Abstract
A spectrum of intrapulmonary airway diseases, for example, cigarette smoke–induced bronchitis, cystic fibrosis, primary ciliary dyskinesia, and non–cystic fibrosis bronchiectasis, can be categorized as “mucoobstructive” airway diseases. A common theme for these diseases appears to be the failure to properly regulate mucus concentration, producing mucus hyperconcentration that slows mucus transport and, importantly, generates plaque/plug adhesion to airway surfaces. These mucus plaques/plugs generate long diffusion distances for oxygen, producing hypoxic niches within adherent airway mucus and subjacent epithelia. Data suggest that concentrated mucus plaques/plugs are proinflammatory, in part mediated by release of IL-1α from hypoxic cells. The infectious component of mucoobstructive diseases may be initiated by anaerobic bacteria that proliferate within the nutrient-rich hypoxic mucus environment. Anaerobes ultimately may condition mucus to provide the environment for a succession to classic airway pathogens, including Staphylococcus aureus, Haemophilus influenzae, and ultimately Pseudomonas aeruginosa. Novel therapies to treat mucoobstructive diseases focus on restoring mucus concentration. Strategies to rehydrate mucus range from the inhalation of osmotically active solutes, designed to draw water into airway surfaces, to strategies designed to manipulate the relative rates of sodium absorption versus chloride secretion to endogenously restore epithelial hydration. Similarly, strategies designed to reduce the mucin burden in the airways, either by reducing mucin production/secretion or by clearing accumulated mucus (e.g., reducing agents), are under development. Thus, the new insights into a unifying process, that is, mucus hyperconcentration, that drives a significant component of the pathogenesis of mucoobstructive diseases promise multiple new therapeutic strategies to aid patients with this syndrome.
Keywords: anaerobes, hydration therapies, IL-1α, mucoobstruction, mucus hyperconcentration
Mucoobstructive pulmonary diseases include a complex of diseases characterized by cough and sputum production, airflow obstruction, airway inflammation, and intermittent/continuous infection. Cystic fibrosis (CF) is the prototype of such diseases, but others would include cigarette smoke–induced chronic bronchitis, primary ciliary dyskinesia, and non-CF bronchiectasis. The common pathophysiological cascade describing these diseases reflects abnormalities of airway epithelial ion transport and/or increased mucin secretion that produce mucus dehydration, mucus stasis, and ultimately airway inflammation and bacterial infection. The ion transport abnormalities that produce airway surface dehydration differ per disease, with CF being perhaps the best-described mechanism (1). Therapies designed to specifically restore function of the mutant cystic fibrosis transmembrane conductance regulator (CFTR) have become available (2–5). However, pharmacological therapies for this disease syndrome/cascade do not exist. Inhaled hypertonic saline and mannitol are “hydrator” therapies used for some of these diseases but not for others (6–8). Elucidation of the mechanisms and pathways that produce this syndrome promises to provide therapeutic targets to aid in the care of this patient population.
Mucus Plugging Triggers Sterile Inflammation in Mucoobstructive Lung Diseases
Mucus plugging has long been recognized as providing a strong stimulus for the neutrophilic airway inflammation and a nutrient-rich nidus for the bacterial infections that are characteristic of mucoobstructive lung diseases such as CF and chronic obstructive pulmonary disease (COPD) (9). Over the past decade, observational studies from the Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST CF) cohort have convincingly shown that neutrophilic airway inflammation is already present in many infants and young children with CF, often in the absence of detectable bacterial infection (10, 11). However, the concept of “inflammation in the absence of bacterial infection” of mucus-obstructed airways has remained controversial.
In this context, emerging data from independent animal models support the concept that mucus plugging per se can trigger airway inflammation in the absence of bacterial infection (12). First, a series of studies in mice with airway-specific overexpression of the β subunit of the epithelial sodium channel (βENaC-Tg), producing a CF-like increase in airway sodium/fluid absorption, demonstrated that airway surface dehydration is sufficient to produce early-onset mucus plugging and the full spectrum of mucoobstructive lung disease, including chronic neutrophilic airway inflammation, goblet cell metaplasia, increased mucin (Muc5b and Muc5ac) production, and emphysema-like structural lung damage (13–18). This mucoobstructive phenotype, including spontaneous airway inflammation, was observed not only under conventional specific pathogen–free conditions but also when βENaC-Tg mice were raised in a germ-free vivarium (19). Second, studies in CF ferrets treated life-long with antibiotics demonstrated that bacterial infection is not required for CF-like mucoinflammatory disease featuring airway mucus plugging, neutrophilic inflammation, and bronchiectasis in this model (20). Third, a comparison of the pulmonary phenotypes of the βENaC-Tg mouse and the Muc5b-deficient mouse indicated that excess mucus/mucus adhesion may be more important than mucociliary dysfunction alone in the in vivo pathogenesis of chronic airway inflammation (21, 22). These studies showed that Muc5b is crucial for mucociliary clearance (MCC) and that Muc5b-deficient mice feature more severe mucociliary dysfunction than βENaC-Tg mice, but no mucus plugging. However, despite a more severe impairment in MCC, Muc5b-deficient mice exhibited modest airway inflammation and structural lung damage compared with βENaC-Tg mice (21).
Studies in βENaC-Tg mice have provided clues regarding the mechanistic links between mucus plugging and sterile airway inflammation. These studies demonstrated that mucus plugging is associated with cellular hypoxia and necrosis of epithelial cells lining the airways (16). Necrotic cell death due to hypoxia is a well-known and potent stimulus of sterile neutrophilic inflammation, and previous studies identified activation of IL-1 receptor (IL-1R) signaling by the alarmin IL-1α that is released from necrotic cells as a key mechanism in this process (23).
These observations triggered more detailed investigations of the role of IL-1R signaling in the pathogenesis of neutrophilic inflammation in mucoobstructive lung disease (24). It was shown that genetic deletion of IL-1R, as well as pharmacological inhibition with the endogenous IL-1 receptor antagonist anakinra, largely inhibited neutrophilic inflammation and structural lung damage in βENaC-Tg mice (24). In addition, evaluation of lung sections from patients with CF and COPD detected necrotic airway epithelial cells in mucus-obstructed airways and found that the numbers of these necrotic cells correlated with the severity of mucus obstruction in the small airways of patients with CF and COPD (24). These findings were also corroborated by an association study in various CF cohorts, suggesting the IL-1R locus as a genetic modifier of CF (25).
Collectively, these data demonstrate that airway surface dehydration plays an important role in the in vivo pathogenesis of mucus plugging and support emerging concepts that 1) accumulated (adherent) airway mucus per se is proinflammatory in the absence of bacterial infection; 2) mucus plugging can trigger the full spectrum of mucoobstructive lung disease in vivo; and 3) hypoxic cell death, triggering IL-1R signaling, may play an important role in the pathogenesis of sterile neutrophilic airway inflammation and may serve as a novel target for antiinflammatory therapy in mucoobstructive lung diseases such as CF and COPD (Figure 1) (12, 26).
Figure 1.
Mucus plugging causes hypoxic epithelial necrosis that triggers sterile inflammation in mucoobstructive lung disease. Mucus plugging produces regional hypoxia and necrosis of a subset of epithelial cells lining the airway surfaces. Dying epithelial cells release the alarmin IL-1α into the airway lumen. Binding of IL-1α to IL-1 receptors (IL-1Rs) on neighboring cells results in activation of the IL-1R/MyD88 signaling pathway, inducing neutrophilic airway inflammation in the absence of bacterial infection. Image courtesy of Joshua Bird. MyD88 = myeloid differentiation primary response 88; NF-κB = nuclear factor-κB; pO2 = partial pressure of oxygen.
