The role of HBeAg in inflammatory responses during the infection of hepatitis B virus (HBV) is not fully understood, and several previous reports with regard to the NF-κB pathway are controversial. In this study, we showed that HBeAg could suppress both Toll-like receptor 2 (TLR2)- and IL-1β-induced activation of NF-κB in cells and clinical samples, and we further revealed novel molecular mechanisms. We found that HBeAg can associate with NEMO, the regulatory subunit for IκB kinase (IKK) that controls the NF-κB signaling pathway, and thereby inhibits TRAF6-mediated K63-linked ubiquitination of NEMO, resulting in downregulation of NF-κB activity and promotion of virus replication. In contrast, the HBeAg-negative HBV mutant can induce higher levels of NF-κB activity. These results are important for understanding the HBV-induced pathogenesis of chronic hepatitis and indicate that different clinical measures should be considered to treat HBeAg-positive and HBeAg-negative infections. Our findings represent a conceptual advance in HBV-related suppression of NF-κB signaling.
KEYWORDS: HBeAg, hepatitis B virus, innate immunity, NEMO, NF-κB, ubiquitination
ABSTRACT
Viruses have adopted diverse strategies to suppress antiviral responses. Hepatitis B virus (HBV), a virus that is prevalent worldwide, manipulates the host’s innate immune system to evade scavenging. It is reported that the hepatitis B e antigen (HBeAg) can interfere with NF-κB activity, which then leads to high viral loads, while HBV with the G1896A mutation remains infectious without the production of HBeAg but can induce more severe proinflammatory response and liver damage. The aim of current work was to study the molecular mechanism by which HBeAg suppresses interleukin-1β (IL-1β)-stimulated NF-κB activity, which leads to the suppression of the innate immune responses to HBV infection. Our study revealed that HBeAg could interact with NEMO, a regulatory subunit associated with IκB kinase, which regulates the activation of NF-κB. HBeAg suppressed the IL-1β-induced tumor necrosis factor (TNF)-associated factor 6 (TRAF6)-dependent K63-linked ubiquitination of NEMO, thereby downregulating NF-κB activity and promoting virus replication. We further demonstrated the inhibitory effect of HBeAg on the NF-κB signaling pathway using primary human hepatocytes, HBV-infected HepG2-NTCP cells, and clinical liver samples. Our study reveals a molecular mechanism whereby HBeAg suppresses IL-1β-induced NF-κB activation by decreasing the TRAF6-dependent K63-linked ubiquitination of NEMO, which may thereby enhance HBV replication and promote a persistent infection.
IMPORTANCE The role of HBeAg in inflammatory responses during the infection of hepatitis B virus (HBV) is not fully understood, and several previous reports with regard to the NF-κB pathway are controversial. In this study, we showed that HBeAg could suppress both Toll-like receptor 2 (TLR2)- and IL-1β-induced activation of NF-κB in cells and clinical samples, and we further revealed novel molecular mechanisms. We found that HBeAg can associate with NEMO, the regulatory subunit for IκB kinase (IKK) that controls the NF-κB signaling pathway, and thereby inhibits TRAF6-mediated K63-linked ubiquitination of NEMO, resulting in downregulation of NF-κB activity and promotion of virus replication. In contrast, the HBeAg-negative HBV mutant can induce higher levels of NF-κB activity. These results are important for understanding the HBV-induced pathogenesis of chronic hepatitis and indicate that different clinical measures should be considered to treat HBeAg-positive and HBeAg-negative infections. Our findings represent a conceptual advance in HBV-related suppression of NF-κB signaling.
INTRODUCTION
Hepatitis B virus (HBV) is one of the leading causes of human liver cancer, with over 350 million chronic infections worldwide (1). HBV itself is not cytopathic, but chronic infection by HBV leads to immune-mediated pathogenesis. The genome of HBV harbors four genes, one of which is the preC-C gene, which is involved in persistent infections in patients (2). Translation of the preC-C gene resulted in production of several products, including precore, core, and hepatitis B e antigen (HBeAg) (2, 3). HBeAg is considered as an auxiliary protein of HBV, which is secreted in the life cycle of HBV (2, 4). Although HBeAg is not essential to HBV replication, it plays an important role in the establishment of chronic infections and modulation of immune responses of human hosts (5, 6). Recent studies showed that HBV infection is modulated by the responses of innate immunity (7). The 25-kDa HBeAg precursor, the precore protein, is translated from the preC transcript and then processed by removal of the N-terminal 19 residues, generating an intermediate protein (p22) which is subsequently processed into mature HBeAg by removal of its C-terminal end (8, 9). There is no unified conclusion on the precise length of the C terminus of HBeAg due to different HBV genotypes (10–12). In this work, we chose the p17 form of HBeAg, in which 34 C-terminal residues are removed to produce the secreted 17-kDa mature form (9, 13). Although a major part of HBeAg is secreted, a fraction of HBeAg and its precursors is retained in the cytoplasm (14, 15); however, the specific role of this cytosolic form of HBeAg has not been fully elucidated.
It has been proposed that HBV establishes chronic infection by antagonizing the innate immune response of the host, thus further limiting HBV clearance by the adaptive immune responses of the host (7). The proper perception and recognition of invading pathogens are the initial steps of an immune response. Sensing of these pathogens is performed by multiple pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs) (16). TLR family members share conserved domain, including a Toll/interleukin-1 (IL-1) receptor (TIR) domain, a leucine-rich repeat domain, and a transmembrane domain (16). All TLRs, except TLR3, recruit adaptor molecules, including tumor necrosis factor (TNF)-associated factor 6 (TRAF6) and MyD88, leading to activation of signaling cascades and subsequent expression of cytokines and chemokines (17). NF-κB represents a regulatory factor of multiple biological processes, such as inflammation, apoptosis, and tumor suppression (17). NF-κB induces production of a number of cytokines such as TNF-α and members of the IL-1 and -6 families, among which IL-1β and TNF-α can further enhance the antiviral responses (18). The inflammatory responses triggered by an HBV infection contribute to the pathogenesis of hepatitis and are also involved in countering viral replication and repairing tissue damage.
The NF-κB essential modulator (NEMO) is a regulatory subunit of IκB kinase (IKK) complex and has a critical function in controlling the activation of NF-κB, which is intimately regulated by IKK and IκB. The IKK heterotrimer consists of NEMO (also called IKKγ), IKKα, and IKKβ (19). In its resting state, NF-κB is strongly bound by IκB to prevent its nuclear localization. In responses to a variety of stimuli, such as viral infection or the presence of IL-1, TNF-α, and bacterial lipopolysaccharide (LPS), IκB is phosphorylated and then undergoes ubiquitination and proteasome-mediated degradation, ultimately releasing NF-κB for nuclear localization, which activates downstream gene transcription (20). However, the results of previous studies are controversial regarding the roles of HBV precore/HBeAg in NF-κB activation pathways. One earlier study showed that HBeAg could associate with IL-1 receptor accessory protein (IL-1RAcP) and activate the downstream NF-κB pathway in HEK293T and human hepatoma HA22T/VGH cells (21). Other studies have demonstrated that HBeAg can suppress TLR signaling pathways and immune responses by inhibiting the expression of TLR2 in hepatocytes and nonparenchymal cells (6, 22). Moreover, Lang and colleagues showed that HBeAg can also suppress TIR-mediated NF-κB activation and beta interferon promoter activity by abrogating TIR-TIR interaction (13). Because the adaptor proteins, Mal/TRAM, that are targeted by HBeAg in TLR2-induced NF-κB activation are not engaged in the IL-1-triggered stimulation of NF-κB, HBeAg and its precursor most likely utilize another unknown strategy to manipulate IL-1β-mediated NF-κB activation.
In this study, we showed that HBV HBeAg could bind with NEMO and inhibit the binding of IKKs with the TAK1 complex. They then suppress the K63-linked ubiquitination of NEMO at different lysine sites, which together further impair IL-1β-mediated activation of NF-κB. Together, the results of the current study suggest a new mechanism for how HBV escapes the innate immune system, and it may provide a therapeutic target for antagonizing latent HBV infections.
RESULTS
Specific interactions of NEMO with HBeAg and its precursor proteins.
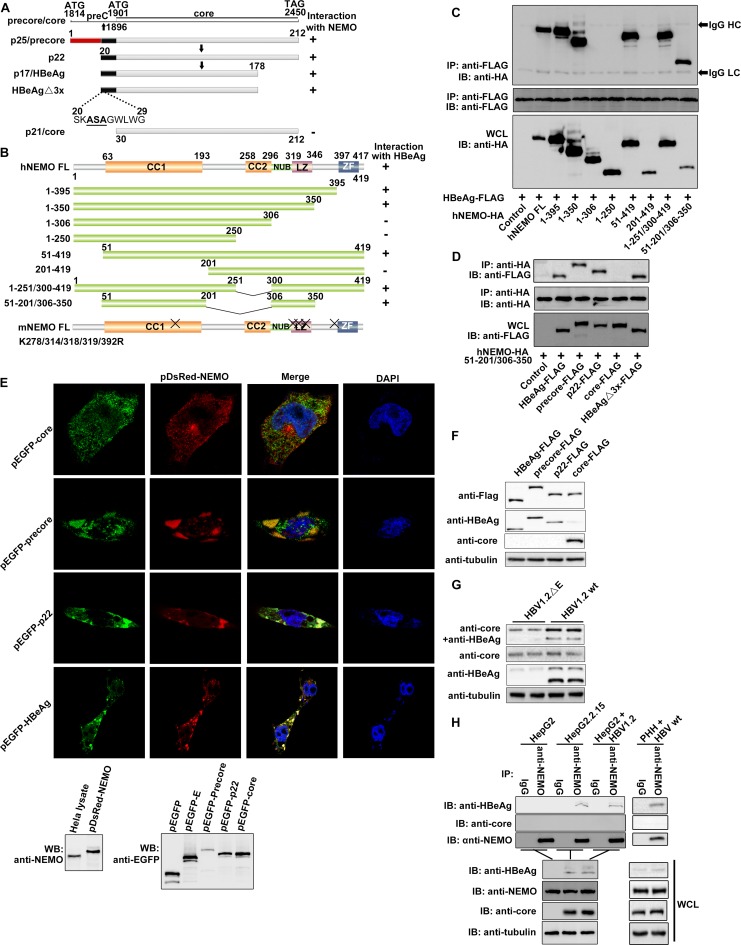
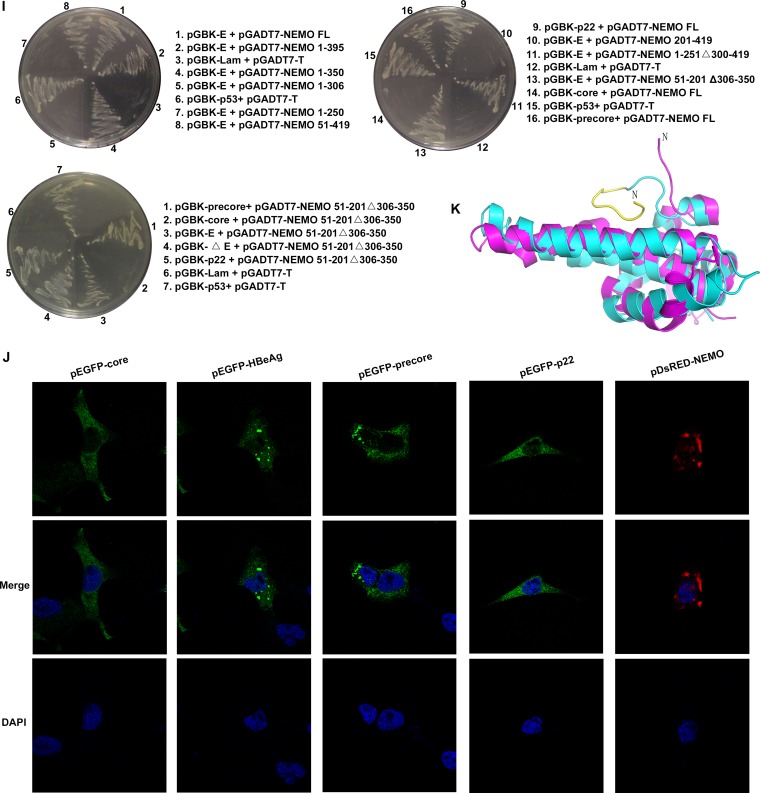
By using a yeast two-hybrid system, we found that HBeAg could interact with NEMO, a modulator of NF-κB activation, and we also observed the same effects for the HBeAg precursor proteins, including precore and p22 (Fig. 1A and I). However, the HBV core protein did not associate with NEMO (Fig. 1A, D, E, and I). To identify the key domains of NEMO involved in the NEMO-HBeAg interaction, we generated a number of NEMO truncations and tested their interactions with HBeAg and its precursor proteins. The HBeAg mutant HBeAgΔ3x, in which three amino-terminal residues, ASA, were substituted for LCL, resulting in loss of its TIRAP/Mal-binding activity, was also constructed and tested (Fig. 1A, D, and I). Interactions between these HBeAg mutants with NEMO were analyzed through coimmunoprecipitation (co-IP) and Western blotting. Amino acid (aa) 51 to 201 and 306 to 350 of NEMO were found to be necessary for binding with HBeAg (Fig. 1B and C). Further experiments revealed that the NEMO mutant that contains just aa 51 to 201 and 306 to 350 could still interact with the precore, p22, and HBeAgΔ3x proteins, thus confirming that these two regions of NEMO are essential and sufficient for the interaction with HBeAg (Fig. 1B, C, and I). A confocal imaging analysis also indicated that HBeAg and its precursors could colocalize with NEMO (Fig. 1E). The expression of the fusion proteins in the transfected cells was confirmed via Western blotting (Fig. 1E and F).
