Abstract
Idiopathic pulmonary fibrosis (IPF) is localized to the lung, is characterized by a pattern of heterogeneous, subpleural patches of fibrotic, remodeled lung, and is associated with a median survival of 3–5 years after diagnosis. A common gain-of-function MUC5B promoter variant, rs35705950, is the strongest risk factor (genetic and otherwise), accounting for at least 30% of the total risk of developing IPF. The MUC5B promoter variant can be used to identify individuals in the preclinical phase of this progressive disease, and, in the IPF lung, we have found that MUC5B is specifically overexpressed in bronchoalveolar epithelium. Thus, MUC5B represents a key molecule to understand the mechanisms that appear to initiate the fibroproliferative process in the bronchoalveolar epithelium. Moreover, focusing on MUC5B may provide a unique opportunity to define the early molecular events that lead to, and potentially prevent, the development of IPF.
Keywords: IPF, MUC5B, idiopathic pulmonary fibrosis
Idiopathic pulmonary fibrosis (IPF) is localized to the lung, is characterized by a pattern of heterogeneous, subpleural patches of fibrotic, remodeled lung, and has a median survival of 3–5 years after diagnosis (1). IPF affects 5 million people worldwide, disproportionately affects men, is associated with cigarette smoking (2, 3), increases with age, is inexplicably increasing in prevalence (4, 5), and is likely underdiagnosed (4, 6). Although IPF takes years to develop, most patients with IPF are diagnosed in the advanced stage, and current therapies slow disease progression with little impact on overall survival (7, 8). Earlier diagnosis of IPF will detect subjects with a lower burden of fibrotic lung disease (9, 10), and may create the opportunity to predict and prevent the progression of pulmonary fibrosis before the lung has been irreversibly compromised by extensive scarring and fibrosis. The scientific premise of this presentation is based on recent data from our laboratories and others that inform an emerging understanding of IPF pathogenesis based on the early clinical phenotype of IPF that is linked to enhanced production of the mucin MUC5B in the bronchoalveolar epithelia. Based on these findings, we now propose to focus on the interaction of MUC5B, the airway epithelia, and repair/regeneration to define the biological signatures and biomarkers that will allow us to identify pulmonary fibrosis in its preclinical phase and enable us to move toward prevention strategies for this progressive disease.
IPF is a complex, heterogeneous genetic disorder that is associated with rare and common sequence variants in many genes (MUC5B, SFTPC, SFTPA2, RTEL1, TERT, and hTR [11–16]), 11 genetic loci (17, 18), and multiple emerging epigenetic (19–23) and transcriptional (24–27) profiles. However, the MUC5B promoter variant, rs35705950, is the strongest and most validated risk factor (genetic and otherwise) for IPF and preclinical pulmonary fibrosis (PrePF). We have found that: 1) a common gain-of-function MUC5B promoter variant rs35705950 accounts for at least 30% of the total risk of developing IPF (28); 2) the contribution of the MUC5B promoter variant, rs35705950, to the risk of IPF has been confirmed in 10 independent studies (28–36), including our genome-wide association study (a standard method to interrogate the entire genome using common genetic variants; odds ratio for T [minor] allele = 4.51; 95% confidence interval = 3.91–5.21; P = 7.2 × 10−95) (17); 3) rs35705950 can potentially be used to identify individuals with PrePF (37, 38), and is predictive of radiographic progression in PrePF (38); and 4) MUC5B appears to be involved in the pathogenesis of IPF. Specifically, MUC5B message and protein are expressed in bronchoalveolar epithelia in IPF (39, 40) and in IPF honeycomb cysts (28, 39).
