Patients with indolent non-Hodgkin lymphoma treated with bendamustine have an increased risk of common infections such as bacterial pneumonia and opportunistic infections such as Pneumocystis jirovecii pneumonia, cytomegalovirus, varicella zoster virus, and histoplasmosis, compared with patients receiving other chemotherapy regimens.
Keywords: infectious complications, non-Hodgkin lymphoma, bendamustine, Pneumocystis jirovecii pneumonia, cytomegalovirus
Abstract
Background
Bendamustine is a potent chemotherapy agent increasingly used to treat indolent non-Hodgkin lymphoma (iNHL). While effective, it causes significant T-cell lymphopenia, which may increase risk of infection. We examined infectious complications associated with bendamustine-containing regimens among older patients with iNHL.
Methods
For this Surveillance, Epidemiology, and End Results (SEER)–Medicare cohort study, we identified 9395 patients with iNHL (follicular, marginal zone, Waldenström macroglobulinemia) treated with chemotherapy from 2006 to 2013. Thirteen percent received bendamustine-containing regimens. We compared baseline characteristics and infection incidence rates between patients treated with and without bendamustine. We conducted multivariate Cox proportional hazards regression (adjusting for demographics, comorbidities, disease and treatment characteristics, risk factors for infection, and antimicrobial prophylaxis) to determine infectious risks associated with bendamustine.
Results
Bendamustine was associated with an increased risk of both common infections such as bacterial pneumonia (hazard ratio [HR], 1.50 [95% confidence interval {CI}, 1.21–4.85]) and opportunistic infections such as cytomegalovirus (HR, 3.98 [95% CI, 1.40–11.26]), varicella zoster virus (HR, 1.49 [95% CI, 1.18–1.89]), histoplasmosis (HR, 3.55 [95% CI, 1.10–11.42]), and Pneumocystis jirovecii pneumonia (when administered as third-line therapy: HR, 3.32 [95% CI, 1.00–11.11]). Risk of infections was more prominent in patients receiving bendamustine as part of later (third-line and above) regimens, and independently associated with well-established factors such as neutropenia and corticosteroid exposure.
Conclusions
Bendamustine is associated with an increased risk of common and opportunistic infections in patients with iNHL. Further prospective investigation into the potential role of antimicrobial prophylaxis is needed in these patients.
Indolent non-Hodgkin lymphomas (iNHL) such as follicular lymphoma, marginal zone lymphoma, Waldenström macroglobulinemia, and chronic lymphocytic leukemia/small lymphocytic lymphoma are slow-growing but incurable B-cell malignancies [1, 2]. They comprise approximately one-third of all non-Hodgkin lymphoma cases, with an anticipated 25000 new diagnoses and 7000 attributable deaths in the United States in 2018 [3]. Traditionally, these patients are not considered at high risk for infectious complications.
Bendamustine is a nitrogen mustard–based chemotherapy drug with alkylating and antimetabolite activity [4, 5]. In 2008, bendamustine was approved as front-line treatment for chronic lymphocytic leukemia and as treatment for rituximab-refractory follicular lymphoma [6]. Bendamustine is frequently used outside of its labeled indication as front-line therapy in follicular lymphoma and other low-grade lymphomas because of presumed safety, tolerability, and efficacy [7–10].
Myelosuppression, particularly CD4+ lymphopenia, is common after bendamustine administration [11–17]. In clinical trials of bendamustine for iNHL, grade 3–4 lymphopenia occurred in up to 74% of patients and appeared to be prolonged [14–18]. In 56 patients with iNHL, for example, the median CD4+ lymphocyte count decreased from 282 to 93 cells/μL after bendamustine, recovering to baseline 7–9 months after last bendamustine administration [18].
The risk of infection associated with bendamustine has yet to be fully characterized. Small series and case reports have reported the occurrence of Pneumocystis jirovecii pneumonia (PCP) and cytomegalovirus (CMV) after bendamustine exposure [19–22]. Some clinical trials suggested that bendamustine plus rituximab (BR) was associated with fewer infections than cyclophosphamide, doxorubicin, vincristine, and prednisone plus rituximab (R-CHOP) [12, 13], but the same risk of infection as fludarabine and rituximab [14]. Another study found that opportunistic infections (OIs) were slightly more common in patients receiving BR (10%) than R-CHOP (7%) or cyclophosphamide, vincristine, and prednisone plus rituximab (R-CVP; 9%) [15]. The recent GALLIUM trial comparing rituximab vs obinutuzumab for follicular lymphoma showed that treatment with bendamustine was associated with higher infection rates, with grade 3–5 infection in 12.8% of patients receiving BR vs 1.6% and 2.3% of those receiving R-CHOP and R-CVP, respectively [23].
Outside of clinical trials, the risk of infection associated with bendamustine-based regimens is not well understood. Here, we examine infectious complications associated with bendamustine exposure among patients with iNHL on a national, population-based level.
METHODS
Data Source
The National Cancer Institute Surveillance, Epidemiology, and End Results (SEER)–Medicare database links data from SEER cancer registries to Medicare enrollment and claims files. SEER registries collect data including site and extent of disease, first-line therapy, sociodemographic characteristics, and dates of diagnosis and death, and currently covers approximately 28% of the US population [24].
For adults at least 65 years of age enrolled in SEER, record linkage to Medicare claims allows for identification of concomitant health conditions and treatments. Medicare is a federally funded health insurance for US citizens ≥65 years of age. Part A coverage includes inpatient care, Part B includes physician and outpatient services, and Part D includes prescription drug costs.
This study was approved by the Partners HealthCare Human Research Committee and reviewed by SEER-Medicare staff for compliance with SEER-Medicare data policies.
