Abstract
Rationale: The San Luis Valley in rural Colorado often has elevated levels of ambient particulate matter. To date little is known about the impact of ambient particulate matter levels and medical care utilization due to asthma exacerbation in rural communities.
Objectives: We investigated the impact of ambient particulate matter concentrations on emergency/urgent visits and hospitalizations for asthma in a rural community.
Methods: Daily ambient particulate matter concentrations from an air quality monitor in the San Luis Valley (2003–2012) were obtained from the state health department. Deidentified data for emergency/urgent visits with a diagnosis code for asthma were collected from the local health care system organization. A generalized linear model using splines and employing generalized estimating equations for correlated measures over time was used to examine the association between daily counts of emergency/urgent visits for asthma and 3- to 5-day averaged ambient particulate matter concentrations.
Results: For each 15-μg/m3 increase in 3-day averaged ambient particulate matter, there was an associated 3.1% increase in hospital counts for all patients with asthma (95% confidence interval, 0.3–5.9%; P = 0.03). When the 3-day average exceeded 50 μg/m3, asthma hospital visits increased by 16.8% (P = 0.03), and when it exceeded 100 μg/m3, visits increased by 65.8% (P = 0.002). In children, the odds of one asthma event requiring an emergency/urgent care visit increased 5.0% with each 15-μg/m3 increase in 3-day averaged ambient particulate matter (P = 0.22).
Conclusions: We observed associations between ambient air levels of particulate matter with a diameter less than 10 μm and emergency/urgent care visits and hospitalization counts in a rural U.S. community prone to dust storms and Environmental Protection Agency exceedances.
Keywords: ambient particulate matter, rural, asthma, children, exacerbations
Particulate matter with a diameter less than 10 μm (PM10) is a heterogeneous mixture composed of hydrocarbons, transition metals, and endotoxins (1). In rural areas, 80% of PM10 is composed of windborne crustal elements, whereas in urban areas it consists primarily of automobile pollution and products of combustion (2).
Long-term exposure to particulate matter air pollution has been associated with premature mortality in those affected by respiratory and cardiovascular diseases (3, 4). Elevated PM10 levels are associated with exacerbations of respiratory conditions including chronic obstructive pulmonary disease and bronchitis (5, 6), and are recognized to worsen asthma symptoms (5, 7–9). Among people with asthma experiencing higher exposure to PM10, studies have found increased physician consultations for worsening asthma (10), greater use of asthma reliever medications, increased emergency room visits for asthma exacerbations (5, 7–9), and increased asthma-related hospitalizations with the association strongest in infants (11). Childhood asthma prevalence in the rural community observed in this study, the San Luis Valley (SLV), is 14% (12, 13), which is higher than the U.S. average of 12% (8), and it is therefore of public health importance to identify potential sources of asthma exacerbations in this community. In addition, PM10 levels in rural areas, similar to the SLV, have more frequent days on which levels exceed the Environmental Protection Agency (EPA) maximum contaminant level of 150 μg/m3 (14).
Research has shown that asthma admission rates are more associated with PM10 levels than with fine particles (15). Studies have reported an increased risk for medical visits for asthma exacerbations of 3–6% for every 10-μg/m3 increase in PM10 (15, 16). PM10 has been identified as an environmental trigger for asthma but in limited studies conducted in populations residing in urban areas and over short periods of time (7, 8, 11, 17). A review by Tzivian (8) calls for rigorous studies over longer periods of time and expanding to include rural and minority populations; this study addresses these identified limitations. Our current research is one of the first to examine the association between outdoor PM10 levels and emergency/urgent visit and hospitalization (EUH) counts related to asthma exacerbation over a 10-year period in a rural, biethnic, underserved community.
Methods
Study Population
The SLV is a high-altitude (∼7,500 ft) rural community located in south central Colorado covering approximately 8,000 square miles. It has a biethnic population of about 48,000 that is both white and Hispanic/Latino. The median income is approximately $29,650 with 25% living below the poverty level (18). The primary economic components are tourism and agriculture (ranching and potato crops) (18). The SLV has an arid climate with an average PM10 level of 25 μg/m3 (range, 6–484 μg/m3) between 2001 and 2011 (19). On average the SLV has two times the number of average daily PM10 levels that exceed the EPA maximum contaminant level of 150 μg/m3 (14), relative to other areas of Colorado, including mountain, industrial, and urban areas (19).
Study Sample
We obtained data for all respiratory-related EUH records from the regional medical center serving all of the SLV population (see the online supplement) for the years 2003 through 2012. We requested visit records limited to those with International Classification of Diseases, version 9 (ICD-9) codes for asthma (493.x), pneumonia (481, 485, 486), acute respiratory illness (460.x, 462.x), and chronic obstructive pulmonary disease with bronchitis (490.x). Each record included patient age at the time of the visit, ICD-9 code, and visit date. Patient sex was not included in the data set because past research has not indicated that sex modifies the association between PM10 levels and asthma exacerbation (20). This study was approved with waiver of consent by the Colorado Multiple Institutional Review Board for human research.
Meteorological Data
The National Oceanic Atmospheric Association’s publicly available meteorological data for 2003 to 2012 were obtained from the one meteorological station in the community, located at the regional airport, 2 miles south of the two monitors in Alamosa. Data included daily average temperature, daily total precipitation, daily average barometric pressure, and daily average wind speed. These variables were selected because of the strong association with elevated PM10 levels in ambient air (21). Each meteorological variable was incorporated into the statistical analysis in the original form (daily average or daily total).
