Abstract
Rationale: The association between obstructive sleep apnea (OSA) and cardiovascular disease (CVD) is complex, bidirectional, and may vary across groups. Understanding which cardiovascular risk factors vary in their relationship to OSA across population groups may improve knowledge of OSA-related CVD susceptibility.
Objectives: To better understand the heterogeneity of associations, we assessed whether associations of OSA with cardiovascular risk factors vary by age, sex, and race/ethnicity.
Methods: We performed cross-sectional analyses of 1,344 Multi-Ethnic Study of Atherosclerosis participants who underwent overnight full polysomnography, assays of fasting blood, and assessments of cardiovascular risk factors. Risk factors considered were blood pressure, glucose/lipid concentrations, white blood cell (WBC) total and subset counts, and cystatin C. The outcome was the apnea–hypopnea index (AHI). Linear regression analyses with tests for interactions were conducted.
Results: The sample had a mean age of 68 ± 9 years. Forty-seven percent of the sample was male, and 32% had moderate or severe OSA (AHI, ≥15). Multivariable adjusted analysis showed significant associations between higher AHI with lower high-density lipoprotein cholesterol and higher diastolic blood pressure and neutrophil counts. Significant interactions with demographic factors were observed. Stronger associations were shown between AHI and higher total WBC count (Pint = 0.006) and glucose concentrations (Pint = 0.006) in younger (<65 yr) than in older individuals, higher triglyceride concentrations in men than in women (Pint = 0.006), and higher total WBC (Pint = 0.07) and monocyte counts (Pint = 0.03) in African American individuals than in other racial groups.
Conclusions: In a multiethnic cohort, we found increased levels of cardiovascular risk factors in association with OSA, including elevated neutrophil counts, a marker of inflammation. Furthermore, several associations were stronger in men, younger individuals, and African American individuals, highlighting pathways for CVD risk that may explain heterogeneity in the associations between CVD and OSA across population groups.
Keywords: sleep apnea, cardiovascular risk factors, neutrophils, leukocytes, granulocytes
Obstructive sleep apnea (OSA) is associated with an increased risk of cardiovascular disease (CVD) (1–3). However, the pathways linking OSA to CVD are complex and include variations in metabolic, vascular, and inflammatory risk factors, and they may vary across population groups (4–7). Some associations may be causal and others bidirectional, and yet others may partly reflect shared risk factors such as obesity (8–11). Although traditional CVD risk factors can vary with OSA (8), prior research has not systematically examined variation of risk factors with OSA by race, age, and sex. Furthermore, limited research has addressed the variation of OSA with nontraditional risk factors, such as inflammation and renal dysfunction, despite strong evidence for the role of inflammation in CVD pathogenesis (11–13).
Given that CVD and OSA associations are generally stronger in younger populations (3, 4, 14, 15) and in men than in women (16–21), we postulated that OSA severity would be more strongly associated with CVD risk factors in these groups. We specifically examined associations between OSA and white blood cell (WBC) counts, given data showing that leukocyte activation and elevated leukocyte counts, direct markers of inflammation, associate with an increased CVD risk (8, 9). By characterizing the associations between CVD risk factors and OSA across age, sex, and racial/ethnic groups, we aimed to identify factors that may explain differences in OSA and CVD across population groups. Some of these results were previously reported in the form of an abstract (22).
Methods
Sample
The sample included participants in the Multi-Ethnic Study of Atherosclerosis (MESA), a multiethnic, community-based, prospective cohort designed to investigate the prevalence and progression of subclinical CVD. Briefly, MESA recruited 6,814 participants aged 45 to 84 years from four ethnic groups. The participants were recruited at six centers, one each in Baltimore, Maryland; Chicago, Illinois; Los Angeles, California; St. Paul, Minnesota; New York, New York; and Forsyth County, North Carolina. Participants were free of clinical CVD at the baseline examination (examination 1 in 2000–2002). Follow-up examinations were performed.
At examination 5 (2010–2012), MESA participants who were not receiving treatment for OSA were invited to participate in a sleep ancillary examination that included overnight in-home polysomnography. Participants using oxygen therapy (n = 4), an oral appliance (n = 4), or continuous positive airway pressure therapy (n = 95) for OSA treatment were excluded. Of 2,261 subjects invited to undergo sleep evaluations, 2,057 had technically acceptable sleep studies. Of those, 1,350 had assays performed for WBC count. The analytical sample included 1,344 individuals with complete data on sleep measures and biomarkers. Table E1 in the online supplement describes the characteristics of the sleep ancillary study population (n = 2,057) and the analytical sample (n = 1,344).
This study was conducted in accordance with the amended Declaration of Helsinki. Written informed consent was obtained from all MESA participants. The study was approved by the institutional review board (IRB) at each field center and the data coordinating center. Each IRB is certified by the U.S. Department of Health and Human Services Office for Human Research Protections: Wake Forest University (IRB number IRB00008492 under Federalwide Assurance FWA00001435); Columbia University (IRB number IRB00002973 under Federalwide Assurance FWA00002636); Johns Hopkins University (IRB number 00001656 under Federalwide Assurance FWA00005752); University of Minnesota (IRB number IRB00000438 under Federalwide Assurance FWA00000312); Northwestern University (IRB number IRB00005003 under Federalwide Assurance FWA00001549); and University of California, Los Angeles (IRB number 00000172 under Federalwide Assurance FWA00004642).
Sleep Measures
Polysomnography was conducted using a 15-channel monitor (Somté System; Compumedics). Recordings included measurement of electroencephalography, electrooculography, chin electromyography, electrocardiography, thoracic and abdominal respiratory inductance plethysmography, airflow (thermocouple and nasal pressure), pulse oximetry, and bilateral limb movements, as described before (23). OSA severity was assessed with the apnea–hypopnea index (AHI), derived as the sum of all apneas (regardless of desaturation) and hypopneas, accompanied by at least a 4% drop in oxygen saturation, divided by sleep duration (23).
