Abstract
Rationale: Poor sleep quality is common in the intensive care unit (ICU) and may be associated with adverse outcomes. Hence, ICU-based efforts to promote sleep are gaining attention, motivating interest in methods to measure sleep in critically ill patients. Actigraphy evaluates rest and activity by algorithmically processing gross motor activity data, usually collected by a noninvasive wristwatch-like accelerometer device. In critically ill patients, actigraphy has been used as a surrogate measure of sleep; however, its use has not been systematically reviewed.
Objectives: To conduct a systematic review of ICU-based studies that used actigraphy as a surrogate measure of sleep, including its feasibility, validity, and reliability as a measure of sleep in critically ill patients.
Methods: We searched PubMed, EMBASE, CINAHL, Proquest, and Web of Science for studies that used actigraphy to evaluate sleep in five or more patients in an ICU setting.
Results: Our search yielded 4,869 citations, with 13 studies meeting eligibility criteria. These 13 studies were conducted in 10 countries, and eight (62%) were published since 2008. Across the 13 studies, the mean total sleep time of patients in the ICU, as estimated using actigraphy, ranged from 4.4 to 7.8 hours at nighttime and from 7.1 to 12.1 hours over a 24-hour period, with 1.4 to 49.0 mean nocturnal awakenings and a sleep efficiency of 61 to 75%. When compared side-by-side with other measures of sleep (polysomnography, nurse assessments, and patient questionnaires), actigraphy consistently yielded higher total sleep time and sleep efficiency, fewer nighttime awakenings (vs. polysomnography), and more overall awakenings (vs. nurse assessment and patient questionnaires). None of the studies evaluated the association between actigraphy-based measures of sleep and outcomes of patients in the ICU.
Conclusions: In critically ill patients, actigraphy is being used more frequently as a surrogate measure of sleep; however, because actigraphy only measures gross motor activity, its ability to estimate sleep is limited by the processing algorithm used. Prior ICU-based studies involving actigraphy were heterogeneous and lacked data regarding actigraphy-based measures of sleep and patient outcomes. Larger, more rigorous and standardized studies are needed to better understand the role of actigraphy in evaluating sleep and sleep-related outcomes in critically ill patients.
Keywords: sleep deprivation, accelerometry, critical care, critical illness, intensive care units
In the intensive care unit (ICU) setting, critically ill patients commonly experience poor nighttime sleep quality, characterized by frequent arousals, fragmentation, and an increased proportion of light stages of sleep (1, 2). Additionally, critically ill patients experience a disproportionate amount of their sleep during the daytime, disrupting circadian rhythms (3). As a consequence, survivors of critical illness frequently cite poor sleep as a source of stress and anxiety in the ICU (4–8) and experience sleepiness after ICU discharge (9).
The role of sleep disturbance in the ICU has gained attention over the past decade, in large part due to interest in the relationship between sleep and delirium, a common ICU syndrome associated with prolonged length of stay and long-term impairments (10–12). Because poor sleep is considered a potentially modifiable risk factor for delirium, ICU-based efforts to promote sleep are recommended by the Society of Critical Care Medicine (11) and listed as a top-five research priority by an expert panel of ICU delirium researchers (13).
Numerous ICU-based sleep-promoting interventions have been investigated, including multifaceted strategies to minimize nocturnal noise, light, and disruptions, as well as trials of medications believed to promote sleep and/or circadian rhythm alignment (14). A key challenge underlying these ICU-based studies is the difficulty of obtaining reliable, valid, and feasible sleep measurements. Polysomnography (PSG), the gold standard for measuring sleep in healthy adults, has provided key knowledge regarding sleep architecture in critically ill patients, but is not feasible during large-scale intervention efforts due to high cost, need for frequent patient monitoring, and difficulty of obtaining recordings beyond 24 hours (15). Hence, other more feasible modes of sleep measurement, such as actigraphy, are gaining attention.
Actigraphy is a noninvasive sensor technology, usually involving a wristwatch-like device, which uses an accelerometer to estimate rest–activity cycles. More specifically, these devices continuously record multiplanar gross motor activity, translating movements into activity counts over a predefined epoch length (i.e., 15, 30, or 60 s). These activity counts are then processed by computer algorithms that use predetermined thresholds to label each epoch as “sleep” or “wake.”
In healthy adults, actigraphy has been validated against PSG and is often used to measure sleep in the outpatient setting (16). When compared with PSG and other sleep modalities, actigraphy has two major theoretical advantages. First, it is affordable and unobtrusive. Second, it collects objective data continuously over prolonged periods, allowing for precise longitudinal evaluations of rest and activity. Furthermore, with current data-management capabilities, actigraphy may prove useful for evaluating sleep-related outcomes as part of large-scale intervention studies (17).
A recent systematic review evaluated the use of actigraphy to measure physical activity in critically ill patients (18). However, for critically ill patients in the ICU setting, the use of actigraphy as a surrogate measure of sleep, particularly with regard to its feasibility, validity, and reliability, has not been systematically evaluated. Therefore, to synthesize knowledge in this area and inform future investigations, we aimed to conduct a systematic review of studies that used actigraphy to evaluate sleep in critically ill patients in the ICU setting.
Methods
This systematic review was performed and reported in accordance with established guidelines (19, 20).
