Abstract
Rationale: Colder temperatures have been shown to increase hospitalization and mortality rates in adults with chronic obstructive pulmonary disease (COPD) and cardiac disease. Seasonal influences on exacerbation rates in adults with severe COPD but without significant cardiovascular disease are unclear. In addition, regional variations in COPD exacerbations in North America have not yet been explored.
Objectives: In this study, we sought to determine the seasonal and regional variability in exacerbation rates in those with COPD but without significant cardiovascular risk factors.
Methods: We studied adults without cardiovascular risk factors from STATCOPE (Simvastatin in the Prevention of COPD Exacerbations) and placebo arm of MACRO (Azithromycin for the Prevention of Exacerbations of COPD) studies. Forty-five study sites were divided into climate regions in Canada and the United States; seasons were defined as winter, spring, summer, and fall. The primary outcome was the rate of COPD exacerbation. Secondary outcomes included time to first exacerbation, severity of exacerbations, all-cause mortality, and antibiotic and steroid use.
Results: We analyzed 1,175 subjects with a mean age of 63.3 ± 8.6 years, forced expiratory volume in 1 second of 41.5 ± 17.1% predicted, and 53.6 ± 29.4 pack-years of smoking history. The COPD exacerbation rate was higher in winter (0.13 exacerbations/person-month) than in spring, summer, and fall (0.11, 0.079, and 0.10 exacerbations/person-month, respectively) (P < 0.001). Summer had the highest proportion of severe exacerbations (40.5%) compared with spring, fall, and winter (32.6%, 34.7%, and 33.1%, respectively) (P = 0.004). Mortality was highest in spring and winter (34% and 30%, respectively). There was significant regional variability in the time to first exacerbation, with the Southeast and West having longer median times to first exacerbation (350 and 342 d, respectively, compared with 184 d in other regions) (P < 0.001).
Conclusions: Significant seasonal and regional variability exist in the rate and severity of exacerbations and overall mortality in adults with COPD without cardiovascular disease.
Keywords: chronic obstructive lung disease, exacerbation, season, region, cardiovascular disease
Chronic obstructive pulmonary disease (COPD) is one of the world’s leading causes of morbidity and mortality and a significant cost to the current healthcare system (1, 2). Exacerbations of COPD have been associated with increased mortality and poorer quality of life (3–5), and much effort has been made to identify and prevent exacerbations. Studies have shown that factors such as low body mass index (BMI) and forced expiratory volume in 1 second (FEV1), smoking status, respiratory infections, and comorbid conditions contribute to worse disease outcomes and more frequent exacerbations.
A few population-based reviews have previously described potential seasonal influences on COPD exacerbations and mortality (6–9). Studies by Jenkins and colleagues (10) and Rabe and colleagues (11) used data from Toward a Revolution in COPD Health (TORCH) and Prevention of Exacerbations with Tiotropium in COPD (POET-COPD) trials, respectively, to show an increased frequency of COPD exacerbations and hospitalizations during winter months. However, these studies were pharmacologic clinical trials that had more restrictive participant populations and included few subjects on current oxygen therapy or with very severe COPD. These studies also included subjects from many different countries, with less homogeneous control for confounding factors. A review paper by Donaldson and Wedzicha (12) also summarized the negative relationship between temperature and COPD morbidity. However, there are limited data on the effects of seasonality and regional variability in adults with severe COPD.
Another factor that clouds the association between seasonality and COPD exacerbations is the presence of comorbid cardiovascular disease (13–15). The seasonal influence of cardiovascular hospitalizations has been reported, with significantly higher rates of myocardial infarction present in the winter months (16–19). It is also known that cardiovascular disease contributes to hospitalization of adults with COPD, both independently and in association with respiratory symptoms (13, 15). Hole and colleagues showed an inverse relationship between FEV1 and death due to ischemic myocardial disease and stroke (20). Sin and colleagues also showed that subjects with worse airflow obstruction had an increased prevalence of myocardial infarction (14). Heightened systemic inflammation present in both COPD and cardiovascular disease likely plays an important role in this relationship (21–23). In addition, concurrent decompensation of heart failure and volume shifts can present as dyspnea in these subjects. Elevated levels of cardiac markers, such as troponins and pro–brain natriuretic peptide are associated with worse mortality in adults with COPD (24). It is difficult to disentangle these two diseases when studying COPD morbidity outcomes, and the impact of seasonality in those with severe COPD but without cardiovascular disease has not been reported.
This study aims to determine seasonal and regional effects on COPD morbidity and mortality in those without cardiovascular risk factors using data from two large randomized controlled trials designed to assess COPD exacerbations. We hypothesize that colder seasons are associated with increased exacerbations and death.
