Summary
Trichomes are specialised structures that originate from the aerial epidermis of plants, and play key roles in the interaction between the plant and the environment. In this study we investigated the trichome phenotypes of four lines selected from the Solanum lycopersicum × Solanum pennellii introgression line (IL) population for differences in trichome density, and their impact on plant performance under water‐deficit conditions. We performed comparative analyses at morphological and photosynthetic levels of plants grown under well‐watered (WW) and also under water‐deficit (WD) conditions in the field. Under WD conditions, we observed higher trichome density in ILs 11‐3 and 4‐1, and lower stomatal size in IL 4‐1 compared with plants grown under WW conditions. The intrinsic water use efficiency (WUE i) was higher under WD conditions in IL 11‐3, and the plant‐level water use efficiency (WUE b) was also higher in IL 11‐3 and in M82 for WD plants. The ratio of trichomes to stomata (T/S) was positively correlated with WUE i and WUE b, indicating an important role for both trichomes and stomata in drought tolerance in tomato, and offering a promising way to select for improved water use efficiency of major crops.
Keywords: trichomes, stomata, water use efficiency, tomato, introgression lines, drought tolerance
Short abstract
Water availability is currently the biggest threat to agricultural productivity worldwide, and improving the water use efficiency of major crops has become essential. We analysed the epidermal features of near‐isogenic tomato lines and their response to drought, and concluded that the trichome‐to‐stomata ratio plays a major role in determining water use efficiency, thereby offering alternative paths for the agricultural improvement of tomato.
Introduction
The epidermis is the outermost tissue of all plant organs and acts as a first contact point with their surroundings. It plays a key role in all plant–environment interactions, and is essential for the maintenance of physiologically favourable conditions (Glover et al., 2016). In the aerial organs of most terrestrial plants, including Solanum lycopersicum (tomato), the epidermis is patterned with trichomes, which are epidermal outgrowths with diverse roles in the defence against biotic and abiotic stresses. The epidermis also contains stomata, which are epidermal pores that regulate gas exchange and contribute directly to the control of water status. The cuticle that covers the surface of the epidermis is a hydrophobic layer, consisting of cutin and waxes, that prevents uncontrolled water loss (Riederer and Schreiber, 2001). As a result of their function in limiting water losses, specialised structures in the epidermis are promising targets to improve the drought tolerance and water use efficiency (WUE) of major crops (Antunes et al., 2012; Galmes et al., 2013; Franks et al., 2015).
Trichomes in Solanum are multicellular and have been classified into eight different types according to the presence or absence of glandular cells, and the shape and number of cells (Luckwill, 1943; McDowell et al., 2011). Research on trichomes has traditionally focused on understanding the specialised metabolic pathways operating in glandular trichomes (Schilmiller et al., 2008; Kang et al., 2014; Spyropoulou et al., 2014); however, trichomes also play a series of important physiological roles, including tolerance to biotic and abiotic stresses, especially in terms of tolerance to insect attack (Bleeker et al., 2012; Tian et al., 2012) and drought (Hauser, 2014).
A role for trichomes in tolerance and adaptation to water stress has been reported for several species. In Arabidopsis lyrata, trichome production has been linked to improved performance under drought conditions (Sletvold and Ågren, 2012). In tomato, SlMX1‐overexpressing plants with high trichome density showed a higher tolerance to water stress compared with unmodified plants (Ewas et al., 2016). In Citrullus lanatus (watermelon), wild, drought‐tolerant genotypes have increased trichome density compared with domesticated, drought‐sensitive varieties (Mo et al., 2016). In addition, trichome formation is increased in plants grown under water stress, such as Hordeum vulgare (barley; Liu and Liu, 2016), Solanum melongena (aubergine; Fu et al., 2013) and Olea europaea (olive; Boughalleb and Hajlaoui, 2011). Trichomes may limit water loss by transpiration through an increase of the leaf–air boundary layer resistance (Palliotti et al., 1994; Guerfel et al., 2009; Mo et al., 2016). Trichomes also protect leaves from UV‐related photoinhibition (Savé et al., 2000; Galmés et al., 2007a), either by reflection of UV radiation or absorption by pigmented molecules (Holmes and Keiller, 2002), and prevent leaf overheating (Ehleringer and Mooney, 1978). These findings indicate an important role for trichomes in plant–water relations.
Stomata consist of two specialised guard cells, which modulate their turgor to regulate the pore aperture in response to environmental stimuli, such as light intensity or CO2 concentration (Hetherington and Woodward, 2003). The effect of stomatal density and size on drought tolerance has been widely studied for many species (Masle et al., 2005; Wentworth et al., 2006; Lawson and Blatt, 2014). Recent studies have shown a link between low stomatal density and improved performance under water‐deficit conditions (Farber et al., 2016; Zhao et al., 2017). In addition, evidence exists that plants adjust their stomatal density under water‐stress conditions (Galmés et al., 2007b; Hamanishi et al., 2012). Reductions in stomatal density in water‐stressed plants have been reported in Triticum aestivum (wheat; Li et al., 2017) and Spondias tuberosa (the umbu tree; Silva et al., 2009). In contrast, for other species, stomatal density increases under drought conditions, as in Phaseolus vulgaris (the common bean; Gan et al., 2010) and Leymus chinensis (Xu and Zhou, 2008). These contradictory observations suggest that the effect of water deficit on stomatal density differs between species, and it should be investigated on a case‐by‐case basis.
Solanum pennellii is a drought‐tolerant wild tomato species that originates from the Peruvian deserts (Rick, 1973; Kahn et al., 1993; Peralta et al., 2008), with important differences at the physiological, morphological and molecular levels to the cultivated tomato, S. lycopersicum, including increased trichome density (Simmons and Gurr, 2005; Moyle, 2008). The S. lycopersicum × S. pennellii introgression line (IL) population (Eshed and Zamir, 1995; Zamir, 2001) consists of near‐isogenic lines with relatively small fragments of the S. pennellii genome in the genetic background of the cultivated tomato, M82. This population has been used successfully before to characterise various aspects of the response of tomato to water stress (Barrios‐Masias et al., 2014; Rigano et al., 2016). Therefore, the S. lycopersicum × S. pennellii IL population provides an excellent platform to investigate the role of differences in epidermal features on performance under water stress.
In this study, we have investigated the effect of differences in trichome density on the response to water stress using several lines from the IL population. We hypothesised that changes in trichome density, introgressed from drought‐tolerant S. pennellii, would lead to changes in WUE, adding to the current understanding of the relationship between water stress and epidermal features, and building a foundation for improvement of tomato under drought stress. We determined that higher trichome densities result in improved WUE, especially under water‐deficit conditions. We also determined the impact of water stress on the phenotype of newly developed tissues to characterise the epidermal responses to drought stress in tomato.
Results
Phenotypic characterisation of tomato lines under glasshouse and field conditions before drought treatment
After a preliminary visual inspection of the 76 lines of the IL population, we chose three ILs (IL 4‐1, 10‐2 and 11‐3) and the parental line M82, for their distinct trichome phenotypes, for further analysis.
We characterised the epidermal features of the three selected ILs (4‐1, 10‐2 and 11‐3) and the parental line M82 in plants grown under full field capacity conditions both in the glasshouse and in the field before the onset of water‐deficit conditions (Figure 1). Under glasshouse conditions, ILs 4‐1 and 10‐2 showed a low trichome density (TD) phenotype, whereas M82 and IL 11‐3 showed a high‐TD phenotype. Although field‐grown plants displayed substantially higher TD than glasshouse‐grown plants, IL 4‐1 had a lower TD than IL 11‐3 and M82, and IL 11‐3 had a higher TD than ILs 10‐2 and 4‐1 (Figure 1a). We also observed differences among lines in their stomatal densities (SD). Under glasshouse conditions, IL 4‐1 had a higher SD than M82 and IL 10‐2, and M82 had a lower SD than ILs 11‐3 and 4‐1. In field‐grown plants, no significant differences were observed between lines for SD (Figure 1b). Similar to the observations for TD, SD values were generally higher under field conditions. When TD and SD were calculated in terms of area units, we observed identical differences between lines for glasshouse‐grown plants, and similar relative values for field‐grown plants (Figure S1). We calculated the ratio of trichomes to stomata (T/S) as an integrative parameter for epidermal anatomy. We observed a substantially higher T/S in M82 compared with the other lines under glasshouse conditions, whereas in field plants, IL 4‐1 showed a lower T/S than M82 and IL 11‐3 (Figure 1c). Stomatal size (SS) showed no significant differences between lines under glasshouse conditions. Under field conditions, however, IL 10‐2 had a higher SS than the other lines under study. Unlike the higher values observed for TD and SD in field‐grown plants compared with glasshouse plants, the SS values were within a similar range under both growth conditions (Figure 1d).
Figure 1.
