Abstract
Rationale: Chronically critically ill patients are often dependent on family members for surrogate decision-making, and these surrogates are at high risk for emotional distress. We hypothesized that patient- and surrogate-specific risk factors for surrogate post-traumatic stress disorder (PTSD) symptoms can be identified early in the course of chronic critical illness.
Objectives: To identify risk factors for PTSD symptoms in surrogate decision-makers of chronically critically ill patients.
Methods: We performed a secondary analysis of the database from a multicenter randomized trial of a communication intervention for chronic critical illness patients and surrogates. Variables preselected for plausible mechanism for increasing PTSD symptoms and identifiable by Day 10 of mechanical ventilation were included in the analysis for association with surrogate PTSD symptoms at 90 days, as measured by the Impact of Events Score–Revised (IES-R). Patient factors included demographics, insurance status, baseline functional status, chronic comorbidities, illness severity, and presence of advance directive. Surrogate variables included demographics, education level and employment, religion, relationship to patient, and Hospital Anxiety and Depression Scale score measured at enrollment. Multivariable linear regression models were then constructed for 26 potential risk factors, including biologically or mechanistically plausible confounders for each, with IES-R score as the outcome. All models were adjusted for multiple respondents, using a mixed model, considering the patients as a random factor.
Results: Our analysis included 306 surrogates for 224 patients. A total of 49% of patients were female, and mean age was 59 years (95% confidence interval [CI], 56.4–60.7). A total of 71% of surrogates were female, and mean age was 51 years (95% CI, 49.3–52.4). After examining each potential risk factor in a separate multivariable model, only Day-10 surrogate Hospital Anxiety and Depression Scale score (β coefficient = 1.02; 95% CI, 0.73–1.30) and patient unresponsiveness (β coefficient = 8.39; 95% CI, 0.83–15.95) were associated with higher IES-R scores.
Conclusions: Among surrogate decision-makers for chronically critically ill patients, high anxiety and depression scores and patient unresponsiveness on or near Day 10 of mechanical ventilation are risk factors for PTSD symptoms at 90 days.
Keywords: critical care, emotional stress, surrogate decision-maker, mechanical ventilation
Chronic critical illness (CCI) is a syndrome characterized by prolonged dependence on life-sustaining therapies after resolution of the acute phase of organ failure (1). Estimates of 1-year mortality range between 50% and 60%, and of those patients who require prolonged mechanical ventilation (PMV) and are alive at the end of 1 year, only 10% will be living at home with functional independence (2). Critical illness represents a major life-altering event, and is often fraught with a series of decisions that must be made regarding invasive, life-sustaining therapies. However, because of ongoing cognitive dysfunction (3), chronically critically ill patients are rarely capable of communication and decision-making in the intensive care unit (ICU). As such, many patients with CCI become dependent upon family members to assist with decision-making about life-sustaining care.
Acting as a family surrogate decision-maker is associated with a number of negative consequences, including a subjective sense of overload and burden, emotional distress, and poor health-related quality of life (4, 5). Specific stressors that surrogate decision-makers frequently identify include uncertainty regarding the patient’s prognosis, perception of conflict with the provider team, and a sense of guilt surrounding medical decisions (6). Perhaps as a result, anxiety, depression, and post-traumatic stress disorder (PTSD) have all been described in family decision-makers of patients with critical illness (4, 7). One longitudinal cohort study found that 35% of family surrogates experienced symptoms of post-traumatic stress 6 months after a loved one’s admission to the ICU, as compared with 15% who experienced anxiety and 6% who experienced depression (8). When comparing patient–caregiver dyads, symptoms of PTSD are higher and persist for longer in the caregivers (9). Identifying potential risk factors for surrogate PTSD symptoms early in the course of a loved one’s CCI could enable physicians, nurses, and social workers to more effectively target support services toward those at highest risk.
Given that PTSD symptoms are common and persistent among surrogates for patients with CCI, we sought to identify potential risk factors present early in the course of CCI that can help identify family members who might benefit from early intervention. We hypothesized that both patient-specific and surrogate decision-maker–specific risk factors for surrogate PTSD symptoms at 90 days can be identified on Day 10 of patient mechanical ventilation.
Methods
We performed a secondary analysis of the data from a multicenter, randomized, controlled trial to determine whether family informational and support meetings would improve emotional outcomes for family surrogates of patients with CCI when compared with routine care (10). We enrolled patients from two tertiary care centers and a community hospital in the Southeastern United States and an urban tertiary care center in the Northeastern United States between October 2010 and November 2014. Participation was offered to patients in four medical ICUs who were 21 years of age or older with at least 7 days of mechanical ventilation, and to two categories of surrogate decision-makers: the primary surrogate with responsibility for health care decision-making if the patient lacked capacity, and additional family decision-makers if they also participated in health care decision-making. Among the exclusions were patients who were ventilated for longer than 7 days at an outside hospital, or had chronic neuromuscular disease, trauma, or burns. We assessed patient and family characteristics measured on or near Day 10 of mechanical ventilation for association with surrogate PTSD symptoms measured 90 days after enrollment in the clinical trial. The protocol for this secondary analysis of existing data was reviewed and approved by the University of North Carolina Institutional Review Board.