Methods
Study Design
Data on participants from the Simvastatin in the Prevention of COPD Exacerbations (STATCOPE) study and the placebo arm from the Azithromycin for the Prevention of Exacerbations of COPD (MACRO) study without significant cardiovascular risk factors were used for analysis (25, 26). Participants had a clinical diagnosis of moderate to severe COPD, with greater than 10 pack-year smoking history, and had to be on supplemental oxygen or have had an exacerbation within the year before enrollment. A total of 1,934 participants, 1,049 in the MACRO study and 885 in the STATCOPE study, were screened. Subjects in the treatment arm of the MACRO study were excluded, as the treatment significantly decreased exacerbation rates. The presence of cardiovascular risks was calculated using the Adult Treatment Panel III. Subjects requiring statin therapy or with 10-year coronary heart disease risk greater than 20% were excluded, as was done in the STATCOPE trial (25). Subjects were also excluded from the study if Adult Treatment Panel III score could not be calculated. In total, 1,175 subjects were included for the analysis (see Figure E1 in the online supplement).
Subjects were enrolled from March 2006 to May 2009 in the MACRO study and March 2010 to October 2013 in the STATCOPE study. Thirty-three participants were enrolled in both studies, and only the data from the MACRO trial were used for analysis in these subjects.
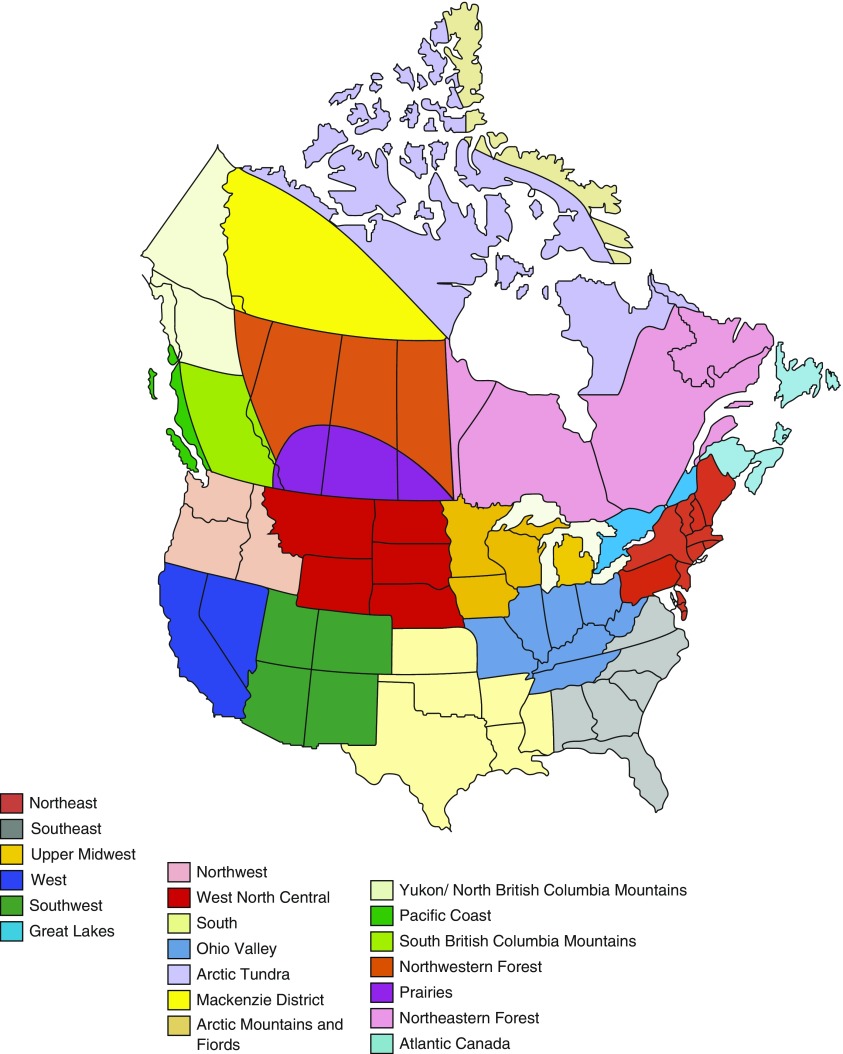
Forty-five study sites were divided into climate regions in Canada and the United States defined by Meteorological Services of Canada and National Oceanic and Atmospheric Administration of the United States on the basis of climate analysis performed by National Centers for Environmental Information scientists (Figure 1). These sites were located in the following cities: Baltimore, Birmingham, Boston, Denver, Los Angeles, Minneapolis, Rochester, San Francisco, Ann Arbor, Philadelphia, Pittsburgh, Ottawa, Vancouver, Winnipeg, Halifax, Calgary, Montreal, and Toronto. They were grouped into the following regions: Northeast, Southeast, Upper Midwest, West, Southwest, Great Lakes, Pacific Coast, and Prairies. Regions with fewer than 35 participants were excluded from data analysis.
Figure 1.
Climate regions in Canada and the United States.
Meteorological definition of season reported by the National Oceanic and Atmospheric Administration was used to identify each season in 3-month periods (27). Winter included December to February, spring included March to May, summer included June to August, and fall included September to November.
The primary outcome of the study was rate of COPD exacerbation. Exacerbations were defined by an increase in severity or new onset of two or more respiratory symptoms (cough, sputum, wheezing, dyspnea, or chest tightness) and requiring treatments with systemic steroids or antibiotics for 3 or more days (26, 28, 29). Poisson regression model was used to estimate rates and rate ratios, and a multivariable model was used to adjust for BMI, age, sex, FEV1% predicted, and current oxygen use status.
