Blastomyces helicus is an emerging dimorphic fungus that causes fatal pulmonary and systemic disease predominantly in immunocompromised humans and companion animals in western and central regions of Canada and the United States.
Keywords: Onygenales, blastomycosis, Emmonsia, endemic mycosis, veterinary
Abstract
Background
Blastomyces helicus (formerly Emmonsia helica) is a dimorphic fungus first isolated from a man with fungal encephalitis in Alberta, Canada. The geographic range, epidemiology, and clinical features of disease are unknown.
Methods
We reviewed human and veterinary isolates of B. helicus identified among Blastomyces and Emmonsia isolates at the University of Alberta Microfungus Collection and Herbarium, University of Texas Health San Antonio’s Fungus Testing Laboratory, and Associated Regional and University Pathologists Laboratories. Isolates were selected based on low Blastomyces dermatitidis DNA probe values and/or atypical morphology. Species identification was confirmed for most isolates by DNA sequence analysis of the internal transcribed spacer with or without D1/D2 ribosomal RNA regions. Epidemiological and clinical data were analyzed.
Results
We identified isolates from 10 human and 5 veterinary cases of B. helicus infection; all were referred from western regions of Canada and the United States. Isolates remained sterile in culture, producing neither conidia nor sexual spores in the mycelial phase, but often producing coiled hyphae. Isolates were most frequently cultured from blood and bronchoalveolar lavage in humans and lungs in animals. Most infected persons were immunocompromised. Histopathological findings included pleomorphic, small or variably sized yeast-like cells, with single or multiple budding, sometimes proliferating to form short, branching, hyphal-like elements. Disease carried a high case-fatality rate.
Conclusions
Blastomyces helicus causes fatal pulmonary and systemic disease in humans and companion animals. It differs from B. dermatitidis in morphological presentation in culture and in histopathology, by primarily affecting immunocompromised persons, and in a geographic range that includes western regions of North America.
Blastomyces helicus is a dimorphic fungal pathogen first isolated in 1970 from the brain and lungs of a man with fungal encephalitis in Alberta, Canada [1, 2]. The patient’s infection was diagnosed as blastomycosis based on histopathology and serological results. However, the fungus isolated failed to sporulate and demonstrated microscopic features that were atypical for Blastomyces dermatitidis. Sigler and Peterson assessed phylogenetic diversity among isolates classified within Emmonsia and Blastomyces and noted that the isolate from the Albertan case grouped with 4 others having similar features and originating from western North America [3]. Sigler named the organism Emmonsia helica [4] as the genus Blastomyces could not be used until conserved according to the rules of fungal nomenclature. Taxonomic validation [5] and recent multilocus phylogenetic analyses have led to a major revision of Blastomyces to include several new taxa previously classified in Emmonsia. These include the type species of Emmonsia, E. parva (now classified as Blastomyces parvus), in addition to E. helica (now B. helicus) and other new Blastomyces species demonstrating varying levels of pathogenicity [6, 7].
The geographic range, epidemiology, and clinical features of disease caused by B. helicus are unknown. Herein we review the epidemiological and clinical data associated with 10 human and 5 veterinary isolates of B. helicus received by 2 reference laboratories in the United States and a fungal collection in Canada. We also review prior reported cases of atypical blastomycosis suggestive of B. helicus based on the fungal morphology in culture and tissue.
METHODS
We reviewed 9 isolates of B. helicus accessioned at the University of Alberta Microfungus Collection and Herbarium (UAMH, now the UAMH Centre for Global Microfungal Biodiversity, University of Toronto, Ontario). We also searched for additional isolates at the Fungus Testing Laboratory at University of Texas Health San Antonio (UTHSA). Isolates referred from 2001 to 2017 and previously identified as Emmonsia or Blastomyces as part of routine clinical workup were selected for review based on atypical morphology or low-positive values of <400000 relative light units (RLUs) on a commercial B. dermatitidis DNA probe (AccuProbe, Hologic, San Diego, California; the manufacturer’s recommended cutoff for the identification of B. dermatitidis is 50000 RLUs [8]). We thus identified an additional 4 isolates. Finally, we searched for additional cases by reviewing isolates identified as Emmonsia species among human clinical fungal isolates submitted since 2008 to Associated Regional and University Pathologists (ARUP) Laboratories, a national reference laboratory located at the University of Utah. This search was prompted by a social media post depicting an Emmonsia isolate cultured at ARUP that appeared suggestive of B. helicus [9], and led to the identification of 2 additional isolates.
