Abstract
Acute myeloid leukemia (AML) was the first malignancy for which immunotherapy, in the form of allogeneic hematopoietic stem cell transplantation (allo-HSCT), was integrated into the standard of care. Allo-HSCT however is an imperfect therapy associated with significant morbidity and mortality while offering only incomplete prevention of AML clinical relapse. These limitations have motivated the search for AML-related antigens that might be used as more specific and effective targets of immunotherapy. While historically such investigations have focused on protein targets expressed uniquely in AML or at significantly higher levels than in normal tissues, this article will review recent discoveries which have identified a novel selection of potential antigen targets for AML immunotherapy, such as non-protein targets including lipids and carbohydrates, neo-antigens created from genetic somatic mutations or altered splicing and post-translational modification of protein targets, together with innovative ways to target overexpressed protein targets presented by cell surface peptide-MHC complexes. These novel antigens represent promising candidates for further development as targets of AML immunotherapy.
Keywords: Acute myeloid leukemia, antigens, immunotherapy
INTRODUCTION
Using the immune system to target acute myeloid leukemia (AML) is an appealing prospect because of the theoretical potential to specifically target the malignant clone while sparing healthy tissues and minimizing the off-target effects typical with conventional cytotoxic chemotherapeutic regimes. Some forms of immunotherapy are already standard practice for eligible patients with AML, and the powerful graft-versus-leukemia (GvL) phenomenon observed after allogeneic hematopoietic stem cell transplantation (allo-HSCT) [1] and donor lymphocyte infusion (DLI) is perhaps the strongest evidence of the ability of the immune system to target the malignant clone in AML [2]. However, both these approaches are associated with significant morbidity and mortality, which highlight the importance of targeting these powerful immunotherapy effects to AML as specifically as possible.
Research efforts in immunotherapy for AML have included both passive immunotherapy approaches including monoclonal antibody (mAb) therapy, adoptive T-cell therapy, and engineered T-cells and antibodies, and with active immunotherapy including cancer vaccines [3]. The success of any of these immune-mediated biologic treatments hinges on appropriate target selection. Antigens can be generally classified into three groups: (1) neoantigens created by genetic events contributing to leukemogenesis (2) leukocyte-restricted antigens, and (3) wild type proteins that are over-expressed [4]. Most of the efforts in antigen-discovery have focused on the latter category of antigen - proteins that are over-expressed in leukemia, or leukemia-associated antigens (LAA). Commonly studied LAAs include WT1, PR3, PRAME, SSX2, MAGE, and many others that have been extensively researched, validated, and in some cases, discarded as a viable target of immunotherapy [1, 5–8]. A protein target that is found on all AMLs across all subtypes of disease has yet to be determined, and indeed, such a target may not exist. AML is a heterogeneous, often oligoclonal disease, and the lack of identification of a single suitable inclusive antigen has motivated the search for alternative targets for immunotherapy in this disease entity.
Several recent studies have examined the antigenic nature of molecules other than just strictly over-expressed protein LAAs in AML - novel lipid antigens, phosphorylated peptides, carbohydrates, neo-antigen epitopes created by acquired somatic mutations, protein isoforms resulting from splice variants, and peptide-MHC complexes recognized by the immune system. Targeting such novel types of antigens in AML has the potential to overcome some of the problems seen in protein-directed immunotherapy, including evolution of the AML clone and the up- or down-regulation of the target of therapy during the course of treatment [9]. This review will survey the recent literature on such novel antigen targets in AML.
TARGETING NON-PROTEIN ANTIGENS: PROOF OF CONCEPT
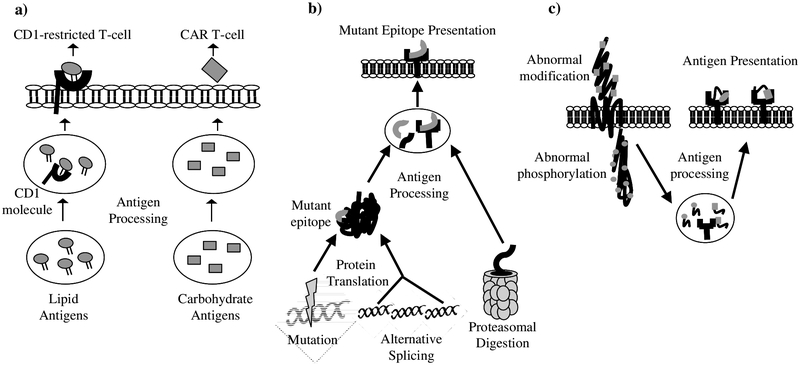
Table 1 presents a list of recently described novel acute leukemia-associated antigens, divided into five categories: non-protein antigens, neoantigens, post-translationally modified antigens, peptide/MHC complexes, and over-expressed antigens. Fig. (1) illustrates potential sources of tumor antigens. Non-protein antigens derived from critical cell machinery are exciting potential targets of immunotherapy because the interference in pathways with lipids and carbohydrates can have immediate, deleterious effects on the cell that are incompatible with survival. Recent studies have identified new non-protein antigens and addressed the immunogenicity and clinical relevance in specifically targeting the leukemia but not normal hematopoietic stem cell (HSC) populations. The most well-known antigen presenting proteins are MHC class I and II molecules that present peptides from endogenous and exogenous protein processing pathways, but other antigen-presenting proteins include the CD1 lineage of molecules that present lipid and glycolipid antigens to CD1-restricted T-cells. The targeting of lipids could allow various immunotherapeutic approaches via these CD1-restricted T-cells in addition to MHC-mediated antigen presentation. Based on previous work that determined that healthy human subjects have a high number of CD1c-reactive T-cells, a group recently examined the ability of hematopoietic-derived tumor cell lines to stimulate CD1c-reactive T-cells by isolating CD1c-reactive T-cells from donors and stimulated them with tumor cell lines expressing CD1c [10, 11]. The production of GM-CSF and IFNγ strongly suggested that there existed within the tumor cell lines a self-lipid antigen capable of stimulating these CD1c-reactive T-cells, and further biochemical experiments to characterize the responsible lipid revealed a novel compound, C16 methyl-lysophosphatidic acid (mLPA). This lipid had the ability in vitro to activate CD1c-reactive T-cells when presented by CD1c+ antigen presenting cells (APCs), and mLPA-specific T-cells were capable of killing CD1c+ primary AML blasts [11]. The novel self-lipid mLPA is very similar in structure to lysophosphatidic acid (LPA), which demonstrates pleomorphic activities on many cell lineages and promotes cell growth, migration, and proliferation [12]. It is conceivable that mLPA has similar functions in leukemia tumor cells, and targeting this lipid could disrupt the proliferation and survival of leukemic blasts. Moreover, the specificity of mLPA to AML blasts and the absence of mLPA in HSCs and other tissues makes mLPA an attractive target for primed CD1c-restricted T-cells.