Search Strategy
We designed our search strategy with the assistance of two university librarians and the use of a computerized search builder program (21). PubMed, EMBASE, CINAHL, Proquest Digital Dissertations, and Web of Science were searched from each database’s start date until December 5, 2016. To capture all actigraphy-based studies in critically ill patients and prevent erroneous exclusion of studies that evaluated sleep as a secondary outcome (i.e., “sleep” term absent in the abstract and keywords), we designed our search strategy a priori without a “sleep” search term (see online supplement) and manually reviewed full-text articles, as necessary, to determine whether sleep was evaluated. Our search strategy had no restrictions based on date, language, or study type.
Study Selection
Two screeners (K.E.S. and B.R.) independently reviewed citation titles and abstracts for the following: 1) publication of primary data in a peer-reviewed journal; 2) measurement of actigraphy in at least five patients in an ICU setting; and 3) use of actigraphy to objectively estimate sleep. All potentially relevant citations were retrieved as full-text articles, which were subsequently evaluated by the two screeners. Disagreements between reviewers were resolved via discussion and, if necessary, input from a third person (B.B.K.). For studies selected for inclusion, we manually searched each study’s reference list to identify other potentially eligible articles.
Data Abstraction and Risk of Bias Assessment
For each included article, data abstraction was independently performed by two reviewers (K.E.S., B.R., or B.B.K.); discordant entries were resolved by a third reviewer (B.B.K. or A.Q.T.). Relevant data collection included study characteristics, patient population, actigraph device characteristics, actigraph-based sleep data (i.e., total sleep time, sleep efficiency, and number of awakenings), and other measures of sleep (i.e., concurrent use of PSG). Risk of bias was assessed using the Newcastle Ottawa Scale for observational studies (22) and the Cochrane Risk of Bias tool for randomized control trials (23) (see online supplement).
Results
Study Selection
Our search strategy identified 4,869 studies, 1,258 of which were duplicates (Figure 1). We screened 3,611 titles and abstracts, yielding 1,037 articles for full-text review. Overall, 13 articles met the criteria for inclusion.
Figure 1.
Flow chart for identifying eligible studies.
Study Characteristics
The 13 studies included 10 observational studies (77%) and three randomized controlled trials (RCTs; 23%) (Table 1). Study locations included North America (n = 3, 23%), Europe (n = 3, 23%), Asia (n = 3, 23%), the Middle East (n = 2, 15%) South America (n = 1, 7%), and Australia (n = 1, 7%). Five studies (38%) were published in or before 2004, and the remaining eight (62%) were published between 2008 and 2015. Seven studies (54%) enrolled patients in medical or medical-surgical ICUs (24–30), two (15%) in cardiothoracic surgical ICUs (31, 32), two (15%) in coronary care units (33, 34), one (7%) in a surgical ICU (35), and one (7%) in a burn ICU (36).
Table 1.
Characteristics of studies that used actigraphy to measure sleep in critically ill patients
| Author Country | Study Population | Study Design | Sleep Outcomes | Key Finding |
|---|---|---|---|---|
| Beecroft and colleagues Canada 2008 (24) | Medical-surgical ICU; stable (n = 12, 67% MV, median age 68, 25% female) | Observational; actigraphy vs. nurse report vs. PSG to measure sleep | TNST, SE, NA | No correlation between actigraphy, nurse report, and PSG measures of sleep |
| Bourne and colleagues United Kingdom 2008 (25) | General ICU; tracheostomized, ICU LOS>5d (n = 24, 100% MV, mean age 64 ± 12, 54% female) | RCT; melatonin (n = 12) vs. placebo (n = 12) to improve sleep | TNST, SE | No correlation between actigraphy and BIS measures of sleep |
| Chen and colleagues Taiwan 2012 (26) | ICU; APS<15 (n = 85, intervention mean age 72 ± 18, control mean age 69 ± 15, 24% female)* | RCT; acupressure (n = 41) vs. control (n = 44) to improve sleep | TNST, TWT, WF | Valerian acupressure increases sleep duration, decreases awake time, and decreases waking frequency |
| Hamze and colleagues Brazil 2015 (27) | General ICU; GCS score of 15 and presentation of disturbed sleep patterns (n = 12, mean age 58 ± 11, 25% female)* | Observational; sleep awakenings in relation to care interventions | NA from care interventions | 4% of care interventions cause awakenings; of these, 38% occur at night |
| Kroon and colleagues Australia 2000 (33) | CCU (n = 13, age not reported, 100% female)* | Observational; actigraphy vs. nurse report vs. patient self-report of sleep | TNST, TWT, SL, NA | No significant difference in TNST, but significant difference in NA and SL when comparing actigraphy vs. nurse report vs. patient self-report |
