Abstract
Few data exist on the incidence or duration of natural Plasmodium falciparum infections in high-transmission settings. School-aged children (SAC) carry a disproportionate burden of infections, suggesting either increased incidence or increased duration. We estimated the incidence and duration of unique infections according to age groups. The Mfera Cohort Study (2014–2017) in Malawi had 2 years of follow-up, with 120 participants tested monthly and during sick visits. Blood samples were collected to detect P. falciparum by microscopy and polymerase chain reaction. Positive samples underwent genotyping. Simulation was used to account for high rates of nondetection of infection among low-parasitemia infections, which increase in frequency with age. Adults had significantly fewer unique infections per person per year (median, 2.5) compared with SAC and children younger than 5 years of age (6.3 and 6.6, respectively). Over half of all genotypes were persistent. Infections lasted significantly longer in adults (median, 180 days) and SAC (median, 163 days) compared with children younger than 5 years of age (median, 97 days), after accounting for age-dependent nondetection of infection. SAC acquired new infections at the same rate as children younger than 5 years, but they maintained these infections for longer periods of time, similar to adults. This study provides new insights into P. falciparum infection dynamics that should be considered when designing malaria control strategies.
Keywords: asymptomatic Plasmodium falciparum infection, Malawi, molecular force of infection, molFOI, persistent infection, Plasmodium incidence
Malaria caused approximately 445,000 deaths in 2016, principally among children younger than 5 years of age in sub-Saharan Africa (1). Despite the high mortality associated with malaria, about 90% of Plasmodium falciparum infections in Malawi are asymptomatic, with the highest infection prevalence occurring in school-aged children (SAC, aged 5–15 years) (2). Despite high uptake of interventions, overall prevalence of P. falciparum infection was 33% in southern Malawi in 2014 (3, 4).
Age is commonly used as a proxy for acquired antimalarial immunity, but cross-sectional surveys in sub-Saharan Africa have demonstrated a disproportionately greater infection burden among SAC (2, 5–7), adding to data suggesting complexity in the association between age and measures of P. falciparum transmission intensity (2, 5–12). Despite evidence that clinical disease and peripheral parasite density are inversely associated with age in areas of high transmission (13), the associations of other measures, including infection incidence and duration, with age are less consistent.
Data on the duration of P. falciparum infection come primarily from studies using intentional infection-induced fever as treatment for neurosyphilis (14, 15). Estimates from controlled experiments, which used high infectious doses administered to previously unexposed individuals, using low-virulence laboratory strains, are unlikely to resemble natural infections in nonnaive individuals (16). The few cohort studies that have examined duration of infection were undertaken in regions with seasonal transmission (16–19). However, infection persistence likely varies with geographic location and transmission pattern (8, 20).
The high burden of malaria in southern Malawi indicates a need for urgent attention. We conducted a longitudinal study in southern Malawi designed to characterize the age-specific patterns of P. falciparum infection in a high-transmission setting. We hypothesized that specific measurements would differ according to age, including duration of time spent with asymptomatic infections, the molecular force of infection (molFOI, defined as the number of new infections per person per year (21)), and the duration of infection with unique infecting genotypes. We hypothesized that SAC would have increased duration of infection overall and with each unique genotype.
METHODS
A longitudinal study with 2 years of follow-up was conducted at the Mfera Health Center in Chikwawa District of rural southern Malawi under the auspices of the Malawi International Center of Excellence for Malaria Research. Human subjects research approvals were granted by the institutional review boards of the University of Maryland School of Medicine, Michigan State University, and the National Health Sciences Research Committee of Malawi. Informed consent was obtained from all participants, or their guardian if under 18 years of age, and assent was obtained from all participants aged 13–17 years. P. falciparum is the dominant Plasmodium species in Malawi, comprising approximately 95% of all infections (22). Symptomatic episodes from this cohort have previously been described (23).
Participants
From June 2014 to March 2015, 120 participants aged 1 year or older were enrolled in an age-stratified manner after presenting to the health center with an episode of uncomplicated malaria. The study was powered to detect immunological outcomes. Participants with symptoms including fever, chills, headaches, myalgia, nausea were screened with a malaria rapid diagnostic test to detect histidine-rich protein-2, which, if positive, prompted microscopic examination of blood to confirm P. falciparum infection. Potential participants were excluded if they tested positive for human immunodeficiency virus, had acute illness requiring hospitalization, had signs or symptoms of severe malaria or severe anemia (24), or were taking medications with antimalarial activity.