Daily PM10 Levels
In the SLV, there are two ambient air monitoring stations, one at Adams State University and the second at a municipal building, located 2 miles apart in the city of Alamosa, which is where the largest portion of the population resides. Monitors are maintained by the Colorado Air Pollution Control Division (CAPCD) of the Colorado Department of Public Health and Environment (CDPHE) (22). PM10 levels were measured daily throughout the 10-year study period (2003–2012) from high-volume canister samples that use quartz filters (8 × 10 in.), using the protocol established by the U.S. National Ambient Air Quality Standards for particulate matter. Filters were collected daily and sent to the CDPHE in a manila envelope for laboratory analysis. The laboratory used the standards of protocol for PM10 gravimetric analysis that determines particulate concentration based on weight difference (22). PM10 level data are reported to the CAPCD and stored in the environmental data warehouse within the CDPHE. The CAPCD analyzes the PM10 levels to identify samples with levels exceeding 150 μg/m3, which are further analyzed for elemental and chemical composition (22). Data from the monitor at the municipal site were more complete (11.2% of daily values missing for Adams, 6.7% for the municipal building, with no apparent trend for missing data for either site), and the PM10 concentrations from these monitors were well correlated (r = 0.88 on untransformed scale and r = 0.81 on natural log of concentrations) over the study period, based on days when both had readings. Because of the strong agreement between monitors and because the municipal monitor data were more complete, the statistical modeling incorporated PM10 concentrations from the municipal monitor only.
PM2.5 is not monitored in the San Luis Valley and therefore was not included in this study.
Statistical Analyses
For each count outcome, a generalized linear model was fit using a log link, including a scale parameter for dispersion, and employing generalized estimating equations to account for serial correlations in hospital counts over time. The m-dependent working covariance structure with m = 4 was selected as the best available structure, allowing a flexible correlation structure between counts for days up to 4 days apart (23). Cubic b-splines were used to model the mean number of counts over time, using evenly spaced knots 1 year apart. Model predictors included class variables for day of the week and month, and continuous variables for daily temperature, pressure, and humidity. Wind speed was initially included but dropped because of weaker significance. Daily visit counts were determined by counting the number of visits per day in total and then by key strata (age category: 5–14, 15–64, 65+; disease category: asthma [493.x]). We examined 1-, 3-, and 5-day moving averages of regular or log-transformed PM10 concentrations. For the 3- and 5-day moving averages, models related the count outcome on a given day to the average PM10 concentration over days up to and including that day (e.g., a count today was related to the average of PM10 today, yesterday, and 2 d ago, for the lagged 3-d moving average [MA]). For individual-day PM10 (i.e., 1-d average), we examined up to lag 4 of the pollutant. Models were also fit using indicators for 1-, 3-, or 5-day averages that exceeded 50- and 100-μg/m3 thresholds. Model-based standard errors were used, as the empirical standard errors could not be estimated for these data. For specific counts of children with asthma, the outcome was modeled as binary (0 vs. at least 1) because counts were rare and a logit link was used. The online supplement describes sensitivity analyses for models using 3- or 5-day MA pollutant variables that consider different ways of handling missing data.
Results
Emergency/Urgent Visit and Hospitalization Counts
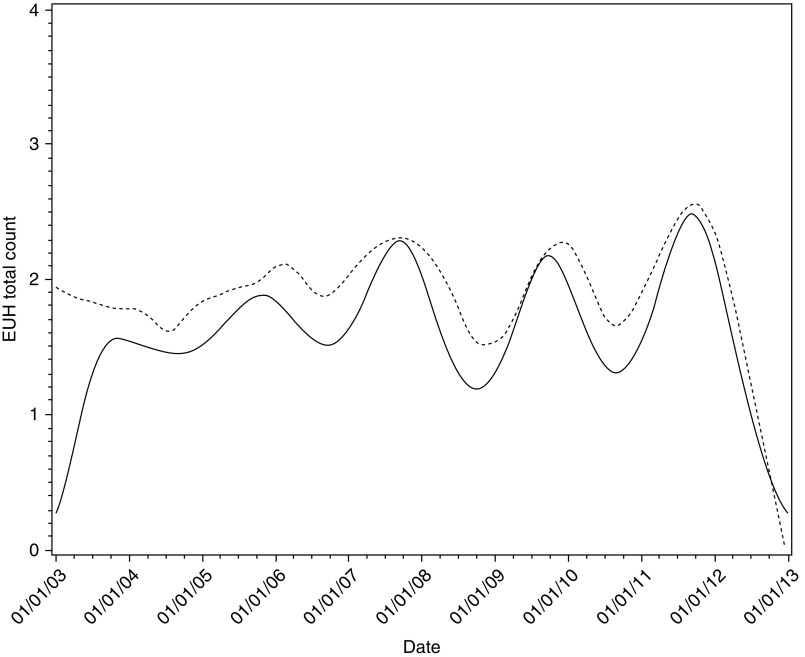
From 2003 through 2012, there were 6,958 total urgent and emergency visits; 3,376 (48.5%) were associated with asthma, 2,006 (28.8%) with pneumonia, 1,345 (19.3%) with pharyngitis/nasopharyngitis, and the remaining 231 (3.3%) with bronchitis. The median number of visits per day was 2 for all respiratory conditions (1 for asthma) with a range of 0 to 11 (0 to 7 for asthma). The mean age of patients was 35.7 years; 15.6% were less than 5 years, 13.1% were 5–14 years, 52.4% were 15–64 years, and 18.9% were 65 years or older. The distribution of patients by age was similar to the age distribution in the SLV (Table 1). Our data set did not include race/ethnicity or sex; however, given that the data set included all EUH counts from the region’s only emergency/urgent care provider and hospital, it is expected that the study cohort is representative of the patient population of the region. Urgent and emergency care visits due to asthma had the highest occurrence on Sunday and Monday and during the months of February, September, and October. Table 2 shows means and SD for hospital counts and PM concentrations for the two monitors, by day of week, month, and year. Preliminarily, we fit the daily all respiratory-related EUH count data and daily PM10 levels using local polynomial regression (a type of nonparametric regression; Figure 1). The data show mean trends that coincided for the most part, and hospital count peaks that tended to occur every other year. Figure 2 shows estimated (mean) visit counts over time, adjusted for PM10, meteorological, and seasonal variables. The fitted curve is consistent with the descriptive curve for counts in Figure 1 and generally reflects a 2-year cycle in count behavior.