We assessed the morphological features of isolates in comparison with those of the ex-type culture of B. helicus (UAMH 7101). Isolates were grown on potato dextrose agar (Difco Laboratories, Detroit, Michigan) at 30°C and 35°C to evaluate colonial features and transition to yeast phase and on other media (cereal agar, oatmeal salts agar, Takashio agar [10]) with incubation at 25°C or 30°C to assess sporulation. Identifications of most isolates were confirmed with DNA sequence analysis of the internal transcribed spacer (ITS) and/or D1/D2 domains of the long subunit of ribosomal RNA performed at the respective laboratories (UAMH, UTHSA, ARUP) using previously described methods [3, 11–13]. Sequences were compared to those of in-house databases at the authors’ respective laboratories and/or to those in GenBank (National Center for Biotechnology Information). The latter includes sequences of 5 Emmonsia species isolates obtained in 2009 [3] that are now reclassified as B. helicus (Supplementary Table 1). Antifungal susceptibility testing was performed on the mold phases of some isolates at UTHSA using the Clinical and Laboratory Standards Institute (CLSI) M38-A2 broth macrodilution method and of another isolate at ARUP using CLSI M38-A2 broth microdilution method with Sensititre Custom Plates (Thermo Fisher Scientific, Waltham, Massachusetts) [14].
Epidemiological and clinical data from submitting laboratories were analyzed. For recent cases, submitting laboratories were contacted for additional, de-identified data. The requirement for review was waived by the institutional ethics review board of UTHSA (protocol 17-229N).
RESULTS
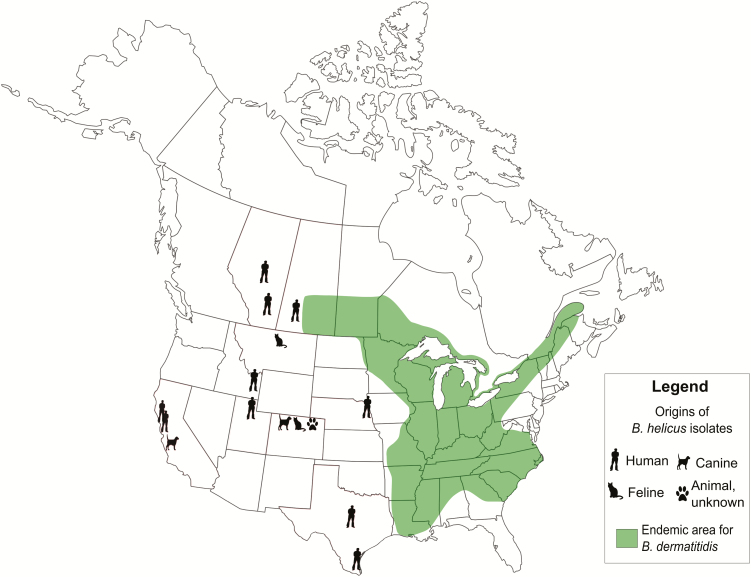
Data for 10 human and 5 veterinary cases of B. helicus are shown in Tables 1 and 2, respectively. Accession numbers for case isolates and for DNA sequences deposited in GenBank are listed in Supplementary Table 1. Three of the confirmed human cases (h1, h5, and h9) were previously reported (as infections caused by B. dermatitidis [1], Emmonsia species [15], and E. helica [16], respectively). All B. helicus isolates were referred from western regions of North America not typically considered endemic for B. dermatitidis [17] (Figure 1). Moreover, no patient history indicated travel to endemic areas. A notable exposure history occurred in 1 human case: Kappagoda et al [15] reported a patient (h5) who had received a liver transplantation, and developed a fatal pulmonary and disseminated B. helicus infection in the early posttransplantation period. He was a snake farmer from rural northern California who, until 1–2 years earlier, trapped small mammals to feed his snakes and for taxidermy.
Table 1.
Epidemiological and Clinicopathological Details of Human Cases of Blastomyces helicus Infection
| Case | Year | Location (Travel History) | Demographics, y | Medical History | Radiology | Histopathology (Sample) | Sample Isolated | Antifungal Treatmenta | Outcome | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| h1 | 1970 | Edmonton, Alberta (no recent travel) |
60 M | Alcoholism, diabetes | CXR: Chronic lesions left upper lung | Small single budding yeasts (lung, brain) | CSF | None | Fatal | [1, 2] |
| h2 | 1992 | Swift Current, Saskatchewan | 35 M | Chronic leukemia, steroids | NA | NA | Blood, BM, liver, sputum | NA | Fatal | |
| h3 | 1999 | Texas (Mexico) | F | Lupus | NA | Small yeasts (lung) | Blood | Fluconazole, amphotericin Bb | Survival | |
| h4 | 2004 | Okotoks, Alberta | 45 M | NA | NA | NA | BAL, blood | NA | NA | |
| h5 | 2010 | Northern California | 55 M | Liver transplant | Chest CT: Diffuse ground glass opacities, centrilobular nodules, pleural effusions | None | Blood, pleural fluid | Voriconazole, liposomal amphotericin B |
Fatal | [15] |
| h6 | 2010 | Pocatello, Idaho | NA | NA | NA | NA | BAL | NA | NA | |
| h7 | 2013 | Cameron County, Texas (Kansas) |
3 F | None | CXR: normal. MRI brain: enhancement of bibasilar cisterns |
Negative (brain) | CSF | Fluconazole | Survival | |
| h8 | 2014 | Salt Lake City, Utah (Idaho) | 50 M | HIV (CD4 160 cells/µL), Hodgkin lymphomac | CXR: Diffuse nodules |
Multiply-budding yeasts (blood, BAL) | Blood, BAL | Voriconazole, amphotericin B lipid complex | Fatal | |
| h9 | 2016 | East Bay, California (Mexico; Central Valley, California) | 40 M | HIV (CD4 count, 5 cells/µL) | CXR: Diffuse nodules. Chest CT: Diffuse nodules, apical cavitation. | Hyphae and multiply-budding yeasts (lung) | BAL | Fluconazole, micafungin, liposomal amphotericin B |
Fatal | [16] |
| h10 | 2017 | Omaha, Nebraska | NA | NA | NA | NA | BAL, pleural fluid | NA | NA |
Abbreviations: BAL, bronchoalveolar lavage; BM, bone marrow; CSF, cerebrospinal fluid; CXR, chest radiograph; CT, computed tomography; F, female; HIV, human immunodeficiency virus; M, male; MRI, magnetic resonance imaging; NA, data not available.
