Abstract
The lung is continuously exposed to particles, toxicants, and microbial pathogens that are cleared by a complex mechanical, innate, and acquired immune system. Mucociliary clearance, mediated by the actions of diverse conducting airway and submucosal gland epithelial cells, plays a critical role in a multilayered defense system by secreting fluids, electrolytes, antimicrobial and antiinflammatory proteins, and mucus onto airway surfaces. The mucociliary escalator removes particles and pathogens by the mechanical actions of cilia and cough. Abnormalities in mucociliary clearance, whether related to impaired fluid secretion, ciliary dysfunction, lack of cough, or the disruption of epithelial cells lining the respiratory tract, contribute to the pathogenesis of common chronic pulmonary disorders. Although mucus and other airway epithelial secretions play a critical role in protecting the lung during acute injury, impaired mucus clearance after chronic mucus hyperproduction causes airway obstruction and infection, which contribute to morbidity in common pulmonary disorders, including chronic obstructive pulmonary disease, asthma, idiopathic pulmonary fibrosis, cystic fibrosis, bronchiectasis, and primary ciliary dyskinesia. In this summary, the molecular and cellular mechanisms mediating airway mucociliary clearance, as well as the role of goblet cell metaplasia and mucus hyperproduction, in the pathogenesis of chronic respiratory diseases are considered.
Keywords: mucus hyperproduction, goblet cell, airway ciliated cells
Human conducting airways are lined primarily by a pseudostratified epithelium consisting of ciliated cells and lesser numbers of secretory (club) and goblet cells, as well as far fewer numbers of “brush” and neuroendocrine cells. Basal cells, located below the surface epithelial cell layer, are found throughout conducting airways, serve as progenitor cells from which ciliated, secretory, and goblet cells differentiate during repair. Cartilaginous airways contain numerous submucosal glands, containing a diversity of serous, secretory, goblet, myoepithelial, and ciliated cells (Figure 1) (1, 2). Epithelial cell types lining the airways are derived from foregut endodermal progenitors that express the transcription factor NKX2-1 (NK2 homeobox 1; thyroid transcription factor 1 [TTF-1]). TTF-1 is required for differentiation of respiratory epithelial cells during lung morphogenesis (3). TTF-1–expressing lung progenitor cells are specified in the early embryo (4) as epithelial cells lining the trachea and esophagus; they differentiate, and the two tubes separate. Conducting airway epithelial progenitors express SOX2 (SRY box 2) and are distinguished from the cells that express SOX9, the latter expressed in cells that form the peripheral or alveolar regions of the lung (5–7). Basal cells, expressing the transcription factors TP63 (tumor protein 63) and SOX2, serve as primary progenitors of goblet, ciliated, and other secretory cells. Ciliated cells, goblet cells, and club cells are derived from basal or other secretory cells via transcriptional networks that are regulated by Notch (8–10). Signaling between airway epithelial cells via Notch ligands (e.g., Jagged1 [JAG1] and Jagged2 [JAG2]) activates Notch receptors that determine epithelial cell fate. In the absence of Notch activity, basal/club progenitors differentiate into ciliated cells; increasing levels of Notch activity cause secretory cell differentiation; and high levels of Notch cause goblet cell differentiation. The diverse epithelial cells lining the lung are readily identified by their structural characteristics, gene and protein expression patterns, and unique functions. Disruption of normal repair processes and loss of normal differentiation of the airway epithelia, causing goblet or squamous cell metaplasia, impair mucociliary clearance in the setting of chronic lung disease.
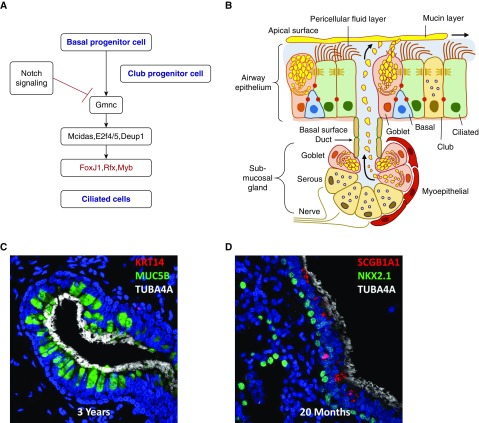
Figure 1.