Study procedures
Upon enrollment, participants were treated with 3 days of artemether/lumefantrine, given insecticide-treated bed nets, and followed for up to 2 years with passive and active malaria surveillance. Participants were scheduled to attend routine monthly visits and were encouraged to return immediately when ill. At each visit, a dried blood spot was preserved on filter paper, and thick blood smears on slides were made. Rapid diagnostic testing was performed only for participants who reported symptoms suggesting malaria illness. If the rapid diagnostic test was positive for P. falciparum, regardless of microscopy results, participants were diagnosed with malaria illness and treated with 3 days of artemether/lumefantrine per Malawi national protocol. Participants were considered lost to follow-up if they could not be traced for 3 consecutive months. Participants who had at least 1 follow-up visit at least 14-days after enrollment were included in this analysis.
Laboratory procedures
DNA was extracted from dried blood spots and subjected to quantitative polymerase chain reaction (qPCR) targeting the lactate dehydrogenase gene to detect P. falciparum DNA (limit of detection, 0.5–5 parasites/μL) (22) in duplicate with any 1 positive result classified as infection detected. Blood smears were stained and examined for Plasmodium parasites. Each slide was read by 2 independent microscopists, and when discrepant, a third reading was done. Samples with at least 2 positive readings were classified as positive.
Parasite genotyping was performed on all samples that were positive for P. falciparum by qPCR or microscopy. Parasite genotypes were differentiated on the basis of polymorphic regions of merozoite surface proteins 1 and 2 (MSP1 and MSP2) and glutamate-rich protein (GLURP) detected through nested PCR (limit of detection, 10 parasites/μL) (25). PCR products were analyzed by polyacrylamide gel electrophoresis and assigned sizes in base pairs by 2 independent readers. When results were discrepant by >15 base pairs, a third reading was done. Bands were included in analysis if at least 2 readers identified the band within 15 base pairs; final band size was an average of all readings. Genotyping was considered successful for samples with bands detected at 2 or more loci. For genotyping failures, the nested PCR reactions were repeated. Parasite density decreases with age and is lower in asymptomatic infections; infections with lower parasite densities are less likely to be successfully genotyped (13).
Definitions
Asymptomatic malaria infection was defined as presence of P. falciparum parasites according to qPCR or microscopy at a time when no symptoms of malaria were reported and more than 2 weeks before or after treatment for symptomatic malaria. Treatment was assumed to prevent new Plasmodium infections for up to 14 days (26).
Persistent genotypes were identified if any samples obtained from a given subject during 2 different visits contained the same alleles in at least 2 loci. Newly detected genotypes were identified if any sample contained alleles in at least 2 loci that had not been detected in any previous infections for a given subject.
The initiation of a new infection was defined as beginning halfway between the previous negative result and the first detection. The end of an infection was defined as halfway between the last detection and next negative visit.
Season was either rainy (December through April) or dry (May through November). The molFOI was the number of newly detected parasite genotypes detected in an individual per year, from all detected infections (21).
The predictor variable for all analyses was age; age was initially explored as a continuous variable and then grouped categorically into young children (younger than 5 years), SAC (ages 5–15 years), or adults (>15 years of age), based on the data. Age was updated after each treatment episode, allowing participants to move into older age categories. For comparability, additional groupings were explored (children aged 5–9 years, adults older than 30 years). Sex and season were identified a priori using causal diagrams (Web Figure 1, available at https://academic.oup.com/aje) as potential confounders or effect modifiers of the association between age and infection. Stratified analyses were initially conducted. There was no evidence of effect modification; thus sex and season were explored as confounders for further analyses. Reported bed-net use was high (>98%) in the population and could not be included as a covariate.
Analysis
Time spent with any asymptomatic infections
Time spent with asymptomatic infections in days (regardless of genotype) per year of follow-up was calculated and compared between age categories using a log-transformed, linear mixed-effect regression model accounting for the nonindependence of repeated observations from each subject.
The molecular force of infection
Mean molFOI was calculated within age category and compared between age groups using a Kruskal-Wallis test. A linear multivariable mixed-effect model for the association between age and log-transformed molFOI was constructed, adjusting for sex. Season was not included in the model as follow-up time included both seasons equally for the majority of participants. To account for low genotyping success, 2 additional estimates of molFOI were calculated: an age-specific conservative estimate and a less-conservative upper limit. The conservative estimate assumed that genotyping failures had the same distribution as the observed asymptomatic infections for a given age group. In addition to this assumption, the upper limit assumed that asymptomatic infections (including those successfully genotyped) had additional genotypes that were not detected, and that the probability of nondetection was equal to the probability of genotyping failure for that age group.
