High glycolytic activity and opportunistic infection load measured by 18F-fluorodeoxyglucose positron emission tomography imaging before antiretroviral therapy initiation are associated with immune reconstitution inflammatory syndrome development in HIV+ patients, highlighting the intersect of metabolism and inflammation in HIV infection.
Keywords: HIV, immune reconstitution inflammatory syndrome, FDG-PET, tuberculosis, glycolytic shift
Abstract
Background
Immune reconstitution inflammatory syndrome (IRIS) represents an unexpected inflammatory response shortly after initiation of antiretroviral therapy (ART) in some human immunodeficiency virus (HIV)–infected patients with underlying neoplasia or opportunistic infections, including tuberculosis. We hypothesized that IRIS is associated with increased glycolysis and that 18F-fluorodeoxyglucose (FDG) positron emission tomography–computed tomography (PET/CT) could help identify high-risk subjects.
Methods
In this prospective cohort study, 30 HIV-infected patients (CD4+ count <100 cells/µL) underwent FDG-PET/CT scans at baseline and 4–8 weeks after ART initiation. Ten patients developed IRIS (6 mycobacterial).
Results
At baseline, total glycolytic activity, total lesion volume, and maximum standardized uptake values (SUVs) of pathologic FDG uptake (reflective of opportunistic disease burden) were significantly higher in IRIS vs non-IRIS (P = .010, .017, and .029, respectively) and significantly correlated with soluble inflammatory biomarkers (interferon-γ, myeloperoxidase, tumor necrosis factor, interleukin 6, soluble CD14). Baseline bone marrow (BM) and spleen FDG uptake was higher in mycobacterial IRIS specifically. After ART initiation, BM and spleen mean SUV decreased in non-IRIS (P = .004, .013) but not IRIS subjects. Our results were supported by significantly higher glucose transporter 1 (Glut-1) expression of CD4+ cells and monocytes after ART initiation in IRIS/mycobacterial IRIS compared with non-IRIS patients.
Conclusions
We conclude that increased pathologic metabolic activity on FDG-PET/CT prior to ART initiation is associated with IRIS development and correlates with inflammatory biomarkers. Abnormally elevated BM and spleen metabolism is associated with mycobacterial IRIS, HIV viremia, and Glut-1 expression on CD4+ cells and monocytes.
Clinical Trials Registration
Treatment of human immunodeficiency virus (HIV)–infected patients with antiretroviral therapy (ART) leads to restoration of CD4+ T cells with recovery of an adequate immune response to pathogens and dramatic improvement of morbidity and mortality. A subset of treated patients (7%–50%), however, will deteriorate clinically despite control of HIV viremia and develop immune reconstitution inflammatory syndrome (IRIS), with worsening manifestations of underlying opportunistic infections (OIs) or neoplasia (paradoxical) or with abrupt and frequently atypical presentation of an occult OI or neoplasia (unmasking) [1]. IRIS is more common in persons starting ART at low CD4 counts and in the presence of an active infection [2]. Studies have also suggested that higher antigen burden may be an important risk factor, especially in mycobacterial IRIS (myco-IRIS) [3].
Standard practice currently calls for immediate ART initiation upon HIV diagnosis regardless of CD4 count [4], and results of randomized controlled trials support concurrent initiation of ART with OI treatment to improve outcomes [5–7]. Based on these recommendations, rates of IRIS especially related to tuberculosis (TB) may rise in places where HIV diagnoses occur at low CD4 counts [8, 9], and elucidation of IRIS immunopathogenesis thus becomes of utmost importance to develop targeted therapies.
Molecular imaging using 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) can provide a metabolic map of various organs, indirectly reflecting immune activation and/or responsiveness to treatment. FDG-PET has already been used to better understand HIV pathogenesis, demonstrating increased metabolic activity in lymph nodes (LNs) of viremic untreated persons [10–12], to follow TB treatment response [13–15] and to potentially identify HIV-infected persons who may reactivate latent infection [16]. The potential utility of FDG-PET is further supported by the role of glucose transporter 1 (Glut-1) in HIV. Glut-1, the most common glucose transporter in humans, is overexpressed in tumors due to increased nutritional demands resulting in a shift from oxidative phosphorylation toward aerobic glycolysis (Warburg effect) [17]. A similar glycolytic shift has been recently recognized in activated macrophages [18] and T lymphocytes [19, 20]. This role is even more important in TB [21, 22], with Glut-1–positive staining observed in 9 of 10 cases of pulmonary TB [23]. Finally, glucose metabolism and glycolysis are favored energy sources in effector CD4 T-cell and inflammatory macrophages, making glycolysis the likely central metabolic pathway in IRIS pathogenesis.
In this report, we prospectively evaluated the association of FDG-PET and IRIS development in ART-naive, HIV-infected patients initiating ART to determine potential predictors of IRIS and explore association of FDG uptake to various inflammatory markers. In addition, we assessed increased metabolic activity in various organs of interest and their changes after ART initiation, to gauge the impact of virologic suppression on those organs that could represent potential metabolically active HIV viral reservoirs.
MATERIALS AND METHODS
Study Design
The study was approved by the Radiation Safety and National Institute of Allergy and Infectious Diseases ethics committees (PET Imaging and Lymph Node Assessment of IRIS in Persons With AIDS [PANDORA], ClinicalTrials.gov registration NCT02147405), and all participants signed informed consent. All procedures were in accordance with the Helsinki Declaration of the World Medical Association.
This was a prospective observational study of HIV-1–infected adults with the primary objective to evaluate PET imaging and tissue biopsies in IRIS. Eligible participants had CD4 T-cell counts <100 cells/µL, were naive to ART, and were willing to start therapy. ART was initiated according to standard treatment guidelines, usually within 2 weeks from study enrollment.
