Abstract
Telemedicine coverage of intensive care units is an organizational innovation that has been touted as a means to improve access to and quality of critical care. The purpose of this narrative review is to discuss the different organizational models of intensive care unit telemedicine and factors that have influenced its adoption and to review the existing literature to consider whether it has lived up to its promise. We conclude by suggesting future directions to fill in some of the existing gaps in the literature.
Keywords: critical care, ICU administration, telemedicine
The intensive care unit (ICU) is traditionally defined as a dedicated area in the hospital where acute care services are provided to patients requiring invasive, life-sustaining therapies and who are at high risk of dying (1, 2). More than 5 million patients are admitted annually to ICUs in the United States, and nearly one in five Americans will die after using ICU services (2, 3). Critical care is both resource intensive and costly, consuming nearly 15% of all hospital costs in the United States, with total costs approaching 1% of the U.S. gross domestic product (4, 5). As a result, substantial efforts have been made in recent years to improve its overall quality, value, and efficiency.
Although it is traditionally defined by what happens in an ICU, modern critical care is less a technologic creation and more of an organizational innovation (6). Therefore, it stands to reason that improvements in ICU quality and efficiency might be maximized through innovations in the reorganization of its care delivery system (6, 7). ICU telemedicine is defined as the provision of care to critically ill patients by healthcare professionals located remotely. It is a particularly appealing strategy because of its potential ability to improve access to trained intensivists, whose in-house presence is associated with lower mortality, shortened ICU length of stay (LOS), and lower costs for critically ill patients (8, 9). The demand for critical care has already and will further outpace the supply of intensivists in the setting of the aging U.S. population (10, 11); ICU telemedicine has been proposed as an intervention that may help alleviate this workforce crisis (12, 13). ICU telemedicine providers typically use electronic medical records combined with audiovisual technologies to assist bedside caregivers in patient care activities, including best practice adherence, monitoring of clinical stability, and the creation and execution of care plans (14–16).
In this review, we describe the history of ICU telemedicine and its different models of implementation in the United States (as very few data on international adoption of ICU telemedicine exist), explore factors influencing its adoption, review the evidence regarding its effectiveness, discuss potential mechanisms for successful implementation, and propose directions for future research.
The History and Organizational Models of ICU Telemedicine
ICU telemedicine was first reported in 1977. Via a two-way audiovisual link, an intensivist at a university hospital remotely conducted daily rounds and once-weekly teaching conferences with staff of a small nonacademic ICU (17). Through their groundbreaking experience, the authors concluded that ICU telemedicine was feasible, though expensive; was generally acceptable to both patients and ICU staff; and had great potential to improve patient care, provide education, and facilitate the establishment of multihospital networks.
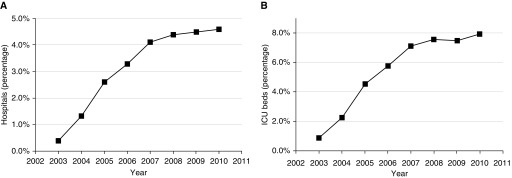
The next two decades saw a substantial improvement in telemedicine technologies, including communication strategies, patient monitoring systems, computerized data collection, and clinical decision support (18). In 1997, a team of investigators at an academic medical center conducted a feasibility study in which they used telemedicine to achieve 24-hour intensivist coverage of a 10-bed surgical ICU at an affiliate hospital over a 16-week period (19, 20). Associated with a marked reduction in severity-adjusted ICU and hospital mortality, ICU LOS, and hospital costs, the ICU telemedicine program was expanded across multiple ICUs in the same healthcare system using commercially available equipment. In a subsequent multisite roll-out, the ICU telemedicine intervention was again associated with significantly reduced hospital mortality (12.9% vs. 9.4%) and ICU LOS (4.35 d vs. 3.63 d) (20). These results ultimately led to the development of the predominant ICU telemedicine technology in the United States, broader commercialization of telecommunication technologies, and subsequent expansion of ICU telemedicine across the United States, from 16 hospitals in 2003 to 213 hospitals in 2010, with most of the growth occurring in the first 4 years of the expansion (14, 21, 22) (Figure 1).
Figure 1.
Growth of intensive care unit (ICU) telemedicine over time, expressed as (A) percentages of all U.S. hospitals using this service, and (B) percentages of ICU beds covered. Reprinted by permission from Reference 21.
Today, ICU telemedicine programs are more widespread, covering at least 15% of ICU beds in the United States (18, 21), but with considerable variation in how and where they have been implemented. For example, ICUs that use telemedicine are widely dispersed geographically and serve communities of all sizes, including both rural and urban settings. They are also located in hospitals that vary substantially in size, from as few as 25 to more than 1,000 licensed beds (23). Despite this variability, certain adoption patterns have emerged (23). Compared with nonadopting hospitals, those with ICU telemedicine programs are more likely to be large, nonprofit, teaching hospitals located in urban settings and with greater access to resources, including major technologies (21).
The most common model is the centralized telemedicine unit, which uses a hub-and-spoke model from which critical care services originate (22, 24). The hub (or center) is the remote site from which a multidisciplinary team (including variable combinations of intensivists, nurses, advanced practitioners, pharmacists, respiratory therapists, and administrative staff) provides off-site monitoring for critically ill patients. Alternatively, the decentralized model uses a reverse hub-and-spoke model, in which there is no central monitoring facility. In this model, computers equipped with audiovisual technology are also located at sites of patient care, but the remote monitoring occurs from sites of convenience for individual remote care providers, such as physician offices or homes (24). Some ICU telemedicine programs have a logistic center that manages patient flow (including ICU admissions and intra- or interhospital transfers) within a hospital or health system (25). Many telemedicine units provide benchmarking data to monitored ICUs, including rates of adherence to evidence-based practices, patient outcomes data, and professional service revenue data; however, how these types of data are reported or used is not well described.