Duration of individual infections
To calculate the duration of infection, it was necessary to calculate the probability of nondetection due to genotyping failure over time. The following calculation was performed separately for each age group to calculate age-specific probabilities of nondetection. Using all genotypes that were detected at both day 0 and day 60, the probability of nondetection at day 30 was calculated as:
This analysis assumed that a genotype detected at both day 0 and day 60 was present at all times between day 0 and day 60, and thus genotype nondetection at day 30 was genotyping failure. This process was repeated for all time frames, generalizing for genotypes detected at both time point t and a later time point t + i. The probabilities of nondetection of the same genotype for all time points between t and t + i (t + 1 through t + i − 1) were calculated. These probabilities gave a probability distribution dependent on the time since detection. Data sets were simulated to impute positives for potential false negatives by randomly sampling from this probability distribution after last detection of a given genotype. Simulated data sets were then randomly sampled to create the final imputed data set of genotype detection by time, accounting for nondetection (Web Figure 2).
Persistent infections were defined as infections detected at least twice with at least 30 days between initial and last detection. The distributions of duration were examined to see if the data fit gamma, Weibull, or lognormal distributions. Wilcoxon rank-sum tests were used to compare the duration of infection according to age and sex. For the final analysis, a mixed-effect linear regression model, adjusting for season, was used to assess the association between age and duration of infection accounting for the nonindependence of repeated observations within individuals. Sex was not included in the final model because it was not associated with duration of infection. All statistical analyses were performed using SAS, version 9.4 (SAS Institute, Inc., Cary, North Carolina).
RESULTS
Of 120 participants enrolled into the study, 114 had at least 14 days of follow-up time and were included in the present analysis. In total, participants accrued 73,514 days of follow-up time, with a median of 720 days (interquartile range, 715–721, range; 24–728). SAC comprised 46% of the final study population, and 25% were under 5 years of age (Table 1).
Table 1.
Median Follow-up Time, the Number of Incident Infections Detected by Genotyping, and the Rate of Treatment With Artemether/Lumefantrine According to Age and Sex, Mfera Cohort Study, Malawi, 2012–2017
| Population Stratum | No. of Participants | % | Days of Follow-up Time, median (IQR) | No. of Incident Infections | % | No. of Treatments per Year |
|---|---|---|---|---|---|---|
| Age category | ||||||
| <5 years | 29 | 25 | 718 (420–721) | 253 | 25 | 2.4 |
| SAC (5–15 years) | 53 | 46 | 720 (720–721) | 629 | 62 | 2.1 |
| Adults (>15 years) | 32 | 28 | 718 (478–720) | 136 | 13 | 0.9 |
| Sex | ||||||
| Male | 49 | 43 | 720 (715–721) | 380 | 37 | 1.8 |
| Female | 65 | 57 | 720 (715–721) | 638 | 63 | 1.9 |
| Total | 114 | 100 | 720 (715–721) | 1,018 | 100 | 1.9 |
Abbreviations: IQR, interquartile range; SAC, school-aged children.
A total of 1,062 blood samples identified as infected by microscopy (613 (58%)) and/or qPCR (1,022 (96%)) were genotyped. Of these, 711 (67%) samples were successfully genotyped for at least 2 of the 3 loci (merozoite surface proteins 1 and 2 and glutamate-rich protein), which were successfully amplified from 66%, 63%, and 64% of the samples, respectively. Genotyping success was lowest (56%) among adults, compared with young children (69%) and SAC (70%). Samples from symptomatic visits were more often successfully genotyped (90%) than those from asymptomatic visits (49%). Samples that failed genotyping were frequently submicroscopic (97%). Geometric mean parasite density was 14,001/μL (standard deviation, 1,437) at malaria illnesses visits and 1,522/μL (standard deviation, 831) at microscopically detectable asymptomatic visits.
Of 711 samples successfully genotyped, 416 (59%) were from symptomatic episodes, and 295 (41%) were from asymptomatic visits; 482 (68%) had more than 1 genotype detected. The incidence of new infections was 5.14 per person-year. Of 1,018 incident infections detected, 36% were asymptomatic. Among adults, 62% (95% confidence interval: 54, 70) of all incident infections were at asymptomatic (as opposed to symptomatic) visits compared with 33% (95% confidence interval: 27, 39) in young children (P < 0.001) and 32% (95% confidence interval: 28, 36) in SAC (P < 0.001).
