Abstract
Chronic obstructive pulmonary disease (COPD) and lung cancer are linked diseases, with both the incidence of and risk of death from non–small cell lung cancer being increased by the presence of COPD. Despite numerous well-performed epidemiological studies having described this link over the past 30 years, the operative mechanisms remain elusive. One of the major obstacles to advancement in the field has been the lack of patient cohorts that have been phenotyped for both COPD and lung cancer. This review discusses several studies performed over the past few years highlighting the impact of COPD on the outcomes of patients with non–small cell lung cancer with respect to lung cancer screening, immune-based therapies, and lung cancer chemoprevention.
Keywords: COPD, lung cancer, immunology
Cigarette smoking is the leading cause of preventable death worldwide, accounting for approximately six million deaths annually (1). Although effective therapies exist for some cigarette smoke–induced diseases (e.g., coronary artery disease), this does not hold true for chronic obstructive pulmonary disease (COPD) and non–small cell lung cancer (NSCLC). The disease burden attributable to these two diseases is not trivial—COPD is the third leading cause of death in the United States, and lung cancer is the leading cause of cancer death worldwide (2, 3). Given the prevalence of COPD and lung cancer, it is not surprising that both diseases frequently occur within the same individual. However, several well-performed epidemiological studies have demonstrated that COPD and lung cancer are linked, with COPD patients displaying a higher incidence of lung cancer than patients without obstructive lung disease (4). Furthermore, this link is independent of cigarette smoke consumption, strongly suggesting that there exist common mechanisms linking COPD/emphysema and lung cancer. Although the exact nature of the mechanistic link between COPD and lung cancer remains obscure, several recent studies have shed light on shared mechanisms between the two diseases (5–7), which are discussed in detail in this review.
COPD Increases Lung Cancer Incidence and Death
Several epidemiological studies have identified an association between the presence of airflow obstruction and the incidence of and risk of death from lung cancer (5, 8–13). The relative contribution of emphysema versus airflow obstruction remains unclear, though both are likely to play a role (Table 1). As such, there are studies that clearly identify both the presence and the severity of radiographic emphysema and airflow limitation as risk factors for lung cancer incidence and death (10–13).
Table 1.
Epidemiological studies linking COPD and lung cancer
| Study | Year | Key Finding | Reference |
|---|---|---|---|
| Skillrud and colleagues | 1986 | First report of increased lung cancer incidence for airflow limitation (FEV1, <70%) | 8 |
| Tockman and colleagues | 1987 | First report of increased lung cancer mortality for airflow limitation (FEV1, <60%) | 9 |
| Wasswa-Kintu and colleagues | 2005 | Meta-analysis of 204,990 subjects demonstrating increased lung cancer incidence with airflow obstruction | 10 |
| de Torres and colleagues | 2007 | First report showing that radiographic emphysema increases lung cancer incidence | 11 |
| Wilson and colleagues | 2008 | Entirety of lung cancer risk attributable to radiographic emphysema | 12 |
| Zulueta and colleagues | 2012 | Increased lung cancer mortality for presence of radiographic emphysema | 13 |
| Young and colleagues | 2015 | Presence of COPD doubles risk of lung cancer diagnosis during screening | 5 |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; FEV1 = forced expiratory volume in 1 second.
The presence of COPD and emphysema has a new practical application in the era of computed tomographic (CT) screening for lung cancer. Results of the National Lung Screening Trial have essentially ushered in the era of CT screening for lung cancer because the study demonstrated a 20% reduction in risk of lung cancer death (14). Young and colleagues reanalyzed the National Lung Screening Trial dataset in an attempt to show the predictive value of the presence of COPD and radiographic incidence in this cohort (5). Notably, patients with COPD were twice as likely to be diagnosed with lung cancer during the screening process as otherwise matched patients without COPD. Furthermore, the COPD group was essentially devoid of premalignant lesions (e.g., adenocarcinoma in situ), suggesting that the presence of COPD nurtures the progression to invasive adenocarcinoma formation versus the persistence of immature lesions. This study supports the notion that all subjects with COPD and/or radiographic emphysema should be candidates for lung cancer screening. This is a relevant issue because just approximately 4% of screen-eligible subjects actually undergo CT screening (15). This is at least in part due to the complicated eligibility criteria (age >55 yr, >30 pack-years, quit <15 yr prior). Adjusting the eligibility criteria to include a diagnosis of COPD would ensure that subjects at particularly high risk for lung cancer are referred for lung cancer screening. It is also likely that the presence of airflow obstruction and radiographic emphysema will feature in novel risk prediction models for pulmonary nodule management, many of which are currently under construction.
