Abstract
Aims
To assess the effect of fractional flow reserve (FFR)-guided percutaneous coronary intervention (PCI) with contemporary drug-eluting stents on the composite of cardiac death or myocardial infarction (MI) vs. medical therapy in patients with stable coronary lesions.
Methods and results
We performed a systematic review and meta-analysis of individual patient data (IPD) of the three available randomized trials of contemporary FFR-guided PCI vs. medical therapy for patients with stable coronary lesions: FAME 2 (NCT01132495), DANAMI-3-PRIMULTI (NCT01960933), and Compare-Acute (NCT01399736). FAME 2 enrolled patients with stable coronary artery disease (CAD), while the other two focused on non-culprit lesions in stabilized patients after acute coronary syndrome. A total of 2400 subjects were recruited from 54 sites world-wide with 1056 randomly assigned to FFR-guided PCI and 1344 to medical therapy. The pre-specified primary outcome was a composite of cardiac death or MI. We included data from extended follow-ups for FAME 2 (up to 5.5 years follow-up) and DANAMI-3-PRIMULTI (up to 4.7 years follow-up). After a median follow-up of 35 months (interquartile range 12–60 months), a reduction in the composite of cardiac death or MI was observed with FFR-guided PCI as compared with medical therapy (hazard ratio 0.72, 95% confidence interval 0.54–0.96; P = 0.02). The difference between groups was driven by MI.
Conclusion
In this IPD meta-analysis of the three available randomized controlled trials to date, FFR-guided PCI resulted in a reduction of the composite of cardiac death or MI compared with medical therapy, which was driven by a decreased risk of MI.
Keywords: Percutaneous coronary intervention, Fractional flow reserve, Prognosis
Introduction
Controversy exists regarding the role of percutaneous coronary intervention (PCI) of stable epicardial coronary lesions to reduce death and myocardial infarction (MI). While American guidelines state that PCI ‘has not been demonstrated to improve survival, […] may increase the short-term risk of MI, […] [and] does not lower the long-term risk of MI’,1 European guidelines admit that they ‘suffer from limitations inherent […] on what is the real benefit from myocardial revascularization’.2 In these discussions, the presence of reversible ischaemia plays a pivotal role. As a result of numerous mechanistic studies, randomized clinical trials, and observational series, fractional flow reserve (FFR) has emerged as the gold standard to guide revascularization.
At least three randomized trials compared FFR-guided PCI vs. medical therapy in haemodynamically stable patients with stable coronary lesions (patients with stable coronary disease or haemodynamically stable patients presenting with acute coronary syndrome (ACS) with clear non-culprit lesions after successful PCI of their culprit lesion).3–5 The primary endpoint of such trials is typically a composite of death, MI, or revascularization. Because of the open label design, patients who did not receive PCI might be more likely to seek medical care, and physicians aware of treatment assignment might be more likely to recommend revascularization in patients without previous PCI, thus introducing a risk of bias for the component of revascularization.6,7 Even though, some trials only included ischaemia-driven4 or urgent revascularizations,3 the inclusion of revascularization in primary composite endpoints continues to be criticized.6,8 The ongoing International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA; ClinicalTrials.gov number, NCT01471522) therefore specified the composite of cardiovascular death or MI as primary endpoint before initiation of the trial but, in view of limited power to detect a clinically relevant difference, subsequently had to fall back to their original intention9 and include resuscitated cardiac arrest and hospitalization for unstable angina or heart failure as additional components of the primary endpoint.10 To resolve a key uncertainty in clinical practice for a frequently performed, invasive and expensive procedure, we did a collaborative individual patient data (IPD) meta-analysis of trials that compared FFR-guided PCI vs. medical therapy in haemodynamically stable patients with stable coronary lesions using cardiac death or MI as pre-specified primary composite endpoint.
Methods
This IPD meta-analysis was performed according to a predefined protocol. We searched MEDLINE, Embase, and the Cochrane Library to identify randomized controlled trials of potential interest without language restriction using the following algorithm: (‘fractional flow reserve’ or FFR) AND (‘percutaneous coronary intervention’ or ‘percutaneous coronary interventions’ or PCI* or stent*) AND (random* or trial* or control*). The search was initially done on 25 March 2017 and last updated on 8 April 2018. We included randomized controlled trials comparing PCI guided by FFR using second-generation drug-eluting stents (DES) vs. medical therapy for patients with stable coronary stenoses. Patients with stable coronary stenoses were defined as patients with stable coronary disease or haemodynamically stable patients presenting with ACS with clear non-culprit lesions after successful PCI of their culprit lesion.
Fractional flow reserve-guided PCI was defined as the performance of PCI based on a positive FFR measurement. We excluded trials where PCI was performed without the use of second-generation DES as well as trials on haemodynamically unstable patients. We checked reference lists of relevant studies and contacted experts in the field to identify additional trials. Two independent reviewers (F.M.Z. and N.P.J.) identified eligible trials and reached consensus in case of discrepancies. After identification of eligible trials, we invited the trials’ principal investigators to contribute to the collaborative analysis, reviewed protocols and publications of each trial, and specified the data requirements in agreement with the principal investigators, including most up-to-date follow-up data. Data were checked for missing values and consistency and queries were resolved through consultation with trialists.