Potential Risk Factors
We selected 26 potential patient and surrogate decision-maker–specific risk factors for surrogate PTSD symptoms from the larger database a priori based on prior literature and a plausible mechanistic role in the development of PTSD symptoms. Potential patient factors included race, functional status, severity of acute illness, selected comorbid diagnoses, and level of alertness. Surrogate factors were age, demographics, religious beliefs, relationship to patient, and symptoms of anxiety and depression. Surrogate race was self-reported using fixed categories during the interviews with family members, and was included because of its association with higher symptoms of depression (11). Functional status before admission was scored as a summary of the total number of activities of daily living (ADLs) and instrumental ADLs that the patient was able to perform as reported by their primary surrogate (12, 13). Severity of illness was defined by generating an estimate of 1-year mortality using the ProVent 14 score (14). Select chronic comorbidities used in the analysis included chronic cerebrovascular disease or hemiplegia, liver disease, history of end-stage renal disease, or cancer. The Richmond Agitation Sedation Scale (RASS) score (15) was measured for each patient by a trained research assistant to assess the patient’s level of alertness at the time of enrollment in the clinical trial. For analyses, the RASS score was categorized as a RASS score of −5 or −4 (unresponsive), −3 to −1 (arousable), and 0 or greater (awake).
Surrogate symptoms of anxiety and depression were assessed at the time of enrollment using the Hospital Anxiety and Depression Scale (HADS) (16, 17). The HADS is a validated instrument designed to measure symptoms of anxiety and depression in hospitalized patients, and has been used for family subjects in the ICU setting (7). The HADS consists of two subscales (anxiety and depression), each containing seven items with a score ranging from 0 (lowest level of symptoms) to 3 (highest level of symptoms). A score of 11 or greater on either subscale suggests the presence of anxiety and/or depression disorder(s); scores from 8 to 10 may represent “borderline” symptom levels. Additional information on available variables can be found in descriptions of the clinical trial (10).
Primary Outcome Measure
The presence and severity of surrogate PTSD symptoms were defined using the Impact of Events Scale–Revised (IES-R), a validated instrument that has been used to evaluate the experience of families of ICU survivors and nonsurvivors (18, 19). The IES-R includes 22 items in 3 subscales, thought intrusion, avoidance, and hyperarousal, rated for “how distressing each difficulty has been” over the past 7 days from 0 (not at all) to 4 (extremely). Subscales are scored individually (mean of item scores) and then summed. Total scores for IES-R range from 0 to 88; a score of 33 or greater is a cut-off used to suggest PTSD-related symptoms at a level consistent with a probable diagnosis of PTSD. The IES-R was administered to all available enrolled surrogate decision-makers beginning 90 days after study enrollment.
Statistical Analyses
Descriptive statistics were analyzed using mean and 95% confidence interval (CI) for continuous variables, and frequency and percentage for categorical variables. To examine the association between each preselected potential risk factor and heightened surrogate PTSD symptoms at 90 days, we constructed individual multiple linear regression models with each potential risk factor as the exposure and the IES-R score as the outcome. Potential confounders were selected for each model by investigators (B.W. and S.S.C.) based on biologic or mechanistic plausibility. All models were adjusted for multiple respondents using a mixed model, considering the patients as a random factor. All tests were two sided, with a significance level of 0.05. Analysis was performed using SAS 9.4 (SAS Institute Inc.).
Results
The original database contained information for 365 surrogate decision-makers and 256 associated patients with CCI. Potential risk factor variables were measured on Day 10 (±3.4) of mechanical ventilation. A 90-day follow-up was complete for 306 (84%) of enrolled surrogates, corresponding to 224 patients. A total of 71% of surrogates were female, and mean age was 51 years. The majority of surrogates were either the spouse/partner or adult child of the patient. Mean HADS score was 16 (95% CI, 15.1–16.9); 91 of 306 family surrogates (30%) had an IES-R score of 33 or greater, consistent with a probable diagnosis of PTSD, at the 90-day follow-up interview. Complete surrogate characteristics are shown in Table 1.
Table 1.