Table 1.
Demographic distribution for the San Luis Valley population (2010) and study cohort (2003–2012)
| San Luis Valley |
Study Cohort |
|
|---|---|---|
| (n = 46,173)* | (3,376 Asthma-related EUH Counts) | |
| Age group | ||
| <5 yr | 1,755 (3.8%) | 384 (11.4%) |
| 5–14 yr | 3,114 (6.7%) | 487 (14.4%) |
| 15–64 yr | 36,086 (78.1%) | 2,062 (61.1%) |
| ≥65 yr | 5,218 (11.3%) | 443 (13.1%) |
| Race/ethnicity | Not available | |
| White non-Hispanic | 23,087 (50%) | |
| Hispanic | 21,701 (47%) | |
| Other | 1,385 (3%) | |
| Sex | ||
| Male | 22,625 (49%) | |
| Female | 23,548 (51%) |
Definition of abbreviation: EUH = emergency/urgent visit and hospitalization.
Entries represent count (percentage).
Data from Reference 18.
Table 2.
Total and asthma-related emergency/urgent and hospital visits: mean (SD)
| PM10 (μg/m3) |
||||
|---|---|---|---|---|
| Total EUH Counts | Asthma EUH Counts | Adams | Municipal | |
|
Day of week | ||||
| Sunday | 2.26 (1.65) | 1.00 (1.13) | 15.4 (1.84) | 18.3 (1.84) |
| Monday | 2.01 (1.59) | 0.99 (1.06) | 18.5 (1.82) | 22.6 (1.70) |
| Tuesday | 1.86 (1.52) | 0.94 (1.09) | 19.2 (1.74) | 24.9 (1.66) |
| Wednesday | 1.86 (1.47) | 0.93 (1.00) | 20.4 (1.81) | 25.4 (1.78) |
| Thursday | 1.74 (1.51) | 0.91 (1.09) | 20.0 (1.84) | 25.2 (1.78) |
| Friday | 1.84 (1.64) | 0.89 (1.09) | 19.1 (1.78) | 24.7 (1.71) |
| Saturday | 1.76 (1.43) | 0.81 (0.96) | 17.3 (1.85) | 21.5 (1.71) |
|
Month | ||||
| January | 2.03 (1.44) | 0.89 (0.97) | 19.9 (1.78) | 24.4 (1.78) |
| February | 2.78 (1.91) | 1.15 (1.22) | 16.1 (1.91) | 22.2 (1.80) |
| March | 2.44 (1.67) | 0.97 (1.09) | 16.6 (1.91) | 21.4 (1.75) |
| April | 1.95 (1.53) | 0.88 (1.03) | 19.8 (2.15) | 24.4 (2.10) |
| May | 1.84 (1.37) | 0.85 (0.92) | 19.2 (1.96) | 24.1 (1.84) |
| June | 1.66 (1.36) | 0.80 (0.93) | 23.2 (1.78) | 28.8 (1.67) |
| July | 1.38 (1.24) | 0.76 (0.93) | 17.9 (1.50) | 22.5 (1.51) |
| August | 1.47 (1.31) | 0.78 (0.90) | 16.6 (1.40) | 19.1 (1.49) |
| September | 1.88 (1.48) | 1.10 (1.13) | 16.5 (1.51) | 19.6 (1.51) |
| October | 1.95 (1.72) | 1.10 (1.29) | 17.2 (1.86) | 20.7 (1.80) |
| November | 1.81 (1.51) | 0.95 (1.13) | 21.2 (1.90) | 27.7 (1.80) |
| December | 1.75 (1.52) | 0.89 (1.09) | 18.8 (1.91) | 23.8 (1.77) |
|
Year | ||||
| 2003 | 1.91 (1.54) | 0.84 (0.98) | 20.5 (1.79) | 22.7 (1.71) |
| 2004 | 1.56 (1.28) | 0.74 (0.90) | 18.5 (1.66) | 20.6 (1.71) |
| 2005 | 2.00 (1.48) | 0.89 (0.94) | 16.9 (1.84) | 20.6 (1.72) |
| 2006 | 1.93 (1.50) | 0.81 (0.98) | 16.8 (1.91) | 21.4 (1.80) |
| 2007 | 2.27 (1.56) | 1.00 (1.06) | 18.3 (1.77) | 24.2 (1.79) |
| 2008 | 1.82 (1.61) | 0.71 (0.95) | 16.8 (1.86) | 23.1 (1.75) |
| 2009 | 2.07 (1.68) | 1.01 (1.15) | 17.2 (1.80) | 21.3 (1.69) |
| 2010 | 1.74 (1.29) | 0.84 (0.94) | 18.4 (1.93) | 22.1 (1.75) |
| 2011 | 2.45 (1.70) | 1.61 (1.36) | 20.5 (1.77) | 30.8 (1.77) |
| 2012 | 1.31 (1.55) | 0.79 (1.01) | 21.3 (1.79) | 26.0 (1.73) |
Definition of abbreviations: EUH = emergency/urgent visit and hospitalization; PM10 = particulate matter with a diameter less than 10 μm, SD = standard deviation.