Interstitial lung abnormalities (ILAs) on high-resolution computed tomography (HRCT) scans were initially reported in asymptomatic relatives of patients with familial IPF (41) and in the elderly (42). Similar to patients with IPF, ILAs in asymptomatic subjects are associated with advanced age (37, 43–46), cigarette smoking (43–49), reduced lung volume (44, 46, 47, 50), and decreased exercise tolerance (51). Moreover, the MUC5B promoter variant, rs35705950, is associated with a higher prevalence of ILAs on HRCT scan (37), and is predictive of radiographic progression (38). These findings suggest that ILAs on HRCT scan are a precursor of IPF. However, ILAs are not specific, and include nonfibrotic and fibrotic HRCT defects, and, consequently, the prevalence of ILAs (>5% in the general population ≥50 yr of age [37, 43–51]) is orders of magnitude higher than the prevalence of IPF.
To address the nonspecificity of ILAs, we have defined a novel entity—PrePF. For our purposes, PrePF will be defined as: abnormalities on chest HRCT consistent with probable or definite fibrosis (e.g., bilateral subpleural reticular changes, honeycombing, or traction bronchiectasis—radiographic findings are concordant with IPF [1, 6, 52]) occurring in asymptomatic subjects of 40 years of age or older recruited from at-risk populations (first-degree relatives of patients with IPF). Our results indicate that 1.8% of the general population aged 50 years or older have PrePF (37), approximately 75% of those with PrePF progress radiographically during a 5- to 6-year period of observation (38), and radiographic progression of PrePF is associated with a decline in lung volumes and increased mortality (38). Kropski and colleagues (53) have shown that PrePF is present in 15–20% of asymptomatic relatives of families with familial IPF (≥two family members with IPF). In aggregate, these findings suggest that PrePF is an early diagnostic sign of IPF and a harbinger of progressive fibrosis, occurring in 1.8% of the general population and 15–20% of high-risk populations of those aged 40 years or greater.
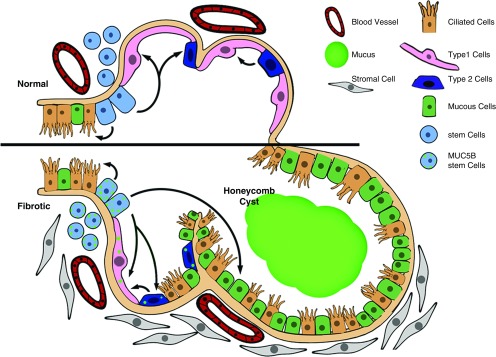
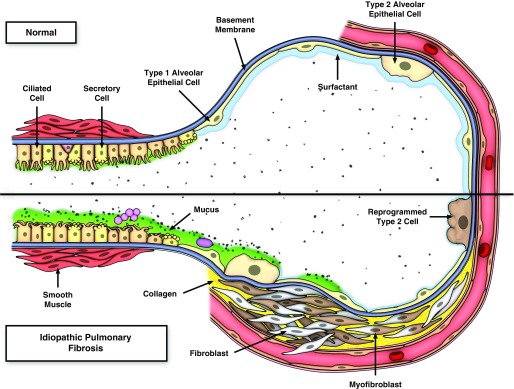
Given the above considerations and the emerging understanding of the pathogenesis of IPF, there are at least two related concepts that link enhanced production of MUC5B in the bronchoalveolar region of the lung to the development of pulmonary fibrosis (54). One line of reasoning focuses on the intracellular relationship between overexpression of MUC5B, metabolic/stress-responsive changes in MUC5B-producing cells, the involvement of the respiratory bronchiole, microscopic honeycomb cyst formation, and repair/regeneration of the distal airspace in IPF (55–58). Based on these considerations, as stem cells attempt to regenerate injured bronchiolar and alveolar epithelium, excess expression of MUC5B may disrupt normal developmental pathways and hijack the normal reparative mechanisms in the distal lung, resulting in chronic fibroproliferation and honeycomb cyst formation (Figure 1). A second line of reasoning focuses on the possibility that IPF is a mucociliary disease caused by recurrent injury/repair at the bronchoalveolar junction, which is initiated and exacerbated by overexpression of MUC5B leading to extracellular effects of reduced mucociliary clearance, retention of particles, and enhanced lung injury. Based on the relationship between the MUC5B promoter variant, rs35705950, and excess production of MUC5B specifically at the bronchoalveolar junction (40), too much MUC5B may impair mucociliary function (59–61), cause excess retention of inhaled substances (air pollutants, cigarette smoke, microorganisms, etc.), and, over time, the foci of lung injury may lead to scar tissue and persistent fibroproliferation that expands and displaces normal lung tissue (Figure 2). Lastly, we have recently found that lung tissue samples from approximately 40% of patients with IPF are highly enriched for transcripts of cilium genes, MUC5B, and MMP7 (27), and this molecular phenotype is associated with the expression of keratin 5+ cells, supporting a role for MUC5B in abnormal repair and aberrant regeneration. Thus, we postulate that: IPF is caused by recurrent injury/repair/regeneration at the bronchoalveolar junction secondary to overexpression of MUC5B, mucociliary dysfunction, retention of particles, ER stress, and disruption of normal reparative and regenerative mechanisms in the distal lung.
