Abstract
Rationale: The prevalence of childhood asthma has been increasing worldwide in parallel with childhood obesity.
Objectives: We investigated whether there is a temporal relationship between early life weight gain (reflecting growth velocity) and early life body mass index (BMI) attained status (reflecting accumulative weight) with future risk of asthma in the Boston Birth Cohort.
Methods: This report includes 1,928 children from the Boston Birth Cohort with a mean age of 7.8 years (standard deviation, 3.3 yr), enrolled at birth and followed prospectively. Asthma was defined using physician diagnosis code (International Classification of Diseases, Ninth Revision, Clinical Modification code 493.xx) in children 2 years and older. We categorized the children by their weight gain trajectory on the basis of changes in z-scores: slow (less than −0.67), on track (−0.67 to 0.67), rapid (0.67–1.28), and extremely rapid (>1.28); and by their BMI attained status (underweight, normal weight, and overweight) during the first 4, 12, and 24 months. Poisson regression models with robust variance estimation were applied to examine the relationship between early life weight gain/attained BMI and asthma.
Results: During the first 4 months of life, 37% had on-track weight grain, 22% had slow weight gain, 15% had rapid weight gain, and 26% had extremely rapid weight gain. At 4 months, 61% were normal weight, 7% were underweight, and 32% were overweight. In adjusted analyses, extremely rapid early life weight gain during the first 4 and 24 months of life were each associated with increased risks of asthma (risk ratio, 1.34 for extremely rapid weight gain at 4 months; 95% confidence interval [CI], 1.06–1.70; risk ratio, 1.32 for extremely rapid weight gain at 24 months; 95% CI, 1.00–1.75) Similarly, overweight at 4, 12, and 24 months were each associated with an increased risk of asthma. Analyses that further adjusted for birthweight or preterm birth showed similar findings.
Conclusions: In this predominantly urban U.S. low-income minority birth cohort, excessive early life weight gain and overweight status were both associated with an increased risk of asthma in childhood.
Keywords: weight gain, asthma, prospective birth cohort
Over the past several decades, the prevalence of childhood asthma has increased worldwide (1). In parallel, an emerging epidemic of obesity has affected all age groups, including young children (2). The combined data reveal that both asthma and obesity disproportionally affect urban, low-income minorities in the United States (1, 3, 4).
A body of cross-sectional and retrospective studies has documented positive associations between low birthweight and/or rapid weight gain in early life and the subsequent development of childhood atopy and asthma, mainly among European populations (5, 6). However, the temporal relationship between early life weight gain and childhood asthma remains inconsistent. For example, a meta-analysis including 147,252 European children in 31 birth cohort studies found that rapid weight gain in infancy was positively associated with childhood asthma outcomes (7). In contrast, a prospective birth cohort study suggested that being overweight in infancy reduced the risk of childhood asthma (8). These inconsistent findings may be partially due to differences in study designs, study populations, and definitions of early life weight gain or asthma outcomes across studies (8, 9). To date, there remains a particular lack of large prospective birth cohort studies on this topic in U.S. populations.
To address this gap, we sought to examine the association between early life weight gain and asthma in an African American–dominant birth cohort, a population with a high risk of obesity and asthma. Our aims were to simultaneously evaluate the associations of both early life weight gain (reflecting growth velocity) and early life BMI attained status (reflecting accumulative weight) with asthma. We also aimed to determine whether these associations differed by the strata of known risk factors for asthma, including preterm birth (PTB), birth weight, and birth weight for gestation.
Methods
Study Population
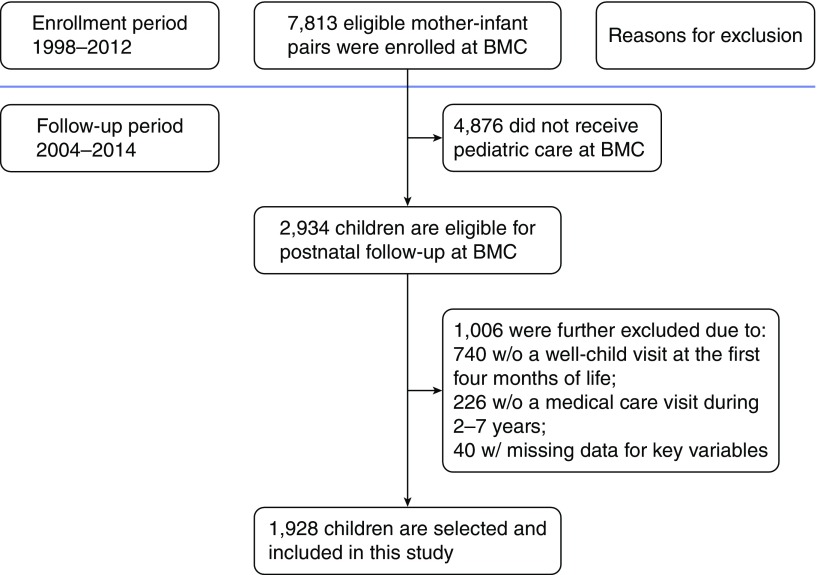
The study population included 1,928 maternal–infant pairs who were part of the Boston Birth Cohort (BBC), a prospective birth cohort study of a predominately African American, urban, low-income minority population initiated in 1998 under rolling enrollment at Boston Medical Center (BMC) (10). Participants were a subset of children born between 1998 and 2012 who received care during at least one postnatal pediatric visit at BMC as documented in their electronic medical record (EMR). Exclusion criteria included: 1) children who were not enrolled in the original BBC at birth, and 2) children who did not receive postnatal pediatric care at BMC. A detailed description of the study exclusion criteria can be found in our previous reports (11, 12). The selection process for study participants is shown in Figure 1. The baseline characteristics of the included and excluded participant samples are provided in Table E1 in the online supplement. Epidemiologic data were collected from the study mothers using a standardized questionnaire that inquired about demographic information, socioeconomic status, maternal asthma history, reproductive history, smoking, and alcohol drinking. Pertinent maternal and child clinical data were obtained from their medical records, including birth outcomes, weight and height measurements, and physician diagnosis and International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. The Institutional Review Boards of BMC, the Massachusetts Department of Public Health, and the Johns Hopkins Bloomberg School of Public Health approved the study protocol. All maternal participants gave written informed consent.