(A) Schematic demonstrating the genetic network controlling ciliated cell differentiation from basal or club cells. In the absence of Notch signaling, Gmnc (geminin coiled-coil domain containing, also called geminin coiled-coil containing protein 1 [gemc1]) controls a transcriptional pathway activity to regulate ciliary proteins. Forkhead box J1 (FoxJ1) regulates their assembly. (B) The airway surface and submucosal gland mediating mucociliary clearance. Reprinted by permission from Reference 1. (C and D) Confocal images of human submucosal gland and airway surfaces. Tubulin α-4A (TUBA4A; white) stains cilia. Mucin 5B (MUC5B; green) stains goblet cells. Reprinted by permission from Reference 2. (D) Club cells (red) are stained for secretoglobin family 1A member 1 (SCGB1A1). NK2 homeobox 1 (NKX2.1; green) stains epithelial cell nuclei. E2F4/5 = E2F transcription factor; DEUP1 = deuterosome assembly protein 1; KRT14 = keratin 14; MCIDAS = multiciliate differentiation and DNA synthesis associated cell cycle protein; Myb = MYB proto-oncogene; Rfx = regulatory factor X.
Ciliated Cell Differentiation and Function
Ciliated cells are readily identified by their multiple apical, motile cilia that are composed of unique structural proteins (e.g., dyneins) and the motor proteins that drive their coordinated directional beating critical for mucociliary clearance. Although the biomechanical roles of multicilia in epithelial cells are well recognized as a critical component of mucociliary clearance, there is increasing recognition that airway ciliated cells sense and respond to both mechanical and irritant stimulation (reviewed in [11]). Airway basal and club cells serve as the primary progenitors of ciliated cells, the latter generally considered as terminally differentiated cells. In the absence of Notch signaling, airway progenitors differentiate into ciliated cells controlled by GMNC (geminin coiled-coil domain containing, also called geminin coiled-coil containing protein 1 [gemc1]), a master regulator of ciliated cell fate. GMNC activates multiciliate differentiation and DNA synthesis associated cell cycle protein (MCDIS), E2F transcription factor (E2F4/5), and deuterosome assembly protein 1 (DEUPI), causing centriole amplification and organization of apical microtubules required for cilia formation. Activation of a transcriptional network regulated by forkhead box J1 (FOXJ1), regulatory factor X (RFX), and MYB proto-oncogene (MYB) activates the transcription and assembly of ciliary proteins (12–16) (Figure 1).
Mutations in more than 35 genes encoding the structural proteins forming cilia (e.g., dyneins and microtubular motor proteins) cause severe chronic lung disorders termed primary ciliary dyskinesias (PCDs). These disorders are diagnosed by clinical symptoms of chronic otitis, sinusitis, pulmonary infections, and bronchiectasis together by identification of causal gene mutations, markedly decreased respiratory nitric oxide, videomicroscopy of airway cell brushings or biopsies, and ultrastructural changes in ciliary ultrastructure (17–19). PCD is variably associated with abnormalities in organ situs (e.g., situs inversus, as seen in Kartagener’s syndrome). The severity of sinus and pulmonary disease caused by mutations in the cilia-related proteins in PCD highlights the critical role of ciliated cell function and mucociliary clearance in innate defense of the respiratory tract. Disruption of ciliated cell functions is seen in many chronic lung diseases (e.g., chronic obstructive pulmonary disease [COPD], cystic fibrosis [CF], and asthma), contributing to infection, morbidity, and mortality in these disorders (20–22).
