Abstract
Asthma, chronic rhinosinusitis, and related incurable allergic afflictions of the upper and lower airways are medically important because of their association with the disabling symptom of dyspnea and, at least for asthma, the potential to cause fatal asphyxiation. Extensive research over the past two decades has uncovered both the physiological basis of airway obstruction in asthma and key governing molecular pathways. Exaggerated airway constriction in response to diverse provocative stimuli, termed airway hyperresponsiveness, is mediated through the cytokines interleukin 4 (IL-4) and IL-13 and the transcription factor signal transducer and activator of transcription 6 (STAT6). Overproduction of mucus has long been known to be an essential second component of airway obstruction and is also mediated in part through the IL-4/IL-13/STAT6 pathway. In this review, we discuss a second major signaling pathway which underlies mucus production that is mediated through proteinase-cleaved fibrinogen signaling through Toll-like receptor 4. Unexpectedly, our analysis of human sputum and paranasal sinus fluid indicates that in most cases of severe allergic airway disease, a unique type of airway fungal infection, termed airway mycosis, is pathogenically linked to these conditions. We further discuss how fungal and endogenous proteinases mediate the fibrinogenolysis that is essential to both Toll-like receptor 4 signaling and fibrin deposition that, together with mucus, contribute to airway obstruction.
Keywords: asthma, chronic rhinosinusitis, airway mycosis, fibrinogen, fungi
Asthma and related allergic disorders of the lower airways (e.g., allergic bronchopulmonary aspergillosis, chronic eosinophilic pneumonitis, Löffler’s syndrome, eosinophilic granulomatosis with polyangiitis) are medically significant conditions often due to their association with the uniquely disabling symptom of dyspnea that is in turn often a result of widespread but usually reversible airway obstruction. In its most severe form, airway obstruction in asthma can lead to lethal asphyxiation, with respiratory arrest resulting in death (1). Accompanying asthma in most cases is chronic rhinosinusitis (CRS), a chronic inflammatory disorder of the paranasal sinuses that occurs either with or without nasal polyposis (2, 3). CRS with nasal polyposis (CRSwNP) most frequently occurs with asthma, whereas CRS without nasal polyposis is often seen as an isolated disorder (4–6).
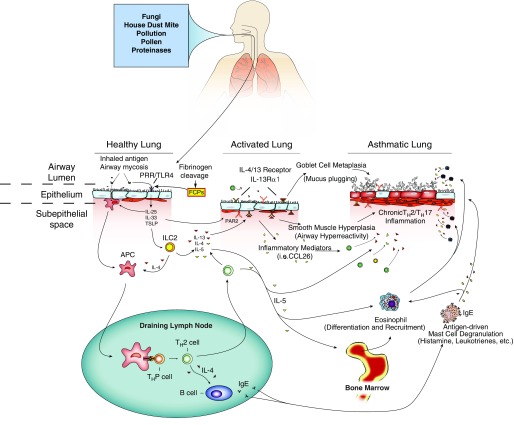
Like asthma, CRSwNP is a type 2 immune disorder marked by local mucosal inflammation that includes abundant eosinophils, T-helper type 2 (Th2) cells, type 2 innate lymphoid cells (ILC2), and immunoglobulin E (IgE)-secreting B cells accompanied by proallergenic cytokines such as interleukin 4 (IL-4), IL-5, IL-13, IL-25, IL-33, and thymic stromal lymphopoietin (6–10) (Figure 1). Asthma and CRSwNP are clearly strongly related conditions, because successful treatment of sinusitis often results in improvement in asthma (11–13).
Figure 1.
Environmental and immune factors mediating airway obstruction in asthma. Many inhaled substances, including mites, various pollutants, and proteases, can either induce or exacerbate asthma. These factors enter the airway lumen to trigger innate immune allergic responses by activating pattern recognition receptors (PRRs), including Toll-like receptor 4 (TLR4) and others expressed on airway epithelial cells. Epithelial cells then secrete cytokines such as interleukin 25 (IL-25), IL-33, and thymic stromal lymphopoietin (TSLP) into the subepithelial space to promote the development of innate lymphoid cell type 2 (ILC2) and dendritic cells that migrate to draining lymph nodes and to promote development of T helper type 2 (TH2) cells and immunoglobulin E (IgE)-secreting B cells. At the same time, inhaled proteinases promote the breakdown of fibrinogen into fibrinogen cleavage products (FCP) that signal through TLR4 to initiate airway hyperreactivity, eosinophilia, and mucus hypersecretion. Proteinases may also interact with protease-activated receptor 2 (PAR2) to either promote or attenuate asthma. With sustained exposure to allergens or establishment of airway mycosis, the low-grade innate allergic inflammation that characterizes healthy lungs converts to an activated lung phenotype characterized by more persistent and intense allergic inflammation. When combined with certain host susceptibility factors, this inflammation evolves further into the asthmatic lung that is dominated by the recruitment and effects of TH2 cells (via chemokine [C-C motif] ligand 26 [CCL26]) and TH17 cells, eosinophils (under the direction of IL-5), and mast cells (under the direction of IL-4 and IgE), which promote severe allergic inflammation, airway hyperreactivity, and mucus hypersecretion. APC = antigen-presenting cell. Image courtesy of J. Morgan Knight. Reprinted by permission from Reference 62.