| Mistraletti and colleagues Italy 2009 (28) | Medical-surgical ICU (n = 13, 100% MV, mean age 60 ± 16, 54% female) | Observational; motor activity and its relation to sleep, agitation, pain, anxiety | Movements per hour | Actigraphy measurements of movements per hour correlate with nurse-reported sleep |
| Ono and colleagues Japan 2011 (35) | SICU; postesophagectomy (n = 22, 0% MV, mean age 64 ± 10, 0% female) | RCT; bright (n = 10) vs. normal (n = 12) light to improve sleep | TST, circadian cycle | Compared with normal light, bright-light therapy better entrains circadian sleep–wake rhythms and decreases nighttime activity |
| Raymond and colleagues Canada 2004 (36) | Burn ICU (n = 16, 0% MV, mean age 35 ± 9, 19% female) | Observational; sleep and its relation to pain and analgesic medication requirement | TST, TNST, TWT, NA | Patients hospitalized for burns experience low sleep durations with highly fragmented sleep |
| Redeker and colleagues United States 1996 (31) | CT-SICU; post-CABG (n = 22, mean age 64 ± 10, 100% female)* | Observational; sleep in women post-CABG over time | TST, TNST, TDST, NA, MSI, MWT, NPER | After CABG, women experience substantial daytime sleep and fragmented nighttime sleep, which improve over time |
| Shilo and colleagues Israel 1999 (29) | Respiratory ICU; stable, conscious, LOS > 4 d (n = 20, mean age 60 ± 11, 55% female)* | Observational case control; sleep and urine melatonin secretion in ICU (n = 14) vs general ward (n = 6) patients | TST, number of sleep periods | Compared with patients in the ward, patients in the ICU obtain less sleep, with short and inconsistent sleep periods throughout the day and night |
| Shilo and colleagues Israel 2000 (30) | Respiratory ICU; stable respiratory-failure (n = 8, 50% MV, mean age 62 ± 14, 62% female) | Observational case control; melatonin vs placebo to improve sleep in ICU vs. general ward (n = 6) patients | TST, TNST, NA | Melatonin improves sleep duration (TNST) and quality (NA) in patients in the ICU |
| Takaesu and colleagues Japan 2015 (34) | CCU (n = 23, 0% MV, age not reported, 30% female) | Observational case control; urine melatonin secretion and sleep in ICU vs. healthy patients | TNST, TDST, SL, WASO, SE | Melatonin secretion is lower and measures of sleep are worse in patients in the CCU than in healthy control subjects |
| van der Kooi and colleagues the Netherlands 2013 (32) | CT-SICU; postoperation (n = 7, median age, 14% female)* | Observational; actigraphy vs. PSG to measure sleep | TST, SE, NA, WASO | Compared with PSG, actigraphy overestimates sleep and underestimates wake time |
Definition of abbreviations: APS = acute physiology score; BIS = bispectral index; CABG = coronary artery bypass graft; CCU = coronary care unit; CT-SICU = cardiothoracic surgery ICU; GCS = Glasgow coma score; ICU = intensive care unit; LOS = length of stay; MSI = mean nighttime sleep interval; MV = mechanically ventilated; MWT = mean wake time; NA = number of awakenings; NPER = percentage of total sleep at night; PSG = polysomnography; RCT = randomized controlled trial; SE = sleep efficiency; SICU = surgical ICU; SL = sleep latency; TDST = total daytime sleep time; TNST = total nighttime sleep time; TST = total sleep time; TWT = total wake time; WASO = wake after sleep onset; WF = waking frequency.
Number of patients on mechanical ventilation not reported.
Overall, the 13 studies enrolled 277 patients, and eight of these studies (62%) enrolled 20 or fewer patients (24, 27–30, 32, 33, 36) (Table 1). Four studies (31%) included mechanically ventilated patients (24, 25, 28, 30), three (23%) enrolled no mechanically ventilated patients (34–36), and six (46%) did not specify the mechanical ventilation status (26, 27, 29, 31–33).
Risk of Bias Assessment
Among the three RCTs, all three had adequate randomized sequence generation and selective reporting, two had adequate allocation concealment, and one had adequate blinding procedures (online supplement). Among the 10 observational studies, only two had adequate outcome assessments (online supplement).
Actigraphy Measurements and Outcomes
Of the 13 studies in this review, 11 (85%) involved actigraph placement on the wrist (24, 25, 27–34, 36) and two (15%) involved placement on the ankle (26, 35) (Table 2). Of the 11 studies involving actigraph placement on the subject’s wrist, three (27%) placed it on the nondominant wrist (30, 31, 34), one (9%) placed it on the dominant wrist (28), five (45%) placed it on the wrist with the least instrumentation or injury (24, 27, 32, 33, 36), and two (18%) did not specify the placement (25, 29). The 13 studies used eight actigraph models from eight different manufacturers, and two of these studies did not report the actigraph model or manufacturer. To estimate sleep from gross activity data, three studies reported that the sleep scoring method used algorithms validated in healthy adults (31, 33, 36), and the other 10 did not report the scoring method. Notably, no study reported the use, development, or validation of an ICU-specific algorithm to estimate sleep from activity data.
Table 2.
