Abstract
Multikinase inhibitors (MKIs), including the tyrosine kinase inhibitors (TKIs), have rapidly become an established factor in daily (hemato)-oncology practice. Although the oral route of administration offers improved flexibility and convenience for the patient, challenges arise in the use of MKIs. As MKIs are prescribed extensively, patients are at increased risk for (severe) drug–drug interactions (DDIs). As a result of these DDIs, plasma pharmacokinetics of MKIs may vary significantly, thereby leading to high interpatient variability and subsequent risk for increased toxicity or a diminished therapeutic outcome. Most clinically relevant DDIs with MKIs concern altered absorption and metabolism. The absorption of MKIs may be decreased by concomitant use of gastric acid-suppressive agents (e.g. proton pump inhibitors) as many kinase inhibitors show pH-dependent solubility. In addition, DDIs concerning drug (uptake and efflux) transporters may be of significant clinical relevance during MKI therapy. Furthermore, since many MKIs are substrates for cytochrome P450 isoenzymes (CYPs), induction or inhibition with strong CYP inhibitors or inducers may lead to significant alterations in MKI exposure. In conclusion, DDIs are of major concern during MKI therapy and need to be monitored closely in clinical practice. Based on the current knowledge and available literature, practical recommendations for management of these DDIs in clinical practice are presented in this review.
Keywords: cytochrome P450 enzyme, drug–drug interaction, drug transporters, gastric acid suppression, metabolism, multikinase inhibitor
Introduction
Although cancer is still the leading cause of death among men and women worldwide, novel treatment options are rapidly evolving. In order to improve treatment efficacy and minimize toxicity more specific targets have been identified. One of the most promising classes of targeted anticancer agents are the multikinase inhibitors (MKIs), including the tyrosine kinase inhibitors (TKIs). MKIs target specific tyrosine kinases within the tumor cell, where they play a key role in signal transduction, gene transcription, and DNA synthesis.1 MKIs like osimertinib (for lung cancer) and cabozantinib (for kidney cancer) rapidly gained a place in standard of care treatment for multiple or new indications [e.g. regorafenib in primary liver cancer, after earlier approvals for gastrointestinal stromal tumor (GIST) and colorectal cancer].
MKIs include both small molecule MKIs and large molecule MKIs. In this review we will solely focus on the small molecule MKIs. Small molecule MKIs are administered orally, which gives them a clear advantage over conventional chemotherapy in terms of flexibility and patient convenience. Many MKIs show a narrow therapeutic window, whereas intra- and interpatient exposure is highly variable and multifactorial.2–4 Also factors like food, beverages, lifestyle, and pharmacogenetic polymorphisms may alter MKI bioavailability significantly.5 For example, as MKIs are predominately metabolized through phase I (e.g. CYP enzymes) or phase II enzymes (e.g. UPD-glucuronyltransferases) or almost exclusively by phase II enzymes (e.g. in the case of afatinib), this makes them highly prone for drug–drug interactions (DDIs) involving drug metabolism.6 Moreover, since cancer patients often use multiple drugs concomitantly with their anticancer therapy, they are even more at risk for DDIs, compared with other patient groups.7
DDIs can be classified as pharmacodynamic or pharmacokinetic.8 Pharmacokinetic DDIs are defined as drug interactions regarding drug absorption, metabolism, distribution and elimination leading to altered plasma concentrations of a drug and possible unfavorable outcomes (e.g. increased toxicity and reduced treatment efficacy). A pharmacodynamic interaction is the altered response in terms of toxicity and efficacy when two or more drugs affect similar molecular targets (e.g. membrane receptors). Pharmacodynamic DDIs can be additive, antagonistic or synergistic. For instance, epidermal growth factor receptor (EGFR) kinase inhibitors often show synergistic antitumor effects when combined with chemotherapy.9
Both the United States Food and Drug Administration (US FDA) and the European Medicines Agency (EMA) present guidelines for the interpretation of DDIs. However, because of discrepancies between recommendations, currently no clear general consensus for the management of DDIs is available. Therefore, the management of DDIs is challenging for clinicians and the need for a general consensus is urgent.
This review article presents an overview of known pharmacokinetic DDIs regarding orally taken MKIs currently approved by the US FDA and EMA. Moreover, if possible, practical recommendations are given for the management of DDIs during MKI therapy in clinical practice.
Methods
We conducted a search in PubMed and the Embase databases for English language studies published until 2 July 2018 for randomized clinical trials, observational studies, and reviews about US FDA and EMA-approved MKIs. We used the following search MESH terms: ‘(Drug interactions) OR (Drug combination) AND (Drug name)’. In Embase, we used ‘clinical studies’, ‘humans’ and ‘only in English’ as additional search limits. The search results were manually screened for relevance. In addition, all MKI (US FDA and EMA) assessment reports were screened on the latest updates regarding DDIs in the scientific updates available at the EMA and US FDA website until 2 July 2018. We included clinical drug–drug interaction studies in human and preclinical pharmacokinetic studies investigating possible interactions. We excluded studies which did not focus on pharmacokinetics or drug interactions. Clinical relevance of the interaction was scored on the basis of the US FDA-classification of the effect of drug interactions and the level of available evidence as a ‘major’, ‘moderate’ or ‘minor’ interaction. If there was no clinical pharmacokinetic study performed, the interaction potential was estimated on the basis of the inhibitory concentration or pKa and the advice in the assessment reports.10
Absorption
Intragastric pH
The absorption of MKIs can be significantly affected by altered intragastric pH. When intragastric pH is elevated (e.g. due to proton pump inhibitors; PPIs), the MKI solubility, bioavailability, and eventually treatment efficacy may be significantly influenced (Figure 1).8,11–13 The impact of this ‘pH effect’ is highly variable per MKI and the clinical relevance of the DDI between MKIs and acid-suppressive agents (e.g. PPIs, H2-antagonists and antacids) must be assessed on an individual basis. A complete overview can be found in Table 1.14–35
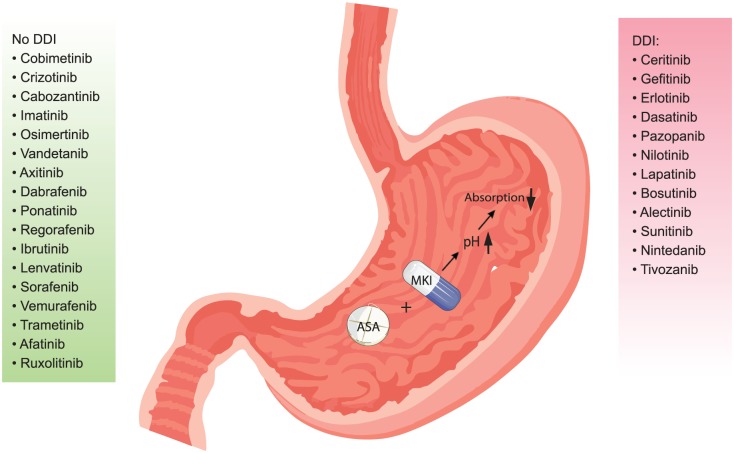
Figure 1.
Working mechanism of the drug–drug interaction with an ASA: MKIs are arranged according to the clinical relevance and magnitude of the interaction in a descending order, with the most relevant interactions on top of the list. A PPI increases stomach pH after intake and thereby decreases absorption of MKIs and therefore bioavailability of MKIs.
ASA, acid-suppressive agent; DDI, drug–drug interaction; MKI, multikinase.
Table 1.