Table 1.
Novel antigens in acute myeloid leukemia.
| Category of Antigen | Antigen | Full Antigen Name | Reference |
|---|---|---|---|
| Non-Protein Antigens | mLPA | methyl-lysophosphatidic acid | [11] |
| a-GalCer | α-galactosylceramide | [13] | |
| LeY | Lewis Y antigen | [16–18] | |
| Neoantigens | IDH1(R132) | isocitrate dehydrogenase 1 | [22, 24, 25] |
| IDH2(R140) | isocitrate dehydrogenase 2 | [23] | |
| NPM1mut | nuclophosmin 1 mutant | [28–30] | |
| NOTCH variants | NOTCH signaling molecule isoform | [46] | |
| FLT3 variants | fms-like tyrosine kinase 3 | [46] | |
| CD44v6 | hyaluronan receptor isoform | [49–50] | |
| Post-translationally Modified Antigens | phosphopeptides | phosphorylated peptides | [52–55] |
| MUC1 | mucin 1 | [59–62] | |
| PRL3 | protein tyrosine phosphatase type Iva member 3 | [65–67] | |
| Peptide-MHC Antigens | PR1/HLA-A2 | proteinase 3 peptide,HLA-A2-restricted | [73] |
| WT1/HLA-A2 | 29 Wilms’ tumor peptides, HLA-A2-restricted | [71, 74, 75] | |
| Over-expressed Antigens | IL12RB1 | interleukin 12 receptor beta 1 | [31] |
| CD96 | member of immunoglobulin superfamily | [31] |
Fig. (1). Sources of tumor antigens.
a) Lipid processing and presentation to CD1-restricted T-cells and carbohydrate processing and cell-surface expression for CAR T-cells. b) Presentation of peptides from neoantigens generated through somatic mutation, alternative splicing, and altered proteasomal digestion. c) Antigen presentation of abnormally modified proteins.
Combining antigen presentation through both the MHC and CD1 pathways is an attractive vaccination approach to boost immune stimulation, and vaccines conjugated with a lipid adjuvant can do just that. In a murine model, Gibbins, et al. administered a vaccine of irradiated leukemia cells loaded with the glycosphingolipid α-galactosylceramide (α-GalCer) [13]. Through protein presentation on MHC and α-GalCer presentation via CD1d molecules to CD1d-restricted NKT-cells, the vaccine was effective at preventing leukemia in murine subjects but not in those mice with active disease, presumably due to immunosuppressive environments exerted by the leukemia. Authors demonstrated compatibility with cytarabine chemotherapy which was first used to reduce tumor burden, after which the vaccine was administered [13]. The effectiveness of this vaccine approach with α-GalCer adjuvant was also previously demonstrated in murine models with AML-ETO9a and acute myelomonocytic leukemia cell lines [14, 15]. The combination of chemotherapy to achieve a minimal residual disease negative status and irradiated leukemia vaccine with lipid adjuvant makes this approach particularly suitable to preventing relapse in a post allogeneic transplant context and provides further evidence for combining T-cell signaling pathways (MHC and CD1) in targeting AML.
The experiences with the tumor-associated carbohydrate antigen Lewis-Y (LeY) antigen in AML serve as excellent proof of concept that targeting non-protein antigens can indeed generate an immune response that might be harnessed in a clinical setting. The LeY antigen is a difucosylated carbohydrate antigen of the blood-group molecule family that has been reported as over-expressed in a variety of tumors [16]. In a study of 33 primary bone marrow (BM) samples from AML patients, 46% of these cases over expressed the LeY antigen while LeY expression was more limited in lymphocytes, both from healthy individuals and those with lymphoid malignancies [17]. A phase I study tested a chimeric antigen receptor (CAR) T-cell against LeY with 4 evaluable patients. The trial reported no acute toxicities and demonstrated a persistence of LeY-specific CAR T-cells for 10 months, though treatment did not produce a durable response in any of the patients. Disappointingly, while some of the patients receiving LeY-directed CAR T-cell immunotherapy saw a reduction in total tumor burden, the patients went on to suffer hematological relapse despite the long-term persistence of the anti-LeY CAR T-cells [18]. While these studies demonstrate effective recognition of the antigen by the immune system, they underscore the suitability of specifically targeting LeY in AML and the need to identify other putative leukemia-associated carbohydrate antigens. Nevertheless, this initial trial of LeY-directed CAR T-cells is an excellent example of translation into clinic and offers strong evidence that targeting carbohydrate antigens in AML can initiate a specific immune response with no severe side effects.
CHANGES IN PRIMARY PROTEIN SEQUENCE: NEOANTIGENS
The dysregulation seen in a cancer cell can generate mutant proteins with changes in primary protein sequence, or neoantigens. Somatic mutations, alternative splicing, and other altered cell activities can generate these neoantigens that may contain extremely immunogenic mutant epitopes. Several gain-of-function mutations in AML can produce functional proteins that contribute to malignant phenotype, and such neoantigens are ideal targets of treatment because of their highly restricted expression in tumor cells. While neoantigens are technically self-antigens, the immune system can be exploited to target them because of the lack of tolerance to these new immunogenic epitopes.