The intensity of interaction between ICU telemedicine program and bedside providers varies widely across three primary domains: time, reactivity, and scope. For example, the ICU telemedicine team may provide services intermittently or in a continuous fashion up to 24 hours per day. ICU telemedicine programs may implement a reactive model, in which telemedicine providers respond to automated alerts for worrisome trends that may not yet be recognized by the bedside providers or to requests for involvement from bedside providers. Such requests may be based on prespecified criteria, such as new admissions, the need for certain therapies indicative of critical illness (such as vasopressors or mechanical ventilation), emergency situations, or questions concerning patient care (22, 26). In contrast, a proactive model typically involves the continuous remote surveillance of patients, including the methodical review of patient data and best-practice adherence (e.g., lung-protective ventilation for patients with acute respiratory distress syndrome) (22). Scheduled (i.e., preemptive) care models also exist, in which virtual visits by the remote provider occur at defined times rather than in response to prompting from the bedside team (24). Importantly, these models of reactivity are not mutually exclusive. Scope of involvement by the ICU telemedicine team also varies substantially, ranging from minimal discretion only (e.g., the ICU telemedicine team only intervenes for life-threatening situations) up to full discretion (e.g., the telemedicine team has full prescribing authority ranging from placing routine orders to changing treatment plans) (27). In this way, bedside providers can adjust the intensity of involvement by the ICU telemedicine program across these domains depending on the local culture and resources available.
The ICU telemedicine team typically expands the number of clinicians involved in patient care by adding a number of remote providers to the bedside provider team (28). However, there are no guidelines to establish the optimal “dose” of remote providers or the ideal composition of the multidisciplinary team. ICU telemedicine teams are staffed with, on average, one nurse per 30 to 35 ICU beds and one intensivist per 100 to 130 patients (28). In one survey, ICU telemedicine centers were staffed an average of 16.5 hours per weekday. Weekday staffing included a median of three nurses, with a range of 1 to 12, and one intensivist, with a range of 1 to 3. Staffing tended to increase with the number of beds being evaluated by the ICU telemedicine program (23).
Factors Influencing ICU Telemedicine Adoption
The rapid expansion of ICU telemedicine in the early 2000s was based on its potential to improve patient outcomes and quality of care (14). Patient safety and increased access to an intensivist workforce that falls short of demand are often cited as primary motivations for adoption (2, 10, 22, 28, 29). Other proposed benefits of ICU telemedicine include the ability to recognize worrisome vital signs or laboratory trends more quickly than bedside providers would alone (28, 30, 31) and to target care processes associated with better outcomes, including more rapid initiation of life-sustaining therapies and shorter response times to alarms (16, 22, 32–34). ICU telemedicine programs may also aid in the identification of patients appropriate for transfer to higher levels of care; alternatively, the supplemental care provided by the remote clinicians may enable patients to remain in their community facilities who would have otherwise been transferred (35). In addition, ICU telemedicine can add to the quality improvement infrastructure within hospitals. Through the ability to capture and analyze large amounts of data, ICU telemedicine programs can provide benchmarking reports to individual hospitals about their overall performance over time and compared with other similar facilities. Finally, some hospitals have viewed ICU telemedicine as a way to build relationships with smaller hospitals, support the development of regional care delivery systems, increase revenue by selling their ICU telemedicine services to unaffiliated hospitals, enable hospitals to provide high-risk procedures to patients with complicated medical histories, or distinguish themselves in competitive markets (28, 36).
Despite its initial rapid expansion, the adoption of ICU telemedicine programs appears to have slowed down over recent years, possibly related to the recent recession across the United States that led to reduced investment in healthcare infrastructure (21). Indeed, cost is a major barrier to adoption (28). A 2013 systematic review estimated the cost to implement an ICU telemedicine program for 1 year was between $50,000 and $123,000 (in 2011 U.S. dollars) per monitored ICU bed, with subsequent operating costs of up to $3 million annually (15, 37, 38). Few studies have assessed the financial return on investment of ICU telemedicine, and those that have reported significant cost benefits often contain limited data on actual financial savings and rarely describe their accounting methods (20, 37, 39). A recent study using an activity-based micro-accounting method at a single academic institution found that implementation of ICU telemedicine led to favorable clinical outcomes and financial benefits that exceeded program capital and operating costs through increased case volume, higher case revenue relative to direct costs, and shorter LOS (40). In contrast, several other studies have shown that ICU telemedicine is associated with higher net costs (41, 42).
ICU telemedicine also faces legal and regulatory challenges. Some states require providers to have a special license to deliver telemedicine services from out of state (36). States also vary in their policies regulating reimbursement for ICU telemedicine services. Because patients may never know that they have been seen by a remote physician, controversy exists as to whether ICU telemedicine services function as a surrogate for in-person care or as an enhanced level of care that augments in-person care (43, 44).
Has ICU Telemedicine Lived up to Its Promise?
There are persistent questions about the benefit of ICU telemedicine for multiple reasons (45). First, the paucity of high-quality evidence prevents strong conclusions about its overall effectiveness. Most existing studies are pre–post studies without concurrent controls (46) and without consistent measurement, reporting, and adjustment for patient severity (47). Furthermore, many studies lack details about how ICU telemedicine was implemented. Because it is such a complex intervention, program structures and local buy-in can vary greatly, likely substantially affecting its overall effectiveness.
With these caveats in mind, a review of the existing literature demonstrates mixed effects of ICU telemedicine on patient outcomes, as summarized in Table 1. Most of the early before–after studies demonstrated significant improvement in patient outcomes associated with implementation of ICU telemedicine (19, 20, 48–51), although the interventions were notably different across these studies in several ways, including (but not limited to) the composition of the ICU telemedicine team, the surveillance models and frequency, and the organization of the target ICUs (Table 1). These positive findings were reinforced by a large study in a single academic center published in 2011 (16). Notably, in this study, remote clinicians worked synergistically with bedside providers to enforce daily goals, respond to bedside alarms, and review adherence to evidence-based practices. Authors reported a significant decrease in risk-adjusted hospital mortality after implementation of the ICU telemedicine program (adjusted odds ratio, 0.40). ICU admission postintervention was also associated with significantly higher rates of best clinical practice adherence, lower rates of ICU-acquired complications, and shorter hospital LOS (16). A subsequent large, multicenter observational study evaluated the relationship between individual processes of care that varied among ICU telemedicine interventions and the outcomes of ICU and hospital mortality and ICU and hospital LOS. The authors found that four individual components of the interventions were associated with better outcomes, including prompt remote intensivist case review, improved adherence to evidence-based practices, reduced response times to alarms, and the real-time use of performance measures (22, 30). By identifying individual domains associated with improved outcomes post–ICU telemedicine implementation, authors offered plausible mechanisms for the potential effectiveness of this intervention (29). Finally, a recent large national study evaluating the effectiveness of ICU telemedicine programs with concurrent controls showed a small relative mortality reduction with wide variation in ICU telemedicine effect across adopting hospitals, although this study used an administrative database that lacked detailed clinical risk adjustment (52).
