Abstract
Rationale: The Scleroderma Lung Study II (SLS II) demonstrated significant improvements in pulmonary function and dyspnea at 24 months compared with baseline when patients with symptomatic scleroderma–related interstitial lung disease (SSc-ILD) were treated with either cyclophosphamide for 1 year (followed for another year on placebo) or mycophenolate mofetil for 2 years in a randomized, double-blind clinical trial. Physiologic and clinical outcomes of SLS II have been published previously.
Objectives: The aim of the study was to assess changes from baseline in the extent of SSc-ILD on high-resolution computed tomography (HRCT) measured in the SLS II participants using quantitative image analysis after 2 years and to determine whether these HRCT changes were correlated with the changes in physiologic and clinical measures over the same time interval.
Methods: Ninety-seven of the 142 randomized subjects (cyclophosphamide group, 47 subjects; mycophenolate mofetil group, 50 subjects) participating in SLS II underwent thoracic volumetric thin-section HRCT at both baseline and 24 months. Quantitative computer-aided diagnosis scores using volumetric HRCT scans were obtained using a previously developed computer-aided system. The scores were quantitative lung fibrosis, quantitative ground glass, quantitative honeycomb, and quantitative interstitial lung disease (QILD), the latter representing the sum of quantitative lung fibrosis, quantitative ground glass, and quantitative honeycomb. These scores were obtained both for the whole lung and for individual lobes. Paired t tests were used for the combined (pooled) cyclophosphamide and mycophenolate mofetil groups to compare 24-month changes from baseline in both the whole lung and the lobe of maximal involvement as determined at baseline (worst lobe).
Results: At the end of the 24-month trial, QILD in the whole lung was significantly reduced by a mean of 2.51% in the pooled groups (adjusted 95% confidence interval, −4.00 to −1.03%; P = 0.001). There was no significant difference in the QILD score improvement between the cyclophosphamide (−2.66%) and mycophenolate (−2.38%) groups when assessed separately (P = 0.88). For the pooled group, the 24-month changes in QILD scores in the whole lung correlated significantly with other outcomes, including 24-month changes in forced vital capacity (ρ = −0.37), single-breath diffusing capacity of the lung for carbon monoxide (ρ = −0.22), and breathlessness as measured by the Transition Dyspnea Index (ρ = −0.26).
Conclusions: Treatment of SSc-ILD with either cyclophosphamide for 1 year, followed by placebo for a second year, or mycophenolate for 2 years was associated with a significant reduction (improvement) in the extent of HRCT SSc-ILD assessed by computer-aided diagnosis scores, which correlated well with one or more other measures of treatment response. These findings demonstrate that actual changes in lung structure accompany improvements in physiologic and/or symptomatic measures in SSc-ILD.
Keywords: Scleroderma Lung Study II, mycophenolate mofetil, cyclophosphamide
Systemic sclerosis (scleroderma) is a complex and life-threatening autoimmune disease associated with tissue fibrosis and small-vessel vasculopathy that targets the skin, lungs, heart, gastrointestinal (GI) tract, peripheral circulation, and musculoskeletal system. Lung involvement has emerged as the leading cause of morbidity and mortality (1–3). In Scleroderma Lung Study I (SLS I), treatment with cyclophosphamide (CYC) led to significant improvements in pulmonary outcomes in symptomatic patients with scleroderma–related interstitial lung disease (SSc-ILD), although with some adverse events (4). Several retrospective studies have shown a moderate benefit following treatment of patients with SSc-ILD with mycophenolate mofetil (MMF) (5). A recent randomized control trial, Scleroderma Lung Study II (SLS-II), demonstrated significant improvements from baseline in lung function and patient-centered outcomes over 24 months in patients receiving either CYC for 1 year (in a dose titrated to 2.0 mg/kg as tolerated followed by placebo for another year) or MMF (in a dose titrated to 1,500 mg twice daily for 2 yr) (6). Pulmonary function test (PFT) results, particularly the forced vital capacity (FVC), have been studied extensively as a primary measure of treatment efficacy in interstitial lung disease (ILD) clinical trials (4–11). Although the response to a 1-year course of treatment with cyclophosphamide in SLS I was statistically significant, the magnitude of the mean changes in FVC percent predicted between patients treated with placebo and those treated with cyclophosphamide was only 2.53% after 12 months of treatment and 4.80% at 18 months (i.e., 6 mo after completing therapy), and there was no difference at 24 months (4, 12). These modest changes raised questions about the clinical significance and durability of cyclophosphamide therapy (12, 13).
High-resolution computed tomography (HRCT) scanning has been used to characterize the nature and extent of SSc-ILD, as well as to predict outcomes (14–24). The use of changes in quantitative computed tomography (CT) as a measure of treatment efficacy in the setting of SSc-ILD is less well known (25–28). In SLS I, the extent of fibrosis at baseline predicted the FVC response to treatment with cyclophosphamide compared with placebo, with those with the most severe fibrosis on the baseline scan showing the greatest response to therapy (29, 30). Moreover, in a subgroup of patients with baseline and 12-month scans, clear improvement in the quantitative score of SSc-ILD was seen in the cyclophosphamide group, whereas worse scores, on average, were found with placebo (30, 31).
In SLS II, significantly positive within-treatment responses in FVC percent predicted were found over 24 months compared with baseline with both cyclophosphamide (mean, 3.0%) and mycophenolate (mean, 3.3%) (6). Similarly, positive responses were found for changes in breathlessness in both treatment arms as demonstrated using the Transition Dyspnea Index (TDI) (2.16 and 1.77 in the cyclophosphamide and mycophenolate arms, respectively [6], 1.5 being the minimal clinically important improvement for the TDI in SSc-ILD) (32).
In the present study, we examined the changes in the extent of quantitative parenchymal abnormalities between baseline and 24-month follow-up HRCT scans using a computer-aided diagnostic method in SLS II participants. We hypothesized that treatment with either cyclophosphamide or mycophenolate is associated with stability and/or improvement in HRCT evidence of ILD and that treatment-associated changes seen on HRCT scans correlate with changes in pulmonary function and patient-reported outcomes.
Methods
Patient Selection
SLS II was a randomized, double-blind, parallel-group trial that enrolled patients from 14 U.S. medical centers with symptomatic SSc-ILD meeting defined criteria for dyspnea (grade 2 on the Mahler Baseline Dyspnea Index) (33), pulmonary function (FVC, ≤80% predicted), and HRCT findings (any ground-glass opacity) (6). There were 142 patients randomly assigned to either mycophenolate (n = 69) or cyclophosphamide (n = 73). PFTs (FVC and single-breath diffusing capacity of the lung for carbon monoxide [DlCO]) and assessments of the level of dyspnea (Mahler TDI) (33) were performed every 6 months for up to 2 years, whereas HRCT scans were performed at baseline and 24 months only. Cyclophosphamide (Roxane Laboratories) was administered once daily (1.8 to 2.3 mg/kg) for 12 months, and placebo was administered during the 12- to 24-month period. Mycophenolate (Roche Laboratories) was administered twice daily up to a maximum total daily dose of 3.0 g for a total of 2 years (6).