Characteristics of surrogate decision-makers at enrollment
| Characteristic | Surrogates (n = 306) |
|---|---|
| Age, mean (95% CI), yr | 51 (49.3–52.4) |
| Female sex, n (%) | 218 (71) |
| Race, n (%) | |
| Black | 72 (24) |
| White | 193 (63) |
| Other | 41 (13) |
| Ethnicity, n (%) | |
| Hispanic or Latino | 38 (12) |
| Not Hispanic or Latino | 267 (88) |
| Marital Status, n (%) | |
| Married/live with partner | 207 (68) |
| Separated/divorced | 38 (12) |
| Single/widowed | 61 (20) |
| Education, n (%) | |
| Advanced degree | 53 (17) |
| College graduate | 82 (27) |
| Some college | 84 (28) |
| High School or less | 86 (28) |
| Employment, n (%) | |
| Disabled from employment | 30 (10) |
| Employed/student | 168 (55) |
| Homemaker | 22 (7) |
| Retired | 58 (19) |
| Unemployed | 27 (9) |
| Primary surrogate income, n (%) | |
| <$15,000 | 31 (14) |
| $15,000–$39,999 | 41 (19) |
| $40,000–$100,000 | 71 (33) |
| >$100,000 | 25 (11) |
| Prefer not to answer | 49 (23) |
| Religion, n (%) | |
| Catholic | 56 (18) |
| Jewish | 17 (6) |
| Protestant | 200 (66) |
| Other | 16 (5) |
| None | 16 (5) |
| Relationship, n (%) | |
| Child | 109 (36) |
| Parent | 41 (13) |
| Sibling | 37 (12) |
| Spouse/partner | 100 (33) |
| Other | 19 (6) |
| No. of decision-makers per patient, n (%) | |
| 1 | 152 (50) |
| 2 or more | 154 (50) |
| Designated as legal healthcare power of attorney, n (%) | 186 (61) |
| Hospital Anxiety and Depression Scale score at Day 10 of mechanical ventilation, mean (95% CI) | |
| Total | 16 (15.1–16.9) |
| Anxiety subscale | 10 (9–10.1) |
| Depression subscale | 7 (6–7) |
Definition of abbreviation: CI = confidence interval.
About one-half of the patients (49%) were female and were slightly older than surrogates, with a mean age of 59 years. Mean ADL score in the 2 weeks before hospital admission was 5 (95% CI, 4.6–5.2), indicating relatively high functionality. Liver disease (24%) and cancer (13%) were the most common comorbid conditions, and 1-year mortality risk, as estimated by the ProVent14 score, was 62% (95% CI, 58.6–64.8). Complete patient characteristics are shown in Table 2.
Table 2.
Characteristics of patients at enrollment
| Characteristic | Patients (n = 224) |
|---|---|
| Age, mean (95% CI), yr | 59 (56.4–60.7) |
| Female sex, n (%) | 109 (49) |
| Race, n (%) | |
| Black | 53 (24) |
| White | 138 (63) |
| Unavailable | 17 (8) |
| Other | 10 (5) |
| Insurance, n (%) | |
| Medicare | 107 (48) |
| Medicaid | 20 (9) |
| Commercial | 76 (34) |
| None | 21 (9) |
| Language | |
| English | 197 (90) |
| Spanish | 12 (6) |
| Other | 9 (4) |
| History of liver disease, n (%) | 29 (13) |
| History of cancer, n (%) | 53 (24) |
| History of stroke, n (%) | 20 (9) |
| History of end-stage renal disease, n (%) | 9 (4) |
| Presence of advance directive at enrollment, n (%) | 31 (14) |
| Activities of daily living score, mean (95% CI)* | 5 (4.6–5.2) |
| Instrumental activities of daily living score, mean (95% CI)† | 16 (14.8–16.9) |
| Hospital length of stay before trial enrollment, mean (95% CI), d | 13 (11.8–13.5) |
| 1-yr mortality as predicted by ProVent score, mean % (95% CI) | 62 (58.6–64.8) |
| Richmond Agitation Sedation Scale at enrollment, n (%) | |
| −5 or −4 (unresponsive) | 95 (44) |
| −3 to −1 (arousable) | 81 (38) |
| ≥0 (awake) | 38 (18) |
Definition of abbreviation: CI = confidence interval.
The range is 0 (dependent) to 6 (independent) in 6 activities.
The range is 8 (dependent) to 31 (independent) in 8 activities.