Three independent reviewers (F.M.Z., N.P.J., and P.J.) assessed trials using the Cochrane Collaboration’s tool for assessing risk of bias in randomized trials,11 discrepancies were resolved by consensus (see Supplementary material online, Appendix). The design of the trials was reported previously.3–5
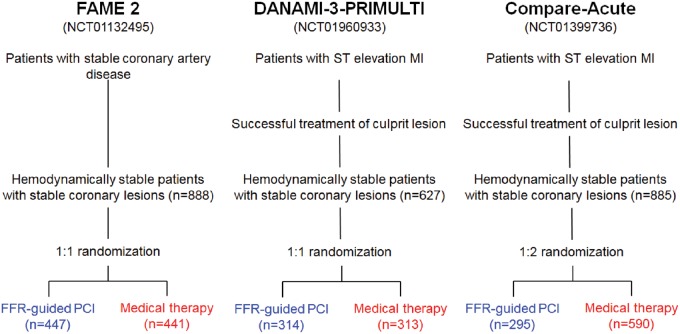
All trials randomized haemodynamically stable patients with stable coronary lesions to FFR-guided PCI or medical therapy (see Figure 1). FAME 2 randomized patients with stable CAD and at least one FFR-positive lesion with an FFR ≤0.80 in 1:1 ratio to either FFR-guided PCI or medical therapy; patients in whom all angiographically significant stenoses were FFR negative did not undergo randomization, received medical therapy and were included in a registry. In DANAMI-3-PRIMULTI and Compare-Acute, haemodynamically stabilized subjects initially admitted with an acute ST-segment elevation myocardial infarction (STEMI) and angiographically significant coronary non-culprit lesions were randomized after successful PCI of the culprit lesion to FFR-guided PCI or medical therapy of non-culprit lesions. Randomization ratios were 1:1 in DANAMI-3-PRIMULTI and 1:2 in Compare-Acute. Ethics committees at each participating institution approved the trial protocols and all subjects signed informed consent before randomization. This report was written in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations.12
Figure 1.
Design of trials included in individual patient data meta-analysis.
Outcomes and definitions
The pre-specified primary outcome for this meta-analysis of IPD was the composite of cardiac death or MI. Secondary endpoints include the composite of all-cause death or MI and individual components of these composites, MI, cardiac death, and all-cause death. Independent clinical events committees adjudicated endpoints in each trial using pre-specified definitions. For the definition of peri-procedural MI, FAME 2 required CK-MB 10-fold above the 99th percentile upper reference limit (URL) or five-fold above URL with clinical evidence of MI; DANAMI-3-PRIMULTI required troponin five-fold above URL with clinical evidence of MI; and Compare-Acute required CK-MB three-fold above URL. For chronic total or subtotal occlusions, a default FFR value of 0.50 was assumed in all trials, consistent with prior studies.3,13
Statistical analysis
Baseline categorical variables are reported as counts and percentages and continuous variables as means and standard deviations, and compared using appropriate regression models stratified by trial. The primary analysis was performed according to the intention-to-treat principle including all randomized patients in the group they were allocated to. Individual patient data were combined in a single data set and analysed using a mixed-effects Cox regression model with baseline hazards stratified by trial and a random intercept to account for variation between trials in baseline risk, and a random slope to account for variation between trials in treatment effect. Treatment effects are presented as hazard ratios (HRs) and 95% confidence intervals (CIs). Heterogeneity of the treatment effect between trials was quantified using the variance of the random slope τ2. Proportional-hazards assumptions were tested after stratification by trial using Schoenfeld residuals. We performed sensitivity analyses of the primary outcome using a mixed-effects Poisson regression model with robust sandwich estimators of standard errors,14 and a mixed-effects flexible parametric model.15 In addition, we used a competing risk model to simultaneously analyse the two components of the primary outcome, and used a conventional two-stage fixed-effect meta-analysis to combine trial-level HRs. Then, we plotted Kaplan–Meier time-to-first-event curves, superimposing estimates of the cumulative incidence per group predicted from the mixed-effects flexible parametric survival model, and used the cumulative incidences and their 95% CIs at 5 years follow-up, as predicted from the mixed-effects flexible parametric survival model, to derive numbers-needed-to-treat, analogous to calculations done for individual trials.16 The use of a mixed-effects flexible parametric survival model to predict cumulative incidences and derive numbers-needed-to-treat avoided Simpson’s paradox17 due to the 1:2 randomization in Compare-Acute. All analyses were based on the same data structure, using time-to-first-event analyses throughout.
Pre-specified subgroup analyses of the primary outcome were performed according to clinical presentation (stable CAD vs. ACS) and FFR status (patients with at least one stable coronary lesion with a positive FFR of ≤0.80 vs. patients with only lesions with a negative FFR of >0.80). In DANAMI-3-PRIMULTI, FFR was only measured in the experimental arm, therefore, the trial had to be excluded from the subgroup analysis according to FFR status. Subgroup analyses of the primary endpoint specified post hoc were performed according to age (>60 years or ≤60 years), sex, diabetes status, previous MI, and smoking. We separated within-trial and across-trial interactions and based tests for subgroup-by-treatment interactions on within-trial interactions,18 except for the subgroup analysis by clinical presentation, which was by design based on an across-trial interaction. Landmark analyses of the primary outcome were performed according to a pre-specified landmark point at 7 days,3 with HRs calculated separately for events that occurred up to 7 days after randomization and events that occurred between 8 days and the end of follow-up. Landmark analyses were accompanied by a test for interaction between treatment and time (first 7 days vs. subsequent period). The power of our meta-analysis to detect a 30% relative risk reduction in the primary composite outcome was calculated as described by Turner et al.19 Between-trial heterogeneity was considered to be low if the between-trial variance τ2 was 0.04 or less.20 Analyses were conducted using R version 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria) and Stata Release 14.2 (Stata Corp, College Station, TX, USA).
Results
We identified 1286 reports, of which 345 were duplicates and removed. After reviewing the remaining 941 unique reports, we found 16 potentially eligible randomized controlled trials (see Supplementary material online, Figure S1 in Appendix), of these five trials were excluded as a wrong comparator was used,13,21–24 seven trials were excluded as PCI was not based on a positive FFR measurement,25–31 and one trial because PCI did not include the use of second-generation DES.32 Three trials met our inclusion criteria: the Fractional Flow Reserve vs. Angiography for Multivessel Evaluation 2 (FAME 2) trial (NCT01132495),3 the Third DANish Study of Optimal Acute Treatment of Patients With STEMI: PRImary PCI in MULTIvessel Disease (DANAMI-3-PRIMULTI, NCT01960933),4 and the Comparison Between FFR-Guided Revascularization vs. Conventional Strategy in Acute STEMI Patients With MVD (Compare-Acute) trial (NCT01399736).5 The principal investigators of all three eligible trials agreed to provide IPD.