aAntifungals were prescribed sequentially for all treated patients except for case h8 who received these concurrently.
bAmphotericin B formulation unknown.
cDay 10 after high-dose chemotherapy with adriamycin, bleomycin, vinblastine, and dacarbazine.
Table 2.
Epizoological and Clinicopathological Details of Veterinary Cases of Blastomyces helicus Infection
| Case | Year | Location (Travel) | Species | Histopathology | Sample | Treatment | Outcome |
|---|---|---|---|---|---|---|---|
| v1 | 2005 | Fort Collins, Colorado (Arizona) | Dog | Small yeasts | Lung | NA | NA |
| v2 | 2007 | Atascadero, California | Dog | NA | Lung | NA | NA |
| v3 | 2009 | Helena, Montana | Cat | Small yeasts | Lung | NA | NA |
| v4 | 2012 | Fort Collins, Colorado | Cat | Small yeasts | Lung | Fluconazole for 11 mo | Survived |
| v5 | 2013 | Fort Collins, Colorado | NA | NA | Lung | NA | NA |
Abbreviation: NA, data not available.
Figure 1.
Geographic origins of human and veterinary Blastomyces helicus isolates, juxtaposed with areas classically considered endemic for B. dermatitidis (modified from [17] based on [18–21]).
Among veterinary cases, animal species could be ascertained for 4 of 5 cases: 2 cats and 2 dogs. Notable exposure histories were also documented for 2 veterinary cases: case v1, a dog from Fort Collins, Colorado, was observed to dig in prairie dog burrows, and case v4, an indoor/outdoor cat from the mountains (at 7000 feet elevation) west of Fort Collins, Colorado, was observed to play with potted plant soil.
Underlying immunocompromising conditions were present for 6 of 7 human patients in whom this information could be obtained (Table 1). Comorbidities in these patients included advanced human immunodeficiency virus (HIV) infection in 2 patients (among whom 1, patient h8, was diagnosed with and recently received chemotherapy for Hodgkin lymphoma) and systemic lupus erythematosus, orthotopic liver transplantation, and leukemia in 1 patient each. Another patient had diabetes mellitus and alcoholism [1]. One patient, h7, a child with fungal meningitis without previous comorbidities, had a normal immunological workup that included HIV testing, lymphocyte subset analysis, lymphocyte function assay, total complement activity (CH50), and pneumococcal vaccine challenge.
In 5 of 10 human cases, B. helicus was isolated from >1 clinical specimen. The most common sites for isolation, in order of decreasing frequency, were blood (n = 5), bronchoalveolar lavage (n = 5), cerebrospinal fluid (n = 2), pleural fluid (n = 2), and from bone marrow, liver, sputum, and lung tissue (n = 1 each) (Table 1). All veterinary isolates were cultured from lung tissue (Table 2).
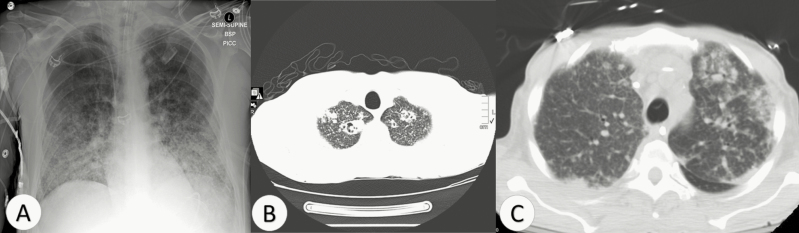
Radiographic results were available for 5 human patients. Abnormalities were noted on chest imaging of 4 of 5 patients, and included diffuse reticulonodular lesions in 2 patients (Figure 2A, 2B), unilateral pleural effusion with diffuse centrilobular nodular lesions in 1 patient [15] (Figure 2C), and a chronic upper lobe lesion in another [2]. All veterinary cases had abnormal chest imaging, but further details were unavailable.
