Abstract
Rationale: Early antibiotics improve outcomes for patients with sepsis. Factors influencing antibiotic timing in emergency department (ED) sepsis remain unclear.
Objectives: Determine the relationship between prehospital level of care of patients with sepsis and ED door-to-antibiotic time.
Methods: This retrospective cohort study comprised patients admitted from the community to an academic ED June 2009 to February 2015 with fluid-refractory sepsis or septic shock. Transfer patients and those whose antibiotics began before ED arrival or after ED discharge were excluded. We used multivariable regression to evaluate the association between the time from ED arrival to antibiotic initiation and prehospital level of care, defined as the highest level of emergency medical services received: none, basic life support (BLS) ambulance, or advanced life support (ALS) ambulance. We measured variation in this association when hypotension was or was not present by ED arrival.
Results: Among 361 community-dwelling patients with sepsis, the level of prehospital care correlated with illness severity. ALS-treated patients received antibiotics faster than patients who did not receive prehospital care (median, 103 [interquartile range, 75 to 135] vs. 144 [98 to 251] minutes, respectively) or BLS-only patients (168 [100–250] minutes; P < 0.001 for each pairwise comparison with ALS). This pattern persisted after multivariable adjustment, where ALS care (−43 min; 95% confidence interval [CI], −84 to −2; P = 0.033) but not BLS-only care (−4 min; 95% CI, −41 to +34; P = 0.97) was associated with less antibiotic delay compared with no prehospital care. ALS-treated patients more frequently received antibiotics within 3 hours of ED arrival (91%) compared with walk-in patients (62%; adjusted odds ratio, 3.11; 95% CI, 1.20 to 8.03; P = 0.015) or BLS-treated patients (56%; adjusted odds ratio, 4.51; 95% CI, 1.89 to 11.35; P < 0.001). ALS-treated patients started antibiotics faster than walk-in patients in the absence of hypotension by ED arrival (−41 min; 95% CI, −110 to −13; P = 0.009) but not when hypotension was present (+25 min; 95% CI, −43 to +92; P = 0.66).
Conclusions: Prehospital ALS but not BLS-only care was associated with faster antibiotic initiation for patients with sepsis without hypotension. Process redesign for non-ALS patients may improve antibiotic timeliness for ED sepsis.
Keywords: emergency medical services, septic shock, ambulance care, emergency medicine, sepsis
Sepsis is a common and costly condition encountered by prehospital and emergency department (ED) clinicians (1). Defined as organ dysfunction resulting from a dysregulated host response to infection (2), the sepsis syndrome accounts for at least 800,000 ED visits and 1.7 million hospitalizations, with 16% mortality and $24 billion in costs in the United States annually (3–6). Prompt sepsis case identification followed by aggressive management promotes optimal patient outcomes (7, 8). In particular, early antibiotic initiation appears to reduce sepsis mortality and progression to septic shock (9–13).
Less than half of patients with sepsis that is present on arrival to the ED currently receive antibiotics within 1 hour, the goal recommended by international guidelines from the Surviving Sepsis Campaign (14–16). Prior studies demonstrated that arriving to the ED via ambulance decreased door-to-antibiotic time but did not distinguish advanced life support (ALS) provided by paramedics from basic life support (BLS) provided by emergency medical technicians (15, 17, 18). Factors likely to influence ED assessment and management of sepsis differ among patients who receive no prehospital care, BLS care, or ALS care, but just one of the three previous studies reported results after adjustment for these potentially confounding factors (18).
Improved knowledge of factors that support or impede prompt antibiotic initiation for sepsis will aid creation of highly reliable sepsis care systems. Integrating prehospital and hospital sepsis care could also speed sepsis diagnosis and treatment. To help clarify whether and how care from emergency medical services (EMS) might influence door-to-antibiotic time for patients with sepsis, we therefore investigated the association between the level of prehospital care and door-to-antibiotic time for ED patients with fluid-refractory sepsis or septic shock.
Methods
Study Design and Setting
We performed a retrospective cohort study of patients admitted to the ED of a university-affiliated county hospital. The region is served by a two-tier system of prehospital emergency care. Emergency medical technicians employed by regional fire departments or private ambulance services and trained in BLS provide initial response to 911 emergency calls. Paramedics trained in ALS provide second-tier response and are assigned calls following protocols and assessments by emergency medical dispatchers and first-tier responders. Example criteria for ALS dispatch include respiratory distress and altered level of consciousness. Dispatch of BLS and ALS providers may occur sequentially (BLS providers request ALS backup) or concurrently (central dispatch of both BLS and ALS simultaneously). In this system, therefore, ALS providers participate in approximately 20% to 25% of all ambulance calls and usually have the assistance of BLS providers (19). On arrival to the study ED, patients arriving without prehospital care and some BLS-transported patients are evaluated by a triage nurse and then placed into a treatment area. BLS-transported patients for whom the ED received advance communication about clinical instability and ALS-transported patients undergo their initial evaluation in an ED treatment area with bedside handoff from the prehospital to the ED team. The University of Washington Institutional Review Board approved this study and approved waiver of informed consent.