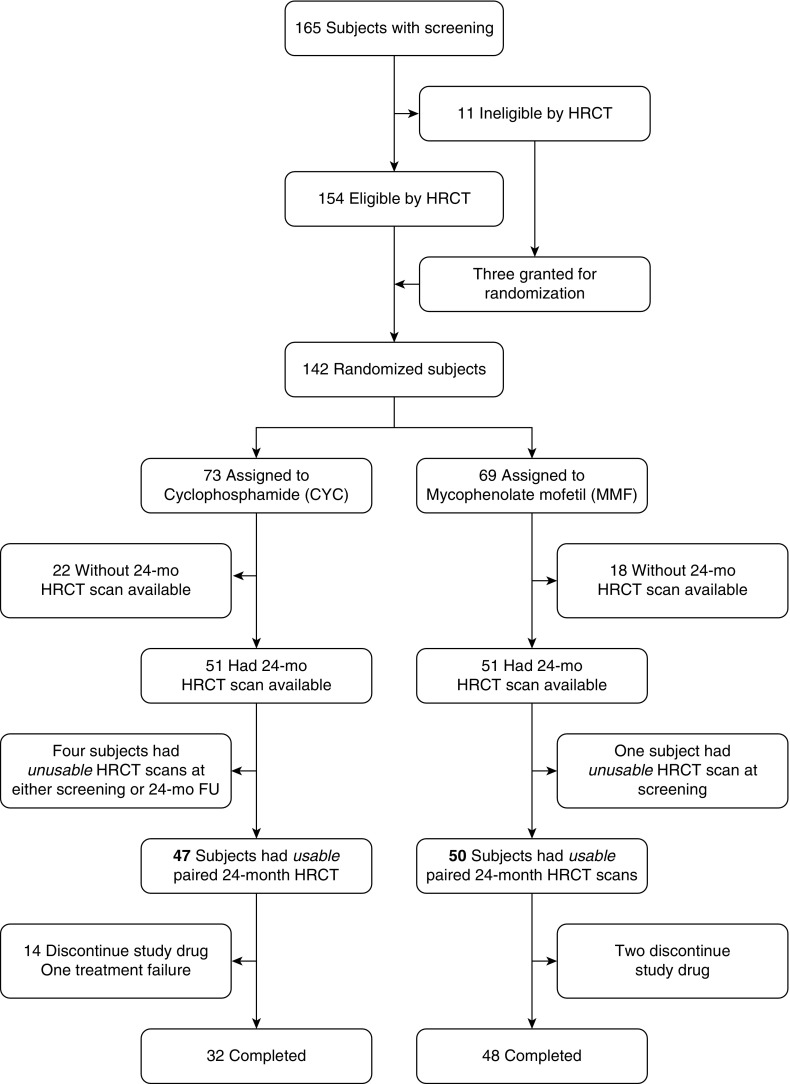
The 126 patients (mycophenolate [n = 63] and cyclophosphamide [n = 63] arms) with acceptable baseline HRCT studies and at least one outcome measure were included in the primary analysis. Acceptable baseline CT studies were defined as being of satisfactory image quality and showing evidence of parenchymal changes associated with SSc-ILD, including any ground-glass opacity (an inclusion criterion), fibrotic reticulations, and honeycombing without evidence of abnormalities indicative of other thoracic disease. Of the 165 screening CT studies, 154 met these criteria (Figure 1). Of the 142 randomized subjects, 97 (25 males, 72 females; mean ± standard deviation [SD] FVC, 66.3 ± 8.9%; mean ± SD DlCO, 55.0 ± 13.1%) had both baseline and 2-year paired HRCT scans. Of the 97 patients with paired HRCT scans, 80 subjects (32 on cyclophosphamide and 48 on mycophenolate) completed treatment at 24 months (Figure 1). The local institutional review board of each participating clinical center approved the use of images and PFTs. All image management was compliant with the Health Insurance Portability and Accountability Act.
Figure 1.
Flowchart of study participants. Eleven subjects were ineligible owing to presence of nodules (n = 6), absence of ground glass (n = 4), and presence of cardiomegaly (n = 1). For those who had baseline and 24-month high-resolution computed tomographic (HRCT) scans available, one of the paired scans from five subjects was not usable, mostly because of incorrect reconstruction interval and slice thickness ranging from 2.5 mm to 5 mm or incomplete coverage of lung. CYC = cyclophosphamide; FU = follow-up; MMF = mycophenolate mofetil.
CT Scanning Protocol
Standardized thin-section (<1.25 mm) reduced-dose (80–100 mAs) volumetric HRCT at suspended full inspiration at total lung capacity (TLC) was performed at all sites. Images were acquired using 12 different multidetector CT scanner models from two manufacturers under strict quality control guidelines, including credentialing the scanners, by showing stability of water and air measures on two phantom scans and stability of phantom measures on phantoms scanned within 24 hours of each subject’s scan. Patients were scanned at suspended TLC, mostly in the prone position. Of the 102 subjects who had baseline and 24-month follow-up scans, 5 were dropped owing to unusable HRCT scans at either screening or 24 months (Figure 1). Of the 80 subjects with paired HRCT scans who completed the study protocol, all were scanned on the same CT platform with the same acquisition protocol and in the same position.
Quantitative CT Image Analysis
Whole lung and lobes on HRCT images were segmented semiautomatically using an in-house analysis workstation (30). An independent thoracic radiologist confirmed lobar segmentation. Quantitative CT texture-based disease extent was derived from a previously described supervised texture classification model (27). Quantitative CT computer-aided diagnostic scores for extent of lung involvement were as follows: quantitative lung fibrosis (QLF), which included fibrotic reticulation patterns only; quantitative honeycomb (QHC); and quantitative ground glass (QGG). Quantitative interstitial lung disease (QILD) scores represented the total ILD pattern and consisted of the sum of all three scores (i.e., QLF + QGG + QHC). These quantitative CT scores were expressed as a percentage of total lung and individual lobe involvement on 194 scans (paired from 97 patients). Based on previous work involving repeated scans (28), decreases or increases of 4% or more in QLF or QILD scores in the lobe of maximal involvement and of 2% or more in the whole lung were considered to represent significant worsening or improvement, respectively, whereas scores that remained within these limits were regarded as representing stable disease.
