Abstract
Exocytosis of secreted mucins is the final step in their intracellular processing, resulting in their release into the airway lumen to interact with water and ions to form mucus. Mucins are secreted at a low baseline rate and a high stimulated rate, and both rates are regulated by second messengers acting on components of the exocytic machinery. The principal physiologic function of the low baseline rate is to support steady-state mucociliary clearance of inhaled particles and pathogens that enter the airways during normal breathing. Even in the setting of mucin hyperproduction, baseline secretion generally does not induce mucus occlusion. The principal physiologic function of the high stimulated rate of secretion from both submucosal glands and surface goblet cells in proximal airways appears to be to sweep away larger particles, whereas in distal airways it appears to act in concert with mucin hyperproduction to induce mucus occlusion to trap migrating helminths. Pathophysiologically, stimulated mucin secretion in the setting of mucin hyperproduction from allergic or other types of airway inflammation in the absence of helminth infection causes airflow obstruction and infection. Molecular components of the mucin exocytic machinery are increasingly being identified, and surprisingly, many components are not shared between baseline and stimulated machines. The physiologic significance of the presence of two distinct molecular machines is not yet known, such as whether these interact selectively with secretory granules of different sizes or contents. A full understanding of the mechanism and regulation of airway mucin secretion will provide further insight into pathophysiologic processes and may identify therapeutic strategies to alleviate obstructive airway diseases.
Keywords: mucin, mucus, secretion, exocytosis
Airway mucin production and secretion are often lumped together, as in the rubric “mucus hypersecretion.” However, mucin production and secretion are distinct phenomena underlaid by separate cell biological processes and regulated by different molecular signals. Mucin production and secretion can themselves each be broken into multiple processes. Other articles in the proceedings of this conference focus on the regulation of mucin gene expression, mucin glycoprotein synthesis, and mucin packaging into secretory granules. This article focuses on the mechanism and regulation of the exocytic release of mucins, including substeps of the assembly and activation of the exocytic machinery, as well as on the roles of different baseline and stimulated mucin secretory rates.
Pathophysiologic Significance of Mucin Secretion
It can be inferred that mucin secretion is required for lung health because deletion of the gene for mucin 5b (Muc5b) in mice results in a profound loss of mucociliary clearance, the accumulation of particulate debris within the lungs, and a high rate of death as a result of infection or airway obstruction (1). Because mucins must be secreted to form extracellular mucus, it seems obvious that mucin secretion is required for lung health. However, an airway epithelial mutation resulting in a complete failure of mucin secretion has not been reported. In fact, such a mutation may not be achievable, owing to cell-autonomous epithelial death resulting from the sharing of key molecular components between regulated and constitutive secretion (2, 3) or the complementation of secretory function in knockout mice by related proteins. Incomplete exocytic defects do not effectively test the necessity for mucin secretion, because increased intracellular mucin stores compensate at least partially for the defect in efficiency (2, 4) also see hydrostatic model on page S166. Despite the possibility that an absolute requirement for mucin secretion in lung health may not be demonstrable, the role of mucin secretion in multiple protective and pathophysiologic processes can be tested with mutant mice with partial defects in secretion.
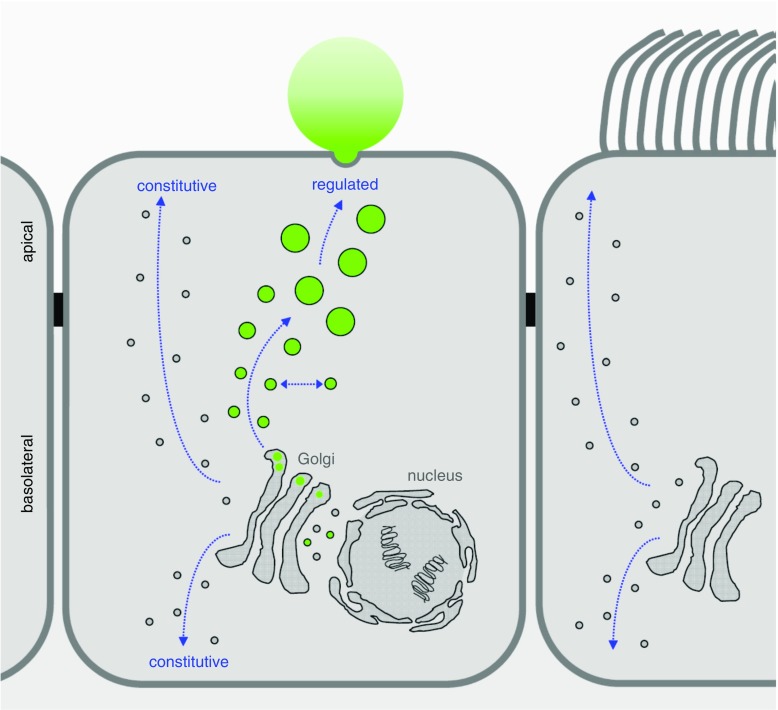
Mucin is secreted in the airways at a slow baseline rate and a high stimulated rate. Both the baseline and stimulated secretory mechanisms are regulated, as indicated by the fact that they both depend on extracellular ligands and second messengers (5) and that the protein Munc13-2 which is a target for second messengers participates in both mechanisms (6). Thus, the pathways for airway mucin secretion are distinct from the apical constitutive secretory pathway (Figure 1), even if some molecular components may be shared.