Study Cohort
In this cohort study, we included patients at least 65 years of age continuously enrolled in Medicare parts A and B from 2006 to 2013 with iNHL treated with chemotherapy. We used the International Classification of Diseases–Oncology, Third Edition and the International Classification of Diseases, Ninth Revision (ICD-9) codes to identify patients with follicular lymphoma, marginal zone lymphoma, and Waldenström macroglobulinemia (Supplementary Appendix A).
Exposure
The primary exposure in this study was treatment with bendamustine. Bendamustine is recommended as first-line and salvage therapy for symptomatic, advanced stage, and/or bulky stage follicular lymphoma and Waldenström macroglobulinemia and conventional chemotherapy for mantle cell lymphoma. When used in combination with rituximab for the treatment of iNHL, bendamustine is considered noninferior to R-CHOP, with a more favorable side effect profile, and is administered as a 90 mg/m2 dose on days 1–2 of a 28-day treatment cycle for 6–8 cycles [5].
We identified bendamustine exposure and other predefined iNHL chemotherapy regimens (R-CHOP; R-CVP; fludarabine and cyclophosphamide plus rituximab (FCR); fludarabine plus rituximab (FR); rituximab monotherapy) by extracting chemotherapy drugs of interest and their administration frequency using Healthcare Common Procedure Coding System (HCPCS) procedure “J” codes and the National Drug Codes (NDC) (Supplementary Appendix B). We then used an established algorithm to identify number of regimens/lines and cycles [25], whereby a new line of therapy was defined by a switch from one predetermined regimen to another regimen via the addition or discontinuation of ≥1 drug.
Outcomes
The primary outcomes were individual and composite bacterial (staphylococcal infection, streptococcal infection, pseudomonal infection, actinomycosis, nocardiosis, tuberculosis, nontuberculous mycobacteria, bacterial pneumonia, bacterial infection not otherwise specified [NOS], sepsis), fungal (PCP, aspergillosis, candidiasis, coccidioidomycosis, histoplasmosis, blastomycosis, cryptococcosis, other mycoses), viral (CMV, varicella zoster virus [VZV], herpes simplex virus [HSV], adenovirus, hepatitis B and C, other viral hepatitis), and parasitic (toxoplasmosis) infections occurring after bendamustine or other chemotherapy regimens. We identified infectious outcomes by ICD-9 codes (Supplementary Appendix C).
Covariates and Potential Confounders
Covariates for analysis included demographics (age, sex, race), comorbidities (Charlson comorbidity index), disease characteristics (lymphoma subtype, stage), treatment characteristics (lines of chemotherapy, cumulative rituximab exposure), other risk factors for infection (human immunodeficiency virus [HIV], allogeneic or autologous stem cell transplantation, solid organ transplantation, corticosteroid exposure, neutropenia, granulocyte stimulating factor use), and antimicrobial prophylaxis, all of which are factors that might confound the relationship between bendamustine exposure and infectious complications.
Demographics, comorbidity, and disease characteristics were derived directly from SEER-Medicare data (Supplementary Appendix D). The Charlson comorbidity index, a score based on the summation of integer weights assigned to age and a number of conditions, was used to assess comorbidity. This score has been validated in elderly and cancer patients and adapted for use with SEER-Medicare administrative data (there are validated algorithms to derive this score directly from SEER-Medicare data) [26, 27]. HIV, stem cell transplantation, and solid organ transplantation were identified via Diagnosis Related Group and ICD-9 diagnosis codes (Supplementary Appendix D). Rituximab administration was extracted from HCPCS and NDC codes, and cumulative rituximab exposure was defined as the total number of rituximab doses received by the start of follow-up time.
Potential time-varying factors (corticosteroid exposure, neutropenia, granulocyte stimulating factor use, and antimicrobial prophylaxis) were assessed using either ICD-9 codes (neutropenia) or HCPCS and NDC codes (corticosteroid exposure, antimicrobial prophylaxis; Supplementary Appendix B). Corticosteroid exposure was defined as receipt of prednisone outside of a predefined chemotherapy regimen. Granulocyte stimulating factor exposure was defined by receipt of filgrastim, peg-filgrastim, or sargramostim. Antimicrobial prophylaxis agents were predefined as trimethoprim-sulfamethoxazole, atovaquone, dapsone, or pentamidine for PCP and acyclovir, valacyclovir, valganciclovir, or famciclovir for herpesviruses. Patients were considered to have these time-varying exposures if they were present within 60 days prior to developing incident infectious complications; patients who did not develop infectious complications were considered exposed if they had any of these exposures during the follow-up period.
Follow-up Time
Follow-up time was calculated from the first administration of bendamustine for the bendamustine group and first administration of any chemotherapy regimen for the nonbendamustine group. Follow-up was censored at 365 days or earlier, at the first occurrence of an infectious outcome. There was no crossover between patients in each group and no analysis of repeated outcomes.
Statistical Analysis
We conducted Pearson χ2 and t tests to compare baseline characteristics between groups. We calculated the incidence (per 100 person-years) of and median time to infectious outcome for patients treated with and without bendamustine.
We estimated the cumulative incidence of composite infectious outcomes using the Kaplan-Meier method. We calculated Mantel-Haenszel rate ratios (RRs) with 95% confidence intervals (CIs) and P values.
Adjusting for differences in baseline characteristics and potential confounders of the relationship between bendamustine exposure and infection, we conducted multivariate Cox proportional hazards regression to examine the relationship between bendamustine exposure and infectious complications. We stratified by line of bendamustine chemotherapy (first-line, second-line, third-line or higher) to assess whether the association between bendamustine and infectious complications was dependent on previously administered chemotherapy regimens. Because bendamustine was considered less toxic and administered to more vulnerable patients earlier in its use for iNHL, we stratified our analysis by patients who received bendamustine before and after 2009. We tested the proportional hazards assumption by graphing survival function by time. Models for PCP and herpesviruses incorporating antimicrobial prophylaxis were restricted to the subset of patients enrolled in Medicare Part D (data available from 2007 to 2013) with outpatient prescription data.