Infectious Component of Mucoobstructive Diseases
The infections associated with mucoobstructive lung diseases typically involve bronchial/bronchiolar airways. Multiple interesting features characterize this form of airway infection. First, the bacterial infections almost exclusively represent infections of mucus adherent to airway surfaces (27). Biofilms may form as a part of the mucus infection, but are usually not found on epithelial surfaces. Second, mucus plaques/plugs on airway surfaces create niches of reduced oxygen concentration, favoring infection by organisms that gain energy efficiently under anaerobic conditions (28–30). Third, many of the infections of airway surfaces are polymicrobial, including a mix of multiple anaerobic and aerobic bacteria in varying densities and distributions (31–34). Fourth, many of these diseases are initiated by aspiration of upper airway commensals into a lower airway environment of static/hypoxic mucus (32). Fifth, the hyperconcentrated adherent mucus generates very “tight” mucus meshes (pores < 10 nm) that tend to shield bacteria growing within mucus masses from penetration, capture, and killing by intraluminal polymorphonuclear cells (35). Finally, airway disease and infection tend to be heterogeneous, with areas of severe disease (e.g., bronchiectasis) juxtaposed to functionally normal airway regions (11, 26).
Data have revealed important new insights into 1) mechanisms for the acquisition of bacterial infection early in the pathogenesis of mucoobstructive lung disease; and 2) the response of airway epithelia in health and disease to the combined stresses of bacterial and host defense products associated with bacterial infection. Both examples have evolved from studies of CF, perhaps the most studied of the mucoobstructive diseases with respect to infection.
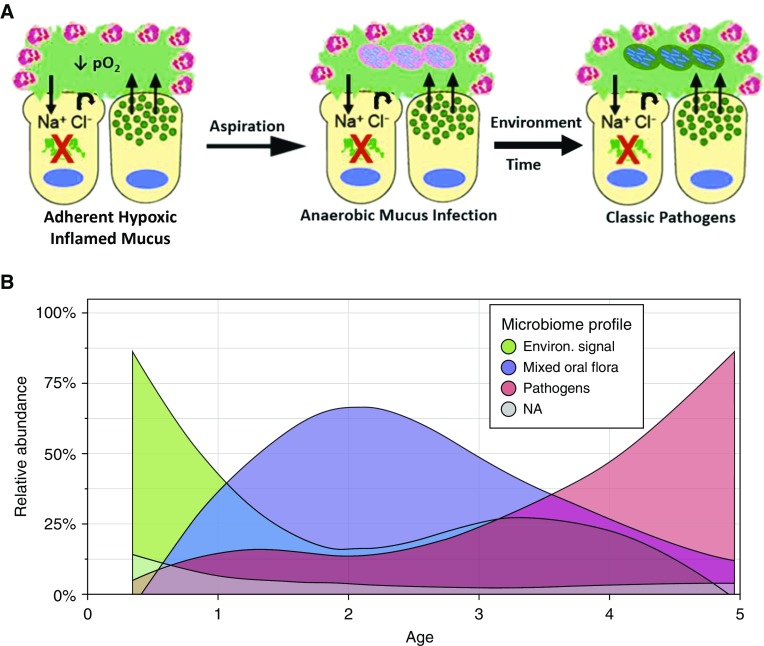
It has been reported that the CF lung environment early in life is characterized by heterogeneous areas of hyperconcentrated, adherent (static) mucus that have zones of frank hypoxia and potentially mucus-stimulated inflammation (Figure 2A). Studies from the AREST-CF cohort suggest that there may be a sequence of bacterial infections that is superimposed on this CF lower airway environment (Figure 2B) (32). Specifically, after a period of relative sterility, perhaps lasting 1–2 years, the environment becomes invaded by strictly anaerobic bacteria that appear to be aspirated from the oropharynx. Subsequently, bacteria known as “classic” CF pathogens invade this environment and likely dominate the environment based on absolute bacterial densities. Early classic CF pathogenic bacteria again may be commensal bacterial in the upper airways, including Staphylococcus, Haemophilus influenzae, and Moraxella. An interesting speculation is that anaerobic bacteria in a hypoxic mucin-rich environment secrete endo/ecto-glycosidases to harvest saccharides from mucin side chains to gain energy by fermentation. Not only does fermentation provide energy for anaerobic bacteria, but it also may provide novel substrates for the growth of the classic CF pathogens (36).
Figure 2.
Early infection in the cystic fibrosis (CF) lung. (A) Predicted sequence of bacterial pathogen acquisition in early CF. The CF airway early in life (left) has heterogeneous areas characterized by a hypoxic mucoinflammatory environment dominated by adherent mucus (green). Aspiration (middle) introduces oral anaerobic pathogens into the lung. Oral anaerobes survive and proliferate in lower airway hypoxic mucus, in part by fermenting sugars cleaved from mucins. With time and environmental exposure (right), classic CF pathogens (Staphylococcus and Pseudomonas) infect adherent CF mucus. Fermentation products of mucins may promote classic pathogen infection. (B) Relative abundance of bacteria in the bronchoalveolar lavage of infants/children with CF, as a function of age. Bacteria were binned into three classes: 1) environmental bacteria (green); 2) oral anaerobes (purple); and 3) classic CF pathogens (pink). Image courtesy of Bryan Zorn and Matthew Wolfgang.
Once established, pathogenic bacterial infections persist in mucus plaques and plugs laden with activated neutrophils and macrophages (17, 37). This mixture of liberated novel bacterial and leukocyte products induces a complex reaction by the host airway epithelium. Experimentally, responses of airway epithelia to bacterial/host products can be measured by exposing normal and CF airway cultures to a supernatant of the mucopurulent material (SMM) extracted from CF lungs excised at transplantation (Figure 3A). Comparisons of the responses of normal airway epithelia with CF airway epithelia to SMM revealed that 1) both genotypes exhibited similar mucin (MUC5AC and MUC5B) secretory responses to SMM; 2) normal airway epithelia exhibited ion transport responses to SMM that included inhibition of ENaC-mediated Na+/fluid absorption and acceleration of CFTR-mediated Cl− secretion, whereas CF airway epithelia responded to SMM with neither an inhibition of ENaC-mediated Na+/fluid absorption nor CFTR-mediated Cl−/fluid secretion; 3) confocal airway surface liquid (ASL) height measurements detected an increase in Cl−/fluid secretion as an increase in ASL height in normal airway epithelia, whereas the absence of ENaC regulation/CFTR-mediated Cl− secretion in CF produced no change in ASL height in CF airway epithelia; and 4) the increase in mucin secretion, accompanied by a relatively larger fluid secretion, diluted the percent solids content of the mucus lining normal human bronchial epithelial culture surfaces, whereas the selective increase in mucin secretion, in the absence of an acceleration in fluid secretion, produced an inappropriate hyperconcentration of CF mucus (38, 39).
Figure 3.