Figure 1.
Regulated versus constitutive secretory pathways of airway epithelial cells. A simplified model of airway surface epithelium is illustrated, showing a secretory (club) cell in the center and a ciliated cell on the right. Proteins destined for secretion, including the secreted mucins (green) that are produced exclusively in club cells and not in ciliated cells, are synthesized in rough endoplasmic reticulum surrounding the nucleus and extending into cytoplasm at the basal pole of the cell. Newly synthesized proteins are transported from the endoplasmic reticulum to the cis-Golgi in coated vesicles and become fully glycosylated as they progress through the Golgi stacks. In the trans-Golgi, mucins are likely segregated from other secreted proteins and packaged into large carrier vesicles as described for other large proteins such as collagen (37). New mucin granules then likely fuse homotypically (laterally) to generate large secretory granules, as occurs in other regulated secretory cells, but this has not yet been directly studied in club cells. Mucin secretory granules can then be released in response to extracellular signals and intracellular second messengers in a classic regulated secretory pathway (see text for details). Distinct apical and basolateral constitutive secretory pathways responsible for the replacement of lipids and proteins in the plasma membrane and secretion of proteins such as cytokines are likely to exist in both club cells and ciliated cells as they do in other polarized cell types, though the constitutive secretory pathways of airway epithelial cells have not yet been delineated at a molecular level. It is important to recognize that the baseline regulated pathway for mucin secretion is not synonymous with the apical constitutive secretory pathway and probably shares few or no molecular components (see text for details).
In surface epithelial cells of all intrapulmonary airways of mice and the distal bronchi and bronchioles of humans, the rate of baseline mucin secretion matches the rate of synthesis in naive (uninflamed) airways such that there is only minimal intracellular mucin (7, 8). In these airways, the small amount of intracellular mucin is not visible by histochemical stains for sugar side chains (Figure 2, left), though sensitive immunohistochemical stains demonstrate intracellular Muc5b/MUC5B throughout the surface epithelium from the trachea distally to the level of (but not including) terminal bronchioles (6, 7, 9, 10). Larger amounts of MUC5B are stored in histochemically visible granules of submucosal glands, and both MUC5B and MUC5AC are stored in visible granules of surface epithelial cells of naive proximal airways of humans (7, 11). With exposure to inflammatory stimuli (e.g., interleukin [IL]-13 for Muc5ac, IL-1β for Muc5b), mucin production in surface epithelial cells can increase 10- to 100-fold, but the efficiency of secretion is not increased, resulting in the accumulation of mucins intracellularly (Figure 2, center). This can be conceived of as a hydrostatic model, such as water in a bucket that contains a hole in the bottom but is continually replenished by inflow from a spigot (2). With increased inflow (i.e., increased mucin production), the water rises to a higher level but eventually stops rising (i.e., comes to a new steady state) because the increased hydrostatic pressure causes increased outflow (i.e., increased mucin secretion), even though the hole at the bottom remains the same size. An increase in the size of the hole at the bottom would correspond to an increase in exocytic efficiency (i.e., stimulated secretion) (see next paragraph). This model has been tested using varying levels of mucin production and mutant mice with loss of function of exocytic proteins (2, 4), but whether the higher steady state of baseline secretion that occurs when mucin production rises is due to the accumulation of more secretory granules or of granules of larger size, or both, is not yet known.
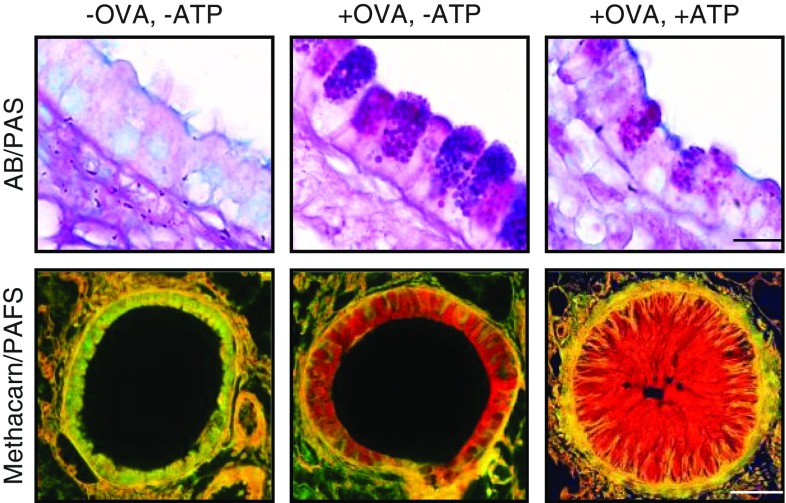
Figure 2.