All three trials had adequate generation of allocation sequences and concealment of allocation using central randomization in two3,4 and sealed opaque sequentially number envelopes in one trial.5 All trials used independent, blinded event adjudication and analysed all randomized patients in the groups they were originally allocated to according to the intention-to-treat principle. By design, blinding of patients and care provider was not possible in any of the trials. As this is unlikely to result in relevant bias for the types of outcomes analysed,33 all trials were classified as having low risk of bias.
A total of 2400 subjects from 54 sites were included in Europe, North America, and Asia, of whom 1056 were randomly assigned to FFR-guided PCI and 1344 to medical therapy. Baseline characteristics are summarized in Supplementary material online, Table S1, discharge medication in Supplementary material online, Table S2. Patients in the two groups were well balanced with regard to most of the baseline demographic, medical history, and discharge medication. Over three quarters of patients were male in both groups. Approximately 19% of patients had diabetes, and about 19% a history of MI. Due to the 1:2 randomization in Compare-Acute, the crude percentages of patients with stable CAD were 42% vs. 33%; after stratification by trial, the difference between groups was 0.0% (95% CI −0.7 to 0.7%, P > 0.99). As expected, more subjects were discharged on dual antiplatelet therapy in the FFR-guided PCI arm than the medical therapy arm (99% vs. 82%, P < 0.001). The percentage of randomized patients with FFR-negative lesions only was 0% in FAME-2 and 49% in Compare-Acute. In DANAMI-3-PRIMULTI, FFR was only measured in the experimental arm, and 31% of randomized patients in this arm had FFR-negative lesions only. Baseline characteristics according to clinical presentation (stable CAD vs. ACS) are presented in Supplementary material online, Table S3.
Primary and secondary endpoints
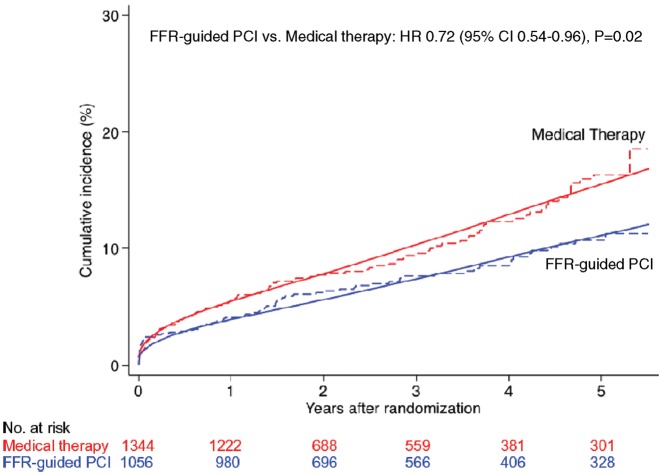
Table 1 presents the cumulative incidence estimates at 5 years of follow-up and HRs of primary and secondary endpoints. The corresponding numbers of events and accumulated observation times are presented in Supplementary material online, Table S4. Figure 2 shows the crude and fitted cumulative incidence curves for the primary composite endpoint of cardiac death or MI. After a median follow-up of 35 months (interquartile range 12–60 months), a 28% relative reduction was observed with FFR-guided PCI as compared with medical therapy (HR 0.72, 95% CI 0.54–0.96; P = 0.02). The estimated cumulative incidence at 5 years was 10.7% (95% CI 8.4–13.6%) for FFR-guided PCI group and 16.4% (95% CI 13.3–20.1%) for medical therapy, which resulted in an estimated number-needed-to-treat to prevent one event up to 5 years of 18 (95% CI 10–72, see Supplementary material online, Table S5 in Appendix). The between-group difference in the primary composite endpoint was driven by a between-group difference in MI (HR 0.70, 95% CI 0.51–0.97; P = 0.03), with an estimated number-needed-to-treat to prevent 1 event up to 5 years of 20 (95% CI 11–87). Conversely, there was little evidence for a difference between groups in cardiac or all-cause deaths. For the secondary composite endpoint of all-cause death or MI, there was 23% relative reduction with FFR-guided PCI (HR 0.76, 95% CI 0.59–0.99; P = 0.04).
Table 1.
Clinical events: primary and secondary endpoints
| Estimated cumulative incidence at 5 years |
Hazard ratio (95% CI) | P-value | ||
|---|---|---|---|---|
| FFR-guided PCI | Medical therapy | |||
| Cardiac death or MIa | 10.7% (8.4–13.6%) | 16.4% (13.3–20.1%) | 0.72 (0.54–0.96) | 0.02 |
| Death or MI | 13.9% (11.2–17.2%) | 19.4% (16.0–23.4%) | 0.76 (0.59–0.99) | 0.04 |
| MI | 8.5% (6.5–11.1%) | 13.4% (10.7–16.8%) | 0.70 (0.51–0.97) | 0.03 |
| Cardiac death | 3.2% (2.1–5.1%) | 3.0% (1.9–4.8%) | 1.04 (0.58–1.78) | 0.89 |
| All-cause mortality | 7.0% (5.2–9.6%) | 6.5% (4.7–8.9%) | 1.03 (0.69–1.54) | 0.89 |
Pre-specified primary outcome. FFR-guided PCI (N = 1056) and medical therapy (N = 1344).
CI, confidence interval; FFR, fractional flow reserve; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Figure 2.
Primary composite endpoint of cardiac death or myocardial infarction. The cumulative incidence of the primary endpoint of cardiac death or myocardial infarction was significantly reduced in subjects randomized to fractional flow reserve-guided percutaneous coronary intervention compared with medical therapy alone. Dashed lines are crude time-to-event curves and solid lines are fitted cumulative incidence curves as predicted from a mixed effects flexible parametric model. Only the fitted curves should be used for inferences about the treatment effect.