Are COPD/Emphysema and Lung Cancer Polar Opposites?
At first glance, emphysema and lung cancer feature pathogenic features on opposite ends of the spectrum. For example, apoptosis is a prominent feature of emphysema, with alveolar cell death serving as a gateway to airspace enlargement. In contrast, apoptosis is a rare (but desired) event in lung cancer, which is characterized by the avoidance of apoptosis and the presence of undeterred cellular proliferation. Similarly, cancers prominently feature angiogenesis, whereas alveolar capillary dropout is frequently encountered in COPD/emphysema. However, when considered from a “cell at risk” perspective, it is easier to comprehend how the emphysematous microenvironment would nurture lung cancer development. In this context, the alveolar capillary dropout and poor vascular supply may induce the expression of genes such as hypoxia-inducible factor-1α, which is typically expressed only by invasive cancers. Similarly, cells residing in fields at risk frequently display evidence of genetic mutation in key genes such as TP53 and CDK2N2A (16, 17). If these cells resided within an emphysematous microenvironment, apoptotic pressure would be high. However, these cells may lack the ability to undergo apoptosis, given their underlying genetic abnormalities, and would therefore be at particularly high risk for malignant transformation.
Immune Responses in COPD and Lung Cancer
Several recent studies have attempted to determine the immune cell composition and function in NSCLC, and a few of these included immune profiling of the lung tissue in these patients. Because COPD is frequently encountered in patients with NSCLC (approximately 70%), the ability to assess the role of COPD in immune response is possible. Although the accumulation of inflammatory cells such as neutrophils and alveolar macrophages has long been known to occur in COPD, these cells do not distinguish COPD by disease severity (using Global Initiative for Chronic Obstructive Lung Disease stage). In contrast, it appears that CD4+ T-cell content and differentiation status (T-helper type 1 cells [Th1] Th17, regulatory T cells [Treg]) optimally distinguishes COPD severity, highlighted by increased CD4+ and Th1 content as the disease progresses (6). A role for Th17 cells in COPD progression has been supported by solid clinical and preclinical studies (interleukin [IL]-17 receptor A–deficient mice are protected from cigarette smoke–induced emphysema) (18). In addition, there is strong evidence from mouse models of lung cancer that IL-17 drives a protumor inflammatory response and fuels lung tumor growth (19). However, it is unclear if Th17-driven inflammation occurs only in the setting of active cigarette smoking, because one study was able to identify increases in Th17 content only by smoking status and not by disease severity (6). There is also evidence that CD8+ T-cell status is impacted by COPD and that PD1 (programmed cell death 1)-expressing populations are associated with disease progression (7).
The immune cell composition present in NSCLC specimens is quite heterogeneous and is different in lung adenocarcinomas as opposed to lung squamous cell carcinomas. Myeloid lineage cells are the most prevalent immune cell populations in NSCLC, with neutrophils being slightly more common than macrophages. Similar to COPD immune cell composition, CD4+ cellular differentiation drives unique subphenotypes in NSCLC, with some cases displaying Th1 predominance and others displaying enhanced Treg immunity. Our group identified a strong inverse correlation between neutrophil (defined as CD66b+) content and CD8+ T-cell content (20). None of the other myeloid populations displayed this feature, nor did the neutrophils in adjacent lung tissue. Notably, this observation was validated by an independent study (21). Additional data to support a deleterious role for neutrophils in NSCLC come from the Alizadeh group that introduced “CIBERSORT,” a method for estimating the cellular content of multicellular tissues using their gene expression profiles (22). Alizadeh and colleagues analyzed microarray gene expression data from more than 18,000 patients spanning 30 cancer types to infer the immune cell content using novel cell type–specific gene expression profiles. They found that neutrophil content predicted mortality better than any other immune cell populations across all cancer types and specifically in lung adenocarcinoma (23).