Shown is the arithmetic mean (SD) for emergency/urgent and hospital visits, and geometric mean (geom. SD) for PM10 concentrations (μg/m3), by day of week, month, and year, 2003–2012.
Figure 1.
Local polynomial regression for daily PM10 (solid line, left y axis) and hospital counts (dashed line, right y axis) over a 10-year period (2003–2012). (First-degree polynomial, with a smoothing parameter of 0.15 for both variables.) EUH = emergency/urgent visit and hospitalization; PM10 = particulate matter with a diameter less than 10 μm.
Figure 2.
Estimated mean emergency/urgent visit and hospitalization (EUH) counts from 2003 to 2012, based on nonparametric regression fit (dashed line) and via generalized linear model (GzLM) fit (solid line). The GzLM fit accounted for variables as described in the section Statistical Analyses.
PM10 and Meteorological Data
The interquartile ranges (IQRs) of 1-, 3-, and 5-day averaged PM10 values from the municipal monitor were 16.0, 14.7, and 14.4, respectively. Both 3- and 5-day averaged PM10 exceeded 50 μg/m3 on 6.0% of days during the study period and exceeded 100 μ/m3 on 0.7% of days. Geometric mean and geometric SD of PM10 concentrations by year, month, and day of week are shown in Table 2, and Table 3 shows a composite summary of pollution and meteorological variables.
Table 3.
Descriptive statistics for particulate matter with a diameter less than 10 μm and meteorological variables
| Variable | Mean | SD | Minimum | 25th Percentile | 50th Percentile | 75th Percentile | Maximum |
|---|---|---|---|---|---|---|---|
| Temperature, °F | 42.7 | 18.2 | −12 | 29 | 44 | 59 | 74 |
| Pressure, altimeter in. | 22.8 | 0.16 | 22.06 | 22.68 | 22.81 | 22.9 | 23.17 |
| Precipitation, in. | 0.019 | 0.074 | 0 | 0 | 0 | 0.005 | 1.49 |
| Wind, mph | 7.2 | 3.91 | 0 | 4.4 | 6.4 | 8.9 | 27.4 |
| PM10, μg/m3 | |||||||
| 1-d | 27.5 | 24.7 | 2 | 16 | 23 | 32 | 635 |
| 3-d | 27.7 | 18.1 | 4 | 17.7 | 24 | 32.3 | 337.5 |
| 5-d | 27.8 | 16.2 | 4.5 | 18.3 | 24.6 | 32.7 | 266 |
| Log 1-d PM10, μg/m3 | 3.14 | 0.57 | 0.69 | 2.77 | 3.14 | 3.47 | 6.45 |
| Log 3-d PM10, μg/m3 | 3.14 | 0.47 | 1.39 | 2.82 | 3.14 | 3.44 | 5.47 |
Definition of abbreviations: PM10 = particulate matter with a diameter less than 10 μm, SD = standard deviation.
Relationships between All Respiratory EUH Counts and PM10
We completed an initial analysis of all respiratory conditions for patients of all ages with PM10 untransformed, which showed that for each IQR increase (15 μg/m3) of 3-day average PM10 in outdoor air there was an associated increase of 1.8% (P = 0.09) in daily hospital visit counts. For 5-day averaged PM10, the findings were similar, with a 2.3% (P = 0.07) increase in daily hospital visit counts for all respiratory disease. When the 3-day average PM10 exceeded 100 μg/m3 (modeling pollutant as binary), the number of hospital visits for all respiratory diseases was 32.6% (P = 0.03) higher than when it did not. Similarly, when the 5-day average exceeded 100 μg/m3, the number of hospital visits for all respiratory diseases was 40.0% (P = 0.01) higher than when it did not. Analyses using individual-day pollutant variables up to lag 4 or log scale of pollutant variables did not yield significant results at the α = 0.05 level.
Relationships between EUH Counts for Asthma and PM10
In models for patients of all ages with PM10 untransformed, we observed that for each IQR increase (15 μg/m3) in lagged 3-day average PM10 there was an associated increase of 3.1% (P = 0.03) in daily EUH counts for asthma (Table 4). For 5-day moving average PM10, the findings were similar, with a 3.1% (P = 0.06) increase in asthma-attributable EUH counts (Table 4). When the 3-day moving average was log transformed, the direction of the findings remained, although it was not statistically significant.
Table 4.