FDG-PET Imaging
FDG-PET scans were performed prior to ART initiation, and 4–8 weeks after ART initiation, on a PET–computed tomography (CT) scanner (Biograph-128 Micro-CT, Siemens Medical Solutions). Approximately 58 minutes following the injection of 10 mCi of 18F-FDG, each patient was positioned on the scanner table and a low-dose CT scan was performed from the base of the skull to the upper thighs for attenuation correction and anatomical localization. Emission scans were then obtained in 3-dimensional mode, in multiple bed positions, each lasting 10–15 minutes. Images were reconstructed with an iterative technique using an ordered subset expectation-maximization algorithm.
Quantification Analysis of FDG PET/CT Studies
Radiologists assessing the images were blinded to the patients’ diagnoses (IRIS vs non-IRIS). Pathologic and organ-specific FDG uptake throughout the body was assessed using MIM Vista workstation (version 6.5.9).
Whole-body Pathologic FDG Uptake Measurement
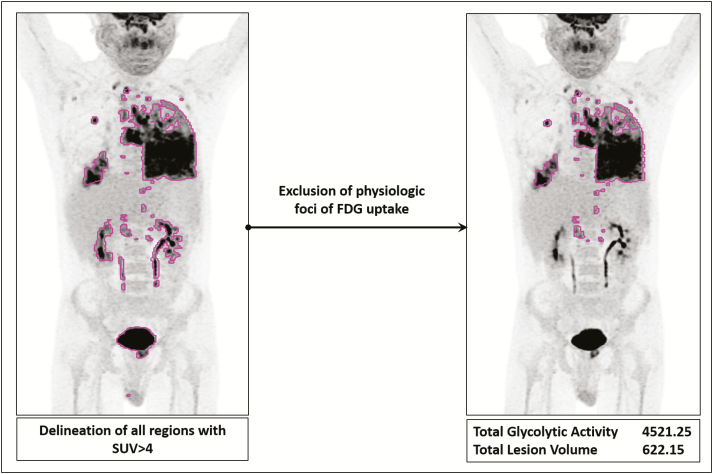
A volume of interest (VOI) encompassing the entire body was drawn and subsequently a standardized uptake value (SUV) threshold-based automatic approach was applied to include all sites of increased metabolic activity. The applied framework enables automatic generation of individual VOIs encircling all areas with FDG uptake above the SUV threshold that is set by the user (Figure 1). We chose an SUV threshold of 4, based on qualitative assessment of best value to include the majority of pathologic uptake in most patients. Afterward, areas with physiologic FDG uptake within the all-inclusive VOI were manually excluded. Finally, the following parameters of the finalized VOI were automatically generated per patient, before and after treatment: maximum SUV (SUVmax), average SUV (SUVmean), total lesion volume (TLV), and total glycolytic activity (TGA = SUVmean × TLV).
Figure 1.
Delineation method for estimating whole-body pathologic uptake values (lesion load). All regions with standardized uptake value (SUV) >4 are first automatically delineated throughout the regions of interest. This is followed by manual exclusion of regions of physiologic uptake, such as the myocardium, kidneys, and bladder. The final contour represents the whole-body pathologic 18F-fluorodeoxyglucose uptake for the patient. Mean SUV, maximum SUV, total glycolytic activity, and total lesion volume are then calculated. Note that in this patient, myocardial SUV values were below the threshold of 4 and thus the heart was not included in the comprehensive volume of interest and did not need to be excluded afterward. Abbreviations: FDG, 18F-fluorodeoxyglucose; SUV, standardized uptake value.
Organ-specific VOIs
VOIs were drawn in the bone marrow (BM), liver, spleen, and axillary LNs and copied to the pre- and posttreatment FDG scans after automatic coregistration of the scans. SUVmax and SUVmean were then generated for the VOIs and compared. Bone marrow values were obtained from VOIs drawn over the L5 vertebra and left iliac wing.
Cytokine Measurements
Cryopreserved plasma was used from baseline and a time point proximal to the second FDG-PET scan. Samples were analyzed by enzyme-linked fluorescent assay on a VIDAS instrument for D-dimer (bioMérieux, Durham, North Carolina) and enzyme-linked immunosorbent assay kits (R&D Systems, Minneapolis, Minnesota and MyBiosource.com, San Diego, California, respectively) for soluble CD14 (sCD14). Plasma levels of interferon gamma (IFN-γ) (lower limit of detection [LLD] = 0.40 pg/mL), tumor necrosis factor (TNF) (LLD = 0.09 pg/mL), myeloperoxidase (MPO) (LLD = 0.07 ng/mL), MCP-1 (LLD = 0.11 pg/mL), sPD1 (LLD = 0.00 pg/mL), sCD14 (LLD = 2.5 mg/L), interleukin 2 (LLD = 0.38 pg/mL), interleukin 6 (IL-6) (LLD = 0.18 pg/mL), interleukin 8 (LLD = 0.16 pg/mL), and interleukin 10 (LLD = 0.09 pg/mL) were measured using a custom multiplex kit and 2 single-plex kits by electrochemiluminescence (Meso Scale Discovery, Gaithersburg, Maryland). Results from interleukin 1β and interleukin 12p70 were at or below the LLD and were excluded.
Flow Cytometry Analysis
Immunophenotyping of cells was performed using fluorochrome-conjugated monoclonal antibodies against CD3, CD4, CD8, and CD14 (BD Bioscience, San Jose, California or eBioscience, San Diego, California). Surface Glut-1 receptor expression was detected by binding to the Glut-1 ligand fused to enhanced green fluorescent protein (GFP) as previously described [24, 25] (Metafora Biosystems, Evry, France). In brief, 106 cells were incubated for 30 minutes with 10 µL of Glut-1–GFP solution prior to Live/Dead staining using LIVE/DEAD Cell Viability Assay (Life Technologies, Carlsbad, California). GFP fluorescence was detected by staining cells with Alexa Fluor 647 anti-GFP antibody at 1:200 (Biolegend). Data were acquired on a LSRII (BD Biosciences) and analyzed with FlowJo 10.0.8 software (Ashland, Oregon).