Increased mucin production followed by stimulated secretion, resulting in airway occlusion. Mouse bronchi fixed with formalin, sectioned longitudinally, and stained with Alcian blue and periodic acid–Schiff stain (AB/PAS, top row) or fixed with methacarn to preserve mucus volume, sectioned transversely, and stained with periodic acid–fluorescent Schiff (PAFS, bottom row). Left: In the naive (uninflamed) state, there is minimal mucin staining inside epithelial cells or in the airway lumen. Center: Mucin hyperproduction (“mucous metaplasia”) was produced by allergic inflammation using an ovalbumin (+OVA) model. Intracellular mucin is visible within numerous purple granules in secretory cells (top) and extensive red staining (bottom) 3 days after OVA aerosol challenge. Right: Sudden mucin release from metaplastic cells was induced by an adenosine triphosphate aerosol (+ATP). Ten minutes later, mucus occludes the airway lumen. Not shown is ATP stimulation of naive airways (OVA−ATP+), because there is no observable difference from unstimulated naive airways (OVA−ATP−). Scale bars = 10 μm in the top row and 50 μm in the bottom row.
Upon stimulation by high levels of extracellular agonists such as ATP, acetylcholine, or histamine, the rate of airway mucin secretion can be increased thousands-fold. For example, by measuring the rate of disappearance of mucin granules from epithelium of perfused canine airways using high-speed video microscopy, Davis and colleagues measured a 1,760-fold increase in rate after the addition of ATP to the perfusate (12), and similar observations were made using the epithelium of healthy subjects and subjects with cystic fibrosis (13). It is possible that the rate of baseline mucin secretion is higher than what has been recorded by video microscopy if mucin-containing granules too small to be observed by this method also undergo exocytosis (5). However, measurement of secreted mucins in the supernatants of cultured cells and perfused tracheas confirms that the rate of stimulated secretion is far greater than the rate of baseline secretion over short time periods (4). Individual mucin granules have been observed to release their contents in approximately 0.1 second (12), and the released mucins absorb several hundred–fold their mass of water to generate mucus in approximately 1 second (14, 15), so the transition from intracellular mucin to extracellular mucus is very rapid. Although the initial burst rate of stimulated secretion exceeds the baseline rate 1,760-fold, it lasts only approximately 10 seconds and results in release of only about one-half of the approximately 30 granules that are released in response to high levels of ATP from naive canine airways. The remaining granules are released during a plateau phase that is approximately 38-fold higher than baseline rate and lasts about 2 minutes. In any case, the stimulation of secretion in an inflamed airway that has stored large amounts of intracellular mucin leads to the rapid release and swelling of the mucin, which can cause occlusion of airways (Figure 2, right).
The principal adaptive value of baseline mucin secretion is presumably to generate a mucus layer that is steadily propelled toward the pharynx by ciliary beating to remove unwanted particles and pathogens that enter the airways during breathing (7, 16). As noted above, absence of the major constitutively expressed airway mucin, Muc5b, results in a severe defect in mucociliary clearance of particles and pathogens in mice (1). Partial reductions in Muc5b result in commensurate reductions in particle clearance, and this may be a factor in the increased susceptibility to lung infection with aging (17). If baseline levels of mucin production and secretion protect against infection, then increased levels of mucin production that result in a proportional increase in baseline secretion might protect against infection. This was tested in a transgenic mouse overexpressing Muc5ac in its airways that demonstrated a thickened airway mucus layer and reduced susceptibility to influenza virus infection (18), and similar studies with a transgenic mouse overexpressing Muc5b in its airways are underway (1). Protection against lung infection is likely to have driven selection of an allele of the MUC5B enhancer in white persons that results in approximately 20-fold overexpression in the airways (19; see article in this issue by David Schwartz pp. S192–S197 [20]). The 12% allele frequency in white persons is similar to the 15% frequency of the sickle hemoglobin allele in areas of hyperendemic malaria, consistent with strong positive selection (21). However, constitutive overexpression of MUC5B comes at a price because it is a major risk factor for development of interstitial lung disease late in life, possibly owing to epithelial progenitor cell depletion resulting from proteostasis stress of producing large amounts of mucin (22).