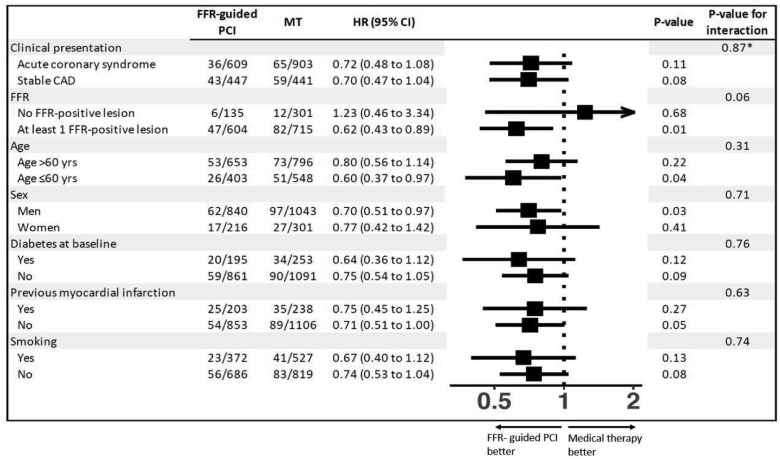
Figure 3 presents subgroup analyses of the primary endpoint. We found little evidence for HRs differing across type of initial presentation (P for interaction = 0.87), but a statistical trend towards an interaction between treatment and FFR (P for interaction = 0.06), with a particularly pronounced benefit of FFR-guided PCI in patients who had at least one lesion with an FFR of 0.80 or less (HR 0.62, 95% CI 0.43–0.89; P = 0.01).
Figure 3.
Subgroup analyses of primary composite endpoint of cardiac death or myocardial infarction. Pre-specified subgroup analyses of the primary outcome are shown according to clinical presentation (stable coronary artery disease vs. acute coronary syndrome) and fractional flow reserve status (patients with at least one stable coronary lesion with a positive fractional flow reserve of ≤0.80 vs. patients with only lesions with a negative fractional flow reserve of >0.80). The Post hoc subgroup analyses of the primary endpoint specified are shown according to age (>60 years or ≤60 years), sex, diabetes status, previous myocardial infarction, and smoking. CAD, coronary artery disease; FFR, fractional flow reserve; HR, hazard ratio; MT, medical therapy; PCI, percutaneous coronary intervention. P-values for interaction are for within-trial interaction unless indicated otherwise. *P-value for interaction is for across-trial interaction.
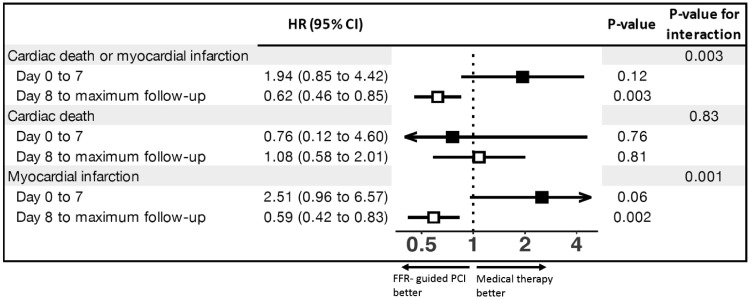
Figure 4 and Supplementary material online, Figure S2 in Appendix present landmark analyses. For the primary composite endpoint of cardiac death or MI we found a strongly positive interaction between treatment and time (P for interaction = 0.003), with a non-significant risk increase with FFR-guided PCI up to 7 days (HR 1.94, 95% CI 0.85–4.42; P = 0.12), but a statistically significant risk reduction with FFR-guided PCI 8 days or more after randomization (HR 0.62, 95% CI 0.46–0.85; P = 0.003). The interaction was entirely driven by MI (P for interaction 0.0015), with a statistical trend towards an increase in events in the FFR-guided PCI arm up to 7 days (HR 2.51 95% CI 0.96–6.57; P = 0.06), but a statistically significant reduction of events 8 days or more after randomization with FFR-guided PCI (HR 0.59, 95% CI 0.42–0.83; P = 0.002). There was no evidence for an interaction between treatment and time for cardiac death (P for interaction = 0.83).
Figure 4.
Landmark analyses of primary composite endpoint and its components. The hazard ratios of the primary composite outcome of cardiac death or myocardial infarction and of components of the primary composite outcome shown according to the time from randomization (7 days or less vs. 8 days or more). The solid boxes represent hazard ratios for 7 days or less after randomization, the open boxes represent hazard ratios for 8 days or more from randomization. Arrows indicate that the ends of the confidence interval are either less than 0.4 or more than 5. FFR, fractional flow reserve; HR, hazard ratio; PCI, percutaneous coronary intervention.
Results of sensitivity analyses were much the same as those of primary analyses (Supplementary material online, Table S6 in Appendix), as were results of competing risk model (Supplementary material online, Table S7 in Appendix) and two-stage meta-analysis (Supplementary material online, Figure S3). Tests of proportional hazards assumption were negative for the primary composite outcome (P = 0.15) and all secondary outcomes (P ≥ 0.09). Heterogeneity in treatment effects between trials was low for all outcomes (τ2 < 0.001). The power of the meta-analysis to detect a 30% relative reduction was 68%. We identified no issues regarding the integrity of the IPD.
Discussion
Our IPD meta-analysis of three randomized trials showed a statistically significant reduction in the pre-specified composite endpoint of cardiac death or MI favouring FFR-guided PCI over medical therapy. Fractional flow reserve-guided PCI also reduced the composite of all-cause death or MI. Differences were driven by a reduction in MI, with little evidence for a reduction in cardiac or all-cause death. The relative reduction of PCI of about 28% corresponds to an estimated 5.7% absolute risk reduction at 5 years and a number-needed-to-treat of 18, which is clinically relevant and in keeping with many other standard treatments.