Study Population
Details of the patient cohort have been previously described (14). Briefly, eligible patients were 18 years of age or older and exhibited fluid-refractory sepsis or septic shock in the ED leading to activation of the study hospital’s sepsis alert protocol. Activation criteria for the protocol—which entails bedside consultation by an intensive care physician and nurse—included suspected infection plus hypotension (mean arterial pressure < 65 mm Hg, systolic blood pressure < 90 mm Hg) and/or hypoperfusion (lactate > 4 mmol/L) despite fluid resuscitation with greater than or equal to 30 ml/kg of crystalloid. ED physicians could also activate a sepsis alert on the basis of clinical judgment. Patients receiving antibiotics before arrival or transferred from another acute care facility were excluded. Patients who did not receive antibiotics in the ED—and who therefore may not have exhibited sufficient signs of infection to prompt the ED clinician to start antibiotics—or who were missing data necessary for multivariable analysis (n = 6) were also excluded.
Data Abstraction
Electronic data on antibiotic initiation, laboratory results, patient demographics, and discharge data were linked to prehospital and hospital clinical data abstracted by trained nurses and physicians. Door-to-antibiotic times were manually verified if missing, more than 6 hours after ED arrival, more than 90 minutes after sepsis protocol activation, or before ED arrival. Reabstraction of 6% of records (randomly selected) demonstrated perfect agreement for antibiotic times and near-perfect agreement for classification of the reason for hospital admission (κ, 0.91; 95% confidence interval [CI], 0.80–1.00).
Exposure and Outcome Measures
The primary outcome was door-to-antibiotic time, defined as the elapsed time between ED arrival and initiation (administration) of an eligible antimicrobial agent (14). Each patient’s prehospital care level was categorized as none (e.g., walk-in or arrival via personal vehicle), BLS, or ALS. For patients receiving prehospital care from both BLS and ALS providers, the highest level of care was assigned. Prehospital air medical transport was considered a form of ALS care. The Charlson Comorbidity Index, Sequential Organ Failure Assessment (SOFA) score, and Mortality in Emergency Department Sepsis (MEDS) score were calculated as previously described (20–22). Systolic blood pressure less than 90 mm Hg or mean arterial pressure less than 65 mm Hg before or at the time of ED arrival defined presenting hypotension. Because of a bimodal distribution and consistent with the full validated Quick SOFA model (23), patients’ first-recorded Glasgow Coma Score was categorized as normal (14–15) or abnormal (≤13). Nocturnal admissions were defined as occurring 11 p.m. to 7:59 a.m. daily. Identification of a discharge diagnosis of sepsis was based on application of modified Angus criteria to the subject’s International Classification of Disease–Clinical Modification version 9 discharge diagnosis codes (24, 25).
Statistical Analysis
For bivariable comparison, we used the chi-square test or Fisher exact test for categorical variables and unpaired t tests with unequal variance or Mann-Whitney tests for continuous variables. For comparisons across more than two categories, we used analysis of variance or Kruskal-Wallis tests. Because essentially all patients brought to the ED from long-term care facilities arrived via EMS, the primary analysis included only community-dwelling patients with sepsis and used robust multivariable linear regression to evaluate the association of prehospital level of care with door-to-antibiotic time after adjustment for potential confounding and precision covariates. A secondary analysis used robust multivariable logistic regression to assess the association between prehospital level of care and antibiotic administration within 3 hours of ED arrival. Significance testing of comparisons between levels of EMS was performed using the Tukey-Kramer method to account for multiple comparisons among groups (26). A secondary analysis compared door-to-antibiotic time for patients arriving from long-term care and nursing facilities via BLS versus ALS ambulance. Adjustment variables were specified a priori on the basis of a plausible or reported confounding relationship between mode of ED arrival and ED care processes and included nighttime ED arrival, illness severity as measured by the MEDS score, source of infection, Hispanic ethnicity or non-white race, age, Charlson Comorbidity Index, first-available Glasgow Coma Score, first-available systolic blood pressure, and first-available respiratory rate.
In a secondary analysis, we used multivariable logistic regression to evaluate whether in-hospital mortality differed between levels of prehospital care after adjustment for the MEDS score, age, Charlson score, source of infection, and initial SOFA score. To explore potential mechanisms of EMS care on door-to-antibiotic time, we used multivariable regression (adjusted as in the primary analysis) to investigate whether prehospital intravenous access or prehospital versus ED endotracheal intubation influenced door-to-antibiotic care for ALS-treated patients, compared the door-to-antibiotic time within each level of prehospital care between patients who did versus did not have a resident physician participate in their ED care and assessed the relationship between prehospital care levels and 1) the elapsed time to other ED care events (e.g., blood draw), and 2) the likelihood that triage evaluations reflected a diagnosis or suspicion of sepsis or infection (see Table E1 in the online supplement). Finally, in a post hoc analysis intended to clarify whether illness severity (i.e., indication bias) drove the observed association between prehospital care and antibiotic timing, we repeated the primary analysis after including 1) stratification on the presence or absence of hypotension by the time the patient was evaluated at ED triage, or 2) adding to the primary model an interaction term between hypotension by ED arrival and the level of prehospital care.
To evaluate the robustness of our findings, we repeated the primary analysis using either stabilized inverse probability of treatment weighting on the basis of a propensity score (27, 28) or adjustment using the propensity score and MEDS score (29). Detailed methods for the propensity-based analyses are described in the online supplement.