FIG 1.
Specific interactions of NEMO with HBeAg and its precursor proteins, precore and p22. (A and B) Schematic drawings of NEMO, HBeAg and its precursors, and their derivatives used in this work. CC1, coiled-coiled domain 1; CC2, coiled-coil domain 2; LZ, leucine zipper; ZF, zinc finger; NUB, ubiquitin binding. “X” indicates the sites of mutagenesis described in the text. (C and D) HEK 293T cells were cotransfected with plasmids as indicated, and coimmunoprecipitation (co-IP) and Western blotting (WB) were performed according to standard protocol mentioned in Materials and Methods. WCL, whole-cell lysate; HC, IgG heavy chain; LC, IgG light chain. (E) HeLa cells were cotransfected with plasmids for pDsRed-NEMO and pEGFP-HBeAg plus their precursors. Nuclear localization was detected using DAPI. Orange signals reveal the specific associations (upper portion). Expressions of NEMO and HBeAg plus its precursors were also confirmed via immunoblotting using anti-enhanced green fluorescent protein (anti-EGFP) and anti-NEMO antibodies, respectively (lower portion). (F and G) HEK 293T cells were cotransfected with plasmids as indicated, and Western blotting was performed by using the proper antibodies as indicated. (H) Interaction of endogenous HBeAg and NEMO was examined when HepG2 cells were cotransfected with plasmids as indicated or primary human hepatocytes (PHHs) were incubated with wild-type HBV and cultured for 8 days. Cells were then harvested and analyzed via co-IP and WB. HepG2.2.15 cells were used as positive controls. (I) The yeast two-hybrid system was used to determine the domains necessary for the interaction of NEMO and HBeAg. (J) Locations of NEMO, HBeAg, and its precursors in HeLa cells. HeLa cells were transfected with the indicated plasmids. Nuclear localization was detected using DAPI. (K) Superimposition of structures of HBeAg (cyan; PDB code 3V6Z) and HBV core (magenta; PDB code 5WTW); residues 20 to 30 of HBeAg (which core lacks) are shown in yellow. N, N terminus.
Coimmunoprecipitation was adopted to detect the interaction of endogenous NEMO and HBeAg. As shown in Fig. 1F and G, the anti-HBeAg antibody could detect both HBeAg and core in Western blots but gave rise to a stronger signal for HBeAg than core. Due to the size differences, HBeAg could be easily distinguished from other derivatives of precore in the Western blot. In contrast, the anti-core antibody we used could detect core but not HBeAg (Fig. 1F). The band of HBeAg could be readily identified in the Western blot. As shown in Fig. 1H, an interaction between HBeAg and endogenous NEMO was detected in HBV1.2-transfected HepG2 cells and the HBV-producing HepG2.2.15 cells and primary human hepatocytes (PHHs) infected by HBV. Together, these results indicate that NEMO can interact with HBeAg and its precursors but not with the highly similar core protein.
HBeAg suppresses IL-1β-induced activation of NF-κB.
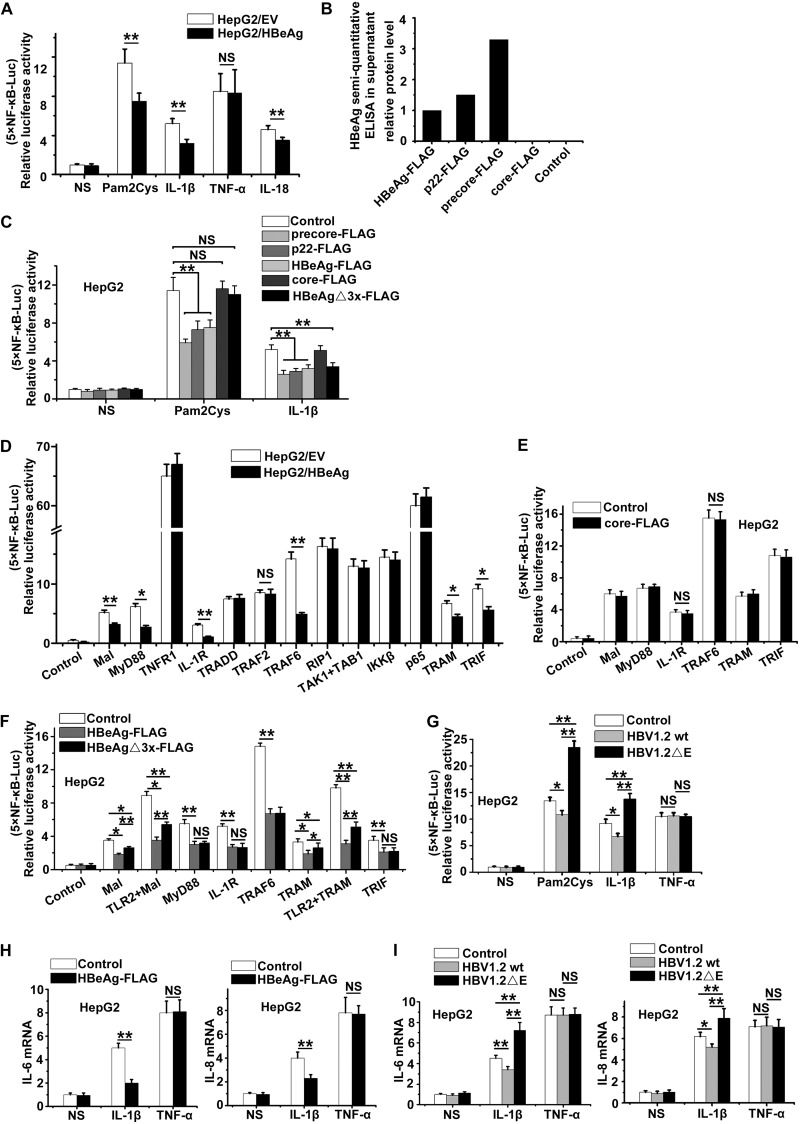
As NEMO is a modulator of NF-κB activation, we further tested whether HBeAg and its precursors could affect NF-κB activity via their interaction with NEMO. The results from previous studies of the effects of HBeAg on NF-κB activity are controversial (6, 13, 21, 23, 24), and thus, we first analyzed the effect HBeAg on the TLR2-, IL-18-, and IL-1β-mediated NF-κB signaling pathways, as well as the TNF-α-induced NF-κB activation pathway. In HepG2 cells transfected with NF-κB–luciferase reporter plasmids, IL-1β-, IL-18-, and TLR2 ligand Pam2Cys-induced activation of NF-κB was lowered when HBeAg was expressed (Fig. 2A), while TNF-α-induced NF-κB activation was not suppressed by HBeAg (Fig. 2A). The supernatants of HepG2 cells transfected with HBeAg and its precursors were also tested by HBeAg enzyme-linked immunosorbent assay (ELISA). The results showed that to some extent, precore, p22, and HBeAg plasmids, but not core, could also produce secreted HBeAg in the corresponding supernatants (Fig. 2B). Further experiments showed that HBeAg and its precursors, but not the core protein, could suppress both IL-1β-induced and Pam2Cys-induced NF-κB activation (Fig. 2C). In contrast, the HBeAg mutant HBeAgΔ3x could inhibit IL-1β-induced but not Pam2Cys-induced NF-κB activation (Fig. 2C).
FIG 2.
HBeAg suppresses the IL-1β-induced activation of NF-κB. 5×NF-κB-Luc reporter plasmids were transfected into the HepG2 cell lines that stably expressed HBeAg (HepG2-HBeAg) or the empty vector control (HepG2-EV), together with pRL-TK plasmids, and 24 h after transfection, cells were treated with Pam2Cys (100 ng/ml), IL-1β (10 ng/ml), TNF-α (10 ng/ml), or 60 ng/ml IL-18 (MBL) for 6 h. Dual-luciferase activities were determined. (B) The HepG2 cells were transfected with the plasmids as indicated for 24 h, and protein levels in the supernatants were determined with semiquantitative HBeAg ELISA kits. (C) 5×NF-κB-Luc was transfected into HepG2 cells with other plasmids as indicated for 24 h. Luciferase activities were then measured after Pam2Cys or IL-1β stimulation for 6 h. (D) 5×NF-κB-Luc plasmids were transfected into stably expressing HepG2-HBeAg or HepG2-EV cells, together with pRL-TK and other plasmids for NF-κB signaling pathway components, for 24 h as indicated, and the luciferase activities were determined as described for panel A. (E and F) HepG2 cells were cotransfected with plasmids as indicated, and the luciferase activities were measured as described above. (G) HepG2 cells were transfected with HBV1.2 wt, HBV1.2 E− or the empty vector, and luciferase activities were measured after treatment with Pam2Cys, IL-1β, or TNF-α for 6 h. (H) The mRNA levels of IL-6 and IL-8 were determined via RT-PCR in stably transfected HepG2-HBeAg or HepG2-EV cells after they were treated with IL-1β or TNF-α for 6 h. (I) The mRNA levels of IL-6 and IL-8 were analyzed via RT-PCR in HepG2 cells after they were transfected with HBV1.2 wt or HBV1.2 E− under the same stimulation as indicated for panel G. The luciferase activities and RT-PCR results represent the averages of results from at least three independent experiments, and data are expressed as means ± SEs. **, P < 0.01; *, P < 0.05. NS, no significant difference.