Secondary outcomes included severity of exacerbations, mortality, antibiotic or steroid use, and time to first exacerbation in each region and season. Exacerbation severity was based on treatment location, with mild exacerbations treated at home, moderate exacerbations resulting in emergency department visits, severe exacerbations requiring hospitalization, and very severe exacerbations requiring intubation and mechanical ventilation in the intensive care unit (25). Percentages of antibiotics only, steroids only, and combined antibiotic and steroid use in each season in different regions were also assessed.
A subgroup analysis correlating frequency of exacerbations and prevalence of positive flu tests in each year was performed in those subjects with at least one exacerbation and in the U.S. regions. Data for prevalence of flu in the United States was obtained from Centers for Disease Control and Prevention (30).
Statistical Analysis
Exacerbation rates were calculated using the number of exacerbation in months divided by the total number of person-months recorded for each season. The rate ratio of exacerbations in the different seasons compared with summer was calculated using the Poisson regression model. Analysis was performed for all regions combined and for each individual region. Seasonal and regional interactions were assessed using the Poisson regression model as well.
The distributions of severity of exacerbation and all-cause mortality were calculated using the number of each exacerbation severity or death during each season over the total number of exacerbations or death.
Univariate comparisons of baseline characteristics and outcomes were conducted using an analysis of variance for continuous variables and a chi-square test for categorical variables. A logistic regression model that included season, region, age, sex, race, BMI, FEV1% predicted, and smoking and oxygen use status at enrollment was used to perform an adjusted analysis of COPD exacerbation. Log-rank tests were used to compare all-cause mortality and time to first exacerbation among different regions and seasons and illustrated using Kaplan-Meier survival curves. Statistical significance was declared if the P value was less than 0.05. Statistical analysis was performed using STATA14.0 and SAS 9.4.
Results
Participant Characteristics
A total of 1,175 participants were included in the study for analysis (Figure 1). Participants were divided into climate regions on the basis of their enrollment centers, and a total of six climate regions were included in the analysis. The Pacific Coast and Prairies regions had fewer than 35 subjects and were not included in the analysis.
At enrollment, participants had a mean age of 63.3 years (standard deviation [SD], 8.6 yr), FEV1 of 41.5% (SD, 17.1%) predicted, and 53.6 pack-years (SD, 29.4 pack-years) of smoking. Fifty percent of participants were on supplemental oxygen at enrollment. There were significant regional differences in baseline FEV1% predicted, BMI, race, current smoking history, and use of oxygen (Table 1).
Table 1.
Characteristics of participants at enrollment
| All Regions (n = 1,175) | Northeast (n = 419) | Southeast (n = 135) | Upper Midwest (n = 254) | West (n = 160) | Southwest (n = 108) | Great Lakes (n = 99) | |
|---|---|---|---|---|---|---|---|
| Age, yr | 63.26 ± 8.6 | 62.4 ± 7.9 | 60.88 ± 8.7 | 64.63 ± 9.2 | 63.19 ± 9.1 | 64.3 ± 9.0 | 65.6 ± 7.1 |
| Smoking history, pack-years | 53.63 ± 29.4 | 54.94 ± 29.6 | 52.99 ± 28 | 55.06 ± 29.4 | 49.68 ± 30.2 | 52.41 ± 31.1 | 53.03 ± 27.5 |
| FEV1, L | 1.18 ± 0.6 | 1.13 ± 0.5 | 1.32 ± 0.6 | 1.16 ± 0.5 | 1.34 ± 0.6 | 1.07 ± 0.53 | 1.14 ± 0.5 |
| FVC, L | 2.67 ± 0.9 | 2.57 ± 0.8 | 2.95 ± 1.0 | 2.65 ± 0.9 | 2.83 ± 0.9 | 2.47 ± 0.9 | 2.58 ± 0.9 |
| FEV1% predicted | 41.50 ± 17.1 | 40.0 ± 16.5 | 1.47 ± 2.0 | 41.04 ± 17.2 | 47.67 ± 18.3 | 37.50 ± 16.2 | 50.53 ± 16.5 |
| BMI, kg/m2 | 27.3 ± 6.6 | 27.9 ± 6.9 | 26.9 ± 5.7 | 27.40 ± 6.8 | 27.8 ± 6.7 | 26.3 ± 6.1 | 25.9 ± 6.1 |
| Male % | 57.79 | 58 | 64.44 | 54.72 | 57.5 | 56.48 | 57.58 |
| Race | |||||||
| White | 77.8 | 71.36 | 65.93 | 87.01 | 70 | 87.04 | 100 |
| African American | 19.1 | 26.49 | 31.11 | 9.06 | 23.13 | 10.19 | 0 |
| Other | 3.15 | 2.15 | 2.96 | 3.94 | 6.88 | 2.78 | 0 |
| Current smoker, % | 28.8 | 27.68 | 43.7 | 25.59 | 38.75 | 17.56 | 17.17 |
| Current O2 use, % | 50.21 | 52.74 | 38.52 | 51.97 | 33.16 | 91.67 | 33.33 |
Definition of abbreviations: BMI = body mass index; FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity.