Generalized estimating equation estimates (95% confidence interval) of change in asthma-related emergency/urgent and hospitalization visit count as a function of particulate matter with a diameter less than 10 μm, (measured in μg/m3)
| Lag Structure for PM10* | Records Available for Analysis | Relative Increase in Hospital Count per IQR Increase in Pollutant† (%) | 95% CI | P Value |
|---|---|---|---|---|
| 3-d MA | 3,445 | 3.1 | 0.3 to 5.9 | 0.03 |
| 3-d MA‡ | 3,444 | 3.9 | 0.9 to 6.8 | 0.01 |
| 5-d MA | 3,512 | 3.1 | −0.1 to 6.4 | 0.06 |
| 1-d, lag 0 | 3,112 | 1.0 | −1.1 to 3.1 | 0.40 |
| 1-d, lag 1 | 3,112 | 1.5 | −0.6 to 3.4 | 0.17 |
| 1-d, lag 2 | 3,111 | 1.5 | −0.6 to 3.6 | 0.17 |
| 1-d, lag 3 | 3,111 | 0.0 | −2.2 to 2.3 | 0.99 |
| 1-d, lag 4 | 3,110 | −0.3 | −2.5 to 2.1 | 0.81 |
Definition of abbreviations: CI = confidence interval; IQR = interquartile range; MA = moving average; PM10 = particulate matter with a diameter less than 10 μm.
Averaged days starting from current day, for example, lagged 3-day MA includes current day plus previous 2 days.
IQR, 16, 14.7, and 14.4 for 1-day, 3-day, and 5-day moving averages, respectively.
Without outlier (1 value that exceeded 300 μg/m3).
When the association was examined using binary PM10 concentrations, where the 3-day average PM10 exceeded 50 μg/m3, there were 16.8% (P = 0.03) more asthma-related EUH counts (Table 5). Similarly, when the 3-day average PM10 exceeded 100 μg/m3, asthma visits were 65.8% (P = 0.002) higher. When the 5-day pollution average exceeded 50 μg/m3, there were 14.1% (P = 0.10) more asthma-related EUH counts than when it did not (Table 5). Similarly, when the 5-day average exceeded 100 μg/m3 asthma-related EUH counts were 45.0% (P = 0.03) higher (Table 5). Analyses using individual-day pollutant variables up to lag 4 or log scale of pollutant variables did not yield significant results at the α = 0.05 level.
Table 5.
Estimates of change in asthma-related emergency/urgent and hospital visit counts for high versus low particulate matter with a diameter less than 10 μm (measured in μg/m3)
| Lag Structure for PM10* | Cut Point for PM10 (μg/m3) | Records Available for Analysis | Relative Increase in Hospital Count for “High” versus “Low” Pollution (%) | 95% CI | P Value |
|---|---|---|---|---|---|
| 3-d MA | 50 | 3,445 | 16.8 | 1.2 to 34.8 | 0.03 |
| 3-d MA | 100 | 3,445 | 65.8 | 20.2 to 128.7 | 0.002 |
| 5-d MA | 50 | 3,512 | 14.1 | −2.3 to 33.3 | 0.10 |
| 5-d MA | 100 | 3,512 | 45.0 | 2.7 to 104.8 | 0.03 |
Definition of abbreviations: CI = confidence interval; MA = moving average; PM10 = particulate matter with a diameter less than 10 μm.
Averaged days start from current day, for example, lagged 3-day MA includes current day plus previous 2 days.
Discussion
In this longitudinal study, increased PM10 levels were associated with an increase in EUH counts related to respiratory conditions, especially asthma, for individuals 5 years of age and older. The greater significance of these binary variables at specific cut points compared with the continuous variables suggests that there may be a threshold effect, below which little or no pollution effect occurs. This was confirmed in follow-up analyses with change-point models, where the linear relationship between hospital counts and PM10 below 50 μg/m3 was very weak and insignificant. Both of the cut points used in our analyses are at an ambient air concentration less than the current EPA air quality standard for PM10 of 150 μg/m3. Our observations in those affected by asthma were supported by similar findings in EUH counts related to all forms of respiratory conditions, suggesting that elevated PM10 levels in ambient air are associated with compromised lung health.
Plausible mechanisms for PM10 provoking respiratory illnesses are related to its proinflammatory properties. In asthma, this proinflammatory response causes increased airway hyperresponsiveness culminating in airway narrowing and the experience of asthma symptoms (24, 25). Experimental studies reveal that exposure to PM10 can cause alveolar cytokine release responsible for bronchospasm and reduced pulmonary function (26) and also known to cause epithelial injury resulting from its oxidative properties (24). It is suggested that this is because PM10, in contrast to gaseous pollutants, can induce oxidative reactions by crossing the fluid in the respiratory tract lining (27).
Epidemiologic research suggests that large (PM10) particles can become lodged in the airways of the lungs, causing compromised breathing inevitably leading to worsening asthma and the possible need for medical intervention. Studies have documented an association between higher PM10 levels in ambient air and an increased number of emergency room visits (5, 11, 20, 28); a systematic review of 25 studies (8) also corroborated an association between PM10 levels in ambient air and an increased number of emergency room visits, and a second review found 2% increases in asthma-related hospitalizations for every 10-μg/m3 increase in PM10 (29), which was substantiated in another large systemic review by Weinmayr and colleagues, which found PM10 significantly increased risk for asthma symptoms (odds ratio, 1.028; 95% confidence interval, 1.006–1.051) (30). Last, a study by Canova and colleagues found that an increase in PM10 levels by 10 μg/m3 was associated with an 35% increase in emergency room visits due to asthma lagged 0–3 days (for lag 0–3: odds ratio, 1.35; 95% confidence interval, 1.04–1.76) similar to findings in this study (29).
