Abstract
Rationale: Previous studies have suggested that acute exacerbations of chronic obstructive pulmonary disease (COPD) may be associated with increased risk of myocardial infarction and ischemic stroke.
Objectives: We aimed to quantify the increased risks of myocardial infarction and ischemic stroke risk associated with both moderate and severe acute exacerbation, and to investigate factors that may modify these risks.
Methods: We performed a self-controlled case series to investigate the rates of myocardial infarction and ischemic stroke after acute exacerbation compared with stable time, within individuals. The participants were 5,696 adults with COPD with a first myocardial infarction (n = 2,850) or ischemic stroke (n = 3,010) and at least one acute exacerbation from the UK Clinical Practice Research Datalink with linked Hospital Episodes Statistics data.
Results: The risks of both myocardial infarction and ischemic stroke were increased in the 91 days after an acute exacerbation. The risks were greater after a severe exacerbation (incidence rate ratio [IRR], 2.58; 95% confidence interval [CI], 2.26–2.95 for myocardial infarction; and IRR, 1.97; 95% CI, 1.66–2.33 for ischemic stroke) than after a moderate exacerbation (IRR, 1.58; 95% CI, 1.46–1.71 for myocardial infarction; and IRR, 1.45; 95% CI, 1.33–1.57 for ischemic stroke). The relative risks of myocardial infarction and ischemic stroke associated with acute exacerbation were lower among those with more frequent exacerbations (IRR, 1.42; 95% CI, 1.24–1.62 vs. IRR, 1.69; 95% CI, 1.50–1.91 for myocardial infarction; and IRR, 1.30; 95% CI, 1.15–1.48 vs. IRR, 1.68; 95% CI, 1.50–1.89 for ischemic stroke). Higher GOLD stage was associated with a lower rate of myocardial infarction (IRR, 1.98; 95% CI, 1.61–2.05 vs. IRR, 1.69; 95% CI, 1.45–1.98) but not for ischemic stroke. Aspirin use at baseline was associated with a lower risk of ischemic stroke (IRR, 1.28; 95% CI, 1.10–1.50 vs. IRR, 1.63; 95% CI, 1.47–1.80) but not with myocardial infarction.
Conclusions: Acute exacerbations of COPD are associated with an increased risk of myocardial infarction and ischemic stroke within 28 days of their onset. Several patient characteristics were identified that are associated with these events.
Keywords: epidemiology, cardiovascular disease, electronic health care records
People with chronic obstructive pulmonary disease (COPD) are at increased risk of myocardial infarction (1) and stroke (2), and up to one-third of patients with COPD die of cardiovascular disease (3). This increased risk cannot be completely explained by smoking (4) and has been attributed to increased systemic inflammation (5).
Acute exacerbations of COPD normally last several days; most are thought to be triggered by bacterial or viral infection (6, 7) and are associated with increased systemic inflammation (8, 9). Previous work has shown that lower respiratory tract infections (LRTIs) are associated with an increased risk of myocardial infarction in the general population (10). In addition, two small studies have suggested that there may be an increased risk of myocardial infarction after periods of acute exacerbation compared with stable periods (11, 12). Frequent exacerbators (people who have two or more treated exacerbations per year) also seem to have a higher long-term risk of myocardial infarction than do infrequent exacerbators (11). The relationship between acute exacerbation and stroke is less clear. A previous study found an increased risk of stroke after acute exacerbation, but this risk was delayed until 49 days after an acute exacerbation (11). Another study found a slightly increased risk of stroke over a 10-year follow-up when comparing exacerbating with nonexacerbating patients with COPD (13).
The risk factors for myocardial infarction and ischemic stroke after acute exacerbation are not known, limiting the strategies or interventions that might mitigate this risk. Improvements in methods to identify acute exacerbation in electronic health records mean that acute exacerbation can now be identified with greater sensitivity and precision (14). In addition, with linkage to secondary care records, the severity of acute exacerbations in patients with COPD can be stratified, using a health care utilization definition, into moderate (general practitioner treated; primary care managed) and severe (hospitalized) events.
The aims of this study were therefore to 1) characterize the magnitude and duration of myocardial infarction and ischemic stroke risks after acute exacerbation, 2) investigate the relationship between severity of acute exacerbation and myocardial infarction and ischemic stroke risk, and 3) investigate whether the associations between exacerbation and both myocardial infarction and ischemic stroke are modified by COPD severity, prior acute exacerbation frequency, myocardial infarction type (ST-elevation myocardial infarction [STEMI] or non-STEMI), comorbid cardiovascular disease, use of cardiovascular medicines, use of inhaled COPD maintenance therapy, or influenza vaccination at baseline.
Methods
Data Sources
We used data from the Clinical Practice Research Datalink (CPRD) linked with Hospital Episodes Statistics (HES) data. The CPRD is a large database of primary care data. It contains details on more than 11 million patients in the UK, with more than 4 million of these being active patients (around 7% of the UK population) (15). The available data include details on symptoms, diagnoses, tests, prescriptions, details on patient demographics and health behaviors, and referrals to secondary care. The diagnostic data in the CPRD are recorded mainly by a system of Read codes, which is a hierarchical classification system. HES is an administrative database containing details of all episodes of admitted patient care in England and Wales. Data are structured into episodes of care by single consultants (“finished consultant episodes”), such that each hospitalization may involve several finished consultant episodes. Data are recorded using ICD-10 (International Statistical Classification of Diseases and Related Health Problems, 10th Revision) codes. Each finished consultant episode may be associated with up to 20 ICD-10 codes, with the first code generally representing the reason for hospitalization. The remaining codes may represent other acute problems, or comorbidities. Data for about 60% of CPRD patients are linked to HES. CPRD–HES data were also linked to Office of National Statistics data to determine the exact date of death.
Study Design
The self-controlled case series is a within-person design developed to reduce confounding in observational studies. The incidence rate of an outcome after an exposure is compared with unexposed periods of time in the same individual, using only data for those who experience the outcome (16). This method has been used widely to investigate the risk of acute cardiovascular events associated with episodes of infection and inflammation (17, 18). We used this design to estimate the incidences of myocardial infarction or ischemic stroke after the onset of acute exacerbation compared with stable periods. As well as being able to estimate the transient effect of an exposure, the major advantage of this design is that within-individual inferences are made because each subject acts as his or her own control. This means that the design implicitly controls for the effects of fixed confounders such as sex, socioeconomic status, and genetic factors, as well as other unknown/unmeasured fixed confounders. Follow-up time is accumulated in various age bands to account for confounding by age.