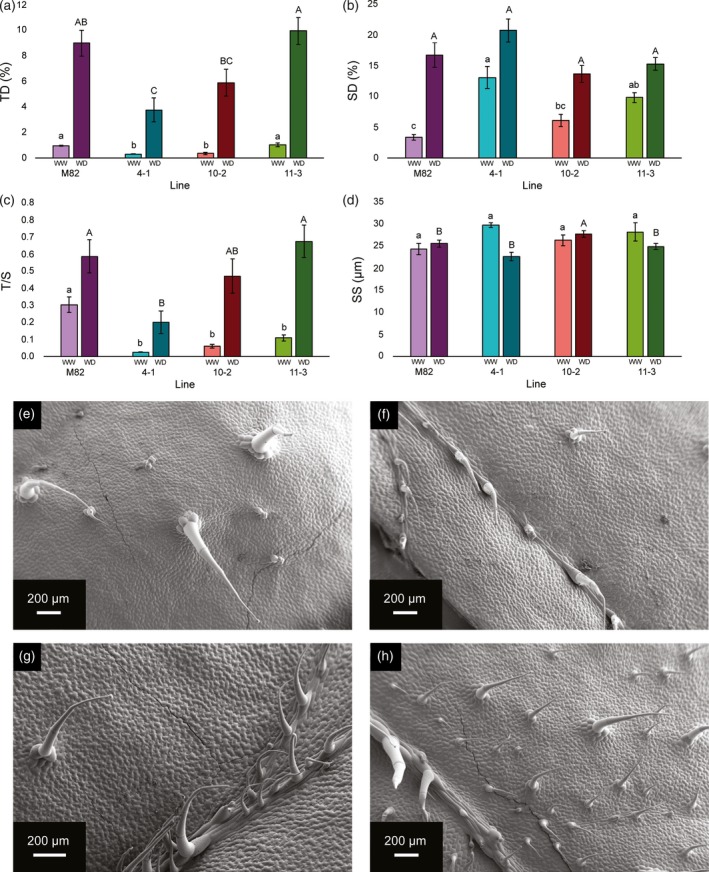
Initial morphological characterization of lines M82, 4‐1, 10‐2 and 11‐3 grown under glasshouse (GH) and field (F) conditions before the onset of drought treatment. (a) Trichome density (TD), (b) stomatal density (SD), (c) trichome‐to‐stomata ratio (T/S) and (d) stomatal size (SS) are expressed as mean ± SE of between three and six replicates per line and treatment. TD and SD were calculated as a percentage of the total number of epidermal cells. SS was calculated as pore length. Different letters denote statistically significant differences by Tukey's analysis (P < 0.05) within glasshouse‐grown plants (lower case) and field‐grown plants (upper case). For panels (a)–(d), purple bars represent M82, turquoise represents 4‐1, red represents 10‐2 and green represents 11‐3, with lighter and darker shades representing GH and F conditions, respectively. Representative scanning electron micrographs for each line: (e) M82, (f) 4‐1, (g) 10‐2 and (h) 11‐3.
We categorised trichomes into different types according to the established classification (Luckwill, 1943; McDowell et al., 2011) and measured the length of type‐I trichomes in glasshouse‐grown plants to determine whether there were differences in other trichome parameters between the ILs (Figure S2). We observed no significant differences in the percentage of different trichome types or trichome length. In general, the analysis of epidermal features and the comparison between glasshouse‐ and field‐grown plants indicated stable genetic components in the determination of trichome density.
Characterisation of photosynthetic parameters under field conditions before drought treatment
We examined whether there were differences in parameters related to gas exchange, carbon fixation and RubisCO kinetics between the lines under study. We observed no significant differences in the net photosynthetic rate (A N), daily carbon fixation rate (C 24h), stomatal conductance (g s), leaf mesophyll conductance (g m), leaf total conductance (g tot), maximum RubisCO carboxylation rate (V cmax) or intrinsic water use efficiency (WUEi) (Table S1), although we observed a trend to lower WUEi values in IL 4‐1 compared with M82 (P < 0.06).
Comparative characterization of epidermal features under well‐watered (WW) and water‐deficit (WD) conditions in the field
In samples collected from lines under WD conditions, IL 4‐1 showed a lower TD than the other lines (Figure 2a). Leaves that developed under WD conditions showed a higher TD in ILs 4‐1 and 11‐3, compared with leaves developed under WW conditions. There were no significant differences for SD between lines under WD conditions (Figure S3). When TD and SD were measured in terms of area unit, we observed a consistently low TD in IL 4‐1 compared with ILs 10‐2 and 11‐3, and there were no differences for SD between lines or treatments (Figure S4). The T/S ratio was significantly lower in IL 4‐1 compared with IL 11‐3 (Figure 2b). Although differences in T/S between WW and WD plants were not significant, there was a trend for higher T/S under WD for IL 11‐3 (P < 0.1). In leaves developed under WD conditions, SS showed no significant differences between lines, but IL 4‐1 had lower SS in leaves developed under WD conditions compared with leaves developed under WW conditions (Figure 2c). For the rest of the lines, no significant differences were observed when comparing WW and WD conditions. We observed no relationship between TD and SD when all data points were considered together, but we observed an inverse association between TD and SD in WD plants (R 2 = 0.94; P = 0.03; Figure 2d).
Figure 2.
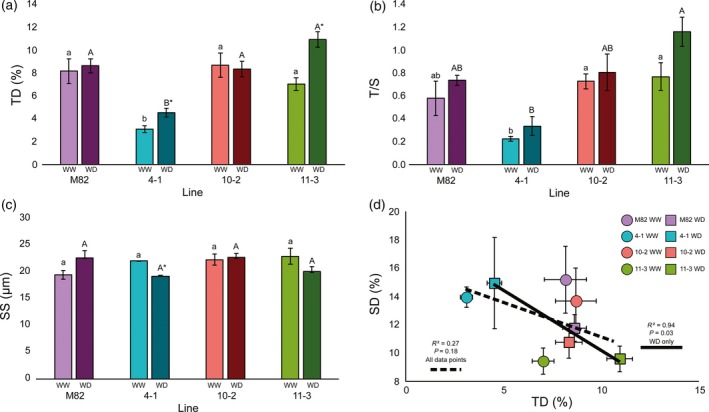
Characterization of epidermal features in lines M82, 4‐1, 10‐2 and 11‐3 under water‐deficit (WD) and well‐watered (WW) conditions in the field. (a) Trichome density (TD) is expressed as a percentage of the number of trichomes per epidermal cell. (b) Trichome‐to‐stomata ratio (T/S) is expressed as the total number of trichomes divided by the total number of stomata in a given area. (c) Stomatal size (SS) is expressed as pore length. Different letters denote statistically significant differences identified by Tukey's analysis (P < 0.05) within WW plants (lower case) and WD plants (upper case). Asterisks represent significant differences for each line between treatments according to the Tukey's test (P < 0.05). (d) A correlation between TD and SD was observed only under WD conditions, according to Pearson's test (P < 0.05), with the correlation index shown in the graph. For panels (a)–(c), purple bars represent M82, turquoise represents 4‐1, red represents 10‐2 and green represents 11‐3, with light and dark colours representing WW and WD treatments, respectively. In panel (d), squares represent WD values and circles represent WW values, and are colour coded as in panels (a)–(c). The dashed line represents the regression line for all data points (P > 0.05) and the solid line represents the regression line for WD data points (P = 0.03). Values are means ± SEs (n = 3).
Comparative analysis of morphological parameters and water status under WW and WD conditions in the field
We evaluated the effects of differences in the densities of trichomes on water status of plants under WW and WD conditions in the field. We observed a lower midday leaf water potential (Ψleaf) in IL 10‐2 compared with M82 and IL 11‐3 under WW conditions (Table 1). Under WD conditions, IL 11‐3 had higher Ψleaf than IL 10‐2. All lines showed a lower Ψleaf under WD conditions except for IL 11‐3, where no difference was observed.
Table 1.
Morphological and water status characterisation of lines M82, 4‐1, 10‐2 and 11‐3 under well‐watered (WW) and water deficit (WD) conditions in the field. Midday leaf water potential (Ψ leaf), leaf mass per area (LMA), leaf thickness (LT) and plant‐level water use efficiency (WUEb) are shown. Values are means ± SE of three to four replicates per line and treatment. Different letters denote statistically significant differences by Tukey analysis (P < 0.05) within each treatment between lines, and asteriks denote statistically significant differences (P < 0.05) between treatments for each line
| Acc. | WW | WD | ||||||
|---|---|---|---|---|---|---|---|---|
| Ψleaf | LMA | LT | WUEb | Ψleaf | LMA | LT | WUEb | |
| MPa | g m−2 | mm | g kg−1 H2O | MPa | g m−2 | mm | g kg−1 H2O | |
| M‐82 | −0.74 ± 0.04a | 79.45 ± 9.56a | 0.70 ± 0.05ab | 0.76 ± 0.12a | −1.01 ± 0.07ab* | 96.01 ± 3.41a | 0.76 ± 0.04ab | 1.14 ± 0.09ab* |
| 4‐1 | −0.86 ± 0.06ab | 58.12 ± 4.33a | 0.63 ± 0.03b | 0.74 ± 0.04a | −1.18 ± 0.05ab* | 63.83 ± 6.69b | 0.66 ± 0.05ab | 0.79 ± 0.02b |
| 10‐2 | −0.99 ± 0.04b | 82.37 ± 6.58a | 0.69 ± 0.01ab | 0.89 ± 0.1a | −1.21 ± 0.07b* | 98.65 ± 5.72a | 0.81 ± 0.02a* | 1.12 ± 0.17ab |
| 11‐3 | −0.74 ± 0.07a | 71.20 ± 9.45a | 0.80 ± 0.03a | 0.85 ± 0.05a | −0.93 ± 0.08a | 64.18 ± 6.35b | 0.63 ± 0.03b* | 1.36 ± 0.06a* |
The leaf mass area (LMA) was lower in ILs 4‐1 and 11‐3 under WD conditions compared with the other lines, but no differences amongst lines under WW conditions or between WW and WD treatments were observed (Table 1). Leaf thickness (LT) was higher in IL 11‐3 compared with IL 4‐1 under WW conditions, but under WD conditions LT in IL 11‐3 was significantly lower than in IL 10‐2 (Table 1).
Whole‐plant water use efficiency (WUEb) showed no differences between lines under WW conditions (Table 1). Under WD conditions, WUEb was lower in IL 4‐1 compared with IL 11‐3. In M82 and IL 11‐3, WUEb was higher in WD plants compared with WW plants.
Comparative analysis of photosynthetic parameters under WW and WD conditions in the field
The parameters A N, C 24h, g s, g m, g tot, V cmax and WUEi showed no significant differences between lines under WW conditions in the field (Table 2). In contrast, under WD conditions, IL 4‐1 had a significantly lower WUEi than the other lines. IL 11‐3 had a significantly higher WUEi in WD compared with WW conditions (Table 2).
Table 2.