Statistical Methods
Comparisons of baseline values and change from baseline to post–ART initiation were performed between IRIS (n = 10) and non-IRIS patients (n = 20), and between myco-IRIS (n = 6) and non-IRIS (n = 20) patients using nonparametric tests (Wilcoxon rank-sum test and Fisher exact test), which are typically more conservative than parametric tests. The primary focus was on the whole-body pathologic FDG uptake (SUVmean, SUVmax, TGA, and TLV). The criteria for statistical significance for this primary comparison were set to P < .05, without adjustment for multiplicity because of the small sample size and the exploratory nature of this pilot study. The hypotheses evaluated here were largely part of a similar process, introducing correlated hypothesis tests and summary measures (eg, SUVmax and SUVmean), making a Bonferroni correction (which assumes independent hypothesis tests) conservative [26]. Organ-specific SUV values were similarly compared, as were clinical laboratory measurements, plasma cytokine levels, and markers of immune activation and inflammation.
Markers of immune activation and inflammation were correlated with whole-body pathologic FDG uptake using a Spearman rank correlation analysis. These correlation estimates were exploratory and considered significant if P < .01, unless the P value was consistently <.05 across >1 of the various imaging parameters evaluated.
Receiver operating characteristic (ROC) curve analyses were conducted using a nonparametric ROC curve estimator, with the corresponding test of statistical significance of the area under the ROC curve (AUC) using pROC in R [27]. All the analyses were conducted using R (version 3.3.2) and SAS (version 9.4) software.
RESULTS
Participant Characteristics and IRIS Events
Thirty ART-naive, HIV-infected patients (22 male, 8 female) were recruited from the greater Washington, D.C., area and their HIV clinical care was provided at the National Institutes of Health Clinical Center. The baseline characteristics of study participants are listed in Table 1.
Table 1.
Baseline Demographic and Clinical Characteristics of Study Participants (Total and by Study Outcome)
| Study Group | All Patients (N = 30) | Non-IRIS (n = 20) | IRIS (n = 10) | P Value, IRIS vs Non-IRIS | Mycobacterial IRIS (n = 6) | P Value, Mycobacterial IRIS vs Non-IRIS |
|---|---|---|---|---|---|---|
| Age, y, median (IQR) | 36 (32–39) | 36 (32–39) | 37 (33–41) | .628 | 37 (33–37) | .714 |
| Female sex, No. (%) | 8 (27) | 4 (20) | 4 (40) | .384 | 3 (50) | .293 |
| Race, No. (%) | .490 | 1 | ||||
| White | 11 (36.7) | 8 (40) | 3 (30) | … | 2 (33.3) | … |
| African American | 18 (60) | 12 (60) | 6 (60) | … | 4 (66.7) | … |
| Asian | 1 (3.3) | 0 (0) | 1 (10) | … | 0 (0) | … |
| Hemoglobin, g/dL, median (IQR) | 10.1 (9.3–11.5) | 10.2 (9.8–11.5) | 9.5 (8.1–10.6) | .201 | 8.8 (8–10.1) | .038a |
| BMI, kg/m2, median (IQR) | 21.8 (19.3–25.1) | 21.8 (19.3–24.8) | 22.1 (18.5–25.5) | .947 | 23.5 (20.8–28.5) | .411 |
| CD4+ T-cell %, median (IQR) | 3 (2–5) | 2 (1–4.5) | 3.5 (3–6) | .119 | 4 (3–6) | .206 |
| CD4+ T cells/μL, median (IQR) | 25 (13–36) | 19 (10–30) | 28 (18–48) | .180 | 28 (14–48) | .361 |
| CD8+ T-cell %, median (IQR) | 69 (63–75) | 66 (60–72) | 70 (65–78) | .118 | 70 (69–76) | .180 |
| CD8+ T cells/μL, median (IQR) | 553 (340–682) | 538 (361–680) | 604 (334–825) | .613 | 604 (318–682) | .927 |
| CD4/CD8 ratio, median (IQR) | 0.04 (0.02–0.07) | 0.03 (0.02–0.06) | 0.05 (0.04–0.09) | .244 | 0.05 (0.04–0.09) | .346 |
| Plasma HIV RNA, log copies/mL, median (IQR) | 5.3 (4.9–5.9) | 5.2 (4.8–5.7) | 5.7 (5.4–6.0) | .068 | 6.0 (5.9–6.1) | .012a |
Abbreviations: BMI, body mass index; HIV, human immunodeficiency virus; IQR, interquartile range; IRIS, immune reconstitution inflammatory syndrome.
aDenotes statistically significant difference.
Participants had a median age of 36 years and low CD4+ T-cell counts (median, 25 cells/μL [interquartile range, 13–36]). Ten of 30 (33%) patients were eventually diagnosed with IRIS based on AIDS Clinical Trials Group criteria (https://actgnetwork.org/IRIS_case_definitions) at a median of 35.5 days after ART initiation. There were no significant differences in the clinical variables between IRIS and non-IRIS patients at baseline. The detailed demographics, clinical presentations, OIs, and relevant infections are shown in Supplementary Tables 1 and 2. Among those with IRIS, 6 patients were diagnosed with myco-IRIS. Two IRIS patients were characterized as having paradoxical TB central nervous system (CNS) IRIS—that is, they had disseminated TB at baseline and manifested CNS involvement during IRIS presentation. The myco-IRIS patients had lower hemoglobin and higher plasma HIV viremia compared with the non-IRIS group (Table 1). All participants experienced CD4 increases and plasma HIV viremia suppression at the time the second FDG-PET (Supplementary Figure 1) was performed.
Whole-body Pathologic (Nonphysiologic) FDG Uptake Across Groups
Whole-body pathologic (nonphysiologic) SUVmax, TGA, and TLV values were significantly higher at baseline in IRIS and in myco-IRIS compared with non-IRIS patients (Table 2).
Table 2.