We performed analyses in Stata version 14.2 (StataCorp LP) and R (R Foundation) and considered a P value (adjusted as appropriate for multiple comparisons using the Tukey-Kramer method) significant if less than 0.05.
Results
Of 481 eligible sepsis alert patients, 361 community-dwelling individuals had complete data and were included in the primary analysis; another 114 patients brought to the ED from long-term care facilities were analyzed separately (Figure E2). Among community-dwelling patients, 101 (28%) did not receive prehospital care, 111 (31%) received BLS-only care, and 149 (41%) received ALS care. Illness severity, organ failure severity (as measured by the SOFA score), shock prevalence, lactate levels, and unadjusted mortality increased as prehospital level of care increased (Table 1). Essentially all patients with sepsis brought to the ED from long-term care facilities arrived by ambulance, and 69 (61%) received ALS care (Table E4). One patient with sepsis residing in a long-term care facility was brought to the ED by private vehicle and was excluded from further analysis.
Table 1.
Demographic and clinical characteristics of community-dwelling patients with sepsis by level of prehospital care
| No Ambulance Care (n = 101) | Basic Life Support (n = 111) | Advanced Life Support (n = 149) | |
|---|---|---|---|
| Age, yr, mean (SD) | 50.2 (14.1) | 56.7 (15.4) | 54.1 (15.3) |
| Female sex | 32 (31.7) | 42 (37.8) | 38 (25.5) |
| Nonwhite race or Hispanic | 53 (52.5) | 51 (45.9) | 57 (38.3) |
| Prehospital care | |||
| Prehospital intravenous access | — | 0 (0) | 125 (84.6) |
| Prehospital shock | — | 35 (31.5) | 77 (51.7) |
| EMS scene to ED arrival, min | — | 35.6 (16.8) | 41.8 (18.6) |
| Infection source | |||
| Pneumonia | 35 (34.6) | 22 (19.8) | 55 (36.9) |
| Soft tissue infection | 12 (11.9) | 18 (16.2) | 16 (10.8) |
| Intraabdominal infection | 10 (9.9) | 15 (13.5) | 4 (2.7) |
| Urinary tract infection | 11 (10.9) | 24 (21.6) | 13 (8.7) |
| Other/unknown | 33 (32.7) | 32 (28.8) | 61 (40.9) |
| ED admission data | |||
| Hypotension by ED arrival* | 21 (20.8) | 40 (36.0) | 88 (59.1) |
| First Glasgow Coma Score* | 15 (15–15) | 15 (15–15) | 12 (3–15) |
| Lactate, mmol/L† | 3.3 (1.9–5.7) | 4.2 (2.7–6.8) | 5.7 (3.5–8.9) |
| White blood cell count, 1,000/dl, mean (SD) | 15.7 (9.5) | 16.8 (10.0) | 15.9 (10.4) |
| SOFA score | 1 (0–4) | 3 (1–5) | 6 (3–9) |
| ED management data | |||
| Total intravenous fluid, L* | 4.0 (3.0–5.0) | 4.0 (3.0–5.0) | 4.1 (3.0–6.0) |
| Vasopressors* | 14 (13.9) | 26 (23.4) | 52 (34.9) |
| Resident physician involved in care | 62 (60.8) | 116 (74.8) | 203 (93.1) |
| Minutes to antibiotic initiation, mean (SD) | 195 (154) | 190 (122) | 114 (65) |
| Antibiotic initiation ≤3 h | 63 (62.4) | 62 (55.9) | 135 (90.6) |
| MEDS score, mean (SD) | 3.8 (3.1) | 5.0 (3.6) | 6.9 (3.5) |
| Charlson Comorbidity Index, mean (SD) | 2.2 (2.2) | 2.3 (2.1) | 2.4 (2.1) |
| Sepsis discharge diagnosis | 73 (72.3) | 83 (74.8) | 115 (77.2) |
| Death before discharge | 10 (9.9) | 21 (18.9) | 33 (22.1) |
| ED length of stay, min | 367 (293–522) | 387 (276–501) | 236 (191–330) |
| Hospital length of stay, d | 5.1 (3.0–11.8) | 6.5 (3.1–15.5) | 5.7 (2.5–12.8) |
Definition of abbreviations: ED = emergency department; EMS = emergency medical services; MEDS score = Mortality in Emergency Department Sepsis score; SD = standard deviation; SOFA = Sequential Organ Failure Assessment.
Values reported as n (%) or median (interquartile range) unless otherwise noted.
Includes all care in both ED and prehospital setting.
Lactate was measured in the hospital’s central laboratory. One patient had no lactate level measured.
Median unadjusted door-to-antibiotic time was 118 (interquartile range [IQR], 79–185) minutes for the overall cohort. Community-dwelling patients with sepsis brought in by ALS ambulance had shorter door-to-antibiotic times (103 [IQR, 75–135] min) compared with either BLS-treated patients (168 [IQR, 100–250] min) or walk-in patients (144 [IQR, 98–251] min; P < 0.001 for overall comparison and for each pairwise comparison vs. ALS). Door-to-antibiotic time did not differ significantly between BLS and walk-in patients (P = 0.93). Among sepsis alert patients brought to the ED from long-term care facilities, median door-to-antibiotic time was again shorter for ALS-treated patients (77 [IQR, 64–119]) versus BLS-treated patients (118 [IQR, 84–203]; P < 0.001).