Figure 2.
Chest radiography findings of 3 patients with pulmonary disease caused by Blastomyces helicus. A, Chest x-ray demonstrating diffuse nodules (case h8). B, Axial view of computed tomography (CT) of chest showing diffuse nodules and bilateral apical consolidation with cavitation (case h9). C, Axial view of CT of chest showing diffuse ground glass opacities, peribronchovascular nodules, and pleural effusions (case h5, from [15]).
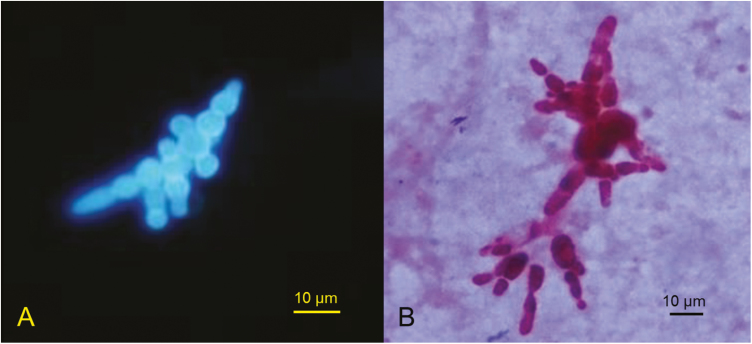
Histopathological or cytopathological results were available for 5 human and 3 veterinary cases (Tables 1 and 2). In case h1, small budding yeasts were noted in brain and lung tissue. In case h9, lung biopsy demonstrated nonnecrotizing granulomas with both hyphae and multiply-budding yeast cells. For 1 human and 3 veterinary cases (h3, v1, v3, and v4), small yeast-like cells were seen in tissue and mistaken for Histoplasma capsulatum. In case h8, yeasts with multiple broad-based buds were observed (Figure 3A). For 1 patient (h7) who had brain involvement proven by culture of B. helicus from cerebrospinal fluid, histopathological examination of brain tissue demonstrated chronic inflammation with histiocytes and focal giant cell formation; fungi were not observed using special stains.
Figure 3.
Multiply-budding yeast-like cells of Blastomyces helicus in calcofluor white stain of bronchoalveolar fluid (A) and in gram stain of growth from a positive blood culture bottle (case h8) (B).
Three isolates were tested with a B. dermatitidis DNA probe (Hologic), and all were reported as positive (h4, 342810 RLUs; v3, positive but values unknown; and v4, 198000 RLUs). In addition, cross-reactivity occurred with a Histoplasma galactomannan urinary antigen (Miravista, Indianapolis, Indiana) in 1 human case (h9; >25 ng/mL [reference < 0.5 ng/mL] [16]) and Blastomyces galactomannan urinary antigen (Miravista) in 1 veterinary case (v4; values unknown).
Results of antifungal susceptibility testing of selected isolates are shown in Table 3. Although breakpoints do not exist, minimum inhibitory concentrations were low for extended-spectrum triazoles (ie, itraconazole, voriconazole, and posaconazole) and amphotericin B, and variable for fluconazole and for the echinocandins micafungin, caspofungin, and anidulafungin.
Table 3.
Results of Antifungal Susceptibility Testing for 4 Blastomyces helicus Isolates From Humans
| Minimum Inhibitory Concentration, µg/mL | ||||||||
|---|---|---|---|---|---|---|---|---|
| Isolate | Fluconazole | Itraconazole | Voriconazole | Posaconazole | Amphotericin B | Micafungin | Caspofungin | Anidulafungin |
| h5 | ND | ND | 0.125 | <0.03 | 0.125 | ND | 0.5 (24 h), 2 (48 h) | ND |
| h7 | 1 | ND | <0.03 | ND | 0.25 | ND | 2 | ND |
| h8 | ND | ≤0.06 | 0.06 | ≤0.06 | 0.5 | 1 | 1 | 1 |
| h9 | 8 | 0.125 | <0.03 | 0.125 | 0.06 | ND | ND | ND |
| h10 | ND | <0.03 | <0.03 | <0.03 | 1 | 2 | ND | ND |
Abbreviation: ND, testing not done.
Management and outcomes could be ascertained for 6 and 7 human patients, respectively. One patient (h1) died without receiving antifungal therapy. In case h7, a child with B. helicus meningitis, treatment with fluconazole for 4 months appeared successful based on clinical response, although the patient was lost to follow-up thereafter. The remaining human patients for whom treatments are known were initially treated with a triazole, which was ultimately replaced with or augmented by a lipid formulation of amphotericin B. Despite these interventions, deaths occurred in all but 1 of these patients, and also in another patient for whom treatment information was unavailable. In total, B. helicus infections were fatal for 5 of 7 human patients for whom outcomes are known. The sole veterinary case for which treatment and outcome are known was a domestic long-hair cat successfully treated with fluconazole for 11 months.