Comparison of Whole-body Pathologic 18F-Fluorodeoxyglucose Uptake Between Study Groups at Baseline
| Study Group | All Patients (N = 30) | Non-IRIS (n = 20) | IRIS (n = 10) | P Value, IRIS vs Non-IRIS | Mycobacterial IRIS (n = 6) | P Value, Mycobacterial IRIS vs Non-IRIS |
|---|---|---|---|---|---|---|
| SUVmean | 5.8 (5.0–6.4) | 5.1 (4.7–6.4) | 6.0 (5.7–6.4) | .108 | 6.2 (5.7–7.7) | .073 |
| SUVmax | 14.1 (11.2–22.6) | 12.1 (8.7–19.2) | 20.0 (14.2–31.6) | .010a | 22.4 (19.7–31.6) | .008a |
| TGA | 182.3 (50.3–559.4) | 106.4 (20.1–328.8) | 404.7 (170.1–3156) | .017a | 1858 (371.4–3821) | .010a |
| TLV | 30.9 (9.8–87.2) | 20.0 (4.7–58.6) | 68.9 (28.4–482.0) | .029a | 284.6 (65.4–536.5) | .014a |
Values are reported as median (interquartile range).
Abbreviations: IRIS, immune reconstitution inflammatory syndrome; SUVmax, maximum standardized uptake value; SUVmean, mean standardized uptake value; TGA, total glycolytic activity; TLV, total lesion volume.
aDenotes statistically significant difference.
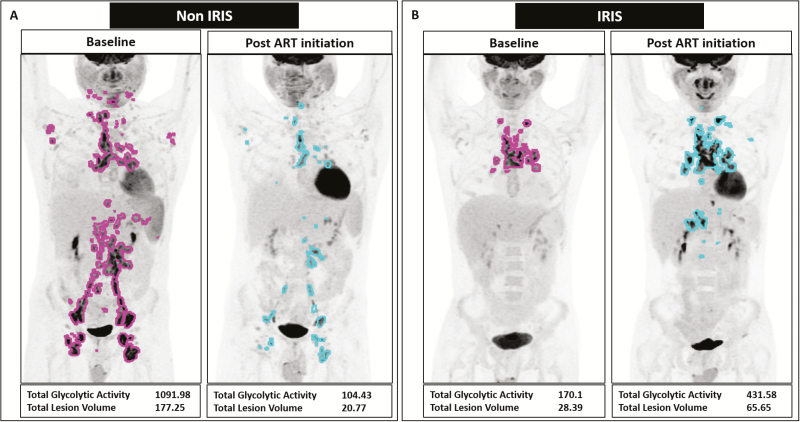
After ART initiation, non-IRIS subjects showed an average drop in TGA and TLV values of 33.7 and 3.96, respectively. In contrast, IRIS and myco-IRIS subjects showed an average increase in TGA and TLV values (increase of 102.2 and 20.27 for IRIS and 439.5 and 84.65 for myco-IRIS, respectively; Figure 2). The differences, however, did not reach statistical significance.
Figure 2.
Changes in whole-body pathologic 18F-fluorodeoxyglucose uptake (total glycolytic activity [TGA] and total lesion volume [TLV]) in representative non–immune reconstitution inflammatory syndrome (IRIS) and IRIS patients. A, A 26-year-old male non-IRIS subject (non-IRIS-05) who presented with diffuse mycobacterium avium complex lymphadenopathy in the neck, chest, abdomen, and pelvis with involvement of the axillary and inguinal lymph nodes (LNs; pink outline). Following antiretroviral therapy (ART) initiation, there was a marked improvement in his lesion load (blue outline) as is reflected in the decrease of both TGA and TLV. B, A 34-year-old male subject diagnosed with varicella zoster virus IRIS, involving >1 dermatome with lesions crossing the midline (IRIS-05). Mediastinal and hilar lymphadenopathy (pink outline) worsened after starting ART, with further involvement of upper abdominal LNs (blue outline). TGA and TLV increased after ART initiation by 153% and 131%, respectively. Abbreviations: ART, antiretroviral therapy; IRIS, immune reconstitution inflammatory syndrome.
Organ-specific Metabolic Activity Measurements at Baseline
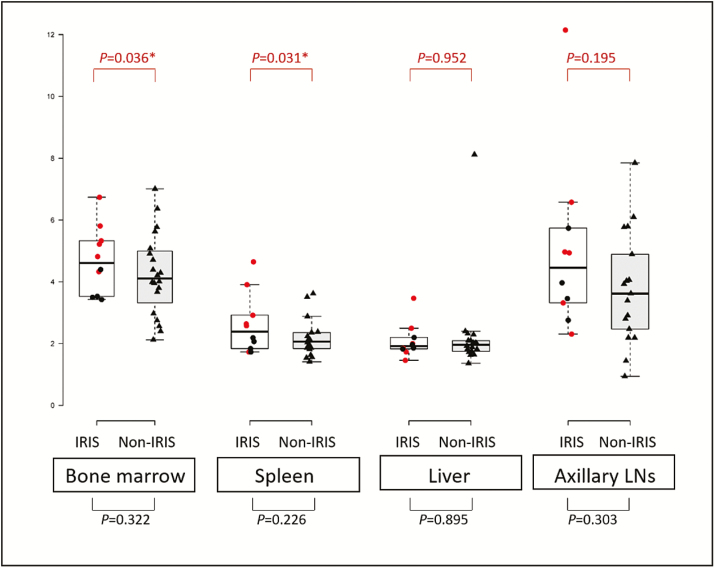
At baseline, myco-IRIS patients had significantly higher SUVmean in the spleen (P = .031) and BM (P = .036) compared with the non-IRIS group (Figure 3). Otherwise, all target organs (BM, spleen, and axillary LNs), except the liver, had higher average SUVmax and SUVmean values in the IRIS group compared with the non-IRIS group; however, this was not statistically significant (Figure 3).