Cardiovascular Risk Factors
At examination 5, systolic and diastolic blood pressures (SBP and DBP, respectively) were calculated as the average of the second and third blood pressure measurements made while the participant was seated. Hypertension was defined as SBP greater than or equal to 140 mm Hg and/or DBP greater than or equal to 90 mm Hg, or use of antihypertensive medication (24). We also analyzed SBP and DBP as continuous measurements. Diabetes was defined as a fasting glucose concentration greater than or equal to 7.0 mmol/L (126 mg/dl) or use of hypoglycemic medications (25). Lipids included fasting concentrations of total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and triglycerides (26). A central laboratory performed assays, as described before (27).
Other laboratory measurements included complete blood count with differential analysis. Cystatin C was measured using a BN II nephelometer (Siemens) with a particle-enhanced immunonephelometric assay (N Latex Cystatin C; Siemens) with fasting plasma specimens stored at −70°C. The intra-assay coefficient of variation for cystatin C ranged from 2.0% to 2.8% (27).
Covariates
Information on demographic and behavioral factors, including age, sex, race/ethnicity, smoking, and medication use, were obtained at examination 5 using a standardized questionnaire. Smoking status was defined as never, former (no cigarettes within the past 30 d), or current. Alcohol drinking was defined as current use (yes/no). Body mass index (BMI) was calculated from measurements of height and weight. Waist circumference was directly measured.
Statistical Analysis
Bivariate associations were assessed using analysis of variance for continuous variables, the Kruskal-Wallis test for nonnormally distributed measures, and the chi-square test for categorical variables. Associations between AHI (with natural logarithm transformation after adding 1) and risk factors were evaluated using multivariable linear regression, adjusting for age, sex, race/ethnicity (white, African American, Hispanic, Chinese), smoking status, statin use, antihypertensive use, hypoglycemic medication use, waist circumference, and BMI. Analyses of glucose excluded individuals using diabetes medications. Tests for multiplicative interaction were performed to assess whether the associations between AHI and CVD risk factors varied across age groups (<65 yr vs. ≥65 yr) (28), sex, and race/ethnicity.
Analyses were conducted using PASW Statistics version 18 (SPSS) or SAS version 9.0 software (SAS Institute). For interpretation of statistical interactions, we used P < 0.05, with suggestive interactions defined as P < 0.10.
Results
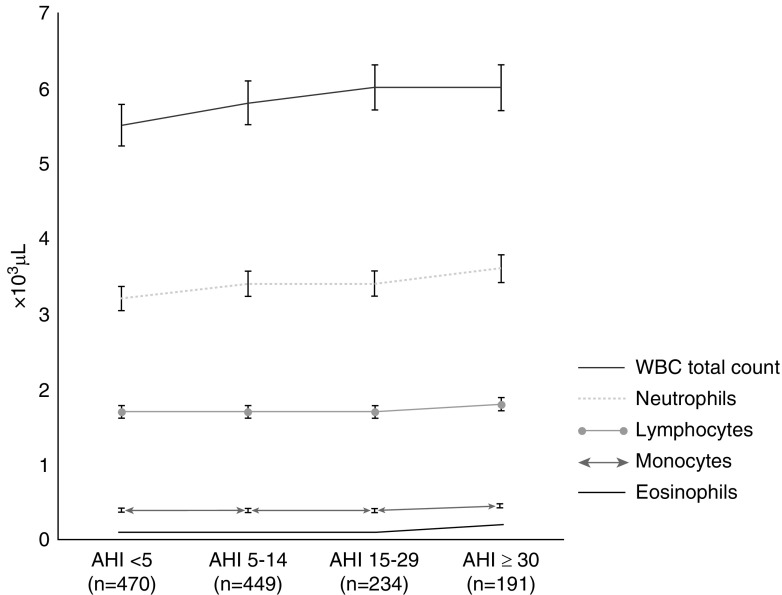
Table 1 shows the distributions of participant characteristics by OSA category. As expected, increasing BMI, increasing waist circumference, and male sex were associated with more severe OSA. Hispanic American individuals were overrepresented in the most severe AHI category. Associated positively with OSA severity were diabetes, fasting glucose and triglyceride concentrations; hypertension prevalence; and DBP. HDL cholesterol and total cholesterol were associated inversely with OSA. No significant associations were observed for LDL or non-HDL cholesterol or for smoking status, alcohol use, or statin use. In unadjusted analyses, higher total WBC counts and all WBC subsets other than lymphocytes, as well as cystatin C concentrations, were associated positively with OSA category, with statistical significance (Figure 1 and Table E2).
Table 1.