For important infections, we calculated the number needed to harm for patients treated with and without bendamustine. For preventable infections (bacterial infection, PCP, CMV, VZV, histoplasmosis), we calculated the number needed to treat with antimicrobial prophylaxis to prevent a case of infection. We assumed antibacterial prophylaxis with levofloxacin with a 44% risk reduction [28], PCP prophylaxis with trimethoprim-sulfamethoxazole with an 85% risk reduction [29], CMV prophylaxis with valganciclovir with a 58% risk reduction [30], VZV/HSV prophylaxis with acyclovir with an 84% risk reduction [31], and histoplasmosis prophylaxis with itraconazole with a 61% risk reduction [32].
All statistical analyses were performed using Stata software version 13 for Windows (StataCorp, College Station, Texas).
RESULTS
We identified 9395 patients with iNHL treated with chemotherapy from 2006 to 2013, of whom 13.2% received bendamustine (Table 1). Patients who received bendamustine had more advanced clinical disease (47.6% vs 39.8% stage IV disease; P < .001), were exposed to more chemotherapy regimens (3.1 vs 2.1 lines of chemotherapy; P < .001), and developed neutropenia more frequently (20.3% vs 18.2%; P = .04).
Table 1.
Baseline Characteristics of Patients With Indolent Non-Hodgkin Lymphoma Treated With and Without Bendamustine
| Characteristic | Bendamustine (n = 1239) |
No Bendamustine (n = 8156) |
P Value |
|---|---|---|---|
| Age, y, mean (SD) | 74.9 (6.1) | 75.0 (6.6) | <.001a |
| Sex, % | <.001 | ||
| Male | 53.2 | 46.6 | |
| Female | 46.8 | 53.4 | |
| Race/ethnicity, % | .08 | ||
| White | 87.7 | 86.1 | |
| African American | 2.7 | 3.0 | |
| Hispanic | 5.4 | 5.6 | |
| Asian | 2.8 | 3.9 | |
| Other | 1.5 | 1.4 | |
| Subtype, % | .24 | ||
| Follicular lymphoma | 74.4 | 76.6 | |
| Waldenström macroglobulinemia | 22.5 | 20.5 | |
| Marginal zone lymphoma | 3.1 | 2.9 | |
| American Joint Committee on Cancer Stage, % | <.001 | ||
| I | 15.7 | 25.7 | |
| II | 13.1 | 16.5 | |
| III | 23.6 | 18.1 | |
| IV | 47.6 | 39.8 | |
| Lines of chemotherapy, mean (SD) | 3.1 (2.0) | 2.1 (1.6) | <.001a |
| First-line chemotherapy, % | <.001 | ||
| R-CHOP | 23.1 | 42.6 | |
| Rituximab monotherapy | 24.1 | 31.5 | |
| R-CVP | 16.4 | 18.1 | |
| Rituximab plus bendamustine | 31.3 | 0.0 | |
| FR or FCR | 2.5 | 3.2 | |
| Other | 2.7 | 4.6 | |
| Cumulative rituximab dose, mean (SD) | 13.8 (10.6) | 9.2 (8.2) | <.001 |
| Infectious risk factor, % | |||
| Stem cell transplantation (allogeneic or autologous) | 1.0 | 0.9 | .76 |
| Human immunodeficiency virus | 0.5 | 0.8 | .12 |
| Solid organ transplantation | 0.3 | 0.4 | .41 |
| Corticosteroid exposure | 36.5 | 40.0 | .02 |
| Neutropenia | 20.3 | 18.2 | .04 |
| Granulocyte stimulating factor use | 24.4 | 22.2 | .05 |
| Charlson comorbidity index score, % | .24 | ||
| 0 | 59.5 | 59.0 | |
| 1 | 24.3 | 24.5 | |
| 2 | 8.7 | 7.6 | |
| ≥3 | 7.5 | 8.9 | |
Unless otherwise specified, P values are determined by Pearson χ2 test.
Abbreviations: FCR, fludarabine and cyclophosphamide plus rituximab; FR, fludarabine plus rituximab; R-CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone plus rituximab; R-CVP, cyclophosphamide, vincristine, and prednisone plus rituximab; SD, standard deviation.
a P value determined by t test.
Patients exposed to bendamustine experienced 2.71 mean infections per person compared with 2.51 in those not exposed (P = .008). The most common infectious complications were bacterial infection NOS, sepsis, VZV, candidiasis, and bacterial pneumonia. Patients treated with bendamustine had higher incidences of bacterial pneumonia (10.5 vs 6.2 per 100 person-years [PY]), CMV (1.0 vs 0.3 per 100 PY), VZV (13.8 vs 8.3 per 100 PY), and histoplasmosis (0.4 vs 0.1 per 100 PY) (Table 2). Median time from chemotherapy to bacterial (82 vs 67 days), viral (134 vs 98 days), and opportunistic (106 vs 75 days) infections was longer for patients receiving bendamustine vs other regimens.
Table 2.