Molecular Control of Goblet Cell Differentiation: Mucus Hyperproduction
Basal and other secretory cells serve as common progenitors of both ciliated and goblet cells. Genetic networks controlling goblet cell identity and mucus production by goblet cells are highly distinct from those regulating basal and ciliated cells. During goblet cell metaplasia, increased Notch signaling suppresses p63 and GMNC and activates SPDEF (Sam-pointed domain Ets-like factor) (Figure 2). SPDEF regulates a transcriptional network required for airway goblet cell differentiation and the synthesis and packaging of mucins (23–25). SPDEF is required for normal airway goblet cell differentiation as well as airway and submucosal gland mucus production in mice. Expression of SPDEF in airway epithelial cells is sufficient to cause goblet cell differentiation (24). Although goblet cells are found throughout the normal airway and in submucosal glands, their differentiation is strongly induced by environmental factors, including exposure to toxicants, irritants, microorganisms, particles, and inflammatory mediators. A schematic of the gene regulatory networks controlling goblet cell differentiation and mucin gene expression is shown in Figure 2. Goblet cell differentiation and mucus hyperproduction accompany and contribute to chronic lung disease. Increased production and secretion of mucus by goblet cells causes airway obstruction and infection, as seen in COPD, CF, PCD, and asthma. Because goblet cells actively secrete diverse inflammatory mediators, they may directly contribute to recruitment and activation of immune and stromal cells.
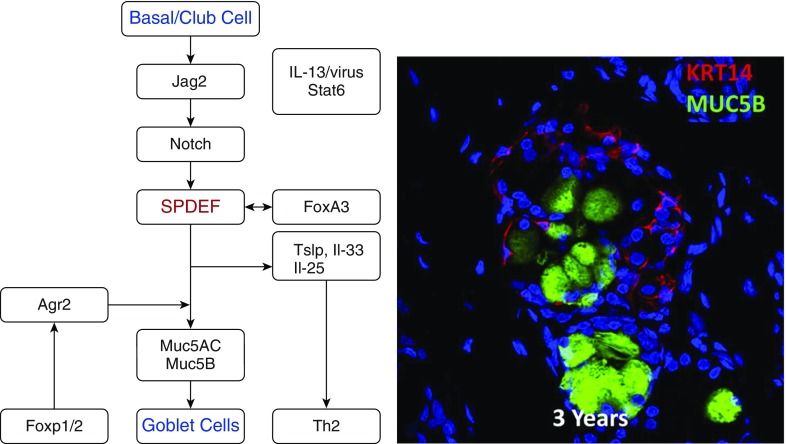
Figure 2.
Left panel shows a network of genes regulating airway goblet cell differentiation. Sam-pointed domain Ets-like factor (SPDEF) is a transcription factor regulating for airway goblet cell differentiation. Increased Notch activates SPDEF, which regulates synthesis of mucins and influences pulmonary T-helper cell type 2 (Th2) inflammation and airway hyperactivity. Right panel is a confocal immunofluorescence image of human submucosal gland. Keratin 14 (KRT14; red) stains myoepithelial cells, MUC5B (green) stains goblet cells. Agr2 = anterior gradient 2; FoxA3 = forkhead box A3; FoxP1/2 = forkhead box protein P1/2; IL = interleukin; Jag2 = Jagged2; Muc5AC = mucin 5AC; Stat6 = signal transducer and activator of transcription 6; Tslp = thymic stromal lymphopoietin.
Submucosal Glands Are Critical for Mucociliary Clearance
The combined surface area of airway submucosal glands is vastly greater than that of the conducting airway surface per se (26). Submucosal glands enter the airways via a single duct lined by a diversity of epithelial cells, including serous cells that regulate fluids in the collecting regions of the ducts. The acinar region of the submucosal gland is lined by goblet cells and serous cells that secrete a variety of innate immune proteins, mucins, and electrolytes that control the hydration of the secretions (Figure 2). Well innervated myoepithelial cells encircle the glands and mediate mucus secretion in response to neural inputs that can activate massive secretory responses from submucosal glands after stimulation by irritants and toxicants (26–29).