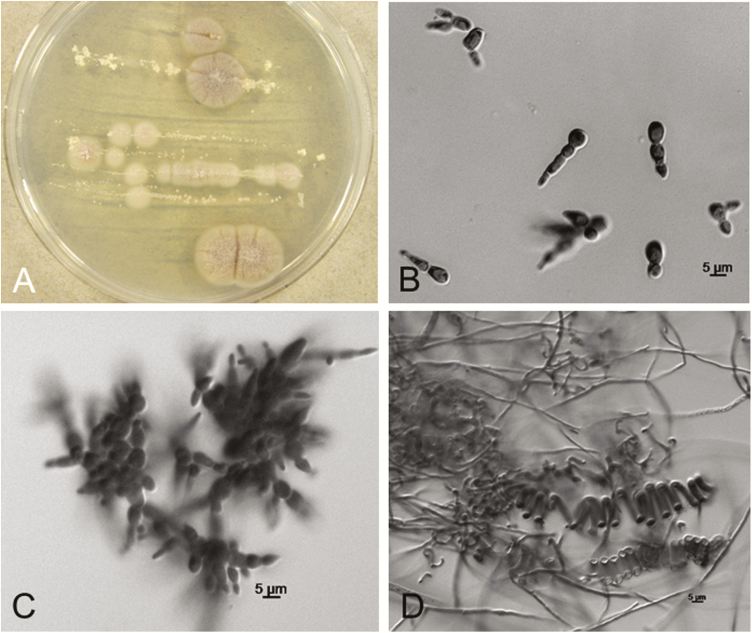
The morphological features of confirmed B. helicus isolates are shown in Figure 4. Isolates produced yeast-like growth on potato dextrose agar at 35°C (eg, Figure 4A). The yeast phase is characterized by pleomorphic variably sized cells (4–9 µm long and 2.5–6.5 µm wide) commonly in short, branched chains, sometimes resulting in complex configurations (seen in vivo in Figure 3A, and in vitro in Figure 3B, Figure 4B and 4C). The mycelial phase is characterized by the absence of conidia and occasional formation of tightly coiled helices (Figure 4D). Morphologies of the mold and yeasts phases were influenced by culture media. All isolates produced yeast phases on potato dextrose agar at 35°C (eg, Figure 4A). Helically coiled hyphae developed primarily on low-carbohydrate media (eg, cereal agar or oatmeal agar incubated at 25°C) and were sometimes accompanied by specialized hyphae usually associated with a sexual stage; however, no sexual spores have been observed when isolates are grown alone or when mated (Sigler, unpublished observation).
Figure 4.
(A-D) Morphological features of Blastomyces helicus in culture. A, Colonies showing mold to yeast transition on potato dextrose agar after 13 days at 35°C (case v1 isolate). B-C. Yeast-like phase at 35°C showing multiply-budding yeast-like cells proliferating in short branched chains (case h4 and h1 isolates, respectively). D, Mycelial phase at 25°C showing typical helically coiled hyphae and absence of conidia (case h2 isolate).
DISCUSSION
Important differences exist between B. helicus and B. dermatitidis with respect to mycological features, apparent geographic range, and in the epidemiology, epizoology, and clinical pathology of the respective mycoses caused by infection. First, none of the B. helicus isolates examined here produced conidia, whereas conidia are usually evident for B. dermatitidis (although these may not be apparent early during culture). Second, B. helicus appears to cause disease in western and mountainous regions of North America not typically considered endemic for B. dermatitidis. Third, human disease caused by B. helicus occurs most commonly in immunocompromised persons, in contrast to B. dermatitidis, which predominantly affects immunocompetent individuals [22]. Fourth, the epizoology of veterinary disease may be different according to Blastomyces species: although the numbers are small, it is notable that half of veterinary B. helicus infections were in cats, whereas B. dermatitidis reportedly affects cats 48–100 times less commonly than dogs [23]. Fifth, human disease caused by B. helicus is frequently systemic. Fungemia was noted for 5 of 10 confirmed human cases. Although B. dermatitidis fungemia has been reported [24, 25], it is extraordinary and, in retrospect, some of these cases may have involved B. helicus [26]. Central nervous system (CNS) disease was proven in 2 of 10 human cases by culturing B. helicus from brain and/or cerebrospinal fluid and a brain lesion was demonstrated radiologically in a third case [16]. In contrast, CNS disease is reported to complicate 5%–10% of cases of extrapulmonary blastomycosis caused by B. dermatitidis [22], although Pappas et al reported a higher rate of CNS involvement among patients with blastomycosis who were coinfected with HIV [25]. Moreover, while skin is the most common site of extrapulmonary blastomycosis involving B. dermatitidis, none of the cases caused by B. helicus were observed to have cutaneous involvement. Finally, histopathological examination in disease caused by B. helicus typically demonstrates small or variably sized yeast-like cells that can be misdiagnosed for either H. capsulatum or B. dermatitidis. However, the presence of multiple budding and/or buds in short chains that may proliferate to form short branching hyphal-like arrangements as illustrated in this report strongly suggest B. helicus. This is in contrast to the classic histopathology of B. dermatitidis: medium to large (8–15 μm) yeast-like cells with single, broad-based buds.