Table 1.
Summary of comparative studies evaluating the effectiveness of intensive care unit telemedicine programs
| Study | Summary of Study Design: No. ICUs/Hospitals; No. of Patients in Control/Telemedicine Groups | Characteristics of ICU Telemedicine Program: Model Timing Reactivity Scope Team Composition | Characteristics of Target ICU: ICU Type(s) Staffing Model ICU Teaching Status |
Major Findings |
|---|---|---|---|---|
| Rosenfeld et al., 2000 (19) | Retrospective observational study with two historical control groups: | Decentralized | SICU | Reduced ICU mortality |
| Continuous | Open | Reduced hospital mortality | ||
| Proactive and reactive | Teaching | Reduced incidence of ICU complications | ||
| Full discretion | Reduced ICU LOS | |||
| 1 Intensivist | Reduced costs | |||
| 1 ICU/1 hospital; | ||||
| 427/201 | ||||
| Breslow et al., 2004 (20) | Retrospective observational study with historical control group: | Centralized | MICU, SICU | Reduced hospital mortality |
| Intermittent (19 h/d) | Open | Reduced ICU LOS | ||
| Proactive and reactive | Mixed teaching and nonteaching patients | Lower variable costs per case | ||
| Variable depending on bedside attending preference | ||||
| 1 Intensivist, 1 RN and 1 clerical staff | ||||
| 2 ICUs/1 hospital; | ||||
| 1,396/744 | ||||
| Thomas et al., 2009 (27) | Pre–post observational study: | Centralized | MICU, SICU, MSICU | No difference in hospital or ICU mortality overall, but reduced hospital and ICU mortality among patients with higher severity of illness |
| Continuous | Variable depending on site | |||
| Proactive and reactive | ||||
| 6 ICUs/5 hospitals; | Variable depending on bedside attending preference | Variable depending on site | ||
| 2,034/2,108 | 2 Intensivists (19 h/d on weekdays), 4 RNs, and 2 clerical staff | No difference in hospital or ICU LOS | ||
| Zawada et al., 2009 (48) | Pre–post observational study: | Centralized | MSICU | Medium-sized regional hospitals: Could not evaluate severity- adjusted mortality, reduced ICU LOS. Tertiary hospital: Reduced observed-to-predicted ICU and hospital mortality, reduced observed-to-predicted ICU and hospital LOS |
| Continuous | Open | |||
| Proactive and reactive | Nonteaching | |||
| 4 ICUs/4 hospitals; | Minimal discretion | |||
| 188/2,445 | 1 physician (not always intensivist) 20 h/d; 1 RN and 1 clerical staff 24 h/d | |||
| McCambridge et al., 2010 (49) | Pre–post observational study: | Centralized | Not specified | Reduced ICU mortality |
| Intermittent (7 p.m. to 7 a.m.) | Closed | Reduced use of mechanical ventilation | ||
| Proactive and reactive | Nonteaching | No difference in ICU or hospital LOS | ||
| 954/959 | Full discretion | |||
| 1 Intensivist and 1 RN | ||||
| 3 ICUs/1 hospital; | ||||
| Morrison et al., 2010 (42) | Retrospective observational study with historical control group, early ICU telemedicine group, and late (well-established) ICU telemedicine group: | Not specified | MICU, SICU, CICU, MSICU | No difference in ICU or hospital mortality |
| Not specified | Open | No difference in ICU or hospital LOS | ||
| Proactive | Teaching (MICU, SICU, CICU), nonteaching (MSCIU) | No difference in costs | ||
| Variable depending on bedside attending preference | ||||
| Not specified | ||||
| 4 ICUs/2 hospitals; | ||||
| 1,371/2,717 | ||||
| Lilly et al., 2011 (16) | Prospective observational study with stepped-wedge implementation: | Centralized | MICU, SICU, CICU | Reduced hospital mortality |
| Continuous | Closed | Reduced hospital LOS | ||
| Proactive and reactive | Teaching hospitals; individual ICU staffing not specified | Higher rates of best practice adherence | ||
| Full discretion | Reduced ICU complications | |||
| 7 ICUs/2 hospitals; | Intensivist present; rest of team not specified | |||
| 1,529/4,761 | ||||
| Kohl et al., 2012 (51) | Pre–post observational study in surgical ICU with medical ICU as temporal control: | Centralized | MICU (control), SICU (intervention) | Reduced ICU and hospital mortality |
| Continuous | MICU closed; SICU open | Reduced ICU LOS | ||
| Proactive and reactive | ||||
| Full discretion | ||||
| 2 RNs during day (7 a.m. to 7 p.m.), and 1 intensivist and 1 RN during night (7 p.m. to 7 a.m.) | Teaching | |||
| 2 ICUs/1 hospital; | ||||
| 466/1,784 | ||||
| Willmitch et al., 2012 (50) | Pre–post observational study with one preimplementation period compared to three consecutive postimplementation periods | Centralized | Not specified | Reduced hospital mortality |
| Variable depending on site | Variable depending on site | Reduced hospital LOS | ||
| Not specified | Not specified | Reduced ICU LOS | ||
| Variable depending on attending preferences | ||||
| 1 intensivist, 3 RNs, 1 clerical staff | ||||
| 10 ICUs/5 hospitals; | ||||
| 6,504/18,152 | ||||
| Lilly et al., 2012 and 2014 (23, 30) | Multicenter retrospective cohort study: | Variable depending on site | Variable depending on site | Reduced ICU mortality |
| Reduced hospital mortality | ||||
| Variable depending on site | Variable depending on site | Reduced ICU LOS | ||
| Reduced hospital LOS | ||||
| Variable depending on site | Variable depending on site | |||
| Variable depending on site | ||||
| 56 ICUs/32 hospitals; | Variable depending on site | |||
| 11,558/107,432 | ||||
| Nassar et al., 2014 (54) | Retrospective observational study including pre–post analysis within ICUs implementing telemedicine programs, in addition to concurrent control ICUs without telemedicine: | Centralized | MICU, SICU, MSICU | No difference in ICU mortality |
| Intermittent (21 h/d) | Variable depending on site | No difference in hospital mortality | ||
| Variable depending on the site | Variable depending on site | No difference in 30-d mortality | ||
| Variable depending on the site | No difference in LOS | |||
| 1 intensivist and 2 RNs | ||||
| 8 telemedicine ICUs with 8 matched control ICUs/7 hospitals; | ||||
| 3,584/3,355 | ||||
| Kahn et al., 2016 (52) | Multicenter retrospective case-control study, matching adopting hospitals (cases) to up to 3 nonadopting hospitals (controls): | Centralized | Not specified | Reduced 90-d mortality |
| Not specified | Not specified | Most effective in large urban hospitals | ||
| Not specified | Variable depending on site | |||
| Not specified | ||||
| Not specified | ||||
| No. ICUs not specified/132 case hospitals with 389 control hospitals; | ||||
| 830,927/292,636 |
Definition of abbreviations: CICU = cardiac intensive care unit; ICU = intensive care unit; LOS = length of stay; MICU = medical intensive care unit; MSICU = medical surgical intensive care unit; RN = registered nurse; SICU = surgical intensive care unit.