Outcomes
Primary outcome
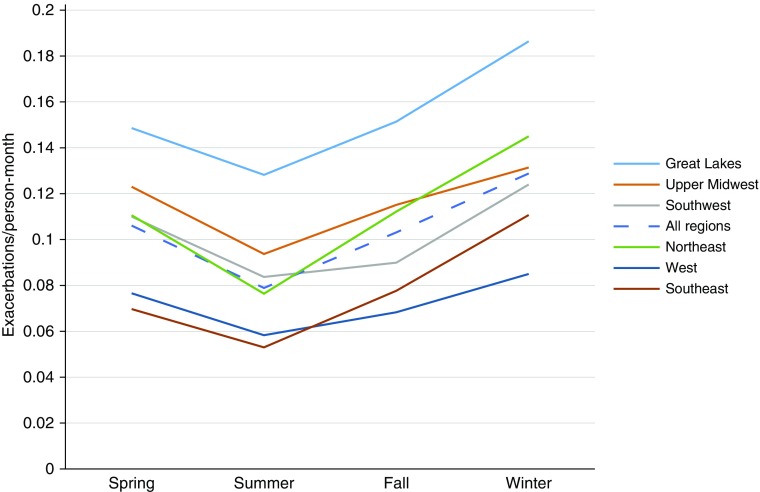
There were a total of 2,179 exacerbations in 787 subjects. The overall rate of COPD exacerbations was higher in the winter (0.13 events/person-month) than in the other seasons (spring: 0.11 events/person-month; summer: 0.08 events/person-month; fall: 0.10 events/person-month) when all regions were combined (P ≤ 0.001) (Figure 2). There was some regional variability in this relationship, but the effect was statistically significant in most regions (Figure 2). The rate ratios of COPD exacerbations were significantly higher in the spring, fall, and winter than in the summer (1.34 [95% confidence interval (CI), 1.20–1.51], 1.31 [1.16–1.47], 1.64 [1.45–1.84], respectively [Table 2]). This relationship was maintained when each region was analyzed separately, with statistical significance in all regions except for Great Lakes when comparing winter and summer (Table 2).
Figure 2.
Rates of exacerbations in each season in each region. Rate ratio (RR) and 95% confidence interval (CI) in the regions with highest and lowest exacerbation rates include: West (RR, 0.65; 95% CI, 0.53–0.81), Southeast (RR, 0.80; 95% CI, 0.56–0.87), and Great Lakes (RR, 1.35; 95% CI, 1.04–1.73) compared with Northeast.
Table 2.
Chronic obstructive pulmonary disease exacerbation rates and adjusted rate ratio in each season in different regions
| Summer | Fall | Winter | Spring | |
|---|---|---|---|---|
| All regions | ||||
| Event rate (events/person-month) | 0.079 | 0.104 | 0.13 | 0.107 |
| 95% CI | 0.072–0.87 | 0.096–0.112 | 0.12–0.139 | 0.098–0.116 |
| Adjusted RR | 1 | 1.31 | 1.64 | 1.34 |
| 95% CI | 1.16–1.47 | 1.45–1.84 | 1.20–1.51 | |
| Northeast | ||||
| Event rate (events/person-month) | 0.077 | 0.11 | 0.15 | 0.11 |
| 95% CI | 0.066–0.089 | 0.10–0.13 | 0.13–0.16 | 0.098–0.13 |
| Adjusted RR | 1 | 1.5 | 1.9 | 1.44 |
| 95% CI | 1.22–1.79 | 1.54–2.29 | 1.19–1.75 | |
| Southeast | ||||
| Event rate (events/person-month) | 0.053 | 0.078 | 0.11 | 0.070 |
| 95% CI | 0.057–0.073 | 0.059–0.10 | 0.087–0.14 | 0.051–0.093 |
| Adjusted RR | 1 | 1.47 | 2.1 | 1.32 |
| 95% CI | 0.95–2.28 | 1.36–3.23 | 0.87–1.99 | |
| Upper Midwest | ||||
| Event rate (events/person-month) | 0.094 | 0.12 | 0.12 | 0.12 |
| 95% CI | 0.078–0.11 | 0.098–0.14 | 0.098–0.13 | 0.1–0.14 |
| Adjusted RR | 1 | 1.23 | 1.39 | 1.31 |
| 95% CI | 0.99–1.53 | 1.11–1.74 | 1.05–1.64 | |
| West | ||||
| Event rate (events/person-month) | 0.59 | 0.69 | 0.086 | 0.078 |
| 95% CI | 0.043–0.079 | 0.052–0.09 | 0.066–0.11 | 0.059–0.1 |
| Adjusted RR | 1 | 1.18 | 1.47 | 1.34 |
| 95% CI | 0.80–1.74 | 1.01–2.13 | 0.91–1.96 | |
| Southwest | ||||
| Event rate (events/person-month) | 0.084 | 0.09 | 0.12 | 0.11 |
| 95% CI | 0.062–0.11 | 0.068–0.12 | 0.097–0.16 | 0.084–0.14 |
| Adjusted RR | 1 | 1.08 | 1.49 | 1.32 |
| 95% CI | 0.72–1.61 | 1.06–2.11 | 0.88–1.98 | |
| Great Lakes | ||||
| Event rate (events/person-month) | 0.13 | 0.15 | 0.19 | 0.15 |
| 95% CI | 0.095–0.17 | 0.12–0.19 | 0.13–0.25 | 0.11–0.2 |
| Adjusted RR | 1.17 | 1.42 | 1.10 | |
| 95% CI | 0.84–1.63 | 0.98–2.05 | 0.76–1.58 | |
Definition of abbreviations: CI = confidence interval; RR = rate ratio.