DDIs regarding gastric acid suppression.
| MKI (year of marketing approval) | Acid-suppressive compound | Decrease in Cmax | Decrease in AUC | Clinical relevance | Recommendations | References |
|---|---|---|---|---|---|---|
| Afatinib (2013) | Not reported yet [a clinical trial is currently ongoing (NTR: 6652)] |
NA | NA | Minor | Based on pKa a nonclinically relevant interaction is expected. | EMA;14 US FDA15 |
| Alectinib (2017) | Esomeprazole at least one hour before a regular breakfast for 5 days. Alectinib was administered 30 min after breakfast | 16% | 22% | Minor | Although the effects are minimal preferably avoid the use of acid-suppressive agents. Otherwise apply separate administration times or consider short-acting antacids. | EMA;14 US FDA;15 Morcos and colleagues16 |
| Axitinib (2012) | Rabeprazole 20 mg for 5 consecutive days 3 h prior to axitinib intake | 42% | 5% | Minor | No interventions needed. Concomitant acid suppression can be used safely. | EMA;14 US FDA;15 Rugo and colleagues17 |
| Bosutinib (2013) | Lansoprazole 60 mg/day for 2 consecutive days | 46% | 26% | Minor | Avoid the use of acid-suppressive agents. Otherwise apply separate administration times or consider short-acting antacids. | EMA;14 US FDA;15 Abbas and colleagues18 |
| Cabozantinib (2016) | Esomeprazole 40 mg delayed release capsule for 6 days 1 h before cabozantinib intake | 10% | 9% | Minor | No interventions needed. Concomitant acid suppression can be used safely. | EMA;14 US FDA;15 Nguyen and colleagues19 |
| Ceritinib (2015) | Esomeprazole 40 mg for 6 consecutive days 1 h before ceritinib intake | 79% (healthy subjects) 25% (patients) |
76% (healthy subjects) 30% (patients) |
Moderate | Avoid the use of acid-suppressive agents. Otherwise separate administration times. Antacids might be used 4 h before or 2 h after ceritinib intake or H2-antagonists can be used 10 h before or 2 h after ceritinib intake. | EMA;14 US FDA;15 Lau and colleagues20 |
| Cobimetinib (2015) | Rabeprazole 20 mg for 5 days prior to cobimetinib administration in a fasted and nonfasted state. In the fasted state concomitantly with cobimetinib and 1 h before cobimetinib in the nonfasted state | 14% in the nonfasted state | <11% | Minor | No interventions needed. Concomitant acid suppression can be used safely. | EMA;14 US FDA;15 Musib and colleagues21 |
| Crizotinib (2012) | Esomeprazole 40 mg for 5 days concomitant with crizotinib | 0% | 10% | Minor | No interventions needed. Concomitant acid suppression can be used safely. | EMA;14 US FDA15 |
| Dabrafenib (2013) | Rabeprazole 40 mg for 4 consecutive days concomitant with dabrafenib | 12% | 3% | Minor | No interventions needed. Concomitant acid suppression is considered safe. | EMA;14 US FDA15 |
| Dasatinib (2006) | Omeprazole 40 mg for 4 consecutive days with dasatinib Maalox 30 ml concomitantly with dasatinib Maalox 30 ml 2 h before dasatinib Famotidine 40 mg 10 h before dasatinib |
42% 58% 26% 63% |
43% 55% NA 61% |
Moderate Moderate Minor Moderate |
Avoid the use of acid-suppressive agents. Otherwise apply separate administration times. H2-antagonists can be used 2 h after dasatinib intake. Antacids can be used 2 h before or after dasatinib intake. | EMA;14 US FDA;15 Eley and colleagues22 |
| Erlotinib (2005) | Omeprazole 40 mg for 7 consecutive days with erlotinib Ranitidine 300 mg once daily concomitantly with erlotinib Ranitidine 150 mg twice daily concomitantly with erlotinib |
61% 54% 17% |
46% 33% 15% |
Moderate Minor Minor |
Avoid the use of acid-suppressive agents. Otherwise apply separate administration times. Or H2-antagonist should be used 2 h after erlotinib intake. Antacids can be used 4 h before or 2 h after erlotinib intake. Furthermore cola may increase erlotinib absorption. | EMA;14 US FDA;15 van Leeuwen and colleagues;23 Kletzl and colleagues24 |
| Gefitinib (2009) | Ranitidine 450 mg twice daily 1 day before gefitinib intake | 71% | 47% | Moderate | Avoid the use of acid-suppressive agents. Otherwise apply separate administration times. Antacids may be used 2 h before or after gefitinib intake. | EMA;14 US FDA;15 Yokota and colleagues25 |
| Ibrutinib (2014) | Omeprazole 40 mg for 5 days in a fasted condition 2 h before ibrutinib intake | 63% | nonsignificant difference | Minor | No interventions needed. Concomitant acid suppression can be used safely. | EMA;14 US FDA;15 de Jong and colleagues26 |
| Imatinib (2001) | Omeprazole 40 mg for 5 consecutive days 15 min before imatinib intake | 3% | 7% | Minor | No interventions needed. Concomitant acid suppression can be used safely. | EMA;14 US FDA;15 Sparano and colleagues;27 Egorin and colleagues28 |
| Lapatinib (2008) | Esomeprazole 40 mg for 7 consecutive days in the evening (12 h before lapatinib intake) | NA | 26% | Minor | Avoid the use of acid-suppressive agents. Otherwise apply separate administration times. Antacids may be used 2 h before or after lapatinib intake. | EMA;14 US FDA15 |
| Lenvatinib (2015) | H2-blockers, antacids, PPIs not further specified in a PBPK analysis | nonsignificant difference | nonsignificant difference | Minor | No clinical studies, but concomitant use with acid-suppressive therapy is considered safe due to a PBPK analysis. | EMA;14 US FDA15 |
| Nilotinib (2007) | Esomeprazole 40 mg for 5 consecutive days 1 h before nilotinib intake | 27% | 34% | Minor | Avoid the use of acid-suppressive agents. Otherwise apply separate administration times. Antacids may be used 2 h before or after nilotinib intake or H2-antagonists can be used 10 h before or 2 h after nilotinib intake. | EMA;14 US FDA;15 Yin and colleagues29–31 |
| Nintedanib (2015) | No clinical study | NA | NA | Moderate | No clinical studies available, however nintedanib bioavailability decreases rapidly with increasing pH so a gastric acid-suppressive drug is likely to give a DDI. | EMA;14 US FDA15 |
| Osimertinib (2016) | Omeprazole 40 mg in a fasted state for 5 consecutive days | 2% | 7% | Minor | No interventions needed. Concomitant acid suppression can be used safely. | EMA;14 US FDA15 |