Statistical Analysis
Baseline demographic and clinical characteristics, PFT results, and quantitative CT texture scores are reported as summary statistics. QLF and QILD scores can range from 0 to 100%, with higher scores indicating greater disease extent. After Box-Cox tests, QLF and QILD scores were transformed to meet the normality assumption. Paired t tests were used to compare the changes at follow-up from baseline in QLF and QILD scores in whole lung and the most severely involved lobe as the primary radiologic endpoints. A mixed-effect model was used to test the treatment-associated changes over 24 months in quantitative HRCT scores after adjusting for the baseline characteristics. In secondary analyses, Spearman rank correlations were used to test the association between changes in QLF and QILD scores and changes in both pulmonary function measures and dyspnea scores (expressed as the TDI). These analyses were performed for both treatment groups combined and for each treatment group separately.
Results
Disposition
The disposition of the study population by the availability of technically usable HRCT scans at both baseline and 24 months is shown by treatment group and completion of 24 months of the assigned study drug in Figure 1. There were 47 and 50 subjects with usable paired 24-month HRCT scans in the cyclophosphamide and mycophenolate arms, respectively.
Descriptive Summary Statistics
Table 1 describes the baseline demographic features, pulmonary function values, dyspnea scores (expressed as the Mahler Baseline Dyspnea Index), skin thickness scores (expressed as the modified Rodnan skin scores (mRSS) (32), and baseline QLF and QILD scores within the whole lung and the lobe of maximal involvement for each treatment arm separately and combined. Baseline characteristics were similar between groups, except for a higher prevalence of males and a higher mean mRSS in the mycophenolate arm. QHC scores were close to 0, indicating that the QGG amount was close to the difference between QILD and QLF scores.
Table 1.
Baseline demographic and clinical characteristics, lung function measures, and radiographic measures
| Cyclophosphamide (n = 47) | Mycophenolate Mofetil (n = 50) | Total (N = 97) | |
|---|---|---|---|
| Age, yr | 51.5 (9.2) | 51.7 (9.0) | 51.6 (9.0) |
| Female sex | 8 (17.0%) | 17 (34.0%) | 25 (25.8%) |
| Race | |||
| White | 30 (63.8%) | 35 (70%) | 65 (67.0%) |
| African American | 12 (25.5%) | 11 (22%) | 23 (23.7%) |
| Asian | 2 (4.3%) | 4 (8%) | 6 (6.2%) |
| Native American | 1 (2.1%) | 0 (0%) | 1 (1.0%) |
| Other | 2 (4.3%) | 0 (0%) | 2 (2.1%) |
| Hispanic ethnicity | 7 (14.9%) | 5 (8.0%) | 11 (11.3%) |
| FVC, % predicted | 66.6 (10.0) | 66.0 (7.8) | 66.3 (8.9) |
| FEV1, % predicted | 69.5 (9.5) | 68.0% (9.3) | 68.7 (9.4) |
| FEV1/FVC percent | 82.8 (5.1) | 81.5 (5.6) | 82.1 (5.4) |
| DlCO, % predicted | 55.4 (14.8) | 54.6 (11.4) | 55.0 (13.1) |
| DlCO/Va ratio | 3.9 (0.8) | 3.9 (0.7) | 3.9 (0.8) |
| TLC, L | 3.6 (0.93) | 3.8 (0.92) | 3.7 (0.9) |
| RV, L | 1.2 (0.51) | 1.3 (0.50) | 1.2 (0.51) |
| mRSS | 11.1 (8.2) | 16.4 (9.9) | 13.8 (9.5) |
| BDI* | 7.3 (2.3) | 7.5 (2.2) | 7.5 (2.2) |
Definition of abbreviations: BDI = Baseline Dyspnea Index, on which the score ranges from 1 to 12, with 1 indicating the most impairment; DlCO = diffusing capacity of the lung for carbon monoxide; DlCO/Va = diffusing capacity of the lung for carbon monoxide adjusted for alveolar volume; FEV1 = forced expiration volume in 1 second; FVC = forced vital capacity; mRSS = modified Rodnan skin score ranging from 0 to 51; RV = residual volume; TLC = total lung capacity.
Continuous data are expressed as mean (standard deviation). Categorical data are expressed as frequency (percent).
n = 45 for cyclophosphamide and n = 48 for mycophenolate mofetil.
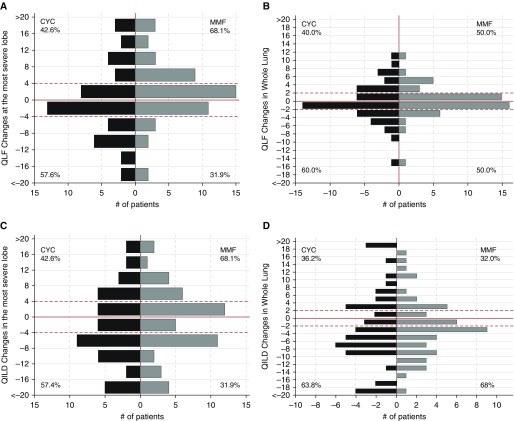
Changes in QLF, QGG, and QILD scores between 24 months and baseline are shown for subjects with paired CT scans at both time points by treatment arm separately and in both arms combined (pooled group) in Table 2. These changes are illustrated for QLF and QILD for each arm separately in Figure 2 by the frequency distribution of these changes at intervals of 4% for the changes in the most severely affected lobe and of 2% for the changes in the whole lung. After adjusting for sex and mRSS at baseline, changes in QLF score were stable (mean, −0.003; adjusted 95% confidence interval [CI], −0.012% to 0.01%), whereas QILD scores decreased significantly by a mean (standard error [SE]) of 2.52% (±0.76) in whole lung (P = 0.001; adjusted 95% CI, −4.00% to −1.03%). Similarly, in the most severe lobe, changes in QLF score were stable (mean, 1.05; adjusted 95% CI, −1.08% to 3.17%), whereas QILD scores decreased significantly by a mean (SE) of 1.8% (±0.85) in whole lung (P = 0.037; mean, −2.51; adjusted 95% CI, −4.19% to −0.13%). Mean duration between the two paired CT scans was 25.1 (SD, 1.9) months. QLF scores of most of the subjects remained stable over the period of the study.
Table 2.