Without careful morphological assessment of an isolate in culture and/or DNA sequence analysis, misidentification might occur because B. helicus may cross-react with a commercial DNA probe for B. dermatitidis. Clinical microbiologists should consider B. helicus if the mold phase of a dimorphic fungal isolate resembles B. dermatitidis (white or tan colony) and lacks sporulation. BLAST (Basic Local Alignment Search Tool) searches with sequences of the ITS and/or D1/D2 regions should elicit matches of 99%–100% similarity with verified B. helicus sequences (Supplementary Table 1) and 96%–97% similarity with authentic B. dermatitidis sequences (eg, strains UAMH 3538 [3] and UAMH 3539 [7]). Pathologists and clinicians should additionally suspect B. helicus when histopathological or cytopathological examination demonstrates the presence of small or variably sized (eg, 4–9 µm), singly or multiply budding yeast cells as well as the presence of pleomorphic hyphal elements.
In retrospect, at least some atypical cases of blastomycosis in the literature attributed to B. dermatitidis may have been due to B. helicus. For example, Tan et al reported fatal disseminated atypical blastomycosis in 2 HIV-infected men from California [26]. Postmortem pathological examination of multiple tissues demonstrated pleomorphic, variably sized yeasts (2–20 µm) with marked polymorphisms (like those demonstrated in their figure 2 and strongly suggestive of B. helicus). The fungi, isolated from multiple ante- and postmortem specimens, were identified as B. dermatitidis on the basis of positive results of a B. dermatitidis DNA probe. However, sporulation was absent. In addition, the isolates lacked the A exoantigen typically expressed by North American strains of B. dermatitidis [26, 27], a result consistent with that reported for a proven isolate of B. helicus (h1) [1]. On the basis of the epidemiological, histopathological, and mycological features atypical for B. dermatitidis but consistent with B. helicus, we conclude that these cases were due to the latter species.
The optimal treatment for B. helicus infection is not established. Although the data are limited by small numbers of isolates tested, the results of in vitro antifungal susceptibility testing suggested that extended-spectrum triazoles and amphotericin B are active against B. helicus. These results are consistent with the findings of Dukik et al [28]. The in vitro antifungal susceptibility profile of B. helicus appears to be similar to that for B. dermatitidis [29] and so it is reasonable that treatment should follow the clinical practice guidelines of the Infectious Diseases Society of America for the treatment of blastomycosis [30]. For immunocompromised patients, treatment should include amphotericin B (lipid formulation, 3–5 mg/kg per day preferred or deoxycholate, 0.7–1.0 mg/kg per day as an alternative) for 1–2 weeks or until clinical improvement is achieved, followed by an extended-spectrum triazole for at least 12 months.
The case-fatality rate of B. helicus disease in this series was high, with deaths occurring in 5 of 7 persons (71%) for whom outcomes are known. The poor outcomes observed may reflect the fact that most patients were immunocompromised and had disseminated disease. Moreover, advanced infections may reflect delayed diagnoses, which can occur with dimorphic fungal diseases outside of areas traditionally considered endemic for such mycoses [31].
The ecological niche of B. helicus is currently unknown, although a clue might be provided from a dog from Fort Collins, Colorado (v1), observed to frequently dig in prairie dog burrows. Two cases of occupationally acquired blastomycosis were reported in workers in Boulder, Colorado, who were excavating prairie dog burrows [32–34]. One patient had isolated pulmonary disease, and the other had pulmonary and cutaneous disease. In both cases, histopathological examination of lung tissue demonstrated small yeasts (2–8 µm) that were considered suggestive of histoplasmosis, coccidioidomycosis, or cryptococcosis [32–34]. Dimorphic fungal isolates were identified as B. dermatitidis based on results of a B. dermatitidis DNA probe. The atypical geographic location for B. dermatitidis and the uncharacteristic histopathological findings suggest that the infections may have been due to B. helicus. Another patient in our series with proven infection due to B. helicus (h5) was a snake farmer from northern California with a remote history of trapping rodents [15]. Together, these observations raise the possibility that B. helicus may be associated with the environments of small mammals. The fact that sporulation does not seem to occur in culture is also intriguing and begs the question of the nature of the infectious propagules in the environment. Our studies suggest the propensity to produce a sexual stage (ie, formation of peridial and helically coiled hyphae), but insufficient isolates have been assessed in mating studies.