To further identify at which step of the NF-κB signaling pathway HBeAg plays an inhibitory role, we tested which of the NF-κB-activating adaptors could be inhibited by HBeAg in HepG2 cells (Fig. 2D). The results showed that Mal-, MyD88-, IL-1R-, TRAM-, TRIF-, and TRAF6-induced activation of NF-κB was suppressed by HBeAg, but HBeAg had no inhibitory effect on the activation of NF-κB induced by other signaling proteins downstream of TRAF6 in the NF-κB signaling pathway (Fig. 2D). In contrast, the core protein had no effect on Mal-, MyD88-, IL-1R-, or TRAF6-induced NF-κB activation (Fig. 2E). However, the HBeAg mutant HBeAgΔ3x, which cannot interact with TIRAP/Mal, still exerted suppressive effects on MyD88-, IL-1R-, and TRAF6-induced NF-κB activation (Fig. 2F), indicating that besides binding with TIRAP/Mal, HBeAg has another way to inhibit NF-κB activation. Collectively, the results show that HBeAg and the HBeAgΔ3x mutant can suppress Mal-, MyD88-, IL-1R-, and TRAF6-induced NF-κB activation.
To validate the above-described results in an HBV replication system, the NF-κB-inhibiting effect of HBeAg was tested by using an HBV replicon (HBV1.2). The replicon of HBV mutant G1896A (HBV1.2 ΔE), which has an HBeAg-negative phenotype, was developed as a control. As shown in Fig. 2G, under the stimulation of Pam2Cys or IL-1β, NF-κB activity was higher in HepG2 cells transfected with the HBeAg-deficient HBV1.2 ΔE replicon than in cells transfected with the wild-type (wt) HBV1.2 replicon, indicating that HBeAg may play a role in the regulation of NF-κB activity during HBV replication.
We also examined whether the presence of HBeAg affects the expression NF-κB-mediated downstream genes induced by IL-1β. We found that HBeAg could significantly reduce the mRNA levels of IL-6 and IL-8 in IL-1β-stimulated HepG2 cells; however, we did not observe a similar inhibitory effect of HBeAg when the HepG2 cells were treated with TNF-α (Fig. 2H). Similar results were obtained in experiments with HBeAg-expressing and HBeAg-deficient HBV replicons (Fig. 2I). These results further confirm the suppressive effect of HBeAg on IL-1β-mediated NF-κB activity.
HBeAg and its precursors inhibit TRAF6-dependent K63-linked polyubiquitination of NEMO.
The activation of the IKK complex, which leads to K48-linked polyubiquitination and degradation of IκBα and subsequent NF-κB activation, depends on the K63-linked polyubiquitination of NEMO (25). Therefore, we next tested whether HBeAg affects the ubiquitination of NEMO. The result showed that the levels of ubiquitin (Ub) conjugation to NEMO were reduced by HBeAg and its precore and p22 precursors but not by the core protein (Fig. 3A, left side). Moreover, these inhibitory effects on the ubiquitination level of NEMO by HBeAg were more prominent when IL-1β was added to stimulate the NF-κB signaling pathway (Fig. 3A, right side). HBeAgΔ3x, which lacks TRAM/TIRAP-binding ability, was also able to inhibit the ubiquitination of NEMO in a manner similar to that of HBeAg (Fig. 3A). Further experiments showed that ubiquitination of NEMO was reduced by the expression of HBeAg in a dose-dependent manner (Fig. 3B), while the mRNA levels of endogenous NEMO and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) remained unchanged (Fig. 3B).
FIG 3.
HBeAg and its precursors inhibit TRAF6-dependent K63-linked polyubiquitination of NEMO. (A) Precore-FLAG, core-FLAG, HBeAg-FLAG, p22-FLAG, and HBeAgΔ3x-FLAG were transfected with NEMO-HA and Myc-Ub for 24 h. Cells were then stimulated with IL-1β (10 ng/ml) for 6 h. IP, immunoprecipitation; IB, immunoblotting. (B) HepG2 cells were cotransfected with plasmids (0 to 2 μg) encoding NEMO-HA, Myc-Ub, and precore-FLAG. Then the cells were stimulated with IL-1β (10 ng/ml) for 6 h. Levels of endogenous NEMO and GAPDH mRNA were examined by quantitative PCR. (C) Transfected cells stably expressing precore-FLAG (HepG2-precore) or its control (HepG2-EV) were cotransfected with plasmids for NEMO-FLAG and HA-Ub (wt, KallR, and K6, -11, -27, -29, -33, -48, and -63 only). Cells were then stimulated with IL-1β (10 ng/ml) for 6 h. (D) Cells stably expressing precore-FLAG (HepG2-precore) or its control (HepG2-EV) were cotransfected with plasmids of NEMO-FLAG and then stimulated with IL-1β (10 ng/ml) for 6 h. Whole-cell lysates were analyzed with anti-HA immunoprecipitation, followed by immunoblotting with an anti-linear polyubiquitin antibody. The anti-linear-ubiquitin antibody recognized linear polyubiquitin (M1-Ub). (E) HepG2 cells stably expressing precore/p22/HBeAg were transfected with plasmids for NEMO-HA and Myc-Ub (WT, K63R, or K48R). (F) The plasmids encoding both the deletion mutant (△N) and the point mutant (C70A) of mTRAF6 were transfected into TRAF6−/− MEFs along with Myc-Ub and NEMO-HA for an ubiquitin conjugation analysis. (G) The mNEMO mutants were transfected into NEMO−/− MEFs along with Myc-Ub and TRAF6 for an ubiquitin conjugation analysis. The cells shown in panels A to F were collected and lysed, and the lysates were immunoprecipitated and immunoblotted using the antibodies as shown. (H) The analysis was carried out as for panel G, using NEMO-deficient MEFs with the indicated NEMO mutants. Cells were treated with IL-1β (10 ng/ml) for 6 h before determining luciferase activity.
To determine what type of ubiquitination modification in NEMO was inhibited by HBeAg, we constructed a series of ubiquitin mutants, as indicated in Fig. 3C. As shown in Fig. 3C, NEMO could be modified by K27-, K29-, and K63-linked polyubiquitination, which is consistent with previous reports (26–28). K6-, K11-, K33-, or K48-linked ubiquitination of NEMO could not be detected. We further showed that precore could dramatically inhibit the K63-linked polyubiquitination of NEMO (Fig. 3C). The linear ubiquitination of NEMO could also lead to the activation of NF-κB, but as shown in Fig. 3D, precore did not regulate the linear ubiquitination of NEMO. We also generated K27R and K63R mutations of the wild-type ubiquitin chain. The K63R mutation significantly reduced the ubiquitination level of NEMO, which reveals that K63 ubiquitination may account for a large proportion of the total ubiquitination of NEMO. When the K27 residue of the wild-type ubiquitin chain was mutated to R, with K63 still present, we found that HBeAg could still downregulate the K27R-mediated ubiquitination of NEMO, which further proves that HBeAg mainly regulates the ubiquitination of NEMO at K63 site (Fig. 3E).
To examine whether the inhibition of NEMO ubiquitination by HBeAg depended on TRAF6, we conducted a ubiquitination assay in TRAF6 knockout (KO) mouse embryo fibroblasts (MEFs) that were reconstituted with wt TRAF6 or its E3 ligase activity-defective mutant (C70A) (29). As shown in Fig. 3E, HBeAg did not further reduce ubiquitination in TRAF6 KO cells or in the cells with the introduced C70A mutant. In contrast, HBeAg markedly reduced NEMO ubiquitination in the cells reconstituted with wt TRAF6 (Fig. 3F). These results imply that HBeAg may specifically target TRAF6-mediated NEMO ubiquitination.
Previous studies have reported that five lysine residues in human NEMO (hNEMO) are responsible for its ubiquitination: hK285, hK399, hK321, hK325, and hK326 (mouse equivalents: mK278, mK392, mK314, mK318, and mK319) (28, 30, 31). To investigate which lysine residues of NEMO are involved in the inhibition of ubiquitination caused by HBeAg, we constructed a single-lysine NEMO mutation (mK392R) and different multiple-lysine NEMO mutations (mK314/318/319R [NEMO 3R], mK314/318/319/278R, and mK314/318/319/278/392R), as shown in Fig. 3G. The mNEMO mutants, mTRAF6, and K63-ubiquitin were cotransfected into NEMO−/− MEF lines. Under IL-1β stimulation, HBeAg strongly decreased but did not completely abolish the ubiquitination of mK392R and mK314/318/319R (Fig. 3G). Because mK278 (hK285) of NEMO is modified in the NOD2/RICK signaling pathway (30), we cotransfected NEMO 4R (3R plus mK278R) with TRAF6 and K63-ubiquitin and found that HBeAg could still inhibit the ubiquitination level of NEMO 4R (Fig. 3G). After the mutation of five lysine residues of NEMO, m278/314/318/319/392R (NEMO 5R), TRAF6-induced ubiquitination was markedly reduced, and we were not able to detect an inhibitory effect of HBeAg on the ubiquitination of NEMO 5R (Fig. 3G). These results suggest the importance of all five lysine residues in the TRAF6-dependent K63-ubiquitin conjugation of NEMO.
We then further investigated whether NF-κB activity was affected when the K63-linked ubiquitination of these five lysine residues of NEMO was inhibited by HBeAg. We examined the NF-κB activity using a luciferase reporting system and transfected the NEMO mutants into NEMO-KO MEFs, as shown in Fig. 3H. The luciferase assay results are in accordance with those of the ubiquitination assays. Together, these results indicate that the TRAF6-dependent K63-linked ubiquitination of NEMO at the five lysine residues, m278 (h285), m314 (h321), m318 (h325), m319 (h326), and m392 (h399), was suppressed by HBeAg.
HBeAg suppresses TAK1-NEMO interaction and phosphorylation of the IKK complex under IL-1β stimulation.
Because HBeAg inhibits the K63-linked ubiquitination of NEMO, we hypothesized that HBeAg may influence the interaction of NEMO with other signaling proteins of the NF-κB signaling pathway, such as IKKα, IKKβ, TAK1, and TRAF6. We cotransfected the constructs into HepG2 cells and examined the interactions. The results indicate that HBeAg did not interrupt the interactions of NEMO with IKKα, IKKβ, and TRAF6 (Fig. 4A).
