Abstract
Despite being a major cause of morbidity and mortality, chronic obstructive pulmonary disease (COPD) is frequently undiagnosed. Yet the burden of disease among the undiagnosed is significant, as these individuals experience symptoms, exacerbations, and excess mortality compared to those without COPD. The U.S. Preventive Services Task Force recommends against routine screening of asymptomatic individuals with spirometry. Hence, case-finding approaches are needed. A recently developed instrument, the five-item COPD Assessment in Primary Care to Identify Undiagnosed Respiratory Disease and Exacerbation Risk questionnaire plus peak expiratory flow, demonstrates good sensitivity and specificity for distinguishing cases from control subjects and is being studied prospectively in primary care settings to determine its impact on patient outcomes. However, finding the undiagnosed is only half the battle. Mounting evidence suggests significant COPD-like respiratory burden among individuals without airflow obstruction. Many experience dyspnea, mucus production, and exacerbation events and have emphysema and airway abnormalities on computed tomographic (CT) imaging of the chest. However, it is still unclear how to best treat these individuals and which individuals go on to develop spirometric obstruction. These challenges underline the importance of defining what constitutes “early disease.” A recently proposed definition characterizes early COPD as either: 1) airflow limitation, 2) compatible CT imaging abnormalities, or 3) accelerated forced expiratory volume in 1 second decline in persons younger than 50 years and with greater than a 10 pack-year smoking history. Although it is recognized that this definition does not encompass all individuals who will develop COPD, it is an attempt to identify a group of individuals with most rapid decline to better understand mechanisms of disease development and where disease-modifying interventions are most likely to be successful. Ultimately, leveraging tools such as chest CT imaging, the electronic medical record, and machine learning algorithms may aid in the identification of such individuals.
Keywords: early COPD, case finding, airflow limitation, lung function decline, chest computed tomography
Chronic obstructive pulmonary disease (COPD) is a major cause of morbidity and mortality in the United States and worldwide (1–4). Although the globally increasing burden of disability associated with the disease is a challenge (5), the problem of frequently undiagnosed COPD is equally concerning. It has been estimated that only one-third to one-half of individuals with chronic airway obstruction carry a formal diagnosis of COPD (6). If we are to ultimately decrease the impact of COPD on individuals and society, early detection will need to play a key role.
The Problem of Undiagnosed COPD
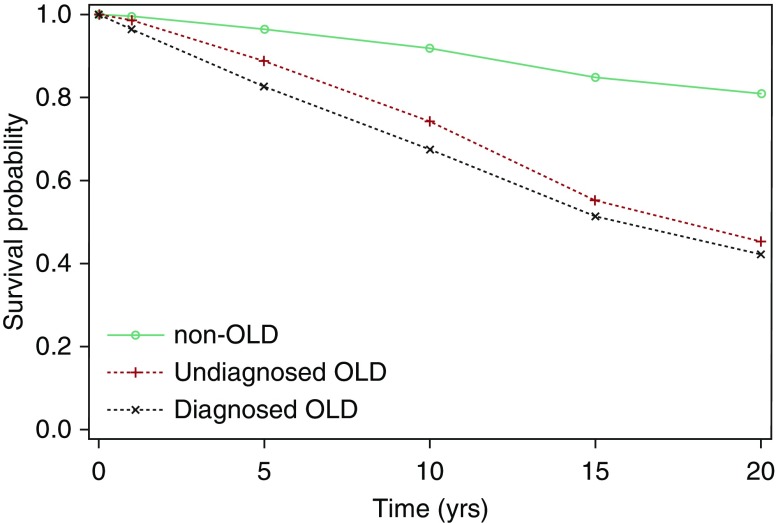
Many barriers contribute to diagnostic delay, particularly in primary care. Respiratory symptoms may be ignored by both patients and clinicians, as dyspnea is often attributed to old age or obesity. Physicians may believe they have little to offer smokers who are not ready or willing to quit and may be less inclined to screen persons who are not active smokers anymore (7). Practice patterns are further reinforced by the U.S. Preventive Services Task Force recommendation against the use of spirometry for routine screening of asymptomatic individuals (8). They cite a lack of evidence that early detection has the ability to alter the course of disease or improve patient outcomes. However, it is worth noting the key word “asymptomatic,” as many individuals at increased risk for COPD self-restrict activity to minimize symptoms. It should not be assumed that undiagnosed COPD is without clinical impact. Analysis of data from the National Health and Nutritional Examination Surveys demonstrated that individuals with undiagnosed chronic airway obstruction had a higher risk for all-cause mortality than those without obstruction (Figure 1) (6). Others have also reported exacerbation-like respiratory events (9), greater health status impairment (10), and increased healthcare costs and resource utilization (11, 12) among undiagnosed individuals. Importantly, appropriate inhaler therapy has been shown to not only improve quality of life but also reduce exacerbations, which are detrimental events in the natural history of the disease (13–15).