Figure 1.
Model of stem cells repopulating bronchioles and alveoli under normal physiologic conditions and when challenged with increased expression of MUC5B. We hypothesize that excessive production of MUC5B by stem cells that attempt to regenerate injured bronchiolar and alveolar epithelium disrupt normal developmental pathways and hijack the normal reparative mechanisms in the distal lung, resulting in chronic fibroproliferation and honeycomb cyst formation. Adapted by permission from Reference 54.
Figure 2.
Model of recurrent injury/repair at the bronchoalveolar junction that is initiated and exacerbated by overexpression of MUC5B, retention of inhaled particles, and enhanced lung injury. The upper panel is the normal bronchoalveolar region and the lower panel represents a bronchoalveolar region affected by idiopathic pulmonary fibrosis (IPF). We hypothesize that IPF is a mucociliary disease that is caused by recurrent injury/repair at the bronchoalveolar junction that is initiated and exacerbated by overexpression of MUC5B leading to reduced ciliary function, retention of particles, and enhanced injury. Reprinted by permission from Reference 54.
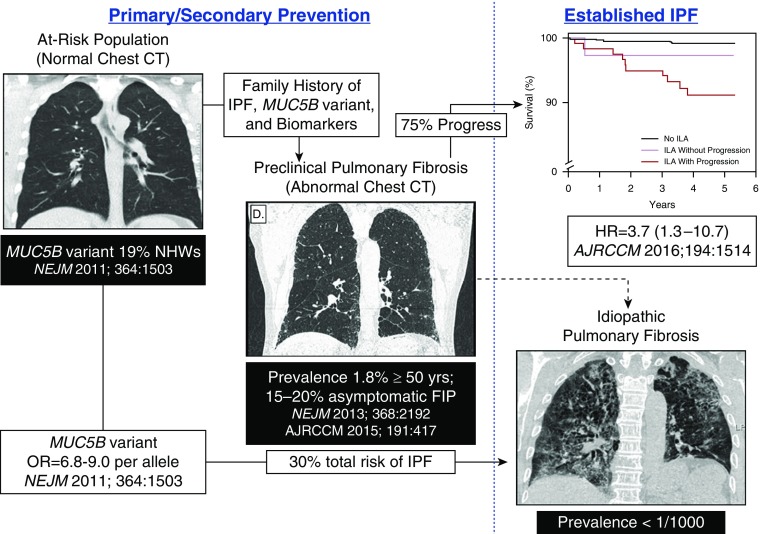
Patients with IPF are usually diagnosed when the fibroproliferative process has caused permanent and extensive lung parenchymal damage. Given the irreversible nature of this disease, even approved treatments for IPF (pirfenidone [7] and nintedanib [8]) only modestly slow progression and have not been shown to alter the 3- to 5-year median survival after diagnosis. However, several common risk factors (age, sex, smoking, and MUC5B promoter variant) and clinical features (physiology, HRCT findings, and disease progression) shared by PrePF and IPF indicate that PrePF may be a harbinger of IPF. The gain-of-function MUC5B promoter variant is the strongest risk factor (genetic and otherwise) for both PrePF (10, 37) and IPF (17, 28–36), and the radiographic features of PrePF and IPF are concordant (10). Moreover, we have recently found that, during a 5- to 6-year period of observation, approximately 75% of subjects with PrePF progressed radiographically, and that radiographic progression of PrePF is associated with a greater decline in forced vital capacity (P = 0.0001) and an increased risk of death (hazard ratio = 3.7 [95% confidence interval = 1.3–10.7]; P = 0.02) (38). Thus, the emerging clinical phenotype of PrePF (≥40 yr of age, asymptomatic → mild respiratory symptoms, and HRCT features of IPF) creates a window of opportunity to identify at-risk individuals with preclinical stages of pulmonary fibrosis before the injury/repair/regenerative process has permanently damaged substantial lung parenchyma (Figure 3). Identification of patients with early or preclinical stages of IPF would allow for the treatment of disease before significant, irreversible loss of functional lung parenchyma has occurred. In addition, the identification of biological pathways active in early stages of IPF will provide important mechanistic clues about the critical pathogenic events involved in the early phases of this complex disease.