Distributions of demographic factors and metabolic and cardiovascular risk factors according to obstructive sleep apnea severity, by apnea–hypopnea index category
| Overall (n = 1,344) | AHI <5 (n = 470) | AHI 5–14 (n = 449) | AHI 15–29 (n = 234) | AHI ≥30 (n = 191) | P Value | |
|---|---|---|---|---|---|---|
| Age, yr | 68 ± 9 | 67 ± 9 | 70 ± 9 | 68 ± 9 | 68 ± 9 | <0.001 |
| Male sex, % | 47 | 35 | 48 | 53 | 68 | <0.001 |
| BMI, kg/m2 | 29 ± 5 | 27 ± 5 | 29 ± 5 | 31 ± 5 | 32 ± 5 | <0.001 |
| Waist circumference, cm | 101 ± 14 | 95 ± 13 | 101 ± 13 | 105 ± 12 | 110 ± 14 | <0.001 |
| Education level, % | 0.007 | |||||
| Less than high school | 17 | 16 | 15 | 18 | 22 | |
| High school | 18 | 16 | 22 | 14 | 18 | |
| College or technical | 49 | 48 | 48 | 56 | 48 | |
| Graduate school | 16 | 20 | 15 | 12 | 12 | |
| Race/ethnicity, % | <0.001 | |||||
| White | 38 | 42 | 41 | 31 | 30.5 | |
| Chinese | 1 | 0 | 0.5 | 4 | 0.5 | |
| African American | 29 | 31 | 26 | 30 | 27 | |
| Hispanic | 32 | 27 | 32.5 | 35 | 42 | |
| Smoking, % | 0.423 | |||||
| Never | 41 | 42 | 42 | 41 | 36 | |
| Former | 51 | 49 | 50 | 53 | 57 | |
| Current | 8 | 9 | 8 | 6 | 7 | |
| Alcohol use (any), % | 46 | 45 | 44 | 45 | 52 | 0.295 |
| Statin use, % | 37 | 33 | 38 | 41 | 40 | 0.137 |
| Any hypertension medication, % | 55 | 49 | 59 | 57 | 59 | 0.011 |
| Oral hypoglycemic use, % | 16 | 14 | 14 | 22 | 20 | 0.005 |
| Insulin use, % | 4 | 3 | 3 | 4 | 8 | 0.003 |
| eGFR, ml/min/1.73 m2 | 81 ± 21 | 82 ± 20 | 80 ± 21 | 80 ± 20 | 84 ± 22 | 0.063 |
| Diabetes mellitus, % | 22 | 18 | 19 | 28 | 31 | <0.001 |
| Fasting glucose*, mg/dl | 96 ± 18 | 94 ± 16 | 95 ± 16 | 99 ± 24 | 101 ± 21 | <0.001 |
| Hypertension, % | 59 | 53 | 62 | 61 | 63 | 0.021 |
| Systolic BP, mm Hg | 123 ± 20 | 121 ± 22 | 124 ± 19 | 123 ± 19 | 126 ± 21 | 0.038 |
| Diastolic BP, mm Hg | 68 ± 10 | 67 ± 10 | 67 ± 9 | 69 ± 10 | 70 ± 10 | 0.001 |
| Total cholesterol, mg/dl | 182 ± 35 | 185 ± 38 | 180 ± 35 | 178 ± 35 | 178 ± 35 | 0.006 |
| HDL cholesterol, mg/dl | 55 ± 15 | 59 ± 18 | 55 ± 15 | 51 ± 14 | 50 ± 13 | <0.001 |
| LDL cholesterol, mg/dl | 105 ± 30 | 107 ± 32 | 105 ± 30 | 105 ± 32 | 102 ± 30 | 0.310 |
| Triglycerides, mg/dl | 110 ± 65 | 103 ± 60 | 107 ± 53 | 115 ± 65 | 130 ± 80 | <0.001 |
| Non-HDL cholesterol, mg/dl | 127 ± 35 | 127 ± 35 | 125 ± 35 | 126 ± 36 | 128 ± 34 | 0.933 |
Definition of abbreviations: AHI = apnea–hypopnea index; AHI <5 = no obstructive sleep apnea; AHI 5–14 = mild obstructive sleep apnea; AHI 15–29 = moderate obstructive sleep apnea; AHI ≥30 = severe obstructive sleep apnea; BMI = body mass index; BP = blood pressure; eGFR = estimated glomerular filtration rate (measurement of kidney function); HDL = high-density lipoprotein; LDL = low-density lipoprotein.
Data are shown as mean ± SD for continuous variables and percents for categorical variables. P values were calculated by analysis of variance for continuous variables and chi-square test for categorical variables. P values in bold typeface highlight the statistical significance. Non-HDL cholesterol was calculated by subtracting the HDL cholesterol value from the total cholesterol reading.
n = 1,104, restricted to those not using diabetes medications.
Figure 1.
Differences across AHI category groups were statistically significant, specifically when we compared the no OSA and severe OSA groups, except for lymphocyte counts. Because more than two groups were used for comparison, we used P values calculated by Kruskal-Wallis test, a nonparametric test. All values are medians, owing to skewed distribution. AHI = apnea–hypopnea index; AHI <5 = no OSA; AHI 5–14 = mild OSA; AHI 15–29 = moderate OSA; AHI ≥30 = severe OSA; OSA = obstructive sleep apnea; WBC = white blood cell.
Analyses of the overall sample that were adjusted for demographic factors, smoking status, medication use, and adiposity showed that DBP, HDL cholesterol concentration, and neutrophil count were each significantly associated with AHI (Table 2). The adjusted associations between AHI and triglyceride concentrations and SBP did not reach statistical significance (P = 0.062 and P = 0.095, respectively).
Table 2.