Photosynthetic characterization of lines M82, 4‐1, 10‐2 and 11‐3 under well‐watered (WW) and water deficit (WD) conditions in the field. Net photosynthetic rate (A N), daily carbon fixation rate (C 24h), stomatal conductance (g s), mesophyll conductance (g m), total leaf conductannce (g tot), maximum velocity of Rubisco carboxylation (V cmax) and intrinsic water use efficiency (WUEi) are shown. Values are means ± standard error of four replicates per line and treatment. Different letters denote statistically significant differences by Tukey analysis (P < 0.05) within each treatment between lines, and asteriks denote statistically significant differences (P < 0.05) between treatments for each line
| Acc. | A N | C 24h | g s | g m | g tot | V cmax | WUEi |
|---|---|---|---|---|---|---|---|
| μmol CO2 m−2 sec−1 | mol m−2 day−1 | mol H2O m−2 sec−1 | mol CO2 m−2 sec−1 | mol CO2 m−2 sec−1 | μmol CO2 m−2 sec−1 | μmol CO2 mol−1 H2O | |
| WW | |||||||
| M‐82 | 13.22 ± 1.16a | 0.34 ± 0.03a | 0.222 ± 0.046a | 0.087 ± 0.015a | 0.049 ± 0.006a | 304.05 ± 57.19a | 66.22 ± 11.52a |
| 4‐1 | 15.31 ± 1.35a | 0.34 ± 0.08a | 0.272 ± 0.027a | 0.088 ± 0.004a | 0.058 ± 0.003a | 349.38 ± 107.21a | 57.09 ± 4.68a |
| 10‐2 | 16.87 ± 1.26a | 0.42 ± 0.04a | 0.229 ± 0.026a | 0.123 ± 0.015a | 0.063 ± 0.004a | 335.96 ± 49.79a | 75.88 ± 7.33a |
| 11‐3 | 15.82 ± 1.43a | 0.44 ± 0.05a | 0.243 ± 0.026a | 0.102 ± 0.017a | 0.061 ± 0.010a | 336.77 ± 38.46a | 65.60 ± 2.11a |
| WD | |||||||
| M‐82 | 11.52 ± 0.80a | 0.31 ± 0.04a | 0.165 ± 0.021a | 0.084 ± 0.010a | 0.046 ± 0.005a | 271.97 ± 22.89a | 71.44 ± 4.26ab |
| 4‐1 | 12.53 ± 0.80a | 0.30 ± 0.03a | 0.233 ± 0.009a | 0.083 ± 0.016a | 0.052 ± 0.006a | 217.25 ± 26.34a | 53.74 ± 2.76b |
| 10‐2 | 13.20 ± 1.59a | 0.38 ± 0.03a | 0.184 ± 0.014a | 0.101 ± 0.018a | 0.052 ± 0.006a | 253.63 ± 49.58a | 71.60 ± 5.29ab |
| 11‐3 | 15.57 ± 2.38a | 0.39 ± 0.03a | 0.187 ± 0.034a | 0.143 ± 0.044a | 0.061 ± 0.013a | 321.88 ± 52.84a | 84.52 ± 6.92a* |
The leaf carbon isotope composition (δ13C) showed no significant differences between lines within each treatment or between treatments for any line; however, leaf δ13C was correlated with WUEi (R 2 = 0.67, P = 0.01; Figure S5a). We also observed a tight positive correlation (R 2 = 0.66, P = 0.01) between WUEi and WUEb (Figure S5b).
Relationships between epidermal features and photosynthetic parameters
We found an inverse correlation between TD and g s (R 2 = 0.58, P = 0.03; Figure 3a), and we also observed a positive correlation between SD per unit area and g s (R 2 = 0.56, P = 0.03; Figure 3b). Therefore, changes in the density of trichomes and stomata had opposite effects at the gas‐exchange level. Importantly, TD was positively correlated with WUEi (R 2 = 0.88, P = 0.00; Figure 3c). SD showed no correlation with WUEi. As expected from the observed relationship between WUEi and WUEb (Figure S5b), TD was positively correlated with WUEb (R 2 = 0.59, P = 0.03; Figure S6a). Interestingly, SD was negatively correlated with WUEb (R 2 = 0.50, P < 0.05; Figure S6b). The correlation between TD and WUEi and WUEb was maintained when TD was expressed per unit area (Figure S7), but this was not the case for the correlation between SD and WUEb, which was only observed when SD was expressed as a percentage of epidermal cells. Finally, a strong positive association was found between T/S and WUEi (R 2 = 0.86, P = 0.00; Figure 4a) and WUEb (R 2 = 0.72, P = 0.01; Figure 4b).
Figure 3.
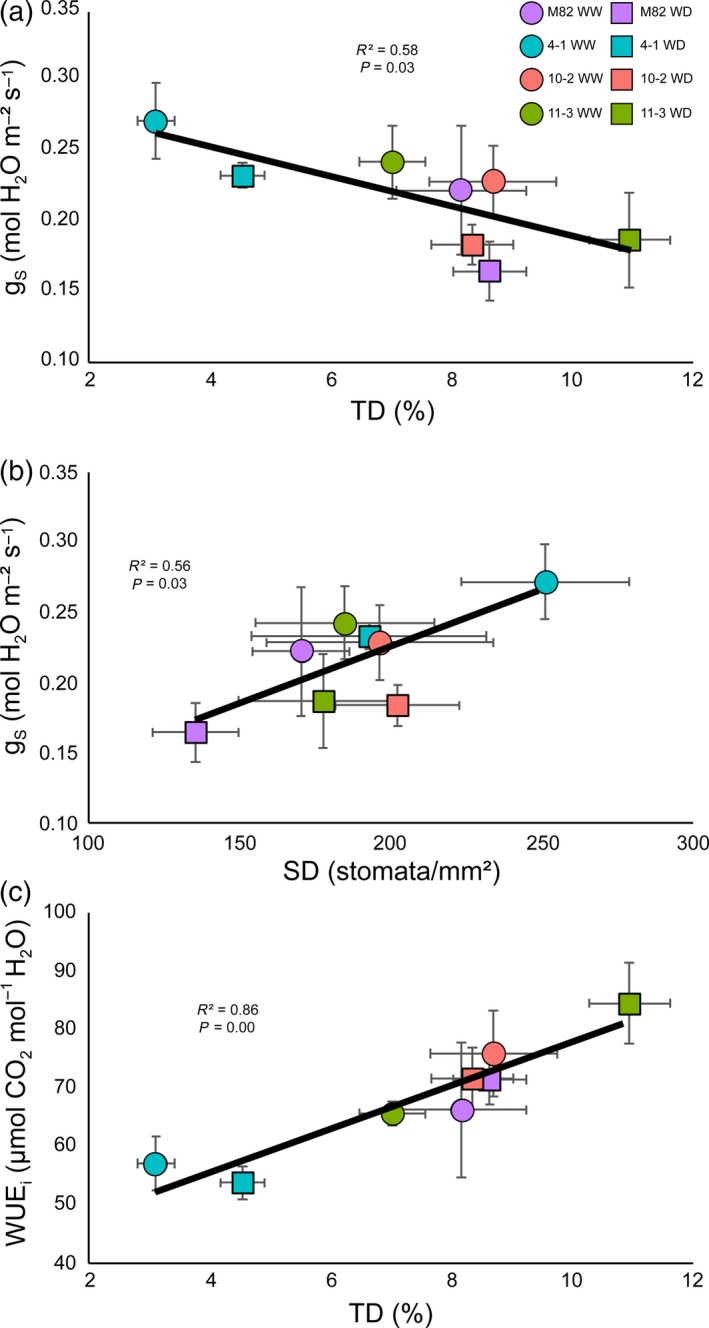
Relationships between trichome and stomatal densities and photosynthetic parameters in lines M82, 4‐1, 10‐2 and 11‐3: (a) inverse correlation between trichome density and stomatal conductance (g s); (b) positive association between stomatal density and stomatal conductance (g s); and (c) positive association between trichome density and intrinsic water use efficiency (WUE i). Correlation coefficients and P values calculated by Pearson's test are displayed in each graph. Purple markers represent M82, turquoise markers represent 4‐1, red markers represent 10‐2 and green markers represent 11‐3. Circles represent values under well‐watered (WW) conditions in the field and squares represent values under water‐deficit (WD) conditions in the field. Values are means ± SEs (n = 3–4).
Figure 4.
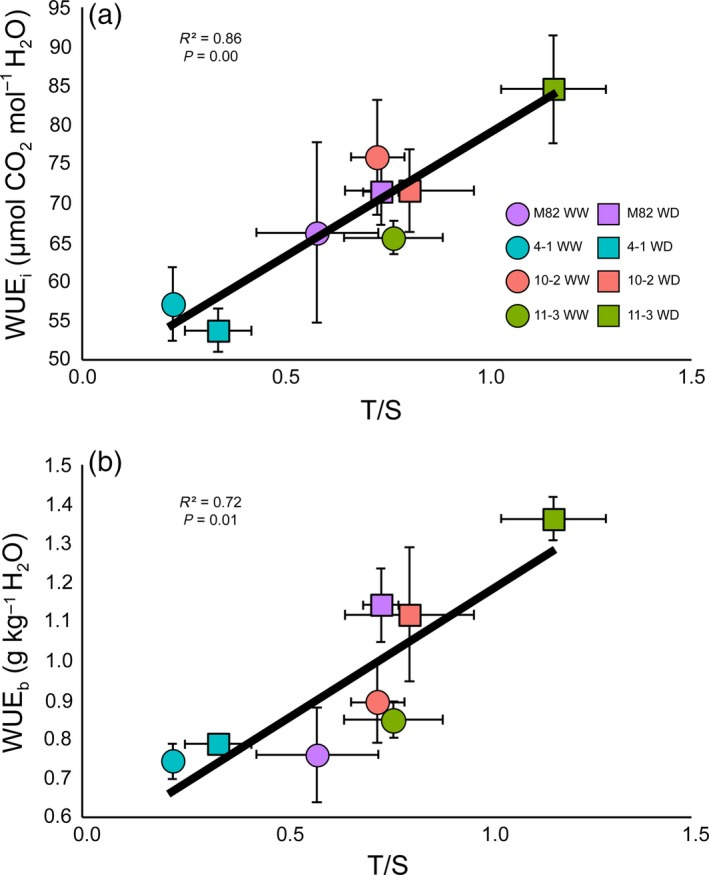
Relationship between trichome/stomata ratios and photosynthetic parameters in lines M82, 4‐1, 10‐2, and 11‐3: (a) correlation between trichome/stomata ratio (T/S) and intrinsic water use efficiency (WUE i); and (b) correlation between T/S and plant‐level water use efficiency (WUE b). Correlation coefficients, calculated by Pearson's test (P < 0.001 for a; P < 0.01 for b), are shown in each graph. Purple markers represent M82, turquoise markers represent 4‐1, red markers represent 10‐2 and green markers represent 11‐3. Circles represent values from well‐watered (WW) conditions in the field and squares represent values from water‐deficit (WD) conditions in the field. Values are means ± SEs (n = 3–4).