The self-controlled case series method relies on three assumptions:
-
1.
That events do not change the probability of future exposures. This assumption should be met in our analysis, as it is not likely that having an myocardial infarction or ischemic stroke changes the future risk of acute exacerbation.
-
2.
That recurrent events are independent. As recurrent myocardial infarctions and strokes are not likely to be independent, we restricted the analysis to first myocardial infarction or stroke only.
-
3.
That the occurrence of the event does not censor or alter observation periods. This assumption may not be met as myocardial infarction and ischemic stroke are associated with considerable mortality. To assess the impact of this assumption, we conducted a sensitivity analysis described in the section Secondary Analyses. In addition, we also stratified the 91-day risk period into smaller time segments to address this potential issue.
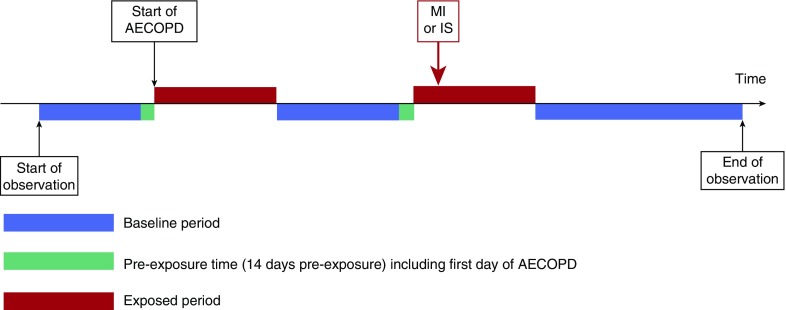
After a previous study (10) we made an a priori decision to include the maximum of 91 days after the onset of acute exacerbation as the exposure period. In addition, we segmented this period into smaller periods of 1–3, 4–7, 8–14, 15–28, and 29–91 days, to determine how the relative risk changes over the exposure period. To reduce misclassification of acute exacerbation with myocardial infarction (or ischemic stroke), we created a 14-day window of preexposure time including the first day of the acute exacerbation, which was not included in either baseline or exposed time. The study design is shown in Figure 1.
Figure 1.
Diagram representing the study design. In this hypothetical example the patient has two exposed periods (acute exacerbation) during follow-up and a first myocardial infarction within 91 days of the start of the second exposed period (acute exacerbation). AECOPD = acute exacerbation of chronic obstructive pulmonary disease; IS = ischemic stroke; MI = myocardial infarction.
Study participants were monitored from January 1, 2004, date of COPD diagnosis, 35th birthdate, or CPRD practice “up to standard” date, whichever was later; follow-up finished on March 31, 2015, date of death, transfer out of practice or practice last collection date, whichever was earlier; the first year of follow-up served as the baseline year.
Study Sample, Exposure, Covariates, and Outcomes
The study sample comprised patients with COPD who had at least one acute exacerbation and a first myocardial infarction or ischemic stroke during the study period. Patients with COPD were identified using a previously validated algorithm (19), and had a diagnostic Read code for COPD, a smoking history (ex or current smoker), and were age 35 years or older. Patients were excluded if their CPRD records could not be linked to HES or the Office of National Statistics.
We characterized acute exacerbation severity according to health care utilization, with those requiring treatment from their general practitioner as “moderate” events and those requiring hospitalization as “severe” events, using previously validated algorithms (14, 20). Acute exacerbations that occurred within 2 weeks of the onset of a previous acute exacerbation were taken to be a continuing event.
Apart from age, sex, and type of myocardial infarction, all potential effect modifiers were defined during the year before start of follow-up using CPRD data. Cardiovascular drug use (β-blocker, aspirin, and statins), inhaled COPD therapy use (long-acting β-agonists, long-acting muscarinic antagonists, and inhaled corticosteroids), and influenza vaccination status were defined by the presence of at least one prescription during the 1-year period before the start of follow-up. Previous cardiovascular disease (stroke, heart failure, and angina for the myocardial infarction analysis; and atrial fibrillation, angina, and previous myocardial infarction for the stroke analysis) was defined as any code suggesting one of these conditions was diagnosed at any time before follow-up start. Global Initiative for Chronic Obstructive Lung Disease (GOLD) grade of airflow limitation was defined on the basis of spirometry results from the year before the start of follow-up. Study participants were dichotomized into exacerbation frequency phenotype categories depending on the number of exacerbations in the 1 year before follow-up start; we also expressed exacerbation frequency during this time in terms of actual number of events (0, 1, 2, ≥3). The main analyses of the associations between acute exacerbation and the risks of myocardial infarction or ischemic stroke within 91 days were stratified by potential effect modifiers.
Myocardial infarction and stroke events were defined using both primary care (CPRD) and hospital data (HES). Read codes were used to define myocardial infarction and ischemic stroke in CPRD (see the online supplement). In HES, myocardial infarctions and ischemic stroke were defined as an ICD-10 code for myocardial infarction or ischemic stroke in the first position of a finished consultant episode. The date of myocardial infarction or ischemic stroke was taken as the date of the start of the finished consultant episode containing the myocardial infarction or ischemic stroke code, rather than the date of admission to hospital. ICD-10 codes I21.0–I21.4 were used to identify myocardial infarction in HES. ICD-10 codes I63.0–I63.9 were used to identify ischemic stroke in HES.
Statistical Analysis
We used conditional Poisson regression to estimate the incidence rate ratio (IRR) of first myocardial infarction or stroke in the 91 days after acute exacerbation compared with stable periods.
We adjusted for age in 1-year age bands. In addition, as weather may be associated with both acute exacerbation (21) and myocardial infarction (22) or stroke (23), we adjusted for season (split into October–March and April–September).
In addition to the main self-controlled case series analysis, we also used a nonparametric spline–based self-controlled case series method (24). The advantages of this method are that the time segments within the total risk period (91 d after an acute exacerbation) do not have to be prespecified, and that this method allows easier visualization of the evolution of the relative risk across the total 91-day risk period.