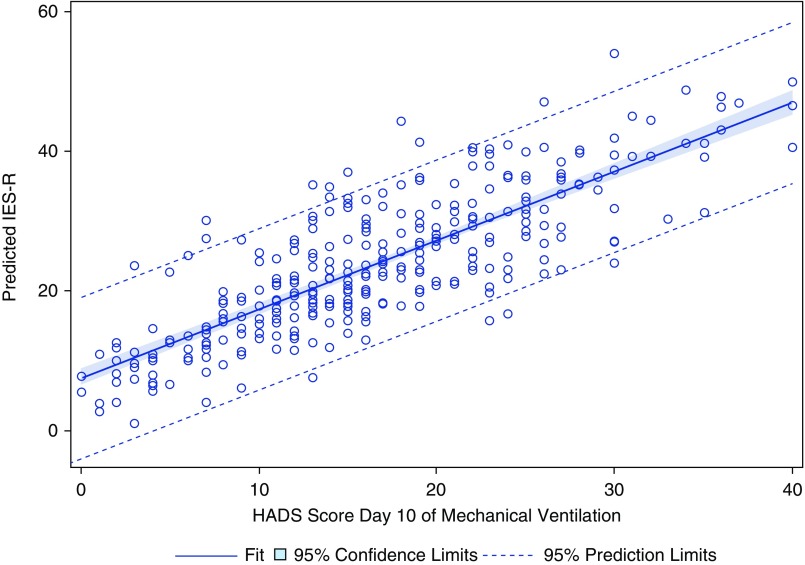
Preselected patient- and surrogate-related characteristics were then assessed for association with 90-day surrogate IES-R score. A complete list of variables included in the analysis is shown in Table 3 (surrogate characteristics) and Table 4 (patient characteristics), along with the associated mean change in IES-R score, as represented by a β coefficient. Covariates included in the individual models are listed in Tables 3 and 4; the associated β coefficient and 95% confidence interval (CI) for each covariate is available in the supplement. Starting with 26 preselected potential risk factors, only two—Day 10 surrogate HADS score and patient unresponsiveness, as indicated by a RASS score of −5 or −4—were significantly associated with caregiver PTSD symptoms at 90 days. Patient RASS score was negatively associated with surrogate IES-R score, with more surrogate PTSD symptoms for patients with a RASS of −5 or −4 (unresponsive) as compared with surrogates of patients with a RASS of 0 or above (awake) (coefficient = 8.39; 95% CI, 0.83–15.95). HADS (measured on Day 10 ± 3.4 of mechanical ventilation) and 90-day PTSD symptoms were positively associated so that each one-point increase in HADS, indicating worse anxiety and depression, was associated with a nearly one-point increase in IES-R (coefficient = 1.02; 95% CI, 0.73–1.30). Predicted surrogate IES-R score by Day 10 HADS score from the linear regression model is shown in Figure 1.
Table 3.
Results of multivariable analysis, surrogate decision-maker–related variables*
| Exposure Variable | β Coefficient (95%CI)† | Potential Confounders |
|---|---|---|
| Surrogate age | −0.08 (−0.25 to 0.08) | Surrogate religion |
| Surrogate employment | ||
| Surrogate education | ||
| Surrogate HADS‡ | ||
| Surrogate race | Surrogate religion | |
| Black | 1.86 (−3.44 to 7.15) | Surrogate employment |
| Other | 3.96 (−2.32 to 10.25) | Surrogate education |
| White | — | Surrogate marital status |
| Surrogate HADS‡ | ||
| Surrogate female sex | 2.47 (−1.82 to 6.75) | Surrogate religion |
| Surrogate employment | ||
| Surrogate education | ||
| Surrogate HADS‡ | ||
| Surrogate Hispanic or Latino | −1.68 (−8.49 to 5.12) | Surrogate religion |
| Surrogate employment | ||
| Surrogate education | ||
| Surrogate HADS‡ | ||
| Surrogate marital status | Surrogate employment | |
| Married/live with partner | −2.34 (−7.38 to 2.71) | Surrogate race |
| Separated/divorced | −0.86 (−7.78 to 6.07) | Surrogate religion |
| Single/widowed | — | Surrogate HADS‡ |
| Surrogate level of education | Surrogate race | |
| Advanced degree | −1.16 (−7.45 to 5.14) | Surrogate ethnicity |
| College graduate | −1.05 (−6.59 to 4.49) | Surrogate sex |
| Some college | 1.24 (−4.2 to 6.69) | Surrogate religion |
| High school or less | — | Surrogate employment |
| Surrogate HADS‡ | ||
| Surrogate marital status | ||
| Patient insurance | ||
| Surrogate employment | Surrogate race | |
| Disabled | 5.79 (−3.22 to 14.8) | Surrogate ethnicity |
| Employed/student | 3.45 (−3.4 to 10.31) | Surrogate sex |
| Homemaker | −1.48 (−11.09 to 8.12) | Surrogate age |
| Retired | 2.95 (−5.63 to 11.53) | Surrogate education |
| Unemployed | — | Surrogate HADS‡ |
| Surrogate marital status | ||
| Patient insurance | ||
| Level of income of primary surrogate§ | Surrogate race | |
| Prefer not to answer | −6.3 (−15.16 to 2.57) | Surrogate ethnicity |
| >$100,000 | −3.4 (−14.24 to 7.43) | Surrogate sex |
| $40,000–$100,000 | −5.44 (−14.18 to 3.3) | Surrogate age |
| $15,000–$39,999 | −7.57 (−16.43 to 1.29) | Surrogate education |
| <$15,000 | — | Surrogate employment |
| Surrogate marital status | ||
| Patient insurance | ||
| Surrogate HADS‡ | ||
| Surrogate religious beliefs | Surrogate race | |
| Catholic | 6.54 (−4.24 to 17.32) | Surrogate ethnicity |
| Jewish | 6.97 (−5.75 to 19.7) | Surrogate sex‡ |
| Protestant | 6.05 (−3.54 to 15.63) | Surrogate age |
| Other | 13.13 (0.64 to 25.62) | Surrogate marital status |
| None | — | |
| Surrogate relationship to patient | Surrogate HADS‡ | |
| Child | −0.65 (−8.79 to 7.5) | Patient age |
| Parent | 2.44 (−6.58 to 11.47) | |
| Sibling | 2.6 (−6.36 to 11.57) | |
| Spouse/partner | 1.89 (−6.12 to 9.9) | |
| Other | — | |