Multivariable linear regression analysis testing associations between risk factors with AHI
| β-Value | SE | P Value | |
|---|---|---|---|
| Metabolic risk factors | |||
| Diabetes mellitus,% | 0.176 | 0.123 | 0.154 |
| Fasting glucose*, mg/dl | 0.001 | 0.002 | 0.540 |
| Hypertension, % | 0.046 | 0.092 | 0.617 |
| Systolic BP, mm Hg | 0.002 | 0.001 | 0.095 |
| Diastolic BP, mm Hg | 0.008 | 0.003 | 0.006 |
| Triglycerides, mg/dl | 0.001 | 4.340 × 10−4 | 0.062 |
| HDL cholesterol, mg/dl | −0.004 | 0.002 | 0.032 |
| Total cholesterol, mg/dl | 0.001 | 0.001 | 0.212 |
| Leukocyte counts and cystatin C levels | |||
| WBC total count, ×103/μl | 0.015 | 0.010 | 0.147 |
| Neutrophils, ×103/μl | 0.046 | 0.018 | 0.009 |
| Monocytes, ×103/μl | 0.181 | 0.112 | 0.108 |
| Lymphocytes, ×103/μl | −0.008 | 0.017 | 0.660 |
| Eosinophils, ×103/μl | 0.052 | 0.200 | 0.795 |
| Cystatin C, mg/L | −0.033 | 0.094 | 0.725 |
Definition of abbreviations: AHI = apnea–hypopnea index [number of events per h of sleep; outcome variable was ln(AHI + 1)]; BMI = body mass index; BP = blood pressure; HDL = high-density lipoprotein; WBC = white blood cells.
Adjustments were made for age, sex, race/ethnicity, smoking status, statin use, any antihypertensive medication, antidiabetes medication (insulin use and/or oral hypoglycemic use), BMI, and waist circumference. Each row represents a unique regression model. Values are natural logarithm transformations. P values in bold typeface highlight the statistical significance.
n = 1,104, restricted to those not using antidiabetes medications. All other analyses are based on n = 1,336 (without missing data).
Evidence for Modification by Age
Adjusted analyses showed statistically significant interactions between age and total WBC count (Pint = 0.006), with stronger associations found in individuals younger than age 65 years than in older individuals (Table 3). More specifically, each increase of 1,000 WBCs per microliter was estimated to be associated with an approximately 8% increase in AHI among individuals younger than 65 years old, with nonsignificant associations in older individuals. The association between neutrophils and AHI also was suggestively stronger in younger than in older subjects (Pint = 0.10). The association between glucose concentrations and AHI was stronger in younger individuals (Pint = 0.006). The association between HDL cholesterol and AHI appeared stronger in older than in younger participants (Pint = 0.09) (Table 3).
Table 3.
Variation of adjusted associations between cardiovascular risk factors by age for AHI
| Age <65 Yr (n = 523) |
Age ≥65 Yr (n = 813) |
P for Interaction | |||
|---|---|---|---|---|---|
| β-Value | 95% CI | β-Value | 95% CI lower, upper | ||
| WBC total count, ×103/μl | 0.078 | 0.024, 0.133 | 0.007 | −0.015, 0.029 | 0.006 |
| Neutrophils, ×103/μl | 0.089 | 0.018, 0.160 | 0.040 | −3.220 × 10−4, 0.080 | 0.098 |
| HDL cholesterol, mg/dl | −0.004 | −0.010, 0.002 | −0.004 | −0.009, 4.645 × 10−6 | 0.088 |
| Fasting glucose, mg/dl* | 0.003 | 0.001, 0.008 | −0.001 | −0.006, 0.003 | 0.006 |
Definition of abbreviations: AHI = apnea–hypopnea index [number of events per h of sleep; outcome variable was ln(AHI + 1)]; BMI = body mass index; CI = confidence interval; HDL = high-density lipoprotein; WBC = white blood cell.
Models shown when there was evidence of a suggestive (P < 0.10) or significant (P < 0.05) interaction with the risk factor and age category (<65 yr vs. ≥65 yr). Each row represents a unique regression model adjusted for sex, race/ethnicity, smoking, statin use, any antihypertensive medication, antidiabetes medication (insulin and/or hypoglycemic), BMI, and waist circumference for each stratified analysis. The P value for interaction terms are based on the full model, including an interaction term for age (<65 yr vs. ≥65 yr) and the exposures. P values in bold typeface highlight the statistical significance. Analyses based on n = 1,336 (without missing data). Values are natural logarithm transformations.
n = 1,104, restricted to those not using diabetes medication.
Evidence for Modification by Sex
A significant sex interaction was observed only for the adjusted association between triglyceride concentrations and AHI (Pint = 0.006), with a stronger positive association in males (Table 4). The association observed between DBP and AHI in the overall sample appeared stronger in men than in women (Pint = 0.08). Women but not men showed a suggested association between monocytes and AHI (Pint = 0.06).
Table 4.
Variation of adjusted associations between cardiovascular risk factors by sex for apnea–hypopnea index
| Female (n = 710) |
Male (n = 626) |
P for Interaction | |||
|---|---|---|---|---|---|
| β-Value | 95% CI | β-Value | 95% CI | ||
| Monocyte count, ×103/μl | 0.469 | 0.030, 0.907 | 0.070 | −0.191, 0.331 | 0.064 |
| Diastolic BP, mm Hg | 0.004 | −0.003, 0.011 | 0.011 | 0.003, 0.019 | 0.084 |
| Triglycerides, mg/dl | −3.940 × 10−4 | −0.002, 0.001 | 0.002 | 3.250 × 10−4, 0.003 | 0.006 |
Definition of abbreviations: BP = blood pressure; CI = confidence interval.
Models are shown when there was evidence of a suggestive (P < 0.10) or significant (P < 0.05) interaction with the risk factor and sex category. Each row represents a unique regression model adjusted for age, race/ethnicity, smoking, statin use, any antihypertensive medication, antidiabetes medication (insulin and/or hypoglycemic), body mass index, and waist circumference for each stratified analysis. P value for interaction was calculated for the full model, including an interaction term for an interaction term for sex and the exposures (monocytes, diastolic BP, triglycerides). P values in bold typeface highlight the statistical significance. Analyses are based on n = 1,336 (without missing data). Values are natural logarithm transformations.