Response of normal versus cystic fibrosis (CF) airway epithelia to bacterial infection. (A, part I) Response of normal (white columns) versus CF (red columns) human bronchial epithelial cultures to luminal application of supernatant of mucopurulent material (SMM) harvested from lumens of CF airways. Shown are total mucin secretion rates in response to SMM. (A, part II) Short-circuit current (Isc) profiles of Na+ absorption (amiloride-sensitive Isc) and cystic fibrosis transmembrane conductance regulator (CFTR) Cl− secretion (forskolin-induced Isc) responses to SMM. (A, part III) Change in airway surface liquid height in response to SMM. (A, part IV) Change in mucus concentration (% solids) in response to SMM (38). (B, part I) Normal epithelia: Airway epithelia in basal state (left) coordinate mucin secretion rates and epithelial Na+ channel–mediated Na+/liquid absorption versus cystic fibrosis transmembrane/Ca2+-activated Cl− channel (CaCC, i.e., TMEM16a) Cl−/liquid secretion to maintain mucus at an approximately 2% solids hydration state commensurate with robust mucociliary clearance (MCC) rates. The balance of liquid transport and mucin secretion is maintained by ATP and adenosine interaction with apical P2Y2 and A2b purinoceptors, respectively. Epithelial responses to bacterial/host products include mucin secretion, which via corelease with mucins of adenosine (and AMP) stimulates a disproportionate increase in CFTR-mediated Cl−/fluid secretion, “super”-hydration of mucus (1.5% solids), and accelerated MCC. The net effect is to flush bacteria off airway surfaces. (B, part II) CF: Under basal, that is, the “nondiseased” but vulnerable, state (left), CF airway epithelia manage to maintain quasi-normal mucus hydration via upregulation of CaCC activity to offset missing CFTR Cl− transport and unregulated Na+ absorption. In response to a bacterial/host product challenge, mucin secretion is upregulated, but the absence of CFTR negates a coupled adenosine-mediated fluid secretory response. The net effect is to increase CF mucus concentration, slow MCC, and lead to spread/worsening of CF airway disease. Image 3B courtesy of Joshua Bird. A2BR = A2B adenosine receptor; ADO = adenosine; ASL = airway surface liquid; ENaC = epithelial Na+ channel; P2Y2R = purinergic receptor P2Y2; PBS = phosphate-buffered saline; PCL = perciliary layer.
These data are informative because they illustrate that the normal airway epithelial response to bacterial infections is dominated by the ability to actively secrete more mucin, trap infecting bacteria, and secrete a large fluid bolus onto epithelial surfaces to accelerate mucociliary clearance and “flush” bacteria off normal pulmonary surfaces (Figure 3B, part I). Of note, a similar coordinate upregulation of epithelial mucin and fluid secretion is also observed in response to allergen challenge, where it may play an important role in efficient allergen clearance to protect the host from chronic allergic airway diseases such as asthma (40–42). In contrast, the paradoxical and inappropriate CF responses to bacterial infection, that is, secreting mucins without secreting fluid, hyperconcentrates mucus and promotes mucus adhesion to CF airway surfaces, limiting the effectiveness of both cilial and cough-dependent mechanisms to clear mucus from airway surfaces (Figure 3B, part II) (38, 39). Thus, this paradoxical CF response can in part explain how the development of bacterial pathogenic infections in CF airways accelerates the rate of decline of lung function in patients with CF.
Therapeutics
Therapeutic Targeting of Mucus Plugging
Current therapeutic approaches to reduce the extent of mucus plugging in mucoobstructive lung diseases fall into three broad categories: 1) hydrating strategies, including both inhaled osmotically active agents (hypertonic saline, mannitol) and ion channel modulators, are designed to “dilute” hyperconcentrated, diseased mucus (43, 44) and to restore effective mucus clearance; 2) mucin production/secretion modifiers are predicted to lower the mucus load and, hence, concentration in the airways (45) and potentially restore the MUC5AC:MUC5B ratio, which has been demonstrated to be significantly altered in disease (46); and 3) improved mucolytics that directly modify the structure of the mucus gel and improve rheological properties of accumulated/adherent mucus to aid clearance of accumulated mucus from the lung.
Hypertonic Saline
Hypertonic saline was established more than a decade ago as being clinically effective in CF, as reflected in modest improvements in pulmonary function and an approximately 50% reduction in pulmonary exacerbations (6, 47). Despite the fact that studies at that time revealed hypertonic saline accelerated mucus clearance in patients with CF, the precise mechanisms of hypertonic saline actions and principles for clinical administration of hypertonic saline have remained controversial. Specifically, questions have arisen as to whether hypertonic saline exhibits actions to 1) electrostatically shield charges on mucins and accelerate their transport (48); 2) produce persistent airway epithelial cell volume reductions with inhibition of ENaC-mediated fluid transport (49); or 3) osmotically draw water onto airway surfaces and dilute/rehydrate adherent hyperconcentrated mucus (47). In part, these contrasting hypotheses emerged from in vitro protocols that typically employed large bolus liquid additions to airway surfaces to simulate the administration of hypertonic saline.
Goralski and colleagues reported the actions of (7%, wt/vol) aerosolized hypertonic saline delivered to human bronchial epithelial cultures covered by a normally hydrated mucus layer (2% solids) versus a CF-like dehydrated mucus layer (12% solids) (50). Aerosol deposition rates were designed to mimic clinical rates of hypertonic saline delivery in vivo. Several points relevant to the mechanism of hypertonic saline emerged from those studies. First, confocal microscopy revealed that administration of hypertonic saline osmotically drew water onto airway surfaces and, indeed, the mucus layer. Interestingly, the relative rates of aerosol deposition versus the rates of passive water movement onto airway surfaces in response to hypertonic saline aerosol deposition produced an ASL osmolality during hypertonic saline administration of approximately 370 mOsm. Second, the hydrating effects of hypertonic saline were maximal at the initiation of aerosol administration and terminated immediately on cessation of delivery. Active epithelial Na+ and fluid absorption were identified as the processes that removed hypertonic saline at the cessation of delivery and, hence, controlled the durability of hypertonic saline hydrating effects. Finally, mucus on airway surfaces produced profound effects on responses to aerosolized hypertonic saline. Particularly striking was the capacity of hyperconcentrated mucus on airway surfaces to substantially extend the durability of hypertonic saline hydrating effects. It is likely that a hyperconcentrated mucus layer, with its high mucus osmotic pressure, provided a counterforce to active sodium transport–generated ion-based osmotic pressures that slowed the net absorption of water from airway surfaces. Interestingly, the relative duration of the effects of hypertonic saline in a normal versus CF-like hypertonic mucus environment mimicked reports from in vivo MCC measurements that described a relatively short period of action of hypertonic saline in normal subjects (<1 h) versus a more durable response (>4 h) in subjects with cystic fibrosis (51, 52).
Ion Channel Modulators
The introduction of CFTR repair therapies has extended the hydration concept and represents one of the most significant advances in the treatment of CF (53). Furthermore, these agents have also confirmed the pathological significance of mucus plugging and failed mucus clearance in CF lung disease. Ivacaftor (VX-770; Kalydeco), a “potentiator” of CFTR function, has demonstrated impressive improvements in lung function and exacerbation frequency in patients with the channel gating mutation G551D (2, 3). In preclinical studies, ivacaftor improved airway epithelial anion secretion, which resulted in an enhanced volume of fluid on airway surfaces (4). This finding translated into improved CFTR channel activity in patients, enhanced rates of mucociliary clearance, and reduced mucus plugging of small airways (54, 55). In the wake of the success of ivacaftor, numerous alternative CFTR therapies have been developed, which include not only potentiators for channel gating mutations, but also CFTR “correctors” to improve trafficking of mutated CFTR channels (e.g., F508del) to the plasma membrane (Table 1). At least 20 candidate drugs, which are described as CFTR modulators, are presently in clinical development for the treatment of CF.
Table 1.