The adaptive value of stimulated mucin secretion is a subject of active investigation. It is hypothesized that the stimulated secretion of fluid and mucins from submucosal glands plays an important role in clearing proximal airways of particles and microbial pathogens (23, 24), and it is likely that stimulated secretion from goblet cells in the surface epithelium of proximal airways performs a similar function. In the intestine, activation of MyD88-dependent innate immune signaling pathways induces rapid mucin secretion from surface epithelial cells to clear bacteria from crypts (25), but there is no evidence of comparable control of mucin secretion by MyD88 in the airways. However, the localized release of ATP or other secretagogues such as proteases in the airway may perform a similar function. In addition, it has been hypothesized that the rapid secretion of hyperproduced mucins in distal airways might trap helminths migrating through the lungs during infection (26). Type 2 inflammation plays a central role in helminth defense, and mucus is a critical component of this defense (27). The type 2 cytokine IL-13 can prime the airways for helminth trapping by increasing Muc5ac production and rendering smooth muscle hyperresponsive to constrictor stimuli. Upon contact with a nematode, the rapid release of mucins together with smooth muscle constriction around the luminal mucus could result in occlusion of the airway lumen and trapping of the helminth (Figure 2, right). There is now evidence for the trapping of helminths in the lungs of primed mice, which was presented at the conference. Presumably, this pathogen defense mechanism goes awry in allergic asthma, in which inhaled substances such as pollens, chitin in insect exoskeletons and fungi, and allergenic proteases falsely signal the presence of helminths, resulting in the release of IL-13 from leukocytes (26). The release of secretagogues such as histamine or ATP can then trigger rapid mucin release and bronchoconstriction, resulting in airway occlusion (Figure 2, right). Thus, an adaptive mechanism that probably evolved to selectively close a limited number of airways to protect against helminth migration becomes maladaptive when multiple airways close simultaneously in response to inhaled stimuli. This is reminiscent of the adaptive localized pulmonary arterial vasoconstriction that helps maintain ventilation–perfusion matching but becomes maladaptive with generalized vasoconstriction during hypoxic conditions such as at altitude.
Molecular Mechanism of Mucin Secretion
Exocytosis has been studied in eukaryotic cells from yeast to neurons and has been found to employ a highly conserved mechanism. Although the core machinery is shared between yeast cells and neurons, a key difference is that neurons add a regulatory machinery that responds to second messengers generated in response to external signals. In this regard, airway epithelial cells more closely resemble neurons than yeast cells.
The core exocytic machinery consists of a four-helix bundle, termed the SNARE complex, that assembles on a Munc18 scaffold to drive fusion between the secretory granule membrane and plasma membrane (5, 7, 28). SNARE proteins contain 60–amino acid coiled-coil domains (shown as black bars in Figure 3). Syntaxin is the central SNARE protein and contains a single SNARE domain together with three SNARE-like domains that form a “closed” four-helix bundle in the resting state (not illustrated). Syntaxin resides on the plasma membrane, and in the “open” state, its SNARE domain pairs with two SNARE domains in a SNAP family protein (synaptosomal-associated protein) that also resides on the plasma membrane, as well as with a SNARE domain in the granule membrane protein VAMP (vesicle-associated membrane protein). Interactions among the SNARE proteins both support the specificity of binding between the apposing membranes and supply the energy to drive membrane fusion. Syntaxin and VAMP proteins have transmembrane domains that anchor them, whereas SNAP proteins are attached to the plasma membrane by palmitoyl groups on a loop between the two SNARE domains.
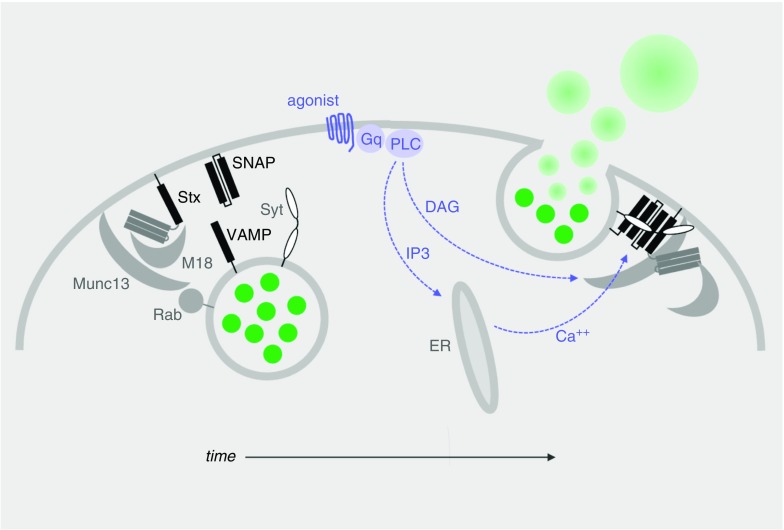
Figure 3.