Figure 1.
Flow chart of enrollment and postnatal follow-up of the Boston Birth Cohort in this study. BMC = Boston Medical Center.
Weight Gain Measurement
We followed study participants who were recruited to the BBC and received pediatric primary care at BMC from birth up to age 16 years during the period from 2004 to 2014. Child’s height and weight at age 4, 12, and 24 months were ascertained from well-child visits based on standard clinical procedures. All study children had weight measured by trained pediatric staff using a standard clinical scale and following the same clinical protocol at all time points. Specifically, older children were measured without shoes and wearing only a t-shirt and underwear, and for infants only a diaper was allowed. Previous related studies have defined early life weight gain during various time intervals (10). To look for consistency of the associations across different early life periods, our study defined change in weight gain z-scores during three different time periods: from birth to the first 4, 12, and 24 months. For each time period, we calculated weight-for-age z-scores, using the Stata program for Child Growth Standards developed by the World Health Organization (13), and the change in weight-for-age z-scores during the corresponding period. For each time period, we further classified change in weight gain z-scores into four groups: 1) slow weight gain, defined as a weight gain z-score less than −0.67; 2) on track, defined as a weight gain z-score between −0.67 and 0.67; 3) rapid weight gain, defined as a weight gain z-score between 0.67 and 1.28; and 4) extremely rapid weight gain, defined as a weight gain z-score greater than 1.28 (10). BMI attained status for the first 4, 12, and 24 months, separately, were grouped as underweight, normal weight, and overweight, corresponding to BMI z-scores of less than fifth, fifth to 85th, and greater than or equal to 85th percentiles, respectively (14).
Outcome Assessment
We identified BBC children with asthma on the basis of physician diagnosis (ICD-9-CM code, 493.xx) as documented in the EMR at the BMC during the period from 2004 to 2014, which included outpatient visits, emergency department visits, and hospitalizations. The main outcome measure in this study was prevalent asthma. Ever asthma was defined as an asthma diagnosis code documented at any time at or after the age of 2 years. In a sensitivity analysis, we used four other definitions of asthma. Recurrent asthma diagnosis was defined based on an asthma diagnosis ICD code documented at two or more clinical visits after 2 years of age (15), ever asthma after 6 years of age was defined as an asthma diagnosis code documented after 6 years of age, recurrent asthma after 6 years of age was defined as an asthma diagnosis code documented two or more times after 6 years of age, and asthma after 6 years of age in 2 consecutive years was defined as an asthma code documented in two consecutive years after 6 years of age.
Potential Confounders
We examined a number of pre-, peri-, and postnatal potential confounding variables. Prenatal variables included maternal age, ethnicity, educational level, smoking status during pregnancy (none, ever, and continuous smokers), parity, prepregnancy BMI, maternal history of asthma, hypertensive disorders, and gestational diabetes. Peri- and postnatal risk variables included preterm birth versus term, birth weight for gestation (small for gestational age, appropriate for gestational age, and large for gestational age), birthweight (<2,500 g vs. ≥2,500 g), breastfeeding status (formula only, exclusively breastfed, and both), child’s sex, and child’s age.
Data Analysis
Demographic and clinical characteristics of the study participants were reported as counts and percentages or as means and standard deviations (SDs). Given that the primary outcome of asthma was common in the BBC, the calculation of an odds ratio would overestimate the relative risk of early life weight gain on asthma. Thus, we performed Poisson regression with robust variance estimation, with and without adjustment for known prenatal and postnatal risk factors as described above, to examine the relationships between early life weight gain and asthma. Early life weight gain was treated as a categorical variable and classified into four groups (slow, on track, rapid, and extremely rapid weight gain) in the models. In recognizing that PTB, gestational age, and birthweight are known risk factors that contribute to early life weight gain and asthma, we performed stratified analyses to explore the potential modifying effects of PTB, birth weight for gestation, and birthweight, respectively. We also examined early life BMI attained status as a categorical variable: underweight, normal weight, and overweight. To examine whether the influence of early life weight gain on asthma was independent of childhood attained BMI, childhood BMI at the last well-child visit was treated as a covariate and adjusted in our models. Sensitivity analyses were applied to examine various asthma definitions, preterm birth, birthweight, and birthweight for gestation. Each measure of association was reported as a risk ratio (RR) with a 95% confidence interval (CI). A P value less than 0.05 was the cutoff for statistical significance. All data analyses were conducted using STATA 14.2 software (StataCorp).
Results
Characteristics of the Study Participants
A total of 1,928 children were included in this study. Table 1 presents the prenatal and postnatal characteristics investigated in this study, grouped by the four early life weight gain categories. During the first 4 months of life, 37% had on-track weight gain, 22% had slow weight gain, 15% had rapid weight gain, and 26% had extremely rapid weight gain. At 4 months, 61% were normal weight, 7% were underweight, and 32% were overweight. In terms of maternal characteristics, the distributions of prepregnancy BMI, hypertensive disorder, and maternal smoking during pregnancy were different across exposure groups. In terms of child characteristics, the distributions of birthweight, gestational age, PTB, birth weight for gestation, and asthma status were different across exposure groups.