Secondary Analyses
One of the assumptions of the self-controlled case series analysis is that the outcomes do not alter the probability of future exposure or result in censoring of the observation time. As myocardial infarction and stroke are associated with death, which would decrease the probability of further acute exacerbation and result in informative censoring, we conducted a sensitivity analysis similar to previous studies (15, 16) to assess the potential impact of breaking this assumption. To do this, we repeated the main analysis in those whose follow-up was not censored due to death first for at least 6 months after myocardial infarction or ischemic stroke, and also in those whose follow-up was not censored due to death for at least 12 months after myocardial infarction or ischemic stroke.
The analysis was conducted using Stata 14.1MP (StataCorp) and R 3.2.4 (R Foundation).
Ethics
The protocol for this research was approved by the Independent Scientific Advisory Committee (ISAC) for the Medicines and Healthcare Products Regulatory Agency Database Research (protocol numbers 15_226A and 17_060). Generic ethical approval for observational research using the CPRD with approval from ISAC has been granted by a Health Research Authority Research Ethics Committee (East Midlands–Derby; REC reference number 05/MRE04/87). The protocol is available on request.
Results
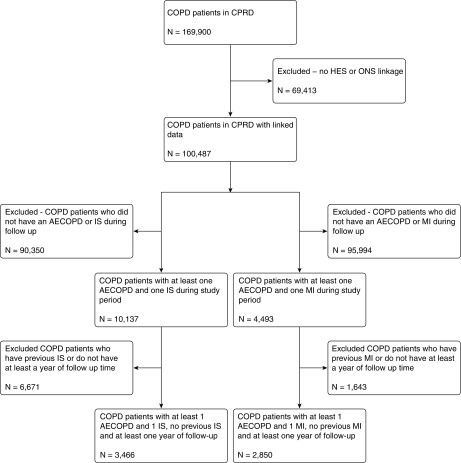
In total, we included 5,696 participants in the study: 2,850 individuals who had a first myocardial infarction and at least one acute exacerbation, during the study period, and 3,466 with a first ischemic stroke and at least one acute exacerbation during the study period (Figure 2). One hundred and sixty-four participants with COPD were included in both analyses. The characteristics of the study participants are summarized in Table 1.
Figure 2.
Patient flow in the study. AECOPD = acute exacerbation of chronic obstructive pulmonary disease; COPD = chronic obstructive pulmonary disease; CPRD = Clinical Practice Research Datalink; HES = Hospital Episodes Statistics; IS = ischemic stroke; MI = myocardial infarction; ONS = Office of National Statistics.
Table 1.
Characteristics of study participants with myocardial infarction or ischemic stroke and acute exacerbation of chronic obstructive pulmonary disease during the study period
| Characteristic | Myocardial Infarction | Ischemic Stroke |
|---|---|---|
| No. of participants | 2,850 | 3,466 |
| Age at index myocardial infarction/ischemic stroke, yr | 73.3 (66.0–80.3) | 75.8 (68.8–81.8) |
| Average observation time from follow-up start, yr | 8.2 (6.0–10.3) | 6.3 (3.9–9.0) |
| Male sex | 59.5% | 55.1% |
| Number with at least one severe event (acute exacerbation requiring hospitalization) | 51.0% | 36.1% |
| Frequent exacerbators, 12 mo before follow-up start | 42.9% | 39.5% |
| Cardiovascular comorbid disease at any time before the start of follow-up | ||
| Angina | 17.9% | 17.2% |
| Heart failure | 7.7% | — |
| Stroke | 5.7% | — |
| Atrial fibrillation | — | 19.0% |
| Myocardial infarction | — | 7.2% |
| Prescribed cardiovascular drug within 12 mo before the start of follow-up | ||
| Statin | 29.4% | 30.5% |
| Aspirin | 28.6% | 30.6% |
| β-Blocker | 20.7% | 23.7% |
| GOLD stage of airflow limitation at start of follow-up (N = 1,476) | ||
| Grade 1–2, n (%) | 976 (64.1) | 978 (65.3) |
| Grade 3–4, n (%) | 547 (35.9) | 519 (34.7) |
| Missing, n | 1,374 | 1,513 |
| Prescribed respiratory drug, 12 mo before the start of follow-up | ||
| ICS users | 60.8% | 42.1% |
| Concomitant LABA | 79.8% | 72.4% |
| Concomitant LAMA | 34.5% | 29.0% |
| Non-ICS users | 39.2% | 57.9% |
| LABA | 14.1% | 34.8% |
| LAMA | 16.0% | 22.8% |
| Baseline influenza vaccination status | 69.3% | 72.6% |
| Type of myocardial infarction (N = 1,872) | ||
| STEMI, n (%) | 734 (37.2) | — |
| Non-STEMI, n (%) | 1,242 (62.9) | — |
| Missing, n | 978 | — |
Definition of abbreviations: GOLD = Global Initiative for Chronic Obstructive Lung Disease; ICS = inhaled corticosteroid; LABA = long-acting β-agonist; LAMA = long-acting muscarinic antagonist; STEMI = ST-elevation myocardial infarction.
Data represent median (interquartile range) or percentage.