Generic model of mucin granule exocytosis progressing over time as illustrated from left to right. This model is based on structural and functional information obtained from the study of neurons and other regulated exocytic cells, as well as functional information obtained from the study of airway secretory cells, as referenced in the text. Left: In the resting state, mucin granules (green) become tethered to the plasma membrane by Rab proteins interacting with effectors (not yet identified or illustrated) and the priming protein Munc13. Center: Agonist binding to heptahelical receptors (blue), such as those for adenosine triphosphate (P2Y2R), adenosine (A3R), and serine proteases (protease-activated receptors 1 and 2), leads to activation of a trimeric G protein (Gq), then phospholipase-Cβ (PLC), resulting in generation of the second messengers diacylglycerol (DAG) and inositol trisphosphate (IP3). DAG activates Munc13 to open syntaxin (Stx) in collaboration with the scaffolding protein Munc18 and promote Stx interactions with the other SNARE proteins (SNAP [synaptosomal-associated protein] and VAMP [vesicle-associated membrane protein], black bars). IP3 induces the release of Ca2+ from apical endoplasmic reticulum (ER) to activate the exocytic calcium sensor synaptotagmin (Syt), which promotes further coiling of the SNARE complex. (Note that in electrically excitable cells such as neurons, Ca2+ instead enters the cytoplasm through plasma membrane channels.) Right: Complete coiling of the SNARE complex induces fusion of the granule and plasma membranes, releasing mucins into the extracellular space, where they absorb water and swell.
Before interacting with the plasma membrane, secretory granules must be transported to its vicinity from the Golgi network by cytoskeletal interactions regulated in part by MARCKS (myristoylated alanine-rich C-kinase substrate) protein (29) (not illustrated). Then, Rab GTPases on the granule surface interact with tethering proteins on the plasma membrane that loosely bind the opposing membranes and initiate interactions with the cytoplasmic proteins Munc13 and Munc18 to open syntaxin (Figure 3, left). Initial interactions among the SNARE proteins result in tight docking of the vesicle to the plasma membrane (not illustrated). Full coiling of the SNARE complex and membrane fusion does not proceed until second messengers bind to regulatory components of the exocytic machinery, particularly calcium binding to the exocytic calcium sensor synaptotagmin (Syt) (Figure 3, center and right).
All of the description above refers to surface airway epithelial cells because these have been studied extensively in mouse models and human tissue culture systems. Mice have only a few submucosal glands in the proximal trachea, and these have not yet been studied for their exocytic mechanism, nor have cultured human submucosal gland mucous cells (11). However, it is likely that the mechanisms are highly conserved among apically secreting cells of respiratory epithelium, based on their close developmental relationship, and as we have confirmed for several exocytic proteins expressed both in surface airway secretory cells and in alveolar type 2 cells (2, 30). In contrast, neuroendocrine cells of the airway epithelium secrete basolaterally and express different exocytic protein isoforms (8).
Two Distinct Molecular Machines Mediate Baseline and Stimulated Mucin Secretion
The molecular mechanism of regulated secretion described above is generic, when in fact multiple paralogs exist for the SNARE proteins and their associated proteins, with specific expression and function in different secretory tissues. Remarkably, distinct molecular machines mediate baseline and stimulated mucin secretion in the airway. For example, Munc18b (30), VAMP8 (31), and Syt2 (32) have major roles in stimulated secretion but only minor roles in baseline secretion, and the Munc18, VAMP, and Syt proteins responsible for the major roles in baseline secretion are not yet known. In contrast to these protein families with selective roles, SNAP23 and Munc13-2 have major roles in both baseline and stimulated secretion (2, 6). Because syntaxins pair selectively with Munc18 isoforms, it is likely that different syntaxins mediate baseline and stimulated mucin secretion, but their identities are not yet known. Rab3D has been found to be associated with mucin granules (8, 32), but the function of Rab proteins in mucin secretion has not yet been analyzed.