Figure 3.
Our overall concept is that family history of idiopathic pulmonary fibrosis (IPF), the MUC5B variant, and biomarkers can identify individuals at high risk for preclinical pulmonary fibrosis (PrePF), establishing the opportunity for primary and secondary prevention of IPF. The “at-risk” population (family history of IPF) and the population with PrePF is large, and the yield of PrePF will be enriched by family history of IPF, the MUC5B variant, other genetic variants, and biomarkers that we discover in this program. Recent findings (38) indicate that PrePF (detected via chest CT scan) is associated with a poor prognosis, suggesting that PrePF may be a harbinger of IPF. CT = computed tomography; FIP = familial interstitial pneumonia; HR = hazard ratio; ILA = interstitial lung abnormality; NHWs = non–Hispanic white individuals; OR = odds ratio. Adapted by permission from Reference 54.
In summary, these findings indicate that: 1) the gain-of-function MUC5B promoter variant, rs35705950, is associated with an elevated risk of developing IPF, accounting for at least 30% of disease risk; 2) in the IPF lung, MUC5B is specifically overexpressed in the bronchoalveolar epithelium; 3) the MUC5B promoter variant can be used to identify individuals in the preclinical phase of this progressive disease; 4) MUC5B represents a key molecule to understand the mechanisms that initiate the fibroproliferative process in the bronchoalveolar epithelium; and 5) focusing on MUC5B may provide a unique opportunity to define the early molecular events that lead to the development of IPF. However, the role of MUC5B (or other genetic variants) in identifying early or more treatable stages of IPF has not been studied, and thus the clinical utility of genetic variants in IPF has yet to be defined. Although most patients with IPF are detected when the disease is advanced, the process takes at least 10 years to develop, and an earlier diagnosis of IPF will detect patients with a lower burden of lung disease (9, 10, 53), providing an opportunity for secondary prevention of this progressive disease. Thus, the overall concept that we propose is that understanding the role of MUC5B in the early molecular stages of lung fibrosis and defining the predictive and prognostic biomarkers in preclinical stages of pulmonary fibrosis will, in aggregate, establish the scientific basis to ultimately prevent the progression of IPF.