Changes in quantitative computer-aided diagnosis scores from baseline, by treatment arm and pooled group
| Cyclophosphamide (n = 47) |
Mycophenolate Mofetil (n = 50) |
Pooled (N = 97) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline Mean (±SE) | 24 Mo Mean (±SE) | Mean Difference (95% CI) | Adjusted Mean Difference (95% CI) | Baseline Mean (±SE) | 24 Mo Mean (±SE) | Mean Difference (95% CI) | Adjusted Mean Difference (95% CI) | Baseline Mean (±SE) | 24 Mo Mean (±SE) | Mean Difference (95% CI) | Adjusted Mean Difference (95% CI) | |
| Whole lung | |
|
|
|||||||||
| QLF | 8.71 (±1.06) | 8.48 (±1.08) | −0.23 (−1.15 to 1.62) | −0.23 (−0.67 to 0.21) | 7.80 (±0.97) | 8.01 (±0.95) | 0.21 (−0.85 to 1.27) | 0.21 (−1.44 to 1.87) | 8.24 (±0.72) | 8.24 (±0.72) | −0.003 (−0.012 to 0.01) | −0.003 (−0.012 to 0.01) |
| QGG | 21.12 (±1.40) | 18.72 (±1.43) | −2.41 (−4.37 to −0.45)* | −2.41 (−4.31 to −0.50)* | 17.98 (±1.08) | 15.29 (±1.07) | −2.69 (−4.40 to −0.98)* | −2.69 (−4.35 to −1.03)* | 19.50 (±0.89) | 16.95 (±0.90) | −2.55 (−3.82 to −1.28)* | −2.55 (−3.80 to −1.30)* |
| QILD | 29.95 (±2.03) | 27.30 (±2.15) | −2.66 (−5.02 to −0.30)* | −2.66 (−4.96 to −0.36)* | 25.82 (±1.81) | 23.44 (±1.76) | −2.38 (−4.66 to −0.10)* | −2.87 (−4.31 to −1.42)* | 27.82 (±1.37) | 25.31 (±1.39) | −2.51 (−4.30 to −0.73)* | −2.52 (−4.00 to −1.03)* |
| Most severe lobe | |
|
|
|||||||||
| QLF | 23.01 (±3.19) | 23.32 (±3.39) | 0.31 (−2.92 to 3.55) | 0.31 (−2.83 to 3.47) | 22.41 (±2.94) | 24.17 (±3.08) | 1.76 (−1.24 to 4.75) | 1.76 (−1.16 to 4.68) | 22.70 (±2.15) | 23.76 (±2.27) | 1.06 (−1.11 to 3.22) | 1.05 (−1.08 to 3.17) |
| QGG | 26.90 (±1.82) | 24.34 (±1.73) | −2.56 (−4.61 to −0.50)* | −2.56 (−4.67 to −0.44)* | 26.46 (±1.36) | 23.12 (1.42) | −3.35 (−5.87 to −0.82)* | −3.35 (−5.80 to −0.88)* | 26.67 (±1.12) | 23.71 (±1.11) | −2.96 (−4.59 to −1.34)* | −2.96 (−4.57 to −1.35)* |
| QILD | 50.1 (±3.19) | 47.96 (±3.52) | −2.16 (−5.86 to 1.54) | −2.16 (−5.76 to 1.44) | 48.94 (±3.06) | 47.51 (±3.31) | −1.43 (−4.32 to 1.46) | −1.43 (−3.55 to 0.69) | 49.51 (±2.20) | 47.72 (±2.40) | −2.51 (−4.88 to −0.14)* | −2.51 (−4.19 to −0.13)* |
Definition of abbreviations: CI = confidence interval; QGG = quantitative ground glass; QILD = quantitative interstitial lung disease; QLF = quantitative lung fibrosis; SE = standard error.
The modified Rodnan skin score 95% CI was rescaled after transformation to meet the normality assumption.
P < 0.05.
Figure 2.
(A and B) Frequency distribution of changes in extent of quantitative lung fibrosis (QLF) and (C and D) quantitative interstitial lung disease (QILD) in both the most severe lobe and the whole lung, by treatment assignment. The horizontal dashed red lines indicate ±4% quantitative changes in the most severe lobe (as defined at baseline) and the ±2% quantitative changes in the whole lung. The solid horizontal red lines indicate the zero percent changes. The gray horizontal lines indicate the changes in QLF and QILD from baseline. The vertical dashed gray lines represent the number of subjects. We defined worse, stable, and better changes in QLF and QILD for the most severe lobe as follows: less than 4% = worse; 4% to −4% = stable; less than −4% = better. Using these cut points, 25%, 45%, and 30% of cyclophosphamide (CYC) patients exhibited worse, stable, and better changes in QLF, respectively, whereas 34%, 52%, and 14% of mycophenolate mofetil (MMF) patients showed worse, stable, and better changes in QLF, respectively. Using the same cut points, 27%, 26%, and 47% of CYC patients exhibited worse, stable, and better changes in QILD, respectively, whereas 26%, 34%, and 40% of MMF patients showed worse, stable, and better changes in QILD, respectively. We defined worse, stable, and better changes in QLF and QILD for the whole lung as follows: greater than 2% = worse; 2% to −2% = stable; less than −2% = better. Using these cut points, 28%, 42%, and 30% of CYC patients exhibited worse, stable, and better changes in QLF, respectively, whereas 34%, 62%, and 18% of MMF patients showed worse, stable, and better changes in QLF, respectively. Using the same cut points, 32%, 11%, and 57% of CYC patients exhibited worse, stable, and better changes in QILD, respectively, whereas 26%, 18%, and 56% of MMF patients showed worse, stable, and better changes in QILD, respectively.
Significant correlations were found between changes in quantitative HRCT scores (QLF and QILD) and changes in percent predicted FVC and TLC over the 24 months of the trial in all subjects with paired HRCT scans (Table 3). In contrast, no meaningful correlation was found between the QLF changes and changes in DlCO, whereas improvement in QILD scores in the whole lung did correlate with DlCO, but not in the most severe lobe, and improvement in DlCO in the whole lung. Associations by treatment arms are shown in Table 4; no apparent between-treatment differences in these associations were found, except for DlCO, for which changes were correlated with changes in QILD in subjects in the cyclophosphamide arm but not in the mycophenolate arm.
Table 3.
Spearman correlation coefficients for changes in quantitative high-resolution computed tomography scores and changes in other physiological and clinical outcome measures at 24 months compared with baseline
| Whole Lung (N = 97) |
Most Severe Lobe (N = 97) |
|||
|---|---|---|---|---|
| QLF | QILD | QLF | QILD | |
| FVC percent predicted | −0.43 (P < 0.001) | −0.37 (P < 0.001) | −0.49 (P < 0.001) | −0.45 (P < 0.001) |
| DlCO percent predicted* | −0.09 (P = 0.42) | −0.22 (P = 0.04) | −0.03 (P = 0.79) | −0.17 (P = 0.10) |
| Percent changes in TLC | −0.33 (P < 0.001) | −0.36 (P < 0.001) | −0.35 (P < 0.001) | −0.34 (P < 0.001) |
| Dyspnea (TDI)† | −0.16 (P = 0.17) | −0.26 (P = 0.03) | −0.07 (P = 0.54) | −0.20 (P = 0.08) |
| mRSS | 0.08 (P = 0.44) | 0.09 (P = 0.37) | 0.09 (P = 0.39) | 0.03 (P = 0.74) |
Definition of abbreviations: DlCO = diffusing capacity of the lung for carbon monoxide; FVC = forced vital capacity; mRSS = modified Rodnan skin score; QILD = quantitative interstitial lung disease; QLF = quantitative lung fibrosis; TDI = Transition Dyspnea Index on 7-point scale from −3 (major deterioration) to +3 (major improvement); TLC = total lung capacity.
n = 93.
n = 72.