The core medical importance of asthma lies in the airway obstruction that is the sine qua non of disease expression (14). In mild disease, airway obstruction can present as a minimally disturbing condition that perhaps impedes maximal athletic performance. In progressively more severe forms of disease, however, airway obstruction exerts progressively greater morbidity and can be lethal in the most severe cases by causing asphyxiation (1). Understanding the causes of airway obstruction in asthma, therefore, becomes paramount to designing optimally effective preventive and therapeutic strategies.
In addition to physical obstruction of the airways due to inflammatory exudate, including often-profound airway eosinophilia and airway wall narrowing resulting from edema, two major mechanisms have been identified that contribute to airway obstruction. The first of these is airway hyperresponsiveness (AHR), in which diverse environmental stimuli lead to exaggerated airway constriction (15). Ultimately a neurological response mediated by vagal parasympathetic efferent impulses, AHR nonetheless is strongly related to the type 2 inflammation occurring within the lungs (16, 17). The second major form of airway obstruction is physical obstruction due to material that is secreted or produced directly in the airways. This airway-blocking material has long been known to consist in part of mucins derived from the Muc5 gene family (18, 19). Parallel research, however, firmly documents that fibrin derived from fibrinogen is a second, and potentially far more important, material that accumulates in and blocks the asthmatic and sinusitic airways (20–22). For example, unlike the loose and gelatinous nature of even crosslinked mucins, fibrin, when crosslinked, becomes much stiffer. When extensively deposited in the majority of the airways, fibrin can produce life-threatening respiratory compromise that is very difficult to resolve (21, 23). In this review, we describe the signaling pathways that contribute to both AHR and airway obstruction and further discuss the surprising role that environmental fungi play in both of these processes.
The IL-4/IL-13 Signaling Pathway in Airway Obstruction
The first major evidence that airway obstruction in allergic asthma is mediated through a distinct signaling pathway came with the discovery that two closely related cytokines, IL-4 and IL-13, were the major mediators of AHR and airway goblet cell metaplasia in mice (24, 25). In part, these cytokines are related because they signal through the same receptor signaling chain, IL-4 receptor-α (IL-4Rα), and the same transcription factor, STAT6 (signal transducer and activator of transcription 6), and both IL-4Rα and STAT6 were also shown to be essential for antigen-induced AHR and goblet cell metaplasia (24, 26), although not in all instances (27). This signaling paradigm has proven to be both relevant and extremely important in asthma because blocking monoclonal antibodies against IL-4Rα have been shown to be effective in controlling symptoms of asthma in late-phase clinical trials (28, 29).
Initial studies indicated that Th2 cells were the dominant sources of IL-4 and IL-13, although eosinophils, basophils, and mast cells are also known to produce these cytokines. More recently, a second major type of IL-13–secreting cell, ILC2, has emerged. ILC2 are innate lymphocytes that rapidly secrete IL-5 and IL-13 at mucosal sites upon initial allergenic challenge (30). Under some experimental conditions, ILC2 can be the dominant source of airway Th2 cytokines controlling allergic airway disease (31, 32), but in others, they appear to be dispensable for full expression of allergic airway disease (33). Nonetheless, discovery of these cells only reinforces the need to neutralize STAT6-activating cytokines at all phases in the evolution of allergic airway disease (Figure 1).
Fibrinogen and Proteinases Are Linked to Airway Obstruction in Experimental Asthma
Although STAT6-activating cytokines are elicited at both innate (early) and adaptive (late) phases of the allergic airway disease response, the main importance of STAT6 is likely to be its requirement to support the maturation of long-lived memory Th2 cells that are capable of sustaining allergic inflammation for extended periods (34). We thus turned our attention to defining further the earliest signals, likely operating at an innate immune level, that were driving allergic airway disease. The first insight came with our discovery that environmental proteinases were essential to the expression of allergic airway disease in mice (35). Even as single molecules, proteinases derived from fungi (e.g., the proteinase derived from Aspergillus oryzae, PAO [35]) as well as from plants (e.g., papain [36]) are potent allergens that are capable of driving robust allergic airway disease and Th2 responses, even when the cognate antigens are not defined. All allergens that are now commonly given intranasally to induce allergic airway disease (e.g., house dust mite allergen, fungus-derived allergens) are potent sources of active proteinases (35, 37, 38).
The one exception to the “proteinase” rule until recently has been ovalbumin. Probably the most widely used experimental allergen, ovalbumin has no proteinase activity, and in fact has no biochemical activity at all. However, immunization with ovalbumin, usually in the context of aluminum-containing adjuvants such as alum, followed by intranasal challenge with adjuvant-free ovalbumin produces robust, if transient (39), allergic airway disease in many experimental systems. To address this paradox, we showed, using the broad-spectrum proteinase antagonist hirudin, that even with this proteinase-free allergen, proteinase activity is still required for robust ovalbumin-dependent allergic airway disease (40). In this unique context, the proteinase is endogenous, likely thrombin itself. Thus, an important insight gained from the detailed analysis of the biochemistry of allergens is that regardless of the allergen, proteinase activity, either exogenously or endogenously derived, is required to induce allergic inflammation and disease (Figure 1).