The landmark study performed by The Cancer Genome Atlas Research Network exhaustively examined the mutational composition of de novo AML and identified recurrent and significantly mutated events that contribute to leukemogenesis [19]. Mutations in the metabolic enzyme isocitrate dehydrogenase (IDH1 and IDH2) are of particular interest because they produce functional proteins with altered enzymatic activity that block the differentiation of hematopoietic progenitors and lead to the uncontrolled proliferation of primitive cells. Gain-of-function mutations in IDH directly interfere with the citric acid cycle and epigenetic state of the cell, preventing the normal function of downstream metabolic enzymes [20–22]. IDH1/2 mutations are present in roughly 20% of de novo AML, and the contribution to leukemogenesis of these proto-oncogenes have been studied and confirmed [19–23]. The most common IDH1 mutation is a substitution at position 132 from an arginine to a histidine (R132H). Conditional knock-in murine models of the IDH1(R132H) mutation yielded a dysfunctional bone marrow niche and a hypermethylation of histones, though IDH1 alone was insufficient to initiate leukemia. The interaction of the mutant proteins with other known leukemia-associated antigens like HOXA9 led to the transformation of an arrested cell to an AML clone [22–24]. Similarly, mutant IDH2 resulting from a substitution of arginine with glutamine at position 140 (R140Q) cooperates with HOXA9, MEIS1, and FLT3 mutations to initiate leukemogenesis [23]. The ability of T-cells to recognize epitopes encompassing the IDH1(R132H) mutation was studied in glioma, and in an MHC-humanized mouse model, vaccination against this mutation was able to induce IDH1(R132H)-specific CD4+ T-cells and a TH1 response through antigen presentation on MHC Class II, indicating endogenous processing of the mutant epitope [25]. Specific inhibitors of the IDH1 and IDH2 mutant proteins are currently being tested in Phase I/II clinical trials (ClinicalTrials.gov identifiers NCT01915498, NCT02074839), and the demonstrated immune recognition of these neoantigens is very promising for the treatment of AML cases with these mutations.
The NPM1 mutation seen in one third of de novo AML patients is a somatic mutation in exon 12 of the NPM1 gene, which causes a substitution of crucial tryptophan residues in the C-terminus of the transcript, creating a nuclear export signal and causing the localization of the mutant protein to the cytoplasm [26, 27]. The mutated cytoplasmic protein has been demonstrated to possess immunogenic epitopes capable of stimulating both CD4+ and CD8+ T-cell responses. In a study by Greiner, et al. assessing recognition of 10 peptides derived from wild type and NPM1 mutated (NPM1mut) proteins, one third of healthy volunteers and AML patients alike mounted immune responses against NPM1 #1 epitope (AIQDLCLAV), and while 20% of healthy volunteers had a response to NPM1 #3 epitope (AIQDLCVAV), almost half of the AML patients had a response both in the CD4+ and CD8+ compartments [28]. Subsequent studies confirmed both the T-cell response against these NPM1mut epitopes in additional cohorts and in a patient who received donor lymphocyte infusion (DLI) after molecular relapse post allo-HSCT [29, 30]. The immunogenicity of NPM1mut epitopes could be harnessed for immunotherapeutic approaches including adoptive T-cell transfer and vaccination. Another source of antigens in NPM1mut is in the leukemic stem cell (LSC) compartment, which differentially express select genes when compared to LSCs from patients who do not harbor an NPM1 mutation. Particularly, the IL-12 receptor beta 1 (IL12RB1) antigen and CD96 were significantly upregulated in a microarray analysis of AML with normal karyotype but with an NMP1 mutation, and other upregulated genes include several members of the stem cell differentiation-mediating family of HOX genes [31]. The expression of these genes does not arise from a change in primary protein sequence of these targets, but the restricted expression in patients harboring an NPM1 mutation makes these antigens feasible targets of specific immunotherapy.
A recent study implicated tyrosine kinase inhibitors (TKIs) in modulating proteasome function in chronic myeloid leukemia (CML), specifically downregulating their proteolytic activity in response to phosphorylation induced by TKIs. This altered proteasome yielded altered epitopes and reduced MHC class I expression, which begs the question of whether these new peptides are capable of initiating an immune response despite less MHC I [32]. A proteasomal digestion of the BCR-ABL fusion protein in CML produced specific HLA-restricted peptides but no T-cells specific to these particular epitopes; however in a lymphoma cell line, a subset of cytotoxic T-cells (CTLs) were identified that recognized peptides generated from altered protea-some activity [33, 34]. Here, the lymphoma cells displayed less MHC class I on their surface, thereby allowing immune escape. However, peptide-dependent CTL activity in response to a protein found in both healthy and TAP-deficient cells was recorded, suggesting immunogenic epitopes are still generated when normal peptide processing is altered [34]. It is conceivable then that altered proteasome function in acute leukemia can produce a repertoire of these neoantigens specific to the tumor cell to be exploited by immunotherapy.
The above evidence demonstrates that genomic instability and dysregulated cellular function of cancer cells indeed creates immunogenic neoantigens, and the discovery of additional repertoires of leukemia-specific neoantigens in murine cancer models really emphasize the “tumor-specific” rather than “cancer-specific” focus of immunotherapeutic approaches against these targets in cancer. They also demonstrate the strong immunoediting phenomenon and the evolution of tumor cells that contain highly potent neoantigens. The clearance of tumor cells by neoantigen-specific CD8+ T-cells favors a cancer cell that loses this antigen expression and downregulates MHC class I, but harnessing this strong anti-tumor response before the immunoediting effect could readily clear tumor cells [35–37]. Several successful inductions of immune response against mutated epitopes have been shown in cancers, and in fact, advanced technology including whole exome sequencing of the tumor cells and screening of tumor-specific mutations can prospectively identify mutated epitopes that can be recognized by tumor-reactive CD4+ T-cells and tumor-infiltrating lymphocytes (TILs) [38–41]. The application of whole exome sequencing to reveal tumor-specific mutations that produce functional tumor-specific neoantigens would result in a more patient-specific immunotherapy that could lead to eradiation of these tumor cells that contain highly antigenic mutant proteins before they have a chance to evade tumor suppressive mechanisms. However, recent observations regarding somatic mutations found with clonal hematopoiesis associated with aging in cancer-free individuals highlight the importance of appropriate patient-specific target validation [42, 43].