However, multiple other observational studies with similar limitations did not find any association of ICU telemedicine with improved patient outcomes (42, 53, 54). Specifically, one study in multiple ICUs across a large U.S. healthcare system evaluated the association of ICU telemedicine with patient outcomes. Of note, ICUs in this study incorporated varying degrees of involvement of the remote ICU telemedicine team and also used a variety of open and closed staffing models (49, 53). The authors reported no difference in severity-adjusted mortality or LOS after intervention. However, they did report decreased mortality for the most severely ill patients after ICU telemedicine implementation, which is consistent with findings from several other studies (41, 53, 55, 56). In another study conducted within the Veterans Affairs Healthcare System using a methodologically rigorous design across seven hospitals with matched controls, authors did not find an association of ICU telemedicine with patient outcomes but noted that their study cohort had an extremely low ICU mortality rate of 2.9%, making it difficult to detect a statistically significant reduction in mortality (54). More recently, one study evaluated whether implementation of an ICU telemedicine program across a regional healthcare system was associated with reduced interhospital transfers (25). Somewhat unexpectedly, they found that interhospital transfers were significantly increased after implementation of the ICU telemedicine intervention, without associated changes in mortality or LOS. This finding was driven primarily by transfers from less specialized to more specialized ICUs and was not readily explained by increased severity of illness (25).
Several systematic reviews and meta-analyses based on these mostly uncontrolled, before–after observational studies concluded that significant associations of ICU telemedicine with reduced ICU mortality and LOS exist, with variable conclusions for hospital mortality and LOS (46, 57). Overall, these data suggest that ICU telemedicine may improve patient outcomes, but likely only when applied in the appropriate setting (29). Unfortunately, the specific characteristics of the target hospital, ICU, and ICU telemedicine unit necessary to optimize the intervention’s effectiveness remain largely undefined. It is also unclear which component of the intervention (e.g., additional providers vs. effects on processes measures vs. the technology itself) may provide greater benefit to patients.
Finally, the cost effectiveness of ICU telemedicine remains uncertain. Multiple studies have demonstrated this intervention to be more cost effective when directed toward the care of sicker patients (41, 56, 58). For example, a simulation analysis on the basis of previously published literature and using hypothetical ICU patients found that the optimal cost effectiveness of ICU telemedicine was achieved when applied to the 30% to 40% highest-risk patients among all ICU patients in urban tertiary hospitals (56). On the other hand, a 2017 study found that implementation of an ICU telemedicine program (including a logistic center that helped manage ICU admissions and discharges) was associated with lower LOS, which translated into greater case volume. This finding, in combination with increased per-case revenue attributed to a structured documentation system that more efficiently captured clinical information, improved financial performance within a single academic medical center (38).
Another recent study of Emory University Critical Care Center’s innovative ICU telemedicine and advanced practice provider training program demonstrated a significant reduction in average Medicare spending per care episode associated with the program’s implementation. These findings were primarily driven by reduced readmissions within 60 days and substitution of home health care for institutional post–acute care for Medicare fee-for-service beneficiaries (59). Despite this, authors noted that sustaining the complex intervention was challenging, because ICU admissions covered by the remote ICU telemedicine providers were reimbursed at the same rate as standard ICU stays despite the added cost of the program (59). Ultimately, the cost effectiveness of ICU telemedicine likely varies between facilities depending on their individual case mix and volume, reimbursement strategy, staffing patterns, presence of existing electronic medical record, and number of beds over which the costs are depreciated (37, 59, 60). For these reasons, the most pressing questions regarding ICU telemedicine intervention at this point is not only whether telemedicine works, but also how, where, and which components of the intervention work best depending on the unique local culture and resources available in a given setting (13).
How and Where Might ICU Telemedicine Be Successfully Implemented?
Certain themes have emerged in the literature over the last two decades that provide some guidance as to how and where ICU telemedicine might be most effective. First, sicker patients may benefit more from ICU telemedicine intervention than less-sick patients (27, 41, 55, 56). Indeed, if patients are not actually critically ill, then having an intensivist involved in their care may not be cost effective or even beneficial (61). Second, although it was originally conceived as a way to expand intensivists’ reach and availability to rural areas, ICU telemedicine may be particularly effective in large urban hospitals (21, 52). ICU capacity strain—when demand on an ICU’s resources exceeds availability—may be the mechanism for this finding (62). Because hospitals located in urban centers are often tertiary care centers with higher daily census and acuity of illness, they may experience greater ICU capacity strain, which has been associated with increased mortality (63). By increasing the number of available care providers, albeit remotely, ICU telemedicine may be one tool that enables ICUs to be more elastic than others during times of high strain, helping to ensure that care remains timely, comprehensive, and accurate (62, 64).