Table 1.
Characteristics of 1,928 mother–infant pairs in the Boston Birth Cohort
| Variables | On Track* (n = 706) | Slow Weight Gain (n = 420) | Rapid Weight Gain (n = 295) | Extremely Rapid Weight Gain (n = 507) |
|---|---|---|---|---|
| Maternal characteristics | ||||
| Maternal age, yr, mean (SD) | 28.8 (6.7) | 28.3 (6.6) | 27.9 (6.2) | 28.9 (6.5) |
| Prepregnancy BMI, kg/m2, mean (SD) | 26.6 (6.7) | 27.2 (6.5) | 25.5 (5.8) | 27.1 (6.9) |
| Prepregnancy BMI† | ||||
| <25.0 | 325 (46.0) | 178 (42.4) | 161 (54.6) | 215 (42.4) |
| 25.0–29.9 | 205 (29.0) | 97 (23.1) | 70 (23.70) | 143 (28.2) |
| ≥30.0 | 144 (20.4) | 119 (28.3) | 49 (16.6) | 123 (24.3) |
| Missing | 32 (4.5) | 26 (6.2) | 15 (5.1) | 26 (5.1) |
| Null parity | 312 (44.3) | 154 (36.8) | 127 (43.2) | 228 (45.0) |
| Breast versus formula feeding |
||||
| Formula only | 172 (25.0) | 114 (28.0) | 61 (21.0) | 120 (24.1) |
| Exclusively breastfed | 60 (8.7) | 27 (6.6) | 23 (7.9) | 40 (8.0) |
| Both | 456 (66.3) | 266 (65.4) | 207 (71.1) | 339 (67.9) |
| Maternal history of asthma, yes | 85 (12.5) | 51 (12.8) | 42 (14.5) | 73 (14.8) |
| Gestational diabetes, yes | 36 (5.1) | 20 (4.8) | 11 (3.7) | 23 (4.6) |
| Hypertensive disorder, yes | 35 (5.0) | 21 (5.1) | 18 (6.1) | 52 (10.3) |
| Education |
||||
| High school or less | 614 (87.0) | 371 (89.2) | 251 (85.7) | 439 (87.1) |
| College or higher | 92 (13.0) | 45 (10.8) | 42 (14.3) | 65 (12.9) |
| Maternal smoking during pregnancy† |
||||
| Never | 581 (83.4) | 357 (85.6) | 243 (83.2) | 385 (76.2) |
| Quitter | 48 (6.9) | 29 (7.0) | 22 (7.5) | 53 (10.5) |
| Current smoker | 68 (9.8) | 31 (7.4) | 27 (9.3) | 67 (13.3) |
| Maternal alcohol drinking during pregnancy, yes | 43 (6.3) | 27 (6.7) | 27 (9.4) | 43 (8.7) |
| Child characteristics | ||||
| Child age, yr, mean (SD)‡ | 7.6 (3.1) | 8.7 (3.8) | 7.5 (3.1) | 7.7 (3.2) |
| Sex, male | 366 (51.8) | 204 (48.6) | 151 (51.2) | 254 (50.1) |
| Birthweight, g, mean (SD)† | 3,146.3 (597.6) | 3,470.0 (586.5) | 2,819.7 (670.1) | 2,220.5 (799.3) |
| Gestational age, wk, mean (SD)† | 38.8 (2.3) | 39.4 (1.6) | 37.8 (2.9) | 34.9 (4.4) |
| Preterm birth (gestational age < 37)† | 99 (14.0) | 25 (6.0) | 80 (27.1) | 303 (59.9) |
| Ever asthma, yes† | 149 (21.1) | 96 (22.9) | 76 (25.8) | 189 (37.3) |
| Birth weight for gestation† | ||||
| Small for gestational age | 71 (10.1) | 35 (8.3) | 54 (18.3) | 85 (16.8) |
| Appropriate for gestational age | 560 (79.3) | 298 (71.0) | 232 (78.6) | 406 (80.1) |
| Large for gestational age | 75 (10.6) | 87 (20.7) | 9 (3.1) | 16 (3.2) |
Definition of abbreviations: BMI = body mass index; SD = standard deviation.
Data presented as n (%) unless otherwise noted.
Weight gain was classified based on the weight gain in the first 4 months.
P < 0.05 across four exposure categories.
Child age was computed based on their last visit.
Association of Early Life Weight Gain Trajectory with Asthma
Extremely rapid early life weight gain group during the first 4, 12, and 24 months of life was found to be associated with an increased risk of asthma compared with being in the on-track group (adjusted RR, 1.44; 95% CI, 1.19–1.75 for the first 4 months; adjusted RR, 1.40; 95% CI, 1.11–1.77 for the first 12 months; and adjusted RR, 1.28; 95% CI, 1.02–1.62 for the first 24 months; Table 2). In addition, early life attained BMI during the first 4, 12, and 24 months of life were each associated with increased risks of asthma (Table 2). Of note, we did not observe a substantial mediation effect of childhood BMI attained status when BMI attained status at the last well-child visit was included in the models, indicating that the associations of early life weight gain and early life BMI attained status with asthma were independent of childhood BMI (Table 2). These findings persisted in adjusted analyses, albeit with smaller effect estimates and, in the case of extremely rapid weight gain at 12 months, loss of statistical significance (Table 2). Sensitivity analyses using different definitions of asthma showed similar findings (Table E2). Additional analyses that further adjusted for birthweight or preterm birth showed similar findings (Table E3). Likewise, similar associations of early life weight gain at the first 4 and 24 months with asthma across each strata were observed (Table E4).