Another source of neoantigens in cancer arise from dys-regulated splicing. Splicing in a healthy individual is a highly regulated process, but abnormal splice variants can lead to abnormal proteins that contribute to malignant pheno-type and are a hallmark of cancer [44]. While a protein may be ubiquitously expressed across a variety of tissues, alternative isoforms arising from dysregulated splicing may distinguish a tumor cell from healthy cells. A recent exhaustive study of the AML genome revealed that almost 30% of expressed genes in AML faced differential splicing that affected nearly all aspects of cell growth and survival, and these splice variants and resulting proteins could be potential targets of therapy [45]. One particular variant of NOTCH2 (NOTCH2-Va) and one of FLT3 (FLT3-Va) are found on the cell surface of AML blasts but not present in healthy donors, and a DNA fragment analysis demonstrated that NOTCH2-Va was present in 73% and FLT3-Va in 50% of patients [46].
Another AML-specific isoform arises from CD44, a class I membrane glycoprotein and the major hyaluronan receptor, overexpressed in some hematological malignancies and important in the phenotype of the tumor cell [47]. Previous studies looked at the activity of a CD44-specific monoclonal antibody (mAb) against AML stem cells, and while this antibody was able to eradicate AML stem cells in xenograft mouse models, CD44 expression is observed in healthy hematopoietic progenitor cells and across other tissues, rendering it a less than ideal target of immunotherapy [48, 49]. However, similar to NOTCH2 and FLT3, CD44 undergoes extensive alternative splicing, and one functionally distinct isoform of CD44, variant 6 (CD44v6), is present in a subset of AML patients. Casucci, et al. showed that CD44v6 was expressed in 16/25 (64%) of their cohort of AML patients with different FAB subtypes, and CD44v6 was absent in HSCs, hematopoietic progenitors, and cells of lymphoid origin. Authors developed a CAR against CD44v6 that demonstrated potent anti-leukemic activity against primary AML cells while sparing HSCs [50]. The relative abundance of NOTCH2-Va, FLT3-Va, and CD44v6 splice variants in AML patients and their specificity to the leukemia makes these isoforms attractive targets of immunotherapy. While the immunogenicity and clinical relevance of NOTCH2-Va and FLT3-Va have yet to be fully elucidated, targeting the CD44v6 isoform has already demonstrated potential therapeutic effects, and CD44v6-directed CAR T-cells hold promise in a clinical setting.
POST-TRANSLATIONALLY MODIFIED PROTEINS: ALTERED EPITOPES
Many proteins undergo post-translational modifications that shape their eventual function in the cell, and aberrations in these modifications, including but not limited to phosphorylation, glycosylation, or prenylation can yield targets of immunotherapy that are specific to the leukemia. Protein phosphorylation is important in the oncogenic signaling process, and phosphorylation is preserved on peptides during antigen processing for both MHC class I and II [51–53]. Phosphorylation can lead to a greater and more stable affinity of the MHC class I molecule HLA-A2 with the phosphorylated peptide, or phosphopeptide. Phosphopeptides that hold a phosphorylation signature characteristic of dysregulated oncogenic signaling can create a huge set of tumor-specific peptide antigens that can be recognized by CD8+ T-cells [54]. A comprehensive study of phosphopeptides in various hematological malignancies identified 10 previously undescribed HLA-A2-restricted and 85 HLA-B7-restricted phosphopeptides. Of these, 56 HLA-B7-restricted phosphopeptides were found on AML, and 36 were not present in other leukemias. Though leukemia patients demonstrate a reduced immunity to these leukemia-associated phosphopep-tides, the partial restoration of immunity in HLA-B7 AML patients can be achieved after allo-HSCT, suggesting a link between the GvL phenomenon and immunity to these phosphopeptides [55].
Aberrant glycosylation is a common condition in cancer that has been associated with tumor invasion and metastasis [56]. Mucins are O-glycosylated proteins that line the apical surfaces of epithelial cells in several organ systems that protect the cell surface. Changes in the glycosylation of mucin 1 (MUC1) are associated with cancer and can contribute to the immune evading characteristics of a tumor cell by allowing survival in hypoxic tumor microenvironments [57]. MUC1 in CML stabilizes the BCR-ABL fusion protein, reducing the sensitivity of tumor cells to imatinib and contributing to pathogenesis [58]. The aberrant expression and oncogenicity of MUC1 was recently studied in AML, and Stroopinsky, et al. reported the high expression of MUC1 on an enriched CD34+Lin-CD38- leukemic stem cell population, with these AML stem cells leading to increased engraftment and leukemogenesis in NOD/SCID mice [59]. Furthermore, MUC1-silenced AML cells injected into irradiated NSG mice had limited AML blast involvement in the bone marrow, suggesting a role of MUC1 in the engraftment and establishment of AML [59]. MUC1 was also shown to associate with FLT3 in AML, and the disruption of the MUC1/FLT3 complex downregulated FLT3 activation [60]. These pieces of evidence point to the integral role of MUC1 in promoting and maintaining the malignant phenotype. MUC1 can bind both MHC class I and II, and passive and active immunotherapies against MUC1 have been explored in other malignancies with varying degrees of success [61]. A recent study generated antibodies against epitopes spanning the insoluble signal peptide (SP) domain of MUC1 and tested the ability of the antibodies in targeting multiple myeloma tumor cells from patients. Functional antibodies against the MUC1-SP domain localized to the tumor cell membrane and induced complement-dependent cytotoxicity (CDC) [62]. The promiscuity of MUC1 in MHC binding allows for a broader immune response through both passive and active immunotherapeutic approaches, and the immunogenicity of the SP domain of MUC1 along with its expression on the cell surface of leukemic stem cells makes MUC1 an exciting potential target of immunotherapy. Further pre-clinical and translational studies are anticipated.