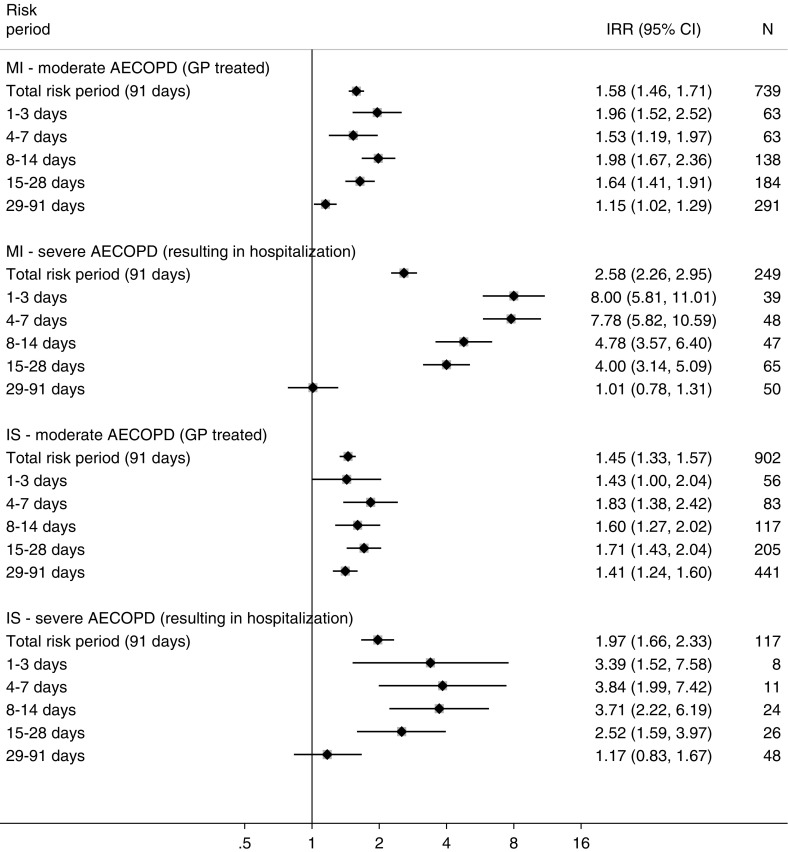
Compared with stable periods, the 91 days after the onset of acute exacerbation were associated with a 65% increased risk of myocardial infarction (IRR, 1.65; 95% CI, 1.50–1.81) and a 51% increased risk of ischemic stroke (IRR, 1.51; 95% CI, 1.39–1.65). The increased risk peaked in the first 3 days after acute exacerbation onset for myocardial infarction and appeared to peak in the 4–7 days after onset for ischemic stroke (Figure 3). The risk gradually fell back to the stable period level after 28 days for myocardial infarction; however, it appeared to remain elevated longer for ischemic stroke (Figure 3).
Figure 3.
Incidence rate ratios of first myocardial infarction or ischemic stroke in risk periods after an acute exacerbation of chronic obstructive pulmonary disease relative to stable periods and stratified by acute exacerbation severity. P value for interaction between moderate and severe acute exacerbation for risk of myocardial infarction, <0.001; P value for interaction between moderate and severe acute exacerbation for risk of ischemic stroke, <0.001. AECOPD = acute exacerbation of chronic obstructive pulmonary disease; CI = confidence interval; GP = general practitioner; IRR = incidence rate ratio; IS = ischemic stroke; MI = myocardial infarction.
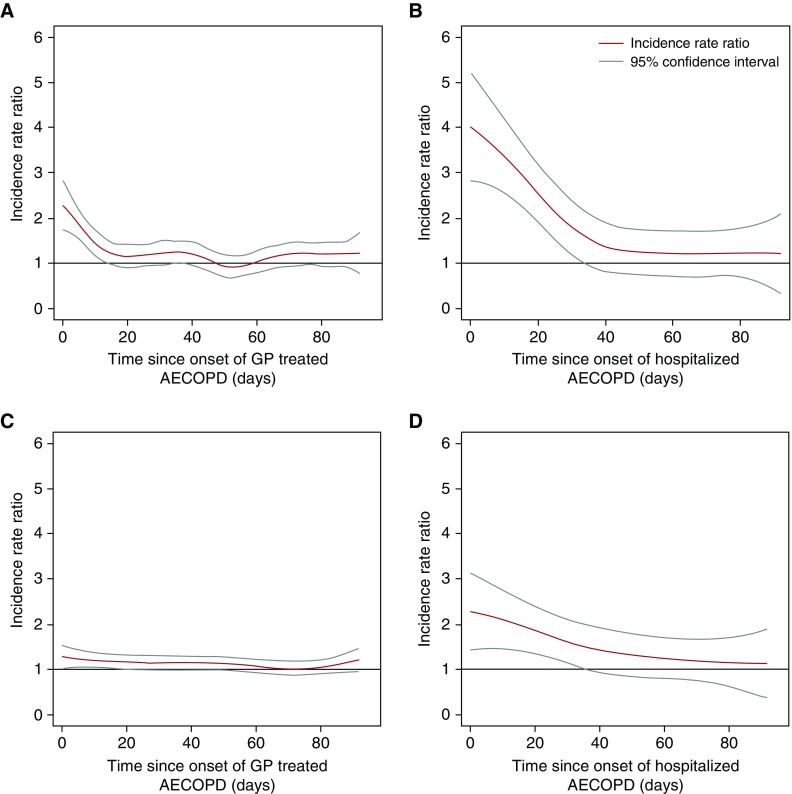
The associations of acute exacerbation and both myocardial infarction and ischemic stroke were modified by the severity of the acute exacerbation (P value for interaction < 0.001 for both myocardial infarction and ischemic stroke), with the risk of myocardial infarction being more than 2.5 times that of stable periods in the 91 days after a severe acute exacerbation, compared with 1.6 times that of stable periods for moderate events. The risk of ischemic stroke was 1.7 times that of stable periods after severe acute exacerbation, and 1.4 times that of stable periods after moderate acute exacerbation. The results of the nonparametric self-controlled case series analysis using spline regression are displayed in Figure 4 and confirm the patterns observed in the segmented analysis (Figure 3).
Figure 4.
(A) Risk of myocardial infarction associated with moderate (general practitioner–treated) acute exacerbation; (B) risk of myocardial infarction associated with severe (hospitalized) acute exacerbation; (C) risk of ischemic stroke associated with moderate (general practitioner–treated) acute exacerbation; and (D) risk of ischemic stroke associated with severe (hospitalized) acute exacerbation. AECOPD = acute exacerbation of chronic obstructive pulmonary disease; GP = general practitioner.