Several factors place children at greater risk for asthma exacerbations due to elevated PM10 levels in ambient air and include the following: rapid growth and development during childhood (31); immature and underdeveloped lungs (32–34); higher inhaled air capacity, which can potentially lead to higher doses of PM10 (35); and a corresponding higher volume-to-body weight ratio than adults (34). Because of their smaller airways the disposition of pollutants, especially PM10, is greater in children than adults (33, 34), thus further supporting the significance of the findings that ambient PM10 levels increase EUH counts in children, especially in rural areas where dust levels are elevated.
This study is not without limitations. It is expected that the use of a single monitor can approximate the individual-level potential for exposure; however, it does not account for individual dose, which is variable due to volume of air intake, spatial variability in dust concentrations, indoor air PM10 concentration, time spent indoors versus outdoors, and time spent at other locations such as school, day care, or work. However, given the concordance between the two PM10 monitors present in Alamosa, the use of the municipal monitor for a population-level approximation of exposure is appropriate. In addition, we considered only PM10 concentrations and did not consider other pollutants with known association with asthma exacerbations in urban populations, including nitrogen oxide (NOx) and sulfur oxide (SOx) (36, 37). These other pollutants were not included because PM10 is the predominant ambient air pollutant of concern in this rural community, with no exceedances of NOx or SOx reported during the 10-year study period, whereas PM10 had 72 exceedances.
Another limitation is that our statistical models did not account for individual-level confounders that could influence the need for an EUH for worsening asthma. Such factors include underlying severity of chronic respiratory illnesses, smoke exposure, adherence to treatment plans, genetic susceptibility, respiratory infections (viral and bacterial), other household exposures, residential distance from clinic or hospital, and access to transportation, all of which can increase the risk of exacerbations and were not included as part of the data ascertainment. These data were not included as part of the data-sharing agreement with the regional medical facility as a means to preserve confidentiality because the data included individual records with some days having only one record. Future research in the area should collect individual-level data that include these other known triggers of asthma exacerbation as part of the prospective study that concurrently tracks air quality and medical care utilization related to asthma.
Another limitation is that PM2.5 was not included in this analysis because it is not measured in the San Luis Valley. PM2.5 has been associated with asthma exacerbation; however, it is inconclusive as to whether asthma outcomes are worse with PM10 or with PM2.5 or whether the mixed effects of both have even worse outcomes (38). It is possible that the exclusion of PM2.5 from the statistical analysis induced bias; however, it is not expected that this bias was differential. In rural areas during dust storms particulate matter of all sizes has been shown to increase; however, PM10 increases the most at a factor of 4.7 whereas PM2.5 and PM1 increase by a factor of only 2, suggesting that in rural dust storms similar to the ones that plague the San Luis Valley, PM10 will increase more than other particulate matter (39). In a pilot study as part of this research, we completed a chemical composition analysis of PM10 quartz filters (n = 75) with daily average levels greater than 75 μg/m3. Results found that 16.5% of the filters had no detectable levels of polyaromatic hydrocarbons, a known pollutant due to traffic and wood burning, and in 85% of samples only one polyaromatic hydrocarbon metabolite was detected. Results suggested that traffic and wood burning were not major contributions to air pollution, which are also common sources of smaller particulate matter.
A final limitation is the defining of our cases on the basis of ICD-9 codes that were listed at time of discharge and then as the primary ICD-9 code during the visit. ICD-9 codes have been shown to underrepresent asthma cases, especially in young children, due to complexity and misdiagnoses with lower respiratory infections, which is why we excluded children under 5 years of age (40). Bias due to case identification through ICD-9 code is more readily noted in children less than 5 years old due to the apprehension of labeling a child of this age with asthma (41). However, a strength of this work is that the study did not involve children under the age of 5, for this reason, and limits this potential issue.
Even with these limitations, this study provides significant results and insights in that asthma exacerbations were associated with PM10 levels in a rural population and the association is strongest in the pediatric population. Our work suggests that exposure to PM10 at thresholds lower than those currently set by the EPA (150 μg/m3) may contribute to greater EUH counts related to asthma over a 24-hour period of time. This suggests the need for continued evaluation of the public health relevance of clean air standards. In conclusion, we observed an association between EUH and hospitalization counts and ambient air PM10 levels in a rural community prone to dust storms and EPA exceedances. Because PM10 in ambient air remains a common exposure in the United States, the impact on asthma and lung health should provide motivation to public health officials to conduct further research to elucidate the role of PM10 in respiratory health for individuals of all ages.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the chemistry laboratory staff at the Colorado Department of Public Health and Environment for their contribution to this research. The authors also thank San Luis Valley Health systems for their involvement in this study.
Footnotes
Supported by National Institute for Environmental Health Sciences (NIEHS) grant 1 PO1 ES018181.