Cystic fibrosis transmembrane conductance regulator potentiators and correctors in drug development pipeline
| Company | Drug Name | Mechanism | Indication | Route | Clinical |
|---|---|---|---|---|---|
| Vertex | Ivacaftor | CFTR potentiator | CF | Oral | Phase 3: EVOLVE (F508del/F508del), >4% FEV1 |
| Tezacaftor | CFTR corrector | Phase 3: EXPAND (residual CFTR mutation/F508del), >6.8% FEV1 | |||
| Novartis | QBW251 | CFTR potentiator | CF, COPD | Oral | Phase 2 CF: Less sweat chloride and more lung function in heterozygous patients with CF with a gating or residual function mutation. F508del/F508del, no evidence of pharmacodynamics effect |
| Phase 2 COPD: (4 wk, 90 patients, LCI) | |||||
| Galapagos | GLPG1837 | CFTR potentiator | CF | Oral | Phase 2: SAPHIRA 1 (G551D), ∼30-mmol reduction in sweat chloride, >5.4% ppFEV1 |
| AbbVie | Phase 2: SAPHIRA 2 (S1251N) completed? | ||||
| Galapagos/AbbVie | GLPG2222 | CFTR corrector (C1) | CF | Oral | Phase 2: ALBATROSS (CFTR gating mutation/F508del, 35 patients, safety, tolerability, sweat chloride) |
| Vertex | VX-152 | CFTR correctors | CF | Oral | Phase 2: Triple combination: VX-152, tezacaftor, ivacaftor in 60 patients with minimal function CFTR mutation/F508del (8 wk), >9.7% FEV1 at 2 wk |
| VX-440 | 2nd generation | Phase 2: Triple combination: VX-440, tezacaftor, ivacaftor in 200 patients with minimal function CFTR mutation/F508del (16 wk), >12.0% FEV1 at 4 wk | |||
| VX-659 | Orkambi | Phase 1: >9.6% FEV1 | |||
| Concert/Vertex | CTP-656 | CFTR potentiator Deuterated ivacaftor | CF | Oral | Phase 2: CFTR gating mutations, 4 wk, 40 patients, sweat chloride, FEV1 |
| ProQR | QR010 | CFTR stimulator RNAi | CF | Inhaled | Phase 1b: Safety, tolerability, and pharmacokinetics, 64 patients with CF, F508del/F508del. Phase 1b: Safety, tolerability, and pharmacokinetics, 18 patients with CF, F508del homozygous or heterozygous |
| Galapagos/AbbVie | GLPG2451 | CFTR potentiator | CF | Oral | Phase 1 |
| Galapagos/AbbVie | GLPG3067 | CFTR potentiator | CF | Oral | Phase 1 |
| Galapagos/AbbVie | GLPG2451 | CFTR potentiator | CF | Oral | Phase 1 |
| Galapagos/AbbVie | GLPG2737 | CFTR corrector (C2) | CF | Oral | Phase 1 |
| Proteostasis | PTI-428 | CFTR amplifier | CF | Oral | Phase 1 |
| Proteostasis | PTI-801 | CFTR corrector | CF | Oral | Phase 1 |
| Flatley Discovery Labs | FDL-169 | CFTR corrector | CF | Oral | Phase 1 |
| Flatley Discovery Labs | FDL-176 | CFTR corrector | CF | Oral | Phase 1 |
| Galapagos/AbbVIe | GLPG2851 | CFTR corrector | CF | Oral | Preclinical |
| Proteostasis | PTI-808 | CFTR potentiator | CF | Oral | Preclinical |
Definition of abbreviations: CF = cystic fibrosis; CFTR = cystic fibrosis transmembrane conductance regulator; COPD = chronic obstructive pulmonary disease; FEV1 = forced expiratory volume in 1 second; LCI = lung clearance index; ppFEV1 = percent predicted FEV1; RNAi = RNA interference.
The utility of CFTR repair therapy may well not be limited to the treatment of CF. At least two CFTR potentiator compounds, ivacaftor and QBW251, have been tested in small, pilot COPD trials (56, 57). The ivacaftor study suggested that there may be the potential for improvements in CFTR function (nasal potential difference and sweat chloride) (56), although the study was underpowered to detect definitive changes. The QBW251 study reported improvements in FEV1 and sweat chloride (58). These are small, early studies, and additional, larger trials will be necessary to understand whether promoting CFTR function will be of benefit to patients with COPD.
Beyond CFTR, additional airway ion channels may represent important drug targets to deliver hydration to the airway, including TMEM16A (ANO-1), SLC26A9, and the epithelial sodium channel, ENaC (59). These CFTR “mutation agnostic” approaches are likely to be important add-on therapies for patients with CF already treated with CFTR repair drugs and also for subjects with CF with mutations not amenable to the current CFTR repair pipeline. Furthermore, these therapies would also have the potential to expand into therapies for the broad class of mucoobstructive lung disease.
TMEM16A is a calcium-activated chloride channel (CaCC) that is expressed in the airway epithelium (60–62). More than 20 years ago, a CaCC was described in the airway that was capable of eliciting a significant anion secretory response in vivo when stimulated with calcium-mobilizing ligands such as ATP and UTP (63, 64). This CaCC-mediated effect regulates anion and fluid secretion in response to the mechanical stresses imposed on the lungs during breathing (65). Furthermore, this system is able to maintain mucus clearance in the absence of CFTR function in the early years of CF. It is also noteworthy that both exercise and chest physical therapy, maneuvers that improve clinical outcomes, enhance calcium signaling in the airway epithelium, thereby regulating ion/fluid transport mechanisms and promoting mucus clearance (66–68). A therapy that would potentiate the activity of TMEM16A is predicted to enhance the fluid secretory capacity of airway epithelia and mimic a CFTR potentiator–like phenotype.
SLC26A9, a member of the solute carrier 26 family, may also contribute to anion and fluid secretion in the airway epithelium (69–71). SLC26A9 has been demonstrated to transport chloride ions through both CFTR-dependent and independent mechanisms, although a paucity of specific pharmacological tools has limited our understanding of its function. Genetic evidence supports SLC26A9 as a disease modifier in CF, and model systems have demonstrated a significant role of this anion channel in the regulation of mucus hydration (72). Upregulation of Slc26a9 expression in airway inflammation protects mice from airway mucus plugging, while knockout animals show a severe plugging phenotype (40, 41). Pharmacological tools are required to enable a better evaluation of the therapeutic potential of activators of this alternative chloride channel.
Inhaled ENaC blockers for the treatment of CF lung disease have been explored for more than 30 years but without robust evidence of clinical efficacy (73). Inhaled amiloride has been tested in several long-term studies and has failed to show reproducible improvements in lung function, potentially due to a short duration of action in the airway combined with the dose-limiting side effect of hyperkalemia (74). So is ENaC an invalid target for the treatment of CF lung disease or have we not yet found the right drug candidate?
What does not appear to be in question is the target validation. Patients with loss-of-function mutations in ENaC subunits have a salt-wasting disease, pseudohypoaldosteronism type 1, but also exhibit accelerated rates of airway mucociliary clearance (75), and inhaled amiloride accelerates mucociliary clearance in clinical studies (76). Further, airway-specific overexpression of βENaC to phenocopy the increased sodium transport characteristic of CF airways resulted in CF-like lung disease in mice (15, 77).
To this end, several groups have explored the discovery of novel inhaled ENaC blockers with a long duration of action in the lung. One example, VX-371, has demonstrated a durable acceleration of MCC in animal models but failed to show an improvement in FEV1 in a 28-day study in CF (78). Unfortunately, this study failed to report any evidence of target engagement at the evaluated dose, precluding any further assessment of the validity of ENaC as a therapeutically useful drug target. In addition to direct blockers of the channel, alternative approaches to negatively regulate ENaC function by either gene silencing (antisense oligonucleotides) or channel internalization (SPX-101) are also being explored (79, 80). SPX-101 is a peptide derivative of a region of SPLUNC-1 (BPIFA1), which has been reported to stimulate the internalization of the ENaC subunits, thereby reducing sodium transport. Inhaled dosing has been reported to increase tracheal mucus velocity in sheep and to improve survival in the βENaC-overexpressing mouse (80), and is currently in phase 2a trials. Critical to understanding whether ENaC is a viable target to deliver clinical benefit will be well-designed clinical studies with biomarkers to establish whether channel function has been attenuated in parallel with the clinical endpoints.