Sleep measures using actigraph devices*
| Study | Device Placement | Device; Epoch† Setting | Recording Time‡ | Total Sleep Time‡ | Total Nighttime Sleep‡§ | Total Wake Time‡ | Wake after Sleep Onset‡ | Total Awakenings‡ | Sleep Latency‡ | Sleep Efficiency, %‡ |
|---|---|---|---|---|---|---|---|---|---|---|
| Beecroft et al. (24) | Wrist | AW64 30 s | 8–12 h | — | 4.4 (3.3) | — | — | 49.0 (34.0) | — | 61 (41) |
| Bourne et al. (25) | Wrist | AW — | 4 n | — | — | — | — | — | — | 73 (53, 93)ǁ¶ 75 (67, 83)**¶ |
| Chen et al. (26) | Ankle | GT1M — | 59 h | — | 7.3 (1.3)†† 7.8 (0.3)‡‡ 7.3 (1.2)§§ 7.1 (1.4)ǁǁ | 0.8 (1.4)†† 0.2 (0.3)‡‡ 0.8 (1.2)§§ 0.9 (1.4)ǁǁ | — | 4.6 (6.2)†† 2.3 (2.8)‡‡ 4.3 (4.4)§§ 6.3 (8.2)ǁǁ | — | — |
| Hamze et al. (27) | Wrist | AS — | 24 h | — | — | — | — | — | — | — |
| Kroon et al. (33) | Wrist | — 60 s | 7 h | — | 5.1 (1.3) | 1.9 (1.3) | — | 14 (8) | 0.4 (1.0) | 74 (19) |
| Mistraletti et al. (28) | Wrist | BT-P 15–20 s | 2–6 d | — | — | — | — | — | — | — |
| Ono et al. (35) | Ankle | AC-210 120 s | 6 d | 7.3 (0.9)ǁ 7.1 (1.4)** | — | — | — | — | — | — |
| Raymond et al. (36) | Wrist | MML 60 s | 12.6 d | 8.3 (2.8) | 5.5 (1.8) | 3.4 (1.7) | — | 26 (9.5) | — | — |
| Redeker et al. (31)¶¶ | Wrist | MML 60 s | 24 h | 12.1 (4.6) | 5.3 (1.9) | 0.3 (0.3) | — | 16.6 (26.4) | — | — |
| Shilo et al. (29) | Wrist | SM — | 72 h | — | — | — | — | — | — | — |
| Shilo et al. (30) | Wrist | SM 20 s | 3 d | — | 6.3 (1.1)ǁ | — | — | 1.4 (3.7)ǁ | — | — |
| Takaesu et al. (34) | Wrist | — — | 24 h | — | 5.6 (1.2)ǁ | — | 1.0 (0.7)ǁ | — | 0.9 (1.2)ǁ | 70 (14)ǁ |
| Van der Kooi et al. (32) | Wrist | AW 30 s | 16 h | — | — | — | — | — | — | — |
Definition of abbreviations: AC-210 = active tracer; AS = ActiSleep actigraph; AW = Actiwatch; AW64 = Actiwatch Model 64; BT-P = BioTrainer-Pro; d = days; GT1M = ActiGraph GT1M; h = hours; ICU = intensive care unit; IQR = interquartile range; MML = MicroMini Motionlogger actigraph; n = nights; s = seconds; SM = Somnitor.
All sleep measures are presented as mean (SD) hours, except for those reported by Beecroft and colleagues (24), who used the median (IQR). All time values are presented in hours, unless otherwise noted, and, as needed, were rounded and/or converted to hours from other time units. Dashes represent unavailable data. Three studies (31, 33, 36) reported the use of a sleep scoring method that used an algorithm validated in healthy adults, and the other 10 did not report the sleep scoring algorithm used.
Epochs are the preset frequency (i.e., every 15 or 30 s) for activity data collection.
“Recording time” is the total time devices recorded data in the ICU per patient. In studies where this was not consistent and ranges were not reported (27, 36), this value was obtained by dividing the total recording time by the number of included patients. “Total sleep time” is the time spent sleeping during both daytime and nighttime, not including the time spent awake during the sleep period. “Total nighttime sleep” is the time spent sleeping during the night period (see footnote §). “Total wake time” is the time spent awake during the nighttime sleeping periods. “Wake after sleep onset” is the time spent awake from sleep onset to final awakening. “Sleep fragmentation index” is the percentage of disruption of sleep during sleep periods. “Total awakenings” is the number of awakenings recorded during nighttime sleep periods. “Sleep latency” is the time spent between the attempt to sleep and the start of sleep. “Sleep efficiency” is the total sleep time divided by the total time spent in the bed (expressed as a percentage).
Defined as 22:00–07:00 (25), 22:00–06:00 (26), 23:00–06:00 (33), 23:00–07:59 (36), 23:00–06:59 (31), 21:00–06:00 (34), 18:00–08:00 (35).
Intervention group.
95% confidence interval.
Control group.
Intervention group on Day 1.
Intervention group on Day 2 with valerian acupressure performed.
Control group on Day 1.
Control group on Day 2.
Data presented for postoperative Day 2 only. The study involved 4 weeks of actigraphy recording, but many patients were not critically ill or in the ICU after postoperative Day 2; hence, only actigraphy data for this single day are presented.
Across these 13 studies, the median (interquartile range [IQR]) duration of actigraphy measurement was 36 (24–72) hours per patient. Nine (69%) captured sleep over a complete 24-hour period, with three (23%) recording critically ill patients for exactly 24 hours (27, 31, 34), and six (46%) recording for more than 24 consecutive hours (26, 28–30, 35, 36). The other four studies primarily evaluated nighttime sleep, with three (23%) measuring for ≤16 hours (24, 32, 33), and one (8%) recording over 4 nights (25). The definition of nighttime sleep also varied among the studies, ranging from 18:00–08:00 (35) to 21:00–06:00 (34), 22:00–06:00 (26), 22:00–07:00 (25), 23:00–06:00 (33), 23:00–06:59 (31), and 23:00–07:59 (36).
Using actigraphy, the minimum mean recorded total nighttime sleep over an 8- to 14-hour period was 4.4 hours and the maximum was 7.8 hours. Over a 24-hour period, the minimum mean sleep time was 7.1 hours and maximum was 12.1 hours. The mean number of nocturnal awakenings ranged from 1.4 to 49.0 per night, and minimum and maximum mean sleep efficiency (total sleep time divided by total time spent in the bed) was 61% and 75%, respectively (Table 2). Mean sleep latency (time to fall asleep) was 39 minutes, and the total time awake during the nighttime sleep period (as defined by each individual sleep study, i.e., 22:00 to 06:00) ranged from 12 minutes to 3.4 hours.