Submucosal glands secrete mucins, predominantly MUC5B (mucin 5B) and lesser amounts of MUC5AC, as well as a variety of innate host defense proteins and antimicrobial peptides, including lysozyme, lactoferrin, β-defensins, surfactant proteins SP-D and SP-A, and others (30). The proteinaceous products of submucosal glands are precisely balanced with fluid and electrolyte transport that enables rapid secretion and dispersal of mucus onto the airway surfaces and the movement of the mucus gel up the airway by ciliary activity. The importance of precise regulation of fluid and ion transport by submucosal glands and airway epithelial cells in lung homeostasis is highlighted by the severe pulmonary disease caused by mutations in cystic fibrosis transmembrane conductance regulator (CFTR) in which secretion of chloride and bicarbonate is impaired. Recurrent sinusitis, airway infection, bronchiectasis, and pulmonary tissue remodeling associated with cystic fibrosis are caused by the lack of chloride, bicarbonate, and fluid transport onto epithelial surfaces, disrupting mucociliary clearance and antimicrobial defenses.
Mucus and Mucins
Mucus is a complex mixture of mucins, diverse antimicrobial proteins, metabolites, fluids, and electrolytes whose abundance and composition vary along the cephalocaudal axis of the lung and in response to environmental exposures and inflammation. Airway mucins represent a family of polymeric, highly glycosylated proteins produced by airway epithelial cells and submucosal glands. Mucins can be subdivided into 1) nonpolymerizing, secreted mucins; 2) cell-associated mucins that are anchored at cell surfaces; and 3) gel-forming mucins, the most abundant of which are MUC5B and MUC5AC. There are at least 21 genes encoding human mucins, of which 14 are expressed in airway epithelial cells (26–30). MUC1, MUC4, MUC16, MUC20, MUC21, and MUC22 are cell-tethered mucins whose precise roles in airway biology are not well established. MUC5B and MUC5AC are secreted and variably expressed by both serous club cells and goblet cells in conducting airways. MUC5B is most highly expressed by goblet cells of submucosal glands, whereas MUC5AC is more highly expressed by airway goblet cells. MUC2, expressed at high levels in the gastrointestinal tract, is not normally present in the human airways, but it is variably detected in patients with CF and COPD. MUC7, a monomeric secreted mucin, and MUC19, a polymeric secreted mucin, are both expressed primarily by salivary glands and are expressed in the airways, where they may play roles in innate host defense (31, 32).
At baseline, both club cells and airway goblet cells produce and secrete mucins. Mucus-containing granules are exocytosed and rapidly hydrated. Mucins undergo rapid expansion during hydration and undergo quaternary structural changes as the mucociliary gel layers are formed. Fluids and secreted proteins, electrolytes, calcium, and metabolites interact with the mucus and create the periciliary fluid layer on which the gel-forming mucins, MUC5B, MUC5AC, and accompanying cargoes move up the airways by ciliary activity. Airway submucosal glands are capable of rapid, massive secretory activity in response to irritants and neural stimulation, cyclic AMP, purinoreceptor and muscarinic activation, and substance P (26, 27). Thus, a precise balance between airway fluid homeostasis and mucus composition is required for normal mucociliary clearance.
Submucosal goblet cells release MUC5B as mucin bundles or extended mucus “strands” that are coated by lesser amounts of MUC5AC that coats the bundles after release of MUC5B from the submucosal glands. MUC5AC coats the strands of MUC5B and may provide anchoring activity that slows the transport of the mucus gel by the movement of periciliary fluids up the airway (30, 33). Recent insights into the role of MUC5B and MUC5AC are derived from studies in transgenic mice in which each gene was deleted. Gene targeting of MUC5B caused severe rhinosinusitis, as well as lung pathology associated with failed mucociliary clearance, accumulation of particles in the nasal cavity and airways, and chronic infection (34). In contrast, deletion of MUC5AC in mice did not cause overt sinus or airway disease but ameliorated allergy-induced airway hyperresponsiveness without changing Th2 (T-helper cell type 2) inflammation, supporting its role in airway plugging and asthma-like inflammation (35). MUC5AC is required for the clearance of nematodes and inhibits neutrophilic inflammation during pulmonary viral infection, supporting its importance in innate immunity. Likewise, MUC1 is immunomodulatory, suppressing responses to bacterial infection, perhaps by inhibition of Toll-like receptor signaling (36).