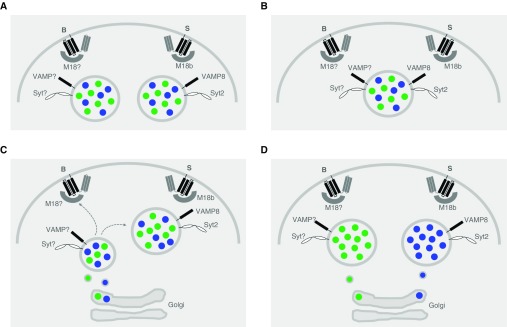
A key open question is whether the baseline and stimulated mucin secretory machines interact selectively with Muc5ac, Muc5b, and other secretory products. Four possible models of interaction between the plasma membrane machines and secretory vesicles are illustrated in Figure 4. The first two of these are considered highly unlikely because there would be no apparent value to the existence of separate plasma membrane machines in these models (Figures 4A and 4B). In the third model (Figure 4C), the baseline exocytic machine is hypothesized to interact selectively with smaller granules early in their maturation after leaving the trans-Golgi network. As the granules further mature and enlarge through lateral fusion, the baseline VAMP and Syt proteins would be removed and replaced by the stimulated machine components VAMP8 and Syt2. This model is reminiscent of the Rab conversion model that directs traffic from early to late endosomes (33). The possible utility of this model could be that baseline secretion of small granules would minimize the chance of airway occlusion during healthy conditions with its adverse consequences for airflow (34), whereas the stimulated secretion of large granules would maximize the chance of airway occlusion to trap migrating helminths during infection (Figure 2). In the fourth model (Figure 4D), the baseline exocytic machine is hypothesized to interact selectively with granules containing Muc5b, whereas the stimulated machine would interact selectively with granules containing Muc5ac. The possible utility of this model could be the selective release of Muc5b for baseline mucociliary clearance, consistent with its demonstrated functional role (1), and the selective explosive release of Muc5ac to trap helminths, again consistent with its demonstrated function (27). Muc5ac and Muc5b have been shown to occupy distinct domains in luminal mucus (10, 24, 35, 36), but it is not yet known whether they are packaged in distinct granules. Resolution of these open questions is an important goal of research in this area.
Figure 4.
Models illustrating the possible interactions of the two regulated exocytic machines of airway secretory cells with mucin granules. In each model, B designates the baseline exocytic machine and S designates the stimulated machine. Munc18 has been shortened to M18 to avoid clutter, and “?” is used to designate an unknown isoform of Munc18, VAMP (vesicle-associated membrane protein), and Syt (synaptotagmin) proteins. Blue dots within the secretory granules designate mucin 5ac (Muc5ac), and green dots designate Muc5b. (A) Two different populations of granules, each containing both Muc5ac and Muc5b, but differentially containing only one of the VAMP and Syt isoforms, are each capable of only interacting with either a baseline or a stimulated plasma membrane machine. (B) A single population of granules containing both Muc5ac and Muc5b also contains both VAMP and Syt isoforms, rendering the granules capable of interacting with both baseline and stimulated plasma membrane machines. (C) A single population of granules containing both Muc5ac and Muc5b derives from small post-Golgi vesicles that contain only the baseline VAMP and Syt isoforms that make them competent for interactions only with the baseline plasma membrane machine. These granules then enlarge by homotypic fusion and mature by losing the VAMP and Syt baseline isoforms and acquiring the VAMP and Syt stimulated isoforms, making them increasingly incompetent for fusion with the baseline plasma membrane machine but increasingly competent for the stimulated plasma membrane machine. (D) Two populations of granules containing only Muc5ac or Muc5b, and differentially containing only one of the VAMP and Syt isoforms, are each capable of only interacting with either a baseline or a stimulated plasma membrane machine.
The existence of distinct components of baseline and stimulated secretory machines suggests a strategy for therapeutic manipulation of mucin secretion. As described above, baseline mucin secretion appears to be primarily responsible for homeostatic clearance function in the airway, whereas stimulated mucin secretion appears to be primarily responsible for mucus occlusion. In societies not burdened by infection with helminths that migrate through the lungs, the advantage of suppressing stimulated mucin secretion might outweigh the disadvantages. Targeting the components of the stimulated exocytic machine that are distinct from the baseline machine is a logical strategy that is currently being tested genetically for Munc18b and VAMP8 (30, 31). Whether such a strategy would result in the selective release of mucins and other secretory products (Figure 4), as well as whether selective release would be advantageous or disadvantageous, needs to be tested.
Conclusions
Mucin secretion is a distinct process from mucin production, mediated by different proteins and regulated by different pathways. Production and secretion interact, such as in an increased rate of mucin production resulting in an increased rate of mucin secretion despite the absence of a change in secretion efficiency. Nonetheless, it is important to specifically elucidate the mechanisms and regulation of baseline and stimulated mucin secretion so that their contributions to airway health and pathophysiology can be understood and optimally managed.
Supplementary Material
Footnotes
Supported by grants HL129795, HL136057, and AI137319 from the National Institutes of Health and grant DICKEY18G0 from the Cystic Fibrosis Foundation.