Figure 1.
Survival curves for participants in the National Health and Nutritional Examination Survey III by obstructive lung disease (OLD) category. Reprinted by permission from Reference 6.
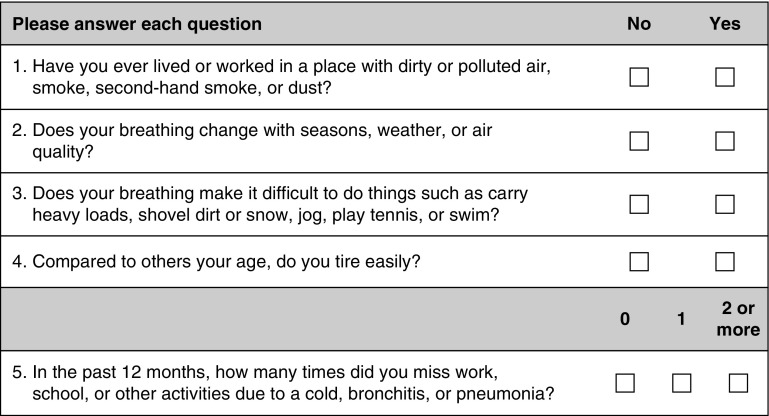
The Global Initiative for Obstructive Lung Disease (GOLD) consensus statement recommends case finding in symptomatic patients (16). However, to date, the development of case-finding approaches has been methodologically limited to tools based on existing epidemiologic literature or expert opinion (17, 18). Moreover, most questionnaires have been designed to identify patients with COPD without reference to disease severity or exacerbation risk, resulting in the selection of a high proportion of individuals with mild or minimally symptomatic disease (19–25). The National Institutes of Health recently sponsored the development of a new approach to case finding in COPD. A systematic analysis of existing databases was performed to provide insight into the best variables for COPD case identification. Machine learning methods were then used to select and validate variables most important in identifying patients with clinically significant COPD. Forty-four candidate items were subsequently selected for a potential instrument and tested in cases (defined as patients with COPD with either at least one exacerbation in the previous year or forced expiratory volume in 1 second [FEV1] < 60% predicted but no exacerbation in the previous year) and control subjects (defined as those without COPD and those with COPD with an FEV1 > 60% predicted and no exacerbation in the previous year) (26). A random forest analysis further reduced the identified variables down to the five-item COPD Assessment in Primary Care to Identify Undiagnosed Respiratory Disease and Exacerbation Risk (CAPTURE) questionnaire (Figure 2). Using CAPTURE plus peak expiratory flow exhibited the best sensitivity (89.7%) and specificity (78.1%) for distinguishing cases from control subjects. These results suggest that a case-finding approach incorporating both a patient questionnaire and peak expiratory flow can be used to identify individuals with clinically significant COPD. The effectiveness of this instrument in primary care settings and its potential to improve patient outcomes are being tested in an ongoing U.S. National Heart, Lung, and Blood Institute (NHLBI)-funded clinical trial (NCT03581227).
Figure 2.