Discussion
The development of leaf trichomes is influenced by water availability
We observed a 10‐ to 15‐fold higher TD in leaves grown under field conditions compared with glasshouse‐grown plants (Figures 1a and S1). These differences were likely to have resulted from differences in the age of the plant as well as changes in environmental conditions between the glasshouse and the field. Trichome development is reported to change with the age of the plant (Telfer et al., 1997; Vendemiatti et al., 2017), with higher TD observed in the late leaves of tomato (Gurr and McGrath, 2001). Moreover, environmental factors, such as temperature, photoperiod, light intensity or soil humidity have direct effects on trichome development in several species, including tomato (Wellso and Hoxie, 1982; Gianfagna et al., 1992; Chien and Sussex, 1996; Souza et al., 2016). Despite the dramatic change between environmental conditions in the glasshouse and the field, the relative differences in TD were conserved between lines (Figure 1a), indicating strong genetic control of trichome development in the selected ILs. We observed a 1.5‐ to 5.0‐fold higher SD in field‐grown plants (Figure 1b). These differences could also be a function of the age of the plant when leaves were sampled (Ceulemans et al., 1995) as well as the environmental conditions (Beerling and Chaloner, 1993; Rogiers et al., 2011). Unlike the observation for TD, relative differences in SD between lines were not conserved in different environments, pointing to the differential regulation of TD and SD.
We assessed the changes in leaf anatomy in WD plants compared with WW plants. We observed a higher TD in ILs 4‐1 and 11‐3 under WD conditions compared with WW conditions (Figure 2a). Increases in TD with herbivore and water stress have been reported previously in several species (Traw and Bergelson, 2003; Bjorkman et al., 2008; Fu et al., 2013), as part of the adaptive stress response. In fact, transcriptomic studies in water‐stressed Arabidopsis thaliana plants showed an upregulation of genes related to trichome initiation and morphogenesis (TT8, BRICK1, KAK), but not of genes involved in stomatal initiation (Bechtold et al., 2016). Not all the lines in this study showed uniform responses, with IL 4‐1 showing bigger changes upon WD treatment (lower SS, higher TD) (Figure 2). This could be explained by a greater inability of IL 4‐1 to control water loss, as indicated by its lower WUEb and WUEi (Tables 1 and 2), leading to more severe physiological stress in this line and, subsequently, a stronger response to WD. In any case, these leaf adaptive changes did not account for an increase in WUE (Tables 1 and 2), probably because of a lower overall TD in IL 4‐1.
We observed an inverse association between trichome density (TD) and stomatal density (SD) only under WD conditions (Figure 2d), in agreement with previous reports in Nicotiana tabacum (tobacco) and tomato (Glover et al., 1998; Glover, 2000). Developmentally, trichomes and stomata originate from protodermal cells (Morohashi and Grotewold, 2009; Pillitteri and Dong, 2013), and the inverse association observed suggests that the regulation of their development might be linked. Similar relationships have been found in trichome mutants of A. thaliana (Bean et al., 2002), where altered trichome phenotypes affected stomatal patterning. In aubergine, increases in TD have been associated with increases in SD (Fu et al., 2013), in contrast to our observations, indicating that there may be different developmental associations even between related Solanum species. The correlation was not observed when TD and SD per area were used, suggesting that TD and SD as percentage of epidermal cells might give a better representation of the developmental changes in the epidermis. The fact that TD and SD were not correlated when both WW and WD plants were considered (Figure 2d) might be a result of the lack of genetic differences in SD, as only TD was clearly different between the assessed lines (Figures 1b and S1b). However, the observed TD–SD association suggests that the developmental response of the leaf to drought stress involves changes in the determination of cell fate in the whole epidermal tissue, simultaneously affecting TD and SD, and this might occur through different regulatory mechanisms under different water regimes. In conclusion, we observed an important effect of water availability on leaf anatomy and the determination of epidermal features.
Variation amongst the ILs and the potential for developing drought‐tolerant varieties
The highly inbred tomato cultivar M82 has traditionally been used as a check variety in breeding programmes (Grandillo et al., 1999) and as a reference cultivar for scientific research, used in relation to the response to water stress as a drought‐sensitive cultivar (Iovieno et al., 2016), in contrast with the drought‐tolerant S. pennellii (Egea et al., 2018). The IL population has been used extensively for functional and physiological studies (Steinhauser et al., 2011; Chitwood et al., 2013; de Oliveira Silva et al., 2018), and the natural variation within the IL population provides an excellent platform to investigate the role of differences in epidermal features on performance under water stress.
The intrinsic water use efficiency (WUEi) is an important target for crop improvement with respect to drought tolerance, although it needs to be considered carefully as it might not be directly related to improved fruit productivity (Blum, 2005, 2009). We observed a lower WUEi for IL 4‐1 under WD conditions compared with the other lines under study (Table 2), whereas WUEi in IL 11‐3 was higher under WD compared with WW conditions (Table 2). This increase in WUEi has been reported in drought‐tolerant varieties in several crops (Guha et al., 2010; Fracasso et al., 2016; Liu et al., 2016), although it might not always have a positive effect on fruit yield. Interestingly, none of the ILs under study were considered before for WUE improvement as they showed no differences in δ13C compared with M82 (Xu et al., 2008), in agreement with our results, so this epidermis‐based analysis provides an alternative path for increased WUE. Therefore, the genomic region introgressed from S. pennellii in IL 11‐3 could be selected to generate more water use efficient tomato cultivars.
The accuracy of WUEi as a measure of drought tolerance has been questioned because of the lack of correlation with whole‐plant measurements of WUE (Medrano et al., 2015). In fact, in newer tomato cultivars, a decrease in WUEi, driven by selection under high light levels and well‐watered conditions, has been reported to be accompanied by increases in agronomic WUE (yield per water used; Barrios‐Masias and Jackson, 2014). Therefore, biomass‐based parameters, specifically plant‐level water use efficiency (WUEb), were also investigated in this study, although specific differences in fruit production or harvest index between ILs were not considered (Caruso et al., 2016). WUEb under WD was lower in IL 4‐1 than in IL 11‐3 (Table 1). Moreover, M82 and IL 11‐3 plants grown under WD conditions had higher WUEb values compared with WW plants (Table 1). The observed WUEi–WUEb correlation (Figure S5b) supports the use of WUEi as a measure of drought tolerance under our experimental conditions. In a similar way, leaf δ13C has been used as a marker of the WUE of a plant (Farquhar and Richards, 1984), and in tomato and S. pennellii it has been reported to correlate with WUEb (Martin and Thorstenson, 1988). In this work, we found a correlation between leaf δ13C and WUEi (Figure S5a), suggesting that leaf δ13C can be used as a tool for selection of high‐WUEi lines in tomato. We did not observe a significant correlation between leaf δ13C and WUEb, however, which might hinder the applicability of our findings.
The role of epidermal features during WD
Water‐stress responses involve changes in leaf anatomy, including changes in the leaf thickness affecting CO2 diffusion (Niinemets et al., 2009; Galmes et al., 2013) and epidermal features, where there has been a focus on the stomata because of their direct role in gas exchange and transpiration (Galmés et al., 2007a; Xu and Zhou, 2008). Although we observed differences in LT in ILs 4‐1 and 11‐3 when WW and WD plants were compared (Table 1), no correlation was found between LT and any gas exchange parameter. Importantly, we observed a significant, inverse correlation between g s and SD per area (Figure 3), but not between g s and SD as a percentage of epidermal cells. This result suggests that SD per area should be used preferentially when assessing gas exchange parameters.
Our findings also support a major role for trichomes in gas exchange and the determination of WUE, however. The correlation observed between TD and WUEi and WUEb (Figures 3b, S6a and S7) suggests that TD has a positive effect in terms of drought tolerance. The lack of differences in trichome types or trichome length in the lines under study (Figure S2) indicates that the observed effects are linked to TD rather than to other trichome parameters. Several roles have been suggested for trichomes in plant drought tolerance (Galmés et al., 2007a; Boughalleb and Hajlaoui, 2011). On the basis that TD is negatively associated with g s (Figure 3a), our data suggest that trichomes in tomato might play a role in avoiding excessive water loss by changing the resistance of the boundary layer, as proposed in previous studies (Guerfel et al., 2009; Mo et al., 2016). Another possible explanation for the correlation between TD and WUE involves the mutually exclusive developmental association between trichomes and stomata. Increased trichome initiation as part of the response to drought (a possibility supported by expression analysis in Arabidopsis; Bechtold et al., 2016), could lead to lower SD that could lead to an improved WUE. Genes involved in ABA signalling, known to play a role in the drought response, are expressed in trichomes (Ren et al., 2010; Daszkowska‐Golec, 2016). For example, the tomato homologue of the WRKY transcription factor ABA OVERSENSITIVE 3 (AtABO3) (Ren et al., 2010) or the bZIP transcription factor ABRE BINDING FACTOR 1 (AtABF1) (Yoshida et al., 2015) are both expressed in trichomes in tomato according to the available RNAseq data (Spyropoulou et al., 2014). Changes in TD could lead to changes in the transcript abundance of these or other ABA‐related factors. In fact, the expression level of SlABO3 is slightly higher in leaves of IL 11‐3 compared with leaves of ILs 4‐1 and 10‐2 according to the available RNAseq data (Chitwood et al., 2013), and SlABF1 is located in the genomic region introgressed from S. pennellii in IL 11‐3, indicating a possible role for trichome‐expressed ABA‐related genes in the observed drought response.