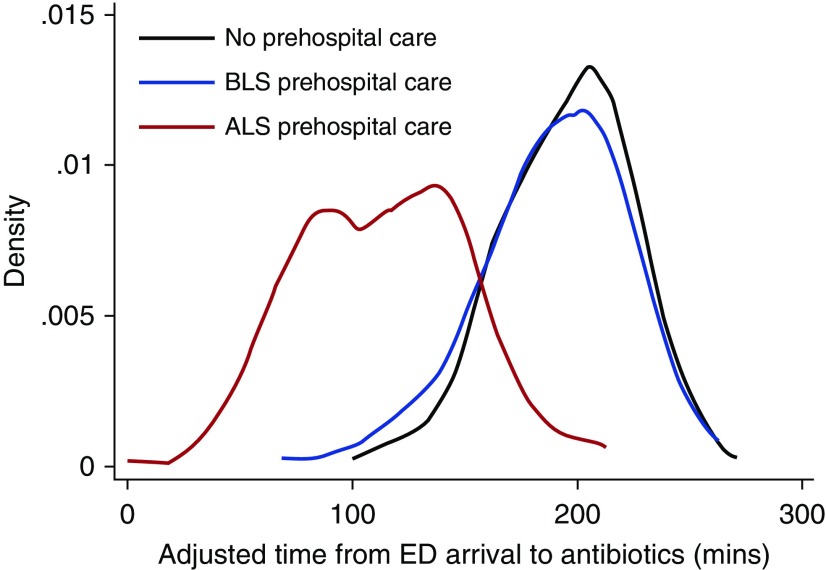
Compared with no prehospital care, adjusted door-to-antibiotic times were faster for community-dwelling patients with sepsis who received ALS (−43 min; 95% CI, −84 to −2 min; P = 0.033) but not BLS-only prehospital care (−4 min; 95% CI, −41 to +34 min; P = 0.97; Table 2). ALS-treated patients also received antibiotics faster (−39 min; 95% CI, −78 to −1; P = 0.046) than BLS-only patients (Figure 1). The sensitivity analyses using propensity score–based inverse probability of treatment weighting and propensity score adjustment yielded similar results (Tables E5 and E6). Among patients with sepsis residing in long-term care facilities, adjusted door-to-antibiotic times were shorter with ALS versus BLS-only prehospital care (−44 min; 95% CI, −85 to −4; P = 0.030).
Table 2.
Adjusted association of prehospital care with door-to-antibiotic time in community-dwelling patients with sepsis
| Comparison | Adjusted Difference in Door-to-Antibiotic Time* (95% CI) | P Value |
|---|---|---|
| Overall comparison | 0.021† | |
| Basic life support vs. no prehospital care, min | −3.9 (−41.7 to 33.9) | 0.98‡ |
| Advanced life support vs. no prehospital care, min | −43.1 (−93.5 to −2.8) | 0.033‡ |
| Advanced life support vs. basic life support, min | −39.2 (−77.8 to −0.6) | 0.046‡ |
Definition of abbreviation: CI = confidence interval.
Adjusted for nighttime emergency department arrival, Mortality in Emergency Department Sepsis score, source of infection, Hispanic ethnicity or nonwhite race, age, Charlson Comorbidity Index, first-available Glasgow Coma Score, first-available systolic blood pressure, and first-available respiratory rate.
Result of F test to assess overall significant effect of prehospital level of care on door-to-antibiotic time.
Confidence intervals and P values adjusted for multiple comparisons using Tukey multiple comparisons correction.
Figure 1.
Density plot of the distribution of adjusted door-to-antibiotic time by level of prehospital care. ALS = advanced life support; BLS = basic life support; ED = emergency department.
Community-dwelling patients with sepsis treated by ALS providers were more likely to receive antibiotics within 3 hours of ED arrival (91%) than both walk-in patients (62%; adjusted odds ratio [aOR], 3.11; 95% CI, 1.20–8.03; P = 0.015) and BLS-treated patients (56%; aOR, 4.51; 95% CI, 1.80–11.35; P < 0.001). BLS prehospital treatment, by contrast, was not significantly associated with the odds of antibiotic initiation within 3 hours compared with no prehospital care (aOR, 0.69; 95% CI, 0.33–1.44; P = 0.46).
Hospital mortality was 18.6% (n = 88) in the overall cohort, with lower mortality among patients who did not receive prehospital care (9.9% vs. 20.9%; P = 0.012). Unadjusted mortality was similar when comparing BLS-treated to ALS-treated patients (19.4% vs. 22.0%). After adjustment, however, prehospital care level was not associated with in-hospital mortality either in the overall cohort or when patients with sepsis residing in the community and at long-term care facilities were analyzed separately (Table 3).
Table 3.