Median time spent with any asymptomatic infections
Participants spent a median of 73 days per year with detectable asymptomatic infections (Table 2). The median time spent with asymptomatic infections did not differ significantly according to age or sex. In final models, age was not associated with significant differences in time spent with asymptomatic infections (Table 3).
Table 2.
Median Time in Days Spent With Detectable Asymptomatic Infections According to Population Characteristics, Mfera Cohort Study, Malawi, 2012–2017
| Population Stratum | Days With Asymptomatic Infection per Year, median (IQR) | P Valuea |
|---|---|---|
| Age category | 0.84 | |
| <5 years | 88 (31–131) | |
| SAC (5–15 years) | 62 (35–140) | |
| Adults (>15 years) | 71 (29–121) | |
| Sex | 0.09 | |
| Male | 63 (15–121) | |
| Female | 85 (41–138) | |
| Overall | 73 (31–131) |
Abbreviations: IQR, interquartile range; SAC, school-aged children.
aP values from Kruskal-Wallis test.
Table 3.
Age-Specific Means From Models for Time Spent With Asymptomatic Infection, Molecular Force of Infection, and Duration of Infection, Mfera Cohort Study, Malawi, 2012–2017
| Predictor and Age Group | Mean (SE)a | P Value | Model Used | Covariates Included |
|---|---|---|---|---|
| Time spent with asymptomatic infection, days | Linear mixed-effect regression | None | ||
| <5 years | 67.5 (12.5) | Referent | ||
| SAC (5–15 years) | 81.7 (14.6) | 0.35 | ||
| Adults (>15 years) | 71.8 (17.8) | 0.81 | ||
| Molecular force of infection | Linear multivariable regression | Sex | ||
| <5 years | 4.1 (1.5) | Referent | ||
| SAC (5–15 years) | 4.3 (1.6) | 0.91 | ||
| Adults (>15 years) | 0.9 (1.7) | 0.006 | ||
| Duration of infection, days | Linear mixed-effect regression | Season of first detection | ||
| <5 years | 85.2 (1.1) | Referent | ||
| SAC (5–15 years) | 124.6 (1.2) | 0.001 | ||
| Adults (>15 years) | 126.8 (1.2) | 0.001 |
Abbreviations: SAC, school-aged children; SE, standard error.
a Outcome variable log-transformed for all models and back transformed to give group-specific mean.
The molecular force of infection
The median number of infections per person per year was 5.3 (interquartile range, 2.5–7.7). Adults had a lower molFOI than both SAC and young children (Table 4). In the final model, age group was significantly associated with molFOI (P = 0.006). Adults had 83% fewer infections detected per person per year compared with young children. On average, SAC had 5% fewer infections, but this was not significantly different from among young children (Table 3).
Table 4.
Median Molecular Force of Infection According to Population Characteristics, Detected by Genotyping and Conservative and Upper-Limit Estimates, Mfera Cohort Study, Malawi, 2012–2017
| Population Stratum | Detected molFOI, median (IQR) | P Valuea | Conservative molFOI, median (IQR) | P Valuea | Upper-Limit molFOI, median (IQR) | P Valuea |
|---|---|---|---|---|---|---|
| Age category | 0.004 | 0.009 | 0.01 | |||
| <5 years | 6.6 (4.6–10.9) | 9.2 (6.4–15.4) | 12.2 (8.4–20.2) | |||
| SAC (5–15 years) | 6.3 (3.2–10.1) | 8.8 (4.5–14.2) | 12.0 (6.1–19.2) | |||
| Adults (>15 years) | 2.5 (1.0–5.1) | 5.3 (2.2–10.7) | 7.6 (3.1–15.2) | |||
| Sex | 0.71 | 0.73 | 0.79 | |||
| Male | 5.4 (1.9–7.7) | 8.6 (3.7–13.5) | 11.4 (5.4–18.2) | |||
| Female | 5.3 (2.6–8.9) | 5.5 (4.2–15.0) | 11.3 (6.0–20.3) | |||
| Overall | 5.3 (2.5–7.7) | 8.5 (4.2–13.4) | 11.4 (6.0–18.1) |
Abbreviations: IQR, interquartile range; molFOI, molecular force of infection; SAC, school-aged children.
aP values from Kruskal-Wallis test compared molFOI by age and by sex for each estimate.
Results did not change after accounting for age-dependent nondetection of genotypes using the conservative and upper-limit estimates (Table 4).