Two factors are likely to explain negative results for analysed outcomes seen in individual trials. First, each trial by itself was underpowered for a composite of cardiac death or MI. Because all trials were powered for a primary composite endpoint that included various definitions of revascularization as one of its components, it cannot be expected that a statistically significant reduction in cardiac death or MI would be found in any single trial. Each component trial in our analysis had recruited less than 1000 patients and ascertained its primary endpoint between 1 year and 2 years of follow-up, and none of the trials had more than 25% power to detect, for example, a 30% relative risk reduction in the composite of cardiac death or MI. Our analysis included 2400 patients at a median of almost 3 years of follow-up, accordingly, its power to detect a 30% relative risk reduction20 is above 65%. While this is still below the optimum of 80–90%, it is considerably above the average observed in meta-analyses of cardiovascular trials.20 Second, prior randomized trials, such as COURAGE,27 used PCI with bare-metal or early generation DES guided by angiography, which was found inferior to modern PCI with modern generation DES34 and FFR-guidance.14
This meta-analysis included stable lesions from subjects presenting with both stable coronary disease and stabilized acute STEMI. While the clinical presentation differs, both physiologic and statistical arguments plus recent guideline recommendations justify combining these studies. First, in contrast to culprit lesions,35 FFR-values in non-culprit vessels usually do not change much between assessments made during the acute phase of a STEMI and assessments weeks or months later during the stable phase of coronary disease.36–39 Therefore, the vast majority of lesions which were classified as FFR-positive during the acute phase will also be classified as FFR-positive during the subsequent stable phase of coronary disease. Second, STEMI patients were only included in the trials after successful opening of the culprit vessel, when they were haemodynamically stable. Third, the heterogeneity in treatment effects between trials was low and there was no evidence for an interaction between clinical presentation and treatment effect, with near identical relative reductions of the primary endpoint of 30% observed in patients with stable CAD and 28% in patients who initially presented with stabilized ACS. Finally, the recent European guidelines on myocardial revascularization40 state that ‘after PCI of the culprit lesion in [an acute coronary syndrome], the choice of further revascularization modality should follow the criteria applied to patients with [stable coronary artery disease]’.
Lesion selection is critically important when assessing the benefit of FFR-guided PCI over medical therapy. If patients who only have FFR-negative lesions are randomized and included in the analysis—as in DANAMI-3-PRIMULTI4 and Compare-Acute5 based on angiographic inclusion criteria—then a substantial proportion of patients receives the same, typically conservative treatment regardless of randomization and the effect of FFR-guided PCI will be diluted in an intention-to-treat analysis. In our meta-analysis, an estimated 26% of randomized patients had only FFR-negative lesions. Nevertheless, a statistically significant 28% reduction in the composite of cardiac death or MI was found. In a subgroup analysis by FFR, a more pronounced, 38% reduction was indeed found in patients with at least one FFR-positive lesion. While we acknowledge that the test for interaction between FFR status and treatment effect showed only a statistical trend (P = 0.06) and DANAMI-3-PRIMULTI could not be included in this analysis as FFR status was unknown for the control group of this trial,4 we consider it likely that the 38% relative reduction of the primary endpoint in the FFR positive subgroup of patients is a true reflection of the benefit of modern FFR-guided PCI in patients with haemodynamically significant stable coronary lesions. Of note, the recent ORBITA trial comparing PCI with a sham intervention included 29% of patients with FFR negative lesions only.26 In contrast to the trials included in our analysis, these patients typically received PCI if they were allocated to the experimental arm, even though PCI is unlikely to improve symptoms or prognosis in patients without haemodynamically significant lesions.7,14,28
Several ongoing trials have the potential to corroborate or refute our findings. In the ISCHEMIA trial, an invasive strategy will be compared with initial medical therapy in patients with stable CAD and moderate ischaemia (NCT01471522), using a primary composite endpoint of cardiovascular death, MI, resuscitated cardiac arrest, or hospitalization for unstable angina or heart failure. ISCHEMIA recently completed recruitment of almost 5200 subjects. Despite its complex design, it may be possible to isolate a subset of patients with FFR-positive lesions randomized to revascularization or medical therapy. In the FULL REVASC trial, FFR-guided PCI will be compared with initial medical therapy of non-culprit lesions in STEMI patients (NCT02862119). The trial will randomize about 4000 patients and use a primary composite endpoint of all-cause mortality or MI. Notably, it will include both patients with FFR-positive and FFR-negative lesions, which will dilute the benefit of the experimental strategy as seen in Compare-Acute and DANAMI-PRIMULTI.4,5
Our analysis should be interpreted in view of several limitations. First, patients and their physicians were not blinded to the allocated strategy. In addition, there was variation between trials in the disclosure of FFR values to patients and physicians. However, knowledge of the allocated strategy and of FFR values is unlikely to bias estimates of our primary composite outcome of cardiac death or MI in favour of the experimental strategy.12 If anything, knowledge of the treatment strategy and FFR values might have rendered patients in control groups more likely to cross over to an invasive strategy. Therefore, an intention- to-treat analysis is likely to underestimate the potential benefit of FFR-guided PCI as compared with medical therapy with regard to our primary outcome of cardiac death or MI.7 The higher use of dual antiplatelet therapy in the FFR-guided PCI group could provide an alternative or complementary explanation for the observed benefit instead of FFR-guided PCI itself, especially since many subsequent events occur in non-target vessels.39 Nevertheless the curves continue to diverge beyond 1 year follow-up, where the majority of patients in both groups is assumed to receive single antiplatelet therapy.
Although FFR provides a lesion- or vessel-specific diagnostic tool, subsequent clinical events were not adjudicated with this level of specificity. Non-target vessel events (e.g. a subsequent MI in a vessel not interrogated with FFR) have little or no mechanistic link to FFR in the target vessel. The distinction between cardiac and non-cardiac death can be complex and subjective. However, all trials had a blind, independent adjudication of events, including death and MI. Finally, we did not distinguish between peri-procedural and spontaneous MI. In addition, information on infarction size or presence of Q waves was not obtained. However, our landmark analysis confirmed an early increase in MI with early FFR-guided PCI within 7 days, followed by a pronounced benefit, with a 41% relative reduction of MI beyond 7 days (P = 0.003), as also seen in FAME 2 alone.3
Conclusion
In this IPD meta-analysis of the three available randomized controlled trials to date, FFR-guided PCI resulted in a reduction of the composite of cardiac death or MI compared with medical therapy, which was driven by a decreased risk of MI.
Funding
This research was completed, in part, with funding from the Canada Research Chairs Programme to P.J.