Author Contributions: K.A.J. drafted the paper, collected air quality data, completed the literature review, and performed basic data analysis. M.S. completed the complex statistical modeling of the medical care utilization and PM10 levels and helped with drafting and editing the manuscript; M.K.H. provided support for K.A.J. and helped edit and prepare the paper; and L.C. was the mentor for K.A.J., provided clinical input, and ascertained the data from SLVHealth.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Sharratt BS, Lauer D. Particulate matter concentration and air quality affected by windblown dust in the Columbia plateau. J Environ Qual. 2006;35:2011–2016. doi: 10.2134/jeq2006.0212. [DOI] [PubMed] [Google Scholar]
- 2.Kundu S, Stone EA. Composition and sources of fine particulate matter across urban and rural sites in the Midwestern United States. Environ Sci Process Impacts. 2014;16:1360–1370. doi: 10.1039/c3em00719g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunekreef B, Holgate ST. Air pollution and health. Lancet. 2002;360:1233–1242. doi: 10.1016/S0140-6736(02)11274-8. [DOI] [PubMed] [Google Scholar]
- 4.Brook RD, Rajagopalan S, Pope CA, III, Brook JR, Bhatnagar A, Diez-Roux AV, et al. American Heart Association Council on Epidemiology and Prevention, Council on the Kidney in Cardiovascular Disease, and Council on Nutrition, Physical Activity and Metabolism. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 5.Lipsett M, Hurley S, Ostro B. Air pollution and emergency room visits for asthma in Santa Clara County, California. Environ Health Perspect. 1997;105:216–222. doi: 10.1289/ehp.97105216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson HR, Atkinson RW, Peacock JL, Sweeting MJ, Marston L. Ambient particulate matter and health effects: publication bias in studies of short-term associations. Epidemiology. 2005;16:155–163. doi: 10.1097/01.ede.0000152528.22746.0f. [DOI] [PubMed] [Google Scholar]
- 7.Galán I, Tobías A, Banegas JR, Aránguez E. Short-term effects of air pollution on daily asthma emergency room admissions. Eur Respir J. 2003;22:802–808. doi: 10.1183/09031936.03.00013003. [DOI] [PubMed] [Google Scholar]
- 8.Tzivian L. Outdoor air pollution and asthma in children. J Asthma. 2011;48:470–481. doi: 10.3109/02770903.2011.570407. [DOI] [PubMed] [Google Scholar]
- 9.Hoek G, Dockery DW, Pope A, Neas L, Roemer W, Brunekreef B. Association between PM10 and decrements in peak expiratory flow rates in children: reanalysis of data from five panel studies. Eur Respir J. 1998;11:1307–1311. doi: 10.1183/09031936.98.11061307. [DOI] [PubMed] [Google Scholar]
- 10.Sinclair AH, Melly S, Tolsma D, Spengler J, Perkins L, Rohr A, et al. Childhood asthma acute primary care visits, traffic, and traffic-related pollutants. J Air Waste Manag Assoc. 2014;64:561–567. doi: 10.1080/10962247.2013.873093. [DOI] [PubMed] [Google Scholar]
- 11.Tolbert PE, Mulholland JA, MacIntosh DL, Xu F, Daniels D, Devine OJ, et al. Air quality and pediatric emergency room visits for asthma in Atlanta, Georgia, USA. Am J Epidemiol. 2000;151:798–810. doi: 10.1093/oxfordjournals.aje.a010280. [DOI] [PubMed] [Google Scholar]
- 12.Cicutto L, Dingae MB, Langmack EL. Improving asthma care in rural primary care practices: a performance improvement project. J Contin Educ Health Prof. 2014;34:205–214. doi: 10.1002/chp.21254. [DOI] [PubMed] [Google Scholar]
- 13.Cicutto L, O’Brien A, DeGolyer J. A school-centered approach for improving the process of asthma care in rural schools [abstract] Am J Respir Crit Care Med. 2015;191:A3807. [Google Scholar]
- 14.Zigler CM, Kim C, Choirat C, Hansen JB, Wang Y, Hund L, et al. HEI Health Review Committee. Causal inference methods for estimating long-term health effects of air quality regulations. Res Rep Health Eff Inst. 2016;187:5–49. [PubMed] [Google Scholar]
- 15.Gordian ME, Ozkaynak H, Xue J, Morris SS, Spengler JD. Particulate air pollution and respiratory disease in Anchorage, Alaska. Environ Health Perspect. 1996;104:290–297. doi: 10.1289/ehp.96104290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crooks JL, Cascio WE, Percy MS, Reyes J, Neas LM, Hilborn ED. The association between dust storms and daily non-accidental mortality in the United States, 1993–2005. Environ Health Perspect. 2016;124:1735–1743. doi: 10.1289/EHP216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atkinson RW, Anderson HR, Strachan DP, Bland JM, Bremner SA, Ponce de Leon A. Short-term associations between outdoor air pollution and visits to accident and emergency departments in London for respiratory complaints. Eur Respir J. 1999;13:257–265. doi: 10.1183/09031936.99.13225799. [DOI] [PubMed] [Google Scholar]
- 18.U.S. Census Bureau. American FactFinder [accessed 1 Sept 2017] Available from: http://factfinder.census.gov/faces/nav/jsf/pages/index.xhtml.
- 19.U.S. Environmental Protection Agency. Air Quality System Data Mart [Internet database] [last updated 11 Feb 2015; accessed 21 Sept 2016] Available from: https://www.epa.gov/airdata.