| Pazopanib (2010) | Esomeprazole 40 mg for 5 consecutive days | 42% | 40% | Minor | Pazopanib should be taken at least 2 h before or 10 h after a dose of an H2-antagonist. Antacids can be used 4 h before or 2 h after pazopanib intake. PPIs should be administered concomitantly with pazopanib in the evening. | EMA;14 US FDA;15 Tan and colleagues32 |
| Ponatinib (2013) | Lansoprazole 60 mg for 2 consecutive days concomitantly with ponatinib | 25% | 1% | Minor | No interventions needed. Concomitant acid-suppressive therapy is considered safe. | EMA;14 US FDA;15
Narasimhan and colleagues33 |
| Regorafenib (2013) | Esomeprazole 40 mg for 5 consecutive days 3 h before and concomitantly with regorafenib. A clinical study was recently finished (De Man et al; Clin Pharmacol Ther in press.) | NA | NA | Minor | No clinical studies available. However regorafenib is considered to be safe since regorafenib pKa is high. | EMA;14 US FDA15 |
| Ruxolitinib (2012) | No clinical study | NA | NA | Minor | No clinical studies available. Concomitant acid-suppressive therapy is considered safe, since pKa of ruxolitinib is high. | EMA;14 US FDA15 |
| Sorafenib (2006) | Omeprazole 40 mg for 5 consecutive days | no significant difference | no significant difference | Minor | No interventions needed. Concomitant acid-suppressive therapy is considered safe. | EMA;14 US FDA15 |
| Sunitinib (2006) | No clinical study | NA | NA | Minor | Sunitinib shows high solubility and therefore concomitant acid-suppressive therapy is considered safe. However survival seems to be lower in patients using ASA. | EMA;14 US FDA;15
Olivier and colleagues34 |
| Tivozanib (2017) | No clinical study | NA | NA | Moderate | No clinical studies available. However adverse event rate was higher in PPI users, which suggests higher tivozanib plasma levels due to a DDI. | EMA;14 US FDA15 |
| Trametinib (2014) | No clinical study | NA | NA | Minor | Trametinib shows consistent solubility over all pH values. Therefore, concomitant acid-suppressive therapy is considered safe. | EMA;14 US FDA15 |
| Vandetanib (2012) | Omeprazole 40 mg for 5 days concomitantly 150 mg ranitidine for 5 days concomitantly with vandetanib |
15% 8% |
6% 1% |
Minor Minor |
No interventions needed. Concomitant acid-suppressive therapy is considered safe. | EMA;14 US FDA;15
Johansson and colleagues35 |
| Vemurafenib (2012) | No clinical study | NA | NA | Minor | No interventions needed. Concomitant acid-suppressive therapy is considered safe. | EMA;14 US FDA15 |
Clinical relevance is scored by means of the US FDA Clinical Drug Interaction Studies, Study Design, Data Analysis, and Clinical Implications Guidance for Industry as a guideline as Major (AUC increase ⩾80%), Moderate (AUC increase ⩾50–<80%), Minor (AUC increase ⩾20–<50%) and by taking into account the performed study and the available evidence regarding pKa and the available assessment report.10,14,15
AUC, area under the curve; DDI, drug–drug interaction; EMA, European Medicines Agency; MKI, multikinase inhibitor; NA, not applicable/unknown; PBPK, physiologically based pharmacokinetic model; PPI, proton pump inhibitor; US FDA, United States Food and Drug Administration.
Indecisive guidelines and the fact that 20–30% of all cancer patients have an indication for the use of acid-suppressive agents (ASAs) complicate the management of this DDI.36 The general consensus is, if possible, to avoid the combination between MKIs and ASAs.37 However, if there is a distinct indication for an ASA (e.g. Barrett’s esophagus), a clear and practical advice to manage the DDI between MKIs and ASAs is essential to safeguard optimal MKI therapy. Based on the pharmacokinetics and pharmacodynamics of both MKIs and ASAs, practical advice can be given for the management of the DDI between MKIs and PPIs, H2-antagonists (H2As) and antacids (see Figure 1 and Table 1).13 This advice may be extrapolated to newly introduced MKIs with a known or suspected drug interaction with gastric suppressive agents and thus with a great impact of the ‘pH effect’ as mentioned in Figure 1 and Table 1.
MKIs and PPIs
Since PPIs do not elevate intragastric pH over the full 24 h-range, a window of relatively low intragastric pH may be used to manage the DDI.38 If there is a hard indication for PPI use, MKIs should be taken at least 2 h before the PPI in the morning in a once-daily regimen, since MKIs can be absorbed completely within this window.13,38 Another possibility is to administer a MKI with an acidic beverage such as cola (pH = 2.5) to manage the DDI, since the acidic beverage temporarily decreases stomach pH resulting in better MKI solubility and absorption.23 Furthermore, the influence of other acidic beverages [e.g. sprite (pH = 3.4) or orange juice (pH = 3.3)] on the absorption of MKIs has not been studied yet.
MKIs and H2-antagonists
Since most H2-antagonists show a short plasma half-life and are administered in a twice daily regimen (e.g. ranitidine), MKIs should be taken at least 2 h before or 10 h after the H2-antagonist intake according to US FDA and EMA guidelines.14,15
Management MKIs and antacids.
Antacids are relatively short-acting agents (e.g. magnesium hydroxide). MKIs should be administered at least 2 h before, or 4 h after antacid intake, to manage this DDI.14,15
Drug transporters and intestinal enzymes
As mentioned previously, MKI absorption is a multifactorial process mediated and affected by passive diffusion, active transport through multiple drug transporters, and intestinal metabolism.7 The activity of these drug transporters and intestinal enzymes may significantly influence MKI bioavailability.
Drug transporters are located throughout the body, especially in the gut, bile ducts, kidneys and the blood–brain barrier (Figure 2).39 The US FDA states: ‘membrane transporters can have clinically relevant effects on the pharmacokinetics and pharmacodynamics of a drug in various organs and tissues by controlling its absorption, distribution, and elimination. In contrast to drug metabolizing enzymes that are largely expressed in the liver and small intestines’.10 Therefore, the effect of a DDI considering drug transporters may be of greater clinical relevance then is assumed so far.
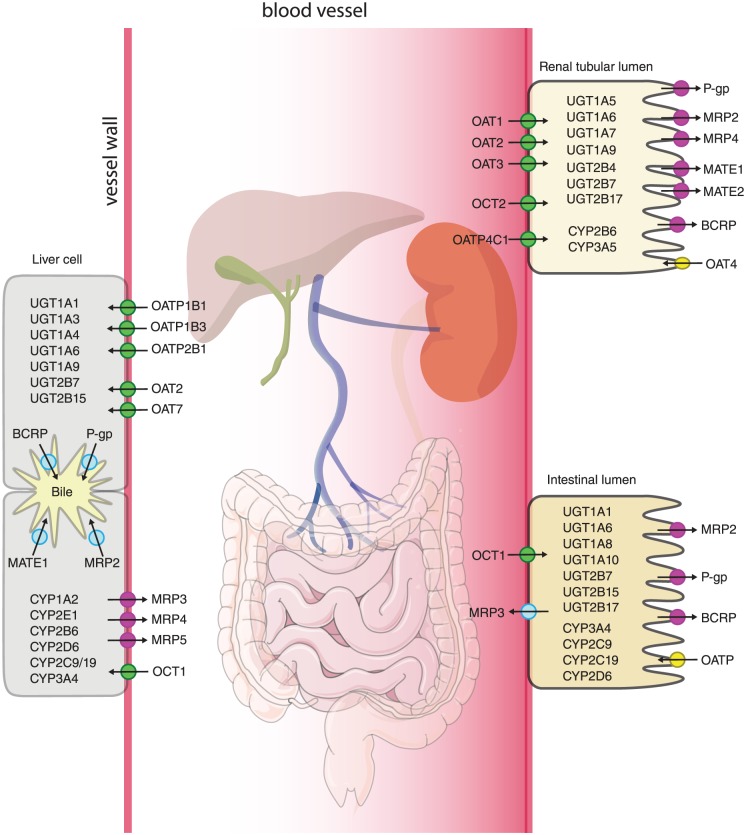
Figure 2.