Incidence Rates of Infectious Complications Among Indolent Non-Hodgkin Lymphoma Patients Treated With and Without Bendamustine With Incidence Rate Ratio Comparing the 2 Groups
| Infection | Incidence Rate (per 100 PY) | ||
|---|---|---|---|
| Bendamustine | No Bendamustine | IRR | |
| Bacterial infection | 53.7 | 42.4 | 1.27 |
| Fungal infection | 22.8 | 17.8 | 1.28 |
| Viral infection | 28.4 | 15.4 | 1.84 |
| Opportunistic infection a | 43.0 | 28.5 | 1.51 |
| Staphylococcal infection | 3.6 | 3.9 | 0.92 |
| Streptococcal infection | 3.6 | 3.9 | 0.92 |
| Pseudomonal infection | 2.4 | 2.0 | 1.19 |
| Actinomycosis | 0.1 | 0.1 | 0.66 |
| Tuberculosis | 0.3 | 0.7 | 0.37 |
| Nontuberculous mycobacteria | 0.9 | 0.5 | 1.68 |
| Bacterial pneumonia | 10.5 | 6.2 | 1.70 |
| Sepsis | 18.4 | 17.6 | 1.05 |
| Bacterial infection NOS | 27.1 | 20.2 | 1.34 |
| Hepatitis B reactivation | 1.1 | 0.9 | 1.14 |
| Hepatitis C | 1.0 | 0.7 | 1.31 |
| Other viral hepatitis | 0.4 | 0.3 | 1.05 |
| Cytomegalovirus | 1.0 | 0.3 | 3.62 |
| Varicella zoster virus | 13.8 | 8.3 | 1.67 |
| Herpes simplex virus | 0.0 | <0.1 | … |
| Adenovirus | 0.4 | 0.1 | 2.63 |
| Nocardiosis | 0.1 | 0.1 | 1.64 |
| Pneumocystis pneumonia | 0.7 | 0.4 | 1.95 |
| Aspergillosis | 0.3 | 0.3 | 1.31 |
| Candidiasis | 12.5 | 11.5 | 1.09 |
| Blastomycosis | 0.1 | 0.0 | … |
| Coccidioidomycosis | 0.1 | 0.1 | 0.94 |
| Histoplasmosis | 0.4 | 0.1 | 3.28 |
| Cryptococcosis | 0.2 | 0.1 | 2.19 |
| Other mycoses NOS | 1.8 | 1.1 | 1.64 |
| Toxoplasmosis | 0.2 | <0.1 | 6.56 |
Boldface terms indicate composite outcomes.
Abbreviations: IRR, incidence rate ratio; NOS, not otherwise specified; PY, person-years; …, unable to perform statistical comparison due to zero values.
aOpportunistic infection defined as composite outcome of pneumocystis pneumonia, varicella zoster virus, cytomegalovirus, herpes simplex virus, adenovirus, toxoplasmosis, nocardiosis, candidiasis, coccidioidomycosis, histoplasmosis, blastomycosis, aspergillosis, cryptococcosis, other mycoses, and opportunistic mycoses.
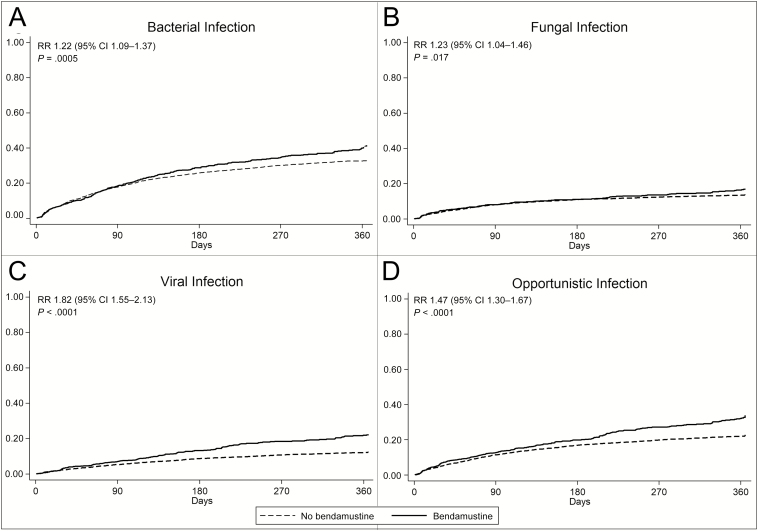
Patients treated with bendamustine had higher cumulative incidences of fungal (RR, 1.23; P = .017), viral (RR, 1.82; P < .0001), and opportunistic (RR, 1.47; P < .0001) infections (Figure 1).
Figure 1.
Cumulative incidence of composite bacterial infections (A), fungal infections (B), viral infections (C), and opportunistic infections (D) among patients with indolent non-Hodgkin lymphoma treated with vs without bendamustine. Mantel-Haenszel rate ratio (RR) with 95% confidence interval (95% CI) and P values are presented. “Opportunistic infection” is composite outcome of pneumocystis pneumonia, varicella zoster virus, cytomegalovirus, herpes simplex virus, adenovirus, toxoplasmosis, nocardiosis, candidiasis, coccidioidomycosis, histoplasmosis, blastomycosis, aspergillosis, cryptococcosis, other mycoses, and opportunistic mycoses.
Adjusting for age, sex, lymphoma subtype and stage, cumulative rituximab exposure, number of chemotherapy lines, corticosteroid exposure, neutropenia, granulocyte stimulating factor use, and antimicrobial prophylaxis, bendamustine exposure was associated with increased hazards of viral infections (hazard ratio [HR], 1.39 [95% CI, 1.17–1.64]) and opportunistic infections (HR, 1.16 [95% CI, 1.02–1.33]), specifically CMV (HR, 3.98 [95% CI, 1.40–11.26]), VZV (HR, 1.49 [95% CI, 1.18–1.89]), and histoplasmosis (HR, 3.55 [95% CI, 1.10–11.42]) (Figure 2 and Table 3). Among common infections, bendamustine was associated with an increased hazard of bacterial pneumonia (HR, 1.50 [95% CI, 1.21–4.85]) and bacterial infection NOS (HR, 1.17 [95% CI, 1.02–1.35]).
Figure 2.
Adjusted hazard ratio (black diamond) with 95% confidence intervals (black bars) of individual and composite infectious complications among indolent non-Hodgkin lymphoma patients treated with vs without bendamustine. Hazard ratios calculated using multivariable Cox proportional hazard models adjusted for age, sex, lymphoma subtype and stage, lines of chemotherapy, cumulative rituximab exposure, corticosteroid exposure, neutropenia, granulocyte stimulating factor use, and antimicrobial prophylaxis when appropriate. “Opportunistic infection” is composite outcome of pneumocystis pneumonia, varicella zoster virus, cytomegalovirus, adenovirus, toxoplasmosis, nocardiosis, candidiasis, coccidioidomycosis, histoplasmosis, blastomycosis, aspergillosis, cryptococcosis, and opportunistic mycoses. Abbreviation: NOS, not otherwise specified.