Role of Goblet Cell Differentiation in the Regulation of Innate Immunity
Although goblet cell metaplasia and mucus production are generally considered a consequence of environmental exposures, goblet cells also play direct roles in the regulation of innate immunity by modulating immunological responses to infections and allergens. Although expression of SPDEF in airway epithelial cells in vivo and in vitro causes goblet cell metaplasia and enhances the expression of mucins, SPDEF directs the expression of Th2 cytokines and chemokines by the goblet cells that, in turn, recruits inflammatory cells (25). SPDEF expression is increased in lung tissue from patients with asthma, CF, and COPD, being associated with goblet cell metaplasia in mucus hyperproduction. SPDEF activates FOXA3 (forkhead box A3), and together these two transcription factors strongly inhibit Th1 and interferon (IFN) responses to viruses and activate Th2 inflammation, at least in part by increasing thymic stromal lymphopoietin (TSLP), interleukin (IL)-25, eotaxin, and IL-33 gene expression in the epithelium to recruit and activate innate lymphocytes (ILC2 cells), dendritic cells, and lymphocytes (23). Thus, SPDEF, expressed selectively in airway goblet cells, activates Th2 inflammation typical of asthma. SPDEF has both nuclear and cytoplasmic functions in goblet cells, inhibiting IFN and nuclear factor-κB (NF-κB) (37). In transgenic mice, SPDEF was required for goblet cell differentiation, airway hyperreactivity, and Th2 inflammation after aeroallergen challenge (25). Thus, goblet cells play important cell-autonomous roles in innate immunity by enhancing Th2 responses and inhibiting NF-κB and IFN signaling. Taken together, SPDEF and FOXA3 interact in a complex genetic network enhancing Th2 inflammation and maintaining goblet cell differentiation and airway hyperreactivity, typical of asthma-like pulmonary responses.
Goblet Cell Metaplasia in Idiopathic Pulmonary Fibrosis
Idiopathic pulmonary fibrosis (IPF) is a relatively common chronic lung disease associated with significant morbidity and mortality. Extensive tissue remodeling in IPF causes the loss of the alveolar gas exchange region of the lung. Normal parenchymal tissue is replaced by extensive fibrosis and epithelial dysplasia, including formation of “honeycombed” cysts. Normal alveolar types I and II (AT1 and AT2, respectively) cells lining alveolar surfaces are replaced by lung epithelial cells with features more closely associated with proximal rather than peripheral alveolar regions of the lung. Immunofluorescence staining of IPF tissue demonstrated extensive goblet cell metaplasia and expression of MUC5B, p63, and cytokeratins typical of conducting airways, consistent with “proximalization” or “bronchiolization” of the lung parenchyma in IPF (38). My research group used RNA sequencing of purified airway epithelial cells from normal and IPF lung tissue and demonstrated the loss of normal AT2 cell identity in IPF (39). Although normal adult human lung cells consisted of well-differentiated AT2 cells expressing genes related to surfactant protein and lipid homeostasis, epithelial cells isolated from IPF lungs contained cells expressing high levels of goblet cell and basal cell markers, including MUC5B, SRYbox2 (SOX2), PAX9 (paired box 9), SPDEF, TP63, and KRT5 (keratin 5). Many cells coexpressing AT1, AT2, and conducting airway markers were observed, findings never seen during normal lung development or at homeostasis. These findings indicate that IPF cells had lost normal epithelial cell–type lineage restrictions. RNA sequencing identified the expression of inflammatory cytokines, chemokines, and growth factors predicted to recruit and activate inflammatory cells that are characteristic of IPF. Bioinformatic analysis of the RNA data demonstrated the increased activity of canonical signaling pathways, including HIPPO/YAP (Yes-associated protein), PI3K (phosphatidylinositol 3-kinase), mTOR (mammalian target of rapamycin), wingless-type MMTV integration site family, member (WNT), and transforming growth factor (TGF)-β/SMAD in the abnormal epithelial cells isolated from IPF lung (39, 40). Taken together, RNA signatures in IPF demonstrate the replacement or transdifferentiation of lung epithelial progenitor cells in IPF, indicating profound loss of normal epithelial cell identity and the loss of alveolar tissues in IPF. Identification of signaling and transcriptional programs active in the abnormal IPF epithelia as well as in other chronic pulmonary diseases may provide the framework for the development of new diagnostic and therapeutic strategies to address currently life-threatening disorders.