FIG 4.
HBeAg suppressesTAK1-NEMO interaction and phosphorylation of the IKK complex under IL-1β stimulation. (A) HepG2 cells were transfected with the plasmids for HA-NEMO, FLAG-IKKα, FLAG-IKKβ, FLAG-TRAF6, and Myc-HBeAg, and co-IP analyses were performed with the antibodies as shown. (B, C, F, G, J, and K). Stably transfected HepG2-HBeAg cells or control HepG2-EV cells were treated with IL-1β (10 ng/ml) or TNF-α (10 ng/ml), and the cells were harvested and lysed at certain time points as indicated. Co-IP and immunoblotting analyses were performed using the proper antibodies. (D, E, H, I, L, and M) Cells were transfected with HBV1.2 wt or HBV1.2 E− and then treated with IL-1β (10 ng/ml) or TNF-α (10 ng/ml). The cells were harvested and lysed at certain time points as indicated. Co-IP and immunoblotting analyses were performed using the proper antibodies. The HepG2 cells were transfected with the plasmids as indicated for 24 h, and the protein levels in the supernatants were determined with semiquantitative HBeAg ELISA kits for panels D and E.
Because TAK1 is an activating kinase for IKKs and can be recruited to the IKK complex under IL-1β and TNF-α stimulation (32), we investigated whether HBeAg affects the interaction between NEMO and TAK1. The results show that HBeAg did disrupt the interaction between TAK1 and NEMO under IL-1β stimulation (Fig. 4B). However, under TNF-α stimulation, HBeAg failed to affect the interaction between TAK1 and NEMO (Fig. 4C), which is consistent with previous observations that HBeAg does not affect TNF-α-mediated NF-κB activation. We also investigated HBeAg’s disruptive effect on the interaction between TAK1 and NEMO by using the HBV wt replicon and the HBV G1896A mutant under IL-1β (Fig. 4D) or TNF-α (Fig. 4E) stimulation, and the results were consistent with the above-described observations. In addition, a semiquantitative ELISA with anti-HBeAg confirmed the expression of HBeAg in the supernatants of the cells transfected with the HBV wt replicon.
As HBeAg could suppress the interaction of NEMO and TAK1, we further tested whether such suppressive effects influence the phosphorylation of IKKs, IκBα, and TAK1. The results show that under IL-1β stimulation, but not that of TNF-α, HBeAg reduced the phosphorylation levels of IKKα/β and IκBα, while the level of phosphorylated TAK1 (pTAK1) was unchanged (Fig. 4F and G). We repeated the above-described experiments using the HBV replicon and its HBV G1896A mutant. Compared with levels associated with wild-type HBV, the phosphorylation levels of IKKα/β and IκBα were relatively high with HBV G1896A transfection in IL-1β-treated cells but not in TNF-α-treated cells (Fig. 4H and I). We then showed that HBeAg could prevent the degradation of IκBα in IL-1β-treated cells but not in TNF-α-treated cells (Fig. 4J and K), which was also verified by using the HBV replicon system (Fig. 4L and M). These results further confirm the role of HBeAg in the inhibition of the IL-1β-mediated NF-κB signaling pathway.
HBeAg suppresses IL-1β-induced NF-κB activity in HBV-infected HepG2-NTCP cells.
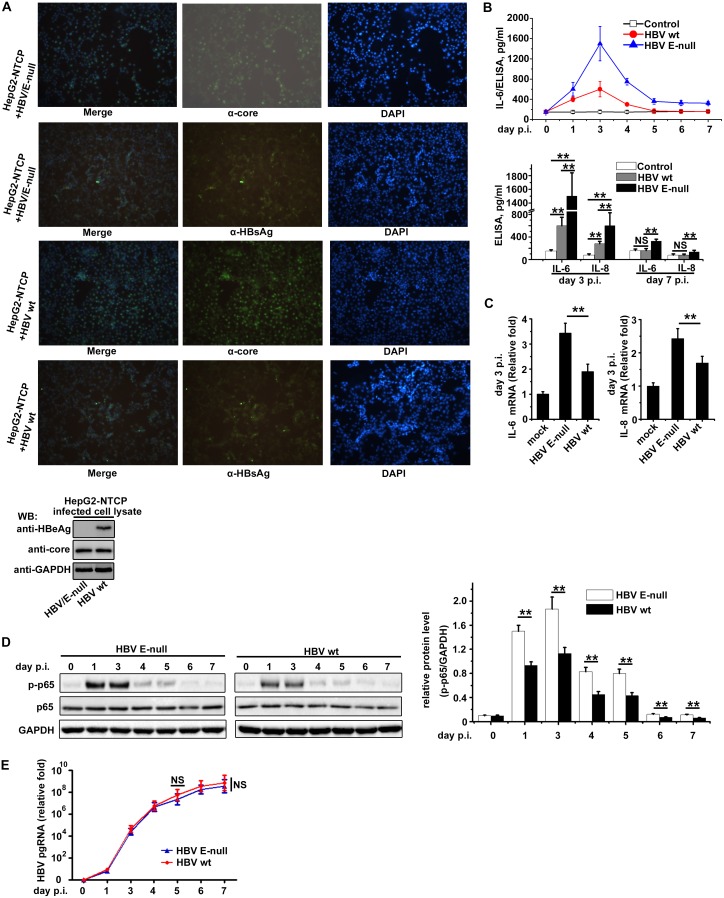
It has been reported that the sodium taurocholate cotransporting polypeptide (NTCP), a multiple-transmembrane transporter, is a functional hepatocyte receptor for HBV and is vital for viral infections (33); therefore, we generated NTCP-expressing HepG2 cells (HepG2-NTCP) for HBV infection. HBV inoculum samples were concentrated from the supernatants of transfected HepG2 cells with plasmid HBV1.2 wt or HBV1.2 G1896A mutant. When HepG2-NTCP cells were infected with the HBV samples, immunofluorescence examinations of core protein and HBsAg revealed that more than 50% of the cells were infected with HBV (Fig. 5A). In HBV-infected PHHs, NF-κB activity increases in the first dozen hours and then becomes quiescent (34); therefore, we tested for NF-κB-mediated IL-6 expression in HBV-infected HepG2-NTCP cells. The results show that the HBV G1896A infection induced higher IL-6 levels than the wild-type HBV infection, and the IL-6 levels in HBV G1896A-infected cells did not return to a quiescent level 5 days postinfection, as was observed with HBV wt infections (Fig. 5B). The results imply that HBeAg plays an important role in inhibiting NF-κB activity during HBV infections. We also examined the mRNA levels of IL-6 and IL-8 from the infected cells at the peak of NF-κB activity, and we found that HBV G1896A induced higher mRNA levels of IL-6 and IL-8 than HBV wt (Fig. 5C). We also analyzed the levels of phosphorylated p65 (p-p65) at specified time points in infected cells; p-p65 is directly associated with activation of NF-κB. The results show that HBV G1896A infection induced higher p-p65 levels than wild-type HBV infection at the corresponding time point during the infection (Fig. 5D). The viral loads at different time points of the infections were measured via reverse transcription-PCR (RT-PCR) analysis of the pregenomic RNA (pgRNA). The replication kinetics curves of wild-type HBV and HBV G1896A in our infection system showed no significant difference (Fig. 5E), which verified that the change in NF-κB activity is due to HBeAg but not the disparity of viral loads between wild-type HBV and HBV G1896A.
FIG 5.
HBeAg suppresses IL-1β-induced NF-κB activity in HBV-infected HepG2-NTCP cells. (A) Three days after the infection of HepG2-NTCP cells with HBV wt or its HBeAg-negative mutant (HBV G1896A), immunofluorescence assays for core protein and HBsAg expression were performed. Images were taken at a magnification of ×200. Expressions of HBeAg and core protein were also confirmed via Western blotting. (B and C) HepG2-NTCP Cells were incubated with a control, HBV wt, or HBV G1896A. The supernatants of the cells were collected at certain time points as indicated, and the protein and mRNA levels of IL-6 and IL-8 in the supernatants were examined via ELISA (B) and RT-PCR (C). p.i., postinfection. (D and E) The infected cells (HBV wild type or E-null) were collected and subjected to Western blotting. The relative protein levels of p-p65 and GAPDH at certain time points between two kinds of infected cells were measured as indicated (D), and the HBV pgRNA levels of the infected cells (HBV wild type or E-null) were also determined by RT-PCR (E). ELISA, RT-PCR, and Western blotting results represent the averages of results from at least three independent experiments, and data are expressed as the means ± SEs. **, P < 0.01; *, P < 0.05.
HBeAg suppresses IL-1β-induced NF-κB signaling in HBV-infected PHHs.
To mimic a physiological infection circumstance, we also used the wild-type and HBeAg-negative (G1896A) HBVs to challenge the PHHs together with the inactivated wild type HBV as a control. The immunofluorescence results in Fig. 6A indicated that both the wild-type and HBeAg-negative (G1896A) HBVs can infect the PHHs within 8 days. When all the three groups of PHHs were incubated with IL-1β, the PHHs from the HBV G1896A group secreted more IL-6 than those from the wild-type HBV group and inactivated HBV control (Fig. 6B). Considering previous reports that HBeAg inhibits TLR2-mediated NF-κB activity (6, 13), we introduced OxPAPC, a TLR2/TLR4 inhibitor, into our experiment to exclude the possible influence of the TLR2 pathway. Our results show that the PHHs from the HBV G1896A group secreted more IL-6 than those from the wild-type HBV group and inactivated HBV control following OxPAPC treatment (Fig. 6B). We also analyzed the IL-8 level under the above-described circumstances, and the results showed that the PHHs from the HBV G1896A group secreted more IL-8 than those from the wild-type HBV group and inactivated HBV control in the presence or absence of OxPAPC (Fig. 6C). These results indicate that HBeAg can inhibit IL-1β-induced NF-κB activation during natural HBV infection, which is consistent with HBeAg’s inhibitory effect on the K63-linked ubiquitination of NEMO.
FIG 6.
HBeAg suppresses IL-1β-induced NF-κB signaling in HBV-infected primary human hepatocytes (PHHs). (A) PHHs were incubated with the wild-type or HBeAg-negative HBV and inactivated HBV as a control and cultured for 8 days. Expressions of HBeAg and core protein of the above samples were confirmed via immunofluorescence using anti-HBeAg and anti-core antibodies, respectively. Nuclear localization was detected using DAPI. (B and C) PHHs were incubated with wild-type or HBeAg-negative HBV and inactivated HBV as a control and cultured for 8 days. Cells were then treated with IL-1β (10 ng/ml) or the combination of IL-1β (10 ng/ml) and OxPAPC (300 μg/ml) for 20 h, and the IL-6 levels in the cell supernatants were determined via ELISA. ***, P < 0.001; **, P < 0.01; *, P < 0.05.