Evidence for Modification by Race
Tests for race/ethnicity interactions showed significant differences in the adjusted association between AHI and monocyte counts in African American individuals (Pint = 0.03 for differences across four racial groups) (Table 5). WBC total counts showed a similar trend (Pint = 0.07). The results estimate an approximately 9% increase in AHI per 1,000 WBCs per microliter and a 2.9-fold increase in AHI per 1,000 monocytes per microliter. After excluding the Chinese group (owing to the small sample; n = 13), the association between AHI and both monocytes (Pint = 0.02) and total WBC counts (Pint = 0.04) were stronger in the African American participants than in white and Hispanic participants (Table E3). Table E4 shows the overall variation of CVD risk factors among the three larger racial/ethnic groups.
Table 5.
Variation of adjusted associations between cardiovascular risk factors by race for apnea–hypopnea index
| White (n = 507) |
Chinese (n = 13) |
African American (n = 382) |
Hispanic (n = 434) |
P for Interaction | |||||
|---|---|---|---|---|---|---|---|---|---|
| β-Value | 95% CI | β-Value | 95% CI | β-Value | 95% CI | β-Value | 95% CI | ||
| WBC total count, ×103/μl | 0.014 | −0.021, 0.049 | −0.454 | −0.708, 0.200 | 0.086 | 0.024, 0.150 | 0.006 | −0.020, 0.033 | 0.07 |
| Monocyte count, ×103/μl | 0.198 | −0.285, 0.680 | −3.735 | −12.261, 4.791 | 1.058 | 0.398, 1.717 | 0.075 | −0.190, 0.340 | 0.03 |
Definition of abbreviations: CI = confidence interval; WBC = white blood cell.
Models are shown when there was evidence of a suggestive (P < 0.10) or significant (P < 0.05) interaction with the risk factor and race categories. Each row represents a unique regression model adjusted for age, sex, smoking, body mass index, waist circumference, statin use, any antihypertensive medication, and antidiabetes use (insulin and/or hypoglycemic), per stratified analysis. P for interaction was calculated for the full model, including an interaction term for race/ethnicity category and exposure. P values in bold typeface highlight the statistical significance. Analyses are based on n = 1,336 (without missing data). Values are natural logarithm transformations.
Sensitivity Analyses
We further adjusted for sleep duration (total sleep time) and allergy (seasonal allergy in the past 2 weeks by self-report), and we observed no appreciable differences in key findings. In addition, we excluded individuals using oral steroids use (n = 17) and likewise observed no significant differences in the findings (Table E5).
Discussion
The emergence of OSA as a risk factor for incident CVD (1–3) has stimulated clinical and public health efforts to improve recognition and treatment of OSA. However, cohort studies indicate significant heterogeneity in the associations between OSA and established CVD across the population (1–4, 8–10). The heterogeneity relating OSA to CVD has raised questions regarding the effects of age, sex, and other factors on underlying phenotypic and mechanistic aspects of OSA and their impact on CVD susceptibility. Differences in associations between OSA and CVD may relate to variations in metabolic and other physiological responses to OSA. Population group differences may partly relate to differences in the severity and duration of OSA exposures (e.g., younger age of onset of OSA in men). Alternatively, genetic and environmental factors that influence levels of traditional and nontraditional CVD risk factors may contribute to differences in associations through causal, bidirectional, and pleiotropic pathways. To help understand this heterogeneity, we analyzed data on CVD risk factors and OSA derived from a large, well-characterized, multiethnic sample of men and women. For the first time in a large and diverse community-based sample, we showed that increasing OSA severity associates with higher WBC counts, suggesting that disturbances in innate immunity may be a pathway linking OSA and CVD and that this pathway may differ by age and race/ethnicity. In particular, the stronger relationship of WBC counts and OSA in middle-aged and African American individuals than in older individuals as well as white and Hispanic individuals suggests that OSA associates with more inflammation in these groups. We also observed significant sex interactions, with stronger associations between triglyceride concentrations and DBP with OSA in men than women, suggesting that metabolic dysfunction and hypertension may contribute to OSA-associated CVD risk in men.
The overall sample showed significant unadjusted associations between OSA severity and multiple measures of metabolic and vascular dysfunction (adiposity, hypertension/blood pressure, diabetes/glucose concentration, and dyslipidemia). In addition, we found significant unadjusted associations between OSA severity and markers of inflammation (total leukocyte counts and all subsets except for lymphocytes) and renal function (cystatin C). After adjusting for multiple potential confounders, significant associations in the overall sample were observed between higher AHI and higher DBP, higher neutrophil counts, and lower HDL cholesterol, supporting the increased prevalence of indices of metabolic syndrome and inflammation in individuals with more severe OSA (29–32).
A novel finding was the association of neutrophil counts with higher AHI in adjusted models. Inflammation contributes to atherogenesis (13), and biomarkers of inflammation predict risk for future CVD (11–13). Elevated markers of inflammation measured by C-reactive protein (33) and inflammatory cytokines (5–7) also have been reported in OSA, although associations often attenuate after adjusting for obesity (7–10). The association of OSA with leukocyte count, a measure of systemic inflammation, has rarely been examined, although several small studies reported abnormalities in leukocytes in patients with OSA (7, 34–36). No prior study has examined variation of WBC counts in large, racially diverse, well-characterized cohorts. The present findings based on total and neutrophil counts implicate innate immunity as a link between OSA and heightened cardiovascular risk. In this regard, even small variations in neutrophil numbers within the normal range strongly associate with the incidence of heart failure, abdominal aortic aneurysm, and nonfatal myocardial infarction (37). Shah and colleagues showed a linear association between neutrophil counts with the risk of developing CVD over a median of 3.8 years of follow-up (37). Increase in CVD was associated with small absolute differences in WBCs: Comparison of neutrophil counts 6–7 × 109/L vs. 2–3 × 109/L (both within the “normal” range) showed strong associations with heart failure (hazard ratio [HR], 2.0), peripheral arterial disease (HR, 1.95), and nonfatal myocardial infarction (HR, 1.58) (37). Notably, low-grade inflammation has been identified as a risk for recurrent cardiovascular events (38). Therefore, our findings point to the potential for future therapies that target underlying inflammatory mechanisms that may link OSA and CVD.