COPD Assessment in Primary Care to Identify Undiagnosed Respiratory Disease and Exacerbation Risk (CAPTURE) chronic obstructive pulmonary disease (COPD) screening questionnaire. Score ranges from 0 (“no” to all five questions) to 6 (“yes” to all questions and at least two respiratory events during the past year). Patients with scores of 0 or 1 are not considered at risk of exacerbation or COPD with a forced expiratory volume in 1 second less than 60% predicted. Those with a score of 5 or 6 have a high likelihood of symptomatic respiratory disease and/or exacerbation. Patients scoring in the middle range (2–4) undergo peak expiratory flow testing for further risk stratification. Reprinted by permission from Reference 26.
COPD without the “O”
Finding the undiagnosed, however, is only half the battle. Mounting evidence suggests “COPD-like” respiratory disease burden among those without airflow obstruction. These symptomatic individuals with “normal” spirometry are a heterogeneous group of individuals, some of whom may never go on to develop airflow obstruction but clearly experience respiratory morbidity and others who have a “pre-COPD” condition associated with more rapid lung function decline that ultimately leads to airflow obstruction. Respiratory symptoms as well as lung parenchymal and airway abnormalities on computed tomographic (CT) imaging of the chest are common among individuals with COPD risk factors but without airflow obstruction. In the Medical Research Council (MRC) National Survey of Health and Development cohort, 34.0% of ever-smokers experienced either chronic cough or chronic sputum expectoration (27). In the COPDGene cohort, 42.3% of smokers with a normal FEV1/forced vital capacity (FVC) ratio had emphysema or airway wall thickening on CT imaging and 23.5% of them had a modified Medical Research Council dyspnea score of 2 or greater compared with 3.7% of never-smokers and 22.4% of individuals with GOLD stage 1 COPD (28). Another analysis of the COPDGene cohort showed that chronic bronchitis symptoms among nonobstructed participants have been associated with worse quality of life, impaired walk distance, increased exacerbation events, and, interestingly, lower likelihood of actively working (29).
In a similar study from the SPIROMICS (SubPopulations and InteRmediate Outcome Measures In COPD Study) cohort, half of nonobstructed smokers had COPD Assessment Test (CAT) scores greater than or equal to 10 (30). These symptomatic smokers with preserved pulmonary function had significantly more COPD-like exacerbations and airway wall thickening on CT scan than both nonobstructed smokers and individuals with GOLD stage 1 or 2 disease with CAT less than 10. Their exacerbation rates were most similar to subjects with GOLD stage 1 or 2 disease with CAT greater than or equal to 10. In a separate analysis of the SPIROMICS cohort, total airway mucin concentrations were found to be elevated among these symptomatic smokers with preserved spirometry, thereby pointing to a pathologic basis for symptoms (31). The burden of exacerbation events was further highlighted by data from the CanCOLD (Canadian Cohort of Obstructive Lung Disease) cohort. In this population-based study, among individuals with FEV1/FVC greater than or equal to 0.7, exacerbation events in the past year were associated with chronic cough, phlegm, and wheezing (32). Furthermore, individuals with exacerbations were more likely to miss social activities, work for income and housework compared to their nonexacerbating counterparts.
Although these data suggest that many symptomatic smokers with preserved pulmonary function experience real morbidity, they do not fit current management guidelines for COPD. Because this population is large, the health burden posed by those with undiagnosed chronic lung disease is significant (33). Importantly, whether they would benefit from therapy is entirely unknown. In the SPIROMICS cohort, 42% of these individuals were on bronchodilators and 23% used inhaled corticosteroids (30). However, we currently have no evidence basis for this. To address this knowledge gap, the NHLBI-funded RETHINC (REdefining THerapy In Early COPD) trial (NCT02867761), which is currently enrolling, will examine whether current and former smokers with respiratory symptoms despite normal spirometry derive benefit from inhaled bronchodilator therapy. Specifically, this is a randomized, double-blind, placebo-controlled trial comparing indacaterol/glycopyrronium to placebo among nonobstructed smokers with CAT score greater than or equal to 10, with a primary outcome of a 4-point improvement in the St. George’s Respiratory Questionnaire. It is hoped that results from this study will provide guidance regarding potential treatment options for these patients.