Cleaved Fibrinogen Signals through TLR4 to Coordinate Allergic Responses
Our studies thus far naturally raised questions about the endogenous substrates that allergenic proteinases presumably act on and the receptors recognizing these proteinase-modified ligands. The first key insight into these important issues came with the discovery that TLR4 was linked to asthma and was required for expression of allergic airway disease induced by diverse allergens (41, 42). We confirmed that TLR4 was indeed required for full expression of AHR, airway eosinophilia, and secretion of IL-5 and IL-13 in response to diverse allergens, including ovalbumin (40). Interestingly, though, we showed that TLR4 was not required for lung IL-4 production and IgE responses, but was needed for robust recruitment of lung ILC2 (40). These findings indicated that although TLR4 was not required for Th2 and IgE responses, it was required for optimal ILC2 responses, without which mice failed to generate a complete allergic airway disease phenotype in response to allergen challenge (Figure 1).
Before these findings by diverse laboratories, TLR4 had been firmly linked to the generation of strong Th1 and Th17 responses after challenge with bacterial lipopolysaccharide (LPS) (43), although under some conditions even LPS can induce allergic responses (41). Linking TLR4 to allergic responses thus suggested that this pattern recognition receptor signaled in a way fundamentally distinct from the well-understood LPS signaling pathway, but few examples of variable receptor signaling existed to suggest possible mechanisms. We reasoned, however, that biased agonism could be occurring, a phenomenon in which the same receptor signals in substantially different ways to effect distinct biological outcomes based on different ligands (44). Biased agonism is perhaps best exemplified by the β2-adrenergic receptor, an allergic disease–relevant G protein–coupled receptor that “normally” activates a cAMP-generating pathway in response to epinephrine and related ligands, but in addition engages a β-arrestin 2–dependent signaling pathway in response to structurally distinct activating ligands (45, 46).
To determine if biased agonism might be occurring through TLR4, we developed an in vitro system to test relevant TLR4-dependent outcomes, ideally pertinent to allergic airway disease. We discovered that bone marrow–derived macrophages (and subsequently airway epithelial cells) respond to proteinases added to the tissue culture media by converting into potent fungus-killing or fungus-arresting cells (40). That is, addition of spores of Aspergillus niger to cultures of naive macrophages resulted in rapid fungal growth and the death of the macrophages, but pretreatment of the macrophages with fungal proteinase resulted in the complete arrest of fungal growth. At first glance, this fungistasis assay might not seem to be highly relevant to asthma, but we previously showed that eosinophils, Th2 cells, IL-13, and IL-5 are either directly fungicidal or contribute in some way to the control of fungal growth in vitro or in vivo (47, 48). This suggests that allergic airway disease, which includes proteinase-activated macrophages, is simply a complex inflammatory and physiologic response that has evolved to control infections caused by proteinase-secreting organisms such as fungi but also helminths.
We used our in vitro fungistasis assay to determine the likely ligands the proteinases were acting on that, after proteolysis, became competent to engage TLR4. On the basis of extensive work conducted by Jules Hoffman’s laboratory that determined the ligand for Toll in Drosophila melanogaster, we reasoned that a novel ligand for TLR4 was likely fibrinogen. Hoffman and colleagues had previously shown that prospaetzle, a protein found in D. melanogaster hemolymph, signaled through Toll to elicit antifungal responses (49). Other investigators had pointed out that prospaetzle, termed coagulogen in horseshoe crabs, was the functional, albeit not structural, equivalent of mammalian fibrinogen (50). We confirmed that fibrinogen alone could induce both fungistatic responses from macrophages and in part elicit allergic airway disease when given to mice intranasally without cognate antigen or proteinases, but these responses absolutely required the presence of TLR4 (40).
In subsequent studies, we showed that the same fungistatic response could be elicited simply by adding to cultured macrophages proteinase activity–free fibrinogen fragments, termed fibrinogen cleavage products (FCP), extracted from the clots that result when thrombin is added to highly purified fibrinogen (40). Again, macrophages derived from mice with an inactivated Tlr4 gene could not be induced to arrest fungal growth in response to FCPs. We further compared other activating stimuli in addition to FCPs to determine their ability to induce macrophage fungistatic responses. Interferon-γ (IFN-γ) induced degrees of fungistatic activity similar to those of FCPs, but macrophages challenged with IL-4 (inducing alternatively activated macrophages; M2) and LPS (inducing classically activated macrophages; M1) failed entirely to induce this response. IFN-γ–dependent fungistasis did not require TLR4, indicating that this response was elicited through a separate, IFN-γ–receptor-dependent pathway. Finally, we showed that FCPs, again without cognate antigen or exogenous proteinases, were sufficient to induce a partial allergic airway disease phenotype marked by airway eosinophilia and mucus gene upregulation (40).
Together, these findings showed that cleaved fibrinogen signaling through TLR4 was important to the induction of both macrophage fungistatic responses and allergic airway disease. Moreover, they demonstrate that macrophages respond entirely differently to two distinct TLR4 ligands, LPS and FCPs, with only the latter inducing fungistasis. Although these studies do not shed light on the downstream signaling events that regulate these distinct biological outcomes, they nonetheless suggest that TLR4 signals in a biased way to integrate diverse microbial signals to direct immune responses tailored to the correct type of infectious challenge (e.g., bacterial or fungal).