Distribution of drug transporters and metabolizing enzymes: A complete overview of all the drug transporters and metabolizing phase I and phase II enzymes are presented in this figure for the main organs involved in the pharmacokinetics of drugs.
BCRP, breast cancer resistance protein (ABCG2); CYP, cytochrome P450 iso-enzyme, MATE, multi-antimicrobial extrusion protein; MRP, multidrug resistance associated protein; OAT, organic anion transporters; OATP, organic anion transporting peptides; OCT, organic cation transporters; P-gp, P-glycoprotein (ABCB1); UGT, UDP-glucuronosyltransferase.
Furthermore, efflux drug transporters like P-glycoprotein, or P-gp (ATP-binding cassette subfamily B member 1, ABCB1) and also breast cancer resistance protein (BCRP; ATP-binding cassette subfamily G member 2, ABCG2) may play a crucial role in drug absorption and enterohepatic recirculation. Enterohepatic recirculation is the process in which foreign chemicals are absorbed into the portal blood stream and metabolized by hepatocytes, secreted into the bile and eventually are reabsorbed after secretion of bile in the gut lumen.40 In this multi-step process drug transporters like P-gp and BCRP play a significant role. Other drug efflux transporters that may influence MKI bioavailability are the multidrug resistance protein subfamily (ATP-binding cassette subfamily C member 1 to 12, ABCC1 to 12, like MRP1) and the multi-antimicrobial extrusion protein (MATE), while several uptake transporters may be involved as well [e.g. organic anion transporting peptides (OATPs), organic anion transporters (OATs), and organic cation transporters (OCTs), see Figure 2].
Many drugs are known P-gp inhibitors (e.g. verapamil) or act as a strong P-gp-inducer (e.g. rifampicin). Drugs like cyclosporine, an inhibitor of several OATPs (e.g. OATP1B1 and BCRP) and cimetidine (OCT2 inhibitor) may influence other drug transporters as well. 41 For example, nintedanib showed a decrease in both area under the curve (AUC) and maximum concentration (Cmax) when co-administered with rifampicin. Since nintedanib is almost exclusively metabolized by phase II enzymes, this effect on AUC and Cmax is most likely due to P-gp induction.42 In general the use of strong P-gp or BCRP inhibitors or inducers is discouraged when MKIs are substrates for these transporters. Furthermore, many MKIs show inhibition of several drug transporters by themselves (Table 2).14,15,18,21,35,41,43–59 When a MKI acts like an inhibitor of these transporters and is co-administered with drug transporter substrates with a narrow therapeutic window (e.g. digoxin), close monitoring of side effects (e.g. severe arrhythmia for digoxin) is warranted. For some MKIs the clinical relevance of DDIs regarding drug transporters is negligible and the combination with inhibitory or inducing compounds is considered to be well tolerated (e.g. bosutinib).14,15
Table 2.
DDIs with drug transporters.
| MKI | Substrate | Inhibits | Cmax | AUC | Clinical implications | Interaction potential | References |
|---|---|---|---|---|---|---|---|
| Afatinib | P-gp, BCRP | in vitro: P-gp, BCRP | Ritonavir: 38% increase Rifampicin: 22% decrease |
Ritonavir: 48% increase Rifampicin: 34% decrease |
For strong P-gp and BCRP inhibitors (e.g. ritonavir, cyclosporine); use staggered dosing, preferably 6 h or 12 h apart from afatinib. When afatinib is administered with a strong P-gp inducer (e.g. rifampicin) increase the afatinib dose with 10 mg with close monitoring of side effects. For substrates of P-gp and BCRP close monitoring of side effects is recommended. | Moderate | EMA;14 US FDA;15
Wind and colleagues43 |
| Alectinib | M4 is a P-gp substrate | in vitro: P-gp, BCRP | NA | NA | When alectinib is co-administered with P-gp or BCRP substrates appropriate monitoring of side effects of these substrates is recommended. | Minor | EMA;14 US FDA;15
Morcos and colleagues44 |
| Axitinib | P-gp, BCRP | in vitro: P-gp, BCRP | NA | NA | appropriate monitoring of side effects is recommended when axitinib is used with P-gp and BCRP substrates or inhibitors and inducers. | Minor, since there is only in vitro evidence and axitinib is only a weak P-gp and BCRP substrate | EMA;14 US FDA15 |
| Bosutinib | P-gp |
in vitro: P-gp, BCRP, OCT1 dabigatran (P-gp substrate): no effect on dabigatran pharmacokinetics |
NA | NA | Clinical relevant interactions with drug transporters are not likely to appear. | Minor | EMA;14 US FDA;15
Abbas and colleagues;18 Hsyu and colleagues45 |
| Cabozantinib | MRP2 | in vitro: P-gp, BCRP, MATE1, MATE2 | NA | NA | Appropriate monitoring is recommended when using substrates of P-gp of BCRP. Interactions with MATE1-2 in clinically relevant concentrations are unlikely. If necessary, a 20 mg dose alteration may be applied. Close monitoring of side effects is warranted when administered with strong MRP2 inhibitors (e.g. cyclosporine). | Moderate | EMA;14 US FDA15 |
| Ceritinib | P-gp | P-gp, BCRP | NA | NA | Concomitant administration with strong inducers or inhibitors of P-gp must be avoided since plasma concentration of ceritinib might be altered. Close monitoring of side effects is warranted when administered with P-gp or BCRP substrates. However CYP DDIs are of greater influence. | Minor, since interactions regarding CYP enzymes are of greater clinical importance | EMA;14 US FDA15 |
| Cobimetinib | P-gp | in vitro: BCRP, OATP1B1, OATP1B3, OCT1 | NA | NA | Concomitant administration with strong P-gp inducers or inhibitors must be avoided. Appropriate monitoring is recommended when using BCRP, OATP1B1, OATP1B3, OCT1 substrates. | Moderate | EMA;14 US FDA;15
Musib and colleagues21 |
| Crizotinib | P-gp | in vitro: P-gp, OCT1, OCT2 | NA | NA | Appropriate monitoring of side effects is recommended when using concomitant P-gp substrates, inhibitors and inducers. Furthermore, close monitoring is recommended when using P-gp, OCT1, OCT2 substrates. | Minor, since CYP interactions are of greater clinical importance | EMA;14 US FDA15 |
| Dabrafenib | P-gp, BCRP | in vitro: OATP1B1, OATP1B3, BCRP | Rosuvastatin: 160% increase | Rosuvastatin: 7% increase | Dabrafenib is not likely to have a clinically relevant interaction with OATP1B1, OATP1B3 and BCRP. Concomitant use with substrates of these transporters is considered safe. The influence of P-gp and BCRP inhibitors or inducers is considered to be small since the bioavailability of dabrafenib is high (95%), therefore only limited pharmacokinetic effects can be expected. | Minor | EMA;14 US FDA15 |
| Dasatinib | P-gp, BCRP | NA | NA | NA | Concomitant administration with strong inducers or inhibitors of P-gp and BCRP must be avoided or side effects must be monitored closely when administered with strong inhibitors. | Minor | EMA;14 US FDA;15
Haouala and colleagues46 |
| Erlotinib | P-gp, BCRP | in vitro: OCT2, OAT3 | NA | NA | Concomitant administration with strong inducers or inhibitors of P-gp or BCRP must be avoided since an altered plasma concentration is possible. Administration with OCT2 and OAT3 substrates should be avoided. | Moderate | EMA;14 US FDA;15
Marchetti and colleagues;47 Sprowl and colleagues; Elmeliegy and colleagues49 |
| Gefitinib | P-gp, BCRP | in vitro: BCRP, P-gp | NA | In vitro Irinotecan: AUC irinotecan 63% increase | Concomitant administration with P-gp and BCRP substrates should be avoided. BCRP inhibition is 10-fold stronger than P-gp inhibition. So especially be careful when gefitinib is combined with BCRP substrates. Avoid the use of strong BCRP or P-gp inhibitors or inducers since gefitinib plasma concentration may be altered. |