In our diverse cohort, we also identified evidence for effect modification by age, sex, and race/ethnicity, providing insight into the variation in the relationship between CVD across groups previously observed (39–41). In agreement with the findings of Newman and coworkers (8), we observed stronger associations between OSA severity and higher glucose concentration in middle-aged than in older individuals. In addition, a stronger association between OSA severity and total WBC count was found in middle-aged individuals. Whether these observations reflect differences in metabolic and inflammatory responses to OSA-related physiological stresses or whether inflammation is a stronger driver of OSA susceptibility in younger than in older individuals requires further study. Regardless of the causal direction, efforts at targeting inflammation may be a particularly promising strategy in middle-aged individuals with OSA.
We also identified significant sex differences in associations. Stronger associations between triglyceride concentrations with increasing AHI were identified in men than in women. Analyses also suggested a stronger association between DBP and AHI in men. These findings suggest a greater aggregation of cardiometabolic risk factors in men than in women with OSA, a pattern that parallels several reports of a greater prevalence of CVD disease in men than in women with OSA (1, 2, 4, 41, 42).
A novel aspect of this study was the ability to explore effect modification by race/ethnicity. We observed a stronger association between WBC counts and OSA severity in African American individuals than in individuals of other racial groups. The elevation in monocyte counts is of particular relevance for CVD, given that macrophages (monocytes that have migrated from the bloodstream to tissues) are integral components of the atherosclerotic plaque, which consists of an accumulation of lipids in the arterial wall, together with infiltration of immune cells, such as macrophages, T cells, and mast cells (13). The roles of multiple aspects of the innate immune system are under active investigation as mediators of chronic inflammation and atherogenesis. Prior research has identified variation in the prevalence of OSA across racial groups (43–45). OSA prevalence risk in African American adults strongly associates with obesity (42), and obesity associates with increased leukocytes (46–48). African American children and young adults, however, have a high prevalence of OSA not explained by obesity (49), possibly reflecting inflammation in upper airway lymphoid tissue. Additional research on differences in the association between inflammation and OSA may provide insight into cardiovascular health disparities.
This study’s strengths include the inclusion of a multiethnic sample recruited from the community. This study used standardized and comprehensive objective sleep measurements and a central laboratory for biomarker assays. A number of CVD risk factors were evaluated, allowing assessment of metabolic, inflammatory, and vascular risk factors. Study limitations include its cross-sectional design, precluding assessment of causality. We modeled the AHI as our outcome to illustrate which CVD risk factors associate with OSA, extending the approach used by Newman and colleagues, who first demonstrated an aggregation of CVD risk factors with OSA (8). Although many of the observed associations likely reflect the contribution of OSA to CVD risk markers, our findings also allow for the possibility that OSA and CVD may be linked by bidirectional and/or common mechanisms, suggested by studies showing associations between variants in inflammatory genes and OSA (49). We adjusted for many known correlates of OSA or CVD; however, there is potential for residual confounding. Markers of inflammation may vary with many factors, including stress. Although we adjusted for multiple factors and conducted sensitivity analyses excluding the small number of individuals using oral steroids and adjusting for seasonal allergies, other factors, such as “stress,” are difficult to measure, and an inability to adjust for these may have confounded the findings. Our sample had a wide range of OSA, but participants were recruited from the community, unselected for the presence of symptoms, and individuals under active treatment for OSA were excluded. It is possible that alternative associations would be observed in a more symptomatic sample. Typical for a community sample of older individuals, a high proportion used statins or antihypertensive medications. Although we statistically adjusted for medication use, it is possible that medication effects attenuated associations between lipids and blood pressure with OSA.
Conclusions
Associations between several cardiovascular risk factors, including WBC count, and OSA varied by age, sex, and race/ethnicity. These differences may underlie population variation in OSA-related CVD susceptibility, and they suggest pathways that may serve as targets for future interventions aimed at reducing CVD in individuals with OSA. Further assessment of causal pathways, including investigation of interindividual differences in response to OSA-related stressors, such as intermittent hypoxia, or to underlying differences in cardiometabolic profile and genetic susceptibility may yield insight into sources of health disparities across the population and their links to OSA.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at www.mesa-nhlbi.org.
Footnotes
Supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 from the National Heart, Lung, and Blood Institute (NHLBI); by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences; by NHLBI grant R01 HL08047 and the RRM Charitable Fund (P.L.); and by the Lemann Foundation (G.R.G.); in addition, S.R. was partly supported by NHLBI grant R35HL135818.