Protein tyrosine phosphatase type IVa member 3 (PRL-3) is an interesting prenylated protein target. The in vivo prenylation of PRL-3 causes an association with the inner leaflet of the plasma membrane, which could have implications in malignant cell migration and proliferation, as many proto-oncogenes require prenylation for their malignant transformation [63, 64]. In a recent study of FLT-ITD negative AML, PRL-3 was found to be over-expressed at the message level in 40% (45/112) of subjects compared to healthy donors and was adversely correlated with survival. The role of PRL-3 in promoting cell proliferation was con firmed in knock-in and knockdown experiments and through xenograft studies where silenced PRL-3 decreased tumor formation. The knockdown of endogenous PRL-3 sensitized ML-1 tumor cells to Ara-C and daunorubicin-induced apoptosis [65]. The over-expression of PRL-3 in the 20 to 30% of FLT3-ITD mutated cases has also been identified as a prognosticator of poor survival, and PRL-3-directed therapy could work synergistically with FLT3 inhibitors [66, 67]. Despite the localization of PRL-3 on the intracellular side of the plasma membrane, antibody recognition of the antigen is still possible. In a murine melanoma model, anti-PRL-3 vaccination therapy inhibited the formation of metastatic PRL-3+ tumors [68]. The elucidation of specific immunogenic peptides of PRL-3 remains, but enhancing AML tumor sensitivity to chemotherapy with therapies targeted against PRL-3 opens an avenue for treating tumor cells that have developed a resistance to standard treatments [69].
PEPTIDE-MHC COMPLEXES: NOVEL EPITOPES
One limitation of T-cell based immunotherapy is the need to identify antigens that are both presented on the cell surface by MHC and necessary for the survival of the tumor cell [7, 70, 71]. The expression of many leukemia-associated antigens is confined to the interior of a tumor cell, so an immune effector cell cannot bind them unless they processed and presented by MHC. These peptide-MHC complexes are potential target of immunotherapy because of the ability of T-cell receptor (TCR)-like antibodies to bind with high affinity to these combined epitopes and mediate antibody-dependent cell cytotoxicity (ADCC) or CDC [72]. Several peptide-MHC complexes and TCR-like mAb against them have shown promise in specifically targeting leukemia while sparing normal hematopoietic stem cell (HSC) populations.
A TCR-like IgG2a antibody (8F4) that only binds a PR1/HLA-A2 combined epitope can lead to CDC of PR1/HLA-A2 positive AML blasts, though some expression of this complex on healthy HSCs and leukocytes has been reported. Nonetheless, researchers were able to identify an appropriate dose of antibody that induced killing of AML blasts but not healthy leukocytes, suggesting a mechanism of preferential killing of leukemia by 8F4 despite expression of PR1/HLA-A2 complex in HSCs [73]. The TCR-like mAbs ESK1 and ESKM bind a combined epitope of WT1 on HLA-A2 (RMF/HLA-A2) and demonstrate an ability to kill RMF/HLA-A2 leukemias in mouse models, with the latter antibody having a defucosylated Fc-region to enhance ADCC [74, 75]. There are no reported off-targeted effects from treatment of mice with ESKM though HSCs also express WT1, again suggesting a mechanism of preferential targeting tumor cells and sparing of normal hematopoietic progenitors [75]. A recent study further mapping potential WT1 epitopes and presenting HLA allele reported 41 new WT1 epitopes. Of the 36 immunogenic WT1 peptides, 29 were processed, presented, and recognized by WT1-specific T-cells [71]. The diverse range of WT1-peptide/MHC complexes are promising potential targets for additional TCR-like mAbs, allowing for the targeting of leukemia even when aberrant WT1 isoforms are expressed in the tumor cells.
PERSPECTIVES
New potential targets of immunotherapy are being found at a rapid pace, but the search for ideal antigens should include more than over-expressed proteins, as the possible repertoire of targets derived from other aspects of cancer cell machinery is vast. It remains an unanswered question in which clinical situation immunotherapy for AML would be most efficacious; however, one central tenet of immunotherapy is the theoretical ability to specifically target the tumor cells while sparing healthy tissues. Many commonly proposed LAAs are not expressed at a level that differentiates the AML from normal tissues [5]. Furthermore, antigen expression, both at the gene and protein levels, is insufficient; the antigen must be processed and actually presented on HLA molecules for immunotherapy to work. The recent mapping of the HLA “ligandome” in AML uncovered thousands of ligands - over a thousand of which were specific to AML [70]. However, these identified naturally processed and presented AML peptides do not match those supposedly proverbial antigens that comprise most of the current attempts at protein-directed immunotherapy - a disconnect which is both sobering and humbling.
Given the gradual accumulation of somatic genomic alterations, including those that produce neoantigens, the establishment of a subclonal architecture where an AML clone will survive initial treatment and continue to accrue mutations will lead to a relapse clone that is resistant to chemotherapy and expresses different antigens compared to the predominant clone at presentation [9]. It is unlikely that a single immunotherapeutic strategy could successfully eliminate all tumor cells, and the immunotherapy of AML in the face of disease evolution could greatly benefit from a multi-pronged attack of non-protein targets and tumor-specific neoantigens essential for tumor cell survival, in addition to those over-expressed proteins known to contribute to leukemic phenotype [76]. These novel, non-protein targets and neoantigens must still satisfy certain criteria to be useful in immunotherapy; namely, they must be leukemia-specific, widely expressed within the AML compartment, important to leukemic phenotype, capable of eliciting an immune response, and translatable into a clinical setting [77, 78]. Furthermore, the optimal timepoint in which to integrate such antigen targeted immunotherapy remains to be determined. Many older adults diagnosed with AML are poorly served with current standard of care therapy and may benefit from early immunotherapy, perhaps in combination with demethylating agents, protea-some inhibitors, immunomodulatory drugs and/or immune checkpoint agents [79–81]. Younger adults and children with AML however may still get significant benefit from initial cytotoxic therapy and potential allo-HSCT followed by immune-based therapy, based on the antigens present on the residual clone remaining after such treatment in a measurable residual disease/maintenance treatment paradigm [82–86]. Finally, the antigens discussed here are not intended to serve as a comprehensive list; rather, they should been viewed as examples of novel types of targets that can bolster the efforts to find productive ways to integrate immunotherapy into the care of patients with AML.