Airway Mycosis Is Linked to Allergic Disease of the Upper and Lower Airways
The ability of proteinases to induce antifungal allergic responses and the well-known association of proteinase activity with fungi raised the intriguing possibility that fungal infection of some type might underlie serious allergic diseases such as severe asthma and CRSwNP. To determine if this association might be genuine, we first examined unstained sputum or tracheal aspirate samples derived from patients with long-standing moderate to severe asthma and discovered that most of these samples contained hyphal and sporelike structures (51). Given these findings, we would have expected that sputum fungal cultures would readily yield fungal growth, but sputum samples cultured under standard conditions are notoriously unreliable, often failing to yield fungi (52).
We reasoned that numerous elements present in asthmatic sputum, including eosinophils, proteinase-activated macrophages, antimicrobial peptides, and mucus, are sufficiently fungistatic or fungicidal to prevent fungal growth under standard culture conditions in which no attempt is made to separate fungal from antifungal elements. We therefore developed a modified sputum culture method that includes a mucolysis step followed by centrifugation that allows us to isolate solid fungal elements (spores, hyphae) from most other sputum elements. After culture under these conditions on antibiotic-impregnated Sabouraud’s media, we succeeded in isolating fungi in greater quantities and diversity from five asthmatic sputum samples than in the reference mycology laboratory (51). We then used these results to guide antifungal therapy in these patients, all of whom were subsequently weaned off mechanical ventilatory support.
We then applied this new culture technique in a controlled context involving patients seeking endoscopic sinus surgery for advanced CRS. After opening the medial wall of the maxillary sinuses, lavage material was cultured using our improved technique. We found that in approximately 75% of patients with CRSwNP with and without asthma, these cultures yielded fungi, whereas cultures were positive for fungi in only 18% of those with no allergic disease (53).
To further distinguish nonconsequential fungal colonization from actual infection, we further determined in these same patients whether they were immunologically sensitized to fungi. Although antifungal IgE responses proved to be relatively insensitive, we found that fungus-specific IL-4 responses from peripheral blood T cells, representing memory recall Th2 responses against any one of a panel of nine fungal allergens, were strongly linked to patients with CRSwNP with and without asthma, occurring in 73% of this subset. In contrast, no patient without allergic disease reacted positively in this new immunological assay. Moreover, if an allergen was derived from a fungus that was actually recovered from a specific patient with CRSwNP and used to restimulate their T cells, positive reactions were found in a remarkable 89% (53). These data support the concept that fungi, which are very commonly isolated from respiratory tract secretions of patients with CRSwNP with and without asthma, are not mere colonizers, but rather are participating in a novel type of fungal airway infection that we term airway mycosis (Figure 1).
Asthma Is Responsive to Antifungal Therapy
To gain further insight into the role of airway mycosis in asthma, we challenged wild-type mice with the spores of many different fungi isolated from house dust and the yeast Candida albicans. These extensive studies demonstrated that all fungi tested are capable of eliciting allergic airway disease, but that some species (e.g., Aspergillus spp., Penicillium spp.) are more potent than others (47). Perhaps surprisingly, C. albicans was just as potent as Aspergillus niger in inducing allergic airway disease (51).
We further confirmed that the vast majority (86%) of the sputa collected from patients with asthma seen at the Michael E. DeBakey VA Medical Center allergy clinic had positive results for fungal growth (54). Over the past five years, we have increasingly emphasized the use of antifungal antibiotics in sputum fungal culture–positive patients with asthma who are otherwise refractory to standard therapy. Our retrospective analysis of these patients indicates that the vast majority respond favorably to antifungal therapy, variously improving lung function (peak flows) and general well-being and reducing use of bronchodilators and inhaled corticosteroids (54). We further observed significant declines in serum IgE levels and blood eosinophils in patients treated with antifungals, especially azole-based antifungals such as voriconazole. These findings are in keeping with some (55), but not all (56), controlled studies of antifungals in asthma.
Conclusions and Future Directions
Recent studies done by diverse groups are painting a more coherent view of the mucoobstructive allergic diseases asthma and CRSwNP. Previously viewed as a collection of immunologically related disorders with distinct etiologies, newer studies now firmly implicate fungi, and specifically airway mycosis, as being the root cause of these disorders in many individuals. Fungi identified from airway specimens are usually interpreted by clinicians as “colonizers” or “commensals,” especially when specific allergic testing fails to reveal hypersensitivity (IgE-specific reactivity) to any fungus. We have shown, however, that the majority (90%) of patients with asthma and CRS exhibit specific Th2 recall responses to fungi directly cultured from their sinus mucus (53). This observation supports an etiologic, and not commensal, role for fungi in asthma/CRSwNP and also suggests a novel diagnostic strategy based on T-cell recall responses to fungal antigens.