In addition, the impact of ICU telemedicine on patient outcomes varies greatly depending on where and how the intervention is applied (45). Table 2 provides some organizational strategies that may maximize the potential benefit of ICU telemedicine. Important factors to consider include the autonomy assigned to the ICU telemedicine team and the degree to which the technology is embraced by bedside clinicians. Studies in which these aspects were limited did not find an association between ICU telemedicine and patient outcomes (27, 54). However, studies in which the remote team was allowed full discretion in the care for all patients showed significant associations with improved mortality and LOS with the intervention (16, 30, 51). Identifying ways to improve collaboration and integration between the ICU remote team and the bedside providers is important for the effective implementation of ICU telemedicine programs.
Table 2.
Strategies to maximize potential benefit of intensive care unit telemedicine
| Ensure adequate autonomy of intensive care unit telemedicine team |
| Build integrated teams between remote providers and bedside clinicians |
| Promote active comanagement with direct intervention by telemedicine team |
| Develop telemedicine-based protocols for care processes and quality improvement |
| Incorporate internal benchmarking practices led by telemedicine team |
A recent qualitative study of staff acceptance after ICU telemedicine implementation found that receipt of training about the technology, staff knowledge about when and how to use it, and perceived need for the program emerged as important themes influencing the acceptance of ICU telemedicine (65). Including bedside nurses as part of the off-site team is another approach to facilitate local acceptance of the ICU telemedicine intervention. Improved integration between the remote and local teams may also be greater when standards for sign-out, collaborative rounding models, and agreement on standard best practice approaches are developed jointly (66). This type of care model helps to encourage trust in the ICU telemedicine team, which in turn may allow them greater autonomy in the direct care of patients and translate into better outcomes. In addition, the use of a direct intervention with timely notification strategy by the remote ICU telemedicine team, rather than a passive monitor and notify approach, has been associated with improved outcomes (67).
Finally, ICU telemedicine programs may be more effective when they are used as tools for population management and are intentionally linked to specific quality-improvement initiatives and care processes (16, 29). For example, ICU telemedicine has been associated with better adherence to evidence-based practices, such as stress ulcer and venous thromboembolism prophylaxis, and lower rates of preventable complications (16, 30, 68, 69). Beyond these traditional approaches to quality improvement, other potentially important care processes associated with ICU telemedicine include the review of daily goal sheets to ensure implementation of care plans, and off-hours, off-site case review by intensivists (16). These care processes may be important contributors to the positive association of ICU telemedicine with patient outcomes observed in multiple studies (16, 30). Indeed, ICU telemedicine’s greatest effectiveness may actually lie in its ability to ensure the consistent implementation of care plans and ICU best practices that have been proven to save lives, rather than in monitoring patients for clinical deterioration (14).
Future Directions
ICU telemedicine is a promising mechanism to improve outcomes for critically ill patients. More insight into why some ICU telemedicine programs are effective and others are less successful is of utmost importance and requires a deeper understanding of how to maximize the value of these additional resources. Additional pre–post observational studies will not be helpful in answering these questions. Rather, future studies should be guided by frameworks of implementation science to allow for their results to be interpreted through the lens of relevance, generalizability, and applicability across health systems (70–72). Such an approach can enable a better understanding of ICU telemedicine’s influence on the organization and structure of the local ICUs and how organizational readiness for telemedicine before implementation may impact its effectiveness (14). For example, using a standardized approach to assess the preimplementation ICU environment is a key first step in efforts to understand the environmental and cultural factors that may influence the program’s success and to allow valid comparisons across centers and sites (14). Mixed-methods research using both qualitative and quantitative approaches will also be necessary to better understand the crucial issue of context when studying if, how, when, and where ICU telemedicine is most effective (45). Finally, more research is needed into the optimal organizational structure of ICU telemedicine programs and staffing models, such as the optimal ratio of off-site providers to patients, and the core competencies required of the personnel staffing the ICU telemedicine units (14, 73).
Conclusions
ICU telemedicine represents an organizational innovation that has the potential to improve access to and quality of critical care. However, its effectiveness is not necessarily assured and likely depends on the characteristics of the environment where it is deployed and the degree of collaboration between remote providers and bedside clinicians. It is crucial to recognize the wide range of implementation strategies for ICU telemedicine when interpreting existing evidence about its effects on the quality and efficiency of critical care. It is also important to account for local culture and resources when deciding whether to implement the intervention in a particular healthcare system. Although several decades of research have suggested many areas of potential benefit, we still lack understanding about how best to apply and leverage this technology to maximize its value and effectiveness. Future research using mixed-methods approaches and validated models for evaluating public health interventions will be essential to understand how, when, and whether ICU telemedicine should be implemented.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Jeremy Kahn and Omar Badawi for providing their input in the preparation of this manuscript.
Footnotes
Supported by National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI) grant 1K12HL133115 (K.C.V.), Collins Medical Trust (K.C.V.), the Medical Research Foundation (K.C.V.), and resources from the VA Portland Health Care System, Portland, Oregon (C.G.S.).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The Department of Veterans Affairs did not have a role in the preparation of the manuscript. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or the U.S. Government.