Our study has limitations. That isolates had been sent to reference laboratories and/or a fungal collection raises the possibility of referral bias. Although the laboratories and collection included in this study were located in western regions of North America, each is routinely referred isolates from throughout Canada and the United States, reducing the likelihood that the observed geographic distribution of B. helicus isolates is due to sampling or referral biases. Another limitation is that the retrospective collection of data, sometimes limited to details provided by referring laboratories, resulted in incomplete information about possible exposures and travel histories. In addition, data were missing for some patients with regard to clinical features, management, and outcomes.
In conclusion, B. helicus caused pulmonary and fatal disseminated disease, mainly in immunocompromised persons, and pulmonary disease in companion animals in regions of Western Canada and the United States not considered endemic for B. dermatitidis. Epidemiological investigations are needed to establish the burden of disease and define the geographic range of this emerging pathogen.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. The authors acknowledge the contributions of Dr Connie Gibas, Carmita Sanders, and the late Dr Deanna Sutton of the Fungus Testing Laboratory, Dr Brian Wickes of the Nucleic Acids Core Facility, and Dr Hongxin Fan of the Molecular Diagnostics Laboratory, UTHSA; and Dr Susan Slechta of the ARUP Institute for Clinical and Experimental Pathology. The authors also wish to acknowledge Dr Robert Odrobina, Dr Jaime Fergie, Dr Martin Rofael, Dr Mary Ann De Groote, Dr Allison Bradley, and Dr Tracey Jensen, for providing case information.
Financial support. This work was supported by a Detweiler Travelling Fellowship from the Royal College of Physicians and Surgeons of Canada to I. S. S.
Potential conflicts of interest. N. P. W. has received grants from Astellas, bioMérieux, F2G, Cidara, Merck, Pfizer, and Viamet, and honorarium and travel grant from Gilead. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Sekhon AS, Jackson FL, Jacobs HJ. Blastomycosis: report of the first case from Alberta, Canada. Mycopathologia 1982; 79:65–9. [DOI] [PubMed] [Google Scholar]
- 2. Sekhon AS, Bogorus MS, Sims HV. Blastomycosis: report of three cases from Alberta with a review of Canadian cases. Mycopathologia 1979; 68:53–63. [DOI] [PubMed] [Google Scholar]
- 3. Sigler L, Peterson SW. Molecular genetic analysis supports recognition of new species among Emmonsia and Blastomyces isolates. Int J Antimicrob Agents 2009; 34:S593. [Google Scholar]
- 4. Sigler L. Emmonsia helica Sigler sp. nov. Index Fungorum 2015; 237:2049–375. Available at: http://www.indexfungorum.org/names/NamesRecord.asp?RecordID=551157. Accessed 26 May 2018. [Google Scholar]
- 5. de Hoog GS, Redhead SA, Feng P, Jiang Y, Dukik K, Sigler L. Proposals to conserve Blastomyces Gilchrist & W. R. Stokes against Blastomyces Costantin & Rolland and Ajellomycetaceae against Paracoccidioidaceae (Ascomycota: Onygenales). Taxon 2016; 65:1167–9. [Google Scholar]
- 6. Dukik K, Muñoz JF, Jiang Y, et al. Novel taxa of thermally dimorphic systemic pathogens in the Ajellomycetaceae (Onygenales). Mycoses 2017; 60:296–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jiang Y, Dukik K, Munoz J, et al. Phylogeny, ecology and taxonomy of systemic pathogens and their relatives in Ajellomycetaceae (Onygenales): Blastomyces, Emergomyces, Emmonsia, Emmonsiellopsis. Fungal Divers 2018. In press 90:245–91. [Google Scholar]
- 8. Hologic Inc. AccuProbe Blastomyces dermatitidis culture identification test [product insert]. 102963 Rev. 001: Hologic Inc, 2016. [Google Scholar]
- 9. Hueth KD. Homeless HIV+, Hodgkin’s lymphoma patient septic with Emmonsia sp. in W. US. Usually found in S. Africa. Available at: https://twitter.com/search?q=emmonsia&src=typd. Accessed 25 April 2018. [Google Scholar]
- 10. University of Alberta Microfungus Collection and Herbarium. Selected media. 2008. Available at: https://www.uamh.ca/_/media/uamh/OrderCultures/Documents/Media.pdf. Accessed 14 April 2018. [Google Scholar]
- 11. Peterson SW, Sigler L. Molecular genetic variation in Emmonsia crescens and Emmonsia parva, etiologic agents of adiaspiromycosis, and their phylogenetic relationship to Blastomyces dermatitidis (Ajellomyces dermatitidis) and other systemic fungal pathogens. J Clin Microbiol 1998; 36:2918–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Romanelli AM, Sutton DA, Thompson EH, Rinaldi MG, Wickes BL. Sequence-based identification of filamentous basidiomycetous fungi from clinical specimens: a cautionary note. J Clin Microbiol 2010; 48:741–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Slechta ES, Hohmann SL, Simmon K, Hanson KE. Internal transcribed spacer region sequence analysis using SmartGene IDNS software for the identification of unusual clinical yeast isolates. Med Mycol 2012; 50:458–66. [DOI] [PubMed] [Google Scholar]