Adjusted association of level of prehospital care with sepsis mortality
| Comparison | Overall Cohort* (n = 474) |
Patients with Sepsis Residing in the Community* (n = 361) |
Patients with Sepsis Residing in Long-Term Care Facilities (n = 113) |
|||
|---|---|---|---|---|---|---|
| Adjusted OR† (95% CI) | P Value | Adjusted OR† (95% CI) | P Value | Adjusted OR† (95% CI) | P Value | |
| Overall comparison | 0.21 | 0.50 | ||||
| Basic life support vs. no prehospital care | 1.23 (0.44–3.44) | 0.89 | 1.24 (0.41–3.77) | 0.90 | ||
| Advanced life support vs. no prehospital care | 0.69 (0.24–1.97) | 0.69 | 0.77 (0.25–2.36) | 0.85 | ||
| Advanced life support vs. basic life support | 0.56 (0.26–1.20) | 0.18 | 0.62 (0.24–1.60) | 0.47 | 0.50 (0.14–1.63) | 0.25 |
Definition of abbreviations: CI = confidence interval; OR = odds ratio.
Confidence intervals and P values adjusted for multiple comparisons using Tukey multiple comparisons correction.
Adjusted for Mortality in Emergency Department Sepsis score, age, Charlson Comorbidity Index, source of infection, and Sequential Organ Failure Assessment score.
In the exploratory analyses investigating potential mechanisms for the observed differences in door-to-antibiotic time, the likelihood of a triage diagnosis suggestive of sepsis or infection decreased as prehospital care level increased (P = 0.019; Table 4). Among ALS-treated patients residing in the community, door-to-antibiotic times did not differ if an intravenous catheter was placed before ED arrival (−12 min; 95% CI, −68 to +44 min; P = 0.82). Similarly, among the 86 ALS-treated community-dwelling patients intubated by the end of their ED stay, prehospital endotracheal intubation was not associated with faster adjusted door-to-antibiotic times than ED intubation (−1 min; 95% CI, −24 to +26; P = 0.94). By contrast, the median times from ED arrival to laboratory draw, blood culture collection, and activation of the sepsis alert were shorter in ALS-treated patients compared with BLS-treated and walk-in patients (Table 4). When patients within each level of prehospital care were compared based on whether a resident physician was involved in their ED care, resident involvement was associated with shorter door-to-antibiotic time for BLS-treated patients but not walk-in or ALS-treated patients (Table 4).
Table 4.
Care processes among community-dwelling patients with sepsis for each level of prehospital care
| No Ambulance Care (n = 101) | Basic Life Support (n = 111) | Advanced Life Support (n = 149) | P Value | |
|---|---|---|---|---|
| Sepsis/infection-related triage diagnosis* | 44 (44) | 61 (40) | 63 (29) | 0.019 |
| Door-to-sepsis alert activation, min* | 199 (138 to 299) | 177 (112 to 281) | 103 (60 to 156) | <0.001 |
| Door-to–blood culture, min* | 75 (39 to 155) | 77 (35 to 164) | 43 (19 to 91) | <0.001 |
| Door-to-lactate draw, min* | 65 (39 to 156) | 61 (35 to 134) | 22 (15 to 50) | <0.001 |
| Difference in door-to-antibiotic time if resident physician involved in care, min | −2 (−62 to 59) | −100 (−156 to −43)† | +8 (−32 to 49) | — |
Values reported as n (%) or median (interquartile range).
Missing values: triage diagnosis, 9; door-to-sepsis alert time, 1; door-to–blood culture time, 13; door-to-lactate time, 1.
P < 0.001.
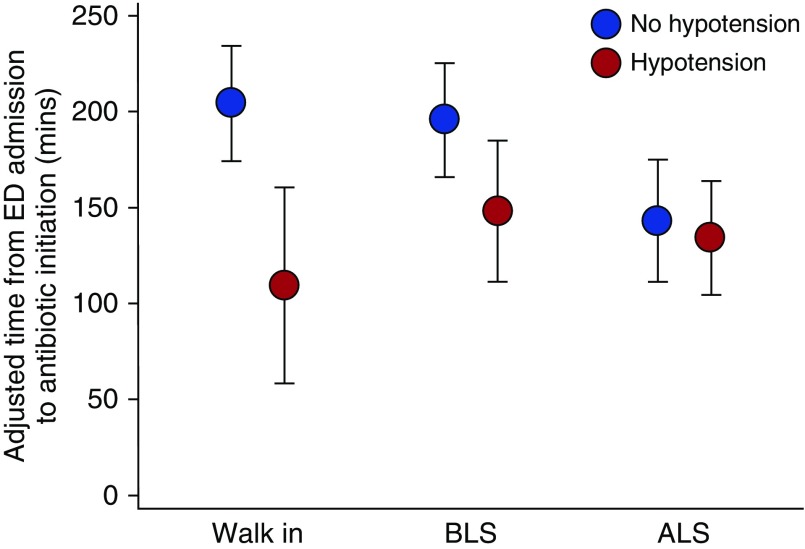
In a post hoc exploratory analysis to determine whether the association of antibiotic timing and prehospital care level differed depending on the presence of hypotension by ED arrival, the adjusted differences in door-to-antibiotic time for ambulance-treated versus walk-in patients without hypotension by ED arrival were similar in magnitude to the primary analysis but not statistically significant (ALS: −46 min; 95% CI, −110 to +19 min; BLS alone: −10 min; 95% CI, −64 to +43 min; Table E7). By contrast, if hypotension was present by ED arrival, ALS versus walk-in patient antibiotic times were similar (+4 min; 95% CI, −39 to +48 min), but BLS-only patients had a trend toward longer door-to-antibiotic times (+42 min; 95% CI, −3 to +88; Table E8). In the parallel analysis using an interaction term, door-to-antibiotic times for BLS-only and walk-in patients without hypotension were significantly longer than ALS-treated patients without hypotension and were also significantly longer than patients with the same prehospital level of care who were hypotensive (Figure 2 and Table E9). When hypotension was present, however, prehospital level of care was not significantly associated with door-to-antibiotic time.