Reduced Mucin Synthesis and Secretion
Reducing the quantity of mucus in the lumen of the airways could theoretically be achieved by either 1) reducing the number of mucin-producing (goblet) cells; 2) inhibiting the pathways regulating mucin biosynthesis; or 3) inhibiting the secretion of packaged mucins, that is, prevent exocytosis. There are currently no such therapies available, although recent data have suggested that the combination of corticosteroids and β2-adrenoreceptor agonists may have a modest effect on airway goblet cell numbers in vitro (81).
Historically, much attention has been afforded to the epidermal growth factor receptor (EGFR) pathway in the regulation of airway goblet cell formation (82). Numerous preclinical models, both in vitro and in vivo, suggested a key role for this pathway in airway epithelial mucin biology. However, more recent clinical data with the EGFR antagonist BIBW 2948 BS failed to show any changes in airway mucin markers in patients with COPD despite evidence of target engagement in a 28-day study (83). The compound was not well tolerated, and it is likely that alternative candidates will be required to further test this target hypothesis.
More recently, the role of the Notch pathway in the regulation of airway epithelial differentiation has become clear. In addition to a developmental role, Notch signaling also appears to regulate the heterogeneity of the cell populations in the adult airway epithelium. Studies using antibodies to antagonize either Notch receptors or ligands have implicated roles for the Notch2 receptor and Jagged ligands in the maintenance of goblet cell populations in both primary human cells and murine models (84, 85). Strategies to control Notch pathway activation may represent a novel approach to attenuate excessive mucus production in the lungs. However, which diseases and patient subpopulations may benefit from this approach will require early proof-of-concept studies.
A number of additional pathways and targets have been proposed to regulate goblet cell formation. Candidates include MAPK13, GABAA antagonists, and CLCA1 inhibitors (45), although the literature and patent filings do not support these being actively pursued for therapeutic benefit.
Reducing goblet cell exocytosis may also provide benefit in a mucus-hyperproducing and -hypersecreting airway (86). Approaches such as purinergic P2Y2 receptor antagonism have been proposed, and candidates such as the MARCKS peptide have shown efficacy in preclinical systems (45). However, to date no candidate compounds or targets have percolated through to clinical testing. As our molecular understanding of the mechanisms controlling goblet cell exocytosis expands, understanding the pathways regulating baseline (tonic) secretion and stimulated secretion may be important for ensuring that the mucociliary apparatus can maintain function while limiting excessive secretion, particularly in the small airways (87). For example, in the Munc13-2−/− mouse, where baseline mucin secretion from club cells was attenuated (88), significant increases in stored mucins, likely available for stimulated secretion, were observed. If suddenly released, this large bolus of mucin could represent a safety concern. Further studies with therapeutic candidates will be required to define the safety implications associated with attenuating goblet cell exocytosis and whether it will be essential to only influence the pathways regulating stimulated secretion to avoid a potential stockpiling of secretory vesicles.
Mucolytics
Inhaled DNase is a “mucolytic” that cleaves extracellular DNA and reduces sputum viscosity (89). It is a mainstay for CF therapy, but its efficacy appears restricted to this patient population. Carbocysteine and N-acetylcysteine, focused on mucin disulfide bond reduction, have been used for many years to treat mucoobstructive lung disease with evidence of only limited efficacy (90, 91). Data have challenged the biochemical efficacy and potency of these agents to remodel the mucus gel to improve its transport properties. To this end, novel, more potent disulfide bond mucin-reducing agents with properties to enable efficient inhaled dosing may have the potential to deliver clinical benefit. Compounds such as P3001, a potent reducing agent with an extended duration of action in the airway lumen, are showing evidence of efficacy both in in vitro mucus transit models as well as in vivo models of mucus obstruction (92, 93).
Conclusion
There is an emerging database describing the causes and consequences of mucus accumulation, adhesion, and obstruction in intrapulmonary airways. A unifying theme appears to be that mucus hyperconcentration, that is, dehydration, produces a failure of mucus transport and, ultimately, the adherent mucus plaques that trigger the inflammation and infection associated with these diseases. Disease-specific therapies aimed at the individual pathogenic processes that initiate mucus hyperconcentration, for example, CFTR-dependent defects in ion transport/airway surface hydration, are models for therapies of this class of diseases. However, until specific disease pathways are identified for each disease, more general strategies to reduce airway mucus concentrations by hydration therapies and/or therapies designed to reduce/slow mucin secretion appear rational. The wide spectrum of approaches to achieve this therapeutic goal provides encouragement that novel and more effective therapies for the mucoobstructive diseases will be available relatively soon.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Joshua Bird for illustration work and Eric Roe for editorial assistance. The authors also thank the UNC Virtual Lung Group and teams at the Marsico Lung Institute, Enterprise Therapeutics, and Charité Hospital, Berlin.
Footnotes
Supported in part by grants from the German Federal Ministry of Education and Research 82DZL004A1 (M.A.M.) and the Einstein Foundation Berlin EP-2017-393 (M.A.M.).