Table 3.
Adjusted Hazard Ratios of Key Infectious Complications Stratified by Line of Bendamustine, With Number Needed to Harm Attributed to Bendamustine and Number Needed to Treat With Antimicrobial Prophylaxis to Prevent a Case of Infection
| Infectious Complication | Overall, HR (95% CI) | P Value | Line of Bendamustine | NNH | NNT | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| First-line, HR (95% CI) | P Value | Second-line, HR (95% CI) | P Value | Third-line, HR (95% CI) | P Value | |||||
| Opportunistic infections | 1.16 (1.02–1.33) | .03 | 1.09 (.86–1.38) | .45 | 1.18 (.94–1.47) | .15 | 1.20 (.99–1.47) | .06 | 17 | NA |
| PCP | 3.26 (1.20–8.84) | .15 | … | … | 2.03 (.46–8.95) | .35 | 3.32 (1.00–11.11) | .05 | 318 | 252a |
| CMV | 3.98 (1.40–11.26) | .01 | 4.89 (.99–24.32) | .05 | … | … | 6.43 (1.90–21.75) | <.01 | 156 | 269b |
| VZV | 1.49 (1.18–1.89) | <.01 | 1.02 (.61–1.73) | .93 | 1.84 (1.29–2.64) | <.01 | 1.52 (1.10–2.10) | .01 | 19 | 23c |
| Bacterial | 0.99 (.88–1.11) | .80 | 0.80 (.63–1.00) | .05 | 0.96 (.78–1.17) | .67 | 1.15 (.98–1.37) | .09 | 31 | 70d |
| Bacterial pneumonia | 1.50 (1.21–4.85) | <.01 | 1.05 (.67–1.65) | .84 | 1.56 (1.09–2.23) | .01 | 1.75 (1.30–2.34) | <.01 | 27 | NA |
| Bacterial infection NOS | 1.17 (1.02–1.35) | .02 | 0.98 (.75–1.28) | .86 | 1.15 (.91–1.46) | .24 | 1.32 (1.10–1.61) | <.01 | 20 | NA |
| NTM | 1.50 (.73–3.09) | .27 | 2.76 (.97–7.89) | .06 | 2.70 (1.06–6.89) | .03 | 0.24 (.03–1.95) | .18 | 304 | NA |
| Fungal | 1.03 (.86–1.23) | .79 | 1.04 (.77–1.42) | .79 | 0.89 (.65–1.23) | .49 | 1.12 (.86–1.46) | .41 | 67 | NA |
| Histoplasmosis | 3.55 (1.10–11.42) | .03 | 2.88 (.35–23.53) | .32 | 4.31 (.90–20.53) | .07 | 3.35 (.57–19.59) | .18 | 355 | 582e |
| Viral | 1.39 (1.17–1.64) | <.01 | 1.11 (.81–1.53) | .51 | 1.52 (1.16–1.99) | <.01 | 1.50 (1.18–1.90) | <.01 | 17 | NA |
Hazard ratios were calculated using multivariable Cox proportional hazard models adjusted for age, sex, lymphoma subtype and stage, lines of chemotherapy, cumulative rituximab exposure, corticosteroid exposure, neutropenia, granulocyte stimulating factor use, and antimicrobial prophylaxis when appropriate. Boldface text indicate statistical significance at a P-value of .05.
Abbreviations: CI, confidence interval; CMV, cytomegalovirus; HR, hazard ratio; NA, not applicable; NNH, number needed to harm attributed to bendamustine; NNT, number needed to treat with prophylaxis to prevent a case of infectious complication; NTM, nontuberculous mycobacteria; PCP, Pneumocystis jirovecii pneumonia; VZV, varicella zoster virus; …, no events in subgroup.
aPCP prophylaxis with trimethoprim-sulfamethoxazole with an 85% risk reduction [29]; NNT is calculated for patients receiving bendamustine as third-line therapy or above.
bCMV prophylaxis with valganciclovir with a 58% risk reduction [30].
cVZV/herpes simplex virus prophylaxis with acyclovir with an 84% risk reduction [31].
dAntibacterial prophylaxis with levofloxacin with a 44% risk reduction [28].
eHistoplasmosis prophylaxis with itraconazole with a 61% risk reduction [32].
In multivariate models stratified by line of bendamustine, there was an increased hazard of PCP in patients receiving bendamustine as third-line therapy (HR, 3.32 [95% CI, 1.00–1.11]). Point estimates for bacterial infections overall (third-line HR, 1.32 [95% CI, 1.10–1.61]), bacterial pneumonia (third-line HR, 1.56 [95% CI, 1.09–2.23]), and VZV (second-line HR, 1.84 [95% CI, 1.29–2.64]; third-line HR, 1.52 [95% CI, 1.10–2.10]) (Table 3) were higher in patients who received bendamustine with later lines of chemotherapy.
Among patients receiving bendamustine as second-line or later therapy, adjusting for type of first-line chemotherapy did not change the overall strength, direction, or significance of the association between bendamustine exposure and infectious risks. Patients with iNHL who received bendamustine before 2009 (when bendamustine was perceived to be more tolerable in terms of side effects than R-CHOP) had statistically similar hazards of CMV (pre-2009: HR, 1.35 [95% CI, 1.06–1.73]; post-2009: HR, 1.25 [95% CI, 1.01–1.57]), VZV (pre-2009: HR, 1.32 [95% CI, 1.04–1.70]; post-2009: HR, 1.21 [95% CI, .96–1.53]), and bacterial pneumonia (pre-2009: HR, 1.68 [95% CI, 1.24–2.28]; post-2009: HR, 1.38 [95% CI, 1.05–1.82]), compared with patients who received bendamustine-containing regimens after 2009. The increased risk of histoplasmosis was only observed in patients who received bendamustine prior to 2009 (HR, 7.32 [95% CI, 1.94–27.58] vs post-2009: HR, 1.10 [95% CI, .13–9.08]).