- 20.Iskandar A, Andersen ZJ, Bønnelykke K, Ellermann T, Andersen KK, Bisgaard H. Coarse and fine particles but not ultrafine particles in urban air trigger hospital admission for asthma in children. Thorax. 2012;67:252–257. doi: 10.1136/thoraxjnl-2011-200324. [DOI] [PubMed] [Google Scholar]
- 21.Ho WC, Hartley WR, Myers L, Lin MH, Lin YS, Lien CH, et al. Air pollution, weather, and associated risk factors related to asthma prevalence and attack rate. Environ Res. 2007;104:402–409. doi: 10.1016/j.envres.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Colorado Air Pollution Control Division (CAPCD), Colorado Department of Public Health and Environment (CDPHE) Report to the Public 2011–2012 [accessed Sept 2014]Available from: www.colorado.gov/cdphe/aqcc
- 23.SAS Institute Inc. SAS/STAT 14.1 user’s guide. Cary, NC: SAS Institute Inc.; 2015. [Google Scholar]
- 24.Li XY, Gilmour PS, Donaldson K, MacNee W. In vivo and in vitro proinflammatory effects of particulate air pollution (PM10) Environ Health Perspect. 1997;105:1279–1283. doi: 10.1289/ehp.97105s51279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donaldson K, Gilmour MI, MacNee W. Asthma and PM10. Respir Res. 2000;1:12–15. doi: 10.1186/rr5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Eeden SF, Tan WC, Suwa T, Mukae H, Terashima T, Fujii T, et al. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM10) Am J Respir Crit Care Med. 2001;164:826–830. doi: 10.1164/ajrccm.164.5.2010160. [DOI] [PubMed] [Google Scholar]
- 27.Kelly FJ. Oxidative stress: its role in air pollution and adverse health effects. Occup Environ Med. 2003;60:612–616. doi: 10.1136/oem.60.8.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pope CA, Dockery DW. Epidemiology of particle effects. In: Holgate ST, Samet JM, Koren HS, Maynard RL, editors. Air pollution and health. London: Academic Press; 1999; pp. 673–706. [Google Scholar]
- 29.Canova C, Dunster C, Kelly FJ, Minelli C, Shah PL, Caneja C, et al. PM10-induced hospital admissions for asthma and chronic obstructive pulmonary disease: the modifying effect of individual characteristics. Epidemiology. 2012;23:607–615. doi: 10.1097/EDE.0b013e3182572563. [DOI] [PubMed] [Google Scholar]
- 30.Weinmayr G, Romeo E, De Sario M, Weiland SK, Forastiere F. Short-term effects of PM10 and NO2 on respiratory health among children with asthma or asthma-like symptoms: a systematic review and meta-analysis. Environ Health Perspect. 2010;118:449–457. doi: 10.1289/ehp.0900844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selgrade MK, Lemanske RF, Jr, Gilmour MI, Neas LM, Ward MD, Henneberger PK, et al. Induction of asthma and the environment: what we know and need to know. Environ Health Perspect. 2006;114:615–619. doi: 10.1289/ehp.8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dietert RR, Etzel RA, Chen D, Halonen M, Holladay SD, Jarabek AM, et al. Workshop to identify critical windows of exposure for children’s health: immune and respiratory systems work group summary. Environ Health Perspect. 2000;108:483–490. doi: 10.1289/ehp.00108s3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bennett WD, Herbst M, Alexis NE, Zeman KL, Wu J, Hernandez ML, et al. Effect of inhaled dust mite allergen on regional particle deposition and mucociliary clearance in allergic asthmatics. Clin Exp Allergy. 2011;41:1719–1728. doi: 10.1111/j.1365-2222.2011.03814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.American Academy of Pediatrics Committee on Environmental Health. 2nd ed. Elk Grove Village, IL: American Academy of Pediatrics; 2003. Pediatric environmental health. [Google Scholar]
- 35.Oyana TJ, Rivers PA. Geographic variations of childhood asthma hospitalization and outpatient visits and proximity to ambient pollution sources at a U.S.–Canada border crossing. Int J Health Geogr. 2005;4:14. doi: 10.1186/1476-072X-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schildcrout JS, Sheppard L, Lumley T, Slaughter JC, Koenig JQ, Shapiro GG. Ambient air pollution and asthma exacerbations in children: an eight-city analysis. Am J Epidemiol. 2006;164:505–517. doi: 10.1093/aje/kwj225. [DOI] [PubMed] [Google Scholar]
- 37.Atkinson RW, Strachan DP, Anderson HR, Hajat S, Emberlin J. Temporal associations between daily counts of fungal spores and asthma exacerbations. Occup Environ Med. 2006;63:580–590. doi: 10.1136/oem.2005.024448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan J, Li S, Fan C, Bai Z, Yang K. The impact of PM2.5 on asthma emergency department visits: a systematic review and meta-analysis. Environ Sci Pollut Res Int. 2016;23:843–850. doi: 10.1007/s11356-015-5321-x. [DOI] [PubMed] [Google Scholar]
- 39.Jaafari J, Naddafi K, Yunesian M, Nabizadeh R, Hassanvand MS, Ghozikali MG, et al. Study of PM10, PM2.5, and PM1 levels in during dust storms and local air pollution events in urban and rural sites in Tehran. Hum Ecol Risk Assess. 2018;24:482–493. [Google Scholar]
- 40.Gibbison B, Griggs K, Mukherjee M, Sheikh A. Ten years of asthma admissions to adult critical care units in England and Wales. BMJ Open. 2013;3:e003420. doi: 10.1136/bmjopen-2013-003420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Miguel-Díez J, Jiménez-García R, Hernández-Barrera V, López de Andrés A, Villa-Asensi JR, Plaza V, et al. National trends in hospital admissions for asthma exacerbations among pediatric and young adult population in Spain (2002–2010) Respir Med. 2014;108:983–991. doi: 10.1016/j.rmed.2014.04.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.