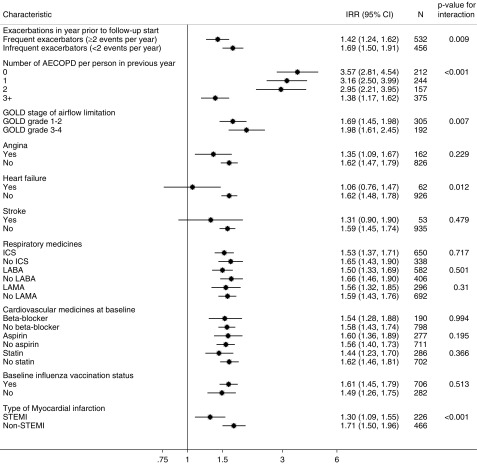
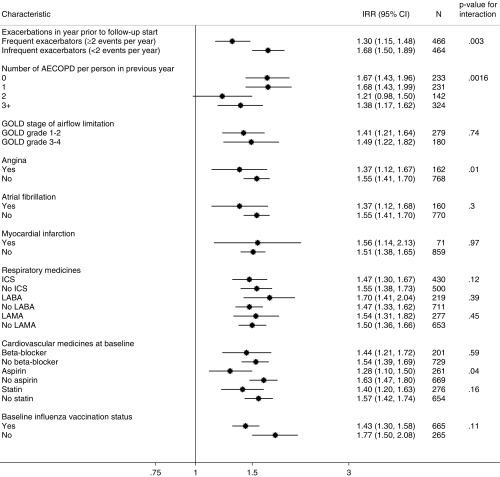
The association of acute exacerbation with myocardial infarction was stronger among infrequent exacerbators compared with frequent exacerbators, with infrequent exacerbators having a 69% higher rate of myocardial infarction in the 91 days after onset of acute exacerbation compared with their stable periods, and frequent exacerbators having a 42% higher rate of myocardial infarction compared with their stable periods (P = 0.009); and infrequent exacerbators having a 68% higher risk of ischemic stroke after acute exacerbation compared with 30% increase for frequent exacerbators (P < 0.001) (Figures 5 and 6). This pattern was also apparent when using the number of baseline acute exacerbations rather than the dichotomous phenotype, although more so for myocardial infarction than ischemic stroke.
Figure 5.
Incidence rate ratios of first myocardial infarction in risk period (91 d) after an acute exacerbation of chronic obstructive pulmonary disease (COPD) relative to stable periods stratified by characteristics of patient with COPD. AECOPD = acute exacerbation of chronic obstructive pulmonary disease; CI = confidence interval; GOLD = Global Initiative for Chronic Obstructive Lung Disease; ICS = inhaled corticosteroid; IRR = incidence rate ratio; LABA = long-acting β-agonist; LAMA = long-acting muscarinic antagonist; STEMI = ST-elevation myocardial infarction.
Figure 6.
Incidence rate ratios of first ischemic stroke in risk period (91 d) after an acute exacerbation of chronic obstructive pulmonary disease (COPD) relative to stable periods stratified by characteristics of patient with COPD. AECOPD = acute exacerbation of chronic obstructive pulmonary disease; CI = confidence interval; GOLD = Global Initiative for Chronic Obstructive Lung Disease; ICS = inhaled corticosteroid; IRR = incidence rate ratio; LABA = long-acting β-agonist; LAMA = long-acting muscarinic antagonist.
The association of acute exacerbation with myocardial infarction was also stronger for those with severe as compared with mild-to-moderate airflow limitation (GOLD grade, 1–2; IRR, 1.69; 95% CI, 1.45–1.98; GOLD grade, 3–4; IRR, 1.98; 95% CI, 1.61–2.05; P = 0.007). However, the association of acute exacerbation with ischemic stroke was not modified by severity of airflow limitation (P = 0.74).
We found that the association of acute exacerbation with myocardial infarction in the 91 days after acute exacerbation was higher for non-STEMIs (IRR, 1.80; 95% CI, 1.56–2.06) than for STEMIs (IRR, 1.39; 95% CI, 1.16–1.68).
Those with previous heart failure had a lower relative risk of myocardial infarction associated with acute exacerbation (IRR, 1.06; 95% CI, 0.76–1.47, compared with IRR, 1.62; 95% CI, 1.48–1.78; P = 0.01), and those with previous angina had a lower risk of ischemic stroke associated with acute exacerbation (IRR, 1.55; 95% CI, 1.41–1.70, compared with IRR, 1.37; 95% CI, 1.12–1.67; P = 0.01). There was some evidence that the association between acute exacerbation and ischemic stroke was lower among those using aspirin (IRR, 1.28; 95% CI, 1.10–1.50) compared with aspirin nonusers (IRR, 1.63; 95% CI, 1.47–1.80; P = 0.04). There was no modification of the associations between acute exacerbation and either myocardial infarction or stroke by other previous cardiovascular disease, cardiovascular drugs, COPD medicines, or influenza vaccine in the baseline period, or by age or sex (data for age and sex not shown).
In the sensitivity analysis among individuals whose observation time was not censored by death after myocardial infarction or ischemic stroke, relative risks were slightly smaller than in the main analysis (see the online supplement).
Discussion
We found that the increased risks of myocardial infarction and ischemic stroke in the weeks after acute exacerbation were of greater magnitude and longer duration than previously estimated (11). These associations were also stronger among those with a severe exacerbation requiring hospitalization, suggesting possible modifiers of the associations between acute exacerbation and both myocardial infarction and ischemic stroke risk. Our data suggest that those with a history of more frequent exacerbations may be at lower risk of myocardial infarction and ischemic stroke after an acute exacerbation, and that the association between acute exacerbation and myocardial infarction may be stronger among those with more severe airflow obstruction. The association between acute exacerbation and ischemic stroke was weaker among aspirin users compared with aspirin nonusers.
We found an eightfold risk of myocardial infarction in the first 3 days after hospitalized acute exacerbation, compared with a twofold risk for moderate events, suggesting a dose–response relationship by severity of acute exacerbation. In addition, our findings suggest that risk of myocardial infarction increases again at around 8–14 days after falling for the first 7 days following moderate but not severe acute exacerbation. This could be a chance finding, but the timing may correspond to secondary bacterial infection in those with a viral exacerbation (25).
For ischemic stroke, we found a 43% increased risk of ischemic stroke in the first 3 days and a twofold risk for severe acute exacerbation compared with stable periods, again suggesting a dose–response relationship. The peak risk of ischemic stroke was in the 4–7 days after acute exacerbation onset at an 80% increased risk of ischemic stroke after general practitioner–treated acute exacerbation and almost a fourfold increased risk of ischemic stroke after hospitalized acute exacerbation.
Previous studies have suggested that frequent exacerbators have a higher risk of myocardial infarction (11). Our study found that the associations of acute exacerbation with the risks of myocardial infarction and ischemic stroke were lower for frequent exacerbators. Crucially, our study compared the relative risk of a myocardial infarction during acute exacerbation with a participant’s own stable period, not risk of myocardial infarction and ischemic stroke between individuals. One explanation could be that frequent exacerbators have a higher risk of myocardial infarction and ischemic stroke during stable periods (due to perhaps increased baseline inflammation [26]), and thus there is less of a relative difference between stable and exacerbation periods for them. Because the self-controlled case series design includes only people who have the outcome of interest, we were not able to measure absolute rates of myocardial infarction or ischemic stroke.