In multivariate Cox models, increasing lines of chemotherapy were independently associated with increased hazards of all OIs (HR, 1.09 [95% CI, 1.07–1.11]) and bacterial infections (HR, 1.09 [95% CI, 1.08–1.11]). Notably, cumulative rituximab exposure was only associated with an increased hazard of VZV (HR, 1.07 [95% CI, 1.01–1.04]). Corticosteroid exposure was associated with increased risk of tuberculosis (HR, 1.84 [95% CI, 1.18–2.89]) and histoplasmosis (HR, 2.91 [95% CI, 1.02–8.26]). Neutropenia was associated with a range of infectious complications including staphylococcal infection (HR, 1.80 [95% CI, 1.31–2.46]), pseudomonal infection (HR, 2.68 [95% CI, 1.74–4.13]), candidiasis (HR, 1.50 [95% CI, 1.25–1.79]), nocardiosis (HR, 10.75 [95% CI, 1.16–99.51]), and nontuberculous mycobacterial infection (HR, 1.94 [95% CI, 1.68–2.25]).
Among patients with iNHL treated with bendamustine vs other chemotherapy regimens, the number needed to harm was 17 for all OIs, 318 for PCP, 156 for CMV, 19 for VZV, and 355 for histoplasmosis (Table 3). The number needed to treat with antimicrobial prophylaxis to prevent a case of infection in patients receiving bendamustine was 70 for all bacterial infections, 374 for PCP, 269 for CMV, and 23 for VZV (Table 3).
DISCUSSION
In this study, we demonstrate that older patients with iNHL treated with bendamustine experience higher rates of both common and opportunistic infections compared to patients receiving other chemotherapies. Among OIs, there is particularly increased risk of infections controlled by cell-mediated immunity such as PCP, herpesvirus reactivation, and histoplasmosis, which is likely secondary to CD4+ lymphopenia induced by this agent [16–22, 33, 34].
To our knowledge, this is the first systematic investigation of bendamustine-associated infectious risks on a population level. The GALLIUM trial reported higher rates of grade 3–5 infection among patients receiving bendamustine compared with CHOP and CVP combined with rituximab and obinutuzumab, but did not delineate specific infectious complications [23]. A meta-analysis assessing grade 3–4 infections in iNHL patients enrolled in clinical trials did not find a significant increase in infections with bendamustine-containing regimens [35]. Clinical trials are designed to assess for oncologic outcomes rather than infectious risks, with inclusion and exclusion criteria that likely select for healthier study participants than those receiving bendamustine in a real-life clinical setting.
Here, we characterize a range of bacterial, viral, fungal, and parasitic infections and assess the risk for developing them among patients with iNHL treated with bendamustine. Patients receiving bendamustine have significant higher risk of both common infections such as bacterial pneumonia and OIs such as PCP, CMV, VZV, and histoplasmosis than patients receiving other chemotherapy regimens.
Within this population, the risk of potentially preventable infections such as bacterial pneumonia, PCP, and CMV appears to be highest among patients receiving bendamustine as part of later (third-line or above) chemotherapy regimens, suggesting that underlying immune dysfunction conferred by more advanced disease and prior immunomodulating chemotherapies may be compounded by the immunosuppressive effects of bendamustine. Additionally, patients who received bendamustine before 2009 for iNHL due to its perceived better tolerability had statistically similar risks of CMV, VZV, and bacterial pneumonia, suggesting that underlying patient vulnerability does not fully explain the infectious risks associated with bendamustine.
These findings are of particular clinical importance as patients with iNHL are traditionally considered to be at low risk of OIs. Our analyses suggest that an OI occurs in 1 of every 17 patients treated with bendamustine, who otherwise would not have developed this OI had they received another chemotherapy regimen. Some patients receiving bendamustine-containing chemotherapy regimens may benefit from antimicrobial prophylaxis against certain preventable OIs associated with substantial morbidity and mortality. We estimate that 1 bacterial infection per 70 patients, 1 PCP case per 374 patients, 1 CMV case per 269 patients, 1 VZV case per 23 patients, and 1 histoplasmosis case per 582 patients with iNHL treated with bendamustine-containing chemotherapy could be prevented with prophylaxis.
This study is limited by the structure of SEER-Medicare administrative and claims data. Although there is potential for undercoding and misclassification of treatment and infectious outcomes, these are unlikely to be differential in nature in the 2 groups, biasing results toward patients treated with bendamustine. Medicare claims have high sensitivity and specificity for identifying chemotherapy administration across all cancer sites [36]. Prior studies have successfully used SEER-Medicare data to characterize common infectious risks among older patients with lymphoid and myeloid malignancies [37, 38]. ICD-9 codes for OIs such as aspergillosis have low positive predictive value but are sufficiently sensitive to identify most cases of invasive aspergillosis [39]. While we recognize limitations of administrative databases for studying the characteristics and severity of infectious complications in detail (eg, whether fungal infections met European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group criteria for proven or probable invasive fungal disease or whether VZV infections had the characteristic rash), we are confident that our analysis captures treatment and significant infectious complications within the limits of the existing data.
As SEER-Medicare only includes patients who are at least 65 years of age, is it unknown whether these findings are generalizable to younger patients with iNHL, or if bendamustine-induced T-lymphocyte depletion on a background of immunosenescence particularly increases the risk of OIs. However, the median age of patients with iNHL falls within the age range of this Medicare cohort, so our findings are directly applicable to many patients with iNHL. Additionally, the use of population-based data means that these findings are more representative than using hospital databases or specialized registers.