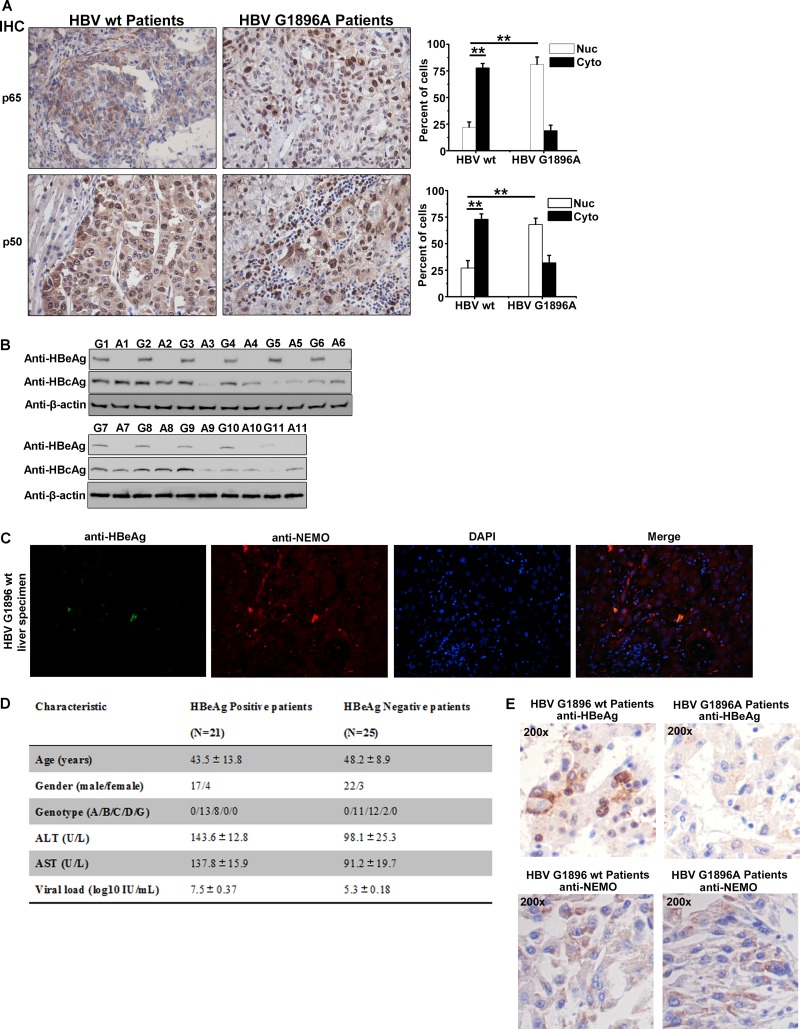
Enhanced NF-κB activity in HBV G1896A bioptic liver tumor tissues from HBV-infected patients.
In HBV infections, the mutation of G to A at genomic position 1896 (G1896A) gives rise to a precore/HBeAg-null variant of HBV (35, 36). We selected bioptic liver samples infected with either the G1896A mutant (precore/HBeAg-null) or G1896 wt (precore/HBeAg positive) by excluding mixed genotypes (Fig. 7F).
FIG 7.
Enhanced NF-κB activity in bioptic liver tissues from HBV-G1896A-infected patients. (A) Immunohistochemical staining of p65 and p50 in HBV wt- or G1896A-infected liver tumor tissues. (B) Eleven pairs of corresponding tissues were lysed and immunoblotted with the indicated antibodies. (C) Immunofluorescence in bioptic liver tissues from HBV wt-infected patients was assessed to determine the colocalization of HBeAg and NEMO. (D) Baseline characteristics of HBV-infected patients whose results are shown in panels A and C. Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase. (E) Immunohistochemical staining of HBeAg or NEMO in HBV wt- or G1896A-infected liver tissues. (F) HBV templates were extracted and then amplified from the liver tissues of the HBV patients. The graphs show the sequencing results for HBV precore regions. (G) Schematic illustration of events underlying HBeAg-regulated NF-κB activity.
We next analyzed NF-κB activation in HBeAg-negative and HBeAg-positive bioptic liver tissues. The translocation of NF-κB p65 and p50 into the nucleus is an indicator for NF-κB activation. As shown in Fig. 7A, the levels of nuclear p65 and p50 in bioptic liver tissues from HBV wt patients were lower than those in tissues from HBV G1896A patients (Fig. 7A). HBV infection was confirmed by Western blotting for HBeAg and core protein (Fig. 7B). We further showed that HBeAg is associated with NEMO in the liver tissue of HBV wt patients by immunofluorescence analysis (Fig. 7C). The clinical information for the sources of the livers tissues is presented in Fig. 7D, and the expression of HBeAg and NEMO in patients is shown in Fig. 7E. The above-described immunofluorescence results indicated that HBeAg could suppress the translocation of p65 and p50 to the nucleus in the clinical bioptic liver tissues, which further demonstrated that HBeAg exerts an inhibitory effect on NF-κB activity.
DISCUSSION
NF-κB plays an important role in human liver homeostasis and pathophysiology. The canonical NF-κB pathway is stimulated by a wide variety of stimuli, like TNF-α and IL-1, as well as viral antigens that act on TLRs (37). Previous studies showed that the NF-κB pathway can be activated by the HBV-encoded proteins HBx and PreS2 (38–40). NF-κB signaling pathway activation eventually induces of the expression of inflammatory cytokines, and NF-κB-triggered IL-1β production can further enhance the expression of cytokines, including IL-6, which is important for the clearance of the virus (41). Surprisingly, as infections continue, NF-κB activity nearly returns to a normal level, together with elevated HBV replication (34). Taken together, previous studies indicate that NF-κB activity increases after the sensing of HBV by the innate immune system, and then HBV can escape the innate immune responses by inhibiting or, more precisely, manipulating NF-κB activity via different strategies. In this report, we reveal a novel mechanism by which HBV HBeAg can associate with NEMO and thereby suppress NF-κB activity.
It has been shown that HBV polymerase inhibits NF-κB signaling by suppressing IKKs via interactions with Hsp90β (42), and other studies have reported that HBeAg suppresses TLR2-induced NF-κB activity (6, 13). However, previous reports contradict each other with regard to the role of HBV precore/HBeAg in IL-1β-induced NF-κB signaling pathways. One earlier study showed that HBeAg could bind with IL-1RAcP and activate the downstream NF-κB pathway in HEK293T and HA22T/VGH cells (21). In contrast, Wilson and coworkers reported that HBeAg suppressed IL-1β-induced NF-κB activation in Huh7 and HepG2 cells and especially in PH5CH8 cells (23). Furthermore, Lang et al. also showed that HBeAg could inhibit IL-1β-induced NF-κB activation in experiments relating to HBeAg and the TLR2 signaling pathway (13). In this study, we revealed that HBeAg can suppress IL-1β-induced NF-κB activation in HepG2 cells, HepG2-NTCP cells, and primary human hepatocytes, and we confirmed its inhibitory role in NF-κB activation in clinical samples. Most importantly, we revealed a novel mechanism by which HBV antagonizes the NF-κB signaling pathway. We found that HBeAg could interact with NEMO and thereby suppress the TRAF6-mediated K63-linked polyubiquitination of NEMO, leading to the suppression of NF-κB activation. Taking into account previous findings on the roles of HBeAg in NF-κB activation, one model was proposed to explain the inhibitory function of HBeAg and its molecular mechanisms (Fig. 7G).
NF-κB can be activated by various inflammatory factors or TLR ligands. In this study, we showed that HBeAg could suppress TLR2- and IL-1β-mediated NF-κB activation but not that of TNF-α. This difference may be explained by the different roles that TRAF6 and TRAF2/5 play in the activation of NF-κB. TRAF6 is indispensable for IL-1β-mediated NF-κB activation (43), while TRAF2/5 play a critical role in TNF-α-induced NF-κB activation (43, 44). A dominant negative mutation of TRAF6, but not of TRAF2/5, would jeopardize IL-1β-mediated activation of the NF-κB signaling pathway (43, 44). Alternatively, TNF-α- but not IL-1-induced NF-κB activation is disrupted in TRAF2−/− TRAF5−/− MEFs. This phenomenon suggests that TRAF2/5, but not TRAF6, are critical in TNF-α-mediated NF-κB activation (45, 46). Here we demonstrated that HBeAg could inhibit TRAF6-dependent K63-linked polyubiquitination of NEMO, and this may explain why HBeAg is not involved in TNF-α-mediated NF-κB activation.
A previous report has indicated that HBeAg acts as a potent inhibitor of pro-IL-1β transcription and IL-1β maturation (47), but the IL-1β level is not constantly low during HBV infection. NF-κB activity increases rapidly at the early stage of HBV infection and then goes back to quiescence (34). It could be envisaged that the level of IL-1β, as a downstream effector of NF-κB, could also be altered in response to the change of NF-κB activity. To some extent, choosing IL-1β as the inducer of the NF-κB activity in our study could also mirror processes during physiological HBV infection. We also tested other stimuli, such as TLR2 agonist Pam2Cys (48) and IL-18 (24), to induce NF-κB activity, and we observed the inhibitory effect of HBeAg on Pam2Cys- and IL-18-induced NF-κB activation (Fig. 2A), which is accordance with downregulation of K63 polyubiquitination of NEMO by HBeAg, since NEMO plays an important role both in TLR2- and IL-18-stimulated NF-κB activation.
Interestingly, although core protein shares ∼90% sequence identity with precore, we did not detect any interaction between core protein and NEMO. Such a discrepancy may be due to the structural and conformational differences of core protein and precore/HBeAg. Previous studies also showed that there are no cross immune reactions of core and HBeAg (49, 50). Structural analysis of HBeAg is shows that the N-terminus of HBeAg is exposed (51), while the corresponding part in core protein is hidden (Fig. 1K). The different orientation of the N termini of HBeAg and core might be the reason why HBeAg but not core could bind to NEMO. These results may partly answer why NEMO can associate with precore and HBeAg but not with core protein.
During HBV infection, the occurrence of mutations in the HBV genome (such as G1896A) and antiviral drug treatments can both lead to HBeAg-negative conversion. The difference is that drug treatments can drastically suppress the replication of HBV, while the HBV G1896A mutant virus can still maintain a relatively high level of virus replication, which may partly explain why many asymptomatic HBV carriers after drug therapy are also HBeAg negative. Emergence of an HBeAg-null mutation in chronic infection is related to more severe liver disease (52). Our current work may provide a mechanistic explanation for this observation. We showed that HBeAg-negative HBV (G1896A) induces a higher level of NF-κB activity than the HBeAg-producing wild-type HBV, with the former consequently leading to the sustained production of higher levels of inflammatory cytokines. Liver inflammation is regulated by many different factors during HBV infection, and one factor may be more dominant than others at specific stages of the infection. The physiological roles and detailed mechanisms of HBeAg and its precursors in the regulation of NF-κB pathway need to be addressed in future studies.