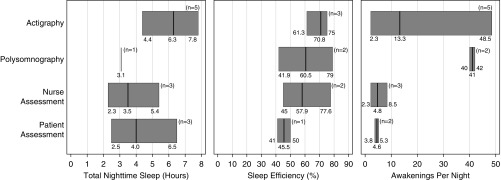
Seven studies (54%) compared actigraphy with one or more other measures of sleep, including PSG (24, 32), nurse report (24–26, 28, 33), patient self-report (25, 26, 33, 36), and the bispectral index (BIS) (25). In each study, actigraphy consistently yielded higher sleep time and efficiency totals than other measures (Figure 2). When nocturnal awakenings were assessed, actigraphy demonstrated fewer mean nighttime awakenings than PSG, but more mean awakenings than nurse- or patient-reported sleep.
Figure 2.
Modified box plots of sleep indices from the seven of 13 included studies (54%) that compared actigraphy against at least one other measure of sleep (polysomnography, nurse assessment, and/or patient assessment) in critically ill patients in the ICU. Dark vertical lines represent medians of actual reported values, and box borders represent minimums and maximums. ICU = intensive care unit.
Four studies (31%) used actigraphy to detect differences in sleep as a part of ICU-based interventions involving acupressure before bedtime (26), nocturnal melatonin use (25, 30), or early-morning bright-light therapy (35). Two of these four studies demonstrated statistically significant improvements: one in night activity and circadian rhythms with early-morning bright-light therapy (35), and the other in sleep quantity with nighttime acupressure (26).
Three studies correlated actigraphic movements with other relevant nonsleep outcomes such as delirium, pain, and agitation (28, 35, 36). Raymond and colleagues concluded that poor sleep, as assessed using actigraphy, was associated with worse pain sensitivity and increased analgesic requirements (36). Mistraletti and colleagues found that actigraphic movements correlated with agitation, sedation, pain, and anxiety (28), and Ono and colleagues demonstrated a trend toward lower postoperative delirium in patients exhibiting decreased actigraph-measured nighttime activity (35). No studies evaluated the association of actigraphic measures of sleep with patient outcomes (i.e., length of stay, mortality, and post-ICU cognitive or physical function).
Discussion
In this systematic review of 13 studies involving actigraphy in critically ill patients, we found that sleep in the ICU, as evaluated using actigraphy, is generally fragmented and decreased in quantity as compared with guideline-based recommendations for sleep (37). This finding is particularly striking given that actigraphy yielded higher sleep quantity and efficiency totals in critically ill patients than other modes of sleep measurement. Nevertheless, given the relative lack of prior research involving actigraphy in critically ill patients, we found that the included studies exhibited marked heterogeneity in the populations and sample sizes enrolled, interventions performed, and sleep-related outcomes measured. Additionally, all studies used actigraphy for descriptive purposes without evaluating for possible associations between actigraphy-based measures of sleep and clinically important patient outcomes. Hence, we were unable to make broad conclusions regarding actigraphy and sleep in critically ill patients in the ICU, and were unable to pool data for a meta-analysis.
The growing interest in sleep in the ICU has highlighted a clear need for a widely available, feasible, and reliable tool to measure sleep in critically ill patients (1, 2, 14, 38). Indeed, increased recognition of the significant morbidity associated with post–intensive care syndrome has heightened interest in minimizing its modifiable risk factors in the ICU (12). Given that poor sleep represents a major risk factor for delirium, and delirium has established associations with the devastating cognitive, physical, and mental health impairments that comprise post–intensive care syndrome, sleep optimization has become a clear priority for both delirium researchers and the Society of Critical Care Medicine (39). Surprisingly, only one study in our review evaluated actigraphy-based measures of sleep and delirium (35), highlighting a key area for future research.
Despite the growing interest in sleep in critically ill patients, research in this area continues to be hindered by the lack of a widely accepted method to measure sleep in the ICU setting. PSG, the well-established gold standard for evaluating sleep and sleep-based interventions, involves simultaneous electroencephalogram (EEG), electromyogram, and electrooculogram recordings, and therefore is cumbersome, labor intensive, and costly to implement. Additionally, PSG interpretation requires a dedicated sleep expert, and in critically ill patients is complicated by EEG derangements caused by common ICU medications and illnesses such as sepsis and renal failure (1, 40). Finally, PSG has been shown to be intolerable for most patients in the ICU beyond 24 hours (15). Hence, in the ICU setting, PSG is considered infeasible for large-scale intervention studies (15).
As a potentially less cumbersome alternative to PSG, BIS involves a single integrated EEG sensor, but few data are available to support its utility for evaluating sleep in the ICU (38). More recently, subjective questionnaires, such as the Richards-Campbell Sleep Questionnaire (RCSQ), have gained popularity for large-scale ICU sleep measurements (41–43). Although it is a validated, inexpensive, feasible, and scalable tool for evaluating sleep, the RCSQ is vulnerable to subject and recall bias, rater fatigue across repeated daily assessments, and lack of completion in the setting of abnormal patient cognition (42). Furthermore, the RCSQ has poor interrater reliability and agreement when it is completed by proxies instead of patients (44). Actigraphy addresses many of the shortcomings of these other modes of evaluating sleep, as it is less expensive and less cumbersome than PSG and BIS, and, in contrast to subjective questionnaires, provides objective and continuous surrogate measurements of sleep.