Supplementary Material
Footnotes
Supported by National Heart, Lung, and Blood Institute grants UH2/3-HL123442, R01-HL097163, R21/R33-HL120770, and P01-HL092870, and by Department of Defense grant W81XWH-17-1-0597.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.American Thoracic Society International consensus statement idiopathic pulmonary fibrosis: diagnosis and treatment. Am J Respir Crit Care Med. 2000;161:646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 2.Steele MP, Speer MC, Loyd JE, Brown KK, Herron A, Slifer SH, et al. Clinical and pathologic features of familial interstitial pneumonia. Am J Respir Crit Care Med. 2005;172:1146–1152. doi: 10.1164/rccm.200408-1104OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumgartner KB, Samet JM, Stidley CA, Colby TV, Waldron JA. Cigarette smoking: a risk factor for idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1997;155:242–248. doi: 10.1164/ajrccm.155.1.9001319. [DOI] [PubMed] [Google Scholar]
- 4.Olson AL, Swigris JJ, Lezotte DC, Norris JM, Wilson CG, Brown KK. Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am J Respir Crit Care Med. 2007;176:277–284. doi: 10.1164/rccm.200701-044OC. [DOI] [PubMed] [Google Scholar]
- 5.Hutchinson JP, McKeever TM, Fogarty AW, Navaratnam V, Hubbard RB. Increasing global mortality from idiopathic pulmonary fibrosis in the twenty-first century. Ann Am Thorac Soc. 2014;11:1176–1185. doi: 10.1513/AnnalsATS.201404-145OC. [DOI] [PubMed] [Google Scholar]
- 6.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King TE, Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, et al. ASCEND Study Group. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 8.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. INPULSIS Trial Investigators. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 9.Ley B, Collard HR, King TE., Jr Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183:431–440. doi: 10.1164/rccm.201006-0894CI. [DOI] [PubMed] [Google Scholar]
- 10.Putman RK, Rosas IO, Hunninghake GM. Genetics and early detection in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2014;189:770–778. doi: 10.1164/rccm.201312-2219PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll RG, Markin C, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356:1317–1326. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 12.Tsakiri KD, Cronkhite JT, Kuan PJ, Xing C, Raghu G, Weissler JC, et al. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci USA. 2007;104:7552–7557. doi: 10.1073/pnas.0701009104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas AQ, Lane K, Phillips J, III, Prince M, Markin C, Speer M, et al. Heterozygosity for a surfactant protein C gene mutation associated with usual interstitial pneumonitis and cellular nonspecific interstitial pneumonitis in one kindred. Am J Respir Crit Care Med. 2002;165:1322–1328. doi: 10.1164/rccm.200112-123OC. [DOI] [PubMed] [Google Scholar]
- 14.Lawson WE, Grant SW, Ambrosini V, Womble KE, Dawson EP, Lane KB, et al. Genetic mutations in surfactant protein C are a rare cause of sporadic cases of IPF. Thorax. 2004;59:977–980. doi: 10.1136/thx.2004.026336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Kuan PJ, Xing C, Cronkhite JT, Torres F, Rosenblatt RL, et al. Genetic defects in surfactant protein A2 are associated with pulmonary fibrosis and lung cancer. Am J Hum Genet. 2009;84:52–59. doi: 10.1016/j.ajhg.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Moorsel CH, van Oosterhout MF, Barlo NP, de Jong PA, van der Vis JJ, Ruven HJ, et al. Surfactant protein C mutations are the basis of a significant portion of adult familial pulmonary fibrosis in a dutch cohort. Am J Respir Crit Care Med. 2010;182:1419–1425. doi: 10.1164/rccm.200906-0953OC. [DOI] [PubMed] [Google Scholar]