In this work, we have tested HBeAg as well as its precursors precore and p22 in the inhibition of NF-κB activation. One important question is which of the different forms is dominant in cells and therefore may exert a physiological function during HBV infection. According to a previous report, HBeAg was detectable in cytoplasm of infected hepatocytes (14). In contrast, precore and p22, which undergo further cleavage to produce mature HBeAg, are unstable and hardly detectable. In accordance with previous observations, one major band of HBeAg size was detected in most of the Western blotting assays from HBV-transfected or -infected samples (Fig. 4H, I, L, M, 5A, and 7B). It can be assumed that HBeAg should be more abundant in cytoplasm than precore and p22 and therefore may play more important roles in the regulation of NF-κB activation.
Tagged or fusion protein are commonly used in biological studies, including those of HBV protein functions (13, 53). In the current work, we used tagged proteins to facilitate the study of protein-protein interaction (Fig. 1). One drawback of this strategy is that the attached tag may influence the conformation and function of the protein, and consequently, the localization and interactions of the tagged protein may be different from those of wild-type proteins. Thus, the results with tagged proteins should be complemented by other functional assays. In the current work, we confirmed the interaction of HBeAg and NEMO by coimmunoprecipitation of endogenous proteins (Fig. 1H) and the inhibitory function of HBeAg on NF-κB activation in HBV-infected cells (Fig. 5 and 6) and clinical samples (Fig. 7). We also noticed the lower HBeAg titer in culture supernatant and weaker phenotype for the HBeAg construct than for the precore construct (Fig. 2B and C). The precore construct may mimic more the process of precore cleavage and HBeAg maturation, whereas the HBeAg construct produces directly a tagged HBeAg protein. Therefore, for functional studies, it is best to use wild-type protein produced during natural infection.
Taken together, our results identify an important mechanism involving HBeAg-mediated suppression of IL-1β-mediated activation of NF-κB via suppression of TRAF6-dependent K63-linked ubiquitination of NEMO. This indicates that HBV evades host’s innate immune response through diversified mechanisms. Together with our recent study showing that HBeAg can promote liver tumorigenesis by enhancing p53 degradation (53), the current work presents a new function of HBeAg in HBV-induced pathogenesis, which may contribute to the development of new therapeutic interventions involving immunoregulation for HBeAg-positive chronic HBV infections.
MATERIALS AND METHODS
Ethics statement.
Liver biopsies for immunofluorescence and immunohistochemical analyses were collected from patients at Zhongnan Hospital (Wuhan, China). Samples were collected for this study from 2006 to 2014. Sample collections were approved by the Institutional Review Board of Wuhan University, and informed consent from all patients was obtained in accord with the Declaration of Helsinki. All subjects were adults, and all provided written consent. All patients were positive for HBsAg for at least 6 months before enrollment, and all patients had serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels that were at least 2-fold greater than normal and a serum HBV DNA level of more than 105 copies/ml (measured via PCR) before enrollment.
The diagnostic criteria of chronic hepatitis B (CHB) complied with the standards of the 2006 diagnostic and treatment guidelines for liver failure (54) issued by the Chinese Society of Infectious Diseases and the Chinese Society of Hepatology, Chinese Medical Association. Figure 7D shows the main clinical and demographic characteristics of the population used in this study.
Cell cultures and transfection.
Human hepatoma cell-derived HepG2 and HepG2-NTCP cells, human cervical carcinoma cell-derived HeLa cells, mouse embryo fibroblast (MEF)-derived cells, and human embryonic kidney (HEK293T) cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (Gibco BRL, Carpinteria, CA) in an incubator with 5% CO2 at 37°C. Female PHHs were purchased from Celsis IVT (Baltimore, MD) and maintained in InVitroGRO CP medium or InVitroGRO HI medium together with Torpedo antibiotic mix (Celsis). TRAF6-null MEFs and NEMO-null MEFs were generous gifts from Xiaolu Lu (University of Southern California). HepG2, HeLa, TRAF6-null MEFs, and NEMO-null MEFs were transfected with Lipofectamine 2000 (Invitrogen), and HEK293T cells were transfected with polyethylenimine (PEI; Polysciences, Warrington, PA) according to the manufacturers’ instructions.
Plasmids, antibodies, and reagents.
The HBeAg, precore, p22, core, and HBeAgΔ3x constructs were derived from pEF-FLAG or pEF-Myc expression vectors. DNA fragments corresponding to the coding sequences were amplified from the template plasmid pUC18/HBV1.3 (kindly provided by Francis V. Chisari, Scripps Research Institute, CA) via PCR. The wild-type pcDNA3.0/HBV1.2 (HBV1.2 wt) was amplified by using pAAV/HBV1.2 as a template, and the mutant HBV1.2 E-null (HBV1.2 G1896A) was constructed by the mutation of G at position 1896 to A from HBV1.2 wt.
hNEMO/mNEMO and their truncations or mutations were constructed with pRK-HA expression vectors. mNEMO and mTRAF6 were amplified from HepG2 cDNA and then cloned into corresponding vectors.
Recombinant TNF-α (PreproTech, Rocky Hill, NJ), IL-1β (PreproTech), Pam2Cys (InvivoGen), and OxPAPC (InvivoGen) were used to stimulate cells as indicated.
The antibodies used in this study included anti-HA (Sigma, USA), anti-FLAG (Sigma), anti-C-Myc (Sungene Biotech, Tianjin, China), anti-β-actin (Proteintech, China), anti-GAPDH (Proteintech), anti-IKKγ (CST, USA), anti-p-p65 (CST), anti-p65 (CST), anti-HBeAg (Santa Cruz, USA), anti-HBsAg (Santa Cruz), anti-core (Santa Cruz), anti-pIKKγ (CST), anti-pIKKα/β (CST), anti-IκBα (CST), anti-pIκBα (CST), anti-pTAK1 (CST), anti-NEMO (Proteintech), and anti-linear-polyubiquitin monoclonal antibody (LUB9; LifeSensors, Inc.).
HBV collection/quantitation and infection.
HBV inoculum samples were concentrated from the supernatants of transfected HepG2 cells with plasmids HBV1.2 wt or HBV1.2 G1896A mutant (HBV1.2 E-null) as described above. The viruses were collected via ultracentrifugation using type70 Ti and SW41 rotors (Beckman) through a 20% (wt/vol) sucrose cushion (1 mM EDTA, 30 mM Tris [pH 7.4], 150 mM NaCl) for 20 h at 70,000 × g and 12°C. HBV DNA was quantitated via PCR using a 7300 real-time PCR system (Applied Biosystems) and an HBV nucleotide kit (KHB, Shanghai, China), with the titers expressed as viral genome copies per milliliter, according to the manufacturer’s instructions. The cells were stimulated with HBeAg-negative or HBeAg-positive inocula or mock agent at concentrations of 1 × 109 viral genome copies per milliliter in 2.5% dimethyl sulfoxide (DMSO).
Immunoprecipitation and Western blotting.
HEK293T cells, HepG2 cells, MEF wt cells, and TRAF6-null MEFs or NEMO-null MEFs were treated with either IL-1β (10 μg/ml) or TNF-α (50 μg/ml) for 6 h before being harvested. Cells were resuspended in phosphate-buffered saline (PBS) and then centrifuged at 1,000 × g and lysed in a buffer (50 mM Tris-HCl [pH 7.6], 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 10 μg/ml of aprotinin, 10 μg/ml of leupeptin, and 1 mM phenylmethylsulfonyl fluoride) on ice. After centrifugation at 12,000 × g, the supernatant was collected and the protein concentration was determined. Proteins were analyzed via Western blotting or coimmunoprecipitation (co-IP). In the co-IP experiments, extracts were incubated for 3 h at 4°C with the relevant antibodies and protein A/G Sepharose (Santa Cruz, USA) or anti-FLAG M2 affinity gel (Sigma, USA). Sepharose beads were centrifuged and washed three times with a wash buffer (20 mM Tris-HCl [pH 7.6] and 150 mM NaCl). The protein samples were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes (GE Healthcare, WI), which were then blocked in skim milk and tested with the desired antibodies. Immunoblot signals were detected by enhanced chemiluminescence (ECL) substrate (Millipore, MA).
In vivo ubiquitination assay.
Cells were cotransfected with NEMO-HA, Myc-Ub (or its mutants), TRAF6-FLAG, and HBeAg-FLAG (or its precursors and HBeAgΔ3x) in various combinations. IL-1β was added 6 h before harvesting of cells.
The cells were lysed with the same buffer as used in the co-IP assay. The cell lysates were centrifuged at 4°C for 10 min and then incubated for 1 h with the desired antibodies. The immune pulldown complexes were adsorbed to the protein A/G Sepharose and coincubated at 4°C for 2 h. After 3 washes, the complexes were eluted by boiling for 5 min and the desired antibodies were added for the second time. Then the immune complexes were adsorbed to protein A/G Sepharose and coincubated again at 4°C for 2 h. The final immune complexes were harvested after three washes and 5 min of boiling in the loading buffer. The subsequent steps for the in vivo ubiquitination assay follow the methods described for the immunoprecipitation and Western blotting assays.
Immunofluorescence microscopy.
For confocal analyses, HeLa cells were grown on 22-mm coverslips in six-well plates and transfected with 2 μg of DNA. After 24 h of transfection, cells were fixed with 4% paraformaldehyde, permeabilized with 0.5% Triton X-100, and stained with 4′,6-diamidino-2-phenylindole (DAPI). The cells were imaged using a fluorescence microscope (Leica, Germany). For the HBV infection analyses, HepG2-NTCP cells were fixed with 4% paraformaldehyde for 30 min and permeabilized with 0.25% Triton X-100 in PBS for 45 min at room temperature. After blocking with 2% bovine serum albumin (BSA) and 0.25% Triton X-100 in PBS for 1 h, the cells were incubated with anti-core and anti-HBsAg, DyLight-488-conjugated goat anti-mouse IgG (Thermo) was used as the secondary antibody. Nuclear staining was performed with DAPI. Images were collected with the same equipment as described above.
Liver biopsies for immunofluorescence assays were collected from patients at Zhongnan Hospital (Wuhan, China). NEMO and HBeAg proteins were examined and visualized using rabbit anti-NEMO and mouse anti-HBeAg antibodies.
Immunohistochemistry.
Liver tissues were collected from patients at Zhongnan Hospital (Wuhan, China). p65 and p50 were examined via immunohistochemical analysis of samples in OCT using anti-p65 or anti-p50 antibodies (CST, USA).
Immunostaining was assessed by two independent pathologists in a blinded manner. A four-level scoring system for staining intensity (0, negative; 1, weak; 2, moderate; and 3, strong) was used, and samples with scores of 1+ were considered positive.
Stable transfection of HepG2 cells with NTCP.
Lentiviruses carrying the appropriate inserts were cotransfected with the packaging plasmids pMD2.G and psPAX2 into HEK293 cells. Transfections were carried out using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. Six hours after transfection, the culture was replaced. Cell culture supernatants were collected at 24, 36, 48, and 60 h posttransfection and pooled. They were then passed through a 0.45-μm-pore-size filter, concentrated via ultracentrifugation, and stored at −80°C until use. Lentiviruses were used to transduce hNTCP into HepG2 cells. Stable cell lines were selected with 2.5 μg/ml of puromycin.