ACKNOWLEDGEMENTS
This work was supported by the Intramural Research Program of the National Heart, Lung, and Blood Institute of the National Institutes of Health. Meghali Goswami is a pre-doctoral student in the Institute for Biomedical Sciences at The George Washington University. This work is from a dissertation to be presented to the above program in partial fulfillment of the requirements for the Ph.D. degree.
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- [1].Barrett AJ, Le Blanc K. Immunotherapy prospects for acute myeloid leukaemia. Clin Exp Immunol 2010; 161(2): 223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Soiffer RJ. Donor lymphocyte infusions for acute myeloid leukaemia. Best Pract Res Clin Haematol 2008; 21(3): 455–66. [DOI] [PubMed] [Google Scholar]
- [3].Smits EL, Berneman ZN, Van Tendeloo VF. Immunotherapy of acute myeloid leukemia: current approaches. Oncologist 2009; 14(3): 240–52. [DOI] [PubMed] [Google Scholar]
- [4].Hourigan CS, Levitsky HI. Evaluation of current cancer immunotherapy: hemato-oncology. Cancer J 2011; 17(5): 309–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Goswami M, Hensel N, Smith BD, et al. Expression of putative targets of immunotherapy in acute myeloid leukemia and healthy tissues. Leukemia 2014; 28(5): 1167–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Snauwaert S, Vanhee S, Goetgeluk G, et al. RHAMM/HMMR (CD168) is not an ideal target antigen for immunotherapy of acute myeloid leukemia. Haematologica 2012; 97(10): 1539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Greiner J, Bullinger L, Guinn BA, Dohner H, Schmitt M. Leukemia-associated antigens are critical for the proliferation of acute myeloid leukemia cells. Clin Cancer Res 2008; 14(22): 7161–6. [DOI] [PubMed] [Google Scholar]
- [8].Greiner J, Schmitt M, Li L, et al. Expression of tumor-associated antigens in acute myeloid leukemia: Implications for specific immunotherapeutic approaches. Blood 2006; 108(13): 4109–17. [DOI] [PubMed] [Google Scholar]
- [9].Ding L, Ley TJ, Larson DE, et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature 2012; 481(7382): 506–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].de Lalla C, Lepore M, Piccolo FM, et al. High-frequency and adaptive-like dynamics of human CD1 self-reactive T cells. Eur J Immunol 2011; 41(3): 602–10. [DOI] [PubMed] [Google Scholar]
- [11].Lepore M, de Lalla C, Gundimeda SR, et al. A novel self-lipid antigen targets human T cells against CD1c(+) leukemias. J Exp Med 2014; 211(7): 1363–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mills GB, Moolenaar WH. The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer 2003; 3(8): 582–91. [DOI] [PubMed] [Google Scholar]
- [13].Gibbins JD, Ancelet LR, Weinkove R, et al. An autologous leukemia cell vaccine prevents murine acute leukemia relapse after cytarabine treatment. Blood 2014; 124(19): 295–363. [DOI] [PubMed] [Google Scholar]
- [14].Mattarollo SR, West AC, Steegh K, et al. NKT cell adjuvant-based tumor vaccine for treatment of myc oncogene-driven mouse B-cell lymphoma. Blood 2012; 120(15): 3019–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Shimizu K, Kurosawa Y, Taniguchi M, Steinman RM, Fujii S. Cross-presentation of glycolipid from tumor cells loaded with alpha-galactosylceramide leads to potent and long-lived T cell mediated immunity via dendritic cells. J Exp Med 2007; 204(11): 2641–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Westwood JA, Murray WK, Trivett M, et al. The Lewis-Y carbohydrate antigen is expressed by many human tumors and can serve as a target for genetically redirected T cells despite the presence of soluble antigen in serum. J Immunother 2009; 32(3): 292–301. [DOI] [PubMed] [Google Scholar]
- [17].Peinert S, Prince HM, Guru PM, et al. Gene-modified T cells as immunotherapy for multiple myeloma and acute myeloid leukemia expressing the Lewis Y antigen. Gene Ther 2010; 17(5): 678–86. [DOI] [PubMed] [Google Scholar]
- [18].Ritchie DS, Neeson PJ, Khot A, et al. Persistence and efficacy of second generation CAR T cell against the LeY antigen in acute myeloid leukemia. Mol Ther 2013; 21(11): 2122–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med 2013; 368(22): 2059–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Fathi AT, Sadrzadeh H, Borger DR, et al. Prospective serial evaluation of 2-hydroxyglutarate, during treatment of newly diagnosed acute myeloid leukemia, to assess disease activity and therapeutic response. Blood 2012; 120(23): 4649–52. [DOI] [PubMed] [Google Scholar]
- [21].DiNardo CD, Propert KJ, Loren AW, et al. Serum 2-hydroxyglutarate levels predict isocitrate dehydrogenase mutations and clinical outcome in acute myeloid leukemia. Blood 2013; 121(24): 4917–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sasaki M, Knobbe CB, Munger JC, et al. IDH1(R132H) mutation increases murine haematopoietic progenitors and alters epigenetics. Nature 2012; 488(7413): 656–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kats LM, Reschke M, Taulli R, et al. Proto-oncogenic role of mutant IDH2 in leukemia initiation and maintenance. Cell Stem Cell 2014; 14(3): 329–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chaturvedi A, Araujo Cruz MM, Jyotsana N, et al. Mutant IDH1 promotes leukemogenesis in vivo and can be specifically targeted in human AML. Blood 2013; 122(16): 2877–87. [DOI] [PubMed] [Google Scholar]
- [25].Schumacher T, Bunse L, Pusch S, et al. A vaccine targeting mutant IDH1 induces antitumour immunity. Nature 2014; 512(7514): 324–7. [DOI] [PubMed] [Google Scholar]
- [26].Falini B, Mecucci C, Tiacci E, et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med 2005; 352(3): 254–66. [DOI] [PubMed] [Google Scholar]
- [27].Grummitt CG, Townsley FM, Johnson CM, Warren AJ, Bycroft M. Structural consequences of nucleophosmin mutations in acute myeloid leukemia. J Biol Chem 2008; 283(34): 23326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Greiner J, Ono Y, Hofmann S, et al. Mutated regions of nucleophosmin 1 elicit both CD4(+) and CD8(+) T-cell responses in patients with acute myeloid leukemia. Blood 2012; 120(6): 1282–9. [DOI] [PubMed] [Google Scholar]