| Two or more surrogate decision-makers per patient | −3.63 (−7.74 to 0.48) | Surrogate HADS‡ |
| Surrogate designation as legal healthcare power of attorney | −2.22 (−6.45 to 2.0) | Patient Cancer |
| Patient ADLs | ||
| Patient iADLs | ||
| Patient HADS‡ | ||
| Surrogate hospital anxiety and depression scale | 1.02 (0.73 to 1.3) | Surrogate race |
| Surrogate ethnicity | ||
| Surrogate sex | ||
| Surrogate age | ||
| Surrogate employment status | ||
| 2 + Surrogates per patient | ||
| Relationship to patient | ||
| Patient age | ||
| Patient Advance directive | ||
| Patient ADLs | ||
| Patient liver disease | ||
| Patient cancer | ||
| Patient ESRD | ||
| Patient ProVent | ||
| Patient RASS |
Definition of abbreviations: ADLs = activities of daily living; CI = confidence interval; ESRD = end-stage renal disease; HADS = Hospital Anxiety and Depression Scale; iADLs = instrumental activities of daily living; IES-R = Impact of Events Score–Revised; RASS = Richmond Agitation Sedation Scale.
Em dash (—) indicates reference group.
All models were adjusted for multiple respondents.
For continuous variables, the coefficient represents mean change in IES-R score per one-unit increase. For categorical variables, the coefficient represents mean difference in IES-R score as compared to the reference group.
P < 0.05.
Model includes primary surrogate decision-makers only.
Table 4.
Results of multivariable analysis, patient-related variables*
| Exposure Variable | β Coefficient (95%CI)† | Potential Confounders |
|---|---|---|
| Patient age | −0.05 (−0.18 to 0.08) | Patient ADLs |
| Patient iADLs | ||
| Patient cancer | ||
| Patient liver disease | ||
| Patient stroke | ||
| Patient ESRD | ||
| Patient advance directive | ||
| Surrogate HADS‡ | ||
| Patient female sex | −2.01 (−6.59 to 2.58) | |
| Patient Insurance | Patient stroke | |
| Commercial | 2.2 (−5.3 to 9.69) | Patient ESRD |
| Medicaid | 1.81 (−7.73 to 11.35) | Patient cancer |
| Medicare | 0.22 (−7.07 to 7.51) | Patient liver disease |
| None | — | Patient advance directive |
| Surrogate HADS‡ | ||
| Patient history of liver disease | 2.48 (−4.29 to 9.25) | Patient age |
| Patient insurance | ||
| Patient ADLs | ||
| Patient iADLs | ||
| Surrogate HADS‡ | ||
| Patient RASS‡ | ||
| Patient history of cancer | −4.45 (−9.53 to 0.62) | Patient age |
| Patient race | ||
| Patient ethnicity | ||
| Patient insurance | ||
| Patient advance directive | ||
| Patient ADLs | ||
| Patient iADLs | ||
| Surrogate HADS‡ | ||
| Patient history of stroke | −1.4 (−9.26 to 6.45) | Patient age |
| Patient race | ||
| Patient insurance | ||
| Patient advance directive | ||
| Patient ADLs | ||
| Patient iADLs | ||
| Surrogate HADS‡ | ||
| Patient history of end-stage renal disease | −8.58 (−20.94 to 3.79) | Patient age |
| Patient race | ||
| Patient insurance | ||
| Patient advance directive | ||
| Patient ADLs | ||
| Patient iADLs | ||
| Surrogate HADS‡ | ||
| Patient RASS‡ | ||
| Presence of advance directive | −4.74 (−9.82 to 0.34) | Patient age |
| Patient race | ||
| Patient ethnicity | ||
| Patient insurance | ||
| Patient cancer | ||
| Patient ESRD | ||
| Patient stroke | ||
| Patient ADLs | ||
| Patient iADLs | ||
| Surrogate HADS‡ | ||
| ADLs | 0.14 (−1.32 to 1.6) | Patient Age |
| Patient insurance | ||
| Patient cancer | ||
| Patient ESRD | ||
| Patient liver disease | ||
| Patient stroke | ||
| Patient iADLs | ||
| Surrogate HADS‡ | ||
| Pre-ICU hospital length of stay | 0.01 (−0.22 to 0.25) | Patient liver disease |
| Surrogate HADS‡ | ||
| Patient age | ||
| 1-year mortality as estimated by ProVent14 score | −0.04 (−0.15 to 0.08) | Patient pre-ICU LOS |
| Surrogate HADS‡ | ||
| Patient RASS‡ | ||
| Patient ADLs | ||
| Richmond Agitation Sedation Scale at enrollment | Patient liver disease | |
| −5 or −4 (unresponsive) | 8.39 (0.83 to 15.95) | Patient ESRD |
| −3 to −1 (arousable) | 2.48 (−5.18 to 10.15) | Patient ProVent |
| 0 and above (awake) | — | Surrogate HADS‡ |
Definition of abbreviations: ADLs = activities of daily living; CI = confidence interval; ESRD = end-stage renal disease; HADS = Hospital Anxiety and Depression Scale; iADLs = instrumental activities of daily living; IES-R = Impact of Events Score–Revised; ICU = intensive care unit; LOS = length of stay; RASS = Richmond Agitation Sedation Scale.