Yeast two-hybrid screens.
HBeAg-binding proteins were identified using the yeast two-hybrid system (Clontech) as recommended by the manufacturer. The precore, core, p22, and HBeAg fragments (subtype ayw) were cloned into the pGBKT7 vector fused into the GAL4 DNA-binding domain. NEMO truncations were constructed in the pGADT7 vector. A human spleen cDNA library (catalogue no. 638824) cloned into the GAL4 activation domain vector pACT2 was screened with the haploid Saccharomyces cerevisiae strain AH109 transformed with pGBKT7-precore construct and selected for tryptophan prototrophy. Individual positive clones were further selected for tryptophan, leucine, histidine, and adenine prototrophy in the presence of 15 mmol/liter of 3-aminotriazole (Sigma). Colonies showing moderate to intense growth were reselected on selective medium and tested via filter assay for β-galactosidase expression. Filters were placed in liquid nitrogen for 30 s and incubated in a buffer containing 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) at 37°C (30 min to 36 h). Yeast plasmid DNA from blue colonies was isolated and transformed into Escherichia coli to rescue positive library plasmids, which were retested in yeast for β-galactosidase activity via cotransformation with pGBKT7-53/pGADT7-T and pGBKT7-lam/pGADT7-T as positive and negative controls, respectively. The positive clones were sequenced and identified.
Dual-luciferase reporting system.
For dual-luciferase reporter assays, HepG2 cells seeded in 24-well culture plates (8 × 104 cells/well) were cotransfected with 5× NF-κB–luciferase plasmids (200 ng) and pRL-TK (Renilla luciferase) (50 ng) together with the desired plasmids. Twenty hours after transfection, the cells were lysed and subjected to a luciferase activity assay using the Dual-Glo luciferase assay system (Promega). All experiments were performed in triplicate.
RT-PCR.
RNA was extracted from the cell lines indicated above using an RNeasy minikit (Qiagen) according to the manufacturer’s specifications. The Rever Tra Ace quantitative PCR (qPCR) RT kit (TOYOBO, Osaka, Japan) was used to produce cDNA from 150 ng of RNA. Quantitative real-time PCR analyses were performed using SYBR master mix (TaKaRa, Japan) on a PCR system (CFX 96; Bio-Rad, USA) with the primers listed below. The relative amounts of PCR product were determined using the threshold cycle (CT) method. All experiments were independently repeated in triplicate.
Specific primers used in the RT-PCR.
Primers used were as follows: for IL-6, 5′-TTCTCCACAAGCGCCTTCGGTC-3′ (forward) and 5′-TCTGTGTGGGGCGGCTACATCT-3′ (reverse); for IL-8, 5′-ATGACTTCCAAGCTGGCCGTGGCT-3′ (forward) and 5′-TCTCAGCCCTCTTCAAAAACTTCT-3′ (reverse); for HBV pgRNA, 5′-CCTAGTAGTCAGTTATGTCAAC-3′ (forward) and 5′-TCTATAAGCTGGAGGAGTGCGA-3′ (reverse); for NEMO, 5′-AAGAGCCAACTGTGTGAGATG-3′ (forward) and 5′-TTCGCCCAGTACGTCCTGA-3′ (reverse); and for GAPDH, 5′-GACAAGCTTCCCGTTCTCAG-3′ (forward) and 5′-GAGTCAACGGATTTGGTCGT-3′ (reverse).
ELISA.
Secreted IL-6 and IL-8 were detected in the supernatants of HBV-infected HepG2-NTCP cells and peripheral blood mononuclear cells (PBMCs) from chronic HBV patients and healthy subjects using specific ELISA kits (Abcam, UK) according to the manufacturer’s instructions. The secreted HBeAg levels in the supernatants of transfected cells were tested by using semiquantitative HBeAg ELISA kits (KHB, Shanghai, China). The animal ELISA kits were also obtained from Abcam. The results were measured using a microplate reader (Biotek, USA). The results are shown as means ± standard deviations, and they correspond to the averages of results from at least three independent experiments.
Molecular modeling of core protein.
HBcAg and HBeAg have a large number of homologous amino acid sequences. We searched for the structure of HBeAg in the Protein Data Bank (PDB) and then carried out molecular modeling of core protein by using the program Swiss-Model and the structure of HBeAg as the template. The structure figure was generated using the program PyMol (http://www.pymol.org).
Statistical analysis.
Differences between mean values were assessed via one-way analysis of variance (ANOVA) and Student-Newman-Keuls multiple-comparisons tests using SPSS version 11.0. Results with a P value less than 0.05 were considered statistically significant.
ACKNOWLEDGMENTS
We thank Xiaoping Chen (Zhongnan Hospital) for his help with the collection of liver biopsies from HBV patients and Xu Yuan and Hui Zhang for their excellent technical assistance.
This study was supported by the National Natural Science Foundation of China (grants 81501758, 81572006, and 81620108020). D.G. is also supported by the Guangdong Zhujiang Talent Recruitment Program and the National Ten-thousand Talents Program.
REFERENCES
- 1.Kao JH, Chen DS. 2002. Global control of hepatitis B virus infection. Lancet Infect Dis 2:395–403. doi: 10.1016/S1473-3099(02)00315-8. [DOI] [PubMed] [Google Scholar]
- 2.Hadziyannis SJ, Vassilopoulos D. 2001. Immunopathogenesis of hepatitis B e antigen negative chronic hepatitis B infection. Antiviral Res 52:91–98. doi: 10.1016/S0166-3542(01)00173-5. [DOI] [PubMed] [Google Scholar]
- 3.Chen RY, Edwards R, Shaw T, Colledge D, Delaney WE IV, Isom H, Bowden S, Desmond P, Locarnini SA. 2003. Effect of the G1896A precore mutation on drug sensitivity and replication yield of lamivudine-resistant HBV in vitro. Hepatology 37:27–35. doi: 10.1053/jhep.2003.50012. [DOI] [PubMed] [Google Scholar]
- 4.Milich DR, Jones JE, Hughes JL, Price J, Raney AK, McLachlan A. 1990. Is a function of the secreted hepatitis B e antigen to induce immunologic tolerance in utero? Proc Natl Acad Sci U S A 87:6599–6603. doi: 10.1073/pnas.87.17.6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen M, Sallberg M, Hughes J, Jones J, Guidotti LG, Chisari FV, Billaud JN, Milich DR. 2005. Immune tolerance split between hepatitis B virus precore and core proteins. J Virol 79:3016–3027. doi: 10.1128/JVI.79.5.3016-3027.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Visvanathan K, Skinner NA, Thompson AJ, Riordan SM, Sozzi V, Edwards R, Rodgers S, Kurtovic J, Chang J, Lewin S, Desmond P, Locarnini S. 2007. Regulation of Toll-like receptor-2 expression in chronic hepatitis B by the precore protein. Hepatology 45:102–110. doi: 10.1002/hep.21482. [DOI] [PubMed] [Google Scholar]
- 7.Durantel D, Zoulim F. 2009. Innate response to hepatitis B virus infection: observations challenging the concept of a stealth virus. Hepatology 50:1692–1695. doi: 10.1002/hep.23361. [DOI] [PubMed] [Google Scholar]
- 8.Chang JJ, Lewin SR. 2007. Immunopathogenesis of hepatitis B virus infection. Immunol Cell Biol 85:16–23. doi: 10.1038/sj.icb.7100009. [DOI] [PubMed] [Google Scholar]
- 9.Garcia PD, Ou JH, Rutter WJ, Walter P. 1988. Targeting of the hepatitis B virus precore protein to the endoplasmic reticulum membrane: after signal peptide cleavage translocation can be aborted and the product released into the cytoplasm. J Cell Physiol 106:1093–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Messageot F, Salhi S, Eon P, Rossignol JM. 2003. Proteolytic processing of the hepatitis B virus e antigen precursor. Cleavage at two furin consensus sequences. J Biol Chem 278:891–895. doi: 10.1074/jbc.M207634200. [DOI] [PubMed] [Google Scholar]
- 11.Ito K, Kim KH, Lok AS, Tong S. 2009. Characterization of genotype-specific carboxyl-terminal cleavage sites of hepatitis B virus e antigen precursor and identification of furin as the candidate enzyme. J Virol 83:3507–3517. doi: 10.1128/JVI.02348-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takahashi K, Machida A, Funatsu G, Nomura M, Usuda S, Aoyagi S, Tachibana K, Miyamoto H, Imai M, Nakamura T, Miyakawa Y, Mayumi M. 1983. Immunochemical structure of hepatitis B e antigen in the serum. J Immunol 130:2903–2907. [PubMed] [Google Scholar]
- 13.Lang T, Lo C, Skinner N, Locarnini S, Visvanathan K, Mansell A. 2011. The hepatitis B e antigen (HBeAg) targets and suppresses activation of the toll-like receptor signaling pathway. J Hepatol 55:762–769. doi: 10.1016/j.jhep.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 14.Mondelli M, Tedder RS, Ferns B, Pontisso P, Realdi G, Alberti A. 1986. Differential distribution of hepatitis B core and E antigens in hepatocytes: analysis by monoclonal antibodies. Hepatology 6:199–204. doi: 10.1002/hep.1840060208. [DOI] [PubMed] [Google Scholar]
- 15.Guidotti LG, Matzke B, Pasquinelli C, Shoenberger JM, Rogler CE, Chisari FV. 1996. The hepatitis B virus (HBV) precore protein inhibits HBV replication in transgenic mice. J Virol 70:7056–7061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowie A, O'Neill LA. 2000. The interleukin-1 receptor/Toll-like receptor superfamily: signal generators for pro-inflammatory interleukins and microbial products. J Leukoc Biol 67:508–514. doi: 10.1002/jlb.67.4.508. [DOI] [PubMed] [Google Scholar]
- 17.Karin M, Lin A. 2002. NF-kappaB at the crossroads of life and death. Nat Immunol 3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 18.Bose S, Banerjee AK. 2003. Innate immune response against nonsegmented negative strand RNA viruses. J Interferon Cytokine Res 23:401–412. doi: 10.1089/107999003322277810. [DOI] [PubMed] [Google Scholar]
- 19.Rothwarf DM, Zandi E, Natoli G, Karin M. 1998. IKK-gamma is an essential regulatory subunit of the IkappaB kinase complex. Nature 395:297–300. doi: 10.1038/26261. [DOI] [PubMed] [Google Scholar]
- 20.Bhoj VG, Chen ZJ. 2009. Ubiquitylation in innate and adaptive immunity. Nature 458:430–437. doi: 10.1038/nature07959. [DOI] [PubMed] [Google Scholar]