Table 2.
Associations of weight gain and body mass index attained status in the first 4, 12, and 24 months with asthma
| Early Life Weight Gain Category |
Early Life BMI Attained Status |
||||||
|---|---|---|---|---|---|---|---|
| On Track | Slow | Rapid | Extremely Rapid | Normal | Underweight | Overweight | |
| First 4 mo, n1/n2* | 149/706 | 96/420 | 76/295 | 189/507 | 258/1,177 | 35/131 | 217/620 |
| Crude RR (95% CI) | Ref | 1.08 (0.86–1.36) | 1.22 (0.96–1.55) | 1.77 (1.47–2.12) | Ref | 1.22 (0.90–1.65) | 1.60 (1.37–1.86) |
| Adjusted RR (95% CI)† | Ref | 1.07 (0.84–1.36) | 1.19 (0.93–1.52) | 1.44 (1.19–1.75) | Ref | 1.07 (0.76–1.51) | 1.27 (1.08–1.50) |
| Adjusted RR (95% CI)‡ | Ref | 0.91 (0.68–1.21) | 1.11 (0.83–1.48) | 1.34 (1.06–1.70) | Ref | 0.95 (0.61–1.48) | 1.22 (1.00–1.50) |
| First 12 mo, n1/n2* | 107/568 | 37/196 | 73/283 | 270/771 | 182/905 | 7/39 | 298/874 |
| Crude RR (95% CI) | Ref | 1.00 (0.72–1.40) | 1.37 (1.05–1.78) | 1.86 (1.53–2.26) | Ref | 0.89 (0.45–1.77) | 1.70 (1.45–1.99) |
| Adjusted RR (95% CI)† | Ref | 1.03 (0.72–1.47) | 1.19 (0.91–1.56) | 1.40 (1.11–1.77) | Ref | 0.94 (0.47–1.90) | 1.30 (1.07–1.59) |
| Adjusted RR (95% CI)‡ | Ref | 0.94 (0.62–1.42) | 1.15 (0.84–1.56) | 1.29 (0.97–1.71) | Ref | 0.89 (0.40–2.00) | 1.29 (1.01–1.64) |
| First 24 mo, n1/n2* | 103/500 | 36/165 | 53/259 | 272/760 | 163/798 | 3/14 | 298/862 |
| Crude RR (95% CI) | Ref | 1.06 (0.76–1.48) | 1.03 (0.77–1.39) | 1.74 (1.42–2.12) | Ref | 1.05 (0.38–2.89) | 1.69 (1.44–2.00) |
| Adjusted RR (95% CI)† | Ref | 1.04 (0.73–1.48) | 0.84 (0.61–1.15) | 1.28 (1.02–1.62) | Ref | 1.37 (0.51–3.71) | 1.30 (1.07–1.59) |
| Adjusted RR (95% CI)‡ | Ref | 0.90 (0.58–1.38) | 0.83 (0.57–1.21) | 1.32 (1.00–1.75) | Ref | 1.02 (0.29–3.62) | 1.34 (1.05–1.71) |
Definition of abbreviations: BMI = body mass index; CI = confidence interval; Ref = reference group; RR = risk ratio.
Significant results are in boldface type.
n1: number of individuals with asthma; n2: number of individuals in each exposure group.
Adjusted for maternal age, education, race, smoking, parity, prepregnancy BMI, maternal history of asthma, hypertensive disorders, gestational diabetes, preterm status, birth weight for gestation, child’s age, child’s sex, and breastfeeding status.
Adjusted for maternal age, education, race, smoking, parity, prepregnancy BMI, maternal history of asthma, hypertensive disorders, gestational diabetes, preterm status, birth weight for gestation, child’s age, child’s sex, breastfeeding status, and BMI attained status at the last well child visit.
Associations of Early Life Weight Gain with Asthma Stratified by Preterm Birth and Birthweight
Analyses stratified on preterm birth and on birthweight showed generally similar findings, although some findings were not statistically significant among preterm children and among those with birthweight less than 2,500 g. (Table 3). Analyses stratified on fetal growth status showed significant associations only in the appropriate for gestational age group (Table E5). In addition, we also stratified study children by various known risk factors, including maternal history of asthma, maternal smoking status during pregnancy, maternal ethnicity, and child’s sex. We found comparable associations between early life weight gain and asthma across those strata, suggesting that those factors did not modify the association between early life weight gain and asthma in our study population.
Table 3.