Whole‐plant water use efficiency (WUEb) was also correlated with SD (Figure S6b), although the impact of SD on WUE was lower than that of TD, because there was no correlation between WUEi and SD at the leaf level, and when correlation coefficients for TD–WUEb and SD–WUEb were compared, the effect of SD on WUEb was lower than that of TD (Figures 3 and S6). The fact that both stomata and trichomes were involved in the drought response was not surprising, given the developmental link that we observed under WD conditions (Figure 2d) and the direct role of stomata in gas exchange (Figure 3b). In fact, the ratio of trichomes to stomata (T/S), which gives information about the relationship between both structures, showed a strong correlation with both WUEi and WUEb (Figure 4). In addition to this, T/S is unitless and is not expressed either in terms of leaf area or percentage of epidermal cells, thereby overcoming the differences observed in correlations between developmental and photosynthetic traits. It is interesting to note that T/S becomes a more prominent parameter under WD conditions, when both TD and SD change together (Figure 2d), whereas under WW conditions, when there is no correlation between them (Figure 2d) or significant changes between lines (Figure S4), the genotype‐specific TD is likely to be the main player in the relation between epidermis and WUE. In conclusion, T/S plays an important role in the efficiency by which water is used by tomato, and differences in T/S could be used to develop more drought‐tolerant tomato varieties.
Experimental procedures
Preliminary characterization of epidermal cells
From a visual inspection of 76 S. lycopersicum cv. M82 × S. pennellii ac. LA716 ILs (Eshed and Zamir, 1995), grown under glasshouse conditions, we selected four lines (ILs 4‐1, 10‐2 and 11‐3, and M82) displaying a clear visual trichome phenotype. IL 4‐1 (LA4048) had an apparent lower trichome density (TD) than the parental line M82, IL 10‐2 (LA4089) had a hairless‐like phenotype, and IL 11‐3 (LA4092) had an apparently higher TD compared with the parental line M82 (LA3475). We characterised the trichome densities of the adaxial and abaxial sides of leaves from the four lines under study. This analysis indicated similar values for TD on both sides of the leaf in all four lines and a strong correlation (R 2 = 0.92, P = 0.04) between the values on both sides of the leaf (Figure S8), which allowed us to work with values on the adaxial surface in all future investigations.
The epidermal features of these four lines were characterised further using scanning electron microscopy (SEM) of plants grown both under glasshouse and field conditions. For glasshouse assays, three or four plants per line were grown under water field capacity at the John Innes Centre (https://www.jic.ac.uk), using natural light with an average temperature of between 20 and 22°C. The terminal leaflet of the first leaf of 4‐week‐old plants was excised, and inter‐vein sections of approximately 0.5 × 0.5 cm were taken as samples. These sections were vacuum‐fixed in a glutaraldehyde 2.5% cacodylate solution and dehydrated through an ethanol series. Samples were dried in a Leica CPD300 critical‐point dryer (Leica Microsystems, http://www.leica.com), to avoid the collapse of trichomes, and were gold‐coated before imaging in a Zeiss Supra 55 VP SEM (Zeiss, https://www.zeiss.com) at 20°C and under high‐vacuum conditions, generating between eight and 15 micrographs of approximately 0.3 mm2 per sample of the adaxial surface.
The epidermal features of these four lines were characterised further using scanning electron microscopy (SEM) of plants grown both under glasshouse and field conditions. For glasshouse assays, three or four plants per line were grown under water field capacity at the John Innes Centre (https://www.jic.ac.uk), using natural light with an average temperature of between 20 and 22°C. The terminal leaflet of the first leaf of 4‐week‐old plants was excised, and inter‐vein sections of approximately 0.5 × 0.5 cm were taken as samples. These sections were vacuum‐fixed in a glutaraldehyde 2.5% cacodylate solution and dehydrated through an ethanol series. Samples were dried in a Leica CPD300 critical‐point dryer (Leica Microsystems, http://www.leica.com), to avoid the collapse of trichomes, and were gold‐coated before imaging in a Zeiss Supra 55 VP SEM (Zeiss, https://www.zeiss.com) at 20°C and under high‐vacuum conditions, generating between eight and 15 micrographs of approximately 0.3 mm2 per sample of the adaxial surface.
Characterisation under field conditions was carried out in the experimental plot at the University of the Balearic Islands (UIB, http://www.uib.eu). The environmental conditions during plant growth are detailed in the next section. Six plants were sampled for each line under study. Terminal leaflets of fully expanded leaves at the same position were excised and sections of 0.5 × 0.5 cm were taken as samples. The adaxial surface of these sections was imaged at 20°C, without coating, inside a Hitachi 3400N variable pressure SEM (Hitachi High‐Tecnhology, https://www.hitachi-hightech.com). We generated between eight and 10 micrographs of approximately 0.3 mm2 per sample, and trichomes, stomata and pavement cells were counted manually. In both analyses, trichome and stomatal densities were expressed both as a percentage of epidermal cells and as density per area. Trichomes were classified in types and had their length measured. For trichome density, all types of trichome were considered together. The ratio of trichomes to stomata was calculated as trichome density divided by stomatal density.
Field growth conditions and drought treatment
Seeds from the four lines were germinated and grown for 4 weeks in glasshouses at the University of the Balearic Islands (UIB) with natural light and average maximum temperatures of 25°C in March–April 2016. Twelve plants per line were placed outdoors in the UIB experimental field for acclimation for 2 weeks before being transferred to 50‐L pots containing a mixture of bog peat‐based horticultural substrate (Prohumin‐Potting Soil Klasmann‐Deilmann; Projar, https://www.projar.es) and perlite (granulometry A13; Projar) in a 4 : 1 proportion (v/v). The environmental conditions from June to September 2016 were those typical for a Mediterranean summer: with average daily temperatures of 22.8 ± 0.3, 25.7 ± 0.3, 25.0 ± 0.2 and 22.7 ± 0.4°C; average daily minimum temperatures of 16.5 ± 0.5, 20.3 ± 0.3, 19.5 ± 0.4 and 17.8 ± 0.4°C; and average daily maximum temperatures of 31.1 ± 0.4, 35.5 ± 0.4, 33.5 ± 0.3 and 34.7 ± 0.6°C, respectively, for June, July, August and September. Plants were watered to field capacity every other day and fertilised weekly with 50% Hoagland's solution for 2 months before beginning the drought treatment.
From 11 July, plants were subjected to two different water regimes: WW and WD. Watering regimes and plant water consumption were monitored by weighing and watering the potted plants every 2 days. For the WW treatment, four plants per line were maintained at field capacity, with a pot water content ranging between 100% field capacity just after irrigation (corresponding to 9.3 L of water per pot) and 69.3 ± 0.1% field capacity (on average throughout the treatment period) (Figure S9). For the WD treatment, the irrigation of four plants was progressively reduced until halving the pot water content compared with the WW plants. Then, WD plants were maintained at a pot water content ranging between 30.3 ± 0.1% field capacity before irrigation and 46.3 ± 0.1% field capacity after irrigation (Figure S9). The four remaining plants per line were used for biomass‐related measurements. The total water supplied and dry biomass of each plant was recorded upon experiment completion (Table S2). Three weeks were allowed from treatment application for the development of new leaves under the new water regime before any measurement was performed.
All leaf‐based measurements and samples were taken from the terminal leaflets of the youngest fully expanded leaves generated after application of the treatment. Epidermal features (trichome and stomatal densities, stomatal size and ratio of trichomes to stomata) were evaluated as described for glasshouse‐grown plants in the ‘Preliminary characterization of plant material’ section.
Plant water status and growth‐related measurements
Plant water status was measured as midday leaf water potential (Ψleaf) (n = 4 per line and treatment) using a Scholander pressure chamber (Soilmoisture Equipment Corp., https://www.soilmoisture.com), as described by Turner (1988).
For the calculation of WUEb, four plants per line were harvested at the time of drought treatment. Seventy‐four days after treatment application, four plants per line and per treatment (WW and WD) were harvested. Leaves, shoots and roots were oven‐dried separately at 60°C and weighed (dry weight, DW). Biomass production during the drought treatment was calculated as the difference between the DW of the plants harvested at the end of the experiment (DWfinal) and the DW of the plants harvested before the treatment (DWinitial). Water consumption was monitored every other day by weighing the pots containing the plants, and the total water consumption (TWC) of each plant was estimated from these values. WUEb was calculated as follows: WUEb = (DWfinal − DWinitial)/TWC.
Leaf morphological determinations
Leaf thickness (LT) was determined for the middle part of the terminal leaflet of a young fully‐expanded leaf with callipers, avoiding regions of the leaf with major veins. Leaf mass area (LMA) was calculated from the same terminal leaflets, as the dry mass to leaf area ratio. Dry mass was measured by weighing after oven‐drying leaves at 60°C for 48 h. The leaf area was digitally measured from pictures of the leaves using imagej 1.49 (National Institutes of Health, https://imagej.nih.gov/ij/) before drying. Both LT and LMA were measured for one leaf per plant (n = 4 per line and treatment).
Leaf gas exchange and chlorophyll a fluorescence
Measurements were performed with an open infrared gas‐exchange analyser equipped with a leaf chamber fluorometer (Li‐6400‐40; LI‐COR, https://www.licor.com) from 09:00 to 12:00 h and from 16:00 to 19:00 h in the first 2 weeks of August 2016. Preliminary tests confirmed non‐significant differences between morning and afternoon measurements. The conditions in the chamber consisted of leaf temperatures of 31–33°C, a vapour pressure deficit of 2.0–3.0 kPa and an air flow of 500 μmol (air) min−1. For net CO2 assimilation rate–substomatal CO2 concentration (A N–C i) curves, the ambient concentration of CO2 in the chamber (C a) was set at 400, 0, 50, 100, 200, 300, 600, 900, 1500, 2000 and 400 μmol CO2 mol−1 air, at a saturating photosynthetic photon flux density (PPFD) of 1500 μmol m−2 sec−1 (with 10% blue light), allowing 4 min between measurements for the chamber to reach a steady state. Corrections for CO2 leakage in and out of the leaf chamber of the Li‐6400‐40 were applied to all gas‐exchange data, as described by Flexas et al. (2007).