Figure 2.
Adjusted door-to-antibiotic time from multivariable regression model incorporating an interaction term between level of prehospital care and the presence or absence of hypotension by emergency department (ED) arrival. Among subjects without hypotension by ED arrival, antibiotic times for advanced life support (ALS)-treated patients were significantly shorter (P < 0.05) versus basic life support (BLS)-only or walk-in patients after multiple comparisons adjustment.
Discussion
Among ED patients with fluid-refractory sepsis and septic shock, we found that patients receiving ALS but not BLS prehospital care received antibiotics more quickly than patients who did not receive prehospital care. Furthermore, ALS prehospital care was associated with significantly shorter door-to-antibiotic times compared with BLS-only prehospital care among patients with sepsis residing both in the community and at long-term care facilities. Although ED arrival diagnoses suggestive of sepsis or infection were actually less commonly recorded for ALS-treated patients, ALS prehospital care was associated with a faster diagnostic evaluation and sepsis alert activation.
Our findings confirm and extend the results of prior studies (15, 17, 18). EMS-treated patients received antibiotics 33 to 36 minutes earlier than walk-in patients in unadjusted analyses, but only one study found a significant association with prehospital care after adjustment for confounding. Furthermore, none of the three prior studies distinguished between levels of prehospital care or accounted for the close linkage demonstrated in our data between origin (home vs. long-term care facility), prehospital care, illness severity, and door-to-antibiotic time. By contrast, we found that the association of door-to-antibiotic time and prehospital care remained significant after thoroughly accounting for severity of illness, place of origin, comorbidities, and other potential confounders.
Our data can also help explain how ALS but not BLS-only care may influence ED door-to-antibiotic time. Prehospital intravenous placement and endotracheal intubation were not associated with shorter door-to-antibiotic time, suggesting faster antibiotics were not the result of ALS care reducing the workload for ED staff. The fact that ALS care was not associated with more ED triage diagnoses suggestive of sepsis or infection suggests that ALS caregivers did not accelerate antibiotic initiation by priming the ED team to make the diagnosis of sepsis either through prehospital diagnosis or through the simple fact of ALS involvement, although these results may be limited as a result of less-detailed triage documentation in patients presenting with higher apparent illness acuity. Rather, the fact that laboratory draws and sepsis alerts occurred faster after ALS but not BLS-only prehospital care suggests triage to ALS but not BLS care resulted in accelerated ED assessment and therefore accelerated therapeutic decisions for patients with sepsis. The analysis examining an interaction between prehospital care and the presence or absence of hypotension by ED arrival, which suggested that only patients without shock before or on ED triage received faster antibiotics when treated by ALS, may indicate that the benefits of ALS versus BLS care primarily accrue to patients with lower apparent illness acuity. A tiered EMS system in which ALS caregivers care for a relatively small proportion of all ambulance-treated patients may, for instance, facilitate ED measures such as patients’ immediate placement into a treatment bed, especially in cases where the signs of sepsis are subtle.
Recent large, well-designed observational studies support the benefit of early initiation of appropriate antibiotics for patients with sepsis. Among the four largest studies, the odds that patients with sepsis would die increased by 4% to 12% for each 1-hour delay in antibiotics (11–13, 30). International guidelines therefore recommend antibiotic initiation within 1 hour, a challenging goal (16). Our results point to potential opportunities to improve sepsis outcomes by 1) increasing the proportion of patients with sepsis who receive ALS rather than BLS prehospital care, 2) better integrating ED and prehospital sepsis care through prehospital notification and potentially prehospital treatment (31), and 3) redesigning early ED patient assessment to ensure brisk patient evaluation for patients at risk of sepsis regardless of the presence or level of prehospital care, perhaps using team-based ED immediate response protocols akin to those common for stroke, myocardial infarction, and trauma. Optimization of prehospital triage and recognition may require improved education for prehospital clinicians (32, 33) and public awareness campaigns for the general public (34). Integrating sepsis prediction tools using data routinely collected into the prehospital medical record could facilitate ALS triage, prehospital notification of the receiving ED, and expedited assessment in the ED, but tools available to date lack validation and likely have inadequate positive predictive value for routine use (35).
Our study’s major limitations relate to confounding by indication (36). Although the magnitude of the observed associations, robustness to comprehensive confounder adjustment, statistical adjustment for multiple comparisons, stability across methods of statistical analysis, and results of the stratified analysis are reassuring, we cannot completely exclude the possibility that the observed association reflects the impact of illness severity rather than prehospital care level on ED antibiotic initiation behaviors. More generally, residual confounding remains possible in our observational analysis. Instrumental variable methods can mitigate concerns about indication bias in research on prehospital care (37, 38) but would require combining data from multiple EDs or regions with varying rates of ALS prehospital care (not available for our single-center study) with granular prehospital and ED care process data (difficult to obtain in large multicenter datasets) and might in any case be difficult to adapt to our analysis including three rather than two levels of prehospital care.