Duration of persistence of individual genotypes
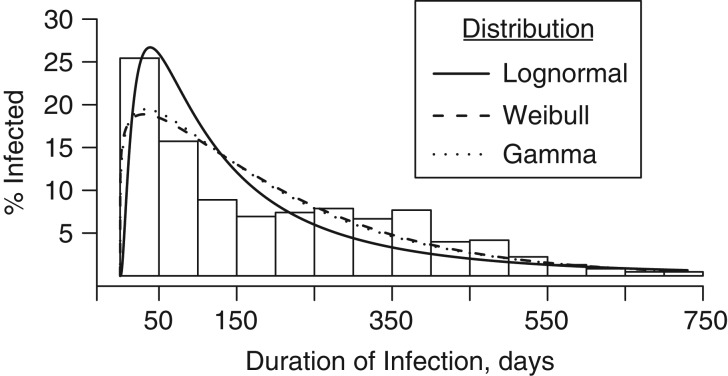
Of 1,220 unique infections (including both incident infections and infections detected at enrollment) successfully genotyped, 693 (57%) were persistent, lasting longer than 1 month. From observed data, median infection duration of a single genotype was 40 days (interquartile range, 36–56). After imputation, median duration of infections was 150 days (Table 5), and distribution of duration was not a good fit to any of the 2-parameter distributions tested (Figure 1). After imputation, median duration of persistent infections was 230 days, with 75% of persistent infections lasting at least 99 days. The longest persistent infection lasted 721 days, the study duration.
Table 5.
Observed and Imputed Median Duration (Days) of Individual Genotypes According to Population Characteristics (n = 1,220), Mfera Cohort Study, Malawi, 2012–2017
| Population Stratum | Duration in Days (Observed Data) | Duration in Days (Imputed Data) | ||
|---|---|---|---|---|
| Median (IQR) | P Valuea | Median (IQR) | P Valuea | |
| Age category | 0.05 | <0.001 | ||
| <5 years | 39 (35–49) | 97 (31–254) | ||
| SAC (5–15 years) | 41 (37–62) | 163 (48–331) | ||
| Adults (>15 years) | 41 (38–43) | 180 (51–339) | ||
| Sex | 0.12 | 0.71 | ||
| Male | 39 (34–45) | 156 (34–310) | ||
| Female | 41 (37–57) | 149 (43–327) | ||
| Season | 0.24 | <0.001 | ||
| Rainy season | 42 (37–56) | 210 (65–340) | ||
| Dry season | 39 (35–55) | 128 (34–304) | ||
| Overall | 40 (36–56) | 150 (38–321) | ||
Abbreviations: IQR, interquartile range; SAC, school-aged children.
aP values from Wilcoxon rank-sum test.
Figure 1.
Distribution of the duration of Plasmodium falciparum infection persistence in days among 114 adults and children in Mfera Cohort Study, Malawi, 2012–2017, after accounting for age-specific nondetection, with fit lines for lognormal (θ = 0, σ = 1.08, ζ = 4.82), Weibull (θ = 0, C = 1.13, σ = 208), and Gamma (θ = 0, α = 1.2, σ = 166) distributions.
Older age was associated with increased proportion of persistent infections (P < 0.001 for linear trend). Age was associated with infection duration in the observed data, using the Wilcoxon rank-sum test. Imputed durations, accounting for nondetection, were significantly shorter among young children than among adults and SAC (Table 5). The imputed results showed no difference in adults versus SAC. After imputation, infections first detected in the rainy season lasted longer than those first detected in the dry season. When we analyzed children aged 5–9 years as a distinct group, we found that they were not different from children aged 10–15 years (P = 0.43), so age categories were maintained. Likewise, adults over 30 years were not significantly different from adults aged 15–30 years (P = 0.10).
In the final model, age was significantly associated with duration of persistent infections, with SAC having infections persisting 38% longer than young children (Table 5). There was no difference between adults and SAC (P = 0.87).
DISCUSSION
We have provided an estimate of natural P. falciparum infection duration using genotyping data collected over a longer period than used in previously published studies. Additionally, this is the first report of incidence and duration of P. falciparum infection in a population with perennial transmission in the context of high bed-net use. We have shown that children have higher infection incidence than adults but that the duration of persistent infections increased with age. In this study, SAC represented an intermediate level of risk for blood-stage P. falciparum infection: They had high incidence of infection, as did young children, and increased duration of infection, similar to adults.