A key finding from these investigations is the diverse importance of fibrinogen to allergic airway disease. Fibrinogen can deposit as fibrin to contribute, together with mucus, to airway obstruction, and FCPs can simultaneously signal through TLR4 to coordinate allergic inflammation, mucus gene induction, and antifungal responses. These findings suggest that interrupting fibrinogenolytic pathways, perhaps through the use of heparin-like products that have previously been shown to be beneficial in asthma (57–59), might be therapeutically useful, perhaps combined with antifungal therapy, in multiple allergic airway diseases. For the most severe cases of status asthmaticus marked by plastic bronchitis with potentially lethal accumulations of fibrin in the airways, future research should also focus on the potential utility of fibrinolytic therapy such as recombinant tissue plasminogen activator (60), particularly in relation to recently approved therapies that target IgE and IL-5.
Many additional questions remain unanswered, however, and should be addressed as part of future studies. It remains unclear how FCPs signal through TLR4 to elicit allergic airway disease and macrophage antifungal immunity. Whether these represent the end result of the same signal or distinct TLR4-derived signals remains an open question. We also do not understand how FCPs engage TLR4: Is this a direct interaction or an indirect process involving intermediate molecules that bind FCPs directly? We are finalizing results of studies designed to address these fundamental issues and hope to report them within the next year.
Clinically, the most important issue suggested by these studies is the precise role that antifungal therapy plays in the management of allergic asthma and CRSwNP. Although we found that airway mycosis is extremely common in our patients seen at a single site (54), it is not clear how widely applicable these findings are. Other than sputum culture using a technique that no commercial laboratories are yet willing to adopt, it will be important to establish acceptable standards for diagnosing airway mycosis as a prelude to beginning potentially toxic antifungal therapy.
Two small prospective clinical trials have assessed the efficacy of oral itraconazole (Fungal Asthma Sensitization Trial [FAST]) or fluconazole in patients with moderate to severe asthma with proven fungal sensitization and found benefit (55, 61). Significant improvements in both objective measures of lung function and subjective symptom and quality-of-life scores were recorded in patients receiving oral itraconazole relative to matched control patients receiving placebo (55).
However, the effectiveness of voriconazole was also assessed for the treatment of Aspergillus fumigatus–associated asthma in the EVITA3 (effectiveness of voriconazole in the treatment of Aspergillus fumigatus–associated asthma) trial over 12 weeks, and this study did not detect a beneficial effect in respiratory symptoms or number of exacerbations (56). However, EVITA3 was underpowered for its primary endpoints and included active and heavy former smokers, which likely confounded data interpretation. Furthermore, none of these studies examined the lung fungal burden in their study subjects, leaving open to speculation the possibility that the beneficial effect of itraconazole might have been secondary to its ability to potentiate corticosteroid antiinflammatory effect, not in reducing fungal burden (56).
Our own retrospective studies indicate that the longer antifungals are continued in asthma, the better patients respond (54). In addition to resolving or markedly reducing eosinophilia and serum IgE levels, patients with asthma treated with antifungals typically stop producing sputum, a clear indication that fungal burdens are lessened, suggesting that the primary effect of the antifungals in asthma is to reduce or eliminate the fundamental cause of disease—fungi—and not to promote the antiinflammatory effects of steroids (54). Nonetheless, firm guidelines on the use of antifungals in asthma and interpretation of their beneficial effects should be based on the results of optimized controlled trials.
Assuming that antifungals are eventually proven to be useful in the primary therapy of asthma and CRS, additional issues needing resolution will include deciding which antifungals are optimal for use in asthma versus CRSwNP, the role that surgery plays in resolving CRSwNP, the duration of an initial course of antifungal therapy, and optimal management of refractory and relapsed disease. Answers to these questions will come at considerable cost and take many years, but they promise to radically transform and improve the management of diverse mucoobstructive airway diseases.
Supplementary Material
Footnotes
This work was supported by National Institutes of Health grants R41AI25007, R01 HL117181, and AI135803; Veterans Administration Office of Research and Development grants I01BX002221 and I01 CX001673; and the Biology of Inflammation Center, Baylor College of Medicine. The views expressed in this article do not communicate an official position of Baylor College of Medicine or the National Institutes of Health.