Each season was defined as follows: winter (December to February), spring (March to May), summer (June to August), and fall (September to November). RR is adjusted for age, sex, forced expiratory volume in 1 second, oxygen use, smoking status, and body mass index
The severity of COPD exacerbations also varied significantly in the different seasons and regions. Even though exacerbations occurred less frequently in the summer, they tended to be more severe than exacerbations that occurred in the other seasons. Forty-three percent of exacerbations were moderate to very severe in the summer compared with 34.8%, 32.1%, and 31.9% in the spring, fall, and winter, respectively (P = 0.001). The total number of severe to very severe exacerbations remained similar throughout the seasons compared with mild to moderate exacerbations. This difference was especially notable in the Northeast, Southeast, Upper Midwest, and Great Lakes regions (Figure E3).
The percentages of subjects receiving steroid only, antibiotic only, and both steroid and antibiotic treatments were calculated for the varying exacerbation severities in each region. There were no significant differences in medication prescription patterns in the different seasons. Most subjects with mild exacerbations received both antibiotics and steroids (50.5%) compared with antibiotics only (33.6%) or steroids only (15.9%). There was a greater proportion of subjects receiving combined antibiotic and steroid treatment as the severity increased (P = 0.001).
Deaths occurred more frequently in the spring and the winter (33.8% and 30.9%) than in the summer and the fall (20.6% and 14.7%), correlating with the patterns observed with the rates of exacerbations (Figure E4).
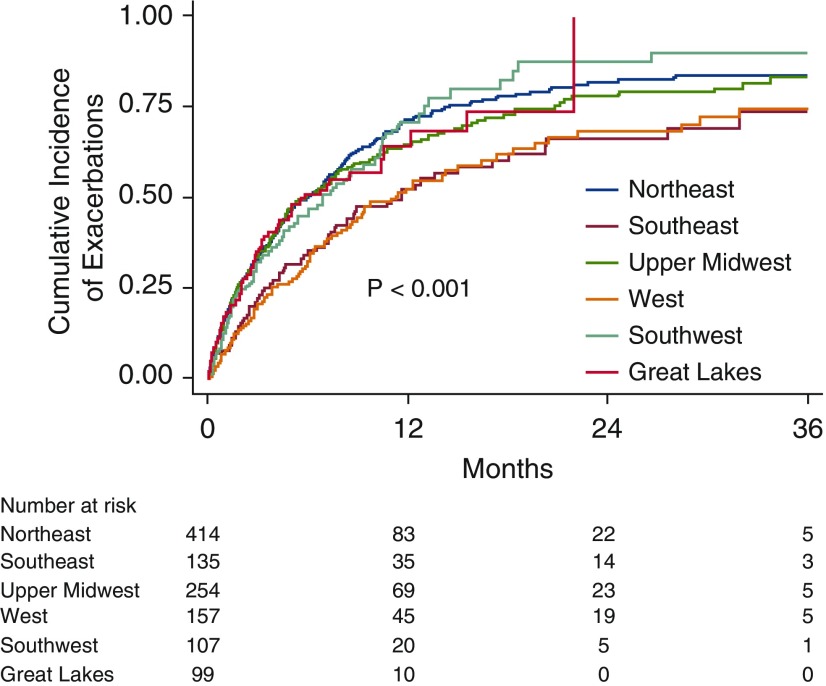
Time to first exacerbation was also assessed from the time of enrollment in each region and season. There was significant seasonal variability for time to first exacerbation within 90 days after enrollment (P < 0.01). Statistically significant regional variability in the time to first exacerbation was also present (Figure 3). Southeast and West climate regions had significantly longer median times to first exacerbation (350 and 342 d, respectively) compared with other climate regions (184 d) (P = 0.001).
Figure 3.
Kaplan-Meier curve of time to first exacerbation in each region.
Subgroup analysis of subjects with at least one exacerbation and the U.S. regions showed that the frequency of exacerbations correlated with changes in flu prevalence (Figure E4).