Table 4.
Associations between 24-month changes in high-resolution computed tomography and changes in pulmonary function tests from baseline, by treatment group
| Absolute Changes in Quantitative HRCT Scores | Absolute Changes in FVC and DlCO (% predicted) and Dyspnea (TDI) |
|||||
|---|---|---|---|---|---|---|
| Cyclophosphamide (n = 47) |
Mycophenolate mofetil (n = 50) |
|||||
| FVC | DlCO | TDI | FVC | DlCO | TDI | |
| QLF in most severe lobe | −0.55 (P < 0.001) | −0.11 (P = 0.47) | 0.16 (P = 0.36) | −0.46 (P = 0.001) | 0.09 (P = 0.55) | 0.02 (P = 0.91) |
| QLF in whole lung | −0.47 (P < 0.001) | −0.15 (n = 0.34) | −0.27 (P = 0.12) | −0.40 (P = 0.004) | −0.03 (P = 0.85) | −0.03 (P = 0.87) |
| QILD in most severe lobe | −0.47 (P < 0.001) | −0.26 (P = 0.09) | −0.36 (P = 0.03) | −0.42 (P = 0.003) | −0.05 (P = 0.72) | −0.03 (P = 0.88) |
| QILD in whole lung | −0.43 (P = 0.003) | −0.32 (P = 0.04) | −0.42 (P = 0.01) | −0.33 (P = 0.02) | −0.10 (P = 0.49) | −0.07 (P = 0.70) |
| QGG in most severe lobe | −0.02 (P = 0.88) | −0.20 (P = 0.19) | −0.36 (P = 0.03) | −0.07 (P = 0.65) | −0.15 (P = 0.31) | −0.03 (P = 0.86) |
| QGG in whole lung | −0.31 (P = 0.04) | −0.32 (P = 0.03) | −0.48 (P = 0.003) | −0.13 (P = 0.36) | −0.15 (P = 0.31) | −0.01 (P = 0.96) |
Definition of abbreviations: DlCO = diffusing capacity of the lung for carbon monoxide; FVC = forced vital capacity; HRCT = high-resolution computed tomography; QGG = quantitative ground glass; QILD = quantitative interstitial lung disease; QLF = quantitative lung fibrosis; TDI = Transition Dyspnea Index on 7-point scale from −3 (major deterioration) to +3 (major improvement).
Data are presented as Spearman’s ρ (P value). n = 97 pairs for FVC and HRCT; n = 93 pairs for DlCO and HRCT for all patients. For DlCO, n = 44 in cyclophosphamide arm and n = 49 in mycophenolate mofetil arm; for TDI, n = 35 in cyclophosphamide arm and n = 37 in mycophenolate mofetil arm.
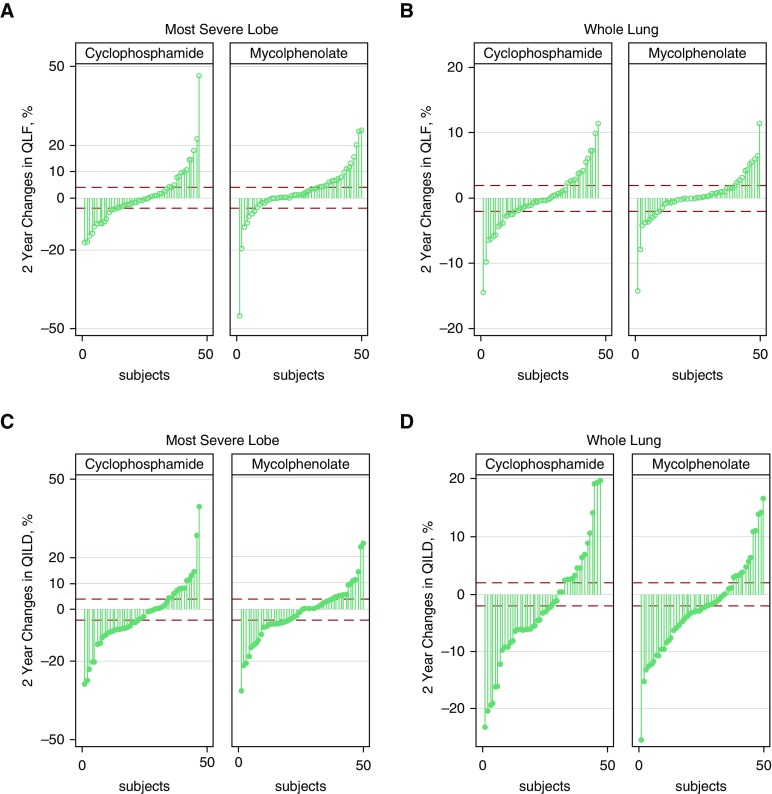
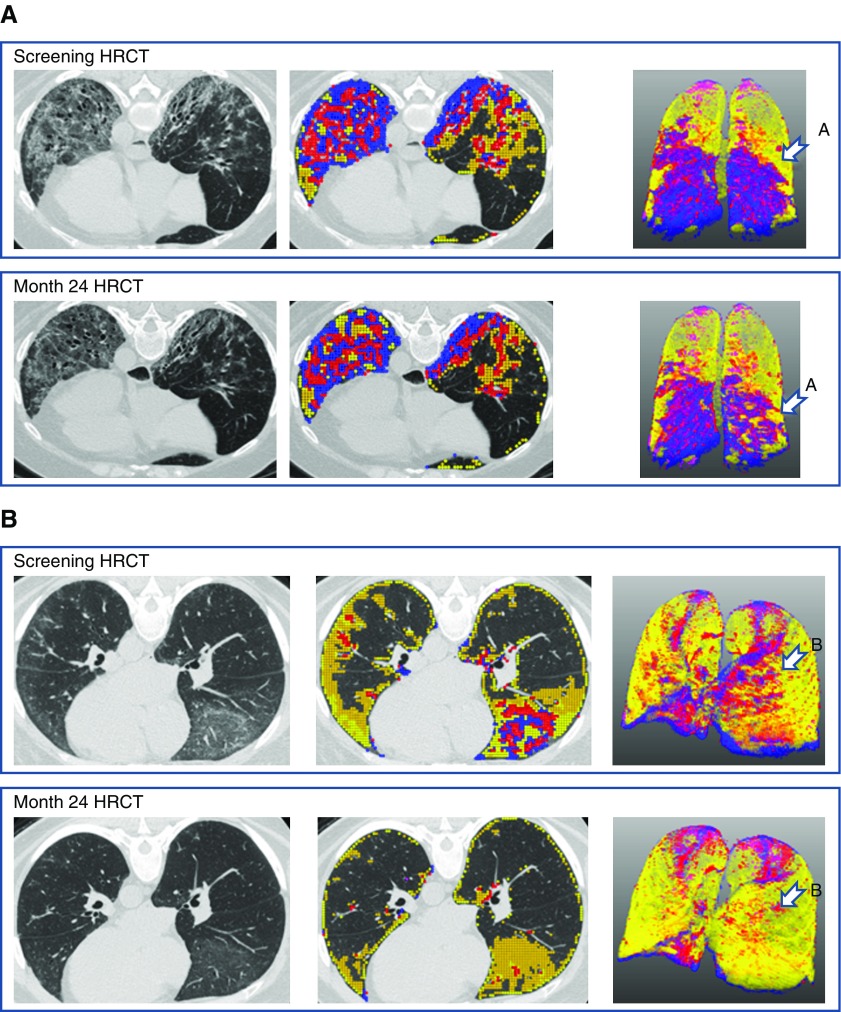
Figure 3 shows the 2-year changes in QILD and QLF scores in both the most severely affected lobe and the whole lung in individual subjects by treatment arm. Approximately half of the subjects in both treatment arms had reductions (improvement) or no changes (stability) in both QLF and QILD scores in the most severe lobe, whereas approximately two-thirds of the subjects had reductions or no changes in QLF and QILD scores in the whole lung (Figure 3). Figure 4 depicts the baseline and 24-month HRCT scans of two representative responders, defined as those with clinically significant reductions in scores, one in the cyclophosphamide arm and the other in the mycophenolate arm. In both subjects, both quantitative radiographic scores and PFTs improved over time.