- 17.Fingerlin TE, Murphy E, Zhang W, Peljto AL, Brown KK, Steele MP, et al. Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis. Nat Genet. 2013;45:613–620. doi: 10.1038/ng.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fingerlin TE, Zhang W, Yang IV, Ainsworth HC, Russell PH, Blumhagen RZ, et al. Genome-wide imputation study identifies novel HLA locus for pulmonary fibrosis and potential role for auto-immunity in fibrotic idiopathic interstitial pneumonia. BMC Genet. 2016;17:74. doi: 10.1186/s12863-016-0377-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanders YY, Ambalavanan N, Halloran B, Zhang X, Liu H, Crossman DK, et al. Altered DNA methylation profile in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;186:525–535. doi: 10.1164/rccm.201201-0077OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pandit KV, Milosevic J, Kaminski N. MicroRNAs in idiopathic pulmonary fibrosis. Transl Res. 2011;157:191–199. doi: 10.1016/j.trsl.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 21.Rabinovich EI, Kapetanaki MG, Steinfeld I, Gibson KF, Pandit KV, Yu G, et al. Global methylation patterns in idiopathic pulmonary fibrosis. PLoS One. 2012;7:e33770. doi: 10.1371/journal.pone.0033770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu G, Friggeri A, Yang Y, Milosevic J, Ding Q, Thannickal VJ, et al. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J Exp Med. 2010;207:1589–1597. doi: 10.1084/jem.20100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang IV, Pedersen BS, Rabinovich E, Hennessy CE, Davidson EJ, Murphy E, et al. Relationship of DNA methylation and gene expression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2014;190:1263–1272. doi: 10.1164/rccm.201408-1452OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang IV, Burch LH, Steele MP, Savov JD, Hollingsworth JW, McElvania-Tekippe E, et al. Gene expression profiling of familial and sporadic interstitial pneumonia. Am J Respir Crit Care Med. 2007;175:45–54. doi: 10.1164/rccm.200601-062OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selman M, Pardo A, Barrera L, Estrada A, Watson SR, Wilson K, et al. Gene expression profiles distinguish idiopathic pulmonary fibrosis from hypersensitivity pneumonitis. Am J Respir Crit Care Med. 2006;173:188–198. doi: 10.1164/rccm.200504-644OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaminski N. Microarray analysis of idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2003;29(3) suppl:S32–S36. [PubMed] [Google Scholar]
- 27.Yang IV, Coldren CD, Leach SM, Seibold MA, Murphy E, Lin J, et al. Expression of cilium-associated genes defines novel molecular subtypes of idiopathic pulmonary fibrosis. Thorax. 2013;68:1114–1121. doi: 10.1136/thoraxjnl-2012-202943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med. 2011;364:1503–1512. doi: 10.1056/NEJMoa1013660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, Noth I, Garcia JG, Kaminski N. A variant in the promoter of MUC5B and idiopathic pulmonary fibrosis. N Engl J Med. 2011;364:1576–1577. doi: 10.1056/NEJMc1013504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stock CJ, Sato H, Fonseca C, Banya WA, Molyneaux PL, Adamali H, et al. Mucin 5B promoter polymorphism is associated with idiopathic pulmonary fibrosis but not with development of lung fibrosis in systemic sclerosis or sarcoidosis. Thorax. 2013;68:436–441. doi: 10.1136/thoraxjnl-2012-201786. [DOI] [PubMed] [Google Scholar]
- 31.Noth I, Zhang Y, Ma SF, Flores C, Barber M, Huang Y, et al. Genetic variants associated with idiopathic pulmonary fibrosis susceptibility and mortality: a genome-wide association study. Lancet Respir Med. 2013;1:309–317. doi: 10.1016/S2213-2600(13)70045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borie R, Crestani B, Dieude P, Nunes H, Allanore Y, Kannengiesser C, et al. The MUC5B variant is associated with idiopathic pulmonary fibrosis but not with systemic sclerosis interstitial lung disease in the European Caucasian population. PLoS One. 2013;8:e70621. doi: 10.1371/journal.pone.0070621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei R, Li C, Zhang M, Jones-Hall YL, Myers JL, Noth I, et al. Association between MUC5B and TERT polymorphisms and different interstitial lung disease phenotypes. Transl Res. 2014;163:494–502. doi: 10.1016/j.trsl.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horimasu Y, Ohshimo S, Bonella F, Tanaka S, Ishikawa N, Hattori N, et al. MUC5B promoter polymorphism in Japanese patients with idiopathic pulmonary fibrosis. Respirology. 2015;20:439–444. doi: 10.1111/resp.12466. [DOI] [PubMed] [Google Scholar]