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Boucher RC. Airway surface dehydration in cystic fibrosis: pathogenesis and therapy. Annu Rev Med. 2007;58:157–170. doi: 10.1146/annurev.med.58.071905.105316. [DOI] [PubMed] [Google Scholar]
- 2.Accurso FJ, Rowe SM, Clancy JP, Boyle MP, Dunitz JM, Durie PR, et al. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med. 2010;363:1991–2003. doi: 10.1056/NEJMoa0909825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Drevinek P, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663–1672. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Goor F, Hadida S, Grootenhuis PD, Burton B, Cao D, Neuberger T, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci USA. 2009;106:18825–18830. doi: 10.1073/pnas.0904709106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wainwright CE, Elborn JS, Ramsey BW, Marigowda G, Huang X, Cipolli M, et al. Lumacaftor–ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med. 2015;373:220–231. doi: 10.1056/NEJMoa1409547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elkins MR, Robinson M, Rose BR, Harbour C, Moriarty CP, Marks GB, et al. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med. 2006;354:229–240. doi: 10.1056/NEJMoa043900. [DOI] [PubMed] [Google Scholar]
- 7.Kellett F, Robert NM. Nebulised 7% hypertonic saline improves lung function and quality of life in bronchiectasis. Respir Med. 2011;105:1831–1835. doi: 10.1016/j.rmed.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 8.Flume PA, Aitken ML, Bilton D, Agent P, Charlton B, Forster E, et al. Optimising inhaled mannitol for cystic fibrosis in an adult population. Breathe (Sheff) 2015;11:39–48. doi: 10.1183/20734735.021414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mall MA, Hartl D. CFTR: cystic fibrosis and beyond. Eur Respir J. 2014;44:1042–1054. doi: 10.1183/09031936.00228013. [DOI] [PubMed] [Google Scholar]
- 10.Sly PD, Brennan S, Gangell C, de Klerk N, Murray C, Mott L, et al. Lung disease at diagnosis in infants with cystic fibrosis detected by newborn screening. Am J Respir Crit Care Med. 2009;180:146–152. doi: 10.1164/rccm.200901-0069OC. [DOI] [PubMed] [Google Scholar]
- 11.Sly PD, Gangell CL, Chen L, Ware RS, Ranganathan S, Mott LS, et al. Risk factors for bronchiectasis in children with cystic fibrosis. N Engl J Med. 2013;368:1963–1970. doi: 10.1056/NEJMoa1301725. [DOI] [PubMed] [Google Scholar]
- 12.Zhou-Suckow Z, Duerr J, Hagner M, Agrawal R, Mall MA. Airway mucus, inflammation and remodeling: emerging links in the pathogenesis of chronic lung diseases. Cell Tissue Res. 2017;367:537–550. doi: 10.1007/s00441-016-2562-z. [DOI] [PubMed] [Google Scholar]
- 13.Gehrig S, Duerr J, Weitnauer M, Wagner CJ, Graeber SY, Schatterny J, et al. Lack of neutrophil elastase reduces inflammation, mucus hypersecretion, and emphysema, but not mucus obstruction, in mice with cystic fibrosis–like lung disease. Am J Respir Crit Care Med. 2014;189:1082–1092. doi: 10.1164/rccm.201311-1932OC. [DOI] [PubMed] [Google Scholar]
- 14.Livraghi A, Grubb BR, Hudson EJ, Wilkinson KJ, Sheehan JK, Mall MA, et al. Airway and lung pathology due to mucosal surface dehydration in β-epithelial Na+ channel–overexpressing mice: role of TNF-α and IL-4Rα signaling, influence of neonatal development, and limited efficacy of glucocorticoid treatment. J Immunol. 2009;182:4357–4367. doi: 10.4049/jimmunol.0802557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mall M, Grubb BR, Harkema JR, O’Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis–like lung disease in mice. Nat Med. 2004;10:487–493. doi: 10.1038/nm1028. [DOI] [PubMed] [Google Scholar]
- 16.Mall MA, Harkema JR, Trojanek JB, Treis D, Livraghi A, Schubert S, et al. Development of chronic bronchitis and emphysema in β-epithelial Na+ channel–overexpressing mice. Am J Respir Crit Care Med. 2008;177:730–742. doi: 10.1164/rccm.200708-1233OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trojanek JB, Cobos-Correa A, Diemer S, Kormann M, Schubert SC, Zhou-Suckow Z, et al. Airway mucus obstruction triggers macrophage activation and matrix metalloproteinase 12–dependent emphysema. Am J Respir Cell Mol Biol. 2014;51:709–720. doi: 10.1165/rcmb.2013-0407OC. [DOI] [PubMed] [Google Scholar]
- 18.Wielputz MO, Eichinger M, Zhou Z, Leotta K, Hirtz S, Bartling SH, et al. In vivo monitoring of cystic fibrosis–like lung disease in mice by volumetric computed tomography. Eur Respir J. 2011;38:1060–1070. doi: 10.1183/09031936.00149810. [DOI] [PubMed] [Google Scholar]
- 19.Livraghi-Butrico A, Kelly EJ, Klem ER, Dang H, Wolfgang MC, Boucher RC, et al. Mucus clearance, MyD88-dependent and MyD88-independent immunity modulate lung susceptibility to spontaneous bacterial infection and inflammation. Mucosal Immunol. 2012;5:397–408. doi: 10.1038/mi.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosen BH, Evans TIA, Moll SR, Gray JS, Liang B, Sun X, et al. Infection is not required for mucoinflammatory lung disease in CFTR-knockout ferrets. Am J Respir Crit Care Med. 2018;197:1308–1318. doi: 10.1164/rccm.201708-1616OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livraghi-Butrico A, Grubb BR, Wilkinson KJ, Volmer AS, Burns KA, Evans CM, et al. Contribution of mucus concentration and secreted mucins Muc5ac and Muc5b to the pathogenesis of muco-obstructive lung disease. Mucosal Immunol. 2017;10:829. doi: 10.1038/mi.2017.29. [DOI] [PubMed] [Google Scholar]
- 22.Roy MG, Livraghi-Butrico A, Fletcher AA, McElwee MM, Evans SE, Boerner RM, et al. Muc5b is required for airway defence. Nature. 2014;505:412–416. doi: 10.1038/nature12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- 24.Fritzsching B, Zhou-Suckow Z, Trojanek JB, Schubert SC, Schatterny J, Hirtz S, et al. Hypoxic epithelial necrosis triggers neutrophilic inflammation via IL-1 receptor signaling in cystic fibrosis lung disease. Am J Respir Crit Care Med. 2015;191:902–913. doi: 10.1164/rccm.201409-1610OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stanke F, Hector A, Hedtfeld S, Hartl D, Griese M, Tummler B, et al. An informative intragenic microsatellite marker suggests the IL-1 receptor as a genetic modifier in cystic fibrosis. Eur Respir J. 2017;50:1700426. doi: 10.1183/13993003.00426-2017. [DOI] [PubMed] [Google Scholar]
- 26.Montgomery ST, Mall MA, Kicic A, Stick SM. Hypoxia and sterile inflammation in cystic fibrosis airways: mechanisms and potential therapies. Eur Respir J. 2017;49:1600903. doi: 10.1183/13993003.00903-2016. [DOI] [PubMed] [Google Scholar]
- 27.Worlitzsch D, Tarran R, Ulrich M, Schwab U, Cekici A, Meyer KC, et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J Clin Invest. 2002;109:317–325. doi: 10.1172/JCI13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newman D. Pathogen growth rates in CF sputum are slow and heterogeneous: implications for research and treatment strategies. Pediatr Pulmonol. 2016;51:162. [Google Scholar]
- 29.DePas WH, Starwalt-Lee R, Van Sambeek L, Ravindra Kumar S, Gradinaru V, Newman DK. Exposing the three-dimensional biogeography and metabolic states of pathogens in cystic fibrosis sputum via hydrogel embedding, clearing, and rRNA labeling. MBio. 2016;7:e00796-16. doi: 10.1128/mBio.00796-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cowley ES, Kopf SH, LaRiviere A, Ziebis W, Newman DK. Pediatric cystic fibrosis sputum can be chemically dynamic, anoxic, and extremely reduced due to hydrogen sulfide formation. MBio. 2015;6:e00767. doi: 10.1128/mBio.00767-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tunney MM, Field TR, Moriarty TF, Patrick S, Doering G, Muhlebach MS, et al. Detection of anaerobic bacteria in high numbers in sputum from patients with cystic fibrosis. Am J Respir Crit Care Med. 2008;177:995–1001. doi: 10.1164/rccm.200708-1151OC. [DOI] [PubMed] [Google Scholar]