Figure 3.
Mean standardized uptake values in individually assessed organs at baseline. Mycobacterial immune reconstitution inflammatory syndrome (IRIS) patient values are presented in red circles. P values between IRIS and non-IRIS (in black triangles) and between mycobacterial IRIS and non-IRIS (in red) are included. *Denotes statistical significance, P < .05. Abbreviations: IRIS, immune reconstitution inflammatory syndrome; LN, lymph node.
Organ-specific Metabolic Activity Changes After ART
In the non-IRIS group, SUVmean in the spleen (Table 3 and Figure 4A) and BM (Table 3 and Supplementary Figure 2A) decreased significantly after ART treatment (P = .009 and P = .003, respectively), whereas in the IRIS group, only SUVmean of the axillary LNs decreased significantly after treatment (P = .027) (Table 3, Figure 4B, and Supplementary Figure 2B).
Table 3.
Mean Standardized Uptake Unit Values Measured in Regions of Interest at Baseline and Post-antiretroviral Therapy Initiation in Study Groups
| Region of Interest | Baseline | Post-ART Initiation | P Value |
|---|---|---|---|
| Non-IRIS | |||
| Bone marrow | 4.1 (3.3–5.0) | 3.4 (3.1–4.2) | .004a |
| Liver | 2.0 (1.8–2.1) | 1.9 (1.7–2.1) | .413 |
| Spleen | 2.1 (1.8–2.4) | 1.9 (1.6–2.2) | .013a |
| R/L axillary LN | 3.6 (2.5–4.9) | 3.7 (2.2–4.7) | .788 |
| IRIS | |||
| Bone marrow | 4.6 (3.5–5.3) | 4.1 (3.5–4.4) | .950 |
| Liver | 1.9 (1.8–2.2) | 1.8 (1.7–2.0) | .287 |
| Spleen | 2.4 (1.8–2.9) | 1.9 (1.7–2.9) | .189 |
| R/L axillary LN | 4.5 (3.3–5.7) | 3.1 (2.1–3.2) | .015a |
Values are reported as median (interquartile range).
Abbreviations: ART, antiretroviral therapy; IRIS, immune reconstitution inflammatory syndrome; L, left; LN, lymph node; R, right.
aDenotes statistically significant difference.
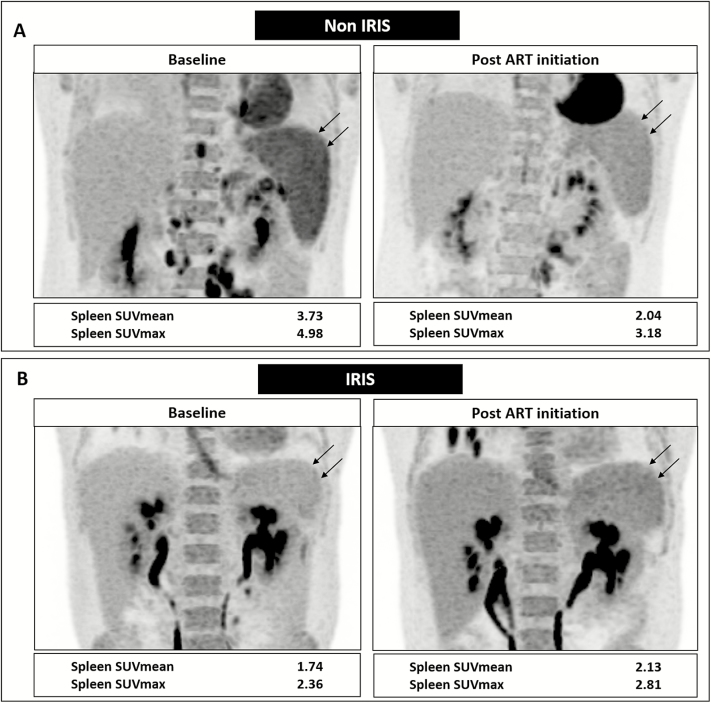
Figure 4.
Changes in spleen 18F-fluorodeoxyglucose (FDG) uptake in representative non–immune reconstitution inflammatory syndrome (IRIS) and IRIS patients. A, A 26-year-old male non-IRIS subject (non-IRIS-05) showing decreased spleen FDG uptake (black arrows) after antiretroviral therapy (ART) on coronal reconstruction positron emission tomography images of the upper abdomen. This is reflected as 45% decrease in mean standardized uptake (SUVmean) and 36% decrease in maximum standardized uptake (SUVmax) values with respect to baseline. B, A 37-year-old male IRIS subject (IRIS-01) with paradoxical mycobacterial IRIS showing slightly increased spleen FDG uptake (black arrows) after ART initiation with 22% increase in SUVmean and 19% increase in SUVmax values with respect to baseline. Abbreviations: ART, antiretroviral therapy; IRIS, immune reconstitution inflammatory syndrome; SUVmax, maximum standardized uptake value; SUVmean, mean standardized uptake value.
Associations of Whole-body Pathologic FDG Uptake With Soluble Biomarkers
Patients with myco-IRIS showed significantly higher D-dimer (P = .019), IFN-γ (P = .026), and borderline elevated IL-6 (P = .042) and MPO (P = .042) at baseline compared with non-IRIS patients (Supplementary Table 3).
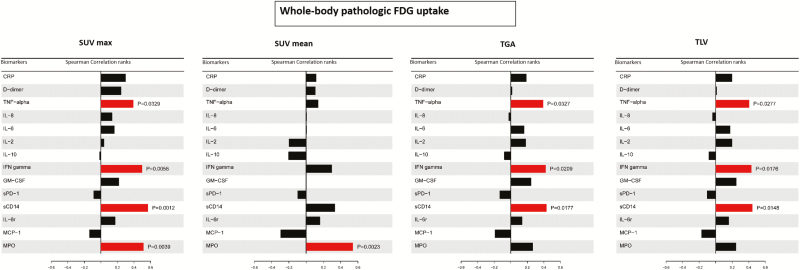
At baseline, whole-body pathologic SUVmax, TGA, and TLV values measured in all 30 patients correlated positively with TNF, IFN-γ, and sCD14 levels whereas SUVmax and SUVmean had also a positive correlation with MPO (Figure 5). When the IRIS group was analyzed separately, whole-body pathologic SUVmax again showed a positive correlation with the levels of TNF, IFN-γ, and sCD14 (Supplementary Figure 3). Whole-body pathologic SUVmax in the myco-IRIS group correlated with C-reactive protein (CRP) and sCD14 (Supplementary Figure 3).