Another possible limitation is the potential for residual confounding. Performance status, bone marrow involvement, and laboratory markers were not available in the SEER-Medicare database and we therefore could not adjust for these factors [40]. We did, however, correct for known prognostic indicators of iNHL including age, stage, comorbidity, and response to therapy. We also conduct stratified analyses by line of bendamustine administration, type of prior chemotherapy regimens, and time period of bendamustine administration, which we believe account for potential confounding to the extent possible using administrative data. Indeed, one of the main strengths of our study is the large, population-based sample of patients with iNHL available from the SEER cancer registries, composing a uniquely large, demographically diverse, multicenter cohort to study.
Further prospective studies of the immune consequences of bendamustine exposure are needed. Meanwhile, clinicians should be aware of the potentially increased risk of both common and opportunistic infections in patients receiving bendamustine-containing chemotherapy regimens, particularly with later lines of chemotherapy, to facilitate timely diagnosis and treatment, and to consider the potential benefit of prophylaxis against OIs.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (grant numbers K23 AI097225, R21 AI119692, R21 AI130669, and R21 AI133330 to S. K.).
Potential conflicts of interest. A. F. is on the drug and safety monitoring board for Axio, Bayer, and Immune Design. D. F. has consulted for Viracor and received research funding from Astellas. E. J. has consulted for Merck, Seattle Genetics, Spectrum, Pharmacyclics, Bayer, and AstraZeneca and received research funding from Celgene. P. L. N. has consulted for Genome DX, Ferring, Astellas, Bayer, Dendreon, Augmenix, Blue Earth, and Nanobiotix and received research funding from Astellas and Janssen. S. K. has consulted for Infinity Pharmaceuticals and GI Windows, received research funding from Wako Diagnostics and Merck, and holds patents on methods for analysis of breath for diagnosis of infectious diseases. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood 2006; 107:265–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shankland KR, Armitage JO, Hancock BW. Non-Hodgkin lymphoma. Lancet 2012; 380:848–57. [DOI] [PubMed] [Google Scholar]
- 3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68:7–30. [DOI] [PubMed] [Google Scholar]
- 4. Kalaycio M. Bendamustine: a new look at an old drug. Cancer 2009; 115:473–9. [DOI] [PubMed] [Google Scholar]
- 5. Rummel MJ, Gregory SA. Bendamustine’s emerging role in the management of lymphoid malignancies. Semin Hematol 2011; 48(Suppl 1):S24–36. [DOI] [PubMed] [Google Scholar]
- 6. National Cancer Institute, National Institutes of Health. FDA approval for bendamustine hydrochloride. Available at: http://www.cancer.gov/cancertopics/druginfo/fda-bendamustine-hydrochloride. Accessed 12 December 2017. [Google Scholar]
- 7. Cheson BD, Wendtner CM, Pieper A, et al. . Optimal use of bendamustine in chronic lymphocytic leukemia, non-Hodgkin lymphomas, and multiple myeloma: treatment recommendations from an international consensus panel. Clin Lymphoma Myeloma Leuk 2010; 10:21–7. [DOI] [PubMed] [Google Scholar]
- 8. Robinson KS, Williams ME, van der Jagt RH, et al. . Phase II multicenter study of bendamustine plus rituximab in patients with relapsed indolent B-cell and mantle cell non-Hodgkin’s lymphoma. J Clin Oncol 2008; 26:4473–9. [DOI] [PubMed] [Google Scholar]
- 9. Kahl BS, Bartlett NL, Leonard JP, et al. . Bendamustine is effective therapy in patients with rituximab-refractory, indolent B-cell non-Hodgkin lymphoma: results from a multicenter study. Cancer 2010; 116:106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moskowitz AJ, Hamlin PA Jr, Perales MA, et al. . Phase II study of bendamustine in relapsed and refractory Hodgkin lymphoma. J Clin Oncol 2013; 31:456–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hoy SM. Bendamustine: a review of its use in the management of chronic lymphocytic leukaemia, rituximab-refractory indolent non-Hodgkin’s lymphoma and multiple myeloma. Drugs 2012; 72:1929–50. [DOI] [PubMed] [Google Scholar]
- 12. Rummel MJ, Niederle N, Maschmeyer G, et al. . Study Group Indolent Lymphomas Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet 2013; 381:1203–10. [DOI] [PubMed] [Google Scholar]
- 13. Mondello P, Steiner N, Willenbacher W, et al. . Bendamustine plus rituximab versus R-CHOP as first-line treatment for patients with indolent non-Hodgkin’s lymphoma: evidence from a multicenter, retrospective study. Ann Hematol 2016; 95:1107–14. [DOI] [PubMed] [Google Scholar]
- 14. Rummel M, Kaiser U, Balser C, et al. . Study Group Indolent Lymphomas Bendamustine plus rituximab versus fludarabine plus rituximab for patients with relapsed indolent and mantle-cell lymphomas: a multicentre, randomised, open-label, non-inferiority phase 3 trial. Lancet Oncol 2016; 17:57–66. [DOI] [PubMed] [Google Scholar]
- 15. Flinn IW, van der Jagt R, Kahl BS, et al. . Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood 2014; 123:2944–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. García Muñoz R, Izquierdo-Gil A, Muñoz A, Roldan-Galiacho V, Rabasa P, Panizo C. Lymphocyte recovery is impaired in patients with chronic lymphocytic leukemia and indolent non-Hodgkin lymphomas treated with bendamustine plus rituximab. Ann Hematol 2014; 93:1879–87. [DOI] [PubMed] [Google Scholar]