- 21.Yang CY, Kuo TH, Ting LP. 2006. Human hepatitis B viral e antigen interacts with cellular interleukin-1 receptor accessory protein and triggers interleukin-1 response. J Biol Chem 281:34525–34536. doi: 10.1074/jbc.M510981200. [DOI] [PubMed] [Google Scholar]
- 22.Wu J, Meng Z, Jiang M, Pei R, Trippler M, Broering R, Bucchi A, Sowa JP, Dittmer U, Yang D, Roggendorf M, Gerken G, Lu M, Schlaak JF. 2009. Hepatitis B virus suppresses toll-like receptor-mediated innate immune responses in murine parenchymal and nonparenchymal liver cells. Hepatology 49:1132–1140. doi: 10.1002/hep.22751. [DOI] [PubMed] [Google Scholar]
- 23.Wilson R, Warner N, Ryan K, Selleck L, Colledge D, Rodgers S, Li K, Revill P, Locarnini S. 2011. The hepatitis B e antigen suppresses IL-1beta-mediated NF-kappaB activation in hepatocytes. J Viral Hepat 18:e499–e507. doi: 10.1111/j.1365-2893.2011.01484.x. [DOI] [PubMed] [Google Scholar]
- 24.Jegaskanda S, Ahn SH, Skinner N, Thompson AJ, Ngyuen T, Holmes J, De Rose R, Navis M, Winnall WR, Kramski M, Bernardi G, Bayliss J, Colledge D, Sozzi V, Visvanathan K, Locarnini SA, Kent SJ, Revill PA. 2014. Downregulation of interleukin-18-mediated cell signaling and interferon gamma expression by the hepatitis B virus e antigen. J Virol 88:10412–10420. doi: 10.1128/JVI.00111-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burns KA, Martinon F. 2004. Inflammatory diseases: is ubiquitinated NEMO at the hub? Curr Biol 14:R1040–R1042. doi: 10.1016/j.cub.2004.11.040. [DOI] [PubMed] [Google Scholar]
- 26.Arimoto K, Funami K, Saeki Y, Tanaka K, Okawa K, Takeuchi O, Akira S, Murakami Y, Shimotohno K. 2010. Polyubiquitin conjugation to NEMO by triparite motif protein 23 (TRIM23) is critical in antiviral defense. Proc Natl Acad Sci U S A 107:15856–15861. doi: 10.1073/pnas.1004621107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zotti T, Uva A, Ferravante A, Vessichelli M, Scudiero I, Ceccarelli M, Vito P, Stilo R. 2011. TRAF7 protein promotes Lys-29-linked polyubiquitination of IkappaB kinase (IKKgamma)/NF-kappaB essential modulator (NEMO) and p65/RelA protein and represses NF-kappaB activation. J Biol Chem 286:22924–22933. doi: 10.1074/jbc.M110.215426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun L, Deng L, Ea CK, Xia ZP, Chen ZJ. 2004. The TRAF6 ubiquitin ligase and TAK1 kinase mediate IKK activation by BCL10 and MALT1 in T lymphocytes. Mol Cell 14:289–301. doi: 10.1016/S1097-2765(04)00236-9. [DOI] [PubMed] [Google Scholar]
- 29.Liu S, Chen J, Cai X, Wu J, Chen X, Wu YT, Sun L, Chen ZJ. 2013. MAVS recruits multiple ubiquitin E3 ligases to activate antiviral signaling cascades. Elife 2:e00785. doi: 10.7554/eLife.00785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abbott DW, Wilkins A, Asara JM, Cantley LC. 2004. The Crohn’s disease protein, NOD2, requires RIP2 in order to induce ubiquitinylation of a novel site on NEMO. Curr Biol 14:2217–2227. doi: 10.1016/j.cub.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 31.Sebban-Benin H, Pescatore A, Fusco F, Pascuale V, Gautheron J, Yamaoka S, Moncla A, Ursini MV, Courtois G. 2007. Identification of TRAF6-dependent NEMO polyubiquitination sites through analysis of a new NEMO mutation causing incontinentia pigmenti. Hum Mol Genet 16:2805–2815. doi: 10.1093/hmg/ddm237. [DOI] [PubMed] [Google Scholar]
- 32.Windheim M, Stafford M, Peggie M, Cohen P. 2008. Interleukin-1 (IL-1) induces the Lys63-linked polyubiquitination of IL-1 receptor-associated kinase 1 to facilitate NEMO binding and the activation of IkappaBalpha kinase. Mol Cell Biol 28:1783–1791. doi: 10.1128/MCB.02380-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, Huang Y, Qi Y, Peng B, Wang H, Fu L, Song M, Chen P, Gao W, Ren B, Sun Y, Cai T, Feng X, Sui J, Li W. 2012. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife 1:e00049. doi: 10.7554/eLife.00049 (Correction, 3:e05570, 2014, doi:.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hosel M, Quasdorff M, Wiegmann K, Webb D, Zedler U, Broxtermann M, Tedjokusumo R, Esser K, Arzberger S, Kirschning CJ, Langenkamp A, Falk C, Buning H, Rose-John S, Protzer U. 2009. Not interferon, but interleukin-6 controls early gene expression in hepatitis B virus infection. Hepatology 50:1773–1782. doi: 10.1002/hep.23226. [DOI] [PubMed] [Google Scholar]
- 35.Chan HL, Hussain M, Lok AS. 1999. Different hepatitis B virus genotypes are associated with different mutations in the core promoter and precore regions during hepatitis B e antigen seroconversion. Hepatology 29:976–984. doi: 10.1002/hep.510290352. [DOI] [PubMed] [Google Scholar]
- 36.Zhand S, Karami C, Hosseinzadeh Adli A, Tabarraei A, Khodabakhshi B, Moradi A. 2015. Correlation between hepatitis B G1896A precore mutations and HBeAg in chronic HBV patients. Jundishapur J Microbiol 8:e17126. doi: 10.5812/jjm.17126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elsharkawy AM, Mann DA. 2007. Nuclear factor-kappaB and the hepatic inflammation-fibrosis-cancer axis. Hepatology 46:590–597. doi: 10.1002/hep.21802. [DOI] [PubMed] [Google Scholar]
- 38.Su F, Schneider RJ. 1996. Hepatitis B virus HBx protein activates transcription factor NF-kappaB by acting on multiple cytoplasmic inhibitors of rel-related proteins. J Virol 70:4558–4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weil R, Sirma H, Giannini C, Kremsdorf D, Bessia C, Dargemont C, Brechot C, Israel A. 1999. Direct association and nuclear import of the hepatitis B virus X protein with the NF-kappaB inhibitor IkappaBalpha. Mol Cell Biol 19:6345–6354. doi: 10.1128/MCB.19.9.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hildt E, Hofschneider PH. 1998. The PreS2 activators of the hepatitis B virus: activators of tumour promoter pathways. Recent Results Cancer Res 154:315–329. doi: 10.1007/978-3-642-46870-4_23. [DOI] [PubMed] [Google Scholar]
- 41.Sun B, Karin M. 2008. NF-kappaB signaling, liver disease and hepatoprotective agents. Oncogene 27:6228–6244. doi: 10.1038/onc.2008.300. [DOI] [PubMed] [Google Scholar]
- 42.Liu D, Wu A, Cui L, Hao R, Wang Y, He J, Guo D. 2014. Hepatitis B virus polymerase suppresses NF-kappaB signaling by inhibiting the activity of IKKs via interaction with Hsp90beta. PLoS One 9:e91658. doi: 10.1371/journal.pone.0091658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lomaga MA, Yeh WC, Sarosi I, Duncan GS, Furlonger C, Ho A, Morony S, Capparelli C, Van G, Kaufman S, van der Heiden A, Itie A, Wakeham A, Khoo W, Sasaki T, Cao Z, Penninger JM, Paige CJ, Lacey DL, Dunstan CR, Boyle WJ, Goeddel DV, Mak TW. 1999. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev 13:1015–1024. doi: 10.1101/gad.13.8.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deng L, Wang C, Spencer E, Yang L, Braun A, You J, Slaughter C, Pickart C, Chen ZJ. 2000. Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell 103:351–361. doi: 10.1016/S0092-8674(00)00126-4. [DOI] [PubMed] [Google Scholar]
- 45.Liu S, Chen ZJ. 2011. Expanding role of ubiquitination in NF-kappaB signaling. Cell Res 21:6–21. doi: 10.1038/cr.2010.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tada K, Okazaki T, Sakon S, Kobarai T, Kurosawa K, Yamaoka S, Hashimoto H, Mak TW, Yagita H, Okumura K, Yeh WC, Nakano H. 2001. Critical roles of TRAF2 and TRAF5 in tumor necrosis factor-induced NF-kappa B activation and protection from cell death. J Biol Chem 276:36530–36534. doi: 10.1074/jbc.M104837200. [DOI] [PubMed] [Google Scholar]
- 47.Yu X, Lan P, Hou X, Han Q, Lu N, Li T, Jiao C, Zhang J, Zhang C, Tian Z. 2017. HBV inhibits LPS-induced NLRP3 inflammasome activation and IL-1beta production via suppressing the NF-kappaB pathway and ROS production. J Hepatol 66:693–702. doi: 10.1016/j.jhep.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 48.Ni CY, Wu ZH, Florence WC, Parekh VV, Arrate MP, Pierce S, Schweitzer B, Van Kaer L, Joyce S, Miyamoto S, Ballard DW, Oltz EM. 2008. Cutting edge: K63-linked polyubiquitination of NEMO modulates TLR signaling and inflammation in vivo. J Immunol 180:7107–7111. doi: 10.4049/jimmunol.180.11.7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schlicht HJ, Wasenauer G, Kock J. 1993. Molecular basis of the diversity of hepatitis B virus core-gene products. Arch Virol Suppl 8:43–52. [DOI] [PubMed] [Google Scholar]
- 50.Milich DR, McLachlan A, Stahl S, Wingfield P, Thornton GB, Hughes JL, Jones JE. 1988. Comparative immunogenicity of hepatitis B virus core and E antigens. J Immunol 141:3617–3624. [PubMed] [Google Scholar]
- 51.DiMattia MA, Watts NR, Stahl SJ, Grimes JM, Steven AC, Stuart DI, Wingfield PT. 2013. Antigenic switching of hepatitis B virus by alternative dimerization of the capsid protein. Structure 21:133–142. doi: 10.1016/j.str.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brunetto MR, Giarin MM, Oliveri F, Chiaberge E, Baldi M, Alfarano A, Serra A, Saracco G, Verme G, Will H. 1991. Wild-type and e antigen-minus hepatitis B viruses and course of chronic hepatitis. Proc Natl Acad Sci U S A 88:4186–4190. doi: 10.1073/pnas.88.10.4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu D, Cui L, Wang Y, Yang G, He J, Hao R, Fan C, Qu M, Liu Z, Wang M, Chen L, Li H, Guo D. 2016. HBV e antigen and its precursors promote the progress of HCC by interacting with NUMB and decreasing p53 activity. Hepatology 64:390–404. doi: 10.1002/hep.28594. [DOI] [PubMed] [Google Scholar]
- 54.Liver Failure and Artificial Liver Group, Chinese Society of Infectious Diseases, Chinese Medical Association, Severe Liver Diseases and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association. 2006. Diagnostic and treatment guidelines for liver failure. Zhonghua Gan Zang Bing Za Zhi 14:643–646. (In Chinese.) [PubMed] [Google Scholar]