Author Contributions: Conception and design: G.R.G., R.W., and S.R.; analysis and interpretation of data: G.R.G., R.W., J.W., and S.R.; drafting the article or revising it critically for important intellectual content: G.R.G., R.W., N.S.J., S.S., M.A., P.L., S.R.; and final approval of the version to be published: all authors.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea–hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 2.Valham F, Mooe T, Rabben T, Stenlund H, Wiklund U, Franklin KA. Increased risk of stroke in patients with coronary artery disease and sleep apnea: a 10-year follow-up. Circulation. 2008;118:955–960. doi: 10.1161/CIRCULATIONAHA.108.783290. [DOI] [PubMed] [Google Scholar]
- 3.Gottlieb DJ, Yenokyan G, Newman AB, O’Connor GT, Punjabi NM, Quan SF, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the Sleep Heart Health Study. Circulation. 2010;122:352–360. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nadeem R, Molnar J, Madbouly EM, Nida M, Aggarwal S, Sajid H, et al. Serum inflammatory markers in obstructive sleep apnea: a meta-analysis. J Clin Sleep Med. 2013;9:1003–1012. doi: 10.5664/jcsm.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Archontogeorgis K, Nena E, Tsigalou C, Voulgaris A, Xanthoudaki M, Froudarakis M, et al. Cystatin C levels in middle-aged patients with obstructive sleep apnea syndrome. Pulm Med. 2016;2016:8081723. doi: 10.1155/2016/8081723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lavie L, Dyugovskaya L, Polyakov A. Biology of peripheral blood cells in obstructive sleep apnea – the tip of the iceberg. Arch Physiol Biochem. 2008;114:244–254. doi: 10.1080/13813450802306701. [DOI] [PubMed] [Google Scholar]
- 8.Newman AB, Nieto FJ, Guidry U, Lind BK, Redline S, Pickering TG, et al. Sleep Heart Health Study Research Group. Relation of sleep-disordered breathing to cardiovascular disease risk factors: the Sleep Heart Health Study. Am J Epidemiol. 2001;154:50–59. doi: 10.1093/aje/154.1.50. [DOI] [PubMed] [Google Scholar]
- 9.Luyster FS, Kip KE, Buysse DJ, Aiyer AN, Reis SE, Strollo PJ., Jr Traditional and nontraditional cardiovascular risk factors in comorbid insomnia and sleep apnea. Sleep (Basel) 2014;37:593–600. doi: 10.5665/sleep.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kasai T, Floras JS, Bradley TD. Sleep apnea and cardiovascular disease: a bidirectional relationship. Circulation. 2012;126:1495–1510. doi: 10.1161/CIRCULATIONAHA.111.070813. [DOI] [PubMed] [Google Scholar]
- 11.Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352:2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 12.Madjid M, Awan I, Willerson JT, Casscells SW. Leukocyte count and coronary heart disease: implications for risk assessment. J Am Coll Cardiol. 2004;44:1945–1956. doi: 10.1016/j.jacc.2004.07.056. [DOI] [PubMed] [Google Scholar]
- 13.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 14.Tantrakul V, Park CS, Guilleminault C. Sleep-disordered breathing in premenopausal women: differences between younger (less than 30 years old) and older women. Sleep Med. 2012;13:656–662. doi: 10.1016/j.sleep.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Miller ME, Williamson JD, Gerstein HC, Byington RP, Cushman WC, Ginsberg HN, et al. ACCORD Investigators. Effects of randomization to intensive glucose control on adverse events, cardiovascular disease, and mortality in older versus younger adults in the ACCORD Trial. Diabetes Care. 2014;37:634–643. doi: 10.2337/dc13-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Appelman Y, van Rijn BB, Ten Haaf ME, Boersma E, Peters SA. Sex differences in cardiovascular risk factors and disease prevention. Atherosclerosis. 2015;241:211–218. doi: 10.1016/j.atherosclerosis.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 17.Stock EO, Redberg R. Cardiovascular disease in women. Curr Probl Cardiol. 2012;37:450–526. doi: 10.1016/j.cpcardiol.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Mazzuca E, Battaglia S, Marrone O, Marotta AM, Castrogiovanni A, Esquinas C, et al. Gender-specific anthropometric markers of adiposity, metabolic syndrome and visceral adiposity index (VAI) in patients with obstructive sleep apnea. J Sleep Res. 2014;23:13–21. doi: 10.1111/jsr.12088. [DOI] [PubMed] [Google Scholar]
- 19.Shepertycky MR, Banno K, Kryger MH. Differences between men and women in the clinical presentation of patients diagnosed with obstructive sleep apnea syndrome. Sleep. 2005;28:309–314. [PubMed] [Google Scholar]
- 20.Puri R, Nissen SE, Shao M, Ballantyne CM, Barter PJ, Chapman MJ, et al. Sex-related differences of coronary atherosclerosis regression following maximally intensive statin therapy: insights from SATURN. JACC Cardiovasc Imaging. 2014;7:1013–1022. doi: 10.1016/j.jcmg.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 21.Mikkola TS, Gissler M, Merikukka M, Tuomikoski P, Ylikorkala O. Sex differences in age-related cardiovascular mortality. PLoS One. 2013;8:e63347. doi: 10.1371/journal.pone.0063347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geovanini GR, Wang R, Weng J, Shea S, Jenny NS, Libby P, et al. Age and sex modify the association between OSA and traditional and novel cardiovascular risk factors: the Multi-Ethnic Study of Atherosclerosis (MESA) [abstract 0447] Sleep. 2017;40(Suppl 1):A166. [Google Scholar]
- 23.Redline S, Budhiraja R, Kapur V, Marcus CL, Mateika JH, Mehra R, et al. The scoring of respiratory events in sleep: reliability and validity. J Clin Sleep Med. 2007;3:169–200. [PubMed] [Google Scholar]
- 24.Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure The Sixth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure Arch Intern Med 19971572413–2446.[Published erratum appears in Arch Intern Med1998;158:573.] [DOI] [PubMed] [Google Scholar]