- 32.Muhlebach MS, Zorn BT, Esther CR, Hatch JE, Murray CP, Turkovic L, et al. Initial acquisition and succession of the cystic fibrosis lung microbiome is associated with disease progression in infants and preschool children. PLoS Pathog. 2018;14:e1006798. doi: 10.1371/journal.ppat.1006798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lynch SV, Bruce KD. The cystic fibrosis airway microbiome. Cold Spring Harb Perspect Med. 2013;3:a009738. doi: 10.1101/cshperspect.a009738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Toole GA. Cystic fibrosis airway microbiome: overturning the old, opening the way for the new. J Bacteriol. 2018;200:e00561-17. doi: 10.1128/JB.00561-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsui H, Verghese MW, Kesimer M, Schwab UE, Randell SH, Sheehan JK, et al. Reduced three-dimensional motility in dehydrated airway mucus prevents neutrophil capture and killing bacteria on airway epithelial surfaces. J Immunol. 2005;175:1090–1099. doi: 10.4049/jimmunol.175.2.1090. [DOI] [PubMed] [Google Scholar]
- 36.Flynn JM, Niccum D, Dunitz JM, Hunter RC. Evidence and role for bacterial mucin degradation in cystic fibrosis airway disease. PLoS Pathog. 2016;12:e1005846. doi: 10.1371/journal.ppat.1005846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dittrich AS, Kuhbandner I, Gehrig S, Rickert-Zacharias V, Twigg M, Wege S, et al. Elastase activity on sputum neutrophils correlates with severity of lung disease in cystic fibrosis. Eur Respir J. 2018;51:1701910. doi: 10.1183/13993003.01910-2017. [DOI] [PubMed] [Google Scholar]
- 38.Abdullah LH, Coakley R, Webster MJ, Zhu Y, Tarran R, Radicioni G, et al. Mucin production and hydration responses to mucopurulent materials in normal versus cystic fibrosis airway epithelia. Am J Respir Crit Care Med. 2018;197:481–491. doi: 10.1164/rccm.201706-1139OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balazs A, Mall MA. Mucopurulent triggering of the airway epithelium. Implications in health and cystic fibrosis. Am J Respir Crit Care Med. 2018;197:418–420. doi: 10.1164/rccm.201712-2554ED. [DOI] [PubMed] [Google Scholar]
- 40.Anagnostopoulou P, Dai L, Schatterny J, Hirtz S, Duerr J, Mall MA. Allergic airway inflammation induces a pro-secretory epithelial ion transport phenotype in mice. Eur Respir J. 2010;36:1436–1447. doi: 10.1183/09031936.00181209. [DOI] [PubMed] [Google Scholar]
- 41.Anagnostopoulou P, Riederer B, Duerr J, Michel S, Binia A, Agrawal R, et al. SLC26A9-mediated chloride secretion prevents mucus obstruction in airway inflammation. J Clin Invest. 2012;122:3629–3634. doi: 10.1172/JCI60429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fritzsching B, Hagner M, Dai L, Christochowitz S, Agrawal R, van Bodegom C, et al. Impaired mucus clearance exacerbates allergen-induced type 2 airway inflammation in juvenile mice. J Allergy Clin Immunol. 2017;140:190–203.e5. doi: 10.1016/j.jaci.2016.09.045. [DOI] [PubMed] [Google Scholar]
- 43.Button B, Anderson WH, Boucher RC. Mucus hyperconcentration as a unifying aspect of the chronic bronchitic phenotype. Ann Am Thorac Soc. 2016;13:S156–S162. doi: 10.1513/AnnalsATS.201507-455KV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mall MA. Unplugging mucus in cystic fibrosis and chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2016;13:S177–S185. doi: 10.1513/AnnalsATS.201509-641KV. [DOI] [PubMed] [Google Scholar]
- 45.Ha EV, Rogers DF. Novel therapies to inhibit mucus synthesis and secretion in airway hypersecretory diseases. Pharmacology. 2016;97:84–100. doi: 10.1159/000442794. [DOI] [PubMed] [Google Scholar]
- 46.Kesimer M, Ford AA, Ceppe A, Radicioni G, Cao R, Davis CW, et al. Airway mucin concentration as a marker of chronic bronchitis. N Engl J Med. 2017;377:911–922. doi: 10.1056/NEJMoa1701632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Donaldson SH, Bennett WD, Zeman KL, Knowles MR, Tarran R, Boucher RC. Mucus clearance and lung function in cystic fibrosis with hypertonic saline. N Engl J Med. 2006;354:241–250. doi: 10.1056/NEJMoa043891. [DOI] [PubMed] [Google Scholar]
- 48.Wills PJ, Hall RL, Chan W, Cole PJ. Sodium chloride increases the ciliary transportability of cystic fibrosis and bronchiectasis sputum on the mucus-depleted bovine trachea. J Clin Invest. 1997;99:9–13. doi: 10.1172/JCI119138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rasgado-Flores H, Krishna Mandava V, Siman H, Van Driessche W, Pilewski JM, Randell SH, et al. Effect of apical hyperosmotic sodium challenge and amiloride on sodium transport in human bronchial epithelial cells from cystic fibrosis donors. Am J Physiol Cell Physiol. 2013;305:C1114–C1122. doi: 10.1152/ajpcell.00166.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goralski JL, Wu D, Thelin WR, Boucher RC, Button B. The in vitro effect of nebulised hypertonic saline on human bronchial epithelium. Eur Respir J. 2018;51:1702652. doi: 10.1183/13993003.02652-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bennett WD, Wu J, Fuller F, Balcazar JR, Zeman KL, Duckworth H, et al. Duration of action of hypertonic saline on mucociliary clearance in the normal lung. J Appl Physiol (1985) 2015;118:1483–1490. doi: 10.1152/japplphysiol.00404.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trimble AT, Whitney Brown A, Laube BL, Lechtzin N, Zeman KL, Wu J, et al. Hypertonic saline has a prolonged effect on mucociliary clearance in adults with cystic fibrosis. J Cyst Fibros. 2018;17:650–656. doi: 10.1016/j.jcf.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gentzsch M, Mall MA. Ion channel modulators in cystic fibrosis. Chest. 2018;154:383–393. doi: 10.1016/j.chest.2018.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Altes TA, Johnson M, Fidler M, Botfield M, Tustison NJ, Leiva-Salinas C, et al. Use of hyperpolarized helium-3 MRI to assess response to ivacaftor treatment in patients with cystic fibrosis. J Cyst Fibros. 2017;16:267–274. doi: 10.1016/j.jcf.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 55.Rowe SM, Heltshe SL, Gonska T, Donaldson SH, Borowitz D, Gelfond D, et al. Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G551D-mediated cystic fibrosis. Am J Respir Crit Care Med. 2014;190:175–184. doi: 10.1164/rccm.201404-0703OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Solomon GM, Hathorne H, Liu B, Raju SV, Reeves G, Acosta EP, et al. Pilot evaluation of ivacaftor for chronic bronchitis. Lancet Respir Med. 2016;4:e32–e33. doi: 10.1016/S2213-2600(16)30047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. ClinicalTrials.gov. A safety, tolerability and efficacy study with QBW251 in COPD patients with QBW251. 2018 [cited 2018 May 14]. Available from: https://clinicaltrials.gov/ct2/show/NCT02449018.
- 58.Novartis Pharmaceuticals. Clinical trial results website: generic drug name: QBW251. 2017 [cited 2018 May 14]. Available from: https://www.novctrd.com/CtrdWeb/displaypdf.nov?trialresultid=16968.