- 17. Yutaka T, Ito S, Ohigashi H, et al. . Sustained CD4 and CD8 lymphopenia after rituximab maintenance therapy following bendamustine and rituximab combination therapy for lymphoma. Leuk Lymphoma 2015; 56:1–3. [DOI] [PubMed] [Google Scholar]
- 18. Saito H, Maruyama D, Maeshima AM, et al. . Prolonged lymphocytopenia after bendamustine therapy in patients with relapsed or refractory indolent B-cell and mantle cell lymphoma. Blood Cancer J 2015; 5:e362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hosoda T, Yokoyama A, Yoneda M, et al. . Bendamustine can severely impair T-cell immunity against cytomegalovirus. Leuk Lymphoma 2013; 54:1327–8. [DOI] [PubMed] [Google Scholar]
- 20. Carter SJ, Bernstein SH, Friedberg JW, Barr PM. Pneumocystis jirovecii pneumonia as a complication of bendamustine in a patient receiving bendamustine plus rituximab for marginal zone lymphoma. Leuk Res 2011; 35:e223–4. [DOI] [PubMed] [Google Scholar]
- 21. Abkur TM, Saeed M, Ahmed SZ, et al. . Pneumocystis jiroveci prophylaxis in patients undergoing bendamustine treatment: the need for a standardized protocol. Clin Case Rep 2015; 3:255–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lim SH, Pathapati S, Langevin J, Hoot A. Severe CMV reactivation and gastritis during treatment of follicular lymphoma with bendamustine. Ann Hematol 2012; 91:643–4. [DOI] [PubMed] [Google Scholar]
- 23. Marcus R, Davies A, Ando K, et al. . Obinutuzumab for the first-line treatment of follicular lymphoma. N Engl J Med 2017; 377:1331–44. [DOI] [PubMed] [Google Scholar]
- 24. Warren JL, Klabunde CN, Schrag D, et al. . Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care 2002; 40:IV3–IV18. [DOI] [PubMed] [Google Scholar]
- 25. Bikov KA, Mullins CD, Seal B, Onukwugha E, Hanna N. Algorithm for identifying chemotherapy/biological regimens for metastatic colon cancer in SEER-Medicare. Med Care 2015; 53:e58–64. [DOI] [PubMed] [Google Scholar]
- 26. Marventano S, Grosso G, Mistretta A, et al. . Evaluation of four comorbidity indices and Charlson comorbidity index adjustment for colorectal cancer patients. Int J Colorectal Dis 2014; 29:1159–69. [DOI] [PubMed] [Google Scholar]
- 27. Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol 2000; 53:1258–67. [DOI] [PubMed] [Google Scholar]
- 28. Gafter-Gvili A, Fraser A, Paul M, et al. . Antibiotic prophylaxis for bacterial infections in afebrile neutropenic patients following chemotherapy. Cochrane Database Syst Rev 2012; 1:CD004386. [DOI] [PubMed] [Google Scholar]
- 29. Stern A, Green H, Paul M, et al. . Prophylaxis for Pneumocystis pneumonia (PCP) in non-HIV immunocompromised patients. Cochrane Database Syst Rev 2014; 10:CD005590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hodson EM, Jones CA, Webster AC, et al. . Antiviral medications to prevent cytomegalovirus disease and early death in recipients of solid-organ transplants: a systematic review of randomised controlled trials. Lancet 2005; 365:2105–15. [DOI] [PubMed] [Google Scholar]
- 31. Glenny AM, Fernandez Mauleffinch LM, Pavitt S, Walsh T. Interventions for the prevention and treatment of herpes simplex virus in patients being treated for cancer. Cochrane Database Syst Rev 2009; 1:CD006706. [DOI] [PubMed] [Google Scholar]
- 32. McKinsey DS, Wheat LJ, Cloud GA, et al. . Itraconazole prophylaxis for fungal infections in patients with advanced human immunodeficiency virus infection: randomized, placebo-controlled, double-blind study. National Institute of Allergy and Infectious Diseases Mycoses Study Group. Clin Infect Dis 1999; 28:1049–56. [DOI] [PubMed] [Google Scholar]
- 33. Layman RM, Ruppert AS, Lynn M, et al. . Severe and prolonged lymphopenia observed in patients treated with bendamustine and erlotinib for metastatic triple negative breast cancer. Cancer Chemother Pharmacol 2013; 71:1183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Penne M, Sarraf Yazdy M, Nair KS, Cheson BD. Extended follow-up of patients treated with bendamustine for lymphoid malignancies. Clin Lymphoma Myeloma Leuk 2017; 17:637–44. [DOI] [PubMed] [Google Scholar]
- 35. Gafter-Gvili A, Gurion R, Raanani P, Shpilberg O, Vidal L. Bendamustine-associated infections-systematic review and meta-analysis of randomized controlled trials. Hematol Oncol 2017; 35:424–31. [DOI] [PubMed] [Google Scholar]
- 36. Lund JL, Stürmer T, Harlan LC, et al. . Identifying specific chemotherapeutic agents in Medicare data: a validation study. Med Care 2013; 51:e27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Anderson LA, Atman AA, McShane CM, Titmarsh GJ, Engels EA, Koshiol J. Common infection-related conditions and risk of lymphoid malignancies in older individuals. Br J Cancer 2014; 110:2796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Titmarsh GJ, McMullin MF, McShane CM, Clarke M, Engels EA, Anderson LA. Community-acquired infections and their association with myeloid malignancies. Cancer Epidemiol 2014; 38:56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chang DC, Burwell LA, Lyon GM, et al. . Comparison of the use of administrative data and an active system for surveillance of invasive aspergillosis. Infect Control Hosp Epidemiol 2008; 29:25–30. [DOI] [PubMed] [Google Scholar]
- 40. Arcaini L, Rattotti S, Gotti M, Luminari S. Prognostic assessment in patients with indolent B-cell lymphomas. ScientificWorldJournal 2012; 2012:107892. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.