Using a unique cohort of 73 patients with NSCLC, 50 of whom had COPD, my colleagues and I assessed the relationships between COPD immune cell content (nonadjacent lung tissue) and NSCLC immune cell content within matched cases. Correlation of immune cell composition between the two diseases correlated most strongly for the CD4+ subsets, specifically Th1 cells (6). In other words, patients with COPD displaying robust Th1-mediated immunity within COPD-affected lung tissue harbored lung cancers that were heavily infiltrated with Th1 cells. Biton and colleagues used a different approach, leveraging formalin-fixed, paraffin-embedded tissues from patients with NSCLC both with and without COPD to show that patients with COPD displayed enhanced PD1 and TIM3 (T-cell immunoglobulin mucin 3) staining on the CD8+ cellular population (7). Thus, immune activity and exhaustion both appear to be enhanced in patients with COPD who have NSCLC compared with no-COPD control subjects.
Impact of COPD on Immunotherapy for NSCLC
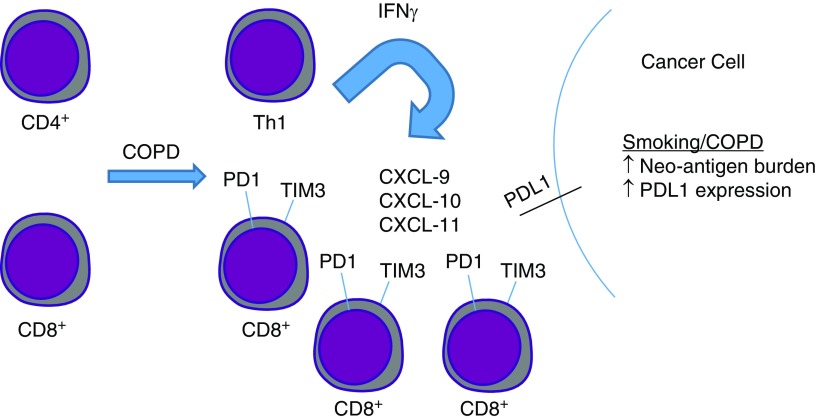
As highlighted in the introductory text above, a diagnosis of COPD leads to increased mortality from lung cancer, such that life survival curves demonstrated reduced survival for patients with NSCLC with COPD compared with those without COPD. Somewhat counterintuitively, cigarette smoking consumption predicts favorable anti-PD1 treatment responses, though this is likely related to increased mutational spectral burden and tumor neoantigen load (24). Surprisingly, two recent studies reported increased survival in anti-PD1 recipient patients with NSCLC with COPD versus those without (6, 7). This represents a highly intriguing and unexpected finding because the survival curves have actually flipped, with the presence COPD favoring survival. Although one of the studies involved relatively small cohorts, the total number of patients between the two studies involved more than 150 patients in each arm (COPD vs. no COPD). The mechanism underlying these findings remains unknown, though the presence of enhance Th1 immunity in a subset of patients with COPD may be responsible because the existence of the “interferon-γ signature” has recently been shown to predict favorable anti-PD1 treatment responses (25). Alternatively, the enhanced expression of the inhibitory receptors PD1 and TIM3 by tumor-infiltrating CD8+ T cells may be the cause of improved anti-PD1 treatment responses (see Figure 1). Prospective studies will be required to validate the presence of COPD as a predictor of favorable treatment response to immune checkpoint blockade and to address the mechanisms by which this occurs.
Figure 1.
Proposed mechanisms of improved anti-PD1 treatment outcomes in COPD. In the setting of COPD, CD4+ cellular differentiation is skewed toward the IFN-γ–producing Th1 phenotype. IFN-γ release induces production of IFN-γ–inducible genes, such as CXCL-9, -10, and -11. These proteins function to recruit additional lymphocytes to sites of tumorigenesis. In the setting of COPD, CD8+ T cells display increased expression of the inhibitory receptors PD1 and TIM3, which also represent T-cell activation. In this setting, in which PDL1 expression is also enhanced, the stage is set for a favorable treatment response when anti-PD1 antibodies are administered, given that the tumor is rich in IFN-γ and activated CD8+ T cells. CD = cluster of differentiation; COPD = chronic obstructive pulmonary disease; CXCL = chemokine (C-X-C motif) ligand; IFN = interferon; PD1 = programmed cell death 1; PDL1 = programmed cell death ligand 1; Th1 = T-helper type cell type 1; TIM3 = T-cell immunoglobulin mucin 3.