Associations of weight gain and body mass index attained status in the first 12 months with asthma stratified by preterm birth status and birthweight
| Early Life Weight Gain Category |
Early Life BMI Attained Status |
||||||
|---|---|---|---|---|---|---|---|
| On Track | Slow | Rapid | Extremely Rapid | Normal | Underweight | Overweight | |
| Term children | |
||||||
| n1/n2* | 101/532 | 36/191 | 60/246 | 92/372 | 170/849 | 7/37 | 112/455 |
| Crude RR (95% CI) | Ref | 0.99 (0.70–1.40) | 1.28 (0.97–1.70) | 1.30 (1.01–1.67) | Ref | 0.94 (0.48–1.87) | 1.23 (1.00–1.52) |
| Adjusted RR (95% CI)† | Ref | 0.99 (0.68–1.44) | 1.19 (0.89–1.60) | 1.30 (1.00–1.70) | Ref | 0.95 (0.47–1.93) | 1.22 (0.97–1.53) |
| Preterm children | |
||||||
| n1/n2* | 6/36 | 1/5 | 13/37 | 177/398 | 12/56 | 0/2 | 185/418 |
| Crude RR (95% CI) | Ref | 1.20 (0.18–8.03) | 2.11 (0.90–4.94) | 2.67 (1.27–5.59) | Ref | NA | 2.06 (1.24–3.45) |
| Adjusted RR (95% CI)† | Ref | 1.23 (0.20–7.74) | 1.74 (0.69–4.37) | 2.26 (0.98–5.25) | Ref | NA | 1.90 (1.08–3.25) |
| Birthweight < 2,500 g | |
||||||
| n1/n2* | 5/25 | 2/5 | 10/35 | 166/399 | 11/49 | 1/2 | 171/413 |
| Crude RR (95% CI) | Ref | 2.00 (0.53–7.57) | 1.43 (0.56–3.67) | 2.19 (0.99–4.83) | Ref | 2.23 (0.51–9.80) | 1.84 (1.08–3.14) |
| Adjusted RR (95% CI)† | Ref | 2.17 (0.57–8.22) | 1.18 (0.45–3.08) | 1.63 (0.71–3.74) | Ref | 2.01 (0.37–10.85) | 1.54 (0.91–2.60) |
| Birthweight ≥ 2,500 g | |
||||||
| n1/n2* | 102/543 | 35/191 | 63/248 | 104/372 | 171/856 | 6/37 | 127/461 |
| Crude RR (95% CI) | Ref | 0.98 (0.69–1.38) | 1.35 (1.03–1.78) | 1.49 (1.17–1.89) | Ref | 0.81 (0.38–1.71) | 1.38 (1.13–1.68) |
| Adjusted RR (95% CI)† | Ref | 0.92 (0.64–1.33) | 1.25 (0.94–1.67) | 1.49 (1.17–1.90) | Ref | 0.72 (0.34–1.52) | 1.39 (1.13–1.70) |
Definition of abbreviations: BMI = body mass index; CI = confidence interval; Ref = reference group; RR = risk ratio.
Significant results are in boldface type.
n1: number of individuals with asthma; n2: number of individuals in each exposure group.
Adjusted for maternal age, education, race, smoking, parity, prepregnancy BMI, maternal history of asthma, hypertensive disorders, gestational diabetes, preterm status, birth weight for gestation, child’s age, child’s sex, and breastfeeding status.
Discussion
To our knowledge, this is one of the largest prospective birth cohort studies of U.S. urban, low-income minority children, an African American–dominant population, to investigate the temporal relationship between early life excessive weight gain and asthma. Our results suggest that excessive weight gain as well as early life BMI attained status in the first 4, 12, and 24 months of life were all associated with an increased risk of childhood asthma in the BBC. The association of excessive weight gain in infancy with asthma remained after adjustment for PTB, birthweight, and birth weight for gestation. In addition, our findings also indicate that early life weight gain is an independent risk factor for asthma, even after adjusting for childhood BMI. Moreover, the findings remained robust when we used different definitions of asthma. Our findings, if further confirmed, underscore the need for pediatricians and parents to pay attention to and ensure optimal early life weight gain in the first 2 years of life.
These findings add to the current knowledge base in several ways: first, most previous relevant studies were conducted in European populations, whereas limited studies have been conducted in minority populations such as blacks, even though there is a plethora of evidence showing that this population is at high risk for obesity and asthma. We have filled in this gap in the data by evaluating the relationship between early life weight gain and asthma in a predominantly urban, low-income minority population. Second, although previous studies observed an association between asthma and obesity in children, the temporal relationship was not clear. As a prospective birth cohort, the BBC allows us to evaluate the prospective associations of early life weight gain (reflecting growth velocity) and early life BMI attained status (reflecting accumulative weight) during the first 4, 12, and 24 months of life with asthma. Third, we further clarified the relative importance of early life weight gain versus childhood BMI status in relation to asthma risk. Our findings provide evidence that early life weight gain categories and/or early life BMI attained status are independently associated with asthma risk, even after adjusting for childhood BMI. If the association between extremely rapid weight gain and childhood asthma is proved to be causal, understanding and preventing extremely rapid early life weight gain would be the next steps in research and translation, which is beneficial not only to future cardiometabolic health but also to mitigation of future asthma risk.
To the contrary, a lack of association between early childhood weight gain patterns and the development of asthma has been reported by other studies (8, 16, 17). For example, in a birth cohort study of 3,756 children enrolled in the Prevention and Incidence of Asthma and Mist Allergy study, Scholtens and colleagues reported that a greater BMI among children younger than 5 years of age was not associated with an elevated risk of asthma symptoms, whereas children 6 years of age or older with a high BMI had an increased risk of developing asthma symptoms (16). Another prospective birth cohort study indicated that being overweight in infancy decreased the risk of childhood asthma (8). However, most studies did not account for the effect of PTB and other potential confounding factors present during the first few years of life.
Accumulating evidence indicates that children born preterm have an increased risk of developing wheezing and asthma in childhood (7, 15). In parallel, it has been shown in previous studies that low birthweight is associated with an increased risk of subsequent asthma (18, 19), but so far the results of these studies have been inconclusive (5, 20). Because PTB is highly correlated with low birthweight and influencing early life weight gain, it is necessary to account for the impact of PTB/low birthweight when investigating the relationship between early life weight gain and asthma. In this study, we examined whether PTB modified the relationship between early life weight gain and asthma. In line with the results reported by Sonnenschein-van der Voort and colleagues (7), we observed a positive association between early life weight gain and asthma even after considering the influence of PTB. In addition to PTB, we also evaluated the potential influence of birthweight, birth weight for gestation, maternal history of asthma, maternal smoking status during pregnancy, maternal ethnicity, and child’s sex, but did not find a significant modifiable effect.