CME will be available for this article at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Nates JL, Nunnally M, Kleinpell R, Blosser S, Goldner J, Birriel B, et al. ICU admission, discharge, and triage guidelines: a framework to enhance clinical operations, development of institutional policies, and further research. Crit Care Med. 2016;44:1553–1602. doi: 10.1097/CCM.0000000000001856. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, Barnato AE, Linde-Zwirble WT, Weissfeld LA, Watson RS, Rickert T, et al. Robert Wood Johnson Foundation ICU End-Of-Life Peer Group. Use of intensive care at the end of life in the United States: an epidemiologic study. Crit Care Med. 2004;32:638–643. doi: 10.1097/01.ccm.0000114816.62331.08. [DOI] [PubMed] [Google Scholar]
- 3.Task Force of the American College of Critical Care Medicine, Society of Critical Care Medicine. Guidelines for intensive care unit admission, discharge, and triage. Crit Care Med. 1999;27:633–638. [PubMed] [Google Scholar]
- 4.Halpern NA, Pastores SM. Critical care medicine in the United States 2000-2005: an analysis of bed numbers, occupancy rates, payer mix, and costs. Crit Care Med. 2010;38:65–71. doi: 10.1097/CCM.0b013e3181b090d0. [DOI] [PubMed] [Google Scholar]
- 5.Halpern NA, Goldman DA, Tan KS, Pastores SM. Trends in critical care beds and use among population groups and medicare and medicaid beneficiaries in the United States: 2000–2010. Crit Care Med. 2016;44:1490–1499. doi: 10.1097/CCM.0000000000001722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scales DC, Rubenfeld GD.editorsOrganization of critical care: an evidence-based approach to improving quality New York: Humana Press; 2016 [Google Scholar]
- 7.Garland A, Gershengorn HB. Staffing in ICUs: physicians and alternative staffing models. Chest. 2013;143:214–221. doi: 10.1378/chest.12-1531. [DOI] [PubMed] [Google Scholar]
- 8.Wallace DJ, Angus DC, Barnato AE, Kramer AA, Kahn JM. Nighttime intensivist staffing and mortality among critically ill patients. N Engl J Med. 2012;366:2093–2101. doi: 10.1056/NEJMsa1201918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pronovost PJ, Angus DC, Dorman T, Robinson KA, Dremsizov TT, Young TL. Physician staffing patterns and clinical outcomes in critically ill patients: a systematic review. JAMA. 2002;288:2151–2162. doi: 10.1001/jama.288.17.2151. [DOI] [PubMed] [Google Scholar]
- 10.Angus DC, Kelley MA, Schmitz RJ, White A, Popovich J, Jr Committee on Manpower for Pulmonary and Critical Care Societies (COMPACCS) Caring for the critically ill patient. Current and projected workforce requirements for care of the critically ill and patients with pulmonary disease: can we meet the requirements of an aging population? JAMA. 2000;284:2762–2770. doi: 10.1001/jama.284.21.2762. [DOI] [PubMed] [Google Scholar]
- 11.Kelley MA, Angus D, Chalfin DB, Crandall ED, Ingbar D, Johanson W, et al. The critical care crisis in the United States: a report from the profession. Chest. 2004;125:1514–1517. doi: 10.1378/chest.125.4.1514. [DOI] [PubMed] [Google Scholar]
- 12.Kahn JM, Rubenfeld GD. The myth of the workforce crisis: why the United States does not need more intensivist physicians. Am J Respir Crit Care Med. 2015;191:128–134. doi: 10.1164/rccm.201408-1477CP. [DOI] [PubMed] [Google Scholar]
- 13.Kahn JM. ICU telemedicine: from theory to practice. Crit Care Med. 2014;42:2457–2458. doi: 10.1097/CCM.0000000000000596. [DOI] [PubMed] [Google Scholar]
- 14.Kahn JM. Intensive care unit telemedicine: promises and pitfalls. Arch Intern Med. 2011;171:495–496. doi: 10.1001/archinternmed.2011.23. [DOI] [PubMed] [Google Scholar]
- 15.Coustasse A, Deslich S, Bailey D, Hairston A, Paul D. A business case for tele-intensive care units. Perm J. 2014;18:76–84. doi: 10.7812/TPP/14-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lilly CM, Cody S, Zhao H, Landry K, Baker SP, McIlwaine J, et al. University of Massachusetts Memorial Critical Care Operations Group. Hospital mortality, length of stay, and preventable complications among critically ill patients before and after tele-ICU reengineering of critical care processes. JAMA. 2011;305:2175–2183. doi: 10.1001/jama.2011.697. [DOI] [PubMed] [Google Scholar]
- 17.Grundy BL, Crawford P, Jones PK, Kiley ML, Reisman A, Pao YH, et al. Telemedicine in critical care: an experiment in health care delivery. JACEP. 1977;6:439–444. doi: 10.1016/s0361-1124(77)80239-6. [DOI] [PubMed] [Google Scholar]
- 18.Lilly CM, Zubrow MT, Kempner KM, Reynolds HN, Subramanian S, Eriksson EA, et al. Society of Critical Care Medicine Tele-ICU Committee. Critical care telemedicine: evolution and state of the art. Crit Care Med. 2014;42:2429–2436. doi: 10.1097/CCM.0000000000000539. [DOI] [PubMed] [Google Scholar]
- 19.Rosenfeld BA, Dorman T, Breslow MJ, Pronovost P, Jenckes M, Zhang N, et al. Intensive care unit telemedicine: alternate paradigm for providing continuous intensivist care. Crit Care Med. 2000;28:3925–3931. doi: 10.1097/00003246-200012000-00034. [DOI] [PubMed] [Google Scholar]
- 20.Breslow MJ, Rosenfeld BA, Doerfler M, Burke G, Yates G, Stone DJ, et al. Effect of a multiple-site intensive care unit telemedicine program on clinical and economic outcomes: an alternative paradigm for intensivist staffing. Crit Care Med. 2004;32:31–38. doi: 10.1097/01.CCM.0000104204.61296.41. [DOI] [PubMed] [Google Scholar]
- 21.Kahn JM, Cicero BD, Wallace DJ, Iwashyna TJ. Adoption of ICU telemedicine in the United States. Crit Care Med. 2014;42:362–368. doi: 10.1097/CCM.0b013e3182a6419f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuhrman SA, Lilly CM. ICU telemedicine solutions. Clin Chest Med. 2015;36:401–407. doi: 10.1016/j.ccm.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Lilly CM, Fisher KA, Ries M, Pastores SM, Vender J, Pitts JA, et al. A national ICU telemedicine survey: validation and results. Chest. 2012;142:40–47. doi: 10.1378/chest.12-0310. [DOI] [PubMed] [Google Scholar]