- [29].Greiner J, Schneider V, Schmitt M, et al. Immune responses against the mutated region of cytoplasmatic NPM1 might contribute to the favorable clinical outcome of AML patients with NPM1 mutations (NPM1mut). Blood 2013; 122(6): 1087–8. [DOI] [PubMed] [Google Scholar]
- [30].Hofmann S, Gotz M, Schneider V, et al. Donor lymphocyte infusion induces polyspecific CD8(+) T-cell responses with concurrent molecular remission in acute myeloid leukemia with NPM1 mutation. J Clin Oncol 2013; 31(3): e44–7. [DOI] [PubMed] [Google Scholar]
- [31].Schneider V, Zhang L, Bullinger L, et al. Leukemic stem cells of acute myeloid leukemia patients carrying NPM1 mutation are candidates for targeted immunotherapy. Leukemia 2014; 28(8): 1759–62. [DOI] [PubMed] [Google Scholar]
- [32].Held SA, Duchardt KM, Tenzer S, et al. Imatinib mesylate and nilotinib affect MHC-class I presentation by modulating the proteasomal processing of antigenic peptides. Cancer Immunol Immunother 2013; 62(4): 715–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Posthuma EF, van Bergen CA, Kester MG, et al. Proteosomal degradation of BCR/ABL protein can generate an HLA-A*0301-restricted peptide, but high-avidity T cells recognizing this leukemia-specific antigen were not demonstrated. Haematologica 2004; 89(9): 1062–71. [PubMed] [Google Scholar]
- [34].van Hall T, Wolpert EZ, van Veelen P, et al. Selective cytotoxic T-lymphocyte targeting of tumor immune escape variants. Nat Med 2006; 12(4): 417–24. [DOI] [PubMed] [Google Scholar]
- [35].Vago L, Perna SK, Zanussi M, et al. Loss of mismatched HLA in leukemia after stem-cell transplantation. N Engl J Med 2009; 361(5): 478–88. [DOI] [PubMed] [Google Scholar]
- [36].Matsushita H, Vesely MD, Koboldt DC, et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature 2012; 482(7385): 400–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].DuPage M, Mazumdar C, Schmidt LM, Cheung AF, Jacks T. Expression of tumour-specific antigens underlies cancer immunoediting. Nature 2012; 482(7385): 405–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lu YC, Yao X, Crystal JS, et al. Efficient identification of mutated cancer antigens recognized by T cells associated with durable tumor regressions. Clin Cancer Res 2014; 20(13): 3401–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Robbins PF, Lu YC, El-Gamil M, et al. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat Med 2013; 19(6): 747–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Tran E, Turcotte S, Gros A, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 2014; 344(6184): 641–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].van Rooij N, van Buuren MM, Philips D, et al. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J Clin Oncol 2013; 31(32): e439–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Genovese G, Kahler AK, Handsaker RE, et al. Clonal Hematopoiesis and Blood-Cancer Risk Inferred from Blood DNA Sequence. N Engl J Med 2014; 371(26): 2477–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med 2014; 371(26): 2488–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ladomery M Aberrant alternative splicing is another hallmark of cancer. Int J Cell Biol 2013; 2013(463786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Adamia S, Haibe-Kains B, Pilarski PM, et al. A genome-wide aberrant RNA splicing in patients with acute myeloid leukemia identifies novel potential disease markers and therapeutic targets. Clin Cancer Res 2014; 20(5): 1135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Adamia S, Bar-Natan M, Haibe-Kains B, et al. NOTCH2 and FLT3 gene mis-splicings are common events in patients with acute myeloid leukemia (AML): new potential targets in AML. Blood 2014; 123(18): 2816–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Zoller M CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nat Rev Cancer 2011; 11(4): 254–67. [DOI] [PubMed] [Google Scholar]
- [48].Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med 2006; 12(10): 1167–74. [DOI] [PubMed] [Google Scholar]
- [49].Erb U, Megaptche AP, Gu X, Buchler MW, Zoller M. CD44 standard and CD44v10 isoform expression on leukemia cells distinctly influences niche embedding of hematopoietic stem cells. J Hematol Oncol 2014; 7: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Casucci M, Nicolis di Robilant B, Falcone L, et al. CD44v6-targeted T cells mediate potent antitumor effects against acute myeloid leukemia and multiple myeloma. Blood 2013; 122(20): 3461–72. [DOI] [PubMed] [Google Scholar]
- [51].Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144(5): 646–74. [DOI] [PubMed] [Google Scholar]
- [52].Depontieu FR, Qian J, Zarling AL, et al. Identification of tumor-associated, MHC class II-restricted phosphopeptides as targets for immunotherapy. Proc Natl Acad Sci USA 2009; 106(29): 12073–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Zarling AL, Ficarro SB, White FM, et al. Phosphorylated peptides are naturally processed and presented by major histocompatibility complex class I molecules in vivo. J Exp Med 2000; 192(12): 1755–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Mohammed F, Cobbold M, Zarling AL, et al. Phosphorylation-dependent interaction between antigenic peptides and MHC class I: a molecular basis for the presentation of transformed self. Nat Immunol 2008; 9(11): 1236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Cobbold M, De La Pena H, Norris A, et al. MHC class I-associated phosphopeptides are the targets of memory-like immunity in leukemia. Sci Transl Med 2013; 5(203): 203ra125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hauselmann I, Borsig L. Altered tumor-cell glycosylation promotes metastasis. Front Oncol 2014; 4: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer 2004; 4(1): 45–60. [DOI] [PubMed] [Google Scholar]