Others have found that increased airflow limitation is associated with increased risk of myocardial infarction (27). This is reflected in our finding that patients with COPD with worse airflow limitation are more susceptible to the effects of acute exacerbation on risk of myocardial infarction than those with lesser limitation. There was no modification of the effect of acute exacerbation on risk of ischemic stroke by severity of airflow limitation, despite increased airflow limitation being associated with stroke (28). Although there may be other differences between these patients, this finding points to the possibility that acutely worsening airflow limitation during acute exacerbation may be involved in the increased risk of myocardial infarction associated with acute exacerbation. This finding lends support to the idea that acute exacerbation may be a risk factor for type 2 myocardial infarction, which is the result of mismatch of myocardial supply and demand of oxygen but is not due to plaque rupture (29). Coupled with our finding that the association between acute exacerbation and ischemic stroke (but not myocardial infarction) was stronger among aspirin nonusers is also suggestive of different mechanisms driving risk of ischemic stroke and myocardial infarction after acute exacerbation.
We found that those with a previous heart failure diagnosis had a lower relative risk of myocardial infarction associated with acute exacerbation. However, this is based on a small number of events in the at-risk period and may be due to misclassification of episodes of breathlessness due to acute heart failure being misclassified as acute exacerbation in these patients, thereby misclassifying episodes of stable time as exposed time. Interestingly, we also found a weaker association between acute exacerbation and ischemic stroke among those with prior angina, which is potentially due to an effect of treatment for angina.
We have previously reported that people with COPD and acute myocardial infarction are more likely to have a non-STEMI than a STEMI, compared with people without COPD with acute myocardial infarction (30). Our finding that the associations of acute exacerbation with myocardial infarction was greater for non-STEMIs may help explain these excess non-STEMIs in those with COPD.
Our finding that acute exacerbation is associated with a transient increased risk of myocardial infarction confirms suggestions from previous work that acute exacerbations are associated with myocardial infarction (11, 12) and myocardial injury (31). Our study also supports previous findings that exacerbating patients with COPD have a higher risk of stroke compared with nonexacerbating patients over a 10-year follow-up (13), and our results extend these findings by precisely identifying the timing and duration of increased risk. In a within-individual analysis of 426 patients with COPD and using prescription antibiotics and oral steroids as a definition of acute exacerbation, Donaldson and colleagues (11) also found an increased risk of myocardial infarction associated with acute exacerbation, but this was limited to the first 5 days after acute exacerbation onset. Donaldson and colleagues also investigated the risk of stroke after acute exacerbation and found a small increased risk for the 49 days after acute exacerbation. We found a significantly higher risk than that previously estimated in previous studies. Our large sample size and validated exposure measures may have allowed us to estimate a more precise effect size and duration of increased risk.
Broadly, our results are comparable with those from Smeeth and colleagues (10), who investigated the relationship between LRTI and risks of myocardial infarction and stroke in 20,921 people from the general population. Smeeth and colleagues reported an IRR of 4.95 (95% CI, 4.43–5.53) for myocardial infarction and an IRR of 3.19 (95% CI, 2.81–3.62) for stroke in the 3 days after LRTI, which declined toward baseline over time, but lasted more than 4 weeks. The higher relative risk of myocardial infarction and ischemic stroke after LRTI in the general population compared with acute exacerbation may be due to a smaller relative difference in inflammation between acute exacerbation/LRTI and stable periods for those with COPD. Alternatively, those with COPD may attend their general practitioner with milder LRTI (in terms of inflammatory burden) than would those from the general population.
We did not find that β-blockers or statins modified the effect of acute exacerbation on risk of first myocardial infarction or ischemic stroke. These findings are not evidence that these medicines do not prevent myocardial infarction or ischemic stroke associated with acute exacerbation, but suggest that the particular risk of myocardial infarction and ischemic stroke associated with acute exacerbation may not be mitigated by use of these medicines. Our findings do not suggest that β-blockers and statins do not attenuate risk overall, because we investigated the relative risk between periods of stability (baseline) and exacerbation. A patient with COPD who has been vaccinated against influenza will have some protection from influenza, but an acute exacerbation can still occur and will still increase the risk of cardiovascular events in the short term. Similarly, although statins and aspirin will reduce the absolute risk of a cardiovascular event in an individual, they will not remove all risk, and a period of increased risk after an acute exacerbation will still occur. However, this finding might also be explained by the definition of medicine use. We defined medicine use at baseline rather than as a time-varying effect modifier as prescription of these drugs is much more likely after acute myocardial infarction or ischemic stroke. Because we included only first myocardial infarctions in the analysis, this period would be associated with an apparent rate of myocardial infarction or ischemic stroke of zero, and as such would have resulted in bias had we used a time-varying definition of cardiovascular medicines. This approach, however, may have resulted in underestimation of any effect modification by these medicines. It is worth noting that those patients in whom primary prevention with these medicines was completely effective would not have been included in the study because of the case-only nature of the design.
Clinicians should be aware that their patients with COPD will be at higher risk of myocardial infarction and ischemic stroke in the weeks after acute exacerbation, and that this risk is much higher for those hospitalized with acute exacerbation. Acute exacerbations are known to be associated with mortality in those with COPD, and our findings are another reason that clinicians should focus on preventing acute exacerbation. We speculate that those patients with higher exacerbation frequency are at lower risk of stroke/myocardial infarction after an exacerbation for two reasons: 1) they have more frequent medical care and are more likely to have relevant comorbidities diagnosed and therefore be receiving more robust risk reduction strategies; and 2) because frequent exacerbators have a heightened inflammatory response even in the stable state, their change in inflammation is less from baseline to exacerbation. This increased response to inflammatory stimuli among infrequent exacerbators may contribute to the increased risk of myocardial infarction and stroke. Such a hypothesis would apply only to relative risk increase between baseline and exacerbation periods; it will not be associated with an overall absolute risk of myocardial infarction and stroke, which is higher among frequent exacerbators.
Our findings suggest that acute exacerbation may explain some of the increased cardiovascular risk in those with COPD. However, the SUMMIT (Study to Understand Mortality and Morbidity in COPD) trial (32), which investigated the effects of vilanterol and fluticasone furoate, did not find a reduction in cardiovascular events despite a reduction in acute exacerbation. However, most of the SUMMIT population had previous coronary artery disease. It is difficult to disentangle the effects of treatment of acute exacerbation on myocardial infarction from the effects of the acute exacerbation itself.