In this systematic review, we observed that studies that used actigraphy to measure sleep reported wide ranges in total sleep time (7.1–10.3 h), nocturnal sleep time (4.4–7.8 h), sleep efficiency (61–75%), and, most dramatically, nocturnal awakenings (1.4–49.0/h). This variance existed even when we compared studies that used the same actigraph model, manufacturer, and epoch setting (31, 36), and when we excluded the two studies that placed the actigraph on the ankle (as opposed to the wrist). This wide range may be explained, in part, by the heterogeneous study populations, different definitions of the “nighttime” sleep period, varying data-processing modes, and the relatively small size of the studies, which enrolled 277 patients in total, or approximately 21 patients per study. These results could be strengthened by larger studies involving longer actigraphy recording times.
As compared with other measures of sleep, we observed that actigraphy tended to estimate higher sleep durations and sleep efficiency in critically ill patients. This observation is likely due to reliance on traditional actigraphy software programs, which score rest and activity (surrogate measures of “sleep” and “wake,” respectively) using algorithms that were validated in healthy, noncritically ill ambulatory adults. In critically ill patients in the ICU who are debilitated, sedated, and/or mechanically ventilated, these traditional actigraphy interpretation algorithms (i.e., those that have been validated to estimate sleep in healthy adults) can therefore miscategorize patients as asleep who are actually awake but exhibiting limited movement (45). To date, actigraphy has not undergone rigorous validation against other measures in the ICU setting, nor have algorithms been developed to account for unique movement characteristics in this patient population. For this reason, actigraphy has generally been considered to be unreliable for sleep measurement in the ICU (46), including by the authors of three studies in this review (24, 25, 32). However, as interest in ICU patient activity and mobility grows, “big data” methods (i.e., machine learning) may be used to develop and test novel actigraphy interpretation algorithms (47), thus expanding the role of actigraphy in evaluating sleep and other important patient outcomes.
Another promising role for actigraphy in sleep ICU studies is suggested by the results of the RCTs included in this review (25, 26, 35). Indeed, given that actigraphy was able to measure the effects of sleep-promoting interventions in the ICU, its greatest utility may be in large-scale interventional studies, where obtaining numerical sleep data is less important than identifying between-group differences and trends.
Finally, this review highlights a growing interest in actigraphy. Fifty percent of the observational studies and all of the RCTs involving actigraphy were published in the last 10 years. Additionally, the significant geographic range of prior studies suggests the widespread appeal and feasibility of actigraphy. Indeed, although current actigraphy interpretation algorithms tend to overestimate sleep duration and efficiency, the ease of use and low cost of actigraphy make it a viable option for any study aiming to evaluate before-and-after trends resulting from ICU-based sleep interventions. Moreover, given the growing interest in ICU outcomes, delirium prevention, and sleep promotion, investigations involving actigraphy in critically ill patients will likely accelerate in the upcoming years.
Strengths and Limitations
Key strengths of this systematic review include a comprehensive search strategy and the use of broad screening criteria to capture all possible studies. Additionally, to our knowledge, this is the first systematic review of actigraphy as a surrogate measure of sleep in the ICU. Key limitations include the fact that by focusing specifically on sleep, our review may have overlooked studies involving sleep-related measures such as circadian rhythm alignment. Additionally, given the varied nomenclature for ICUs, it is possible that some studies that evaluated sleep in specialty ICUs were not captured by our search string, despite our comprehensive search strategy. Nevertheless, our review represents an up-to-date and extensive review of the use of actigraphy as a surrogate measure of sleep in critically ill patients in the ICU.
Conclusions
Actigraphy is an increasingly popular surrogate measure for sleep in critically ill patients. Although actigraphy-based studies reinforce the understanding that critically ill patients, in general, experience poor sleep in the ICU setting, they also report wide ranges of sleep quality and quantity, tend to estimate higher sleep durations than other measurement modalities, and are limited by a lack of ICU-specific actigraphy data-processing algorithms. With rising interest in efforts to promote sleep in critically ill patients, further investigation is needed to better understand the role of actigraphy in evaluating sleep and sleep-related outcomes in critically ill patients in the ICU.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Bethany Myers and Carrie Price for their assistance with the literature search, as well as Sne Kanji for her help in obtaining article text files.
Footnotes
B.B.K. was supported by a grant through the UCLA Clinical Translational Research Institute and the National Center for Advancing Translational Sciences, National Institutes of Health (UL1TR000124).