Mesophyll conductance to CO2 (g m) was estimated according to (Harley et al., 1992) as:
where Γ* is the chloroplast CO2 compensation point in the absence of day respiration, ETR is the electron transport rate and R L is the rate of non‐photorespiratory CO2 evolution under light. ETR and R L were calculated as described by Galmes et al. (2011). Γ* was retrieved from in vitro‐based measurements for S. lycopersicum by Hermida‐Carrera et al. (2016), but adjusted for the leaf temperature during the measurement.
Total leaf conductance (g tot) was calculated assuming the stomatal conductance (g s) and mesophyll conductance (g m) were in series, such that: g tot = 1/(1/g s + 1/g m).
A N–C i curves were transformed into A N‐chloroplastic CO2 concentration (C c) curves using estimated values of g m. From A N–C C curves, the maximum velocity of Rubisco carboxylation (V cmax) was calculated as described by (Bernacchi et al., 2002), but using specific values of Rubisco kinetics for S. lycopersicum adjusted to the leaf temperature during the measurement (Hermida‐Carrera et al., 2016). The intrinsic water use efficiency (WUEi) was calculated as the ratio between the net photosynthetic rate (A N) and the stomatal conductance (g s), A N/g s.
We determined the daily carbon fixation rate (C 24h) by measuring the net CO2 exchange rate at 2‐h intervals over 24 h. These measurements were performed after drought treatment (n = 4 per line and per treatment) using an open infrared gas‐exchange analyser equipped with a clear chamber (Li‐6400‐40; LI‐COR). Three measurements were performed under ambient CO2 and light levels, averaged per plant and per time point. The daily fixation rate was calculated as the integral value for the curve generated by the point measurements.
Leaf δ13C isotope composition
The dried leaves used to calculate LMA were ground to fine dust for the determination of carbon isotopic composition. Samples were subjected to combustion in an elemental analyser (Thermo Flash EA 1112 Series; ThermoFisher Scientific, http://www.thermofisher.com) and CO2 was injected into a continuous‐flow isotope ratio mass spectrometer (Thermo‐Finnigan Delta XP; ThermoFisher Scientific). Peach leaf standards (NIST 1547) were run every six samples. The standard deviation of the analysis was <0.2%.
Statistical analysis
The differences between lines, treatments and interactions were assessed by univariate analysis of variance (anova). Significant differences between means were determined by a post‐hoc Tukey's test (P < 0.05). The relationship between variables in each experiment was determined by correlation coefficient (R 2). The analyses were performed using r 3.2.2 (R Core Team, https://www.r-project.org).
Conflict of interest
The authors declare no conflicts of interest.
Supporting information
Figure S1. Initial morphological characterization of lines M82, 4‐1, 10‐2 and 11‐3 grown under glasshouse (GH) and field conditions (F) before the onset of drought treatment.
Figure S2. Percentage of trichome types and trichome length in plants grown under glasshouse conditions.
Figure S3. Stomatal density in lines M82, 4‐1, 10‐2 and 11‐3 under water‐deficit (WD) and well‐watered (WW) conditions in the field.
Figure S4. Trichome and stomatal densities in lines M82, 4‐1, 10‐2 and 11‐3 under water‐deficit (WD) and well‐watered (WW) conditions in the field, expressed in terms of area.
Figure S5. Correlations between carbon isotope composition and intrinsic water use efficiency, and between intrinsic water use efficiency and plant‐level water use efficiency, in lines M82, 4‐1, 10‐2 and 11‐3.
Figure S6. Relationship between epidermal features and plant‐level water use efficiency (WUEb) in plants under well‐watered (WW) and water‐deficit (WD) conditions in the field.
Figure S7. Correlations between trichome density expressed per unit area and water use in lines M82, 4‐1, 10‐2 and 11‐3 under WW and WD conditions.
Figure S8. Trichome densities on abaxial and adaxial sides of leaves of lines M82, 4‐1, 10‐2 and 11‐3 grown under glasshouse conditions.
Figure S9. Evolution of the pot water content during the experiment for the well‐watered (WW, blue) and water‐deficit (WD, red) plants.
Table S1. Leaf morphological traits and photosynthetic characterization of the lines M82, 4‐1, 10‐2 and 11‐3 under field conditions before the onset of the drought treatment.
Table S2. Dry biomass and total water supplied to plants upon completion of the experiment for lines M82, 4‐1, 10‐2 and 11‐3.
Acknowledgements
We thank Kim Findlay, Elaine Barclay and Ferran Hierro for the technical support in SEM image acquisition. We are grateful to Cyril Douthe for LI‐COR technical assistance. This article benefitted from insightful discussions with Prof. James Brown (JIC). We appreciate the financial support of the Rotation Studentship from the John Innes Foundation, the Institute Strategic Program Understanding and Exploiting Plant and Microbial Secondary Metabolism (BB/J004596/1) from the UK Biotechnology and Biological Sciences Research Council (BBSRC) to JIC, as well as the project AGL2013–42364R (Plan Nacional, Spain) awarded to Dr Galmés and the European funded COST ACTION FA1106 QualityFruit. We are grateful to Mr Miquel Truyols and collaborators of the UIB Experimental Field and Glasshouses (UIB Grant 15/2015) for their support of our experiments.
[The copyright line for this article was changed on 21 December 2018 after original online publication].
References
- Antunes, W.C. , Provart, N.J. , Williams, T.C.R. and Loureiro, M.E. (2012) Changes in stomatal function and water use efficiency in potato plants with altered sucrolytic activity. Plant Cell Environ. 35, 747–759. [DOI] [PubMed] [Google Scholar]
- Barrios‐Masias, F.H. and Jackson, L.E. (2014) California processing tomatoes: morphological, physiological and phenological traits associated with crop improvement during the last 80 years. Eur. J. Agron. 53, 45–55. [Google Scholar]
- Barrios‐Masias, F.H. , Chetelat, R.T. , Grulke, N.E. and Jackson, L.E. (2014) Use of introgression lines to determine the ecophysiological basis for changes in water use efficiency and yield in California fprocessing tomatoes. Funct. Plant Biol. 41, 119–132. [DOI] [PubMed] [Google Scholar]
- Bean, G.J. , Marks, M.D. , Hülskamp, M. , Clayton, M. and Croxdale, J.L. (2002) Tissue patterning of Arabidopsis cotyledons. New Phytol. 153, 461–467. [DOI] [PubMed] [Google Scholar]
- Bechtold, U. , Penfold, C.A. , Jenkins, D.J. , et al (2016) Time‐series transcriptomics reveals that AGAMOUS‐LIKE22 affects primary metabolism and developmental processes in drought‐stressed Arabidopsis . Plant Cell, 28, 345–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beerling, D.J. and Chaloner, W.G. (1993) The impact of atmospheric CO2 and temperature changes on stomatal density: observation from Quercus robur lammas leaves. Ann. Bot. 71, 231–235. [Google Scholar]
- Bernacchi, C.J. , Portis, A.R. , Nakano, H. , von Caemmerer, S. and Long, S.P. (2002) Temperature response of mesophyll conductance. Implications for the determination of rubisco enzyme kinetics and for limitations to photosynthesis in vivo . Plant Physiol. 130, 1992–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkman, C. , Dalin, P. and Ahrne, K. (2008) Leaf trichome responses to herbivory in willows: induction, relaxation and costs. New Phytol. 179, 176–184. [DOI] [PubMed] [Google Scholar]
- Bleeker, P.M. , Mirabella, R. , Diergaarde, P.J. , VanDoorn, A. , Tissier, A. , Kant, M.R. , Prins, M. , de Vos, M. , Haring, M.A. and Schuurink, R.C. (2012) Improved herbivore resistance in cultivated tomato with the sesquiterpene biosynthetic pathway from a wild relative. Proc. Natl Acad. Sci. USA, 109, 20124–20129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum, A. (2005) Drought resistance, water‐use efficiency, and yield potential ‐ are they compatible, dissonant, or mutually exclusive? Aust. J. Agric. Res. 56, 1159–1168. [Google Scholar]
- Blum, A. (2009) Effective use of water (EUW) and not water‐use efficiency (WUE) is the target of crop yield improvement under drought stress. Field. Crop. Res. 112, 119–123. [Google Scholar]
- Boughalleb, F. and Hajlaoui, H. (2011) Physiological and anatomical changes induced by drought in two olive cultivars (cv Zalmati and Chemlali). Acta Physiol. Plant. 33, 53–65. [Google Scholar]
- Caruso, G. , Gomez, L.D. , Ferriello, F. , et al (2016) Exploring tomato Solanum pennellii introgression lines for residual biomass and enzymatic digestibility traits. BMC Genet. 17, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceulemans, R. , Van Praet, L. and Jiang, X.N. (1995) Effects of CO2 enrichment, leaf position and clone on stomatal index and epidermal cell density in poplar (Populus). New Phytol. 131, 99–107. [DOI] [PubMed] [Google Scholar]
- Chien, J.C. and Sussex, I.M. (1996) Differential regulation of trichome formation on the adaxial and abaxial leaf surfaces by Gibberellins and photoperiod in Arabidopsis thaliana (L) Heynh . Plant Physiol. 111, 1321–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitwood, D.H. , Kumar, R. , Headland, L.R. , et al (2013) A quantitative genetic basis for leaf morphology in a set of precisely defined tomato introgression lines. Plant Cell, 25, 2465–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daszkowska‐Golec, A. (2016) The role of abscisic acid in drought stress: how ABA helps plants to cope with drought stress In: Drought Stress Tolerance in Plants, Vol 2: Molecular and Genetic Perspectives (Hossain M.A., Wani S.H., Bhattacharjee S., Burritt D.J. and Tran L.‐S.P., eds). Cham: Springer International Publishing, pp. 123–151. [Google Scholar]