Other limitations include the fact that, although this study was neither designed nor powered to assess this outcome, we did not observe an association between risk-adjusted mortality and the level of prehospital care. Our study was restricted to patients for whom the study hospital’s sepsis alert protocol was activated, so we cannot be certain whether prehospital care influences sepsis care similarly for patients who had less severe sepsis, in whom ED caregivers failed to recognize sepsis, or for whom the ED team failed to activate the ED sepsis protocol. We were not able to evaluate if ED treatment areas, care intervals preceding room assignment, or fluid resuscitation practices varied across prehospital care levels. It is possible that paramedics diagnosed sepsis but were unable to help ED providers reach the same diagnosis more quickly, but we believe this is unlikely in an ED where physicians and nurses enjoy a close working relationship with ALS providers and value their expertise. Triage diagnosis data were similarly detailed across levels of prehospital care, but it is possible that accelerated ED assessment prompted by ALS prehospital care may reduce the effort nurses spend recording triage diagnoses or complaints suggestive of infection and sepsis despite recognizing these factors clinically. Finally, although our findings are consistent with those from other university hospitals (15, 17, 18), it is possible that our results would not generalize to other ED types, to settings without a tiered system of prehospital care, or to EMS systems where a larger fraction of ambulance-transported patients receive ALS care.
Prehospital care by ALS but not BLS providers was associated with decreased door-to-antibiotic times for ED patients with sepsis who did not have hypotension present on ED arrival. Our findings suggest possible opportunities to optimize ED care processes to aid delivery of high-quality sepsis care in the ED.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Emily Wilson, M.S., for her input on this study’s analysis and interpretation and the anonymous reviewers for providing insightful input that helped us improve this manuscript.
Footnotes
Supported by the National Institutes of Health grants T32 HL007287, T32 DK007467, and UL1 TR000423. The funding source had no role in design, conduct, analysis, or reporting of this study.
Author Contributions: I.D.P., K.H.M., T.D.R., C.L.H., and S.M.B. conceived the study. I.D.P., K.H.M., K.E.R., B.A.M., and D.J.C. acquired the data. I.D.P. and A.M.B. analyzed the data. I.D.P., K.H.M., K.E.R., T.D.R., A.M.B., C.L.H., and S.M.B. interpreted the results. I.D.P. authored the manuscript. All authors were involved in manuscript revision and final approval.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Seymour CW, Rea TD, Kahn JM, Walkey AJ, Yealy DM, Angus DC. Severe sepsis in pre-hospital emergency care: analysis of incidence, care, and outcome. Am J Respir Crit Care Med. 2012;186:1264–1271. doi: 10.1164/rccm.201204-0713OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang HE, Jones AR, Donnelly JP. Revised national estimates of emergency department visits for sepsis in the United States. Crit Care Med. 2017;45:1443–1449. doi: 10.1097/CCM.0000000000002538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lagu T, Rothberg MB, Shieh M-S, Pekow PS, Steingrub JS, Lindenauer PK. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med. 2012;40:754–761. doi: 10.1097/CCM.0b013e318232db65. [DOI] [PubMed] [Google Scholar]
- 5.Kaukonen K-M, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA. 2014;311:1308–1316. doi: 10.1001/jama.2014.2637. [DOI] [PubMed] [Google Scholar]
- 6.Rhee C, Dantes R, Epstein L, Murphy DJ, Seymour CW, Iwashyna TJ, et al. CDC Prevention Epicenter Program. Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009–2014. JAMA. 2017;318:1241–1249. doi: 10.1001/jama.2017.13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 8.Angus DC, Barnato AE, Bell D, Bellomo R, Chong CR, Coats TJ, et al. A systematic review and meta-analysis of early goal-directed therapy for septic shock: the ARISE, ProCESS and ProMISe Investigators. Intensive Care Med. 2015;41:1549–1560. doi: 10.1007/s00134-015-3822-1. [DOI] [PubMed] [Google Scholar]
- 9.Kalil AC, Johnson DW, Lisco SJ, Sun J. Early goal-directed therapy for sepsis: a novel solution for discordant survival outcomes in clinical trials. Crit Care Med. 2017;45:607–614. doi: 10.1097/CCM.0000000000002235. [DOI] [PubMed] [Google Scholar]