Supplementary Material
Footnotes
Supported by National Institutes of Health grants U01HL110964, R01HL095580, and U01HL122642.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Whitsett JA, Alenghat T. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat Immunol. 2015;16:27–35. doi: 10.1038/ni.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Du Y, Kitzmiller JA, Sridharan A, Perl AK, Bridges JP, Misra RS, et al. Lung Gene Expression Analysis (LGEA): an integrative web portal for comprehensive gene expression data analysis in lung development. Thorax. 2017;72:481–484. doi: 10.1136/thoraxjnl-2016-209598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kimura S, Hara Y, Pineau T, Fernandez-Salguero P, Fox CH, Ward JM, et al. The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 1996;10:60–69. doi: 10.1101/gad.10.1.60. [DOI] [PubMed] [Google Scholar]
- 4.Perl AK, Wert SE, Nagy A, Lobe CG, Whitsett JA. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc Natl Acad Sci USA. 2002;99:10482–10487. doi: 10.1073/pnas.152238499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danopoulos S, Alonso I, Thornton ME, Grubbs BH, Bellusci S, Warburton D, et al. Human lung branching morphogenesis is orchestrated by the spatiotemporal distribution of ACTA2, SOX2, and SOX9. Am J Physiol Lung Cell Mol Physiol. 2018;314:L144–L149. doi: 10.1152/ajplung.00379.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nikolić MZ, Caritg O, Jeng Q, Johnson JA, Sun D, Howell KJ, et al. Human embryonic lung epithelial tips are multipotent progenitors that can be expanded in vitro as long-term self-renewing organoids. Elife. 2017;6:e26575. doi: 10.7554/eLife.26575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Que J, Luo X, Schwartz RJ, Hogan BL. Multiple roles for Sox2 in the developing and adult mouse trachea. Development. 2009;136:1899–1907. doi: 10.1242/dev.034629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morimoto M, Liu Z, Cheng HT, Winters N, Bader D, Kopan R. Canonical Notch signaling in the developing lung is required for determination of arterial smooth muscle cells and selection of Clara versus ciliated cell fate. J Cell Sci. 2010;123:213–224. doi: 10.1242/jcs.058669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morimoto M, Nishinakamura R, Saga Y, Kopan R. Different assemblies of Notch receptors coordinate the distribution of the major bronchial Clara, ciliated and neuroendocrine cells. Development. 2012;139:4365–4373. doi: 10.1242/dev.083840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siebel C, Lendahl U. Notch signaling in development, tissue homeostasis, and disease. Physiol Rev. 2017;97:1235–1294. doi: 10.1152/physrev.00005.2017. [DOI] [PubMed] [Google Scholar]
- 11.Bloodgood RA. Sensory reception is an attribute of both primary cilia and motile cilia. J Cell Sci. 2010;123:505–509. doi: 10.1242/jcs.066308. [DOI] [PubMed] [Google Scholar]