Discussion
Our study illustrates significant seasonal and regional variability in the rates and severity of COPD exacerbations in those without significant cardiovascular disease. This finding is especially important because cardiovascular disease is an independent risk factor for mortality and hospital admissions in colder seasons (8, 16–19, 31, 32). Admissions for COPD exacerbations are often due to combined respiratory and cardiac etiology, and heightened systemic inflammation in these diseases likely plays a role in worsening the cardiopulmonary status in those with COPD (14, 15, 33). In fact, low FEV1 is associated with all-cause and cardiovascular mortality, stroke, and atrial fibrillation (13, 14, 34). Therefore, it is very difficult to independently study the effects of seasonality without separating the comorbid conditions. Our study is unique in that we effectively excluded those with significant cardiovascular disease in adults with COPD and thereby provide epidemiologic control to assess effects seasonality may have on COPD exacerbations.
Many of the prior studies that examined the seasonal influences on COPD exacerbation rates were retrospective analyses of pharmacologic studies and included subjects with less severe disease and followed for a shorter duration. For example, the POET-COPD trial, which was designed to study the effects of tiotropium compared with those of salmeterol in adults with COPD over a 1-year follow-up period, showed a twofold increase in COPD exacerbations and greater all-cause mortality during winter months compared with those during summer months (11). Data from the TORCH study, designed to study the effects of salmeterol–fluticasone propionate combination in participants with COPD, showed a higher prevalence of exacerbations in winter months and that older age, lower FEV1% predicted, and prior exacerbations portended increased exacerbation frequency (10). Both studies included participants from more than 25 countries, making it difficult to account for confounding regional variables. Participants were also followed for a significantly shorter period compared with our study. Single-center data from the London COPD cohort showed an increased occurrence and duration of COPD exacerbations in colder months (9). Our study population comprised adults with more severe COPD, with a greater percentage of participants requiring oxygen and having lower FEV1% predicted and with a history of exacerbations 1 year before enrollment. Such subjects are often more prone to and negatively affected by recurrent exacerbations, and identification and prevention of risk factors contributing to exacerbations are especially important in this group. Our group of subjects also represented various climate regions throughout North America and included data that spanned a period of more than 9 years. This helps to better assess regional differences and minimize any potential bias from year-to-year variability in climate and respiratory pathogen prevalence.
Our study also showed significant regional differences in COPD exacerbation rates (Figure 2). Greater differences in rates were seen in the Northeast, Southeast, and Upper Midwest regions. The reasons for this are unknown, but the potential effects of humidity and precipitation in the coastal regions on respiratory symptoms, as well as a potential role of indoor and outdoor pollution, may be important. Further analysis of climate, including humidity and precipitation data in the different regions, will help better characterize the regions and potentially shed light on the effects of weather on exacerbation rates.
Even though the exacerbations occurred less frequently in the warmer months, the exacerbations tended to be more severe than those occurring in the winter. This was especially striking in the Northeast, Southeast, Upper Midwest, and Great Lakes, where more than 50% of exacerbations in the summer were moderate to very severe exacerbations. One possible explanation for this finding is that exacerbations in the winter months are triggered by respiratory infections that had potentially fewer consequences for the participants. Prior studies have shown that COPD exacerbations are often triggered by viral infections (35–39). In fact, our subgroup analysis looking at the prevalence of flu reported by the U.S. Centers for Disease Control and Prevention (30) in those with at least one exacerbation showed a relationship between increased prevalence of flu and an increase in exacerbation rate (Figure E5). We postulate that exacerbations in the summer occurred in those with more severe disease who are prone to more frequent exacerbations because of significant underlying lung disease, independent of the exposure to infectious pathogens. Humidity, air pollution, exposure to indoor air conditioning, and changes in activity patterns may all have contributed to the type of exacerbations these adults with COPD experience (22, 40).
One of the limitations of our study is that many participants were excluded from the initial screening because of inability to retrospectively assess cardiovascular risk factors. However, this exclusion ensured that those who were included in the study represented adults with COPD without comorbid cardiovascular disease. Even though we have excluded regions with fewer than 35 subjects, certain regions, such as the Great Lakes (n = 99), had fewer participants than other regions, such as the Northeast (n = 419). This may limit our ability to detect seasonal effects in those regions with fewer subjects. In addition, our study used location of treatment to categorize the severity of exacerbations. Although this severity definition has been used in many prior clinical trials, including those by Albert and colleagues (26) and Criner and colleagues (25), other definitions have been used in other studies, such as medication or symptom changes, and may present different severity outcomes.
In conclusion, there is a clear regional, as well as seasonal, variability in rates, severity, and time to first COPD exacerbation in adults with COPD without cardiovascular risk factors in North America. To our knowledge, no other study has effectively controlled for cardiovascular disease in assessing seasonal and regional variability in COPD exacerbations. This study is a stepping stone to further characterizing climate and environmental factors, as well as regional access to care, that may play a role in rates and severity of COPD exacerbations. Further analysis must be performed to better characterize these regions and subject characteristics.
Supplementary Material
Footnotes
Supported by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health grants U10 HL074407, U10 HL074408, U10HL074409, U10 HL074416, U10 HL074418, U10 HL074422, U10 HL074424, U10 HL074428, U10 HL074431, U10 HL074439, and U10 HL074441, and the Canadian Institutes of Health Research grant 115074.