- Egea, I. , Albaladejo, I. , Meco, V. , Morales, B. , Sevilla, A. , Bolarin, M.C. and Flores, F.B. (2018) The drought‐tolerant Solanum pennellii regulates leaf water loss and induces genes involved in amino acid and ethylene/jasmonate metabolism under dehydration. Sci. Rep. 8, 2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehleringer, J.R. and Mooney, H.A. (1978) Leaf hairs: effects on physiological activity and adaptive value to a desert shrub. Oecologia, 37, 183–200. [DOI] [PubMed] [Google Scholar]
- Eshed, Y. and Zamir, D. (1995) An introgression line population of Lycopersicon pennellii in the cultivated tomato enables the identification and fine mapping of yield‐associated QTL. Genetics, 141, 1147–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewas, M. , Gao, Y.Q. , Wang, S.C. , et al (2016) Manipulation of SlMXl for enhanced carotenoids accumulation and drought resistance in tomato. Sci. Bull. 61, 1413–1418. [DOI] [PubMed] [Google Scholar]
- Farber, M. , Attia, Z. and Weiss, D. (2016) Cytokinin activity increases stomatal density and transpiration rate in tomato. J. Exp. Bot. 67, 6351–6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar, G. and Richards, P. (1984) Isotopic composition of plant carbon correlates with water‐use efficiency of wheat genotypes. Funct. Plant Biol. 11, 539–552. [Google Scholar]
- Flexas, J. , Diaz‐Espejo, A. , Berry, J.A. , Cifre, J. , Galmes, J. , Kaldenhoff, R. , Medrano, H. and Ribas‐Carbo, M. (2007) Analysis of leakage in IRGA's leaf chambers of open gas exchange systems: quantification and its effects in photosynthesis parameterization. J. Exp. Bot. 58, 1533–1543. [DOI] [PubMed] [Google Scholar]
- Fracasso, A. , Trindade, L.M. and Amaducci, S. (2016) Drought stress tolerance strategies revealed by RNA‐Seq in two sorghum genotypes with contrasting WUE. BMC Plant Biol. 16, 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks, P.J. , Doheny‐Adams T, W. , Britton‐Harper, Z.J. and Gray, J.E. (2015) Increasing water‐use efficiency directly through genetic manipulation of stomatal density. New Phytol. 207, 188–195. [DOI] [PubMed] [Google Scholar]
- Fu, Q.S. , Yang, R.C. , Wang, H.S. , Zhao, B. , Zhou, C.L. , Ren, S.X. and Guo, Y.‐D. (2013) Leaf morphological and ultrastructural performance of eggplant (Solanum melongena L.) in response to water stress. Photosynthetica, 51, 109–114. [Google Scholar]
- Galmes, J. , Conesa, M.A. , Ochogavia, J.M. , Perdomo, J.A. , Francis, D.M. , Ribas‐Carbo, M. , Save, R. , Flexas, J. , Medrano, H. and Cifre, J. (2011) Physiological and morphological adaptations in relation to water use efficiency in Mediterranean accessions of Solanum lycopersicum . Plant Cell Environ. 34, 245–260. [DOI] [PubMed] [Google Scholar]
- Galmes, J. , Ochogavia, J.M. , Gago, J. , Roldan, E.J. , Cifre, J. and Conesa, M.A. (2013) Leaf responses to drought stress in Mediterranean accessions of Solanum lycopersicum: anatomical adaptations in relation to gas exchange parameters. Plant Cell Environ. 36, 920–935. [DOI] [PubMed] [Google Scholar]
- Galmés, J. , Medrano, H. and Flexas, J. (2007a) Photosynthesis and photoinhibition in response to drought in a pubescent (var. minor) and a glabrous (var. palaui) variety of Digitalis minor . Environ. Exp. Bot. 60, 105–111. [Google Scholar]
- Galmés, J. , Flexas, J. , Savé, R. and Medrano, H. (2007b) Water relations and stomatal characteristics of Mediterranean plants with different growth forms and leaf habits: responses to water stress and recovery. Plant Soil, 290, 139–155. [Google Scholar]
- Gan, Y. , Zhou, L. , Shen, Z.‐J. , Shen, Z.‐X. , Zhang, Y.‐Q. and Wang, G.‐X. (2010) Stomatal clustering, a new marker for environmental perception and adaptation in terrestrial plants. Bot. Stud. 51, 325–336. [Google Scholar]
- Gianfagna, T.J. , Carter, C.D. and Sacalis, J.N. (1992) Temperature and photoperiod influence trichome density and sesquiterpene content of Lycopersicon‐ hirsutum F. hirsutum . Plant Physiol. 100, 1403–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover, B.J. (2000) Differentiation in plant epidermal cells. J. Exp. Bot. 51, 497–505. [DOI] [PubMed] [Google Scholar]
- Glover, B.J. , Perez‐Rodriguez, M. and Martin, C. (1998) Development of several epidermal cell types can be specified by the same MYB‐related plant transcription factor. Development, 125, 3497–3508. [DOI] [PubMed] [Google Scholar]
- Glover, B.J. , Airoldi, C.A. and Moyroud, E. (2016). Epidermis: outer cell layer of the plant. In eLS. Chichester, UK: John Wiley & Sons, Ltd. 10.1002/9780470015902.a0002072.pub3 [DOI] [Google Scholar]
- Grandillo, S. , Zamir, D. and Tanksley, S.D. (1999) Genetic improvement of processing tomatoes: a 20 years perspective. Euphytica, 110, 85–97. [Google Scholar]
- Guerfel, M. , Baccouri, O. , Boujnah, D. , Chaïbi, W. and Zarrouk, M. (2009) Impacts of water stress on gas exchange, water relations, chlorophyll content and leaf structure in the two main Tunisian olive (Olea europaea L.) cultivars. Sci. Hortic. 119, 257–263. [Google Scholar]
- Guha, A. , Sengupta, D. , Kumar Rasineni, G. and Ramachandra Reddy, A. (2010) An integrated diagnostic approach to understand drought tolerance in mulberry (Morus indica L.). Flora – Morphol Distrib Funct Ecol Plants, 205, 144–151. [Google Scholar]
- Gurr, G.M. and McGrath, D. (2001) Effect of plant variety, plant age and photoperiod on glandular pubescence and host‐plant resistance to potato moth (Phthorimaea operculella) in Lycopersicon spp. Ann. Appl. Biol. 138, 221–230. [Google Scholar]
- Hamanishi, E.T. , Thomas, B.R. and Campbell, M.M. (2012) Drought induces alterations in the stomatal development program in Populus . J. Exp. Bot. 63, 4959–4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley, P.C. , Loreto, F. , Di Marco, G. and Sharkey, T.D. (1992) Theoretical considerations when estimating the mesophyll conductance to co2 flux by analysis of the response of photosynthesis to CO2. Plant Physiol. 98(4), 1429–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser, M.‐T. (2014) Molecular basis of natural variation and environmental control of trichome patterning. Front. Plant Sci. 5, 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermida‐Carrera, C. , Kapralov, M.V. and Galmés, J. (2016) Rubisco catalytic properties and temperature response in crops. Plant Physiol. 171(4), 2549–2561. 10.1104/pp.16.01846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington, A.M. and Woodward, F.I. (2003) The role of stomata in sensing and driving environmental change. Nature, 424, 901–908. [DOI] [PubMed] [Google Scholar]
- Holmes, M.G. and Keiller, D.R. (2002) Effects of pubescence and waxes on the reflectance of leaves in the ultraviolet and photosynthetic wavebands: a comparison of a range of species. Plant Cell Environ. 25, 85–93. [Google Scholar]
- Iovieno, P. , Punzo, P. , Guida, G. , et al (2016) Transcriptomic changes drive physiological responses to progressive drought stress and rehydration in tomato. Front. Plant Sci. 7, 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn, T.L. , Fender, S.E. , Bray, E.A. and O'Connell, M.A. (1993) Characterization of expression of drought‐ and abscisic acid‐regulated tomato genes in the drought‐resistant species Lycopersicon pennellii . Plant Physiol. 103, 597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, J.H. , McRoberts, J. , Shi, F. , Moreno, J.E. , Jones, A.D. and Howe, G.A. (2014) The flavonoid biosynthetic enzyme chalcone isomerase modulates terpenoid production in glandular trichomes of tomato. Plant Physiol. 164, 1161–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson, T. and Blatt, M.R. (2014) Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol. 164, 1556–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Li, H. , Li, Y. and Zhang, S. (2017) Improving water‐use efficiency by decreasing stomatal conductance and transpiration rate to maintain higher ear photosynthetic rate in drought‐resistant wheat. Crop J. 5, 231–239. [Google Scholar]
- Liu, X. and Liu, C. (2016) Effects of Drought‐Stress on Fusarium Crown Rot Development in Barley. PLoS ONE, 11, e0167304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, E.K. , Mei, X.R. , Yan, C.R. , Gong, D.Z. and Zhang, Y.Q. (2016) Effects of water stress on photosynthetic characteristics, dry matter translocation and WUE in two winter wheat genotypes. Agric. Water Manag. 167, 75–85. [Google Scholar]
- Luckwill, L. (1943) The genus Lycopersicon: a historical, biological, and taxonomic survey of the wild and cultivated tomatoes. Aberdeen: The University Press. [Google Scholar]
- Martin, B. and Thorstenson, Y.R. (1988) Stable carbon isotope composition (δ13C), water use efficiency, and biomass productivity of Lycopersicon esculentum, Lycopersicon pennellii, and the F(1) hybrid. Plant Physiol. 88, 213–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masle, J. , Gilmore, S.R. and Farquhar, G.D. (2005) The ERECTA gene regulates plant transpiration efficiency in Arabidopsis . Nature, 436, 866–870. [DOI] [PubMed] [Google Scholar]