Moderate | EMA;14 US FDA;15
Stewart and colleagues50 |
| Ibrutinib | NA | in vitro: P-gp, BCRP | NA | NA | When P-gp or BCRP substrates are used, they should be taken at least 6 h before or after ibrutinib intake. Inhibitors or inducers of transporters are not likely to result in clinically meaningful changes in ibrutinib pharmacokinetics and can be used concomitantly. | Minor | EMA;14 US FDA;15
de Jong and colleagues51 |
| Imatinib | P-gp, BCRP | in vitro: BCRP | NA | NA | A clinical relevant interaction with P-gp or BCRP inhibitors or inducers may be possible. Close monitoring of substrate specific side effects is advised when used concomitantly with BCRP substrates. Although the interaction potential is considered to be low. | Minor | EMA;14 US FDA;15
Eechoute and colleagues52 |
| Lapatinib | P-gp, BCRP | in vitro: P-gp, BCRP, OATP1B1 | Digoxin (P-gp substrate): 100% increase (digoxin) | Digoxin (P-gp substrate): 60–80% increase (digoxin) | Lapatinib is highly susceptible for interactions regarding drug transporters. When using P-gp, BCRP, OATP1B1 substrates close monitoring of side effects is recommended. The use of strong P-gp and BCRP inhibitors or inducers should be avoided. | Major | EMA;14 US FDA;15
Koch and colleagues53 |
| Lenvatinib | P-gp, BCRP, MDR1 | in vitro: P-gp, BCRP, OATP1B3 | Ketoconazole: 19% increase single-dose rifampicin: 33% increase |
Ketoconazole: 15% increase single-dose rifampicin: 31% increase |
Clinical relevant interactions with strong inhibitors or inducers of P-gp, BCRP are not likely to appear, but close monitoring for lenvatinib specific side effects is recommended. Concomitant administration with P-gp, BCRP and OATP1B3 substrates should be avoided. | Minor | EMA;14 US FDA;15
Shumaker and colleagues 54,55 |
| Nilotinib | P-gp, BCRP | in vitro: P-gp, BCRP | NA | Imatinib (CYP3A4/P-gp inhibitor): nilotinib AUC increased with 18–40% | Concomitant administration with strong P-gp or BCRP inducers or inhibitors must be avoided since an altered plasma concentration is possible otherwise side effects should be monitored closely. | Minor | EMA;14 US FDA;15
Lemos and colleagues56 |
| Nintedanib | P-gp | in vitro: P-gp, OCT1, BCRP | Ketoconazole: 83% increase Rifampicin: 60% decrease |
Ketoconazole: 61% increase Rifampicin: 50% decrease |
when administered with strong P-gp inhibitors a 100 mg step-wise dose reduction must be considered. The duration of therapy with strong inducers must be minimized since inadequate plasma levels of nintedanib might occur. Concomitant administration with P-gp, BCRP and OCT1 substrates should be avoided. | Major | EMA;14 US FDA15 |
| Osimertinib | P-gp, BCRP | in vitro: P-gp, BCRP | Rosuvastatin (BCRP substrate): 72% increase | Rosuvastatin (BCRP substrate): 35% increase | Concomitant administration with strong P-gp and BCRP inducers or inhibitors must be avoided since an altered plasma concentration is likely. When co-administered with BCRP or P-gp substrates close monitoring of side effects is recommended. | Minor | EMA;14 US FDA15 |
| Pazopanib | P-gp, BCRP | in vitro: OATP1B1, P-gp, BCRP | Lapatinib (P-gp and BCRP inhibitor) 60% Increase | Lapatinib (P-gp and BCRP inhibitor): 50% increase | Co-administration with strong P-gp or BCRP inhibitors must be avoided. Close monitoring of side effects is advised when used concomitantly with P-gp or BCRP substrates. | Moderate | EMA;14 US FDA15 |
| Ponatinib | P-gp, BCRP | in vitro: P-gp, BCRP | NA | NA | Appropriate monitoring is recommended when co-administered with P-gp or BCRP substrates. Also, the use of strong inhibitors or inducers of P-gp, BCRP must be avoided, although DDI potential is considered to be low since ponatinib is only a weak substrate for P-gp and BCRP. | Minor | EMA;14 US FDA15 |
| Regorafenib | P-gp, BCRP |
in vitro: BCRP regorafenib has no effect on digoxin AUC |
Rosuvastatin (BCRP substrate): 360% increase | Rosuvastatin (BCRP substrate): 280% increase | BCRP substrates should be used with caution. When administered with strong inhibitors or inducers of P-gp and BCRP close observation of side effects is warranted. | Major | EMA;14 US FDA15 |
| Ruxolitinib | NA | in vitro: P-gp, BCRP | NA | NA | When ruxolitinib is administered with P-gp or BCRP substrates close monitoring of side effects is advised for these substrates. DDI potential can be minimized if time between administration is kept apart as long as possible. | Minor | EMA;14 US FDA15 |
| Sorafenib | P-gp, OATP1B1, OATP1B3, MRP2-3 | P-gp | NA | NA | Concomitant administration with strong inhibitors or inducers of P-gp, OATP1B1, OATP1B3 and MRP2-3 should be avoided. Administration with P-gp substrates should be done with caution. | Moderate | EMA;14 US FDA;15
Bins and colleagues57 |
| Sunitinib | P-gp |
in vitro: P-gp, BCRP co-administration with gefitinib (BCRP inhibitor) did not result in significant AUC changes of sunitinib |
NA | NA | Appropriate monitoring is recommended when co-administered with P-gp or BCRP substrates. Also, the use of strong inhibitors or inducers of P-gp must be avoided. | Minor | EMA;14 US FDA15 |
| Tivozanib | NA | in vitro: BCRP | NA | NA | Co-administration with BCRP substrates must be avoided or side effects must be monitored closely. | Minor | EMA;14 US FDA15 |
| Trametinib | P-gp | in vitro: P-gp, BCRP, OAT1, OAT3, OATP1B1, OATP1B3, OATP2B1, OCT2, and MATE1 | NA | NA | Co-administration of strong inhibitors or inducers of P-gp must be avoided. When P-gp, BCRP, OAT1, OAT3, OATP1B1, OATP1B3, OCT2 and MATE1 substrates are used, staggered dosing must be applied (at least 2 h apart) to minimize DDI risk. However, based on the low dose and low clinical systemic exposure relative to the in vitro inhibition or induction potential this is not expected to be of in vivo significance. | Minor | EMA;14 US FDA15 |
| Vandetanib | NA | in vitro: P-gp, BCRP, OCT2 | Metformin (OCT-2 substrate) increased with 50% Digoxin (P-gp substrate) increased with 29% |
Metformin (OCT-2 substrate) increased with 74% Digoxin (P-gp substrate) increased with 23% |
Co-administration with P-gp, BCRP, OCT2 substrates must be avoided and side effects must be monitored closely. Concomitant intake with strong inhibitors or inducers of drug transporters is safe. | Moderate | EMA;14 US FDA;15
Johansson and colleagues35 |
| Vemurafenib | P-gp, BCRP | in vitro: P-gp, BCRP | Digoxin (P-gp substrate) increased 50% | Digoxin (P-gp substrate) increased 80% | Concomitant administration with strong inhibitors or inducers of P-gp and BCRP should be avoided. Appropriate monitoring is recommended when co-administered with P-gp or BCRP substrates. | Major | EMA;14 US FDA;15
Zhang and colleagues59 |
Clinical relevance is scored by means of the US FDA Clinical Drug Interaction Studies, Study Design, Data Analysis, and Clinical Implications Guidance for Industry as a guideline as major (AUC increase ⩾80%), moderate (AUC increase ⩾50 to <80%), minor (AUC increase ⩾20 to <50%) taken into account the available evidence for both change in AUC of MKI and change in AUC for transporter substrates, since there is no separate scoring system for drug transporter interactions. If there was no clinical evidence, clinical relevance was estimated on the basis of available evidence regarding inhibitory concentrations and the assessment report. Interaction potential was then scored as minor or at most moderate.