Em dash (—) indicates reference group.
All models were adjusted for multiple respondents.
For continuous variables, the coefficient represents mean change in IES-R score per one-unit increase. For categorical variables, the coefficient represents mean difference in IES-R score as compared to the reference group.
P < 0.05.
Figure 1.
Predicted Impact of Events Score–Revised score from logistic regression by Hospital Anxiety and Depression Scale score, adjusted for multiple respondents. HADS = Hospital Anxiety and Depression Scale; IES-R = Impact of Events Score–Revised.
In a post hoc sensitivity analysis, Day 10 HADS score remained a risk factor for surrogate PTSD symptoms even after adjusting for events that occurred later in the ICU stay or after hospital discharge, including death by the time of 90-day surrogate interview and study group from the clinical trial (coefficient = 1.08; 95% CI, 0.81–1.35).
Discussion
In this analysis of potential risk factors for PTSD symptoms in surrogate decision-makers of patients with CCI, surrogate anxiety and depression (HADS score) and patient unresponsiveness on or near Day 10 of mechanical ventilation were risk factors for increased surrogate PTSD symptoms at 90 days. No variables pertaining to surrogate or patient demographics, premorbid patient health status, or events of the hospitalization before ICU admission were found to be significant. HADS is a simple scale that can be administered to family members in a few minutes, and level of arousal and responsiveness is routinely assessed in all patients.
Family surrogate decision-makers with higher HADS scores near Day 10 of mechanical ventilation, especially when their loved one is unresponsive while they are at the bedside, can be targeted for early interventions to help them cope with severe illness and decision-making. Interventions to reduce long-term emotional distress in surrogate decision-makers have met with limited success in rigorous clinical trials to date (10, 19–22). Targeting those individuals who are at highest risk and implementing interventions early in the course of CCI could enhance the success of such interventions, and this could be facilitated by using the risk factors identified in this study. A simple means to identify higher-risk family decision-makers could also help to deploy intervention resources in a more focused and efficient manner.
The finding that unresponsiveness remained a risk factor even after adjusting for HADS is an interesting one for which there are several possible explanations. A RASS score of −4 or −5 at Day 10 of mechanical ventilation could reflect deep sedation due to severe hypoxemia or ventilator dyssynchrony, or due to advanced encephalopathies related to underlying acute diseases. Trauma patients were excluded from the clinical trial, and acute cerebral vascular events were unusual in this medical ICU population. One explanation for this finding is that unresponsiveness is a surrogate for advanced illness and poor prognosis. However, other illness severity measures were not significantly associated. Another explanation is that a patient’s unresponsiveness over days is an extremely distressing experience for the family, secondary to either the fact that it is an overt and constant reminder of the critical nature of their loved one’s illness, and/or the fact that it places a heavier burden on the surrogate decision-maker without guidance from the patient (23).
Our findings provide novel insight into the identification of surrogates at highest risk for emotional distress after a loved one’s discharge from the ICU. A 2005 study of caregivers in the general ICU population in France measured a limited number of variables, and found that female sex, cancer in the ICU patient, and being a child of the ICU patient were independently associated with increased risk for post-traumatic stress symptoms, but, as CCI represents a distinct syndrome with a potentially separate profile for long-term surrogate distress, these findings may not be generalizable to the CCI population. Reliance on these demographic factors alone to identify surrogates at risk for heightened PTSD symptoms could result in omission of high-risk surrogates in other categories, such as high-risk male surrogates. Furthermore, some of the factors identified in that study, such as surrogate perception that information was incomplete or patient death in the ICU, occur later in the course of critical illness, and thus represent less useful targets for screening and intervention during the patient’s ICU course. Another study of a Greek cohort of family caregivers for patients admitted to the ICU found that female sex and baseline anxiety were significant joint predictors of the development of PTSD symptoms before hospital discharge, although this study was limited by small sample size with just 32 caregivers (24). Investigation of a German cohort found that higher relationship satisfaction between the patient and the surrogate was protective against post-traumatic stress in the CCI population, but this study was limited by low follow-up rate with only 12% of enrolled patient–surrogate dyads available for 6 month follow-up (25). A recent study by Torke and colleagues (26) demonstrated that baseline distress, as measured by the Kessler six-item Psychological Distress Scale, was positively associated with post-traumatic stress at 6–8 weeks after hospitalization for family surrogate decision-makers of older adults admitted to either the ICU or the general wards. As compared with the population in this study, our patients had a much higher severity of illness and worse long-term outcomes, and family members had a more significant degree of PTSD symptoms. This secondary analysis is based on the largest completed randomized, controlled trial of patients with CCI and surrogates to date, thus providing a rich dataset and a unique opportunity to explore potential risk factors for surrogate PTSD in this population.