The conclusions from our investigation are limited by low sensitivity of the genotyping methods used. Using whole blood would have increased sensitivity but would have required collecting blood via venipuncture, which was not feasible given the frequency of sampling and age range of participants. Smaller blood volumes and high numbers of submicroscopic infections contributed to low genotyping success, which was related to both parasite density and age: A lower proportion of asymptomatic infections were successfully genotyped compared with symptomatic infections. Decreasing parasitemia with age means qPCR-positive samples from adults were less likely to be successfully genotyped than those from children, and decreased detectability of infection in adults might have biased our estimates of molFOI and duration of infection. Additionally, the use of gel rather than capillary electrophoresis might have reduced our sensitivity for the detection all genotypes present in a sample (27).
To address this, the probability of nondetection was calculated in an age-dependent manner. Our upper limit estimates of molFOI suggests that, even after accounting for the high probability of nondetection among adults, molFOI is significantly lower in adults than in children. In estimating infection duration, the observed data suggested an increased frequency of longer-duration infections among SAC; however, after accounting for nondetection, SAC and adults were similar in this respect. After accounting for nondetection, rainy season infections were significantly longer than those first detected in the dry season. It is likely that some infections first detected in the dry season were previously present but undetected due to low parasitemia. We did not take into account nondetection before first detection in simulations, and thus this difference might be a result of methods used.
Most data on the age dependence of P. falciparum infection have come from cross-sectional analyses, with few studies having prospectively examined the association between age and molecularly-detected incidence of infection (10, 21). We found that molFOI remained stable with increasing age in children but declined significantly after age 15 years. In contrast, a study in Mali among individuals aged 4–25 years found no age differences in infection incidence based on PCR positivity, but this group did not distinguish between parasite genotypes (10).
Our results are similar to those in publications from a 1-year cohort study in Ghana (8, 16). After accounting for imperfect detection of infection, investigators estimated a mean infection duration of 124 days for young children, 179 days for children 5–9 years, and <90 days for individuals aged 10 years or older (8, 16). Although our estimates are in a similar range, we did not detect a significantly longer duration of infection among SAC (nor among children aged 5–9 years), compared with adults. The availability of study clinicians to attend to all illnesses among our study participants might have led to more frequent treatment of malaria infections compared with the study conducted in Ghana, in which clinical care was not provided to participants. Frequency of treatment likely affects duration of infection. If access to care were lowest among SAC in Ghana, as we have found in cross-sectional studies in Malawi (2), SAC would have been less likely than adults to receive treatment for persistent nfections, perhaps contributing to the observed difference in duration.
Other published results on the duration of natural infection come from 2 cohort studies (18, 19) and differ from our findings on the duration of follow-up and management of all detected infections, even in the absence of symptoms. Both studies were shorter and might have been unable to identify long-lasting persistent infections due to insufficient follow-up and informing participants of asymptomatic infections, potentially prompting treatment (19). We did not report to participants or treat asymptomatic infections because they were not detected in real-time, allowing observations of natural infections for longer periods than would have been possible otherwise.
Our findings suggest that high prevalence of P. falciparum infection among SAC detected in cross-sectional studies is driven by increased rates of new infections and a long duration of persistent infections. We found that the incidence of infection among SAC was equivalent to that among younger children, while the duration of persistence among SAC was equivalent to that in adults. New blood-stage infections are as likely to be detected in SAC as in children younger than 5 years, but can they persist without causing symptoms for a long period of time, in a pattern similar to that of adults. Young children were more likely to be treated for malaria over the course of the study, potentially decreasing the duration of otherwise persistent infections. The combination of long duration of infection and apparent high incidence of new blood-stage infections might contribute to increased infection prevalence among SAC. This combination could be related to decreased access to care, decreased access to interventions, or incomplete immunity that limits symptoms but does not clear infections. In our prospective cohort, all participants were provided with bed nets and had access to prompt treatment with effective antimalarial medication. This contrasts with community-based studies, where SAC are significantly less likely to access care or use bed nets (28–32), suggesting that our findings might underestimate typical prevalence and persistence among SAC.