Therapeutic Goal: Identify a COPD Agent that Doubles as a Lung Cancer Chemopreventive
When considering an exhaustive study of a potential therapeutic target for patients with COPD, it is imperative that the investigative team consider the impact of such a therapeutic on lung cancer pathogenesis. Although a particular target may hold promise as a COPD therapeutic, if this strategy would promote tumorigenesis, then subclinical lesions might develop into invasive cancers. Matrix metalloproteinase 12 (MMP-12 or macrophage elastase) serves as an excellent example in this regard. Extensive preclinical and clinical data support a deleterious role for MMP-12 in the progression of COPD and emphysema, including the fact that it is one of the most highly induced genes in patients with COPD (26, 27). Developing antagonists against MMP-12 would be a logical therapeutic strategy for COPD/emphysema. Unfortunately, MMP-12 is one of the rare prohost MMPs with respect to tumorigenesis. MMP-12 generates angiostatic peptides from matrix protein precursors (28, 29). In other words, MMP-12 inhibition in patients with COPD would likely slow the progression of COPD at the expense of speeding up the progression of lung cancer.
Because the majority of lung cancer–associated mortality resides within the COPD populations (>20 million patients in the United States), the clear goal of the field should be to develop a disease-modifying therapy for COPD that also functions as a lung cancer chemopreventive. Although this may be a lofty goal (disease-modifying therapies for COPD do not exist), the existence of potential therapeutic targets that drive COPD progression and lung cancer growth makes this theoretically possible. Many of the potential therapeutic targets in this regard involve the proinflammatory nature of both diseases, such as IL-6 and tumor necrosis factor-α, which have been shown to drive disease pathogenesis in relevant mouse models. One of the most attractive candidates is IL-1α/β, for which there are data to support deleterious roles in both COPD and lung cancer (30, 31). A recent study using canakinumab, an IL-1β antagonist, serves as an inadvertent proof of concept in this regard (32–34).
Canakinumab was recently studied in a group at high risk for cardiac events because there was ample preclinical data suggesting that IL-1β blockade would limit atherosclerotic disease. Therefore, more than 10,000 subjects with prior cardiac events and elevated C-reactive protein levels were randomized to receive either placebo or one of three doses of canakinumab. The primary endpoint was a recurrent cardiac event. The initial report of this study was somewhat inconclusive because only the middle dose of canakinumab tested was able to reduce subsequent cardiac events (35). However, as described in a follow-up report, the investigative team found that the subsequent development of lung cancer incidence and death was reduced in a dose-dependent manner (32). Notably, only lung cancer incidence was affected, with the incidence of nonlung cancer being unchanged. These results strongly suggest that inhibiting IL-1β specifically in the lung is able to impact tumorigenesis, which would be consistent with the concept that the proinflammatory COPD disease environment promotes tumor formation. Although COPD status was not tracked in this cohort, given the age and smoking demographics of the cohort, it is likely that more than 50% of these subjects harbored the disease. Additional controlled studies will be required to prove the efficacy of IL-1β in this regard. Currently, canakinumab is being tested in combination with anti-PD1 antibody therapy for the treatment of NSCLC.
Conclusions
COPD and lung cancer are linked diseases, with the incidence and risk of death from lung cancer increased by the presence of COPD. This link has a new practical application, which lies in the recognition that patients with COPD are at particularly high risk for the subsequent development of lung cancer. This knowledge should lead to the addition of COPD diagnosis to prioritize patients to undergo potentially lifesaving CT screening for lung cancer. Although the exact nature of the link(s) between COPD and lung cancer remain difficult to elucidate, the interrelationships of immune system function in the two diseases are beginning to take shape. Enhanced Th1-mediated immunity and CD8+ cellular activation (PD1 and TIM3 expression) in patients with COPD harboring lung cancer may explain why patients with COPD surprisingly display prolonged survival with administration of immune checkpoint inhibitor therapy. Last, the recent report that an IL-1β antagonist (canakinumab) was capable of reducing lung cancer development in high-risk patients serves as an important proof of concept that lung cancer chemoprevention is a viable strategy to combat the high morbidity and mortality associated with lung cancer in patients with COPD.