Author Contributions: E.L., C.T.L., H.-Y.T., J.M.K., Z.M., A.U.L., A.R., F.K., and D.B.C. contributed equally to the writing and intellectual input of the manuscript. J.M.K. prepared Figure 1.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Molfino NA, Nannini LJ, Martelli AN, Slutsky AS. Respiratory arrest in near-fatal asthma. N Engl J Med. 1991;324:285–288. doi: 10.1056/NEJM199101313240502. [DOI] [PubMed] [Google Scholar]
- 2.Bresciani M, Paradis L, Des Roches A, Vernhet H, Vachier I, Godard P, et al. Rhinosinusitis in severe asthma. J Allergy Clin Immunol. 2001;107:73–80. doi: 10.1067/mai.2001.111593. [DOI] [PubMed] [Google Scholar]
- 3.Langdon C, Mullol J. Nasal polyps in patients with asthma: prevalence, impact, and management challenges. J Asthma Allergy. 2016;9:45–53. doi: 10.2147/JAA.S86251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachert C, Claeys SE, Tomassen P, van Zele T, Zhang N. Rhinosinusitis and asthma: a link for asthma severity. Curr Allergy Asthma Rep. 2010;10:194–201. doi: 10.1007/s11882-010-0096-0. [DOI] [PubMed] [Google Scholar]
- 5.Pakdaman MN, Luong A. The links between chronic rhinosinusitis and asthma. Curr Opin Otolaryngol Head Neck Surg. 2011;19:218–223. doi: 10.1097/MOO.0b013e32834500a8. [DOI] [PubMed] [Google Scholar]
- 6.Porter PC, Ongeri V, Luong A, Kheradmand F, Corry DB. Seeking common pathophysiology in asthma, atopy and sinusitis. Trends Immunol. 2011;32:43–49. doi: 10.1016/j.it.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luong A, Marple BF. Allergic fungal rhinosinusitis. Curr Allergy Asthma Rep. 2004;4:465–470. doi: 10.1007/s11882-004-0013-5. [DOI] [PubMed] [Google Scholar]
- 8.Luong A, Davis LS, Marple BF. Peripheral blood mononuclear cells from allergic fungal rhinosinusitis adults express a Th2 cytokine response to fungal antigens. Am J Rhinol Allergy. 2009;23:281–287. doi: 10.2500/ajra.2009.23.3311. [DOI] [PubMed] [Google Scholar]
- 9.Pakdaman MN, Corry DB, Luong A. Fungi linking the pathophysiology of chronic rhinosinusitis with nasal polyps and allergic asthma. Immunol Invest. 2011;40:767–785. doi: 10.3109/08820139.2011.596876. [DOI] [PubMed] [Google Scholar]
- 10.Shaw JL, Fakhri S, Citardi MJ, Porter PC, Corry DB, Kheradmand F, et al. IL-33-responsive innate lymphoid cells are an important source of IL-13 in chronic rhinosinusitis with nasal polyps. Am J Respir Crit Care Med. 2013;188:432–439. doi: 10.1164/rccm.201212-2227OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikeda K, Tanno N, Tamura G, Suzuki H, Oshima T, Shimomura A, et al. Endoscopic sinus surgery improves pulmonary function in patients with asthma associated with chronic sinusitis. Ann Otol Rhinol Laryngol. 1999;108:355–359. doi: 10.1177/000348949910800407. [DOI] [PubMed] [Google Scholar]
- 12.Tosca MA, Cosentino C, Pallestrini E, Caligo G, Milanese M, Ciprandi G. Improvement of clinical and immunopathologic parameters in asthmatic children treated for concomitant chronic rhinosinusitis. Ann Allergy Asthma Immunol. 2003;91:71–78. doi: 10.1016/s1081-1206(10)62062-5. [DOI] [PubMed] [Google Scholar]
- 13.Tsao CH, Chen LC, Yeh KW, Huang JL. Concomitant chronic sinusitis treatment in children with mild asthma: the effect on bronchial hyperresponsiveness. Chest. 2003;123:757–764. doi: 10.1378/chest.123.3.757. [DOI] [PubMed] [Google Scholar]
- 14.Lambrecht BN, Hammad H. The airway epithelium in asthma. Nat Med. 2012;18:684–692. doi: 10.1038/nm.2737. [DOI] [PubMed] [Google Scholar]
- 15.Boushey HA, Holtzman MJ, Sheller JR, Nadel JA. Bronchial hyperreactivity. Am Rev Respir Dis. 1980;121:389–413. doi: 10.1164/arrd.1980.121.2.389. [DOI] [PubMed] [Google Scholar]
- 16.Corry DB, Folkesson HG, Warnock ML, Erle DJ, Matthay MA, Wiener-Kronish JP, et al. Interleukin 4, but not interleukin 5 or eosinophils, is required in a murine model of acute airway hyperreactivity. J Exp Med. 1996;183:109–117. doi: 10.1084/jem.183.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corry DB, Grünig G, Hadeiba H, Kurup VP, Warnock ML, Sheppard D, et al. Requirements for allergen-induced airway hyperreactivity in T and B cell-deficient mice. Mol Med. 1998;4:344–355. [PMC free article] [PubMed] [Google Scholar]
- 18.Ordoñez CL, Khashayar R, Wong HH, Ferrando R, Wu R, Hyde DM, et al. Mild and moderate asthma is associated with airway goblet cell hyperplasia and abnormalities in mucin gene expression. Am J Respir Crit Care Med. 2001;163:517–523. doi: 10.1164/ajrccm.163.2.2004039. [DOI] [PubMed] [Google Scholar]
- 19.Fahy JV. Goblet cell and mucin gene abnormalities in asthma. Chest. 2002;122(6) Suppl:320S–326S. doi: 10.1378/chest.122.6_suppl.320s. [DOI] [PubMed] [Google Scholar]
- 20.Fahy JV, Liu J, Wong H, Boushey HA. Cellular and biochemical analysis of induced sputum from asthmatic and from healthy subjects. Am Rev Respir Dis. 1993;147:1126–1131. doi: 10.1164/ajrccm/147.5.1126. [DOI] [PubMed] [Google Scholar]