Conflict of interest: E.O. reports institutional research grant and lecturing fee from AstraZeneca, and is on the advisory board for Bayer, outside the submitted work. N.P.J. reports an institutional licensing agreement with Boston Scientific, Volcano/Philips, and St Jude Medical outside the submitted work. In addition, he has a patent pending on quantification of aortic valve stenosis (SAVI). E.B. reports grants from Abbott Vascular, Boston Scientific, outside the submitted work. W.F.F. reports grants from Abbott Vascular, Medtronic, CathWorks, ACIST Medical, personal fees from Boston Scientific, and minor stock options from HeartFlow, outside the submitted work. L.K. reports grants from Danish Agency for Science, Technology and Innovation and by the Danish Council for Strategic Research supported the Danami-3 study, during the conduct of the study. P.C.S. reports grants from Abbott Vascular, St. Jude Medical, during the conduct of the study; grants and personal fees from Abbott Vascular, personal fees from St. Jude Medical, outside the submitted work. B.D.B. reports grants from Abbott, Boston Scientific, and Biotronik, and institutional consultancy for Opsens, Boston Scientific, Abbott, outside the submitted work. N.H.J.P. reports grants and personal fees from Abbott and Opsens, outside the submitted work. P.J. has received research grants to the institution from Astra Zeneca, Biotronik, Biosensors International, Eli Lilly and The Medicines Company, and serves as unpaid member of the steering group of trials funded by Astra Zeneca, Biotronik, Biosensors, St. Jude Medical and The Medicines Company. T.E. reports personal fees from Abbott, during the conduct of the study and personal fees from Boston Scientific, Bayer AS, and Astra Zeneca, outside the submitted work.
Supplementary Material
Footnotes
See page 187 for the editorial comment on this article (doi: 10.1093/eurheartj/ehy859)
References
- 1. Fihn SD, Blankenship JC, Alexander KP, Bittl JA, Byrne JG, Fletcher BJ, Fonarow GC, Lange RA, Levine GN, Maddox TM, Naidu SS, Ohman EM, Smith PK.. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2014;130:1749–1767. [DOI] [PubMed] [Google Scholar]
- 2. Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C, Ferreira JR, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabaté M, Senior R, Taggart DP, van der Wall EE, Vrints CJ ESC Committee for Practice Guidelines Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Document Reviewers Knuuti J, Valgimigli M, Bueno H, Claeys MJ, Donner-Banzhoff N, Erol C, Frank H, Funck-Brentano C, Gaemperli O, Gonzalez-Juanatey JR, Hamilos M, Hasdai D, Husted S, James SK, Kervinen K, Kolh P, Kristensen SD, Lancellotti P, Maggioni AP, Piepoli MF, Pries AR, Romeo F, Rydén L, Simoons ML, Sirnes PA, Steg PG, Timmis A, Wijns W, Windecker S, Yildirir A, Zamorano JL.. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 3. De Bruyne B, Fearon WF, Pijls NH, Barbato E, Tonino P, Piroth Z, Jagic N, Mobius-Winckler S, Rioufol G, Witt N, Kala P, MacCarthy P, Engström T, Oldroyd K, Mavromatis K, Manoharan G, Verlee P, Frobert O, Curzen N, Johnson JB, Limacher A, Nüesch E, Jüni P.. Fractional flow reserve-guided PCI for stable coronary artery disease. N Engl J Med 2014;371:1208–1217. [DOI] [PubMed] [Google Scholar]
- 4. Engstrøm T, Kelbæk H, Helqvist S, Høfsten DE, Kløvgaard L, Holmvang L, Jørgensen E, Pedersen F, Saunamäki K, Clemmensen P, De Backer O, Ravkilde J, Tilsted HH, Villadsen AB, Aarøe J, Jensen SE, Raungaard B, Køber L.. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3—PRIMULTI): an open-label, randomized controlled trial. Lancet 2015;386:665–671. [DOI] [PubMed] [Google Scholar]
- 5. Smits PC, Abdel-Wahab M, Neumann FJ, Boxma-de Klerk BM, Lunde K, Schotborgh CE, Piroth Z, Horak D, Wlodarczak A, Ong PJ, Hambrecht R, Angerås O, Richardt G, Omerovic E, Compare AI.. Fractional flow reserve-guided multivessel angioplasty in myocardial infarction. N Engl J Med 2017;376:1234–1244. [DOI] [PubMed] [Google Scholar]
- 6. Boden WE. Which is more enduring–FAME or COURAGE? N Engl J Med 2012;367:1059–1061. [DOI] [PubMed] [Google Scholar]
- 7. Xaplanteris P, Fournier S, Pijls NHJ, Fearon WF, Barbato E, Tonino PAL, Engstrøm T, Kääb S, Dambrink J-H, Rioufol G, Toth GG, Piroth Z, Witt N, Fröbert O, Kala P, Linke A, Jagic N, Mates M, Mavromatis K, Samady H, Irimpen A, Oldroyd K, Campo G, Rothenbühler M, Jüni P, De Bruyne B; FAME 2 Investigators. Five-year outcomes with PCI guided by fractional flow reserve. N Eng J Med 2018;379:250–259. [DOI] [PubMed] [Google Scholar]
- 8. Rajkumar CA, Nijjer SS, Cole GD, Al-Lamee R, Francis DP.. Moving the goalposts into unblinded territory: the larger lessons of DEFER and FAME 2 and their implications for shifting end points in ISCHEMIA. Circ Cardiovasc Qual Outcome 2018;11:e004665. [DOI] [PubMed] [Google Scholar]
- 9. Hochman JS, Maron DJ.. Letter by Hochman and Maron Regarding Article, “‘Faith Healing’ and ‘Subtraction Anxiety’ in Unblinded Trials of Procedures: lessons From DEFER and FAME-2 for End Points in the ISCHEMIA Trial”. Circ Cardiovasc Qual Outcomes 2018;11:e004742. [DOI] [PubMed] [Google Scholar]