Author Contributions: J.Y.S. contributed to the study design, data analysis and interpretation, and writing and editing of the paper. H.Z. contributed to the analysis of data and revision of the paper. H.V., R.M.R., D.S., and N.M. contributed to the data interpretation and revision of the paper. G.J.C. contributed to the study design, data interpretation, and revision of the paper.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: on behalf of the Chronic Obstructive Pulmonary Disease CRN Investigators
References
- 1.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Connors AF, Jr, Dawson NV, Thomas C, Harrell FE, Jr, Desbiens N, Fulkerson WJ, et al. Outcomes following acute exacerbation of severe chronic obstructive lung disease: the SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments) Am J Respir Crit Care Med. 1996;154:959–967. doi: 10.1164/ajrccm.154.4.8887592. [DOI] [PubMed] [Google Scholar]
- 3.Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1418–1422. doi: 10.1164/ajrccm.157.5.9709032. [DOI] [PubMed] [Google Scholar]
- 4.Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57:847–852. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soler-Cataluña JJ, Martínez-García MÁ, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:925–931. doi: 10.1136/thx.2005.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donaldson GC, Seemungal T, Jeffries DJ, Wedzicha JA. Effect of temperature on lung function and symptoms in chronic obstructive pulmonary disease. Eur Respir J. 1999;13:844–849. doi: 10.1034/j.1399-3003.1999.13d25.x. [DOI] [PubMed] [Google Scholar]
- 7.Vilkman S, Keistinen T, Tuuponen T, Kivelä SL. Seasonal variation in hospital admissions for chronic obstructive pulmonary disease in Finland. Arctic Med Res. 1996;55:182–186. [PubMed] [Google Scholar]
- 8.Lim Y-H, Hong Y-C, Kim H. Effects of diurnal temperature range on cardiovascular and respiratory hospital admissions in Korea. Sci Total Environ. 2012;417-418:55–60. doi: 10.1016/j.scitotenv.2011.12.048. [DOI] [PubMed] [Google Scholar]
- 9.Donaldson GC, Goldring JJ, Wedzicha JA. Influence of season on exacerbation characteristics in patients with COPD. Chest. 2012;141:94–100. doi: 10.1378/chest.11-0281. [DOI] [PubMed] [Google Scholar]
- 10.Jenkins CR, Celli B, Anderson JA, Ferguson GT, Jones PW, Vestbo J, et al. Seasonality and determinants of moderate and severe COPD exacerbations in the TORCH study. Eur Respir J. 2012;39:38–45. doi: 10.1183/09031936.00194610. [DOI] [PubMed] [Google Scholar]
- 11.Rabe KF, Fabbri LM, Vogelmeier C, Kögler H, Schmidt H, Beeh KM, et al. Seasonal distribution of COPD exacerbations in the Prevention of Exacerbations with Tiotropium in COPD trial. Chest. 2013;143:711–719. doi: 10.1378/chest.12-1277. [DOI] [PubMed] [Google Scholar]
- 12.Donaldson GC, Wedzicha JA. The causes and consequences of seasonal variation in COPD exacerbations. Int J Chron Obstruct Pulmon Dis. 2014;9:1101–1110. doi: 10.2147/COPD.S54475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sidney S, Sorel M, Quesenberry CP, Jr, DeLuise C, Lanes S, Eisner MD. COPD and incident cardiovascular disease hospitalizations and mortality: Kaiser Permanente Medical Care Program. Chest. 2005;128:2068–2075. doi: 10.1378/chest.128.4.2068. [DOI] [PubMed] [Google Scholar]
- 14.Sin DD, Man SFP. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation. 2003;107:1514–1519. doi: 10.1161/01.cir.0000056767.69054.b3. [DOI] [PubMed] [Google Scholar]
- 15.Sin DD, Man SFP. Chronic obstructive pulmonary disease as a risk factor for cardiovascular morbidity and mortality. Proc Am Thorac Soc. 2005;2:8–11. doi: 10.1513/pats.200404-032MS. [DOI] [PubMed] [Google Scholar]
- 16.Spencer FA, Goldberg RJ, Becker RC, Gore JM. Seasonal distribution of acute myocardial infarction in the second National Registry of Myocardial Infarction. J Am Coll Cardiol. 1998;31:1226–1233. doi: 10.1016/s0735-1097(98)00098-9. [DOI] [PubMed] [Google Scholar]
- 17.Bhaskaran K, Hajat S, Haines A, Herrett E, Wilkinson P, Smeeth L. Effects of ambient temperature on the incidence of myocardial infarction. Heart. 2009;95:1760–1769. doi: 10.1136/hrt.2009.175000. [DOI] [PubMed] [Google Scholar]
- 18.Näyhä S. Cold and the risk of cardiovascular diseases: a review. Int J Circumpolar Health. 2002;61:373–380. doi: 10.3402/ijch.v61i4.17495. [DOI] [PubMed] [Google Scholar]
- 19.Danet S, Richard F, Montaye M, Beauchant S, Lemaire B, Graux C, et al. Unhealthy effects of atmospheric temperature and pressure on the occurrence of myocardial infarction and coronary deaths: a 10-year survey: the Lille-World Health Organization MONICA project (Monitoring trends and determinants in cardiovascular disease) Circulation. 1999;100:E1–E7. doi: 10.1161/01.cir.100.1.e1. [DOI] [PubMed] [Google Scholar]