- 24.Reynolds HN, Rogove H, Bander J, McCambridge M, Cowboy E, Niemeier M. A working lexicon for the tele-intensive care unit: we need to define tele-intensive care unit to grow and understand it. Telemed J E Health. 2011;17:773–783. doi: 10.1089/tmj.2011.0045. [DOI] [PubMed] [Google Scholar]
- 25.Pannu J, Sanghavi D, Sheley T, Schroeder DR, Kashyap R, Marquez A, et al. Impact of telemedicine monitoring of community ICUs on interhospital transfers. Crit Care Med. 2017;45:1344–1351. doi: 10.1097/CCM.0000000000002487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vespa PM, Miller C, Hu X, Nenov V, Buxey F, Martin NA. Intensive care unit robotic telepresence facilitates rapid physician response to unstable patients and decreased cost in neurointensive care. Surg Neurol. 2007;67:331–337. doi: 10.1016/j.surneu.2006.12.042. [DOI] [PubMed] [Google Scholar]
- 27.Thomas EJ, Lucke JF, Wueste L, Weavind L, Patel B. Association of telemedicine for remote monitoring of intensive care patients with mortality, complications, and length of stay. JAMA. 2009;302:2671–2678. doi: 10.1001/jama.2009.1902. [DOI] [PubMed] [Google Scholar]
- 28.Berenson RA, Grossman JM, November EA. Does telemonitoring of patients--the eICU--improve intensive care? Health Aff (Millwood) 2009;28:w937–w947. doi: 10.1377/hlthaff.28.5.w937. [DOI] [PubMed] [Google Scholar]
- 29.Kahn JM. The use and misuse of ICU telemedicine. JAMA. 2011;305:2227–2228. doi: 10.1001/jama.2011.716. [DOI] [PubMed] [Google Scholar]
- 30.Lilly CM, McLaughlin JM, Zhao H, Baker SP, Cody S, Irwin RS UMass Memorial Critical Care Operations Group. A multicenter study of ICU telemedicine reengineering of adult critical care. Chest. 2014;145:500–507. doi: 10.1378/chest.13-1973. [DOI] [PubMed] [Google Scholar]
- 31.Kahn JM, Gunn SR, Lorenz HL, Alvarez J, Angus DC. Impact of nurse-led remote screening and prompting for evidence-based practices in the ICU. Crit Care Med. 2014;42:896–904. doi: 10.1097/CCM.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phillips J. Clinical alarms: complexity and common sense. Crit Care Nurs Clin North Am. 2006;18:145–156, ix. doi: 10.1016/j.ccell.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 34.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 35.Tyler PD, Celi LA. Tele-ICU increases interhospital transfers: does big brother know better? Crit Care Med. 2017;45:1417–1419. doi: 10.1097/CCM.0000000000002510. [DOI] [PubMed] [Google Scholar]
- 36.Adler-Milstein J, Kvedar J, Bates DW. Telehealth among US hospitals: several factors, including state reimbursement and licensure policies, influence adoption. Health Aff (Millwood) 2014;33:207–215. doi: 10.1377/hlthaff.2013.1054. [DOI] [PubMed] [Google Scholar]
- 37.Kumar G, Falk DM, Bonello RS, Kahn JM, Perencevich E, Cram P. The costs of critical care telemedicine programs: a systematic review and analysis. Chest. 2013;143:19–29. doi: 10.1378/chest.11-3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lilly CM, Motzkus C, Rincon T, Cody SE, Landry K, Irwin RS UMass Memorial Critical Care Operations Group. ICU telemedicine program financial outcomes. Chest. 2017;151:286–297. doi: 10.1016/j.chest.2016.11.029. [DOI] [PubMed] [Google Scholar]
- 39.Ries M. Evaluating tele-ICU cost--an imperfect science. Crit Care Med. 2016;44:441–442. doi: 10.1097/CCM.0000000000001506. [DOI] [PubMed] [Google Scholar]
- 40.Lilly CM, Motzkus CA. ICU telemedicine: financial analyses of a complex intervention. Crit Care Med. 2017;45:1558–1561. doi: 10.1097/CCM.0000000000002535. [DOI] [PubMed] [Google Scholar]
- 41.Franzini L, Sail KR, Thomas EJ, Wueste L. Costs and cost-effectiveness of a telemedicine intensive care unit program in 6 intensive care units in a large health care system. J Crit Care. 2011;26:329.e1–329.e6. doi: 10.1016/j.jcrc.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morrison JL, Cai Q, Davis N, Yan Y, Berbaum ML, Ries M, et al. Clinical and economic outcomes of the electronic intensive care unit: results from two community hospitals. Crit Care Med. 2010;38:2–8. doi: 10.1097/CCM.0b013e3181b78fa8. [DOI] [PubMed] [Google Scholar]
- 43.McCambridge MM, Tracy JA, Sample GA. Point: should tele-ICU services be eligible for professional fee billing? Yes. Tele-ICUs and the triple aim. Chest. 2011;140:847–849. doi: 10.1378/chest.11-1555. [DOI] [PubMed] [Google Scholar]
- 44.Hoffmann S. Counterpoint: should tele-ICU services be eligible for professional fee billing? No. Chest. 2011;140:849–851. doi: 10.1378/chest.11-1560. [DOI] [PubMed] [Google Scholar]
- 45.Kahn JM. Virtual visits--confronting the challenges of telemedicine. N Engl J Med. 2015;372:1684–1685. doi: 10.1056/NEJMp1500533. [DOI] [PubMed] [Google Scholar]
- 46.Wilcox ME, Adhikari NK. The effect of telemedicine in critically ill patients: systematic review and meta-analysis. Crit Care. 2012;16:R127. doi: 10.1186/cc11429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Young LB, Chan PS, Lu X, Nallamothu BK, Sasson C, Cram PM. Impact of telemedicine intensive care unit coverage on patient outcomes: a systematic review and meta-analysis. Arch Intern Med. 2011;171:498–506. doi: 10.1001/archinternmed.2011.61. [DOI] [PubMed] [Google Scholar]
- 48.Zawada ET, Jr, Herr P, Larson D, Fromm R, Kapaska D, Erickson D. Impact of an intensive care unit telemedicine program on a rural health care system. Postgrad Med. 2009;121:160–170. doi: 10.3810/pgm.2009.05.2016. [DOI] [PubMed] [Google Scholar]