Figure 3.
Changes in quantitative lung fibrosis (QLF) by treatment arm (A) in the most severe lobe and (B) in whole lung, as well as changes in quantitative interstitial lung disease (QILD) by treatment arm (C) in the most severe lobe and (D) in whole lung, are shown.
Figure 4.
Representative subjects with favorable treatment response by high-resolution computed tomography (HRCT). (A) A 53-year-old woman assigned to the cyclophosphamide arm had forced vital capacity (FVC) of 67% predicted at baseline and 79% predicted at 24-month follow-up. The sum of the blue and red areas represents the extent of quantitative lung fibrosis (QLF), and the yellow area represents the extent of quantitative ground glass. The extent of quantitative interstitial lung disease (QILD) is represented by the entire colored area. After 24 months, the extent of QLF was reduced, indicating improvement in fibrosis (arrow in A). (B) A 51-year-old woman assigned to the mycophenolate mofetil arm had FVC of 69% predicted at baseline and 81% predicted at 24-month follow-up. The sum of the blue and red areas represents the extent of QLF, and the yellow area represents the extent of quantitative ground glass. The extent of QILD is represented by the entire colored area. After 24 months, the areas representing QLF were reduced (arrow in B).
Discussion
This double-blind, randomized, placebo-controlled clinical trial examined the efficacy of oral cyclophosphamide for 1 year or of mycophenolate mofetil for 2 years for SSc-ILD. Our findings indicate a significant treatment effect of both cyclophosphamide and mycophenolate mofetil on changes in prespecified exploratory outcome quantitative image analysis biomarkers of QLF and QILD scores over a period of 2 years. There was no significant benefit of one treatment over the other in HRCT measures, however, a finding that was supported by similar results in the primary study outcome measures of FVC change over the course of the study (6). The HRCT changes in structural measures of ILD support the potential clinical effectiveness of both cyclophosphamide and mycophenolate mofetil for progressive SSc-ILD.
Both oral cyclophosphamide and mycophenolate have been shown to be effective in one or more randomized controlled trials using FVC as the primary outcome measure (4, 6, 7). The present analysis provides further evidence that mycophenolate and cyclophosphamide are effective therapeutic agents for SSc-ILD on the basis of observed improvements in parenchymal structure as measured by the changes in the quantitative assessment of the extent of fibrosis and ILD. The response to cyclophosphamide is consistent with that reported in SLS I, in which significant improvements in both QLF and QILD scores were demonstrated for the cyclophosphamide group, whereas scores for fibrosis and ILD generally worsened in the placebo arm, consistent with disease progression in the majority of the latter subjects (28, 30, 31).
In the present study of the SLS II population, there was significant improvement in the extent of ILD on HRCT as measured using the QILD score in those subjects who completed treatment with either mycophenolate or cyclophosphamide in comparison with the scores at baseline, suggesting that both drugs offered therapeutic benefit. In contrast, among those subjects who failed to complete treatment, the extent of fibrosis and ILD tended to worsen, consistent with a significant association of HRCT evidence of changes in lung structure with clinical outcomes with respect to both favorable and unfavorable responses of SSc-ILD to immunosuppressive therapy. Our observations of significant associations between changes in both FVC and TLC and changes in the quantitative radiographic measures of extent of ILD in both treatment arms provide validating evidence that the improvements in the physiologic measures are surrogate indicators of structural improvement in pulmonary fibrosis.
An interesting finding was the relationship between fibrosis and the response to treatment as measured by QLF and QILD scores. Although the ground-glass opacity on HRCT may represent acute alveolitis, it is nonspecific because it might also represent fine intralobular fibrosis (20, 34, 35). Although in this study QGG did decrease in both the cyclophosphamide and mycophenolate mofetil groups, by −2.56 (1.05) (−4.61 to −0.50; P = 0.015) and −3.35 (1.25) (−5.80 to −0.88; P = 0.008), respectively, the direction of change could have been progression of either inflammation or fine fibrosis to more obvious fibrosis rather than resolution to normal lung. Thus, no change or actual decreases in QLF appear to be more useful indicators of stabilization or improvement, respectively, in ILD.
An important limitation of this study is the absence of a follow-up HRCT scan at 12 months, thereby preventing our ability to detect changes in CT measures of SSc-ILD during the active phase of one of the treatment arms, namely the cyclophosphamide arm, wherein the active study drug was administered for only 1 year. Moreover, a 12-month follow-up HRCT scan would have allowed a direct comparison of our present findings with those previously reported concerning the impact of cyclophosphamide versus placebo in SLS I on quantitative radiographic measures of fibrosis and ILD assessed at 1 year. The absence of a placebo arm in SLS II is another limitation of the SLS II study design in the interpretation of the clinical significance of the changes over time after active treatment with two immunosuppressive agents. However, the natural history of SSc-ILD would lead one to expect either slow progression or stability rather than actual improvement in the extent of ILD. A further limitation of the present study is the relatively small number of randomized subjects with a second HRCT scan (47 of 73 assigned to cyclophosphamide and 50 of 69 assigned to mycophenolate), together with the withdrawal of a substantial number of randomized subjects from the treatment phase of the trial, such that only 32 and 48 subjects in the cyclophosphamide and mycophenolate arms, respectively, both completed their assigned treatment and had follow-up scans. In addition, four subjects and one subject in the cyclophosphamide and mycophenolate groups, respectively, had at least one unusable scan at either baseline or 24 months, owing to nonvolumetric scans with thick slices or incomplete coverage of the lung on the scan. Furthermore, the fact that a larger number of subjects in the cyclophosphamide arm than in the mycophenolate arm failed to receive a follow-up scan may have affected our ability to assess potential treatment differences in the HRCT findings, although the results of the physiologic measures were comparable between the two treatments (6). A final limitation is the lack of histopathologic correlation for follow-up changes in quantitative imaging measures. The availability of direct radiologic–pathologic correlation would strengthen the validation of the imaging-based biomarkers beyond the correlations with physiologic measures.