Supplementary Material
Footnotes
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Samet JM. Tobacco smoking: the leading cause of preventable disease worldwide. Thorac Surg Clin. 2013;23:103–112. doi: 10.1016/j.thorsurg.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Miniño AM, Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2008. Natl Vital Stat Rep. 2011;59:1–126. [PubMed] [Google Scholar]
- 4.Houghton AM. Mechanistic links between COPD and lung cancer. Nat Rev Cancer. 2013;13:233–245. doi: 10.1038/nrc3477. [DOI] [PubMed] [Google Scholar]
- 5.Young RP, Duan F, Chiles C, Hopkins RJ, Gamble GD, Greco EM, et al. Airflow limitation and histology shift in the National Lung Screening Trial: the NLST-ACRIN cohort substudy. Am J Respir Crit Care Med. 2015;192:1060–1067. doi: 10.1164/rccm.201505-0894OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mark NM, Kargl J, Busch SE, Yang GHY, Metz HE, Zhang H, et al. Chronic obstructive pulmonary disease alters immune cell composition and immune checkpoint inhibitor efficacy in non-small cell lung cancer. Am J Respir Crit Care Med. 2018;197:325–336. doi: 10.1164/rccm.201704-0795OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biton J, Ouakrim H, Dechartres A, Alifano M, Mansuet-Lupo A, Si H, et al. Impaired tumor-infiltrating T cells in patients with chronic obstructive pulmonary disease impact lung cancer response to PD-1 blockade. Am J Respir Crit Care Med. 2018;198:928–940. doi: 10.1164/rccm.201706-1110OC. [DOI] [PubMed] [Google Scholar]
- 8.Skillrud DM, Offord KP, Miller RD. Higher risk of lung cancer in chronic obstructive pulmonary disease: a prospective, matched, controlled study. Ann Intern Med. 1986;105:503–507. doi: 10.7326/0003-4819-105-4-503. [DOI] [PubMed] [Google Scholar]
- 9.Tockman MS, Anthonisen NR, Wright EC, Donithan MG. Airways obstruction and the risk for lung cancer. Ann Intern Med. 1987;106:512–518. doi: 10.7326/0003-4819-106-4-512. [DOI] [PubMed] [Google Scholar]
- 10.Wasswa-Kintu S, Gan WQ, Man SF, Pare PD, Sin DD. Relationship between reduced forced expiratory volume in one second and the risk of lung cancer: a systematic review and meta-analysis. Thorax. 2005;60:570–575. doi: 10.1136/thx.2004.037135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Torres JP, Bastarrika G, Wisnivesky JP, Alcaide AB, Campo A, Seijo LM, et al. Assessing the relationship between lung cancer risk and emphysema detected on low-dose CT of the chest. Chest. 2007;132:1932–1938. doi: 10.1378/chest.07-1490. [DOI] [PubMed] [Google Scholar]
- 12.Wilson DO, Weissfeld JL, Balkan A, Schragin JG, Fuhrman CR, Fisher SN, et al. Association of radiographic emphysema and airflow obstruction with lung cancer. Am J Respir Crit Care Med. 2008;178:738–744. doi: 10.1164/rccm.200803-435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zulueta JJ, Wisnivesky JP, Henschke CI, Yip R, Farooqi AO, McCauley DI, et al. Emphysema scores predict death from COPD and lung cancer. Chest. 2012;141:1216–1223. doi: 10.1378/chest.11-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jemal A, Fedewa SA. Lung cancer screening with low-dose computed tomography in the United States—2010 to 2015. JAMA Oncol. 2017;3:1278–1281. doi: 10.1001/jamaoncol.2016.6416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franklin WA, Gazdar AF, Haney J, Wistuba II, La Rosa FG, Kennedy T, et al. Widely dispersed p53 mutation in respiratory epithelium: a novel mechanism for field carcinogenesis. J Clin Invest. 1997;100:2133–2137. doi: 10.1172/JCI119748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spira A, Beane JE, Shah V, Steiling K, Liu G, Schembri F, et al. Airway epithelial gene expression in the diagnostic evaluation of smokers with suspect lung cancer. Nat Med. 2007;13:361–366. doi: 10.1038/nm1556. [DOI] [PubMed] [Google Scholar]