Characterizing the incidence and duration of P. falciparum infection is essential to understanding the drivers of transmission and is necessary to design informed public health policy. This study provides detailed understanding of P. falciparum infection dynamics in a region with perennially high rates of transmission. While many infections in this setting were transient, 57% of all infections lasted longer than 1 month, with a maximum duration of 2 years. Incidence of new infections remained high among teenagers, and effects of acquired immunity on developing new infections were not apparent until after 15 years of age. Our results could inform strategies for malaria control and elimination in Africa. These results also expand on growing evidence that SAC are substantial contributors to the P. falciparum infection burden and should be a focus of future malaria control measures.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore, Maryland (Andrea G. Buchwald, Miriam K. Laufer); Geriatrics Research, Education, and Clinical Center, Baltimore Veterans Affairs Medical Center, Baltimore, Maryland (John D. Sorkin); Division of Geriatrics and Gerontology, Department of Medicine, University of Maryland School of Medicine, Baltimore, Maryland (John D. Sorkin); Malaria Alert Center, University of Malawi College of Medicine, Blantyre, Malawi (Alick Sixpence¸ Mabvuto Chimenya, Milius Damson, Don Mathanga); Department of Epidemiology, University of Michigan School of Public Health, Ann Arbor, Michigan (Mark L. Wilson); College of Osteopathic Medicine, Michigan State University, East Lansing, Michigan (Karl Seydel, Terrie E. Taylor); and Department of Medicine, New York University School of Medicine, New York, New York (Sarah Hochman).
This work was supported by the National Institutes of Health (Malawi International Center of Excellence for Malaria Research grant U19AI089683), the National Institute of Allergy and Infectious Diseases (grant K24AI114996), and the National Institute on Aging (grant P30AG028747).
We thank the following persons, who were instrumental in the completion of this work: Miriam Ismail, Courtney Aceto, Alaina Halbach, Dominique Earland, Dr. Ankur Sharma, Sudhaunshu Joshi, Syze Gama, and Biraj Shrestha.
Some of the data presented in this paper were previously presented at the American Society for Tropical Medicine and Hygiene 66th Annual Meeting, November 5–9, 2017, Baltimore, Maryland (presentation 1934).
Conflict of interest: none declared.
Abbreviations
- molFOI
molecular force of infection
- PCR
polymerase chain reaction
- qPCR
quantitative polymerase chain reaction
- SAC
school-aged children
REFERENCES
- 1. World Health Organization World Malaria Report: 2016. 2016. Geneva, Switzerland: World Health Organization. http://apps.who.int/iris/bitstream/handle/10665/252038/9789241511711-eng.pdf?sequence=1. Accessed August 20, 2018.
- 2. Walldorf JA, Cohee LM, Coalson JE, et al. . School-age children are a reservoir of malaria infection in Malawi. PLoS One. 2015;10(7):e0134061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. National Malaria Control Program II Malawi Malaria Indicator Survey (MIS) 2014. https://dhsprogram.com/pubs/pdf/MIS18/MIS18.pdf 2014. Lilongwe, Malawi, and Rockville, Maryland: Ministry of Health and United States Agency for International Development. Accessed August 20, 2018.
- 4. Buchwald AG, Coalson JE, Cohee LM, et al. . Insecticide-treated net effectiveness at preventing Plasmodium falciparum infection varies by age and season. Malar J. 2017;16:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mawili-Mboumba DP, Bouyou Akotet MK, Kendjo E, et al. . Increase in malaria prevalence and age of at risk population in different areas of Gabon. Malar J. 2013;12:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pullan RL, Bukirwa H, Staedke SG, et al. . Plasmodium infection and its risk factors in eastern Uganda. Malar J. 2010;9:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wilson S, Booth M, Jones FM, et al. . Age-adjusted Plasmodium falciparum antibody levels in school-aged children are a stable marker of microgeographical variations in exposure to Plasmodium infection. BMC Infect Dis. 2007;7:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bretscher MT, Maire N, Felger I, et al. . Asymptomatic Plasmodium falciparum infections may not be shortened by acquired immunity. Malar J. 2015;14:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vafa M, Troye-Blomberg M, Anchang J, et al. . Multiplicity of Plasmodium falciparum infection in asymptomatic children in Senegal: relation to transmission, age and erythrocyte variants. Malar J. 2008;7:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tran TM, Li S, Doumbo S, et al. . An intensive longitudinal cohort study of Malian children and adults reveals no evidence of acquired immunity to Plasmodium falciparum infection. Clin Infect Dis. 2013;57(1):40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bereczky S, Liljander A, Rooth I, et al. . Multiclonal asymptomatic Plasmodium falciparum infections predict a reduced risk of malaria disease in a Tanzanian population. Microbes Infect. 2007;9(1):103–110. [DOI] [PubMed] [Google Scholar]