Changes in quantitative HRCT scores of SSc-ILD provide independent validation of the favorable treatment effect produced by both cyclophosphamide and mycophenolate in SLS II, supporting the concept that treatment-related improvements in the 2-year course of FVC percent predicted by both forms of immunosuppressive therapy (cyclophosphamide and mycophenolate) reflect structural changes in lung disease. The HRCT scan findings therefore support the interpretation that cyclophosphamide and mycophenolate, on average, slowed the progression of ILD over a 2-year period and in a substantial minority of the subjects actually resulted in partial resolution of the ILD.
Conclusions
Changes in HRCT outcomes over 24 months were significantly correlated with changes in FVC percent predicted and TLC percent predicted, providing structural evidence of stabilization or improvement in lung fibrosis in support of the physiologic changes in patients with symptomatic SSc-ILD when treated with either mycophenolate for 2 years or cyclophosphamide for 1 year. Results of the present study suggest that the incorporation of follow-up HRCT scans in controlled clinical trials of interventions in patients with SSc-ILD (and potentially in other interstitial pneumonitides) might prove to be a useful adjunct to conventional physiologic and patient-centered endpoints.
Supplementary Material
Footnotes
Supported by the National Heart, Lung and Blood Institute, National Institutes of Health. Drug supply was provided by Hoffmann-La Roche and Genentech.
Author Contributions: J.G.G.: study design; data collection; statistical analysis; and manuscript preparation, review, and editing; G.H.J.K.: collection of data, statistical analysis, data analysis, and manuscript review; C.-H.T., E.V., D.F., P.C., M.B., M.R., and D.K.: data collection, data analysis, and manuscript review; and D.P.T.: collection of data, data analysis, and manuscript review and editing.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Steen VD, Conte C, Owens GR, Medsger TA., Jr Severe restrictive lung disease in systemic sclerosis. Arthritis Rheum. 1994;37:1283–1289. doi: 10.1002/art.1780370903. [DOI] [PubMed] [Google Scholar]
- 2.Steen VD, Medsger TA., Jr Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum. 2000;43:2437–2444. doi: 10.1002/1529-0131(200011)43:11<2437::AID-ANR10>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 3.Medsger TA., Jr . Classification, prognosis. In: Clements PJ, Furst DE, editors. Systemic sclerosis. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2004. pp. 17–28. [Google Scholar]
- 4.Tashkin DP, Elashoff R, Clements PJ, Goldin J, Roth MD, Furst DE, et al. Scleroderma Lung Study Research Group. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006;354:2655–2666. doi: 10.1056/NEJMoa055120. [DOI] [PubMed] [Google Scholar]
- 5.Tzouvelekis A, Galanopoulos N, Bouros E, Kolios G, Zacharis G, Ntolios P, et al. Effect and safety of mycophenolate mofetil or sodium in systemic sclerosis-associated interstitial lung disease: a meta-analysis. Pulm Med. 2012;2012:143637. doi: 10.1155/2012/143637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tashkin DP, Roth MD, Clements PJ, Furst DE, Khanna D, Kleerup EC, et al. Sclerodema Lung Study II Investigators. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir Med. 2016;4:708–719. doi: 10.1016/S2213-2600(16)30152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoyles RK, Ellis RW, Wellsbury J, Lees B, Newlands P, Goh NS, et al. A multicenter, prospective, randomized, double-blind, placebo-controlled trial of corticosteroids and intravenous cyclophosphamide followed by oral azathioprine for the treatment of pulmonary fibrosis in scleroderma. Arthritis Rheum. 2006;54:3962–3970. doi: 10.1002/art.22204. [DOI] [PubMed] [Google Scholar]
- 8.Bérezné A, Ranque B, Valeyre D, Brauner M, Allanore Y, Launay D, et al. Therapeutic strategy combining intravenous cyclophosphamide followed by oral azathioprine to treat worsening interstitial lung disease associated with systemic sclerosis: a retrospective multicenter open-label study. J Rheumatol. 2008;35:1064–1072. [PubMed] [Google Scholar]
- 9.Silver RM, Warrick JH, Kinsella MB, Staudt LS, Baumann MH, Strange C. Cyclophosphamide and low-dose prednisone therapy in patients with systemic sclerosis (scleroderma) with interstitial lung disease. J Rheumatol. 1993;20:838–844. [PubMed] [Google Scholar]
- 10.Akesson A, Scheja A, Lundin A, Wollheim FA. Improved pulmonary function in systemic sclerosis after treatment with cyclophosphamide. Arthritis Rheum. 1994;37:729–735. doi: 10.1002/art.1780370518. [DOI] [PubMed] [Google Scholar]
- 11.White B, Moore WC, Wigley FM, Xiao HQ, Wise RA. Cyclophosphamide is associated with pulmonary function and survival benefit in patients with scleroderma and alveolitis. Ann Intern Med. 2000;132:947–954. doi: 10.7326/0003-4819-132-12-200006200-00004. [DOI] [PubMed] [Google Scholar]
- 12.Tashkin DP, Elashoff R, Clements PJ, Roth MD, Furst DE, Silver RM, et al. Scleroderma Lung Study Research Group. Effects of 1-year treatment with cyclophosphamide on outcomes at 2 years in scleroderma lung disease. Am J Respir Crit Care Med. 2007;176:1026–1034. doi: 10.1164/rccm.200702-326OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez FJ, McCune WJ. Cyclophosphamide for scleroderma lung disease [editorial] N Engl J Med. 2006;354:2707–2709. doi: 10.1056/NEJMe068095. [DOI] [PubMed] [Google Scholar]
- 14.Fujita J, Yoshinouchi T, Ohtsuki Y, Tokuda M, Yang Y, Yamadori I, et al. Non-specific interstitial pneumonia as pulmonary involvement of systemic sclerosis. Ann Rheum Dis. 2001;60:281–283. doi: 10.1136/ard.60.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim DS, Yoo B, Lee JS, Kim EK, Lim CM, Lee SD, et al. The major histopathologic pattern of pulmonary fibrosis in scleroderma is nonspecific interstitial pneumonia. Sarcoidosis Vasc Diffuse Lung Dis. 2002;19:121–127. [PubMed] [Google Scholar]