Sex difference in relation to childhood overweight/obesity and asthma has been reported by other studies (21, 22). For example, Chen and colleagues performed a meta-analysis and found that sex might modify the relationship between childhood overweight/obesity and asthma (21). A recent study reported by Ekström and colleagues also found a sex difference in the association between BMI development and asthma status (22). However, most studies focused on childhood BMI, overweight, or obesity, rather than early life weight gain. Our study did not observe a significant sex difference in the early life weight gain–asthma association. Future studies should further investigate the role of sex on the association between early life weight gain and asthma.
Our findings are in line with several previous studies (23–25). For example, in a study of 9,723 children included in a British prospective birth study, Sonnenschein-van der Voort and colleagues demonstrated that rapid weight gain in early life is associated with asthma among children at age 8 years (23). In addition to asthma, previous reports have documented that respiratory morbidity may be influenced in part by early life origins and results from abnormal fetal and/or infant growth (26, 27). Similar to the positive association with childhood asthma, rapid weight gain in early life has been shown to be consistently associated with lung function across studies (28, 29). For example, Sonnenschein-van der Voort and colleagues assessed the associations of childhood growth patterns with asthma and lung function in adolescence and found positive associations of early childhood weight gain with asthma, bronchial hyperresponsiveness, and higher lung volume but also found a positive association with increased measures of airway obstruction (23).
The results of this study may be explained at several levels. First, extremely rapid weight gain, especially in the first few months of life, might adversely affect structural remodeling of the lung with changes in alveolar formation and maturation of immune responses (30). Turner and colleagues indicated that infants born with normal birth weight experienced excessive early life weight gain due to a calorie-rich dietary intake that might lead to less favorable growth of the airway wall and, as a result, impair their pulmonary function (29). Second, previous reports showed that adverse changes in maturation of the immune system during early life development due to extremely rapid weight gain might increase the risk of childhood asthma (30, 31). In parallel, previous studies documented that childhood obesity was related to dysfunction of the immune response via altering the release of adipokines and cytokines (32). Because early weight gain increased the risk of childhood obesity, it is possible that excessive weight gain in infancy may adversely modify immunological development and, subsequently, lead to childhood asthma. Third, early life weight gain is a risk factor for overweight and obesity in later life, which is known to be associated with asthma and compromised lung function in both children and adults (16, 33).
Several limitations of this study should be noted. Asthma usually is diagnosed by physicians in clinical practice but is not based on the results of a precise biochemical test. In this study, we defined incident asthma on the basis of EMR data; hence, the reliability and accuracy of the asthma diagnoses were dependent on the accurate recording of physicians. To examine the robustness of the diagnoses, we performed sensitivity analyses on the basis of various diagnostic algorithms for asthma. The comparable results from our sensitivity analyses support the robustness of our study findings across various asthma diagnoses used in this study. Although some outcome misclassification may have occurred, it would be undifferentiated by weight gain status and would reduce the estimated risk. Although we adjusted for several important confounding factors, residual confounding by unmeasured factors, such as individual genetic susceptibility, early life living environment, maternal diet during pregnancy, age of introduction of solids, and early life dietary intake, might still remain (34, 35). We also accounted for potential confounding period effects, but this might not have been fully controlled for even if we included birth year in the analyses (Table E6). In addition, it was difficult to measure weight precisely in the clinic, especially for infants, although measurement error should be random in terms of outcome and as a result would likely decrease the power but would not change the association direction, and any measurement errors should be nondifferential. Metabolic biomarkers, for example, leptin and adiponectin, were not examined in this study, and further investigation into whether similar associations could be found between metabolic biomarkers and asthma in childhood should be considered. We only investigated the influence of early life weight gain on asthma, but because asthma is commonly comorbid with other atopic diseases, such as eczema and allergic rhinitis, it would be of interest to further investigate the relationship between early life weight gain and other atopic diseases in addition to asthma.
As noted, in contrast to previous studies conducted mostly in European populations, our study was conducted in an African American–dominant, high-risk population for obesity and asthma. In this context, our findings have research, clinical, and public health implications. Our study lent further support to similar findings in European populations, strengthening the evidence of a true association. If so, our findings link two major U.S. health disparities (obesity and asthma) under a life course perspective and underscore the importance of ensuring optimal early childhood weight gain, which is important both for future cardiometabolic health and for reducing the risk of asthma. Our findings are directly relevant to high-risk U.S. populations and may be relevant to other populations with characteristics similar to the BBC.
In summary, the findings from this study suggest that excessive weight gain in the first 4, 12, and 24 months of life was significantly and independently associated with childhood asthma in this predominantly urban, low-income minority prospective U.S. birth cohort. Our findings warrant additional investigation in independent birth cohorts, especially U.S. minority cohorts. It would also be of importance to examine biological mechanisms underlying the link between excessive weight gain in early life and asthma. This line of research has important clinical and public health implications, given that weight gain before 2 years of age is a modifiable risk factor.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank all of the study participants for their support and help with the study. They also thank the field team at the Department of Pediatrics, Boston University School of Medicine, for the dedication and hard work and the obstetric nursing staff at Boston Medical Center for their help and support. They also thank Tami R. Bartell at Ann & Robert H. Lurie Children’s Hospital of Chicago, Stanley Manne Children’s Research Institute for English editing.