Author Contributions: A.M.J. and B.F.D. wrote the article. All authors contributed to the conceptualization of the article.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Roy MG, Livraghi-Butrico A, Fletcher AA, McElwee MM, Evans SE, Boerner RM, et al. Muc5b is required for airway defence. Nature. 2014;505:412–416. doi: 10.1038/nature12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ren B, Azzegagh Z, Jaramillo AM, Zhu Y, Pardo-Saganta A, Bagirzadeh R, et al. SNAP23 is selectively expressed in airway secretory cells and mediates baseline and stimulated mucin secretion. Biosci Rep. 2015;35:e00220. doi: 10.1042/BSR20150004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaul S, Mittal SK, Feigenbaum L, Kruhlak MJ, Roche PA. Expression of the SNARE protein SNAP-23 is essential for cell survival. PLoS One. 2015;10:e0118311. doi: 10.1371/journal.pone.0118311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu Y, Abdullah LH, Doyle SP, Nguyen K, Ribeiro CM, Vasquez PA, et al. Baseline goblet cell mucin secretion in the airways exceeds stimulated secretion over extended time periods, and is sensitive to shear stress and intracellular mucin stores. PLoS One. 2015;10:e0127267. doi: 10.1371/journal.pone.0127267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis CW, Dickey BF. Regulated airway goblet cell mucin secretion. Annu Rev Physiol. 2008;70:487–512. doi: 10.1146/annurev.physiol.70.113006.100638. [DOI] [PubMed] [Google Scholar]
- 6.Zhu Y, Ehre C, Abdullah LH, Sheehan JK, Roy M, Evans CM, et al. Munc13-2−/− baseline secretion defect reveals source of oligomeric mucins in mouse airways. J Physiol. 2008;586:1977–1992. doi: 10.1113/jphysiol.2007.149310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med. 2010;363:2233–2247. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans CM, Williams OW, Tuvim MJ, Nigam R, Mixides GP, Blackburn MR, et al. Mucin is produced by Clara cells in the proximal airways of antigen-challenged mice. Am J Respir Cell Mol Biol. 2004;31:382–394. doi: 10.1165/rcmb.2004-0060OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seibold MA, Smith RW, Urbanek C, Groshong SD, Cosgrove GP, Brown KK, et al. The idiopathic pulmonary fibrosis honeycomb cyst contains a mucociliary pseudostratified epithelium. PLoS One. 2013;8:e58658. doi: 10.1371/journal.pone.0058658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonser LR, Zlock L, Finkbeiner W, Erle DJ. Epithelial tethering of MUC5AC-rich mucus impairs mucociliary transport in asthma. J Clin Invest. 2016;126:2367–2371. doi: 10.1172/JCI84910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Widdicombe JH, Wine JJ. Airway gland structure and function. Physiol Rev. 2015;95:1241–1319. doi: 10.1152/physrev.00039.2014. [DOI] [PubMed] [Google Scholar]
- 12.Davis CW, Dowell ML, Lethem M, Van Scott M. Goblet cell degranulation in isolated canine tracheal epithelium: response to exogenous ATP, ADP, and adenosine. Am J Physiol. 1992;262:C1313–C1323. doi: 10.1152/ajpcell.1992.262.5.C1313. [DOI] [PubMed] [Google Scholar]
- 13.Lethem MI, Dowell ML, Van Scott M, Yankaskas JR, Egan T, Boucher RC, et al. Nucleotide regulation of goblet cells in human airway epithelial explants: normal exocytosis in cystic fibrosis. Am J Respir Cell Mol Biol. 1993;9:315–322. doi: 10.1165/ajrcmb/9.3.315. [DOI] [PubMed] [Google Scholar]
- 14.Verdugo P. Supramolecular dynamics of mucus. Cold Spring Harb Perspect Med. 2012;2:a009597. doi: 10.1101/cshperspect.a009597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shumilov D, Popov A, Fudala R, Akopova I, Gryczynski I, Borejdo J, et al. Real-time imaging of exocytotic mucin release and swelling in Calu-3 cells using acridine orange. Methods. 2014;66:312–324. doi: 10.1016/j.ymeth.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dickey BF. Walking on solid ground: a gel-on-brush model of airway mucosal surfaces. Science. 2012;337:924–925. doi: 10.1126/science.1227091. [Published erratum appears in Science 2012;338:604.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grubb BR, Livraghi-Butrico A, Rogers TD, Yin W, Button B, Ostrowski LE. Reduced mucociliary clearance in old mice is associated with a decrease in Muc5b mucin. Am J Physiol Lung Cell Mol Physiol. 2016;310:L860–L867. doi: 10.1152/ajplung.00015.