- 14. Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard. 2nd edition. CLSI document M38-A2. Wayne, PA: CLSI, 2008. [Google Scholar]
- 15. Kappagoda S, Adams JY, Luo R, Banaei N, Concepcion W, Ho DY. Fatal Emmonsia sp. infection and fungemia after orthotopic liver transplantation. Emerg Infect Dis 2017; 23:346–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rofael M, Schwartz IS, Sigler L, Kong LK, Nelson N. Emmonsia helica infection in HIV-infected man, California, USA. Emerg Infect Dis 2018; 24:166–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Centers for Disease Control and Prevention. Sources of blastomycosis. 2017. Available at: https://www.cdc.gov/fungal/diseases/blastomycosis/causes.html. Accessed 27 May 2018. [Google Scholar]
- 18. Davies JL, Epp T, Burgess HJ. Prevalence and geographic distribution of canine and feline blastomycosis in the Canadian prairies. Can Vet J 2013; 54:753–60. [PMC free article] [PubMed] [Google Scholar]
- 19. Crampton TL, Light RB, Berg GM, et al.. Epidemiology and clinical spectrum of blastomycosis diagnosed at Manitoba hospitals. Clin Infect Dis 2002; 34:1310–6. [DOI] [PubMed] [Google Scholar]
- 20. Morris SK, Brophy J, Richardson SE, et al. Blastomycosis in Ontario, 1994-2003. Emerg Infect Dis 2006; 12:274–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Litvinov IV, St-Germain G, Pelletier R, Paradis M, Sheppard DC. Endemic human blastomycosis in Quebec, Canada, 1988–2011. Epidemiol Infect 2013; 141:1143–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bradsher RW. Blastomycosis. In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett’s principles and practice of infectious diseases. Elsevier, 2015:2963–73. [Google Scholar]
- 23. Legendre AM. Blastomycosis. In: Greene CE, ed. Infectious diseases of the dog and cat. Elsevier, 2012:606–14. [Google Scholar]
- 24. Musial CE, Wilson WR, Sinkeldam IR, Roberts GD. Recovery of Blastomyces dermatitidis from blood of a patient with disseminated blastomycosis. J Clin Microbiol 1987; 25:1421–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pappas PG, Pottage JC, Powderly WG, et al. Blastomycosis in patients with the acquired immunodeficiency syndrome. Ann Intern Med 1992; 116:847–53. [DOI] [PubMed] [Google Scholar]
- 26. Tan G, Kaufman L, Peterson EM, de la Maza LM. Disseminated atypical blastomycosis in two patients with AIDS. Clin Infect Dis 1993; 16:107–11. [DOI] [PubMed] [Google Scholar]
- 27. Kaufman L, Standard PG, Weeks RJ, Padhye AA. Detection of two Blastomyces dermatitidis serotypes by exoantigen analysis. J Clin Microbiol 1983; 18:110–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dukik K, Al-Hatmi AMS, Curfs-Breuker I, Faro D, De Hoog S, Meis JF. Antifungal susceptibility of emerging dimorphic pathogens in the family Ajellomycetaceae. Antimicrob Agents Chemother 2018; 62:e01886–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li RK, Ciblak MA, Nordoff N, Pasarell L, Warnock DW, McGinnis MR. In vitro activities of voriconazole, itraconazole, and amphotericin B against Blastomyces dermatitidis, Coccidioides immitis, and Histoplasma capsulatum. Antimicrob Agents Chemother 2000; 44:1734–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chapman SW, Dismukes WE, Proia LA, et al. Infectious Diseases Society of America Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis 2008; 46:1801–12. [DOI] [PubMed] [Google Scholar]
- 31. Benedict K, Thompson GR III, Deresinski S, Chiller T. Mycotic infections acquired outside areas of known endemicity, United States. Emerg Infect Dis 2015; 21:1935–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Centers for Disease Control and Prevention. Blastomycosis acquired occupationally during prairie dog relocation—Colorado, 1998. MMWR Morb Mortal Wkly Rep 1999; 48:98–100. [PubMed] [Google Scholar]
- 33. De Groote MA, Bjerke R, Smith H, Rhodes III LV. Expanding epidemiology of blastomycosis: clinical features and investigation of 2 cases in Colorado. Clin Infect Dis 2000; 30:582–4. [DOI] [PubMed] [Google Scholar]
- 34. Hannah EL, Bailey AM, Hajjeh R, Gershman K, Lindsley M, Hoffman RE. Public health response to 2 clinical cases of blastomycosis in Colorado residents. Clin Infect Dis 2001; 32:E151–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.