Strong drug transporter inhibitors: P-gp: amiodarone, carvedilol, clarithromycin, dronedarone, itraconazole, lapatinib, lopinavir, propafenone, quinidine, ranolazine, ritonavir, saquinavir, telaprevir, tipranavir and ritonavir, verapamil. BCRP: curcumin, cyclosporine, eltrombopag OATP1B1/OATP1B3: atazanavir, ritonavir, clarithromycin, cyclosporine, erythromycin, gemfibrozil, lopinavir, rifampin (single dose), simeprevir OAT1/OAT3: p-aminohippuric acid (PAH), probenecid, teriflunomide, MATE1/MATE2-K: cimetidine, dolutegravir, isavuconazole, ranolazine, trimethoprim, vandetanib strong drug transporter inducers: P-gp: rifampicin, carbamazepine, phenytoin, St. John’s wort, ritonavir.10,41,58
AUC, area under the curve; BCRP, breast cancer resistance protein (ABCG2); DDI, drug–drug interaction; EMA, European Medicines Agency; MATE; multi-antimicrobial extrusion protein; MKI, multikinase inhibitor; MRP, multidrug resistance associated protein; NA, not applicable or only preclinical data available; OAT, organic anion transporters; OATP, organic anion transporting peptides; OCT, organic cation transporters; P-gp, P-glycoprotein (ABCB1); US FDA, United States Food and Drug Administration.
In contrast with the above mentioned unwanted adverse effects, mostly found in preclinical studies, DDIs concerning drug transporters and MKIs may also be used in a beneficial way. For example, MKIs may potentially increase chemotherapy concentrations through P-gp or BCRP inhibition (e.g. increased paclitaxel plasma concentration resulting from P-gp inhibition by nilotinib or increased nilotinib concentrations as a result of P-gp inhibition by imatinib).60,61
In conclusion, we found only a limited number of clinical studies, which investigated the effects of inhibition or induction of drug transporters by MKIs, since this is a relatively novel field of DDI research. Combinations between strong drug transporter inhibitory or inducing compounds should be avoided for most MKIs as mentioned in Table 2.
Intestinal metabolism
Another important factor in drug absorption is intestinal metabolism. Many MKIs are metabolized in the gut wall through intestinal CYP3A4, which is often in close proximity of drug transporters, such as P-gp. When a MKI is given concomitantly with an intestinal CYP3A4 inducer (e.g. rifampicin) or inhibitor (e.g. grapefruit juice) this may significantly change MKI bioavailability.62 However, in contrast, Van Erp and colleagues failed to show a significant increase in sunitinib exposure, when co-administered with grapefruit juice.63 Moreover, since many MKIs undergo extensive first-pass metabolism and are thus dependent of both intestinal and hepatic metabolism, it is difficult to determine whether intestinal metabolism or hepatic metabolism is the main contributor to an altered drug bioavailability.
Metabolism
In the liver, MKIs are predominately metabolized by CYP enzymes into either active or inactive metabolites. For some MKIs, like nintedanib, phase II metabolism through UDP-glycosyltransferases (UGTs), glutathione S-transferases and sulfotransferases (SULTs) is dominant in their metabolism.6,64,65 Inhibition or induction of these phase I and II enzymes by co-administered medication may lead to either (severe) toxicity or loss of effective MKI therapy, respectively.
As DDIs with strong CYP3A4 inhibitors and inducers (e.g. ketoconazole and rifampicin, respectively) play a significant role in MKI therapy, they are usually well described and clear recommendations for the management of these DDIs are presented in the assessment report. There are many (strong) inducers or inhibitors of CYP enzymes for which a complete overview can be found at the FDA and EMA websites.41,66 Moreover, some MKIs (e.g. imatinib, pazopanib) also displayed inhibitory or inducing activity by themselves.67–70 The general advice is to avoid concomitant administration with strong inhibitors or inducers of CYP enzymes. If this is not possible, a MKI dose adjustment, based on the advice given in the assessment report is recommended. For strong inducers a gradual dose escalation of the prescribed dose is advised with close monitoring of MKI-specific side effects. For an overview of clinically relevant DDIs and for practical recommendations see Table 3.14,15,41,43,44,67-69,71–93
Table 3.
DDIs regarding drug metabolism.
| MKI | Major CYP | Minor CYPs and others | Inhibitory activity | Inducing activity | Inhibitory compound | Inducing compound | Change in Cmax | Change in AUC | Clinical recommendations | Clinical relevance | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Afatinib | mainly due to nonenzyme-catalyzed Michael adduct formation | FMO3, CYP3A4 | NA | NA | ritonavir | 38 % increase | 48 % increase | No DDI is expected, combination with CYP inducers or inhibitors is considered safe. The effect is most likely through P-gp induction and inhibition. | Minor | EMA;14 US FDA;15
Wind and colleagues43 |
|
| rifampicin | 22 % decrease | 34% decrease | |||||||||
| Alectinib | CYP3A4 | CYP2C8, CYP3A5 | There was no influence on midazolam (CYP3A4 substrate) pharmacokinetics | CYP1A2, CYP2B6, CYP3A4 (in vitro) | Posaconazole | 18% increase | 75% increase | Since alectinib metabolites are equally effective as alectinib strong inhibitors or inducers of CYP3A4 can be safely combined with close monitoring of side effects from alectinib. | Minor (since alectinib metabolites are equally active) | EMA;14 US FDA;15
Morcos and colleagues44 |
|
| rifampicin | 51% decrease | 73% decrease | |||||||||
| Axitinib | CYP3A4 | CYP3A5, CYP1A2, CYP2C19, UGT1A1 | UGT1A4, UGT1A7, UGT1A9, CYP1A2 | NA | ketoconazole | 50% increase | 106% increase | 50% dose reduction of axitinib is recommended when concomitantly used with strong inhibitors of CYP3A4 and slow dose escalation is advised for strong inducers of CYP3A4. Smoking is not allowed since it might alter CYP1A2 metabolism. | Moderate | EMA;14 US FDA;15
Pithavala and colleagues71,72 |
|
| rifampicin | 71% decrease | 79% decrease | |||||||||
| Bosutinib | CYP3A4 | Mono-oxygenase enzymes (FMO) | NA | NA | ketoconazole aprepitant (moderate CYP3A4 inhibitor) |
420% increase 50% increase |
760% increase 100% increase |