Our study had several strengths. First, the within-individual nature of the study design minimizes confounding by factors such as sex, genetics, long-term medicine use, and socioeconomic status. Our study also used data from the CPRD, which is broadly generalizable to the UK population. In addition, compared with previous studies, we used a validated definition of acute exacerbation in electronic health records which enabled us to accurately identify acute exacerbation, and we used linked secondary care HES data to categorize them as moderate or severe. In addition, we obtained data on myocardial infarction and ischemic stroke events from both primary and linked HES data, which allowed us to identify more myocardial infarction (33) and ischemic stroke. Another strength of our study was that, compared with previous work in similar populations, our sample size was significantly larger.
Our study also has some weaknesses. To deal with time-varying confounders, we split time up into 1-year age bands and adjusted for these. In addition, we specifically adjusted for the effects of season. However, our study could still be susceptible to time-varying confounders if these correlated closely in time with acute exacerbation, such as the use of treatments for acute exacerbation. In addition, our study may have been susceptible to misclassification of acute exacerbation and myocardial infarction. We have previously demonstrated that people with COPD have delayed diagnosis of myocardial infarction (30); if these events are originally diagnosed as acute exacerbation, this may result in a spurious association between acute exacerbation and myocardial infarction. However, to reduce the impact of this bias we excluded the first day of acute exacerbation from the analysis, and used a validated algorithm for identifying acute exacerbation (14). We are aware that we may not have eliminated this bias completely, however; such a bias is unlikely to explain a substantial proportion of the effect however, as the effect of acute exacerbation in the risk of myocardial infarction lasted for several weeks. We find it unlikely that stroke would be misclassified as acute exacerbation. Although there is evidence that troponin may rise at the time of an acute exacerbation, we are also aware that people with COPD do not have as great a troponin rise at myocardial infarction as do people without COPD. Thus, the misclassification may occur in both directions. We have used validated definitions for acute exacerbation and validated myocardial infarction codes based on the work of others, and so what we have defined as “events” are likely to be true exacerbations or myocardial infarction/stroke events.
Conclusions
Compared with stable periods, we found associations between acute exacerbation and increased risks of myocardial infarction and ischemic stroke in the 4 weeks after the exacerbation. The increased risk of acute vascular events after acute exacerbation was significantly higher for severe compared with moderate acute exacerbation. The association of acute exacerbation with myocardial infarction risk was higher for infrequent exacerbators, and for those with more severe airflow limitation, and was more strongly associated with non-STEMIs. The association of acute exacerbation with ischemic stroke risk was higher for infrequent exacerbators and weaker among aspirin nonusers.
Supplementary Material
Footnotes
Supported by GlaxoSmithKline and the Medical Research Council (MRC) as part of an MRC Industry Collaboration Agreement (G0902135).
Author Contributions: Conception and design: K.J.R., J.K.Q., H.M., L.S., I.D., and N.P.; analysis and interpretation: K.J.R., O.C., J.K.Q., H.M., L.S., I.D., and N.P.; drafting the manuscript for important intellectual content: K.J.R., O.C., J.K.Q., H.M., L.S., I.D., and N.P. K.J.R. is the guarantor of the article, taking responsibility for the integrity of the work as a whole, from inception to published article.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Rothnie KJ, Yan R, Smeeth L, Quint JK. Risk of myocardial infarction (MI) and death following MI in people with chronic obstructive pulmonary disease (COPD): a systematic review and meta-analysis. BMJ Open. 2015;5:e007824. doi: 10.1136/bmjopen-2015-007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morgan AD, Sharma C, Rothnie KJ, Potts J, Smeeth L, Quint JK. Chronic obstructive pulmonary disease and the risk of stroke. Ann Am Thorac Soc. 2017;14:754–765. doi: 10.1513/AnnalsATS.201611-932SR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sin DD, Anthonisen NR, Soriano JB, Agusti AG. Mortality in COPD: role of comorbidities. Eur Respir J. 2006;28:1245–1257. doi: 10.1183/09031936.00133805. [DOI] [PubMed] [Google Scholar]
- 4.Feary JR, Rodrigues LC, Smith CJ, Hubbard RB, Gibson JE. Prevalence of major comorbidities in subjects with COPD and incidence of myocardial infarction and stroke: a comprehensive analysis using data from primary care. Thorax. 2010;65:956–962. doi: 10.1136/thx.2009.128082. [DOI] [PubMed] [Google Scholar]
- 5.Barnes PJ. Chronic obstructive pulmonary disease: effects beyond the lungs. PLoS Med. 2010;7:e1000220. doi: 10.1371/journal.pmed.1000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sethi S. Bacteria in exacerbations of chronic obstructive pulmonary disease: phenomenon or epiphenomenon? Proc Am Thorac Soc. 2004;1:109–114. doi: 10.1513/pats.2306029. [DOI] [PubMed] [Google Scholar]
- 7.Wedzicha JA. Role of viruses in exacerbations of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2004;1:115–120. doi: 10.1513/pats.2306030. [DOI] [PubMed] [Google Scholar]
- 8.Wedzicha JA, Seemungal TAR, MacCallum PK, Paul EA, Donaldson GC, Bhowmik A, et al. Acute exacerbations of chronic obstructive pulmonary disease are accompanied by elevations of plasma fibrinogen and serum IL-6 levels. Thromb Haemost. 2000;84:210–215. [PubMed] [Google Scholar]
- 9.Dev D, Wallace E, Sankaran R, Cunniffe J, Govan JR, Wathen CG, et al. Value of C-reactive protein measurements in exacerbations of chronic obstructive pulmonary disease. Respir Med. 1998;92:664–667. doi: 10.1016/s0954-6111(98)90515-7. [DOI] [PubMed] [Google Scholar]