- 21.Brogan TV, Finn LS, Pyskaty DJ, Jr, Redding GJ, Ricker D, Inglis A, et al. Plastic bronchitis in children: a case series and review of the medical literature. Pediatr Pulmonol. 2002;34:482–487. doi: 10.1002/ppul.10179. [DOI] [PubMed] [Google Scholar]
- 22.Kim DY, Cho SH, Takabayashi T, Schleimer RP. Chronic rhinosinusitis and the coagulation system. Allergy Asthma Immunol Res. 2015;7:421–430. doi: 10.4168/aair.2015.7.5.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Do TB, Chu JM, Berdjis F, Anas NG. Fontan patient with plastic bronchitis treated successfully using aerosolized tissue plasminogen activator: a case report and review of the literature. Pediatr Cardiol. 2009;30:352–355. doi: 10.1007/s00246-008-9312-2. [DOI] [PubMed] [Google Scholar]
- 24.Grünig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 26.Kuperman D, Schofield B, Wills-Karp M, Grusby MJ. Signal transducer and activator of transcription factor 6 (Stat6)-deficient mice are protected from antigen-induced airway hyperresponsiveness and mucus production. J Exp Med. 1998;187:939–948. doi: 10.1084/jem.187.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blease K, Schuh JM, Jakubzick C, Lukacs NW, Kunkel SL, Joshi BH, et al. Stat6-deficient mice develop airway hyperresponsiveness and peribronchial fibrosis during chronic fungal asthma. Am J Pathol. 2002;160:481–490. doi: 10.1016/S0002-9440(10)64867-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wenzel S, Castro M, Corren J, Maspero J, Wang L, Zhang B, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting β2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet. 2016;388:31–44. doi: 10.1016/S0140-6736(16)30307-5. [DOI] [PubMed] [Google Scholar]
- 29.Wenzel S, Ford L, Pearlman D, Spector S, Sher L, Skobieranda F, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368:2455–2466. doi: 10.1056/NEJMoa1304048. [DOI] [PubMed] [Google Scholar]
- 30.Hoyler T, Klose CS, Souabni A, Turqueti-Neves A, Pfeifer D, Rawlins EL, et al. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity. 2012;37:634–648. doi: 10.1016/j.immuni.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drake LY, Iijima K, Kita H. Group 2 innate lymphoid cells and CD4+ T cells cooperate to mediate type 2 immune response in mice. Allergy. 2014;69:1300–1307. doi: 10.1111/all.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christianson CA, Goplen NP, Zafar I, Irvin C, Good JT, Jr, Rollins DR, et al. Persistence of asthma requires multiple feedback circuits involving type 2 innate lymphoid cells and IL-33. J Allergy Clin Immunol. 2015;136:59–68.e14. doi: 10.1016/j.jaci.2014.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li BW, de Bruijn MJ, Tindemans I, Lukkes M, KleinJan A, Hoogsteden HC, et al. T cells are necessary for ILC2 activation in house dust mite-induced allergic airway inflammation in mice. Eur J Immunol. 2016;46:1392–1403. doi: 10.1002/eji.201546119. [DOI] [PubMed] [Google Scholar]
- 34.Finkelman FD, Morris SC, Orekhova T, Mori M, Donaldson D, Reiner SL, et al. Stat6 regulation of in vivo IL-4 responses. J Immunol. 2000;164:2303–2310. doi: 10.4049/jimmunol.164.5.2303. [DOI] [PubMed] [Google Scholar]
- 35.Kheradmand F, Kiss A, Xu J, Lee SH, Kolattukudy PE, Corry DB. A protease-activated pathway underlying Th cell type 2 activation and allergic lung disease. J Immunol. 2002;169:5904–5911. doi: 10.4049/jimmunol.169.10.5904. [DOI] [PubMed] [Google Scholar]
- 36.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kiss A, Montes M, Susarla S, Jaensson EA, Drouin SM, Wetsel RA, et al. A new mechanism regulating the initiation of allergic airway inflammation. J Allergy Clin Immunol. 2007;120:334–342. doi: 10.1016/j.jaci.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 38.Porter PC, Yang T, Luong A, Delclos GL, Abramson SL, Kheradmand F, et al. Proteinases as molecular adjuvants in allergic airway disease. Biochim Biophys Acta. 2011;1810:1059–1065. doi: 10.1016/j.bbagen.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yiamouyiannis CA, Schramm CM, Puddington L, Stengel P, Baradaran-Hosseini E, Wolyniec WW, et al. Shifts in lung lymphocyte profiles correlate with the sequential development of acute allergic and chronic tolerant stages in a murine asthma model. Am J Pathol. 1999;154:1911–1921. doi: 10.1016/S0002-9440(10)65449-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Millien VO, Lu W, Shaw J, Yuan X, Mak G, Roberts L, et al. Cleavage of fibrinogen by proteinases elicits allergic responses through Toll-like receptor 4. Science. 2013;341:792–796. doi: 10.1126/science.1240342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002;196:1645–1651. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009;15:410–416. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McAleer JP, Vella AT. Understanding how lipopolysaccharide impacts CD4 T-cell immunity. Crit Rev Immunol. 2008;28:281–299. doi: 10.1615/critrevimmunol.v28.i4.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kenakin T. Biased agonism. F1000 Biol Rep. 2009;1:87. doi: 10.3410/B1-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drake MT, Violin JD, Whalen EJ, Wisler JW, Shenoy SK, Lefkowitz RJ.β-Arrestin-biased agonism at the β2-adrenergic receptor J Biol Chem 20082835669–5676. [DOI] [PubMed] [Google Scholar]