- 10. Bangalore S, Maron DJ, Reynolds HR, Stone GW, O'Brien SM, Alexander KP, Hochman JS.. ISCHEMIA: establishing the Primary End Point. Circ Cardiovasc Qual Outcomes 2018;11:e004791.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA.. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stewart LA, Clarke M, Rovers M, Riley RD, Simmonds M, Stewart G, Tierne JF; PRISMA-IPD Development Group. Preferred reporting items for systematic review and meta-analyses of individual participant data: the PRISMA-IPD Statement. JAMA 2015;313:1657–1665. [DOI] [PubMed] [Google Scholar]
- 13. Pijls NH, Fearon WF, Tonino PA, Siebert U, Ikeno F, Bornschein B, van't Veer M, Klauss V, Manoharan G, Engstrøm T, Oldroyd KG, Ver Lee PN, MacCarthy PA, De Bruyne B.. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease: 2-year follow-up of the FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) study. J Am Coll Cardiol 2010;56:177–184. [DOI] [PubMed] [Google Scholar]
- 14. Crowther MJ, Riley RD, Staessen JA, Wang J, Gueyffier F, Lambert PC.. Individual patient data meta-analysis of survival data using Poisson regression models. BMC Med Res Methodol 2012;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Crowther MJ, Look MP, Riley RD.. Multilevel mixed effects parametric survival models using adaptive Gauss-Hermite quadrature with application to recurrent events and individual participant data meta-analysis. Stat Med 2014;33:3844–3858. [DOI] [PubMed] [Google Scholar]
- 16. Altman DG, Andersen PK.. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ 1999;319:1492–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hernán MA, Clayton D, Keiding N.. The Simpson's paradox unraveled. Int J Epidemiol 2011;40:780–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hua H, Burke DL, Crowther MJ, Ensor J, Tudur Smith C, Riley RD.. One-stage individual participant data meta-analysis models: estimation of treatment-covariate interactions must avoid ecological bias by separating out within-trial and across-trial information. Stat Med 2017;36:772–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Turner RM, Bird SM, Higgins JP.. The impact of study size on meta-analyses: examination of underpowered studies in Cochrane reviews. PLoS One 2013;8:e59202.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. da Costa BR, Juni P.. Systematic reviews and meta-analyses of randomized trials: principles and pitfalls. Eur Heart J 2014;35:3336–3345. [DOI] [PubMed] [Google Scholar]
- 21. Chen SL, Ye F, Zhang JJ, Xu T, Tian NL, Liu ZZ, Lin S, Shan SJ, Ge Z, You W, Liu YQ, Qian XS, Li F, Yang S, Kwan TW, Xu B, Stone GW.. Randomized comparison of FFR-guided and angiography-guided provisional stenting of true coronary bifurcation lesions: the DKCRUSH-VI trial (Double Kissing Crush Versus Provisional Stenting Technique for Treatment of Coronary Bifurcation Lesions VI). JACC Cardiovasc Interv 2015;8:536–546. [DOI] [PubMed] [Google Scholar]
- 22. Layland J, Oldroyd KG, Curzen N, Sood A, Balachandran K, Das R, Junejo S, Ahmed N, Lee MMY, Shaukat A, O'Donnell A, Nam J, Briggs A, Henderson R, McConnachie A, Berry C, Hannah A, Stewart A, Metcalfe M, Norrie J, Chowdhary S, Clark A, Henderson R, Balachandran K, Berry C, Baird G, O'Donnell A, Sood A, Curzen N, Das R, Ford I, Layland J, Junejo S, Oldroyd K.. Fractional flow reserve vs. angiography in guiding management to optimize outcomes in non-ST-segment elevation myocardial infarction: the British Heart Foundation FAMOUS-NSTEMI randomized trial. Eur Heart J 2015;36:100–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Davies JE, Sen S, Dehbi HM, Al-Lamee R, Petraco R, Nijjer SS, Bhindi R, Lehman SJ, Walters D, Sapontis J, Janssens L, Vrints CJ, Khashaba A, Laine M, Van Belle E, Krackhardt F, Bojara W, Going O, Härle T, Indolfi C, Niccoli G, Ribichini F, Tanaka N, Yokoi H, Takashima H, Kikuta Y, Erglis A, Vinhas H, Canas Silva P, Baptista SB, Alghamdi A, Hellig F, Koo BK, Nam CW, Shin ES, Doh JH, Brugaletta S, Alegria-Barrero E, Meuwissen M, Piek JJ, van Royen N, Sezer M, Di Mario C, Gerber RT, Malik IS, Sharp ASP, Talwar S, Tang K, Samady H, Altman J, Seto AH, Singh J, Jeremias A, Matsuo H, Kharbanda RK, Patel MR, Serruys P, Escaned J.. Use of the instantaneous wave-free ratio or fractional flow reserve in PCI. N Engl J Med 2017;376:1824–1834. [DOI] [PubMed] [Google Scholar]
- 24. Götberg M, Christiansen EH, Gudmundsdottir IJ, Sandhall L, Danielewicz M, Jakobsen L, Olsson SE, Öhagen P, Olsson H, Omerovic E, Calais F, Lindroos P, Maeng M, Tödt T, Venetsanos D, James SK, Kåregren A, Nilsson M, Carlsson J, Hauer D, Jensen J, Karlsson AC, Panayi G, Erlinge D, Fröbert O.. Instantaneous wave-free ratio versus fractional flow reserve to guide PCI. N Engl J Med 2017;376:1813–1823. [DOI] [PubMed] [Google Scholar]
- 25. Al-Lamee R, Thompson D, Dehbi H-M, Sen S, Tang K, Davies J, Keeble T, Mielewczik M, Kaprielian R, Malik IS, Nijjer SS, Petraco R, Cook C, Ahmad Y, Howard J, Baker C, Sharp A, Gerber R, Talwar S, Assomull R, Mayet J, Wensel R, Collier D, Shun-Shin M, Thom SA, Davies JE, Francis DP, Al-Lamee R, Thompson D, Sen S, Tang K, Davies J, Keeble T, Kaprielian R, Malik IS, Nijjer SS, Petraco R, Cook C, Ahmad Y, Howard J, Shun-Shin M, Sethi A, Baker C, Sharp A, Ramrakha P, Gerber R, Talwar S, Assomull R, Foale R, Mayet J, Wensel R, Thom SA, Davies JE, Francis DP, Khamis R, Hadjiloizou N, Khan M, Kooner J, Bellamy M, Mikhail G, Clifford P, O'Kane P, Levy T, Swallow R.. Percutaneous coronary intervention in stable angina (ORBITA): a double-blind, randomised controlled trial. Lancet 2018;391:31–40. [DOI] [PubMed] [Google Scholar]