- 20.Hole DJ, Watt GCM, Davey-Smith G, Hart CL, Gillis CR, Hawthorne VM.Impaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective population study BMJ 1996313711–715. [Discussion, pp. 715–716.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agustí A, Faner R. Systemic inflammation and comorbidities in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2012;9:43–46. doi: 10.1513/pats.201108-050MS. [DOI] [PubMed] [Google Scholar]
- 22.Gan WQ, Man SFP, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004;59:574–580. doi: 10.1136/thx.2003.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sin DD, Man SFP. Systemic inflammation and mortality in chronic obstructive pulmonary disease. Can J Physiol Pharmacol. 2007;85:141–147. doi: 10.1139/y06-093. [DOI] [PubMed] [Google Scholar]
- 24.Chang CL, Robinson SC, Mills GD, Sullivan GD, Karalus NC, McLachlan JD, et al. Biochemical markers of cardiac dysfunction predict mortality in acute exacerbations of COPD. Thorax. 2011;66:764–768. doi: 10.1136/thx.2010.155333. [DOI] [PubMed] [Google Scholar]
- 25.Criner GJ, Connett JE, Aaron SD, Albert RK, Bailey WC, Casaburi R, et al. COPD Clinical Research Network; Canadian Institutes of Health Research. Simvastatin for the prevention of exacerbations in moderate-to-severe COPD. N Engl J Med. 2014;370:2201–2210. doi: 10.1056/NEJMoa1403086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Albert RK, Connett J, Bailey WC, Casaburi R, Cooper JA, Jr, Criner GJ, et al. COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689–698. doi: 10.1056/NEJMoa1104623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Oceanic and Atmospheric Administration. Meteorological versus astronomical seasons [accessed 2017 Sep]. Available from: https://www.ncdc.noaa.gov/news/meteorological-versus-astronomical-seasons.
- 28.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 29.Niewoehner DE, Rice K, Cote C, Paulson D, Cooper JA, Jr, Korducki L, et al. Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once-daily inhaled anticholinergic bronchodilator: a randomized trial. Ann Intern Med. 2005;143:317–326. doi: 10.7326/0003-4819-143-5-200509060-00007. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. FluView Interactive. 2014 [accessed 2017 Sep]. Available from: http://www.cdc.gov/flu/weekly/fluviewinteractive.htm.
- 31.Pan W-H, Li LA, Tsai MJ. Temperature extremes and mortality from coronary heart disease and cerebral infarction in elderly Chinese. Lancet. 1995;345:353–355. doi: 10.1016/s0140-6736(95)90341-0. [DOI] [PubMed] [Google Scholar]
- 32.Mapel DW, Dedrick D, Davis K. Trends and cardiovascular co-morbidities of COPD patients in the Veterans Administration Medical System, 1991-1999. COPD. 2005;2:35–41. doi: 10.1081/copd-200050671. [DOI] [PubMed] [Google Scholar]
- 33.Thomsen M, Ingebrigtsen TS, Marott JL, Dahl M, Lange P, Vestbo J, et al. Inflammatory biomarkers and exacerbations in chronic obstructive pulmonary disease. JAMA. 2013;309:2353–2361. doi: 10.1001/jama.2013.5732. [DOI] [PubMed] [Google Scholar]
- 34.Buch P, Friberg J, Scharling H, Lange P, Prescott E. Reduced lung function and risk of atrial fibrillation in the Copenhagen City Heart Study. Eur Respir J. 2003;21:1012–1016. doi: 10.1183/09031936.03.00051502. [DOI] [PubMed] [Google Scholar]
- 35.Ko FWS, Ip M, Chan PKS, Chan MC, To KW, Ng SS, et al. Viral etiology of acute exacerbations of COPD in Hong Kong. Chest. 2007;132:900–908. doi: 10.1378/chest.07-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wedzicha JA. Role of viruses in exacerbations of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2004;1:115–120. doi: 10.1513/pats.2306030. [DOI] [PubMed] [Google Scholar]
- 37.Kurai D, Saraya T, Ishii H, Takizawa H. Virus-induced exacerbations in asthma and COPD. Front Microbiol. 2013;4:293. doi: 10.3389/fmicb.2013.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walter RE, Wilk JB, Larson MG, Vasan RS, Keaney JF, Jr, Lipinska I, et al. Systemic inflammation and COPD: the Framingham Heart Study. Chest. 2008;133:19–25. doi: 10.1378/chest.07-0058. [DOI] [PubMed] [Google Scholar]
- 39.Rohde G, Wiethege A, Borg I, Kauth M, Bauer TT, Gillissen A, et al. Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: a case-control study. Thorax. 2003;58:37–42. doi: 10.1136/thorax.58.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Di Q, Wang Y, Zanobetti A, Wang Y, Koutrakis P, Choirat C, et al. Air Pollution and Mortality in the Medicare Population. N Engl J Med. 2017;376:2513–2522. doi: 10.1056/NEJMoa1702747. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.