Figure 5.
Correlation of whole-body pathologic 18F-fluorodeoxyglucose uptake with inflammatory cellular and soluble biomarkers measured in all patients at baseline. Correlations for maximum standardized uptake, mean standardized uptake, total glycolytic activity, and total lesion values are shown. Abbreviations: CRP, C-reactive protein; GM-CSF, granulocyte macrophage–colony-stimulating factor; FDG, 18F-fluorodeoxyglucose; IFN, interferon; IL, interleukin; MCP-1, monocyte chemoattractant protein 1; MPO, myeloperoxidase; sCD14, soluble CD14; sPD-1, soluble programmed death receptor-1; SUVmax, maximum standardized uptake; SUVmean, mean standardized uptake; TGA, total glycolytic activity; TLV, total lesion value; TNF, tumor necrosis factor.
After the initiation of ART, the change in TGA and TLV of whole-body pathologic FDG uptake correlated with IFN-γ (P = .002 and P = .007, respectively) and TNF (P = .004 and P = .009, respectively).
Defining Threshold for Development of IRIS/Myco-IRIS Based on Baseline SUVmax Values
Using SUVmax values of whole-body pathologic uptake at baseline, we found that the optimal cutoff value for differentiating IRIS from non-IRIS, in this dataset, was 11.98, with 100% sensitivity and 50% specificity. We also found that the optimal SUVmax cutoff value for differentiating myco-IRIS from non-IRIS was 18.07, with 100% sensitivity and 70% specificity. The cutoff values were chosen to minimize the combined false-negative and false-positive rates. The AUC values for SUVmax were 0.80 and 0.87, reflecting good accuracy in respectively separating IRIS and myco-IRIS from non-IRIS subjects. The AUC for SUVmax was higher (albeit not statistically significant) than that for CRP, IL-6, MPO, IFN-γ, TNF, and sCD14 (Supplementary Figure 4).
Expression of Glut-1 in T Cells and Monocytes
The proportion of CD4+ T cells (Figure 6A and 6C) and monocytes (Figure 6B and 6D) expressing Glut-1 was not significantly different between groups pre-ART. Post–ART initiation, however, non-IRIS subjects had significantly lower proportions of CD4+ T cells and monocytes expressing Glut-1 compared with IRIS (P = .022 and P = .008, respectively) and myco-IRIS participants (P = .009 for both; Figure 6). In the subset of participants who underwent PET scans and had Glut-1 staining (n = 11), CD4+ T-cell Glut-1 expression pre-ART positively correlated with TGA (r = 0.65, P = .034) and monocyte Glut-1 expression post-ART positively correlated with SUVmax (r = 0.66, P = .03).
Figure 6.
Immunophenotyping examples from mycobacterial immune reconstitution inflammatory syndrome (IRIS) and non-IRIS subjects. A, Glucose transporter 1 (Glut-1+) cells within the CD4+ cell population. B, Glut-1+ cells within the CD14+ cell population (monocytes). C and D, Distribution of Glut-1 percentage positive staining in CD4 and CD14 cells, respectively, at baseline and post–antiretroviral therapy initiation. Median values and interquartile ranges are shown. *xxx; **xxx. Abbreviations: ART, antiretroviral therapy; Glut-1, glucose transporter 1; HC, healthy control; IRIS, immune reconstitution inflammatory syndrome; myco-IRIS, mycobacterial immune reconstitution inflammatory syndrome.
DISCUSSION
In our study, we used FDG-PET/CT to prospectively evaluate ART-naive HIV-infected patients with severe lymphopenia and underlying infections prior to treatment initiation, to assess potential predictive value for IRIS development. Testing the hypothesis that lesion load and its colocalizing immune response, both of which are associated with increased metabolic activity, could be a predisposing factor for IRIS [3], we measured quantitatively whole-body pathologic FDG uptake. Whole-body pathologic total glycolytic activity values and total lesion volumes were significantly higher at baseline in patients who eventually developed IRIS, suggesting higher immune response possibly driven by more active or disseminated infection. TGA and TLV differences were more striking in mycobacterial IRIS, probably due to the pathogenic involvement of activated macrophages in mycobacterial infections. Despite the small sample size, ROC curves for SUVmax seemed promising to identify patients at risk for IRIS (especially mycobacterial), performing overall better than other inflammatory biomarkers, but larger studies are needed to validate this finding. A sensitive test could be useful to exclude patients from IRIS prevention clinical trials, for example, or from more intense monitoring.
Considering that only a small fraction of T lymphocytes are present in peripheral blood while most reside in lymphoid tissues, imaging modalities that can capture the activity of immune cells in lymphoid tissues or effector sites could be helpful in better understanding the pathogenesis of IRIS [28]. In addition, antigen-presenting cells are not represented in peripheral blood and can be highly active metabolically in lymphoid organs and BM. We found that mean organ metabolic activities in the BM, axillary LNs, and spleen were uniformly higher at baseline in IRIS compared with non-IRIS patients, reaching statistical significance in the BM and spleen in the mycobacterial IRIS group. This is consistent with a prominent role of myeloid cells in IRIS pathogenesis, potentially reflecting abundant activated macrophages. Findings in the BM of IRIS patients could also partially reflect increased numbers of proliferating and maturing erythrocytes (IRIS patients were more anemic), although involvement by the infectious/neoplastic ongoing process cannot be excluded. In fact, in 1 patient with mycobacterial IRIS who underwent a BM biopsy, there was evidence of TB involvement with nonnecrotizing granulomas.