- 10.Whiles BB, Deis AS, Simpson SQ. Increased time to initial antimicrobial administration is associated with progression to septic shock in severe sepsis patients. Crit Care Med. 2017;45:623–629. doi: 10.1097/CCM.0000000000002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 12.Liu VX, Fielding-Singh V, Greene JD, Baker JM, Iwashyna TJ, Bhattacharya J, et al. The timing of early antibiotics and hospital mortality in sepsis. Am J Respir Crit Care Med. 2017;196:856–863. doi: 10.1164/rccm.201609-1848OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seymour CW, Gesten F, Prescott HC, Friedrich ME, Iwashyna TJ, Phillips GS, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. 2017;376:2235–2244. doi: 10.1056/NEJMoa1703058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peltan ID, Mitchell KH, Rudd KE, Mann BA, Carlbom DJ, Hough CL, et al. Physician variation in time to antimicrobial treatment for septic patients presenting to the emergency department. Crit Care Med. 2017;45:1011–1018. doi: 10.1097/CCM.0000000000002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Femling J, Weiss S, Hauswald E, Tarby D. EMS patients and walk-in patients presenting with severe sepsis: differences in management and outcome. South Med J. 2014;107:751–756. doi: 10.14423/SMJ.0000000000000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levy MM, Evans LE, Rhodes A. The Surviving Sepsis Campaign Bundle: 2018 update. Intensive Care Med. 2018;44:925–928. doi: 10.1007/s00134-018-5085-0. [DOI] [PubMed] [Google Scholar]
- 17.Studnek JR, Artho MR, Garner CL, Jr, Jones AE. The impact of emergency medical services on the ED care of severe sepsis. Am J Emerg Med. 2012;30:51–56. doi: 10.1016/j.ajem.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Band RA, Gaieski DF, Hylton JH, Shofer FS, Goyal M, Meisel ZF. Arriving by emergency medical services improves time to treatment endpoints for patients with severe sepsis or septic shock. Acad Emerg Med. 2011;18:934–940. doi: 10.1111/j.1553-2712.2011.01145.x. [DOI] [PubMed] [Google Scholar]
- 19.Public Health-Seattle & King County Division of Emergency Medical Services. 2015 Annual Report to the King County Council. 2015 [accessed 2018 Mar 7]. Available from: https://www.kingcounty.gov/depts/health/emergency-medical-services/reports.
- 20.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi J-C, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 21.Vincent J-L, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 22.Shapiro NI, Wolfe RE, Moore RB, Smith E, Burdick E, Bates DW. Mortality in Emergency Department Sepsis (MEDS) score: a prospectively derived and validated clinical prediction rule. Crit Care Med. 2003;31:670–675. doi: 10.1097/01.CCM.0000054867.01688.D1. [DOI] [PubMed] [Google Scholar]
- 23.Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:762–774. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Iwashyna TJ, Odden A, Rohde J, Bonham C, Kuhn L, Malani P, et al. Identifying patients with severe sepsis using administrative claims: patient-level validation of the angus implementation of the international consensus conference definition of severe sepsis. Med Care. 2014;52:e39–e43. doi: 10.1097/MLR.0b013e318268ac86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kramer CY. Extension of multiple range tests to group means with unequal numbers of replications. Biometrics. 1956;12:307–310. [Google Scholar]
- 27.Robins JM, Hernán MÁ, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661–3679. doi: 10.1002/sim.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 30.Ferrer R, Martin-Loeches I, Phillips G, Osborn TM, Townsend S, Dellinger RP, et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med. 2014;42:1749–1755. doi: 10.1097/CCM.0000000000000330. [DOI] [PubMed] [Google Scholar]
- 31.Alam N, Oskam E, Stassen PM, Exter PV, van de Ven PM, Haak HR, et al. PHANTASi Trial Investigators and the ORCA (Onderzoeks Consortium Acute Geneeskunde) Research Consortium the Netherlands. Prehospital antibiotics in the ambulance for sepsis: a multicentre, open label, randomised trial. Lancet Respir Med. 2018;6:40–50. doi: 10.1016/S2213-2600(17)30469-1. [DOI] [PubMed] [Google Scholar]
- 32.Seymour CW, Carlbom D, Engelberg RA, Larsen J, Bulger EM, Copass MK, et al. Understanding of sepsis among emergency medical services: a survey study. J Emerg Med. 2012;42:666–677. doi: 10.1016/j.jemermed.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Green RS, Travers AH, Cain E, Campbell SG, Jensen JL, Petrie DA, et al. Paramedic recognition of sepsis in the prehospital setting: a prospective observational study. Emerg Med Int. 2016;2016:6717261. doi: 10.1155/2016/6717261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubulotta FM, Ramsay G, Parker MM, Dellinger RP, Levy MM, Poeze M Surviving Sepsis Campaign Steering Committee; European Society of Intensive Care Medicine; Society of Critical Care Medicine. An international survey: public awareness and perception of sepsis. Crit Care Med. 2009;37:167–170. doi: 10.1097/ccm.0b013e3181926883. [DOI] [PubMed] [Google Scholar]
- 35.Smyth MA, Brace-McDonnell SJ, Perkins GD. Identification of adults with sepsis in the prehospital environment: a systematic review. BMJ Open. 2016;6:e011218. doi: 10.1136/bmjopen-2016-011218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kyriacou DN, Lewis RJ. Confounding by indication in clinical research. JAMA. 2016;316:1818–1819. doi: 10.1001/jama.2016.16435. [DOI] [PubMed] [Google Scholar]
- 37.Iwashyna TJ, Kennedy EH. Instrumental variable analyses: exploiting natural randomness to understand causal mechanisms. Ann Am Thorac Soc. 2013;10:255–260. doi: 10.1513/AnnalsATS.201303-054FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanghavi P, Jena AB, Newhouse JP, Zaslavsky AM. Outcomes of basic versus advanced life support for out-of-hospital medical emergencies. Ann Intern Med. 2015;163:681–690. doi: 10.7326/M15-0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.