- 16.Bouros D, Wells AU, Nicholson AG, Colby TV, Polychronopoulos V, Pantelidis P, et al. Histopathologic subsets of fibrosing alveolitis in patients with systemic sclerosis and their relationship to outcome. Am J Respir Crit Care Med. 2002;165:1581–1586. doi: 10.1164/rccm.2106012. [DOI] [PubMed] [Google Scholar]
- 17.Desai SR, Veeraraghavan S, Hansell DM, Nikolakopolou A, Goh NS, Nicholson AG, et al. CT features of lung disease in patients with systemic sclerosis: comparison with idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia. Radiology. 2004;232:560–567. doi: 10.1148/radiol.2322031223. [DOI] [PubMed] [Google Scholar]
- 18.Goldin JG, Lynch DA, Strollo DC, Suh RD, Schraufnagel DE, Clements PJ, et al. Scleroderma Lung Study Research Group. High-resolution CT scan findings in patients with symptomatic scleroderma-related interstitial lung disease. Chest. 2008;134:358–367. doi: 10.1378/chest.07-2444. [DOI] [PubMed] [Google Scholar]
- 19.Wells AU, Rubens MB, du Bois RM, Hansell DM. Serial CT in fibrosing alveolitis: prognostic significance of the initial pattern. AJR Am J Roentgenol. 1993;161:1159–1165. doi: 10.2214/ajr.161.6.8249719. [DOI] [PubMed] [Google Scholar]
- 20.Wells AU. High-resolution computed tomography and scleroderma lung disease. Rheumatology (Oxford) 2008;47(Suppl 5):v59–v61. doi: 10.1093/rheumatology/ken271. [DOI] [PubMed] [Google Scholar]
- 21.Goh NSL, Desai SR, Veeraraghavan S, Hansell DM, Copley SJ, Maher TM, et al. Interstitial lung disease in systemic sclerosis: a simple staging system. Am J Respir Crit Care Med. 2008;177:1248–1254. doi: 10.1164/rccm.200706-877OC. [DOI] [PubMed] [Google Scholar]
- 22.Giacomelli R, Valentini G, Salsano F, Cipriani P, Sambo P, Conforti ML, et al. Cyclophosphamide pulse regimen in the treatment of alveolitis in systemic sclerosis. J Rheumatol. 2002;29:731–736. [PubMed] [Google Scholar]
- 23.Griffiths B, Miles S, Moss H, Robertson R, Veale D, Emery P. Systemic sclerosis and interstitial lung disease: a pilot study using pulse intravenous methylprednisolone and cyclophosphamide to assess the effect on high resolution computed tomography scan and lung function. J Rheumatol. 2002;29:2371–2378. [PubMed] [Google Scholar]
- 24.Pakas I, Ioannidis JPA, Malagari K, Skopouli FN, Moutsopoulos HM, Vlachoyiannopoulos PG. Cyclophosphamide with low or high dose prednisolone for systemic sclerosis lung disease. J Rheumatol. 2002;29:298–304. [PubMed] [Google Scholar]
- 25.Davas EM, Peppas C, Maragou M, Alvanou E, Hondros D, Dantis PC. Intravenous cyclophosphamide pulse therapy for the treatment of lung disease associated with scleroderma. Clin Rheumatol. 1999;18:455–461. doi: 10.1007/s100670050138. [DOI] [PubMed] [Google Scholar]
- 26.Kim HJ, Li G, Gjertson D, Elashoff R, Shah SK, Ochs R, et al. Classification of parenchymal abnormality in scleroderma lung using a novel approach to denoise images collected via a multicenter study. Acad Radiol. 2008;15:1004–1016. doi: 10.1016/j.acra.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim HG, Tashkin DP, Clements PJ, Li G, Brown MS, Elashoff R, et al. A computer-aided diagnosis system for quantitative scoring of extent of lung fibrosis in scleroderma patients. Clin Exp Rheumatol. 2010;28(5) Suppl 62:S26–S35. [PMC free article] [PubMed] [Google Scholar]
- 28.Kim HJ, Brown MS, Elashoff R, Li G, Gjertson DW, Lynch DA, et al. Quantitative texture-based assessment of one-year changes in fibrotic reticular patterns on HRCT in scleroderma lung disease treated with oral cyclophosphamide. Eur Radiol. 2011;21:2455–2465. doi: 10.1007/s00330-011-2223-2. [DOI] [PubMed] [Google Scholar]
- 29.Khanna D, Tseng CH, Farmani N, Steen V, Furst DE, Clements PJ, et al. Scleroderma Lung Study Research Group. Clinical course of lung physiology in patients with scleroderma and interstitial lung disease: analysis of the Scleroderma Lung Study placebo group. Arthritis Rheum. 2011;63:3078–3085. doi: 10.1002/art.30467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldin J, Elashoff R, Kim HJ, Yan X, Lynch D, Strollo D, et al. Treatment of scleroderma-interstitial lung disease with cyclophosphamide is associated with less progressive fibrosis on serial thoracic high-resolution CT scan than placebo: findings from the scleroderma lung study. Chest. 2009;136:1333–1340. doi: 10.1378/chest.09-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim HJ, Tashkin DP, Gjertson DW, Brown MS, Kleerup E, Chong S, et al. Transitions to different patterns of interstitial lung disease in scleroderma with and without treatment. Ann Rheum Dis. 2016;75:1367–1371. doi: 10.1136/annrheumdis-2015-208929. [DOI] [PubMed] [Google Scholar]
- 32.Khanna D, Tseng CH, Furst DE, Clements PJ, Elashoff R, Roth M, et al. for Scleroderma Lung Study Investigators. Minimally important differences in the Mahler’s Transition Dyspnoea Index in a large randomized controlled trial—results from the Scleroderma Lung Study. Rheumatology (Oxford) 2009;48:1537–1540. doi: 10.1093/rheumatology/kep284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahler DA, Ward J, Fierro-Carrion G, Waterman LA, Lentine TF, Mejia-Alfaro R, et al. Development of self-administered versions of modified baseline and transition dyspnea indexes in COPD. COPD. 2004;1:165–172. doi: 10.1081/copd-120030829. [DOI] [PubMed] [Google Scholar]
- 34.Elicker BM, Webb WR. Fundamentals of high-resolution lung CT: common findings, common patterns, common diseases, and different diagnosis. Philadelphia: Lippincott Williams & Wilkins; 2013. [Google Scholar]
- 35.Shah RM, Jimenez S, Wechsler R. Significance of ground-glass opacity on HRCT in long-term follow-up of patients with systemic sclerosis. J Thorac Imaging. 2007;22:120–124. doi: 10.1097/01.rti.0000213572.16904.40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.