- 49.McCambridge M, Jones K, Paxton H, Baker K, Sussman EJ, Etchason J. Association of health information technology and teleintensivist coverage with decreased mortality and ventilator use in critically ill patients. Arch Intern Med. 2010;170:648–653. doi: 10.1001/archinternmed.2010.74. [DOI] [PubMed] [Google Scholar]
- 50.Willmitch B, Golembeski S, Kim SS, Nelson LD, Gidel L. Clinical outcomes after telemedicine intensive care unit implementation. Crit Care Med. 2012;40:450–454. doi: 10.1097/CCM.0b013e318232d694. [DOI] [PubMed] [Google Scholar]
- 51.Kohl BA, Fortino-Mullen M, Praestgaard A, Hanson CW, Dimartino J, Ochroch EA. The effect of ICU telemedicine on mortality and length of stay. J Telemed Telecare. 2012;18:282–286. doi: 10.1258/jtt.2012.120208. [DOI] [PubMed] [Google Scholar]
- 52.Kahn JM, Le TQ, Barnato AE, Hravnak M, Kuza CC, Pike F, et al. ICU telemedicine and critical care mortality: a national effectiveness study. Med Care. 2016;54:319–325. doi: 10.1097/MLR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thomas JT, Moeckli J, Mengeling MA, Goedken CC, Bunch J, Cram P, et al. Bedside critical care staff use of intensive care unit telemedicine: comparisons by intensive care unit complexity. Telemed J E Health. 2017;23:718–725. doi: 10.1089/tmj.2016.0243. [DOI] [PubMed] [Google Scholar]
- 54.Nassar BS, Vaughan-Sarrazin MS, Jiang L, Reisinger HS, Bonello R, Cram P. Impact of an intensive care unit telemedicine program on patient outcomes in an integrated health care system. JAMA Intern Med. 2014;174:1160–1167. doi: 10.1001/jamainternmed.2014.1503. [DOI] [PubMed] [Google Scholar]
- 55.Yoo BK, Kim M, Sasaki T, Melnikow J, Marcin JP. Economic evaluation of telemedicine for patients in ICUs. Crit Care Med. 2016;44:265–274. doi: 10.1097/CCM.0000000000001426. [DOI] [PubMed] [Google Scholar]
- 56.Yoo BK, Kim M, Sasaki T, Hoch JS, Marcin JP. Selected use of telemedicine in intensive care units based on severity of illness improves cost-effectiveness. Telemed J E Health. 2018;24:21–36. doi: 10.1089/tmj.2017.0069. [DOI] [PubMed] [Google Scholar]
- 57.Chen J, Sun D, Yang W, Liu M, Zhang S, Peng J, et al. Clinical and economic outcomes of telemedicine programs in the intensive care unit: a systematic review and meta-analysis. J Intensive Care Med. 2018;33:383–393. doi: 10.1177/0885066617726942. [DOI] [PubMed] [Google Scholar]
- 58.Yoo EJ, Dudley RA. Evaluating telemedicine in the ICU. JAMA. 2009;302:2705–2706. doi: 10.1001/jama.2009.1924. [DOI] [PubMed] [Google Scholar]
- 59.Trombley MJ, Hassol A, Lloyd JT, Buchman TG, Marier AF, White A, et al. The impact of enhanced critical care training and 24/7 (tele-ICU) support on medicare spending and postdischarge utilization patterns Health Serv Res[online ahead of print] 27 Dec 2017. DOI: 10.1111/1475-6773.12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buchman TG, Coopersmith CM, Meissen HW, Grabenkort WR, Bakshi V, Hiddleson CA, et al. Innovative interdisciplinary strategies to address the intensivist shortage. Crit Care Med. 2017;45:298–304. doi: 10.1097/CCM.0000000000002209. [DOI] [PubMed] [Google Scholar]
- 61.Wilcox ME, Wiener-Kronish JP. Telemedicine in the intensive care unit: effect of a remote intensivist on outcomes. JAMA Intern Med. 2014;174:1167–1169. doi: 10.1001/jamainternmed.2014.289. [DOI] [PubMed] [Google Scholar]
- 62.Halpern SD. ICU capacity strain and the quality and allocation of critical care. Curr Opin Crit Care. 2011;17:648–657. doi: 10.1097/MCC.0b013e32834c7a53. [DOI] [PubMed] [Google Scholar]
- 63.Gabler NB, Ratcliffe SJ, Wagner J, Asch DA, Rubenfeld GD, Angus DC, et al. Mortality among patients admitted to strained intensive care units. Am J Respir Crit Care Med. 2013;188:800–806. doi: 10.1164/rccm.201304-0622OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vranas KC, Kerlin MP. ICU physician workflow: inside the balloon. Crit Care Med. 2016;44:1607–1608. doi: 10.1097/CCM.0000000000001784. [DOI] [PubMed] [Google Scholar]
- 65.Moeckli J, Cram P, Cunningham C, Reisinger HS. Staff acceptance of a telemedicine intensive care unit program: a qualitative study. J Crit Care. 2013;28:890–901. doi: 10.1016/j.jcrc.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 66.Lilly CM, Thomas EJ. Tele-ICU: experience to date. J Intensive Care Med. 2010;25:16–22. doi: 10.1177/0885066609349216. [DOI] [PubMed] [Google Scholar]
- 67.Hawkins HA, Lilly CM, Kaster DA, Groves RH, Jr, Khurana H. ICU telemedicine comanagement methods and length of stay. Chest. 2016;150:314–319. doi: 10.1016/j.chest.2016.03.030. [DOI] [PubMed] [Google Scholar]
- 68.Lilly CM, Zuckerman IH, Badawi O, Riker RR. Benchmark data from more than 240,000 adults that reflect the current practice of critical care in the United States. Chest. 2011;140:1232–1242. doi: 10.1378/chest.11-0718. [DOI] [PubMed] [Google Scholar]
- 69.Pronovost PJ, Berenholtz SM, Goeschel C, Thom I, Watson SR, Holzmueller CG, et al. Improving patient safety in intensive care units in Michigan. J Crit Care. 2008;23:207–221. doi: 10.1016/j.jcrc.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 70.Green LW, Glasgow RE. Evaluating the relevance, generalization, and applicability of research: issues in external validation and translation methodology. Eval Health Prof. 2006;29:126–153. doi: 10.1177/0163278705284445. [DOI] [PubMed] [Google Scholar]
- 71.Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89:1322–1327. doi: 10.2105/ajph.89.9.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gaglio B, Shoup JA, Glasgow RE. The RE-AIM framework: a systematic review of use over time. Am J Public Health. 2013;103:e38–e46. doi: 10.2105/AJPH.2013.301299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Afessa B. Tele-intensive care unit: the horse out of the barn. Crit Care Med. 2010;38:292–293. doi: 10.1097/CCM.0b013e3181b9d4dc. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.