Avoid strong and moderate CYP3A4 inhibitors or inducers. Otherwise stop bosutinib treatment or reduce bosutinib dose by 20%. Dose escalation is often not useful since adequate plasma levels are not reached with a maximum dose of 600 mg qd. | Major | EMA;14 US FDA;15
Hsyu and colleagues;73 Abbas and colleagues74 |
|
| rifampicin | 86% decrease | 92% decrease | |||||||||
| Cabozantinib | CYP3A4 | CYP2C9 | CYP2C9, CYP3A, CYP2C19 (in vitro) No significant effect on Rosiglitazone AUC (CYP2C8 substrate) |
NA | ketoconazole | no significant difference | 38% increase | (Chronic) co-administration of strong inhibitors and inducers of CYP3A4 must be avoided. If necessary, a 20 mg dose alteration may be applied. For CYP2C9, CYP2C19 or CYP3A4 substrates with a narrow therapeutic window close monitoring of side effects is recommended, however the inhibitory and inducing potential of cabozantinib is likely to be low. | Moderate | EMA;14 US FDA;15
Nguyen and colleagues75 |
|
| rifampicin | no significant difference | 77% decrease | |||||||||
| Ceritinib | CYP3A4 | NA | CYP3A4, CYP2C9, CYP2A6, CYP2E1 (in vitro) | CYP3A4 | ketoconazole | 20% increase | 190% increase | A 30% dose reduction may be applied when ceritinib is administered with strong inhibitors of CYP3A4. Concomitant use of strong inducers should be avoided. When administered with CYP2C9, CYP2A6, CYP2E1 or CYP3A4 substrates close monitoring of side effects is recommended. | Moderate | EMA;14 US FDA15 | |
| rifampicin | 44% decrease | 70% decrease | |||||||||
| Cobimetinib | CYP3A4 | CYP2C19, CYP2D6, UGT2B7 | Dextromethorphan (CYP2D6 substrate) and midazolam exposure was not altered by cobimetinib. | CYP1A2 (in vitro) | itraconazole | 220% increase | 570% increase | Avoid the (chronic) use of strong CYP3A4 inhibitors or inducers (especially treatment with strong inhibitors). If treatment is necessary monitoring of side effects must be applied and the use must be limited. Also, a 20 mg dose adjustment may be made. Concomitant administration with CYP1A2 substrates must be avoided or side effects must be monitored closely. | Major | EMA;14 US FDA;15
Budha and colleagues76 |
|
| rifampicin (PBPK model) | 63% decrease | 83% decrease | |||||||||
| Crizotinib | CYP3A4 | CYP3A5, CYP2C8, CYP2C19, CYP2D6 | CYP3A4, CYP2B6, UGT1A1, UGT2B7 Midazolam AUC increased with 270% |
UGT1A1, CYP2B6, CYP2C8, CYP2C9 | ketoconazole | 40% increase | 220% increase | Avoid the (chronic) use of strong CYP3A4 inhibitors or inducers. If treatment is necessary monitoring of side effects is recommended. When administered with CYP3A4, UGT1A1, UGT2B7, CYP2C8, CYP2C9 or CYP2B6 substrates close monitoring is recommended. | Major | EMA;14 US FDA;15
Xu and colleagues77 |
|
| rifampicin | 79% decrease | 84% decrease | |||||||||
| Dabrafenib | CYP2C8 | CYP3A4 | CYP1A2, CYP2D6 R-warfarin (CYP2C19 substrate) AUC decreased with 33% and Cmax increased with 19% S-warfarin (CYP2C9 substrate) AUC decreased with 37% and Cmax increased with 17% |
CYP3A4, CYP2B6 midazolam (a CYP3A4 substrate) AUC and Cmax decreased with 47% and 65% respectively |
ketoconazole gemfibrozil |
33% increase no significant difference |
71% increase 47% increase |
Avoid the (chronic) use of strong CYP3A4 and CYP2C8 inhibitors or inducers. If there is a hard indication for the use of strong inhibitors or inducers, the duration of use must be limited. When used with CYP3A4, CYP1A2, CYP2B6, CYP2C9 and CYP2C19 substrates side effects must be monitored closely, especially in the first 3 days of use. |
Minor | EMA;14 US FDA;15
Suttle and colleagues78 |
|
| rifampicin | 27% decrease | 34% decrease | |||||||||
| Dasatinib | CYP3A4 | FMO, UGT | CYP2C8, CYP3A4 simvastatin (CYP3A4 substrate) AUC and Cmax increased with 20% and 37% respectively. |
NA | Ketoconazole | 384% increase | 256% increase | Avoid strong CYP3A4 inducers or inhibitors. When administered with strong inhibitors dasatinib dose must be reduced with 20–40 mg. When administered with strong inducers a dose escalation must be applied with close monitoring of side effects. When administered with CYP2C8 or CYP3A4 substrates close monitoring of side effects is recommended. | Major | EMA;14 US FDA;15
Johnson and colleagues79 |
|
| rifampicin | 81% decrease | 82% decrease | |||||||||
| Erlotinib | CYP3A4 | CYP1A2, CYP1A1, CYP1B1, CYP3A5 | CYP1A1, CYP3A4, CYP2C8 and UGT1A1 Midazolam AUC decreased with 24% Paclitaxel (CYP2C8) AUC was unchanged |
NA | Ketoconazole Ciprofloxacin (CYP1A2 inhibitor) |
69% increase No significant difference |
86% increase 39% increase |
When strong CYP3A4, CYP1A2 inducers are used dose increase up to 300 mg is advised with monitoring of side effects. For strong inhibitors a 50 mg dose reduction is recommended. Use of CYP1A2 inducers or inhibitors (e.g. smoking) is discouraged. When administered with CYP3A4, CYP1A1, and UGT1A1 substrates close monitoring of side effects is recommended. | Moderate | EMA;14 US FDA;15
Hamilton and colleagues80 |
|
| rifampicin | 29% decrease | 69% decrease | |||||||||
| Gefitinib | CYP3A4 | CYP3A5, CYP2C19 CYP2D6 |
CYP2D6 and CYP2C19 Metoprolol (a CYP2D6 substrate) AUC increased with 35% |
NA | itraconazole | 61% increase | 78% increase | Dose reduction is not necessary, when combined with strong CYP3A4 inhibitors, since gefitinib has a good tolerability profile. The use of strong CYP3A4 inducers needs to be avoided. When combined with CYP2D6 or CYP2C19 substrates close monitoring of side effects is recommended. |
Major | EMA;14 US FDA;15
Swaisland and colleagues81 |
|
| rifampicin | 65% decrease | 83% decrease | |||||||||
| Ibrutinib | CYP3A4 | CYP2D6 | CYP3A4 | CYP2B6 | ketoconazole grapefruit juice erythromycin voriconazole |
2800% increase 250% increase 240% increase 570% increase |
2300% increase 120% increase 200% increase 470% increase |
If the use of strong CYP3A4 inhibitors is necessary reduce ibrutinib dose to 140 mg or temporarily (<7 days) stop ibrutinib therapy. For moderate inhibitors reduce ibrutinib dose to 280 mg. Minimize the time of use for strong inducers of CYP3A4. Strong inhibitors or inducers of CYP2D6 must be used with caution. |
Major | EMA;14 US FDA;15 de Jong and colleagues82 | |
| Rifampicin | 92% decrease | 90% decrease | |||||||||
| Imatinib | CYP3A4 | CYP3A5, CYP1A2, CYP2D6, CYP2C9, CYP2C19 | CYP2C9 Cyclosporin (a CYP3A4/CYP2C8 substrate) concentration raised with 26% during imatinib therapy metoprolol (CYP2D6 substrate) AUC increased with 23% simvastatin (CYP3A4 substrate) AUC increased with 250% |