However, whether these symptoms herald a condition that will ultimately progress to airflow obstruction (i.e., “early COPD”) is unclear. Before 2006, the GOLD guidelines included a “GOLD 0” class, which consisted of symptomatic, at-risk individuals who did not have airflow obstruction (34). However, in the 2007 GOLD Revision, GOLD 0 was discarded because of incomplete evidence that these individuals necessarily progress to GOLD 1 (35). This decision was based in part on data from the population-based Copenhagen City Heart Study. Although there was an association between symptoms of chronic mucous hypersecretion and more rapid FEV1 decline, particularly among men, many individuals without symptoms still progressed to airflow obstruction (36). In another analysis from the same study, 13.2% and 20.5% of symptomatic, nonobstructed smokers at enrollment developed COPD after 5 and 15 years of follow-up, respectively (37). By contrast, 11.6% and 18.5% of asymptomatic, nonobstructed smokers at enrollment had COPD after 5 and 15 years, respectively. Therefore, a significant proportion of incident airflow obstruction was actually observed among subjects without symptoms of cough and sputum production, suggesting that the GOLD 0 classification may be of little help in identifying individuals at risk for COPD.
Defining Early COPD
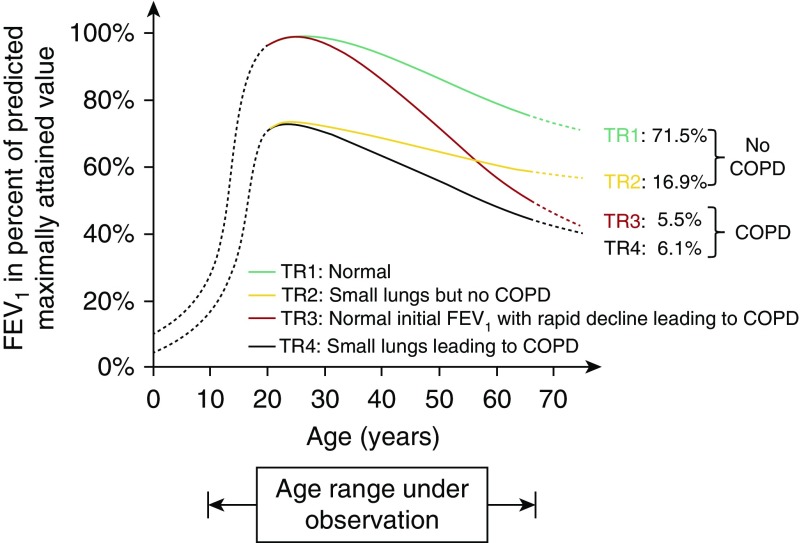
This subsequently leads us to the question of how to define early COPD. Up until recently there has been no accepted definition. In thinking about this, it is essential to distinguish “early disease,” which refers to evidence of a pathologic process in a young individual, from “late mild disease,” which refers to pathology that may be mild in severity but in an older individual who may have had it for decades. A recent review by Martinez and colleagues proposed a definition for early COPD as an individual younger than 50 years with 10 pack-years or more smoking history and either: 1) signs of airflow limitation (post-bronchodilator FEV1/FVC less than the lower limit of normal); or 2) emphysema or abnormal airways on CT imaging, or 3) evidence of accelerated FEV1 decline compared with FVC (38). These criteria were chosen with the intent to best identify individuals on a pathway toward more rapid lung function decline that would ultimately result in airflow obstruction. However, it is recognized that this is not an all-inclusive definition, because more than 20% of individuals with COPD never smoked (39, 40) and because individuals progress toward airflow obstruction by different pathways. Recent data from large cohort studies suggest that factors impacting development of airflow obstruction include failure to achieve peak lung function, more rapid lung function decline in adulthood, or a combination of both (Figure 3) (41, 42). Factors that have been implicated in failure to achieve peak lung function include maternal smoking and a history of childhood respiratory infections and asthma. Prevention of the development of COPD will ultimately need to address these factors in addition to the cornerstone intervention of tobacco cessation in smokers. It is entirely possible that the pathologic basis for low achievement of peak lung function may be different from that of accelerated decline in adulthood. The definition for early COPD proposed by Martinez and colleagues focuses primarily on those with accelerated lung function decline in adulthood, with the goal of identifying a group of patients most likely to help us understand disease pathogenesis and in whom both preventive measures and disease-modifying therapies are most likely to be successful (38).