- 12. Sondén K, Doumbo S, Hammar U, et al. . Asymptomatic multiclonal Plasmodium falciparum infections carried through the dry season predict protection against subsequent clinical malaria. J Infect Dis. 2015;212(4):608–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bousema T, Okell L, Felger I, et al. . Asymptomatic malaria infections: detectability, transmissibility and public health relevance. Nat Rev Microbiol. 2014;12(12):833–840. [DOI] [PubMed] [Google Scholar]
- 14. Eyles DE, Young MD. The duration of untreated or inadequately treated Plasmodium falciparum infections in the human host. J Natl Malar Soc. 1951;10(4):327–336. [PubMed] [Google Scholar]
- 15. Jeffery GM, Eyles DE. Infectivity to mosquitoes of Plasmodium falciparum as related to gametocyte density and duration of infection. Am J Trop Med Hyg. 1955;4(5):781–789. [DOI] [PubMed] [Google Scholar]
- 16. Felger I, Maire M, Bretscher MT, et al. . The dynamics of natural Plasmodium falciparum infections. PLoS One. 2012;7(9):e45542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roca-Feltrer A, Schellenberg JR, Smith L, et al. . A simple method for defining malaria seasonality. Malar J. 2009;8:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Franks S, Koram KA, Wagner GE, et al. . Frequent and persistent, asymptomatic Plasmodium falciparum infections in African infants, characterized by multilocus genotyping. J Infect Dis. 2001;183(5):796–804. [DOI] [PubMed] [Google Scholar]
- 19. Baliraine FN, Afrane YA, Amenya DA, et al. . A cohort study of Plasmodium falciparum infection dynamics in Western Kenya Highlands. BMC Infect Dis. 2010;10:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hofmann NE, Wampfle R, Silkey M, Robinson LJ, Mueller I, Smith T, Felger I, eds. Infection dynamics of natural Plasmodium infections in Papua New Guinean children. American Society of Tropical Medicine and Hygiene 64th Annual Meeting; 2015; Philadelphia, Pennsylvania, USA: ASTMH.
- 21. Mueller I, Schoepflin S, Smith TA, et al. . Force of infection is key to understanding the epidemiology of Plasmodium falciparum malaria in Papua New Guinean children. Proc Natl Acad Sci U S A. 2012;109(25):10030–10035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rantala AM, Taylor SM, Trottman PA, et al. . Comparison of real-time PCR and microscopy for malaria parasite detection in Malawian pregnant women. Malar J. 2010;9:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Buchwald AG, Sixpence A, Chimenya M, et al. . Clinical implications of asymptomatic Plasmodium falciparum infections in Malawi [published online ahead of print August 10, 2005]. Clin Infect Dis. (doi: 10.1093/cid/ciy427). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Trampuz A, Jereb M, Muzlovic I, et al. . Clinical review: severe malaria. Crit Care. 2003;7(4):315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Network WAR. Molecular Testing for Malaria Standard Operating Procedure (SOP). Manual size estimation of MSP1, MSP2 and GLURP PCR products on gel. 2015. http://www.wwarn.org/sites/default/files/attachments/procedures/maunual-size-estimation-of-msp1-msp2-and-glurp-pcr-products-on-gel.doc. Accessed August 20, 2018.
- 26. Four Artemisinin-Based Combinations (4ABC) Study Group A head-to-head comparison of four artemisinin-based combinations for treating uncomplicated malaria in African children: a randomized trial. PLoS Med. 2011;8(11):e1001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liljander A, Wiklund L, Falk N, et al. . Optimization and validation of multi-coloured capillary electrophoresis for genotyping of Plasmodium falciparum merozoite surface proteins (MSP1 and 2). Malar J. 2009;8:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mutuku FM, Khambira M, Bisanzio D, et al. . Physical condition and maintenance of mosquito bed nets in Kwale County, coastal Kenya. Malar J. 2013;12:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Buchwald AG, Walldorf JA, Cohee LM, et al. . Bed net use among school-aged children after a universal bed net campaign in Malawi. Malar J. 2016;15:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Skarbinski J, Mwandama D, Luka M, et al. . Impact of health facility-based insecticide treated bednet distribution in Malawi: progress and challenges towards achieving universal coverage. PLoS One. 2011;6(7):e21995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Garley AE, Ivanovich E, Eckert E, et al. . Gender differences in the use of insecticide- treated nets after a universal free distribution campaign in Kano State, Nigeria: post-campaign survey results. Malar J. 2013;12:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Holtz TH, Marum LH, Mkandala C, et al. . Insecticide-treated bednet use, anaemia, and malaria parasitaemia in Blantyre District, Malawi. Trop Med Int Health. 2002;7(3):220–230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.