- 18.Chen K, Pociask DA, McAleer JP, Chan YR, Alcorn JF, Kreindler JL, et al. IL-17RA is required for CCL2 expression, macrophage recruitment, and emphysema in response to cigarette smoke. PLoS One. 2011;6:e20333. doi: 10.1371/journal.pone.0020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang SH, Mirabolfathinejad SG, Katta H, Cumpian AM, Gong L, Caetano MS, et al. T helper 17 cells play a critical pathogenic role in lung cancer. Proc Natl Acad Sci USA. 2014;111:5664–5669. doi: 10.1073/pnas.1319051111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kargl J, Busch SE, Yang GH, Kim KH, Hanke ML, Metz HE, et al. Neutrophils dominate the immune cell composition in non-small cell lung cancer. Nat Commun. 2017;8:14381. doi: 10.1038/ncomms14381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lizotte PH, Ivanova EV, Awad MM, Jones RE, Keogh L, Liu H, et al. Multiparametric profiling of non-small-cell lung cancers reveals distinct immunophenotypes. JCI Insight. 2016;1:e89014. doi: 10.1172/jci.insight.89014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12:453–457. doi: 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21:938–945. doi: 10.1038/nm.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR, et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. 2017;127:2930–2940. doi: 10.1172/JCI91190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science. 1997;277:2002–2004. doi: 10.1126/science.277.5334.2002. [DOI] [PubMed] [Google Scholar]
- 27.Hunninghake GM, Cho MH, Tesfaigzi Y, Soto-Quiros ME, Avila L, Lasky-Su J, et al. MMP12, lung function, and COPD in high-risk populations. N Engl J Med. 2009;361:2599–2608. doi: 10.1056/NEJMoa0904006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Houghton AM, Grisolano JL, Baumann ML, Kobayashi DK, Hautamaki RD, Nehring LC, et al. Macrophage elastase (matrix metalloproteinase-12) suppresses growth of lung metastases. Cancer Res. 2006;66:6149–6155. doi: 10.1158/0008-5472.CAN-04-0297. [DOI] [PubMed] [Google Scholar]
- 29.Acuff HB, Sinnamon M, Fingleton B, Boone B, Levy SE, Chen X, et al. Analysis of host- and tumor-derived proteinases using a custom dual species microarray reveals a protective role for stromal matrix metalloproteinase-12 in non-small cell lung cancer. Cancer Res. 2006;66:7968–7975. doi: 10.1158/0008-5472.CAN-05-4279. [DOI] [PubMed] [Google Scholar]
- 30.Botelho FM, Bauer CM, Finch D, Nikota JK, Zavitz CC, Kelly A, et al. IL-1α/IL-1R1 expression in chronic obstructive pulmonary disease and mechanistic relevance to smoke-induced neutrophilia in mice. PLoS One. 2011;6:e28457. doi: 10.1371/journal.pone.0028457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lappalainen U, Whitsett JA, Wert SE, Tichelaar JW, Bry K. Interleukin-1β causes pulmonary inflammation, emphysema, and airway remodeling in the adult murine lung. Am J Respir Cell Mol Biol. 2005;32:311–318. doi: 10.1165/rcmb.2004-0309OC. [DOI] [PubMed] [Google Scholar]
- 32.Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ CANTOS Trial Group. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:1833–1842. doi: 10.1016/S0140-6736(17)32247-X. [DOI] [PubMed] [Google Scholar]
- 33.Caetano MS, Zhang H, Cumpian AM, Gong L, Unver N, Ostrin EJ, et al. IL6 blockade reprograms the lung tumor microenvironment to limit the development and progression of K-ras-mutant lung cancer. Cancer Res. 2016;76:3189–3199. doi: 10.1158/0008-5472.CAN-15-2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gong L, da Silva Caetano M, Cumpian AM, Daliri S, Garza Flores A, Chang SH, et al. Tumor necrosis factor links chronic obstructive pulmonary disease and K-ras mutant lung cancer through induction of an immunosuppressive pro-tumor microenvironment. Oncoimmunology. 2016;5:e1229724. doi: 10.1080/2162402X.2016.1229724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.