- 10.Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351:2611–2618. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 11.Donaldson GC, Hurst JR, Smith CJ, Hubbard RB, Wedzicha JA. Increased risk of myocardial infarction and stroke following exacerbation of COPD. Chest. 2010;137:1091–1097. doi: 10.1378/chest.09-2029. [DOI] [PubMed] [Google Scholar]
- 12.Halpin DMG, Decramer M, Celli B, Kesten S, Leimer I, Tashkin DP. Risk of nonlower respiratory serious adverse events following COPD exacerbations in the 4-year UPLIFT trial. Hai. 2011;189:261–268. doi: 10.1007/s00408-011-9301-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin C-S, Shih C-C, Yeh C-C, Hu CJ, Chung CL, Chen TL, et al. Risk of stroke and post-stroke adverse events in patients with exacerbations of chronic obstructive pulmonary disease. PLoS One. 2017;12:e0169429. doi: 10.1371/journal.pone.0169429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rothnie KJ, Müllerová H, Hurst JR, Smeeth L, Davis K, Thomas SL, et al. Validation of the recording of acute exacerbations of COPD in UK primary care electronic healthcare records. PLoS One. 2016;11:e0151357. doi: 10.1371/journal.pone.0151357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrett E, Gallagher AM, Bhaskaran K, Forbes H, Mathur R, van Staa T, et al. Data resource profile: Clinical Practice Research Datalink (CPRD) Int J Epidemiol. 2015;44:827–836. doi: 10.1093/ije/dyv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitaker HJ, Farrington CP, Spiessens B, Musonda P. Tutorial in biostatistics: the self-controlled case series method. Stat Med. 2006;25:1768–1797. doi: 10.1002/sim.2302. [DOI] [PubMed] [Google Scholar]
- 17.Minassian C, Thomas SL, Smeeth L, Douglas I, Brauer R, Langan SM. Acute cardiovascular events after herpes zoster: a self-controlled case series analysis in vaccinated and unvaccinated older residents of the united states. PLoS Med. 2015;12:e1001919. doi: 10.1371/journal.pmed.1001919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas SL, Minassian C, Ganesan V, Langan SM, Smeeth L. Chickenpox and risk of stroke: a self-controlled case series analysis. Clin Infect Dis. 2014;58:61–68. doi: 10.1093/cid/cit659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quint JK, Müllerova H, DiSantostefano RL, Forbes H, Eaton S, Hurst JR, et al. Validation of chronic obstructive pulmonary disease recording in the Clinical Practice Research Datalink (CPRD-GOLD) BMJ Open. 2014;4:e005540. doi: 10.1136/bmjopen-2014-005540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rothnie KJ, Müllerová H, Thomas SL, Chandan JS, Smeeth L, Hurst JR, et al. Recording of hospitalizations for acute exacerbations of COPD in UK electronic health care records. Clin Epidemiol. 2016;8:771–782. doi: 10.2147/CLEP.S117867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tseng C-M, Chen Y-T, Ou S-M, Hsiao YH, Li SY, Wang SJ, et al. The effect of cold temperature on increased exacerbation of chronic obstructive pulmonary disease: a nationwide study. PLoS One. 2013;8:e57066. doi: 10.1371/journal.pone.0057066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhaskaran K, Hajat S, Haines A, Herrett E, Wilkinson P, Smeeth L. Short term effects of temperature on risk of myocardial infarction in England and Wales: time series regression analysis of the Myocardial Ischaemia National Audit Project (MINAP) registry. BMJ. 2010;341:c3823. doi: 10.1136/bmj.c3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kyobutungi C, Grau A, Stieglbauer G, Becher H. Absolute temperature, temperature changes and stroke risk: a case-crossover study. Eur J Epidemiol. 2005;20:693–698. doi: 10.1007/s10654-005-0703-x. [DOI] [PubMed] [Google Scholar]
- 24.Ghebremichael-Weldeselassie Y, Whitaker HJ, Farrington CP. Spline-based self-controlled case series method. Stat Med. 2017;36:3022–3038. doi: 10.1002/sim.7311. [DOI] [PubMed] [Google Scholar]
- 25.George SN, Garcha DS, Mackay AJ, Patel AR, Singh R, Sapsford RJ, et al. Human rhinovirus infection during naturally occurring COPD exacerbations. Eur Respir J. 2014;44:87–96. doi: 10.1183/09031936.00223113. [DOI] [PubMed] [Google Scholar]
- 26.Wedzicha JA, Brill SE, Allinson JP, Donaldson GC. Mechanisms and impact of the frequent exacerbator phenotype in chronic obstructive pulmonary disease. BMC Med. 2013;11:181. doi: 10.1186/1741-7015-11-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sin DD, Wu L, Man SFP. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest. 2005;127:1952–1959. doi: 10.1378/chest.127.6.1952. [DOI] [PubMed] [Google Scholar]
- 28.Truelsen T, Prescott E, Lange P, Schnohr P, Boysen G. Lung function and risk of fatal and non-fatal stroke: the Copenhagen City Heart Study. Int J Epidemiol. 2001;30:145–151. doi: 10.1093/ije/30.1.145. [DOI] [PubMed] [Google Scholar]
- 29.Saaby L, Poulsen TS, Hosbond S, Larsen TB, Pyndt Diederichsen AC, Hallas J, et al. Classification of myocardial infarction: frequency and features of type 2 myocardial infarction. Am J Med. 2013;126:789–797. doi: 10.1016/j.amjmed.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 30.Rothnie KJ, Smeeth L, Herrett E, Pearce N, Hemingway H, Wedzicha J, et al. Closing the mortality gap after a myocardial infarction in people with and without chronic obstructive pulmonary disease. Heart. 2015;101:1103–1110. doi: 10.1136/heartjnl-2014-307251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McAllister DA, Maclay JD, Mills NL, Leitch A, Reid P, Carruthers R, et al. Diagnosis of myocardial infarction following hospitalisation for exacerbation of COPD. Eur Respir J. 2012;39:1097–1103. doi: 10.1183/09031936.00124811. [DOI] [PubMed] [Google Scholar]
- 32.Vestbo J, Anderson JA, Brook RD, Calverley PM, Celli BR, Crim C, et al. SUMMIT Investigators. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet. 2016;387:1817–1826. doi: 10.1016/S0140-6736(16)30069-1. [DOI] [PubMed] [Google Scholar]
- 33.Herrett E, Shah AD, Boggon R, Denaxas S, Smeeth L, van Staa T, et al. Completeness and diagnostic validity of recording acute myocardial infarction events in primary care, hospital care, disease registry, and national mortality records: cohort study. BMJ. 2013;346:f2350. doi: 10.1136/bmj.f2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.