Our study has several limitations. The observational nature of the analysis limits our ability to fully elucidate the cause-and-effect nature of the observed risk factors and the outcome of interest. In addition, because we are unable to measure PTSD symptoms before patients’ hospital admission, we do not know whether surrogates experienced significant PTSD symptoms at baseline due to other life events. However, inclusion of anxiety and depression as a variable in our analysis at least partially accounts for this possibility, as there is some overlap between anxiety symptoms and PTSD symptoms. We chose to evaluate 26 variables selected from an existing database based on clinical intuition and previously published literature, and it is possible that there are additional risk factors that were not measured or analyzed. Finally, our database consists of family members who consented to participation in a clinical trial and patients who were admitted to medical ICUs at mostly large academic medical centers. Therefore, results may not be generalizable to all chronically critically ill patients and surrogates.
In conclusion, among surrogate decision-makers of patients with CCI, high surrogate anxiety and depression scores and patient unresponsiveness on or near Day 10 of mechanical ventilation are risk factors for PTSD symptoms that are identifiable early in the course of CCI. Identifying risk factors for surrogate PTSD symptoms early in the course of a loved one’s CCI could ultimately enable physicians, nurses, and social workers to more effectively target support services toward those at highest risk. Further work is needed to determine whether there are elements of clinician support and communication that mitigate PTSD risk in surrogates, and to use this knowledge to develop targeted interventions.
Supplementary Material
Footnotes
Supported by National Institutes of Health institutional training grants 2Y32HL007106-41 and R01-NR012413.
Author Contributions: B.W., A.C., and S.S.C. had full access to all data, and are responsible for the integrity and the accuracy of the data analysis; B.W., J.E.N., C.E.C., L.C.H., M.D., J.A.T., and S.S.C. were responsible for study design; B.W., A.C., S.C., J.E.N., C.E.C., L.C.H., M.D., J.A.T., and S.S.C. were responsible for analysis, or interpretation of the data; B.W., A.C., S.C., J.E.N., C.E.C., L.C.H., M.D., J.A.T., and S.S.C. critically revised the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Nelson JE, Cox CE, Hope AA, Carson SS. Chronic critical illness. Am J Respir Crit Care Med. 2010;182:446–454. doi: 10.1164/rccm.201002-0210CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carson SS, Bach PB, Brzozowski L, Leff A. Outcomes after long-term acute care: an analysis of 133 mechanically ventilated patients. Am J Respir Crit Care Med. 1999;159:1568–1573. doi: 10.1164/ajrccm.159.5.9809002. [DOI] [PubMed] [Google Scholar]
- 3.Nelson JE, Tandon N, Mercado AF, Camhi SL, Ely EW, Morrison RS. Brain dysfunction: another burden for the chronically critically ill. Arch Intern Med. 2006;166:1993–1999. doi: 10.1001/archinte.166.18.1993. [DOI] [PubMed] [Google Scholar]
- 4.Cameron JI, Chu LM, Matte A, Tomlinson G, Chan L, Thomas C, et al. RECOVER Program Investigators (Phase 1: towards RECOVER); Canadian Critical Care Trials Group. One-year outcomes in caregivers of critically ill patients. N Engl J Med. 2016;374:1831–1841. doi: 10.1056/NEJMoa1511160. [DOI] [PubMed] [Google Scholar]
- 5.Douglas SL, Daly BJ. Caregivers of long-term ventilator patients: physical and psychological outcomes. Chest. 2003;123:1073–1081. doi: 10.1378/chest.123.4.1073. [DOI] [PubMed] [Google Scholar]
- 6.Wendler D, Rid A. Systematic review: the effect on surrogates of making treatment decisions for others. Ann Intern Med. 2011;154:336–346. doi: 10.7326/0003-4819-154-5-201103010-00008. [DOI] [PubMed] [Google Scholar]
- 7.Azoulay E, Pochard F, Kentish-Barnes N, Chevret S, Aboab J, Adrie C, et al. FAMIREA Study Group. Risk of post-traumatic stress symptoms in family members of intensive care unit patients. Am J Respir Crit Care Med. 2005;171:987–994. doi: 10.1164/rccm.200409-1295OC. [DOI] [PubMed] [Google Scholar]