- 12.Arbi M, Pefani DE, Kyrousi C, Lalioti ME, Kalogeropoulou A, Papanastasiou AD, et al. GemC1 controls multiciliogenesis in the airway epithelium. EMBO Rep. 2016;17:400–413. doi: 10.15252/embr.201540882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campbell EP, Quigley IK, Kintner C. Foxn4 promotes gene expression required for the formation of multiple motile cilia. Development. 2016;143:4654–4664. doi: 10.1242/dev.143859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Didon L, Zwick RK, Chao IW, Walters MS, Wang R, Hackett NR, et al. RFX3 modulation of FOXJ1 regulation of cilia genes in the human airway epithelium. Respir Res. 2013;14:70. doi: 10.1186/1465-9921-14-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stubbs JL, Vladar EK, Axelrod JD, Kintner C. Multicilin promotes centriole assembly and ciliogenesis during multiciliate cell differentiation. Nat Cell Biol. 2012;14:140–147. doi: 10.1038/ncb2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou F, Narasimhan V, Shboul M, Chong YL, Reversade B, Roy S. Gmnc is a master regulator of the multiciliated cell differentiation program. Curr Biol. 2015;25:3267–3273. doi: 10.1016/j.cub.2015.10.062. [DOI] [PubMed] [Google Scholar]
- 17.Lucas JS, Barbato A, Collins SA, Goutaki M, Behan L, Caudri D, et al. European Respiratory Society guidelines for the diagnosis of primary ciliary dyskinesia. Eur Respir J. 2017;49:1601090. doi: 10.1183/13993003.01090-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenfeld M, Ostrowski LE, Zariwala MA. Primary ciliary dyskinesia: keep it on your radar. Thorax. 2018;73:101–102. doi: 10.1136/thoraxjnl-2017-210776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shapiro AJ, Zariwala MA, Ferkol T, Davis SD, Sagel SD, Dell SD, et al. Genetic Disorders of Mucociliary Clearance Consortium. Diagnosis, monitoring, and treatment of primary ciliary dyskinesia: PCD Foundation consensus recommendations based on state of the art review. Pediatr Pulmonol. 2016;51:115–132. doi: 10.1002/ppul.23304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bustamante-Marin XM, Ostrowski LE. Cilia and mucociliary clearance. Cold Spring Harb Perspect Biol. 2017;9:a028241. doi: 10.1101/cshperspect.a028241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Button B, Anderson WH, Boucher RC. Mucus hyperconcentration as a unifying aspect of the chronic bronchitic phenotype. Ann Am Thorac Soc. 2016;13(Suppl 2):S156–S162. doi: 10.1513/AnnalsATS.201507-455KV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou-Suckow Z, Duerr J, Hagner M, Agrawal R, Mall MA. Airway mucus, inflammation and remodeling: emerging links in the pathogenesis of chronic lung diseases. Cell Tissue Res. 2017;367:537–550. doi: 10.1007/s00441-016-2562-z. [DOI] [PubMed] [Google Scholar]
- 23.Chen G, Korfhagen TR, Karp CL, Impey S, Xu Y, Randell SH, et al. Foxa3 induces goblet cell metaplasia and inhibits innate antiviral immunity. Am J Respir Crit Care Med. 2014;189:301–313. doi: 10.1164/rccm.201306-1181OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen G, Korfhagen TR, Xu Y, Kitzmiller J, Wert SE, Maeda Y, et al. SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J Clin Invest. 2009;119:2914–2924. doi: 10.1172/JCI39731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajavelu P, Chen G, Xu Y, Kitzmiller JA, Korfhagen TR, Whitsett JA. Airway epithelial SPDEF integrates goblet cell differentiation and pulmonary Th2 inflammation. J Clin Invest. 2015;125:2021–2031. doi: 10.1172/JCI79422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Widdicombe JH, Wine JJ. Airway gland structure and function. Physiol Rev. 2015;95:1241–1319. doi: 10.1152/physrev.00039.2014. [DOI] [PubMed] [Google Scholar]