The prominent role of activated myeloid cells in IRIS was also supported by the fact that while BM and spleen SUVmean values decreased significantly after treatment in non-IRIS patients, consistent with HIV viremia induced immune activation as previously described [10], they did not change significantly in IRIS patients. In addition, correlations of the whole-body pathologic FDG uptake with IFN-γ, TNF, sCD14, and MPO, were also noted in our patients at baseline, shedding more light on the inflammatory pathophysiology of IRIS and its relationship to metabolic activity of mostly T lymphocytes and myeloid cells.
Increased glycolysis, initially described in cancer (Warburg effect), is the metabolic signature of effector and T-helper 1 cell responses as well as inflammatory macrophages driven by IFN-γ [21, 29, 30]. Upregulation of key glycolytic enzymes and increased glucose transport in the setting of TB especially has also been recently described [21, 22]. In support of increased glycolysis in activated immune cells in IRIS and mycobacterial IRIS, we found significantly higher CD4+ T-cell and monocyte Glut-1 expression in IRIS/myco-IRIS subjects compared to non-IRIS subjects after ART initiation. Further study of the metabolic pathways involved in IRIS inflammation is thus warranted to identify possible targets for intervention.
The limitations of our study include a small sample size that restricted a more robust assessment of predictive utility of FDG-PET in IRIS, despite being a relatively large study for FDG-PET/CT imaging in HIV-infected patients. In addition, the number of hypotheses tested may have led to a higher proportion of false-positive findings. Another limitation is the lack of direct evaluation of infection sites such as lungs or involved LNs by biopsy to confirm the findings of Glut-1 expression, which should be further explored.
The ability to predict IRIS based on clinical and imaging biomarkers prior to initiating treatment can allow for closer monitoring and earlier treatment (corticosteroid administration), to curtail potential morbidity and mortality. A beneficial effect of prednisone in myco-IRIS has already been demonstrated, reducing hospitalization rate and hastening recovery [31, 32], paving the road for an ongoing large randomized, double-blind, placebo-controlled trial of prophylactic prednisone in patients with risk for paradoxical TB-IRIS [33]. In addition, understanding the role of metabolism in HIV-associated inflammation may unravel new therapeutic targets, such as the mammalian target of rapamycin pathway, with more specificity and less immunosuppressive potential compared to the standard of care.
In conclusion, FDG-PET could represent a valuable imaging modality in HIV-infected patients at risk of developing IRIS upon starting treatment. An activated immune system along with higher lesion load and metabolic activity appeared to be linked to inflammation and IRIS development. Patients with such findings on FDG-PET might benefit from closer observation, more intensified treatment of underlying infection, and earlier treatment initiation to decrease IRIS-associated morbidity. Further studies evaluating the intersections of metabolism and immunity and their topography in HIV inflammatory responses may be informative in deciphering the involved pathways and potential therapeutic targets.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Author contributions. D. A. H. and I. S. conceived and planned the study, contributed to data collection and analysis, and drafted the final manuscript. D. A. H., C. M., and G. Z. P. evaluated and analyzed the imaging data. A. B., J. W., and L. E. D. performed the statistical analysis and prepared the graphs. A. R., J. H., G. R., D. M., E. L., J. M. M., A. L., A. P., M. M., V. S., and C. M. performed experiments and collected and analyzed the corresponding data. D. A. H., A. B., and I. S. prepared the first version of the manuscript. All authors reviewed and approved the final version of the manuscript
Acknowledgments. The study authors acknowledge the incredible support from all the clinical staff in the National Institute of Allergy and Infectious Diseases (NIAID) inpatient ward; the OP8 HIV clinic staff; and Angela Kibiy, April Poole, and Megan Anderson for study coordination efforts.
Disclaimer. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does the mention of trade names, commercial products, or organizations imply endorsement by the US government.
Financial support. This work was supported in part by the Intramural Research Program of the NIAID, National Institutes of Health (NIH), and by the Center for Infectious Diseases Imaging, NIH Clinical Center. This project has also been funded in part with federal funds from the National Cancer Institute of the NIH (contract number HHSN261200800001E).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Chang CC, Sheikh V, Sereti I, French MA. Immune reconstitution disorders in patients with HIV infection: from pathogenesis to prevention and treatment. Curr HIV/AIDS Rep 2014; 11:223–32. [DOI] [PubMed] [Google Scholar]
- 2. Boulougoura A, Sereti I. HIV infection and immune activation: the role of coinfections. Curr Opin HIV AIDS 2016; 11:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andrade BB, Singh A, Narendran G, et al. Mycobacterial antigen driven activation of CD14++CD16– monocytes is a predictor of tuberculosis-associated immune reconstitution inflammatory syndrome. PLoS Pathog 2014; 10:e1004433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Geneva, Switzerland: WHO, 2015. [PubMed] [Google Scholar]