- 25.Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, et al. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 26.Cui Y, Blumenthal RS, Flaws JA, Whiteman MK, Langenberg P, Bachorik PS, et al. Non-high-density lipoprotein cholesterol level as a predictor of cardiovascular disease mortality. Arch Intern Med. 2001;161:1413–1419. doi: 10.1001/archinte.161.11.1413. [DOI] [PubMed] [Google Scholar]
- 27.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 28.Aronow WS, Fleg JL, Pepine CJ, Artinian NT, Bakris G, Brown AS, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents J Am Coll Cardiol 2011572037–2114.[Published errata appear in J Am Coll Cardiol 2011;57:2125; J Am Coll Cardiol 2011;58:2037; and J Am Coll Cardiol 2016;68:135.] [DOI] [PubMed] [Google Scholar]
- 29.Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, et al. Sleep Heart Health Study. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 30.Bakker JP, Weng J, Wang R, Redline S, Punjabi NM, Patel SR. Associations between obstructive sleep apnea, sleep duration, and abnormal fasting glucose: the Multi-Ethnic Study of Atherosclerosis. Am J Respir Crit Care Med. 2015;192:745–753. doi: 10.1164/rccm.201502-0366OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim J, Yoon DW, Lee SK, Lee S, Choi KM, Robert TJ, et al. Concurrent presence of inflammation and obstructive sleep apnea exacerbates the risk of metabolic syndrome: A KoGES 6-year follow-up study. Medicine (Baltimore) 2017;96:e4488. doi: 10.1097/MD.0000000000004488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Destors M, Tamisier R, Galerneau LM, Lévy P, Pepin JL. Pathophysiology of obstructive sleep apnea syndrome and its cardiometabolic consequences [in French] Presse Med. 2017;46:395–403. doi: 10.1016/j.lpm.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 33.Ridker PM. A test in context: high-sensitivity C-reactive protein. J Am Coll Cardiol. 2016;67:712–723. doi: 10.1016/j.jacc.2015.11.037. [DOI] [PubMed] [Google Scholar]
- 34.Eisele HJ, Markart P, Schulz R. Obstructive sleep apnea, oxidative stress, and cardiovascular disease: evidence from human studies. Oxid Med Cell Longev. 2015;2015:608438. doi: 10.1155/2015/608438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yenigun A, Karamanli H. Investigation of the relationship between neutrophil-to-lymphocyte ratio and obstructive sleep apnoea syndrome. J Laryngol Otol. 2015;129:887–892. doi: 10.1017/S0022215115001747. [DOI] [PubMed] [Google Scholar]
- 36.Dyugovskaya L, Lavie P, Lavie L. Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am J Respir Crit Care Med. 2002;165:934–939. doi: 10.1164/ajrccm.165.7.2104126. [DOI] [PubMed] [Google Scholar]
- 37.Shah AD, Denaxas S, Nicholas O, Hingorani AD, Hemingway H. Neutrophil counts and initial presentation of 12 cardiovascular diseases: a CALIBER cohort study. J Am Coll Cardiol. 2017;69:1160–1169. doi: 10.1016/j.jacc.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ridker PM. Residual inflammatory risk: addressing the obverse side of the atherosclerosis prevention coin. Eur Heart J. 2016;37:1720–1722. doi: 10.1093/eurheartj/ehw024. [DOI] [PubMed] [Google Scholar]
- 39.Lin CM, Davidson TM, Ancoli-Israel S. Gender differences in obstructive sleep apnea and treatment implications. Sleep Med Rev. 2008;12:481–496. doi: 10.1016/j.smrv.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Javaheri S, Sharma RK, Wang R, Weng J, Rosen BD, Bluemke DA, et al. Association between obstructive sleep apnea and left ventricular structure by age and gender: the Multi-Ethnic Study of Atherosclerosis. Sleep (Basel) 2016;39:523–529. doi: 10.5665/sleep.5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gaines J, Vgontzas AN, Fernandez-Mendoza J, Kritikou I, Basta M, Bixler EO. Gender differences in the association of sleep apnea and inflammation. Brain Behav Immun. 2015;47:211–217. doi: 10.1016/j.bbi.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mokhlesi B, Ham SA, Gozal D. The effect of sex and age on the comorbidity burden of OSA: an observational analysis from a large nationwide US health claims database. Eur Respir J. 2016;47:1162–1169. doi: 10.1183/13993003.01618-2015. [DOI] [PubMed] [Google Scholar]
- 43.Redline S, Tishler PV, Hans MG, Tosteson TD, Strohl KP, Spry K. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med. 1997;155:186–192. doi: 10.1164/ajrccm.155.1.9001310. [DOI] [PubMed] [Google Scholar]
- 44.Fiorentino L, Marler M, Stepnowsky C, Johnson S, Ancoli-Israel S. Sleep in older African Americans and Caucasians at risk for sleep-disordered breathing. Behav Sleep Med. 2006;4:164–178. doi: 10.1207/s15402010bsm0403_3. [DOI] [PubMed] [Google Scholar]
- 45.Ruiter ME, DeCoster J, Jacobs L, Lichstein KL. Sleep disorders in African Americans and Caucasian Americans: a meta-analysis. Behav Sleep Med. 2010;8:246–259. doi: 10.1080/15402002.2010.509251. [DOI] [PubMed] [Google Scholar]
- 46.Ryder E, Diez-Ewald M, Mosquera J, Fernández E, Pedreañez A, Vargas R, et al. Association of obesity with leukocyte count in obese individuals without metabolic syndrome. Diabetes Metab Syndr. 2014;8:197–204. doi: 10.1016/j.dsx.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 47.Mraz M, Haluzik M. The role of adipose tissue immune cells in obesity and low-grade inflammation. J Endocrinol. 2014;222:R113–R127. doi: 10.1530/JOE-14-0283. [DOI] [PubMed] [Google Scholar]
- 48.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patel SR, Goodloe R, De G, Kowgier M, Weng J, Buxbaum SG, et al. Association of genetic loci with sleep apnea in European Americans and African-Americans: the Candidate Gene Association Resource (CARe) PLoS One. 2012;7:e48836. doi: 10.1371/journal.pone.0048836. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.