Figure 3.
Distribution of participants into four trajectories according to baseline forced expiratory volume in 1 second (FEV1) level (below or above 80% predicted) and presence or absence of Global Initiative for Obstructive Lung Disease grade 2 or greater chronic obstructive pulmonary disease (COPD) at the final examination. The solid lines represent the schematic natural history of FEV1 for the age range of the study, and the broken lines represent hypothetical trajectories (TR). Reprinted by permission from Reference 41.
If we wish to identify individuals at risk for rapid lung function decline regardless of whether they have preserved lung function at baseline, CT scan abnormalities may be particularly helpful in this regard. Although patients without airflow obstruction typically do not have significant emphysema, they do have evidence of gas trapping indicative of small airway abnormality. Accumulating data suggest that small airway abnormality precedes the development of emphysema (43). Advanced analytic methods applied to CT images can quantitate “functional” small airway disease as defined by voxels less than −856 Hounsfield units on the expiration scan and greater than or equal to −950 Hounsfield units on the inspiration scan (44). This type of CT imaging abnormality can identify individuals without airflow obstruction who are at risk for both FEV1 decline and further disease progression on imaging (45).
Conclusions
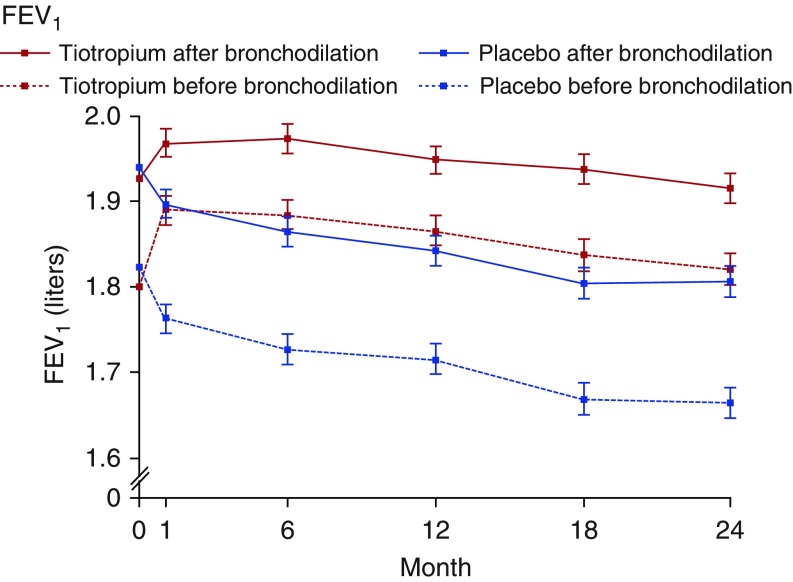
Additional research will, it is hoped, allow this “working” definition of early COPD to be further refined. It may ultimately be that a combination of factors provides better predictive value than a single metric. Regardless, we need a better understanding of the causes of morbidity as well as the mechanisms that contribute to both resilience to and triggering of disease progression. Disease modification studies may have failed in part because of a focus on late-stage disease. For example, although the UPLIFT (Understanding Potential Long-Term Impacts on Function with Tiotropium) study showed that tiotropium did not reduce the rate of FEV1 decline in participants with moderate to advanced COPD (14), a recent smaller and shorter trial showed evidence of reduction in lung function decline using the same drug in subjects with milder COPD (Figure 4) (46). However, replicating such studies on a broader scale may be challenging, given that the majority of individuals with early disease go undiagnosed. This has resulted in a vicious cycle, as the lack of disease-modifying therapies further reinforces recommendations against screening. CT imaging has the potential to help us identify individuals with early disease, even in the absence of airflow obstruction. For example, presence of emphysema noted on a low-dose chest CT scan ordered for lung cancer screening should trigger suspicion for the presence of early smoking-related lung disease if not already diagnosed. Leveraging additional tools, such as the electronic medical record used in conjunction with machine learning algorithms, to develop predictive analytics may also aid in identifying at-risk individuals.