Footnotes
The Boston Birth Cohort (the parent study) is supported in part by the March of Dimes PERI grants 20-FY02-56 and #21-FY07-605 and National Institutes of Health grants R21ES011666, R21HD066471, R01HD086013, and 2R01HD041702. H.-J.T. is supported by National Science Council grant 101-2314-B-400-009-MY2, Ministry of Science and Technology grant 103-2314-B-400-004-MY3, and the Fulbright Taiwan Foundation for Scholarly Exchange. X.H. is partially supported by Hopkins Population Center National Institute of Child Health and Human Development grant R24HD042854.
Author Contributions: H.-J.T. conceptualized, designed, and supervised the study, and drafted the manuscript. G.W., X.H., and Y.J. assisted in data analysis, and interpreted the results. T.-C.Y., S.R., H.J., and T.L.C. provided intellectual inputs and assisted in data analysis and results interpretation. X.W. supervised the study, and raised funding for the study. All authors contributed to the interpretation and discussion of the results, and read and approved the final article.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Devereux G. The increase in the prevalence of asthma and allergy: food for thought. Nat Rev Immunol. 2006;6:869–874. doi: 10.1038/nri1958. [DOI] [PubMed] [Google Scholar]
- 2.Chen Z, Salam MT, Alderete TL, Habre R, Bastain TM, Berhane K, et al. Effects of childhood asthma on the development of obesity among school-aged children. Am J Respir Crit Care Med. 2017;195:1181–1188. doi: 10.1164/rccm.201608-1691OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asher MI, Montefort S, Björkstén B, Lai CK, Strachan DP, Weiland SK, et al. ISAAC Phase Three Study Group. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases one and three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–743. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- 4.Pulgarón ER. Childhood obesity: a review of increased risk for physical and psychological comorbidities. Clin Ther. 2013;35:A18–A32. doi: 10.1016/j.clinthera.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kindlund K, Thomsen SF, Stensballe LG, Skytthe A, Kyvik KO, Backer V, et al. Birth weight and risk of asthma in 3-9-year-old twins: exploring the fetal origins hypothesis. Thorax. 2010;65:146–149. doi: 10.1136/thx.2009.117101. [DOI] [PubMed] [Google Scholar]
- 6.Wandalsen GF, Chong-Neto HJ, de Souza FS, Solé D, Bacharier LB. Early weight gain and the development of asthma and atopy in children. Curr Opin Allergy Clin Immunol. 2014;14:126–130. doi: 10.1097/ACI.0000000000000037. [DOI] [PubMed] [Google Scholar]
- 7.Sonnenschein-van der Voort AM, Arends LR, de Jongste JC, Annesi-Maesano I, Arshad SH, Barros H, et al. Preterm birth, infant weight gain, and childhood asthma risk: a meta-analysis of 147,000 European children. J Allergy Clin Immunol. 2014;133:1317–1329. doi: 10.1016/j.jaci.2013.12.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Z, Lai HJ, Roberg KA, Gangnon RE, Evans MD, Anderson EL, et al. Early childhood weight status in relation to asthma development in high-risk children. J Allergy Clin Immunol. 2010;126:1157–1162. doi: 10.1016/j.jaci.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.To T, Vydykhan TN, Dell S, Tassoudji M, Harris JK. Is obesity associated with asthma in young children? J Pediatr. 2004;144:162–168. doi: 10.1016/j.jpeds.2003.09.047. [DOI] [PubMed] [Google Scholar]
- 10.Wang G, Johnson S, Gong Y, Polk S, Divall S, Radovick S, et al. Weight gain in infancy and overweight or obesity in childhood across the gestational spectrum: a prospective birth cohort study. Sci Rep. 2016;6:29867. doi: 10.1038/srep29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang G, Divall S, Radovick S, Paige D, Ning Y, Chen Z, et al. Preterm birth and random plasma insulin levels at birth and in early childhood. JAMA. 2014;311:587–596. doi: 10.1001/jama.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Zuckerman B, Pearson C, Kaufman G, Chen C, Wang G, et al. Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight. JAMA. 2002;287:195–202. doi: 10.1001/jama.287.2.195. [DOI] [PubMed] [Google Scholar]
- 13.WHO Multicentre Growth Reference Study Group. WHO child growth standards: head circumference-for-age, arm circumference-for-age, triceps skinfold-for-age and subscapular skinfold-for-age: methods and development. Geneva, Switzerland: World Health Organization; 2007. [Google Scholar]
- 14.Roy SM, Spivack JG, Faith MS, Chesi A, Mitchell JA, Kelly A, et al. Infant BMI or weight-for-length and obesity risk in early childhood. Pediatrics. 2016;137:e20153492. doi: 10.1542/peds.2015-3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He H, Butz A, Keet CA, Minkovitz CS, Hong X, Caruso DM, et al. Preterm birth with childhood asthma: the role of degree of prematurity and asthma definitions. Am J Respir Crit Care Med. 2015;192:520–523. doi: 10.1164/rccm.201503-0522LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scholtens S, Wijga AH, Seidell JC, Brunekreef B, de Jongste JC, Gehring U, Postma DS, Kerkhof M, Smit HA. Overweight and changes in weight status during childhood in relation to asthma symptoms at 8 years of age. J Allergy Clin Immunol. 2009;123:1312–1318, e2. doi: 10.1016/j.jaci.2009.02.029. [DOI] [PubMed] [Google Scholar]