- McDowell, E.T. , Kapteyn, J. , Schmidt, A. , et al (2011) Comparative functional genomic analysis of Solanum glandular trichome types. Plant Physiol. 155, 524–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrano, H. , Tomás, M. , Martorell, S. , Flexas, J. , Hernández, E. , Rosselló, J. , Pou, A. , Escalona, J.‐M. and Bota, J. (2015) From leaf to whole‐plant water use efficiency (WUE) in complex canopies: limitations of leaf WUE as a selection target. Crop J. 3, 220–228. [Google Scholar]
- Mo, Y. , Yang, R. , Liu, L. , Gu, X. , Yang, X. , Wang, Y. , Zhang, X. and Li, H. (2016) Growth, photosynthesis and adaptive responses of wild and domesticated watermelon genotypes to drought stress and subsequent re‐watering. Plant Growth Regul. 79, 229–241. [Google Scholar]
- Morohashi, K. and Grotewold, E. (2009) A systems approach reveals regulatory circuitry for Arabidopsis trichome initiation by the GL3 and GL1 selectors. PLoS Genet. 5, e1000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyle, L.C. (2008) Ecological and evolutionary genomics in the wild tomatoes (Solanum sect. Lycopersicon). Evolution, 62, 2995–3013. [DOI] [PubMed] [Google Scholar]
- Niinemets, U. , Diaz‐Espejo, A. , Flexas, J. , Galmes, J. and Warren, C.R. (2009) Role of mesophyll diffusion conductance in constraining potential photosynthetic productivity in the field. J. Exp. Bot. 60, 2249–2270. [DOI] [PubMed] [Google Scholar]
- de Oliveira Silva, F.M. , Lichtenstein, G. , Alseekh, S. , et al (2018) The genetic architecture of photosynthesis and plant growth‐related traits in tomato. Plant Cell Environ. 41, 327–341. [DOI] [PubMed] [Google Scholar]
- Palliotti, A. , Bongi, G. and Rocchi, P. (1994) Peltate trichomes effects on photosynthetic gas exchange of Olea Europea L. leaves. Life Sci. Adv. Plant Physiol. 13, 35–44. [Google Scholar]
- Peralta, I.E. , Spooner, D.M. and Knapp, S. (2008) Taxonomy of wild tomatoes and their relatives (Solanum sect Lycopersicoides, sect, Juglandifolia, sect. Lycopersicon; Solanaceae). Syst. Bot. Monographs, 84, 186. [Google Scholar]
- Pillitteri, L.J. and Dong, J. (2013) Stomatal development in Arabidopsis. Arabidopsis Book, 11, e0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, X. , Chen, Z. , Liu, Y. , Zhang, H. , Zhang, M. , Liu, Q. , Hong, X. , Zhu, J.K. and Gong, Z. (2010) ABO3, a WRKY transcription factor, mediates plant responses to abscisic acid and drought tolerance in Arabidopsis . Plant J. 63, 417–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rick, C.M. (1973) Potential genetic resources in tomato species: clues from observations in native habitats In Genes, Enzymes, and Populations (Srb A.M. ed.). Boston, MA: Springer US, pp. 255–269. [DOI] [PubMed] [Google Scholar]
- Riederer, M. and Schreiber, L. (2001) Protecting against water loss: analysis of the barrier properties of plant cuticles. J. Exp. Bot. 52, 2023–2032. [DOI] [PubMed] [Google Scholar]
- Rigano, M.M. , Arena, C. , Di Matteo, A. , Sellitto, S. , Frusciante, L. and Barone, A. (2016) Eco‐physiological response to water stress of drought‐tolerant and drought‐sensitive tomato genotypes. Plant Biosystems, 150, 682–691. [Google Scholar]
- Rogiers, S.Y. , Hardie, W.J. and Smith, J.P. (2011) Stomatal density of grapevine leaves (Vitis vinifera L.) responds to soil temperature and atmospheric carbon dioxide. Aust. J. Grape Wine Res. 17, 147–152. [Google Scholar]
- Savé, R. , Biel, C. and de Herralde, F. (2000) Leaf Pubescence, Water Relations and Chlorophyll Fluorescence in Two Subspecies of Lotus Creticus L. Biol. Plant. 43, 239–244. [Google Scholar]
- Schilmiller, A.L. , Last, R.L. and Pichersky, E. (2008) Harnessing plant trichome biochemistry for the production of useful compounds. Plant J. 54, 702–711. [DOI] [PubMed] [Google Scholar]
- Silva, E.C. , Nogueira, R.J.M.C. , Vale, F.H.A. , Araújo, F.P.D. and Pimenta, M.A. (2009) Stomatal changes induced by intermittent drought in four umbu tree genotypes. Braz. J. Plant. Physiol. 21, 33–42. [Google Scholar]
- Simmons, A.T. and Gurr, G.M. (2005) Trichomes of Lycopersicon species and their hybrids: effects on pests and natural enemies. Agric. For. Entomol. 7, 265–276. [Google Scholar]
- Sletvold, N. and Ågren, J. (2012) Variation in tolerance to drought amongst Scandinavian populations of Arabidopsis lyrata . Evol. Ecol. 26, 559–577. [Google Scholar]
- Souza, M.A.A.D. , Santos, L.A.D. , Brito, D.M.C.D. , Rocha, J.F. , Castro, R.N. , Fernandes, M.S. and Souza, S.R.D. (2016) Influence of light intensity on glandular trichome density, gene expression and essential oil of menthol mint (Mentha arvensis L.). J. Essent. Oil Res. 28, 138–145. [Google Scholar]
- Spyropoulou, E. , Haring, M. and Schuurink, R. (2014) RNA sequencing on Solanum lycopersicum trichomes identifies transcription factors that activate terpene synthase promoters. BMC Genom. 15, 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhauser, M.C. , Steinhauser, D. , Gibon, Y. , Bolger, M. , Arrivault, S. , Usadel, B. , Zamir, D. , Fernie, A.R. and Stitt, M. (2011) Identification of enzyme activity Quantitative Trait Loci in a Solanum lycopersicum X Solanum pennellii Introgression Line population. Plant Physiol. 157, 998–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telfer, A. , Bollman, K.M. and Poethig, R.S. (1997) Phase change and the regulation of trichome distribution in Arabidopsis thaliana . Development, 124, 645–654. [DOI] [PubMed] [Google Scholar]
- Tian, D. , Tooker, J. , Peiffer, M. , Chung, S. and Felton, G. (2012) Role of trichomes in defense against herbivores: comparison of herbivore response to woolly and hairless trichome mutants in tomato (Solanum lycopersicum). Planta, 236, 1053–1066. [DOI] [PubMed] [Google Scholar]
- Traw, M.B. and Bergelson, J. (2003) Interactive effects of jasmonic acid, salicylic acid, and gibberellin on induction of trichomes in Arabidopsis . Plant Physiol. 133, 1367–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, N.C. (1988) Measurement of plant water status by the pressure chamber technique. Irrig. Sci. 9, 289–308. [Google Scholar]
- Vendemiatti, E. , Zsögön, A. , Silva, G.F.F.E. , de Jesus, F.A. , Cutri, L. , Figueiredo, C.R.F. , Tanaka, F.A.O. , Nogueira, F.T.S. and Peres, L.E.P. (2017) Loss of type‐IV glandular trichomes is a heterochronic trait in tomato and can be reverted by promoting juvenility. Plant Sci. 259, 35–47. [DOI] [PubMed] [Google Scholar]
- Wellso, S.G. and Hoxie, R.P. (1982) The Influence of environment on the expression of trichomes in wheat. Crop Sci. 22, 879–886. [Google Scholar]
- Wentworth, M. , Murchie, E.H. , Gray, J.E. , Villegas, D. , Pastenes, C. , Pinto, M. and Horton, P. (2006) Differential adaptation of two varieties of common bean to abiotic stress: acclimation of photosynthesis. J. Exp. Bot. 57, 699–709. [DOI] [PubMed] [Google Scholar]
- Xu, Z. and Zhou, G. (2008) Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J. Exp. Bot. 59, 3317–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, X. , Martin, B. , Comstock, J.P. , Vision, T.J. , Tauer, C.G. , Zhao, B. , Pausch, R.C. and Knapp, S. (2008) Fine mapping a QTL for carbon isotope composition in tomato. Theor. Appl. Genet. 117, 221. [DOI] [PubMed] [Google Scholar]
- Yoshida, T. , Fujita, Y. , Maruyama, K. , Mogami, J. , Todaka, D. , Shinozaki, K. and Yamaguchi‐Shinozaki, K. (2015) Four Arabidopsis AREB/ABF transcription factors function predominantly in gene expression downstream of SnRK2 kinases in abscisic acid signalling in response to osmotic stress. Plant Cell Environ. 38, 35–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir, D. (2001) Improving plant breeding with exotic genetic libraries. Nat. Rev. Genet. 2, 983–989. [DOI] [PubMed] [Google Scholar]
- Zhao, L. , Li, Y. , Xie, Q. and Wu, Y. (2017) Loss of CDKC;2 increases both cell division and drought tolerance in Arabidopsis thaliana . Plant J. 91, 816–828. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Initial morphological characterization of lines M82, 4‐1, 10‐2 and 11‐3 grown under glasshouse (GH) and field conditions (F) before the onset of drought treatment.
Figure S2. Percentage of trichome types and trichome length in plants grown under glasshouse conditions.
Figure S3. Stomatal density in lines M82, 4‐1, 10‐2 and 11‐3 under water‐deficit (WD) and well‐watered (WW) conditions in the field.
Figure S4. Trichome and stomatal densities in lines M82, 4‐1, 10‐2 and 11‐3 under water‐deficit (WD) and well‐watered (WW) conditions in the field, expressed in terms of area.
Figure S5. Correlations between carbon isotope composition and intrinsic water use efficiency, and between intrinsic water use efficiency and plant‐level water use efficiency, in lines M82, 4‐1, 10‐2 and 11‐3.
Figure S6. Relationship between epidermal features and plant‐level water use efficiency (WUEb) in plants under well‐watered (WW) and water‐deficit (WD) conditions in the field.
Figure S7. Correlations between trichome density expressed per unit area and water use in lines M82, 4‐1, 10‐2 and 11‐3 under WW and WD conditions.
Figure S8. Trichome densities on abaxial and adaxial sides of leaves of lines M82, 4‐1, 10‐2 and 11‐3 grown under glasshouse conditions.
Figure S9. Evolution of the pot water content during the experiment for the well‐watered (WW, blue) and water‐deficit (WD, red) plants.
Table S1. Leaf morphological traits and photosynthetic characterization of the lines M82, 4‐1, 10‐2 and 11‐3 under field conditions before the onset of the drought treatment.
Table S2. Dry biomass and total water supplied to plants upon completion of the experiment for lines M82, 4‐1, 10‐2 and 11‐3.