Author Contributions: Conception and design: K.E.S., B.R., D.M.N., J.L.M., and B.B.K. Analysis and interpretation: K.E.S., B.R., D.M.N., A.Q.T., J.L.M, and B.B.K. Drafting the manuscript for important intellectual content: K.E.S. and B.B.K. Final approval of the version to be published: K.E.S., B.R., D.M.N., A.Q.T., J.L.M., and B.B.K.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Kamdar BB, Needham DM, Collop NA. Sleep deprivation in critical illness: its role in physical and psychological recovery. J Intensive Care Med. 2012;27:97–111. doi: 10.1177/0885066610394322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knauert MP, Malik V, Kamdar BB. Sleep and sleep disordered breathing in hospitalized patients. Semin Respir Crit Care Med. 2014;35:582–592. doi: 10.1055/s-0034-1390080. [DOI] [PubMed] [Google Scholar]
- 3.Oldham MA, Lee HB, Desan PH. Circadian rhythm disruption in the critically ill: an opportunity for improving outcomes. Crit Care Med. 2016;44:207–217. doi: 10.1097/CCM.0000000000001282. [DOI] [PubMed] [Google Scholar]
- 4.Huang HW, Zheng BL, Jiang L, Lin ZT, Zhang GB, Shen L, et al. Effect of oral melatonin and wearing earplugs and eye masks on nocturnal sleep in healthy subjects in a simulated intensive care unit environment: which might be a more promising strategy for ICU sleep deprivation? Crit Care. 2015;19:124. doi: 10.1186/s13054-015-0842-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freedman NS, Kotzer N, Schwab RJ. Patient perception of sleep quality and etiology of sleep disruption in the intensive care unit. Am J Respir Crit Care Med. 1999;159:1155–1162. doi: 10.1164/ajrccm.159.4.9806141. [DOI] [PubMed] [Google Scholar]
- 6.Novaes MA, Knobel E, Bork AM, Pavão OF, Nogueira-Martins LA, Ferraz MB. Stressors in ICU: perception of the patient, relatives and health care team. Intensive Care Med. 1999;25:1421–1426. doi: 10.1007/s001340051091. [DOI] [PubMed] [Google Scholar]
- 7.Rotondi AJ, Chelluri L, Sirio C, Mendelsohn A, Schulz R, Belle S, et al. Patients’ recollections of stressful experiences while receiving prolonged mechanical ventilation in an intensive care unit. Crit Care Med. 2002;30:746–752. doi: 10.1097/00003246-200204000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Simini B. Patients’ perceptions of intensive care. Lancet. 1999;354:571–572. doi: 10.1016/S0140-6736(99)02728-2. [DOI] [PubMed] [Google Scholar]
- 9.Dhooria S, Sehgal IS, Agrawal AK, Agarwal R, Aggarwal AN, Behera D. Sleep after critical illness: Study of survivors of acute respiratory distress syndrome and systematic review of literature. Indian J Crit Care Med. 2016;20:323–331. doi: 10.4103/0972-5229.183908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Figueroa-Ramos MI, Arroyo-Novoa CM, Lee KA, Padilla G, Puntillo KA. Sleep and delirium in ICU patients: a review of mechanisms and manifestations. Intensive Care Med. 2009;35:781–795. doi: 10.1007/s00134-009-1397-4. [DOI] [PubMed] [Google Scholar]
- 11.Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, Dasta JF, et al. American College of Critical Care Medicine. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41:263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 12.Needham DM, Davidson J, Cohen H, Hopkins RO, Weinert C, Wunsch H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med. 2012;40:502–509. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
- 13.Pandharipande PP, Ely EW, Arora RC, Balas MC, Boustani MA, La Calle GH, et al. The intensive care delirium research agenda: a multinational, interprofessional perspective. Intensive Care Med. 2017;43:1329–1339. doi: 10.1007/s00134-017-4860-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamdar BB, Martin JL, Needham DM, Ong MK. Promoting sleep to improve delirium in the ICU. Crit Care Med. 2016;44:2290–2291. doi: 10.1097/CCM.0000000000001982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knauert MP, Yaggi HK, Redeker NS, Murphy TE, Araujo KL, Pisani MA. Feasibility study of unattended polysomnography in medical intensive care unit patients. Heart Lung. 2014;43:445–452. doi: 10.1016/j.hrtlng.2014.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van de Water ATM, Holmes A, Hurley DA. Objective measurements of sleep for non-laboratory settings as alternatives to polysomnography--a systematic review. J Sleep Res. 2011;20:183–200. doi: 10.1111/j.1365-2869.2009.00814.x. [DOI] [PubMed] [Google Scholar]
- 17.Morgenthaler T, Alessi C, Friedman L, Owens J, Kapur V, Boehlecke B, et al. Standards of Practice Committee; American Academy of Sleep Medicine. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep. 2007;30:519–529. doi: 10.1093/sleep/30.4.519. [DOI] [PubMed] [Google Scholar]
- 18.Verceles AC, Hager ER. Use of accelerometry to monitor physical activity in critically ill subjects: a systematic review. Respir Care. 2015;60:1330–1336. doi: 10.4187/respcare.03677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamdar BB, Shah PA, Sakamuri S, Kamdar BS, Oh J. A novel search builder to expedite search strategies for systematic reviews. Int J Technol Assess Health Care. 2015;31:51–53. doi: 10.1017/S0266462315000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Ottawa Hosp Res Inst. 2013;3:1–4. [Google Scholar]
- 23.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beecroft JM, Ward M, Younes M, Crombach S, Smith O, Hanly PJ. Sleep monitoring in the intensive care unit: comparison of nurse assessment, actigraphy and polysomnography. Intensive Care Med. 2008;34:2076–2083. doi: 10.1007/s00134-008-1180-y. [DOI] [PubMed] [Google Scholar]