- 5. Luetkemeyer AF, Kendall MA, Nyirenda M, et al. Adult AIDS Clinical Trials Group A5221 Study Team Tuberculosis immune reconstitution inflammatory syndrome in A5221 STRIDE: timing, severity, and implications for HIV-TB programs. J Acquir Immune Defic Syndr 2014; 65:423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blanc FX, Sok T, Laureillard D, et al. CAMELIA (ANRS 1295–CIPRA KH001) Study Team Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med 2011; 365:1471–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abdool Karim SS, Naidoo K, Grobler A, et al. Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med 2011; 365:1492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Post FA, Szubert AJ, Prendergast AJ, et al. Reduction of Early Mortality in HIV-Infected Adults and Children Starting Antiretroviral Therapy (REALITY) Trial Team Causes and timing of mortality and morbidity among late presenters starting antiretroviral therapy in the REALITY trial. Clin Infect Dis 2018; 66:132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ford N, Doherty M. The enduring challenge of advanced HIV infection. N Engl J Med 2017; 377:283–4. [DOI] [PubMed] [Google Scholar]
- 10. Belkhir L, Jonckheere S, Lhommel R, Vandercam B, Yombi JC. High FDG uptake on FDG-PET scan in HIV-1 infected patient with advanced disease. Acta Clin Belg 2011; 66:419–21. [DOI] [PubMed] [Google Scholar]
- 11. Bertagna F, Biasiotto G, Rodella R, Giubbini R, Alavi A. 18F-fluorodeoxyglucose positron emission tomography/computed tomography findings in a patient with human immunodeficiency virus-associated Castleman’s disease and Kaposi sarcoma, disorders associated with human herpes virus 8 infection. Jpn J Radiol 2010; 28:231–4. [DOI] [PubMed] [Google Scholar]
- 12. Martin C, Castaigne C, Tondeur M, Flamen P, De Wit S. Role and interpretation of fluorodeoxyglucose-positron emission tomography/computed tomography in HIV-infected patients with fever of unknown origin: a prospective study. HIV Med 2013; 14:455–62. [DOI] [PubMed] [Google Scholar]
- 13. Sathekge M, Maes A, Van de Wiele C. FDG-PET imaging in HIV infection and tuberculosis. Semin Nucl Med 2013; 43:349–66. [DOI] [PubMed] [Google Scholar]
- 14. Sathekge M, Maes A, Kgomo M, Stoltz A, Van de Wiele C. Use of 18F-FDG PET to predict response to first-line tuberculostatics in HIV-associated tuberculosis. J Nucl Med 2011; 52:880–5. [DOI] [PubMed] [Google Scholar]
- 15. Vorster M, Sathekge MM, Bomanji J. Advances in imaging of tuberculosis: the role of 18F-FDG PET and PET/CT. Curr Opin Pulm Med 2014; 20:287–93. [DOI] [PubMed] [Google Scholar]
- 16. Esmail H, Lai RP, Lesosky M, et al. Characterization of progressive HIV-associated tuberculosis using 2-deoxy-2-[18F]fluoro-D-glucose positron emission and computed tomography. Nat Med 2016; 22:1090–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ganapathy V, Thangaraju M, Prasad PD. Nutrient transporters in cancer: relevance to Warburg hypothesis and beyond. Pharmacol Ther 2009; 121:29–40. [DOI] [PubMed] [Google Scholar]
- 18. Palsson-McDermott EM, O’Neill LA. The Warburg effect then and now: from cancer to inflammatory diseases. Bioessays 2013; 35:965–73. [DOI] [PubMed] [Google Scholar]
- 19. Palmer CS, Ostrowski M, Balderson B, Christian N, Crowe SM. Glucose metabolism regulates T cell activation, differentiation, and functions. Front Immunol 2015; 6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Palmer CS, Anzinger JJ, Zhou J, et al. Glucose transporter 1-expressing proinflammatory monocytes are elevated in combination antiretroviral therapy-treated and untreated HIV+ subjects. J Immunol 2014; 193:5595–603. [DOI] [PubMed] [Google Scholar]
- 21. Appelberg R, Moreira D, Barreira-Silva P, et al. The Warburg effect in mycobacterial granulomas is dependent on the recruitment and activation of macrophages by interferon-γ. Immunology 2015; 145:498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shi L, Salamon H, Eugenin EA, Pine R, Cooper A, Gennaro ML. Infection with Mycobacterium tuberculosis induces the Warburg effect in mouse lungs. Sci Rep 2015; 5:18176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mamede M, Higashi T, Kitaichi M, et al. [18F]FDG uptake and PCNA, Glut-1, and Hexokinase-II expressions in cancers and inflammatory lesions of the lung. Neoplasia 2005; 7:369–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cretenet G, Clerc I, Matias M, et al. Cell surface Glut1 levels distinguish human CD4 and CD8 T lymphocyte subsets with distinct effector functions. Sci Rep 2016; 6:24129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kinet S, Swainson L, Lavanya M, et al. Isolated receptor binding domains of HTLV-1 and HTLV-2 envelopes bind Glut-1 on activated CD4+ and CD8+ T cells. Retrovirology 2007; 4:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Proschan MA. Multiple comparisons. In: D’ Agostino RB, Sullivan LM, Massaro J, eds. Encyclopedia of clinical trials. New York: Wiley, 2008. [Google Scholar]
- 27. Robin X, Turck N, Hainard A, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 2011; 12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nowak M, Carrasquillo JA, Yarboro CH, et al. A pilot study of the use of 2-[18F]-fluoro-2-deoxy-D-glucose-positron emission tomography to assess the distribution of activated lymphocytes in patients with systemic lupus erythematosus. Arthritis Rheum 2004; 50:1233–8. [DOI] [PubMed] [Google Scholar]
- 29. Peng M, Yin N, Chhangawala S, Xu K, Leslie CS, Li MO. Aerobic glycolysis promotes T helper 1 cell differentiation through an epigenetic mechanism. Science 2016; 354:481–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mills EL, O’Neill LA. Reprogramming mitochondrial metabolism in macrophages as an anti-inflammatory signal. Eur J Immunol 2016; 46:13–21. [DOI] [PubMed] [Google Scholar]
- 31. Meintjes G, Skolimowska KH, Wilkinson KA, et al. Corticosteroid-modulated immune activation in the tuberculosis immune reconstitution inflammatory syndrome. Am J Respir Crit Care Med 2012; 186:369–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Meintjes G, Wilkinson RJ, Morroni C, et al. Randomized placebo-controlled trial of prednisone for paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS 2010; 24:2381–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stek C, Schutz C, Blumenthal L, et al. Preventing paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome in high-risk patients: protocol of a randomized placebo-controlled trial of prednisone (PredART Trial). JMIR Res Protoc 2016; 5:e173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.