- 26. Boden WE, O'Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, Chaitman BR, Shaw L, Gosselin G, Nawaz S, Title LM, Gau G, Blaustein AS, Booth DC, Bates ER, Spertus JA, Berman DS, Mancini GB, Weintraub WS.. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007;356:1503–1516. [DOI] [PubMed] [Google Scholar]
- 27. Zimmermann FM, Ferrara A, Johnson NP, van Nunen LX, Escaned J, Albertsson P, Erbel R, Legrand V, Gwon HC, Remkes WS, Stella PR, van Schaardenburgh P, Bech GJ, De Bruyne B, Pijls NH.. Deferral vs. performance of percutaneous coronary intervention of functionally non-significant coronary stenosis: 15-year follow-up of the DEFER trial. Eur Heart J 2015;36:3182–3188. [DOI] [PubMed] [Google Scholar]
- 28. Park SH, Jeon KH, Lee JM, Nam CW, Doh JH, Lee BK, Rha SW, Yoo KD, Jung KT, Cho YS, Lee HY, Youn TJ, Chung WY, Koo BK.. Long-term clinical outcomes of fractional flow reserve-guided versus routine drug-eluting stent implantation in patients with intermediate coronary stenosis: five-year clinical outcomes of DEFER-DES trial. Circ Cardiovasc Interv 2015;8:e002442. [DOI] [PubMed] [Google Scholar]
- 29. Thiele H, Akin I, Sandri M, Fuernau G, de Waha S, Meyer-Saraei R, Nordbeck P, Geisler T, Landmesser U, Skurk C, Fach A, Lapp H, Piek JJ, Noc M, Goslar T, Felix SB, Maier LS, Stepinska J, Oldroyd K, Serpytis P, Montalescot G, Barthelemy O, Huber K, Windecker S, Savonitto S, Torremante P, Vrints C, Schneider S, Desch S, Zeymer U.. PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med 2017;377:2419–2432. [DOI] [PubMed] [Google Scholar]
- 30. Wald DS, Morris JK, Wald NJ, Chase AJ, Edwards RJ, Hughes LO, Berry C, Oldroyd KG.. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med 2013;369:1115–1123. [DOI] [PubMed] [Google Scholar]
- 31. Gershlick AH, Khan JN, Kelly DJ, Greenwood JP, Sasikaran T, Curzen N, Blackman DJ, Dalby M, Fairbrother KL, Banya W, Wang D, Flather M, Hetherington SL, Kelion AD, Talwar S, Gunning M, Hall R, Swanton H, McCann GP.. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol 2015;65:963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dambrink JH, Debrauwere JP, van't Hof AW, Ottervanger JP, Gosselink AT, Hoorntje JC, de Boer MJ, Suryapranata H.. Non-culprit lesions detected during primary PCI: treat invasively or follow the guidelines? EuroIntervention 2010;5:968–975. [PubMed] [Google Scholar]
- 33. Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, Als-Nielsen B, Balk EM, Gluud C, Gluud LL, Ioannidis JP, Schulz KF, Beynon R, Welton NJ, Wood L, Moher D, Deeks JJ, Sterne JA.. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Ann Intern Med 2012;157:429–438. [DOI] [PubMed] [Google Scholar]
- 34. Windecker S, Stortecky S, Stefanini GG, da Costa BR, daCosta BR, Rutjes AW, Di Nisio M, Silletta MG, Siletta MG, Maione A, Alfonso F, Clemmensen PM, Collet J-P, Cremer J, Falk V, Filippatos G, Hamm C, Head S, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann F-J, Richter D, Schauerte P, Sousa Uva M, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A, Kolh P, Jüni P.. Revascularisation versus medical treatment in patients with stable coronary artery disease: network meta-analysis. BMJ 2014;348:g3859.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cuculi F, De Maria GL, Meier P, Dall'Armellina E, de Caterina AR, Channon KM, Prendergast BD, Choudhury RP, Forfar JC, Kharbanda RK, Banning AP.. Impact of microvascular obstruction on the assessment of coronary flow reserve, index of microcirculatory resistance, and fractional flow reserve after ST-segment elevation myocardial infarction. J Am Coll Cardiol 2014;64:1894–1904. [DOI] [PubMed] [Google Scholar]
- 36. Ntalianis A, Sels JW, Davidavicius G, Tanaka N, Muller O, Trana C, Barbato E, Hamilos M, Mangiacapra F, Heyndrickx GR, Wijns W, Pijls NH, De Bruyne B.. Fractional flow reserve for the assessment of nonculprit coronary artery stenoses in patients with acute myocardial infarction. JACC Cardiovasc Interv 2010;3:1274–1281. [DOI] [PubMed] [Google Scholar]
- 37. Musto C, De Felice F, Rigattieri S, Chin D, Marra A, Nazzaro MS, Cifarelli A, Violini R.. Instantaneous wave-free ratio and fractional flow reserve for the assessment of nonculprit lesions during the index procedure in patients with ST-segment elevation myocardial infarction: the WAVE study. Am Heart J 2017;193:63–69. [DOI] [PubMed] [Google Scholar]
- 38. Lee JM, Kim HK, Lim KS, Park JK, Choi KH, Park J, Hwang D, Rhee TM, Yang JH, Shin ES, Nam CW, Doh JH, Hahn JY, Koo BK, Jeong MH.. Influence of local myocardial damage on index of microcirculatory resistance and fractional flow reserve in target and nontarget vascular territories in a porcine microvascular injury model. JACC Cardiovasc Interv 2018;11:717–724. [DOI] [PubMed] [Google Scholar]
- 39. Yeh RW, Kereiakes DJ, Steg PG, Windecker S, Rinaldi MJ, Gershlick AH, Cutlip DE, Cohen DJ, Tanguay JF, Jacobs A, Wiviott SD, Massaro JM, Iancu AC, Mauri L.. Benefits and risks of extended duration dual antiplatelet therapy after PCI in patients with and without acute myocardial infarction. J Am Coll Cardiol 2015;65:2211–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferovi PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO.. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2018; doi: 10.1093/eurheartj/ehy394. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.