- [58].Kawano T, Ito M, Raina D, et al. MUC1 oncoprotein regulates Bcr-Abl stability and pathogenesis in chronic myelogenous leukemia cells. Cancer Res 2007; 67(24): 11576–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Stroopinsky D, Rosenblatt J, Ito K, et al. MUC1 is a potential target for the treatment of acute myeloid leukemia stem cells. Cancer Res 2013; 73(17): 5569–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Liu S, Yin L, Stroopinsky D, et al. MUC1-C oncoprotein promotes FLT3 receptor activation in acute myeloid leukemia cells. Blood 2014; 123(5): 734–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Roulois D, Gregoire M, Fonteneau JF. MUC1-specific cytotoxic T lymphocytes in cancer therapy: induction and challenge. Biomed Res Int 2013; 2013: 871936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Kovjazin R, Horn G, Smorodinsky NI, Shapira MY, Carmon L. Cell surface-associated anti-MUC1-derived signal peptide antibodies: implications for cancer diagnostics and therapy. PLoS One 2014; 9(1): e85400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Berndt N, Hamilton AD, Sebti SM. Targeting protein prenylation for cancer therapy. Nat Rev Cancer 2011; 11(11): 775–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Zeng Q, Si X, Horstmann H, et al. Prenylation-dependent association of protein-tyrosine phosphatases PRL-1, −2, and −3 with the plasma membrane and the early endosome. J Biol Chem 2000; 275(28): 21444–52. [DOI] [PubMed] [Google Scholar]
- [65].Qu S, Liu B, Guo X, et al. Independent oncogenic and therapeutic significance of phosphatase PRL-3 in FLT3-ITD-negative acute myeloid leukemia. Cancer 2014; 120(14): 2130–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Zhou J, Bi C, Chng WJ, et al. PRL-3, a metastasis associated tyrosine phosphatase, is involved in FLT3-ITD signaling and implicated in anti-AML therapy. PLoS One 2011; 6(5): e19798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Park JE, Yuen HF, Zhou JB, et al. Oncogenic roles of PRL-3 in FLT3-ITD induced acute myeloid leukaemia. EMBO Mol Med 2013; 5(9): 1351–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Guo K, Li J, Tang JP, et al. Targeting intracellular oncoproteins with antibody therapy or vaccination. Sci Transl Med 2011; 3(99): 99ra85. [DOI] [PubMed] [Google Scholar]
- [69].Swords R, Freeman C, Giles F. Targeting the FMS-like tyrosine kinase 3 in acute myeloid leukemia. Leukemia 2012; 26(10): 2176–85. [DOI] [PubMed] [Google Scholar]
- [70].Berlin C, Kowalewski DJ, Schuster H, et al. Mapping the HLA ligandome landscape of acute myeloid leukemia: a targeted approach toward peptide-based immunotherapy. Leukemia 2015; 29(3): 647–59. [DOI] [PubMed] [Google Scholar]
- [71].Doubrovina E, Carpenter T, Pankov D, et al. Mapping of novel peptides of WT-1 and presenting HLA alleles that induce epitope-specific HLA-restricted T cells with cytotoxic activity against WT-1(+) leukemias. Blood 2012; 120(8): 1633–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Dahan R, Reiter Y. T-cell-receptor-like antibodies - generation, function and applications. Expert Rev Mol Med 2012; 14: e6. [DOI] [PubMed] [Google Scholar]
- [73].Sergeeva A, Alatrash G, He H, et al. An anti-PR1/HLA-A2 T-cell receptor-like antibody mediates complement-dependent cytotoxicity against acute myeloid leukemia progenitor cells. Blood 2011; 117(16): 4262–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Dao T, Yan S, Veomett N, et al. Targeting the intracellular WT1 oncogene product with a therapeutic human antibody. Sci Transl Med 2013; 5(176): 176ra33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Veomett N, Dao T, Liu H, et al. Therapeutic efficacy of an Fc-enhanced TCR-like antibody to the intracellular WT1 oncoprotein. Clin Cancer Res 2014; 20(15): 4036–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Weber G, Gerdemann U, Caruana I, et al. Generation of multi-leukemia antigen-specific T cells to enhance the graft-versus-leukemia effect after allogeneic stem cell transplant. Leukemia 2013; 27(7): 1538–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Cheever MA, Allison JP, Ferris AS, et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res 2009; 15(17): 5323–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Anguille S, Van Tendeloo VF, Berneman ZN. Leukemia-associated antigens and their relevance to the immunotherapy of acute myeloid leukemia. Leukemia 2012; 26(10): 2186–96. [DOI] [PubMed] [Google Scholar]
- [79].Hourigan CS, Karp JE. Development of therapeutic agents for older patients with acute myelogenous leukemia. Curr Opin Investig Drugs 2010; 11(6): 669–77. [PMC free article] [PubMed] [Google Scholar]
- [80].Zeidner JF, Foster MC. Immunomodulatory Drugs: IMiDs in acute myeloid leukemia (AML). Curr Drug Targets 2015; (In press): [DOI] [PubMed] [Google Scholar]
- [81].Knaus HA KC, Luznik L, Gojo I Immunomodulatory drugs II: Immune Checkpoint Agents in Acute Leukemia . Current Drug Targets 2015; (Submitted). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Hourigan CS, Karp JE. Minimal residual disease in acute myeloid leukaemia. Nat Rev Clin Oncol 2013; 10(8): 460–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Hokland P, Cotter F. Readying the minimal residual disease concept in acute myeloid leukaemia for prime time - the American way. Br J Haematol 2013; 162(4): 429–30. [DOI] [PubMed] [Google Scholar]
- [84].Roug AS, Hansen MC, Nederby L, Hokland P. Diagnosing and following adult patients with acute myeloid leukaemia in the genomic age. Br J Haematol 2014; 167(2): 162–76. [DOI] [PubMed] [Google Scholar]
- [85].Hourigan CS, McCarthy P, de Lima M. Back to the future! The evolving role of maintenance therapy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2014; 20(2): 154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Oran B, de Lima M. Prevention and treatment of acute myeloid leukemia relapse after allogeneic stem cell transplantation. Curr Opin Hematol 2011; 18(6): 388–94. [DOI] [PubMed] [Google Scholar]