- 17.Paul IM, Camera L, Zeiger RS, Guilbert TW, Bacharier LB, Taussig LM, et al. Childhood Asthma Research and Education (CARE) Network. Relationship between infant weight gain and later asthma. Pediatr Allergy Immunol. 2010;21:82–89. doi: 10.1111/j.1399-3038.2009.00926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brooks AM, Byrd RS, Weitzman M, Auinger P, McBride JT. Impact of low birth weight on early childhood asthma in the United States. Arch Pediatr Adolesc Med. 2001;155:401–406. doi: 10.1001/archpedi.155.3.401. [DOI] [PubMed] [Google Scholar]
- 19.Dik N, Tate RB, Manfreda J, Anthonisen NR. Risk of physician-diagnosed asthma in the first 6 years of life. Chest. 2004;126:1147–1153. doi: 10.1378/chest.126.4.1147. [DOI] [PubMed] [Google Scholar]
- 20.Caudri D, Wijga A, Gehring U, Smit HA, Brunekreef B, Kerkhof M, et al. Respiratory symptoms in the first 7 years of life and birth weight at term: the PIAMA Birth Cohort. Am J Respir Crit Care Med. 2007;175:1078–1085. doi: 10.1164/rccm.200610-1441OC. [DOI] [PubMed] [Google Scholar]
- 21.Chen YC, Dong GH, Lin KC, Lee YL. Gender difference of childhood overweight and obesity in predicting the risk of incident asthma: a systematic review and meta-analysis. Obes Rev. 2013;14:222–231. doi: 10.1111/j.1467-789X.2012.01055.x. [DOI] [PubMed] [Google Scholar]
- 22.Ekström S, Magnusson J, Kull I, Andersson N, Bottai M, Besharat Pour M, et al. Body mass index development and asthma throughout childhood. Am J Epidemiol. 2017;186:255–263. doi: 10.1093/aje/kwx081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sonnenschein-van der Voort AM, Howe LD, Granell R, Duijts L, Sterne JA, Tilling K, et al. Influence of childhood growth on asthma and lung function in adolescence. J Allergy Clin Immunol. 2015;135:1435–1443, e7. doi: 10.1016/j.jaci.2014.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flexeder C, Thiering E, Brüske I, Koletzko S, Bauer CP, Wichmann HE, et al. GINIplus and LISAplus Study Group. Growth velocity during infancy and onset of asthma in school-aged children. Allergy. 2012;67:257–264. doi: 10.1111/j.1398-9995.2011.02748.x. [DOI] [PubMed] [Google Scholar]
- 25.Rzehak P, Wijga AH, Keil T, Eller E, Bindslev-Jensen C, Smit HA, et al. GA2LEN-WP 1.5 Birth Cohorts. Body mass index trajectory classes and incident asthma in childhood: results from 8 European birth cohorts--a Global Allergy and Asthma European Network initiative. J Allergy Clin Immunol. 2013;131:1528–1536. doi: 10.1016/j.jaci.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Lucas JS, Inskip HM, Godfrey KM, Foreman CT, Warner JO, Gregson RK, et al. Small size at birth and greater postnatal weight gain: relationships to diminished infant lung function. Am J Respir Crit Care Med. 2004;170:534–540. doi: 10.1164/rccm.200311-1583OC. [DOI] [PubMed] [Google Scholar]
- 27.Turner S, Prabhu N, Danielan P, McNeill G, Craig L, Allan K, et al. First- and second-trimester fetal size and asthma outcomes at age 10 years. Am J Respir Crit Care Med. 2011;184:407–413. doi: 10.1164/rccm.201012-2075OC. [DOI] [PubMed] [Google Scholar]
- 28.van der Gugten AC, Koopman M, Evelein AM, Verheij TJ, Uiterwaal CS, van der Ent CK. Rapid early weight gain is associated with wheeze and reduced lung function in childhood. Eur Respir J. 2012;39:403–410. doi: 10.1183/09031936.00188310. [DOI] [PubMed] [Google Scholar]
- 29.Turner S, Zhang G, Young S, Cox M, Goldblatt J, Landau L, et al. Associations between postnatal weight gain, change in postnatal pulmonary function, formula feeding and early asthma. Thorax. 2008;63:234–239. doi: 10.1136/thx.2006.064642. [DOI] [PubMed] [Google Scholar]
- 30.Martinez FD. Maturation of immune responses at the beginning of asthma. J Allergy Clin Immunol. 1999;103:355–361. doi: 10.1016/s0091-6749(99)70456-2. [DOI] [PubMed] [Google Scholar]
- 31.Rao L, Tiller C, Coates C, Kimmel R, Applegate KE, Granroth-Cook J, et al. Lung growth in infants and toddlers assessed by multi-slice computed tomography. Acad Radiol. 2010;17:1128–1135. doi: 10.1016/j.acra.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Magrone T, Jirillo E. Childhood obesity: immune response and nutritional approaches. Front Immunol. 2015;6:76. doi: 10.3389/fimmu.2015.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sonnenschein-van der Voort AM, Jaddoe VW, Raat H, Moll HA, Hofman A, de Jongste JC, et al. Fetal and infant growth and asthma symptoms in preschool children: the Generation R Study. Am J Respir Crit Care Med. 2012;185:731–737. doi: 10.1164/rccm.201107-1266OC. [DOI] [PubMed] [Google Scholar]
- 34.Flaherman V, Rutherford GW. A meta-analysis of the effect of high weight on asthma. Arch Dis Child. 2006;91:334–339. doi: 10.1136/adc.2005.080390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szczepankiewicz A, Breborowicz A, Sobkowiak P, Popiel A. Are genes associated with energy metabolism important in asthma and BMI? J Asthma. 2009;46:53–58. doi: 10.1080/02770900802460514. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.