Figure 4.
Mean forced expiratory volume in 1 second (FEV1) before and after bronchodilator use in tiotropium versus placebo among subjects with chronic obstructive pulmonary disease Global Initiative for Obstructive Lung Disease stages 1 or 2. Reprinted by permission from Reference 46.
Supplementary Material
Footnotes
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Brown DW, Croft JB, Greenlund KJ, Giles WH. Trends in hospitalization with chronic obstructive pulmonary disease-United States, 1990-2005. COPD. 2010;7:59–62. doi: 10.3109/15412550903499548. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) Deaths from chronic obstructive pulmonary disease--United States, 2000-2005. MMWR Morb Mortal Wkly Rep. 2008;57:1229–1232. [PubMed] [Google Scholar]
- 3.Kochanek KD, Xu J, Murphy SL, Miniño AM, Kung HC. Deaths: final data for 2009. Natl Vital Stat Rep. 2011;60:1–116. [PubMed] [Google Scholar]
- 4.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez CH, Mannino DM, Jaimes FA, Curtis JL, Han MK, Hansel NN, et al. Undiagnosed obstructive lung disease in the United States: associated factors and long-term mortality. Ann Am Thorac Soc. 2015;12:1788–1795. doi: 10.1513/AnnalsATS.201506-388OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yawn BP, Wollan PC. Knowledge and attitudes of family physicians coming to COPD continuing medical education. Int J Chron Obstruct Pulmon Dis. 2008;3:311–317. doi: 10.2147/copd.s2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siu AL, Bibbins-Domingo K, Grossman DC, Davidson KW, Epling JW, Jr, García FA, et al. US Preventive Services Task Force (USPSTF) Screening for chronic obstructive pulmonary disease: U.S. Preventive Services Task Force recommendation statement. JAMA. 2016;315:1372–1377. doi: 10.1001/jama.2016.2638. [DOI] [PubMed] [Google Scholar]
- 9.Labonté LE, Tan WC, Li PZ, Mancino P, Aaron SD, Benedetti A, et al. Canadian Respiratory Research Netword; CanCOLD Collaborative Research Group. Undiagnosed COPD contributes to the burden of health care utilization: data from the CanCOLD study. Am J Respir Crit Care Med. 2016;194:285–298. doi: 10.1164/rccm.201509-1795OC. [DOI] [PubMed] [Google Scholar]
- 10.Miravitlles M, Soriano JB, García-Río F, Muñoz L, Duran-Tauleria E, Sanchez G, et al. Prevalence of COPD in Spain: impact of undiagnosed COPD on quality of life and daily life activities. Thorax. 2009;64:863–868. doi: 10.1136/thx.2009.115725. [DOI] [PubMed] [Google Scholar]
- 11.Mapel DW, Robinson SB, Dastani HB, Shah H, Phillips AL, Lydick E. The direct medical costs of undiagnosed chronic obstructive pulmonary disease. Value Health. 2008;11:628–636. doi: 10.1111/j.1524-4733.2007.00305.x. [DOI] [PubMed] [Google Scholar]
- 12.Akazawa M, Halpern R, Riedel AA, Stanford RH, Dalal A, Blanchette CM. Economic burden prior to COPD diagnosis: a matched case-control study in the United States. Respir Med. 2008;102:1744–1752. doi: 10.1016/j.rmed.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 13.Calverley P, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, et al. TRial of Inhaled STeroids ANd long-acting beta2 agonists study group. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003;361:449–456. doi: 10.1016/S0140-6736(03)12459-2. [DOI] [PubMed] [Google Scholar]
- 14.Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, et al. UPLIFT Study Investigators. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543–1554. doi: 10.1056/NEJMoa0805800. [DOI] [PubMed] [Google Scholar]
- 15.Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 16.Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 report: GOLD executive summary. Am J Respir Crit Care Med. 2017;195:557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 17.Calverley PM, Nordyke RJ, Halbert RJ, Isonaka S, Nonikov D. Development of a population-based screening questionnaire for COPD. COPD. 2005;2:225–232. [PubMed] [Google Scholar]