- 35.Peljto AL, Selman M, Kim DS, Murphy E, Tucker L, Pardo A, et al. The MUC5B promoter polymorphism is associated with idiopathic pulmonary fibrosis in a Mexican cohort but is rare among Asian ancestries. Chest. 2015;147:460–464. doi: 10.1378/chest.14-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Vis JJ, Snetselaar R, Kazemier KM, ten Klooster L, Grutters JC, van Moorsel CH. Effect of Muc5b promoter polymorphism on disease predisposition and survival in idiopathic interstitial pneumonias. Respirology. 2016;21:712–717. doi: 10.1111/resp.12728. [DOI] [PubMed] [Google Scholar]
- 37.Hunninghake GM, Hatabu H, Okajima Y, Gao W, Dupuis J, Latourelle JC, et al. MUC5B promoter polymorphism and interstitial lung abnormalities. N Engl J Med. 2013;368:2192–2200. doi: 10.1056/NEJMoa1216076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Araki T, Putman RK, Hatabu H, Gao W, Dupuis J, Latourelle JC, et al. Development and progression of interstitial lung abnormalities in the Framingham Heart Study. Am J Respir Crit Care Med. 2016;194:1514–1522. doi: 10.1164/rccm.201512-2523OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seibold MA, Smith RW, Urbanek C, Groshong SD, Cosgrove GP, Brown KK, et al. The idiopathic pulmonary fibrosis honeycomb cyst contains a mucocilary pseudostratified epithelium. PLoS One. 2013;8:e58658. doi: 10.1371/journal.pone.0058658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakano Y, Yang IV, Walts AD, Watson AM, Helling BA, Fletcher AA, et al. MUC5B promoter variant rs35705950 affects MUC5B expression in the distal airways in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2016;193:464–466. doi: 10.1164/rccm.201509-1872LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosas IO, Ren P, Avila NA, Chow CK, Franks TJ, Travis WD, et al. Early interstitial lung disease in familial pulmonary fibrosis. Am J Respir Crit Care Med. 2007;176:698–705. doi: 10.1164/rccm.200702-254OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Copley SJ, Wells AU, Hawtin KE, Gibson DJ, Hodson JM, Jacques AE, et al. Lung morphology in the elderly: comparative CT study of subjects over 75 years old versus those under 55 years old. Radiology. 2009;251:566–573. doi: 10.1148/radiol.2512081242. [DOI] [PubMed] [Google Scholar]
- 43.Washko GR, Hunninghake GM, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, et al. COPDGene Investigators. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med. 2011;364:897–906. doi: 10.1056/NEJMoa1007285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsushima K, Sone S, Yoshikawa S, Yokoyama T, Suzuki T, Kubo K. The radiological patterns of interstitial change at an early phase: over a 4-year follow-up. Respir Med. 2010;104:1712–1721. doi: 10.1016/j.rmed.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 45.Sverzellati N, Guerci L, Randi G, Calabrò E, La Vecchia C, Marchianò A, et al. Interstitial lung diseases in a lung cancer screening trial. Eur Respir J. 2011;38:392–400. doi: 10.1183/09031936.00201809. [DOI] [PubMed] [Google Scholar]