- 59.Mall MA, Galietta LJ. Targeting ion channels in cystic fibrosis. J Cyst Fibros. 2015;14:561–570. doi: 10.1016/j.jcf.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 60.Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, et al. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322:590–594. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- 61.Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008;134:1019–1029. doi: 10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455:1210–1215. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
- 63.Knowles MR, Clarke LL, Boucher RC. Activation by extracellular nucleotides of chloride secretion in the airway epithelia of patients with cystic fibrosis. N Engl J Med. 1991;325:533–538. doi: 10.1056/NEJM199108223250802. [DOI] [PubMed] [Google Scholar]
- 64.Boucher RC, Cheng EH, Paradiso AM, Stutts MJ, Knowles MR, Earp HS. Chloride secretory response of cystic fibrosis human airway epithelia: preservation of calcium but not protein kinase C– and A–dependent mechanisms. J Clin Invest. 1989;84:1424–1431. doi: 10.1172/JCI114316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tarran R, Button B, Boucher RC. Regulation of normal and cystic fibrosis airway surface liquid volume by phasic shear stress. Annu Rev Physiol. 2006;68:543–561. doi: 10.1146/annurev.physiol.68.072304.112754. [DOI] [PubMed] [Google Scholar]
- 66.Button B, Picher M, Boucher RC. Differential effects of cyclic and constant stress on ATP release and mucociliary transport by human airway epithelia. J Physiol. 2007;580:577–592. doi: 10.1113/jphysiol.2006.126086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dodd ME, Prasad SA. Physiotherapy management of cystic fibrosis. Chron Respir Dis. 2005;2:139–149. doi: 10.1191/1479972305cd078ra. [DOI] [PubMed] [Google Scholar]
- 68.Wheatley CM, Baker SE, Morgan MA, Martinez MG, Liu B, Rowe SM, et al. Moderate intensity exercise mediates comparable increases in exhaled chloride as albuterol in individuals with cystic fibrosis. Respir Med. 2015;109:1001–1011. doi: 10.1016/j.rmed.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Avella M, Loriol C, Boulukos K, Borgese F, Ehrenfeld J. SLC26A9 stimulates CFTR expression and function in human bronchial cell lines. J Cell Physiol. 2011;226:212–223. doi: 10.1002/jcp.22328. [DOI] [PubMed] [Google Scholar]
- 70.Bertrand CA, Zhang R, Pilewski JM, Frizzell RA. SLC26A9 is a constitutively active, CFTR-regulated anion conductance in human bronchial epithelia. J Gen Physiol. 2009;133:421–438. doi: 10.1085/jgp.200810097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Salomon JJ, Spahn S, Wang X, Fullekrug J, Bertrand CA, Mall MA. Generation and functional characterization of epithelial cells with stable expression of SLC26A9 Cl− channels. Am J Physiol Lung Cell Mol Physiol. 2016;310:L593–L602. doi: 10.1152/ajplung.00321.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li H, Salomon JJ, Sheppard DN, Mall MA, Galietta LJ. Bypassing CFTR dysfunction in cystic fibrosis with alternative pathways for anion transport. Curr Opin Pharmacol. 2017;34:91–97. doi: 10.1016/j.coph.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 73.Burrows EF, Southern KW, Noone PG. Sodium channel blockers for cystic fibrosis. Cochrane Database Syst Rev. 2014;4:CD005087. doi: 10.1002/14651858.CD005087.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hirsh AJ. Altering airway surface liquid volume: inhalation therapy with amiloride and hyperosmotic agents. Adv Drug Deliv Rev. 2002;54:1445–1462. doi: 10.1016/s0169-409x(02)00161-8. [DOI] [PubMed] [Google Scholar]
- 75.Kerem E, Bistritzer T, Hanukoglu A, Hofmann T, Zhou Z, Bennett W, et al. Pulmonary epithelial sodium-channel dysfunction and excess airway liquid in pseudohypoaldosteronism. N Engl J Med. 1999;341:156–162. doi: 10.1056/NEJM199907153410304. [DOI] [PubMed] [Google Scholar]
- 76.Kohler D, App E, Schmitz-Schumann M, Wurtemberger G, Matthys H. Inhalation of amiloride improves the mucociliary and the cough clearance in patients with cystic fibroses. Eur J Respir Dis Suppl. 1986;146:319–326. [PubMed] [Google Scholar]
- 77.Zhou Z, Duerr J, Johannesson B, Schubert SC, Treis D, Harm M, et al. The ENaC-overexpressing mouse as a model of cystic fibrosis lung disease. J Cyst Fibros. 2011;10:S172–S182. doi: 10.1016/S1569-1993(11)60021-0. [DOI] [PubMed] [Google Scholar]
- 78.Vertex. Vertex reports third-quarter 2017 financial results. 2017 [cited 2018 May 14]. Available from: http://investors.vrtx.com/releasedetail.cfm?ReleaseID=1045401.
- 79.Crosby JR, Zhao C, Jiang C, Bai D, Katz M, Greenlee S, et al. Inhaled ENaC antisense oligonucleotide ameliorates cystic fibrosis–like lung disease in mice. J Cyst Fibros. 2017;16:671–680. doi: 10.1016/j.jcf.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 80.Scott DW, Walker MP, Sesma J, Wu B, Stuhlmiller TJ, Sabater JR, et al. SPX-101 is a novel epithelial sodium channel–targeted therapeutic for cystic fibrosis that restores mucus transport. Am J Respir Crit Care Med. 2017;196:734–744. doi: 10.1164/rccm.201612-2445OC. [DOI] [PubMed] [Google Scholar]
- 81.Lachowicz-Scroggins ME, Finkbeiner WE, Gordon ED, Yuan S, Zlock L, Bhakta NR, et al. Corticosteroid and long-acting β-agonist therapy reduces epithelial goblet cell metaplasia. Clin Exp Allergy. 2017;47:1534–1545. doi: 10.1111/cea.13015. [DOI] [PubMed] [Google Scholar]
- 82.Nadel JA, Burgel PR. The role of epidermal growth factor in mucus production. Curr Opin Pharmacol. 2001;1:254–258. doi: 10.1016/s1471-4892(01)00045-5. [DOI] [PubMed] [Google Scholar]
- 83.Woodruff PG, Wolff M, Hohlfeld JM, Krug N, Dransfield MT, Sutherland ER, et al. Safety and efficacy of an inhaled epidermal growth factor receptor inhibitor (BIBW 2948 BS) in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181:438–445. doi: 10.1164/rccm.200909-1415OC. [DOI] [PubMed] [Google Scholar]
- 84.Danahay H, Pessotti AD, Coote J, Montgomery BE, Xia D, Wilson A, et al. Notch2 is required for inflammatory cytokine-driven goblet cell metaplasia in the lung. Cell Reports. 2015;10:239–252. doi: 10.1016/j.celrep.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 85.Lafkas D, Shelton A, Chiu C, de Leon Boenig G, Chen Y, Stawicki SS, et al. Therapeutic antibodies reveal Notch control of transdifferentiation in the adult lung. Nature. 2015;528:127–131. doi: 10.1038/nature15715. [DOI] [PubMed] [Google Scholar]
- 86.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med. 2010;363:2233–2247. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Adler KB, Tuvim MJ, Dickey BF. Regulated mucin secretion from airway epithelial cells. Front Endocrinol (Lausanne) 2013;4:129. doi: 10.3389/fendo.2013.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhu Y, Ehre C, Abdullah LH, Sheehan JK, Roy M, Evans CM, et al. Munc13-2−/− baseline secretion defect reveals source of oligomeric mucins in mouse airways. J Physiol. 2008;586:1977–1992. doi: 10.1113/jphysiol.2007.149310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fuchs HJ, Borowitz DS, Christiansen DH, Morris EM, Nash ML, Ramsey BW, et al. Pulmozyme Study Group. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. N Engl J Med. 1994;331:637–642. doi: 10.1056/NEJM199409083311003. [DOI] [PubMed] [Google Scholar]
- 90.Tam J, Nash EF, Ratjen F, Tullis E, Stephenson A. Nebulized and oral thiol derivatives for pulmonary disease in cystic fibrosis. Cochrane Database Syst Rev. 2013;7:CD007168. doi: 10.1002/14651858.CD007168.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zeng Z, Yang D, Huang X, Xiao Z. Effect of carbocisteine on patients with COPD: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2017;12:2277–2283. doi: 10.2147/COPD.S140603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ehre C, Rushton Z, Yu J, Gentzsch M, Esther CR, Hill DB, et al. Pharmacological approaches to clear mucus from the lungs. Pediatr Pulmonol. 2015;50:225–226. [Google Scholar]
- 93.Ehre C, Rushton ZL, Wang B, Hothem LN, Morrison CB, Fontana NC, et al. An improved inhaled mucolytic to treat airway muco-obstructive diseases. Am J Respir Crit Care Med. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.