- 18.van Schayck CP, Halbert RJ, Nordyke RJ, Isonaka S, Maroni J, Nonikov D. Comparison of existing symptom-based questionnaires for identifying COPD in the general practice setting. Respirology. 2005;10:323–333. doi: 10.1111/j.1440-1843.2005.00720.x. [DOI] [PubMed] [Google Scholar]
- 19.Freeman D, Nordyke RJ, Isonaka S, Nonikov DV, Maroni JM, Price D, et al. Questions for COPD diagnostic screening in a primary care setting. Respir Med. 2005;99:1311–1318. doi: 10.1016/j.rmed.2005.02.037. [DOI] [PubMed] [Google Scholar]
- 20.Yawn BP, Mapel DW, Mannino DM, Martinez FJ, Donohue JF, Hanania NA, et al. Lung Function Questionnaire Working Group. Development of the Lung Function Questionnaire (LFQ) to identify airflow obstruction. Int J Chron Obstruct Pulmon Dis. 2010;5:1–10. [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez FJ, Raczek AE, Seifer FD, Conoscenti CS, Curtice TG, D’Eletto T, et al. COPD-PS Clinician Working Group. Development and initial validation of a self-scored COPD Population Screener Questionnaire (COPD-PS) COPD. 2008;5:85–95. doi: 10.1080/15412550801940721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Price DB, Tinkelman DG, Halbert RJ, Nordyke RJ, Isonaka S, Nonikov D, et al. Symptom-based questionnaire for identifying COPD in smokers. Respiration. 2006;73:285–295. doi: 10.1159/000090142. [DOI] [PubMed] [Google Scholar]
- 23.Raghavan N, Lam YM, Webb KA, Guenette JA, Amornputtisathaporn N, Raghavan R, et al. Components of the COPD Assessment Test (CAT) associated with a diagnosis of COPD in a random population sample. COPD. 2012;9:175–183. doi: 10.3109/15412555.2011.650802. [DOI] [PubMed] [Google Scholar]
- 24.Kotz D, Nelemans P, van Schayck CP, Wesseling GJ. External validation of a COPD diagnostic questionnaire. Eur Respir J. 2008;31:298–303. doi: 10.1183/09031936.00074307. [DOI] [PubMed] [Google Scholar]
- 25.Haroon S, Jordan R, Takwoingi Y, Adab P. Diagnostic accuracy of screening tests for COPD: a systematic review and meta-analysis. BMJ Open. 2015;5:e008133. doi: 10.1136/bmjopen-2015-008133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez FJ, Mannino D, Leidy NK, Malley KG, Bacci ED, Barr RG, et al. High-Risk-COPD Screening Study Group. A new approach for identifying patients with undiagnosed chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;195:748–756. doi: 10.1164/rccm.201603-0622OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allinson JP, Hardy R, Donaldson GC, Shaheen SO, Kuh D, Wedzicha JA. The presence of chronic mucus hypersecretion across adult life in relation to chronic obstructive pulmonary disease development. Am J Respir Crit Care Med. 2016;193:662–672. doi: 10.1164/rccm.201511-2210OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Regan EA, Lynch DA, Curran-Everett D, Curtis JL, Austin JH, Grenier PA, et al. Genetic Epidemiology of COPD (COPDGene) Investigators. Clinical and radiologic disease in smokers with normal spirometry. JAMA Intern Med. 2015;175:1539–1549. doi: 10.1001/jamainternmed.2015.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez CH, Kim V, Chen Y, Kazerooni EA, Murray S, Criner GJ, et al. COPDGene Investigators. The clinical impact of non-obstructive chronic bronchitis in current and former smokers. Respir Med. 2014;108:491–499. doi: 10.1016/j.rmed.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woodruff PG, Barr RG, Bleecker E, Christenson SA, Couper D, Curtis JL, et al. SPIROMICS Research Group. Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med. 2016;374:1811–1821. doi: 10.1056/NEJMoa1505971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kesimer M, Ford AA, Ceppe A, Radicioni G, Cao R, Davis CW, et al. Airway mucin concentration as a marker of chronic bronchitis. N Engl J Med. 2017;377:911–922. doi: 10.1056/NEJMoa1701632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan WC, Bourbeau J, Hernandez P, Chapman KR, Cowie R, FitzGerald JM, et al. CanCOLD Collaborative Research Group. Exacerbation-like respiratory symptoms in individuals without chronic obstructive pulmonary disease: results from a population-based study. Thorax. 2014;69:709–717. doi: 10.1136/thoraxjnl-2013-205048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bowler RP, Kim V, Regan E, Williams AAA, Santorico SA, Make BJ, et al. Prediction of acute respiratory disease in current and former smokers with and without COPD. Chest. 2014;146:941–950. doi: 10.1378/chest.13-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) workshop summary. Am J Respir Crit Care Med. 2001;163:1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 35.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 36.Vestbo J, Prescott E, Lange P Copenhagen City Heart Study Group. Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Am J Respir Crit Care Med. 1996;153:1530–1535. doi: 10.1164/ajrccm.153.5.8630597. [DOI] [PubMed] [Google Scholar]
- 37.Vestbo J, Lange P. Can GOLD Stage 0 provide information of prognostic value in chronic obstructive pulmonary disease? Am J Respir Crit Care Med. 2002;166:329–332. doi: 10.1164/rccm.2112048. [DOI] [PubMed] [Google Scholar]
- 38.Martinez FJ, Han MK, Allinson JP, Barr RG, Boucher RC, Calverley PMA, et al. At the root: defining and halting progression of early chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;197:1540–1551. doi: 10.1164/rccm.201710-2028PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lamprecht B, McBurnie MA, Vollmer WM, Gudmundsson G, Welte T, Nizankowska-Mogilnicka E, et al. BOLD Collaborative Research Group. COPD in never smokers: results from the population-based burden of obstructive lung disease study. Chest. 2011;139:752–763. doi: 10.1378/chest.10-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomsen M, Nordestgaard BG, Vestbo J, Lange P. Characteristics and outcomes of chronic obstructive pulmonary disease in never smokers in Denmark: a prospective population study. Lancet Respir Med. 2013;1:543–550. doi: 10.1016/S2213-2600(13)70137-1. [DOI] [PubMed] [Google Scholar]
- 41.Lange P, Celli B, Agustí A, Boje Jensen G, Divo M, Faner R, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med. 2015;373:111–122. doi: 10.1056/NEJMoa1411532. [DOI] [PubMed] [Google Scholar]
- 42.Bui DS, Lodge CJ, Burgess JA, Lowe AJ, Perret J, Bui MQ, et al. Childhood predictors of lung function trajectories and future COPD risk: a prospective cohort study from the first to the sixth decade of life. Lancet Respir Med. 2018;6:535–544. doi: 10.1016/S2213-2600(18)30100-0. [DOI] [PubMed] [Google Scholar]
- 43.McDonough JE, Yuan R, Suzuki M, Seyednejad N, Elliott WM, Sanchez PG, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med. 2011;365:1567–1575. doi: 10.1056/NEJMoa1106955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galbán CJ, Han MK, Boes JL, Chughtai KA, Meyer CR, Johnson TD, et al. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat Med. 2012;18:1711–1715. doi: 10.1038/nm.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bhatt SP, Soler X, Wang X, Murray S, Anzueto AR, Beaty TH, et al. COPDGene Investigators. Association between functional small airways disease and FEV1 decline in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2016;194:178–184. doi: 10.1164/rccm.201511-2219OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou Y, Zhong NS, Li X, Chen S, Zheng J, Zhao D, et al. Tiotropium in early-stage chronic obstructive pulmonary disease. N Engl J Med. 2017;377:923–935. doi: 10.1056/NEJMoa1700228. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.