- 46.Shukla AK, Violin JD, Whalen EJ, Gesty-Palmer D, Shenoy SK, Lefkowitz RJ. Distinct conformational changes in β-arrestin report biased agonism at seven-transmembrane receptors. Proc Natl Acad Sci USA. 2008;105:9988–9993. doi: 10.1073/pnas.0804246105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Porter PC, Roberts L, Fields A, Knight M, Qian Y, Delclos GL, et al. Necessary and sufficient role for T helper cells to prevent fungal dissemination allergic lung disease. Infect Immun. 2011;79:4459–4471. doi: 10.1128/IAI.05209-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Porter P, Susarla SC, Polikepahad S, Qian Y, Hampton J, Kiss A, et al. Link between allergic asthma and airway mucosal infection suggested by proteinase-secreting household fungi. Mucosal Immunol. 2009;2:504–517. doi: 10.1038/mi.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 50.Gokudan S, Muta T, Tsuda R, Koori K, Kawahara T, Seki N, et al. Horseshoe crab acetyl group-recognizing lectins involved in innate immunity are structurally related to fibrinogen. Proc Natl Acad Sci USA. 1999;96:10086–10091. doi: 10.1073/pnas.96.18.10086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mak G, Porter PC, Bandi V, Kheradmand F, Corry DB. Tracheobronchial mycosis in a retrospective case-series study of five status asthmaticus patients. Clin Immunol. 2013;146:77–83. doi: 10.1016/j.clim.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pashley CH, Fairs A, Morley JP, Tailor S, Agbetile J, Bafadhel M, et al. Routine processing procedures for isolating filamentous fungi from respiratory sputum samples may underestimate fungal prevalence. Med Mycol. 2012;50:433–438. doi: 10.3109/13693786.2011.615762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Porter PC, Lim DJ, Maskatia ZK, Mak G, Tsai CL, Citardi MJ, et al. Airway surface mycosis in chronic TH2-associated airway disease. J Allergy Clin Immunol. 2014;134:325–331. doi: 10.1016/j.jaci.2014.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li E, Tsai CL, Maskatia ZK, Kakkar E, Porter P, Rossen RD, et al. Benefits of antifungal therapy in asthma patients with airway mycosis: a retrospective cohort analysis. Immun Inflamm Dis. 2018;6:264–275. doi: 10.1002/iid3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Denning DW, O’Driscoll BR, Powell G, Chew F, Atherton GT, Vyas A, et al. Randomized controlled trial of oral antifungal treatment for severe asthma with fungal sensitization: the Fungal Asthma Sensitization Trial (FAST) study. Am J Respir Crit Care Med. 2009;179:11–18. doi: 10.1164/rccm.200805-737OC. [DOI] [PubMed] [Google Scholar]
- 56.Agbetile J, Bourne M, Fairs A, Hargadon B, Desai D, Broad C, et al. Effectiveness of voriconazole in the treatment of Aspergillus fumigatus-associated asthma (EVITA3 study) J Allergy Clin Immunol. 2014;134:33–39. doi: 10.1016/j.jaci.2013.09.050. [DOI] [PubMed] [Google Scholar]
- 57.Ahmed T, Garrigo J, Danta I. Preventing bronchoconstriction in exercise-induced asthma with inhaled heparin. N Engl J Med. 1993;329:90–95. doi: 10.1056/NEJM199307083290204. [DOI] [PubMed] [Google Scholar]
- 58.Ceyhan B, Celikel T. Effect of inhaled heparin on methacholine-induced bronchial hyperreactivity. Chest. 1995;107:1009–1012. doi: 10.1378/chest.107.4.1009. [DOI] [PubMed] [Google Scholar]
- 59.Ahmed T, Gonzalez BJ, Danta I. Prevention of exercise-induced bronchoconstriction by inhaled low-molecular-weight heparin. Am J Respir Crit Care Med. 1999;160:576–581. doi: 10.1164/ajrccm.160.2.9812076. [DOI] [PubMed] [Google Scholar]
- 60.Heath L, Ling S, Racz J, Mane G, Schmidt L, Myers JL, et al. Prospective, longitudinal study of plastic bronchitis cast pathology and responsiveness to tissue plasminogen activator. Pediatr Cardiol. 2011;32:1182–1189. doi: 10.1007/s00246-011-0058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ward GW, Jr, Woodfolk JA, Hayden ML, Jackson S, Platts-Mills TA. Treatment of late-onset asthma with fluconazole. J Allergy Clin Immunol. 1999;104:541–546. doi: 10.1016/s0091-6749(99)70321-0. [DOI] [PubMed] [Google Scholar]
- 62.Tung HY, Li E, Landers C, Nguyen A, Kheradmand F, Knight JM, et al. Advances and evolving concepts in allergic asthma. Semin Respir Crit Care Med. 2018;39:64–81. doi: 10.1055/s-0037-1607981. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.