- 27.Ma J, Rubin BK, Voynow JA. Mucins, mucus, and goblet cells. Chest. 2018;154:169–176. doi: 10.1016/j.chest.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Oh EJ, Mazzone SB, Canning BJ, Weinreich D. Reflex regulation of airway sympathetic nerves in guinea-pigs. J Physiol. 2006;573:549–564. doi: 10.1113/jphysiol.2005.104661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tos M. Distribution of mucus producing elements in the respiratory tract: differences between upper and lower airway. Eur J Respir Dis Suppl. 1983;128:269–279. [PubMed] [Google Scholar]
- 30.Bonser LR, Erle DJ. Airway mucus and asthma: the role of MUC5AC and MUC5B. J Clin Med. 2017;6:112. doi: 10.3390/jcm6120112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Culp DJ, Robinson B, Cash MN, Bhattacharyya I, Stewart C, Cuadra-Saenz G. Salivary mucin 19 glycoproteins: innate immune functions in Streptococcus mutans-induced caries in mice and evidence for expression in human saliva. J Biol Chem. 2015;290:2993–3008. doi: 10.1074/jbc.M114.597906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan H, Bobek LA. Regulation of human MUC7 mucin gene expression by cigarette smoke extract or cigarette smoke and Pseudomonas aeruginosa lipopolysaccharide in human airway epithelial cells and in MUC7 transgenic mice. Open Respir Med J. 2010;4:63–70. doi: 10.2174/1874306401004010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ermund A, Meiss LN, Rodriguez-Pineiro AM, Bähr A, Nilsson HE, Trillo-Muyo S, et al. The normal trachea is cleaned by MUC5B mucin bundles from the submucosal glands coated with the MUC5AC mucin. Biochem Biophys Res Commun. 2017;492:331–337. doi: 10.1016/j.bbrc.2017.08.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roy MG, Livraghi-Butrico A, Fletcher AA, McElwee MM, Evans SE, Boerner RM, et al. Muc5b is required for airway defence. Nature. 2014;505:412–416. doi: 10.1038/nature12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Evans CM, Raclawska DS, Ttofali F, Liptzin DR, Fletcher AA, Harper DN, et al. The polymeric mucin Muc5ac is required for allergic airway hyperreactivity. Nat Commun. 2015;6:6281. doi: 10.1038/ncomms7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim KC, Lillehoj EP. MUC1 mucin: a peacemaker in the lung. Am J Respir Cell Mol Biol. 2008;39:644–647. doi: 10.1165/rcmb.2008-0169TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Korfhagen TR, Kitzmiller J, Chen G, Sridharan A, Haitchi HM, Hegde RS, et al. SAM-pointed domain ETS factor mediates epithelial cell-intrinsic innate immune signaling during airway mucous metaplasia. Proc Natl Acad Sci USA. 2012;109:16630–16635. doi: 10.1073/pnas.1208092109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Plantier L, Crestani B, Wert SE, Dehoux M, Zweytick B, Guenther A, et al. Ectopic respiratory epithelial cell differentiation in bronchiolised distal airspaces in idiopathic pulmonary fibrosis. Thorax. 2011;66:651–657. doi: 10.1136/thx.2010.151555. [DOI] [PubMed] [Google Scholar]
- 39.Xu Y, Mizuno T, Sridharan A, Du Y, Guo M, Tang J, et al. Single-cell RNA sequencing identifies diverse roles of epithelial cells in idiopathic pulmonary fibrosis. JCI Insight. 2016;1:e90558. doi: 10.1172/jci.insight.90558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gokey JJ, Sridharan A, Xu Y, Green J, Carraro G, Stripp BR, et al. Active epithelial Hippo signaling in idiopathic pulmonary fibrosis. JCI Insight. 2018;3:e98738. doi: 10.1172/jci.insight.98738. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.