- 8.Anderson WG, Arnold RM, Angus DC, Bryce CL. Posttraumatic stress and complicated grief in family members of patients in the intensive care unit. J Gen Intern Med. 2008;23:1871–1876. doi: 10.1007/s11606-008-0770-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fumis RR, Ranzani OT, Martins PS, Schettino G. Emotional disorders in pairs of patients and their family members during and after ICU stay. PLoS One. 2015;10:e0115332. doi: 10.1371/journal.pone.0115332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carson SS, Cox CE, Wallenstein S, Hanson LC, Danis M, Tulsky JA, et al. Effect of palliative care–led meetings for families of patients with chronic critical illness: a randomized clinical trial. JAMA. 2016;316:51–62. doi: 10.1001/jama.2016.8474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunlop DD, Song J, Lyons JS, Manheim LM, Chang RW. Racial/ethnic differences in rates of depression among preretirement adults. Am J Public Health. 2003;93:1945–1952. doi: 10.2105/ajph.93.11.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged: the index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 13.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 14.Hough CL, Caldwell ES, Cox CE, Douglas IS, Kahn JM, White DB, et al. ProVent Investigators and the National Heart Lung and Blood Institute’s Acute Respiratory Distress Syndrome Network. Development and validation of a mortality prediction model for patients receiving 14 days of mechanical ventilation. Crit Care Med. 2015;43:2339–2345. doi: 10.1097/CCM.0000000000001205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han JH, Vasilevskis EE, Shintani A, Graves AJ, Schnelle JF, Dittus RS, et al. Impaired arousal at initial presentation predicts 6-month mortality: an analysis of 1,084 acutely ill older patients. J Hosp Med. 2014;9:772–778. doi: 10.1002/jhm.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 17.Pochard F, Darmon M, Fassier T, Bollaert P-E, Cheval C, Coloigner M, et al. French FAMIREA Study Group. Symptoms of anxiety and depression in family members of intensive care unit patients before discharge or death. A prospective multicenter study. J Crit Care. 2005;20:90–96. doi: 10.1016/j.jcrc.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Creamer M, Bell R, Failla S. Psychometric properties of the Impact of Event Scale–Revised. Behav Res Ther. 2003;41:1489–1496. doi: 10.1016/j.brat.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 19.White DB, Angus DC, Shields AM, Buddadhumaruk P, Pidro C, Paner C, et al. PARTNER Investigators. A randomized trial of a family-support intervention in intensive care units. N Engl J Med. 2018;378:2365–2375. doi: 10.1056/NEJMoa1802637. [DOI] [PubMed] [Google Scholar]
- 20.Curtis JR, Back AL, Ford DW, Downey L, Shannon SE, Doorenbos AZ, et al. Effect of communication skills training for residents and nurse practitioners on quality of communication with patients with serious illness: a randomized trial. JAMA. 2013;310:2271–2281. doi: 10.1001/jama.2013.282081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curtis JR, Treece PD, Nielsen EL, Gold J, Ciechanowski PS, Shannon SE, et al. Randomized trial of communication facilitators to reduce family distress and intensity of end-of-life care. Am J Respir Crit Care Med. 2016;193:154–162. doi: 10.1164/rccm.201505-0900OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cox CE, Hough CL, Carson SS, White DB, Kahn JM, Olsen MK, et al. Effects of a telephone- and web-based coping skills training program compared with an education program for survivors of critical illness and their family members: a randomized clinical trial. Am J Respir Crit Care Med. 2018;197:66–78. doi: 10.1164/rccm.201704-0720OC. [DOI] [PubMed] [Google Scholar]
- 23.Nelson JE, Hanson LC, Keller KL, Carson SS, Cox CE, Tulsky JA, et al. The voice of surrogate decision-makers. family responses to prognostic information in chronic critical illness. Am J Respir Crit Care Med. 2017;196:864–872. doi: 10.1164/rccm.201701-0201OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paparrigopoulos T, Melissaki A, Efthymiou A, Tsekou H, Vadala C, Kribeni G, et al. Short-term psychological impact on family members of intensive care unit patients. J Psychosom Res. 2006;61:719–722. doi: 10.1016/j.jpsychores.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 25.Wintermann GB, Weidner K, Strauß B, Rosendahl J, Petrowski K. Predictors of posttraumatic stress and quality of life in family members of chronically critically ill patients after intensive care. Ann Intensive Care. 2016;6:69. doi: 10.1186/s13613-016-0174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torke AM, Callahan CM, Sachs GA, Wocial LD, Helft PR, Monahan PO, et al. Communication quality predicts psychological well-being and satisfaction in family surrogates of hospitalized older adults: an observational study. J Gen Intern Med. 2018;33:298–304. doi: 10.1007/s11606-017-4222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.