- 46.Putman RK, Hatabu H, Araki T, Gudmundsson G, Gao W, Nishino M, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators; COPDGene Investigators. Association between interstitial lung abnormalities and all-cause mortality. JAMA. 2016;315:672–681. doi: 10.1001/jama.2016.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lederer DJ, Enright PL, Kawut SM, Hoffman EA, Hunninghake G, van Beek EJ, et al. Cigarette smoking is associated with subclinical parenchymal lung disease: the Multi-Ethnic Study of Atherosclerosis (MESA)-lung study. Am J Respir Crit Care Med. 2009;180:407–414. doi: 10.1164/rccm.200812-1966OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Washko GR, Lynch DA, Matsuoka S, Ross JC, Umeoka S, Diaz A, et al. Identification of early interstitial lung disease in smokers from the COPDGene Study. Acad Radiol. 2010;17:48–53. doi: 10.1016/j.acra.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jin GY, Lynch D, Chawla A, Garg K, Tammemagi MC, Sahin H, et al. Interstitial lung abnormalities in a CT lung cancer screening population: prevalence and progression rate. Radiology. 2013;268:563–571. doi: 10.1148/radiol.13120816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ho JE, Gao W, Levy D, Santhanakrishnan R, Araki T, Rosas IO, et al. Galectin-3 is associated with restrictive lung disease and interstitial lung abnormalities. Am J Respir Crit Care Med. 2016;194:77–83. doi: 10.1164/rccm.201509-1753OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Doyle TJ, Washko GR, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, et al. COPDGene Investigators. Interstitial lung abnormalities and reduced exercise capacity. Am J Respir Crit Care Med. 2012;185:756–762. doi: 10.1164/rccm.201109-1618OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Travis W, King T, Bateman E, Lynch D, Capron F, Center D, et al. American Thoracic Society; European Respiratory Society. American Thoracic Society/European Respiratory Society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias: this joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 53.Kropski JA, Pritchett JM, Zoz DF, Crossno PF, Markin C, Garnett ET, et al. Extensive phenotyping of individuals at risk for familial interstitial pneumonia reveals clues to the pathogenesis of interstitial lung disease. Am J Respir Crit Care Med. 2015;191:417–426. doi: 10.1164/rccm.201406-1162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Evans CM, Fingerlin TE, Schwarz MI, Lynch D, Kurche J, Warg L, et al. Idiopathic pulmonary fibrosis: a genetic disease that involves mucociliary dysfunction of the peripheral airways. Physiol Rev. 2016;96:1567–1591. doi: 10.1152/physrev.00004.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raghu G. Idiopathic pulmonary fibrosis: guidelines for diagnosis and clinical management have advanced from consensus-based in 2000 to evidence-based in 2011. Eur Respir J. 2011;37:743–746. doi: 10.1183/09031936.00017711. [DOI] [PubMed] [Google Scholar]
- 56.Travis WD, Costabel U, Hansell DM, King TE, Jr, Lynch DA, Nicholson AG, et al. ATS/ERS Committee on Idiopathic Interstitial Pneumonias. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188:733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Selman M, Pardo A, Kaminski N. Idiopathic pulmonary fibrosis: aberrant recapitulation of developmental programs? PLoS Med. 2008;5:e62. doi: 10.1371/journal.pmed.0050062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Plantier L, Crestani B, Wert SE, Dehoux M, Zweytick B, Guenther A, et al. Ectopic respiratory epithelial cell differentiation in bronchiolised distal airspaces in idiopathic pulmonary fibrosis. Thorax. 2011;66:651–657. doi: 10.1136/thx.2010.151555. [DOI] [PubMed] [Google Scholar]
- 59.Button B, Cai LH, Ehre C, Kesimer M, Hill DB, Sheehan JK, et al. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science. 2012;337:937–941. doi: 10.1126/science.1223012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boucher RC. Idiopathic pulmonary fibrosis—a sticky business. N Engl J Med. 2011;364:1560–1561. doi: 10.1056/NEJMe1014191. [DOI] [PubMed] [Google Scholar]
- 61.Livraghi-Butrico A, Grubb BR, Wilkinson KJ, Volmer AS, Burns KA, Evans CM, et al. Contribution of mucus concentration and secreted mucins Muc5ac and Muc5b to the pathogenesis of muco-obstructive lung disease. Mucosal Immunol. 2017;10:395–407. doi: 10.1038/mi.2016.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.