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ehre C, Worthington EN, Liesman RM, Grubb BR, Barbier D, O’Neal WK, et al. Overexpressing mouse model demonstrates the protective role of Muc5ac in the lungs. Proc Natl Acad Sci USA. 2012;109:16528–16533. doi: 10.1073/pnas.1206552109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med. 2011;364:1503–1512. doi: 10.1056/NEJMoa1013660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartz DA. Idiopathic pulmonary fibrosis is a genetic disease involving mucus and the peripheral airways. Ann Am Thorac Soc. 2018;15(Supp 3):S192–S197. doi: 10.1513/AnnalsATS.201802-144AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piel FB, Patil AP, Howes RE, Nyangiri OA, Gething PW, Williams TN, et al. Global distribution of the sickle cell gene and geographical confirmation of the malaria hypothesis. Nat Commun. 2010;1:104. doi: 10.1038/ncomms1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dickey BF, Whitsett JA. Understanding interstitial lung disease: it’s in the mucus. Am J Respir Cell Mol Biol. 2017;57:12–14. doi: 10.1165/rcmb.2017-0116ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ermund A, Meiss LN, Rodriguez-Pineiro AM, Bähr A, Nilsson HE, Trillo-Muyo S, et al. The normal trachea is cleaned by MUC5B mucin bundles from the submucosal glands coated with the MUC5AC mucin. Biochem Biophys Res Commun. 2017;492:331–337. doi: 10.1016/j.bbrc.2017.08.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ostedgaard LS, Moninger TO, McMenimen JD, Sawin NM, Parker CP, Thornell IM, et al. Gel-forming mucins form distinct morphologic structures in airways. Proc Natl Acad Sci USA. 2017;114:6842–6847. doi: 10.1073/pnas.1703228114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Birchenough GM, Nyström EE, Johansson ME, Hansson GC. A sentinel goblet cell guards the colonic crypt by triggering Nlrp6-dependent Muc2 secretion. Science. 2016;352:1535–1542. doi: 10.1126/science.aaf7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dickey BF. Exoskeletons and exhalation. N Engl J Med. 2007;357:2082–2084. doi: 10.1056/NEJMe0706634. [DOI] [PubMed] [Google Scholar]
- 27.Hasnain SZ, Evans CM, Roy M, Gallagher AL, Kindrachuk KN, Barron L, et al. Muc5ac: a critical component mediating the rejection of enteric nematodes. J Exp Med. 2011;208:893–900. doi: 10.1084/jem.20102057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoon TY, Munson M. SNARE complex assembly and disassembly. Curr Biol. 2018;28:R397–R401. doi: 10.1016/j.cub.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Adler KB, Tuvim MJ, Dickey BF. Regulated mucin secretion from airway epithelial cells. Front Endocrinol (Lausanne) 2013;4:129. doi: 10.3389/fendo.2013.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim K, Petrova YM, Scott BL, Nigam R, Agrawal A, Evans CM, et al. Munc18b is an essential gene in mice whose expression is limiting for secretion by airway epithelial and mast cells. Biochem J. 2012;446:383–394. doi: 10.1042/BJ20120057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones LC, Moussa L, Fulcher ML, Zhu Y, Hudson EJ, O’Neal WK, et al. VAMP8 is a vesicle SNARE that regulates mucin secretion in airway goblet cells. J Physiol. 2012;590:545–562. doi: 10.1113/jphysiol.2011.222091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tuvim MJ, Mospan AR, Burns KA, Chua M, Mohler PJ, Melicoff E, et al. Synaptotagmin 2 couples mucin granule exocytosis to Ca2+ signaling from endoplasmic reticulum. J Biol Chem. 2009;284:9781–9787. doi: 10.1074/jbc.M807849200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rink J, Ghigo E, Kalaidzidis Y, Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 2005;122:735–749. doi: 10.1016/j.cell.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 34.Evans CM, Raclawska DS, Ttofali F, Liptzin DR, Fletcher AA, Harper DN, et al. The polymeric mucin Muc5ac is required for allergic airway hyperreactivity. Nat Commun. 2015;6:6281. doi: 10.1038/ncomms7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lachowicz-Scroggins ME, Yuan S, Kerr SC, Dunican EM, Yu M, Carrington SD, et al. Abnormalities in MUC5AC and MUC5B protein in airway mucus in asthma. Am J Respir Crit Care Med. 2016;194:1296–1299. doi: 10.1164/rccm.201603-0526LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kesimer M, Ehre C, Burns KA, Davis CW, Sheehan JK, Pickles RJ. Molecular organization of the mucins and glycocalyx underlying mucus transport over mucosal surfaces of the airways. Mucosal Immunol. 2013;6:379–392. doi: 10.1038/mi.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pakdel M, von Blume J. Exploring new routes for secretory protein export from the trans-Golgi network. Mol Biol Cell. 2018;29:235–240. doi: 10.1091/mbc.E17-02-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.