):219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang H, Haiman CA, Kolonel LN, et al. . Self-reported ethnicity, genetic structure and the impact of population stratification in a multiethnic study. Hum Genet. 2010;128(2):165–177. [DOI] [PMC free article] [PubMed] [Google Scholar]