- 25.Bourne RS, Mills GH, Minelli C. Melatonin therapy to improve nocturnal sleep in critically ill patients: encouraging results from a small randomised controlled trial. Crit Care. 2008;12:R52. doi: 10.1186/cc6871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen JH, Chao YH, Lu SF, Shiung TF, Chao YF. The effectiveness of valerian acupressure on the sleep of ICU patients: a randomized clinical trial. Int J Nurs Stud. 2012;49:913–920. doi: 10.1016/j.ijnurstu.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 27.Hamze FL, de Souza CC, Chianca TC. The influence of care interventions on the continuity of sleep of intensive care unit patients. Rev Lat Am Enfermagem. 2015;23:789–796. doi: 10.1590/0104-1169.0514.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mistraletti G, Taverna M, Sabbatini G, Carloni E, Bolgiaghi L, Pirrone M, et al. Actigraphic monitoring in critically ill patients: preliminary results toward an “observation-guided sedation.”. J Crit Care. 2009;24:563–567. doi: 10.1016/j.jcrc.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Shilo L, Dagan Y, Smorjik Y, Weinberg U, Dolev S, Komptel B, et al. Patients in the intensive care unit suffer from severe lack of sleep associated with loss of normal melatonin secretion pattern. Am J Med Sci. 1999;317:278–281. doi: 10.1097/00000441-199905000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Shilo L, Dagan Y, Smorjik Y, Weinberg U, Dolev S, Komptel B, et al. Effect of melatonin on sleep quality of COPD intensive care patients: a pilot study. Chronobiol Int. 2000;17:71–76. doi: 10.1081/cbi-100101033. [DOI] [PubMed] [Google Scholar]
- 31.Redeker NS, Mason DJ, Wykpisz E, Glica B. Sleep patterns in women after coronary artery bypass surgery. Appl Nurs Res. 1996;9:115–122. doi: 10.1016/s0897-1897(96)80206-0. [DOI] [PubMed] [Google Scholar]
- 32.van der Kooi AW, Tulen JH, van Eijk MM, de Weerd AW, van Uitert MJ, van Munster BC, et al. Sleep monitoring by actigraphy in short-stay ICU patients. Crit Care Nurs Q. 2013;36:169–173. doi: 10.1097/CNQ.0b013e318283cff3. [DOI] [PubMed] [Google Scholar]
- 33.Kroon K, West S. ‘Appears to have slept well’: assessing sleep in an acute care setting. Contemp Nurse. 2000;9:284–294. doi: 10.5172/conu.2000.9.3-4.284. [DOI] [PubMed] [Google Scholar]
- 34.Takaesu Y, Futenma K, Kobayashi M, Komada Y, Tanaka N, Yamashina A, et al. A preliminary study on the relationships between diurnal melatonin secretion profile and sleep variables in patients emergently admitted to the coronary care unit. Chronobiol Int. 2015;32:875–879. doi: 10.3109/07420528.2015.1048869. [DOI] [PubMed] [Google Scholar]
- 35.Ono H, Taguchi T, Kido Y, Fujino Y, Doki Y. The usefulness of bright light therapy for patients after oesophagectomy. Intensive Crit Care Nurs. 2011;27:158–166. doi: 10.1016/j.iccn.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Raymond I, Ancoli-Israel S, Choiniere M. Sleep disturbances, pain and analgesia in adults hospitalized for burn injuries. Sleep Med. 2004;5:551–559. doi: 10.1016/j.sleep.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 37.Ohayon M, Wickwire EM, Hirshkowitz M, Albert SM, Avidan A, Daly FJ, et al. National Sleep Foundation’s sleep quality recommendations: first report. Sleep Health. 2017;3:6–19. doi: 10.1016/j.sleh.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 38.Bourne RS, Minelli C, Mills GH, Kandler R. Clinical review: sleep measurement in critical care patients: research and clinical implications. Crit Care. 2007;11:226. doi: 10.1186/cc5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barr J, Pandharipande PP. The pain, agitation, and delirium care bundle: synergistic benefits of implementing the 2013 Pain, Agitation, and Delirium Guidelines in an integrated and interdisciplinary fashion. Crit Care Med. 2013;41(9) Suppl 1:S99–S115. doi: 10.1097/CCM.0b013e3182a16ff0. [DOI] [PubMed] [Google Scholar]
- 40.Watson PL, Pandharipande P, Gehlbach BK, Thompson JL, Shintani AK, Dittus BS, et al. Atypical sleep in ventilated patients: empirical electroencephalography findings and the path toward revised ICU sleep scoring criteria. Crit Care Med. 2013;41:1958–1967. doi: 10.1097/CCM.0b013e31828a3f75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kamdar BB, King LM, Collop NA, Sakamuri S, Colantuoni E, Neufeld KJ, et al. The effect of a quality improvement intervention on perceived sleep quality and cognition in a medical ICU. Crit Care Med. 2013;41:800–809. doi: 10.1097/CCM.0b013e3182746442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richards KC, O’Sullivan PS, Phillips RL. Measurement of sleep in critically ill patients. J Nurs Meas. 2000;8:131–144. [PubMed] [Google Scholar]
- 43.Patel J, Baldwin J, Bunting P, Laha S. The effect of a multicomponent multidisciplinary bundle of interventions on sleep and delirium in medical and surgical intensive care patients. Anaesthesia. 2014;69:540–549. doi: 10.1111/anae.12638. [DOI] [PubMed] [Google Scholar]
- 44.Kamdar BB, Shah PA, King LM, Kho ME, Zhou X, Colantuoni E, et al. Patient-nurse interrater reliability and agreement of the Richards-Campbell sleep questionnaire. Am J Crit Care. 2012;21:261–269. doi: 10.4037/ajcc2012111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin JL, Hakim AD. Wrist actigraphy. Chest. 2011;139:1514–1527. doi: 10.1378/chest.10-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma AJ, Rawat N, Reiter A, Shrock C, Zhan A, Stone A, et al. Measuring patient mobility in the ICU using a novel noninvasive sensor. Crit Care Med. 2017;45:630–636. doi: 10.1097/CCM.0000000000002265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Renevey P, Sola J, Theurillat P, Bertschi M, Krauss J, Andries D, et al. Validation of a wrist monitor for accurate estimation of RR intervals during sleep. Conf Proc IEEE Eng Med Biol Soc. 2013;2013:5493–5496. doi: 10.1109/EMBC.2013.6610793. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.