NA | Ketoconazole | 26% increase | 40% increase | No intervention is needed for strong CYP3A4 inhibitors but monitoring for toxic effects is recommended and duration of strong CYP3A4 inhibitor compounds needs to be minimized. For CYP3A4 inducers a 50% imatinib dose increase may be applied. Also, close monitoring is recommended for concomitant use of CYP3A4, CYP2C9 and CYP2B6 substrates with narrow therapeutic windows. | Moderate | EMA;14 US FDA;15 Wang and colleagues;67 O’Brien and colleagues; Atiq and colleagues69 | |
| rifampicin | 54% decrease | 74% decrease | |||||||||
| Lapatinib | CYP3A4 | CYP3A5, CYP1A2, CYP2D6, CYP2C8, CYP2C9, CYP2C19 | CYP3A4, CYP2C8 Midazolam (CYP3A4 substrate) AUC increased with 45% Paclitaxel (CYP2C8 substrate) AUC increased with 37% concomitant with pazopanib |
NA | ketoconazole | 114% increase | 257% increase | For strong inhibitors lapatinib dose must be lowered to 500 mg. For strong inducers a gradual increase of lapatinib dose must be administered with close monitoring of side effects. When administered with CYP3A4 or CYP2C8 substrates close monitoring of side effects is recommended. | Moderate | EMA;14 US FDA;15 Tan and colleagues83 Koch and colleagues84 | |
| carbamazepine | 59% decrease | 72% decrease | |||||||||
| Lenvatinib | Oxidase by aldehyde oxydase and conjugation by glutathione | CYP3A4 | NA | NA | ketoconazole | 19% increase | 15% increase | Lenvatinib administration with CYP3A4 inducers or inhibitors is considered safe. | Minor | EMA;14 US FDA15 | |
| rifampicin | no significant difference | 18% decrease | |||||||||
| Nilotinib | CYP3A4 | CYP2C8, CYP1A1, CYP1A2, CYP1B1 | CYP2D6, CYP2C9, CYP3A4, CYP2C8, UGT1A1 (in vitro) Midazolam AUC increased 160% and Cmax 100% Warfarin (CYP2C9 substrate) AUC did nog change |
CYP2B6, CYP2C8, CYP2C9 (in vitro) | ketoconazole | 84% increase | 201% increase | For strong CYP3A4 inhibitors nilotinib dose must be lowered to 400 mg once daily. For strong inducers nilotinib dose must be gradually increased depending on toxic side effects. When administered with CYP2D6, CYP2C8 or CYP3A4, CYP2C9, UGT1A1 substrates close monitoring of side effects is recommended. | Major | EMA;14 US FDA;15 Zhang and colleagues85 | |
| rifampicin | 64% decrease | 80% decrease | |||||||||
| Nintedanib | Hydrolysis due to esterases | UGT1A1, UGT1A7, UGT1A8, UGT1A10, CYP’s (5%) | NA | NA | ketoconazole | 83% increase | 61% increase | Nintedanib co-administration with strong CYP inducers or inhibitors is considered safe since only a small part is metabolized by CYP enzymes and the interaction is more likely through P-gp inhibition or induction. | Minor | EMA;14 US FDA15 | |
| rifampicin | 60% decrease | 50% decrease | |||||||||
| Osimertinib | CYP3A4 | CYP3A5, CYP1A2, CYP2A6, CYP2C9, CYP2E1 | CYP1A2, CYP2C8, UGT1A1(in vitro) CYP3A4, CYP3A5 Simvastatin AUC and Cmax decreased with 9% and 23% respectively |
CYP3A4, CYP1A2 | itraconazole | 20% decrease | 24% increase | Administration with strong inhibitors of CYP3A4 is considered safe. Strong inducers of CYP3A4 must be used with caution and the duration must be minimized. When administered with CYP3A4/3A5, CYP1A2, CYP2C8 and UGT1A1 substrates close monitoring of side effects is recommended. | Moderate | EMA;14 US FDA;15 Vishwanathan and colleagues;86 Harvey and colleagues87 | |
| Rifampicin | 73% decrease | 78% decrease | |||||||||
| Pazopanib | CYP3A4 | CYP1A2, CYP2C8 |
in vitro: CYP3A4, CYP2B6, CYP2C8, CYP2D6, CYP2E1, UGT1A1 midazolam AUC and Cmax increased both with 30% respectively dextromethorphan (CYP2D6 substrate) AUC and Cmax increased with 33% an 64% respectively paclitaxel (a CYP2C8 substrate) AUC and Cmax increased with 26% and 31% respectively Caffeine (CYP1A2 substrate), Warfarin (CYP2C9 substrate) and omeprazole (CYP2C19 substrate) AUC did not change |
NA | ketoconazole | 45% increase | 66% increase | When a strong CYP3A4 inhibitor is administered a 50% pazopanib dose reduction may be applied for strong inducers duration of therapy must be limited. Close observations for CYP2C8, CYP2D6, CYP2E1, UGT1A1 and CYP3A4 substrates with narrow therapeutic windows must be applied when co-administered with pazopanib. |
Minor | EMA;14 US FDA;15 Tan and colleagues83 | |
| Phenytoin or carbamazepine | 50% decrease | 30% decrease | |||||||||
| Ponatinib | CYP3A4 | CYP2D6, CYP2C8, CYP3A5 | NA | NA | ketoconazole | 47% increase | 78% increase | When administered with strong CYP3A4 inhibitors a dose reduction to 30 mg may be administered. The co-administration of strong inducers should be avoided or therapy duration should be minimized. | Moderate | EMA;14 US FDA;15 Narasimhan and colleagues88,89 | |
| Rifampicin | 42% decrease | 62% decrease | |||||||||
| Regorafenib | CYP3A4 | UGT1A9 |
in vitro: UGT1A1, UGT1A9, CYP2C8, CYP2B6, CYP2C9, CYP2C19, CYP3A4 Irinotecan metabolite (SN-38) (substrate of UGT1A1) AUC increased with 44% |
NA | ketoconazole | 40% increase | 33% increase | Co-administration with strong inhibitors or inducers of CYP3A4 and UGT1A9 should be avoided. Influence on regorafenib plasma levels is relatively small. Regorafenib dose must be gradually increased when administered with strong CYP3A4 inhibitors and close monitoring of side effect with a 40mg dose escalation may be applied when administered with strong CYP3A4 inducers and the use must be minimized. Toxicity must be monitored for UGT1A1, UGT1A9, CYP2C8, CYP2C9, CYP2C19 or CYP3A4 substrates; however, pharmacokinetic data did not result in clinically meaningful interactions. | Moderate | EMA;14 US FDA15 | |
| Rifampicin | 20% decrease | 50% decrease | |||||||||
| Ruxolitinib | CYP3A4 | CYP2C9 | Intestinal CYP3A4 | NA | ketoconazole erythromycin |
33% increase 8% increase |
91% increase 27% increase |
When administered with strong inhibitors of CYP3A4 and CYP2C9 a 50% dose reduction may be applied if there is relevant toxicity. For moderate inhibitors a dose reduction is not necessary. For strong CYP3A4 and CYP2C9 inducers the use must be minimized. |
Moderate | EMA;14 US FDA;15 Shi and colleagues90 | |
| Rifampicin | 52% decrease | 71% decrease | |||||||||
| Sorafenib | CYP3A4 | UGT1A9 | UGT1A9, UGT1A1 Administration with cyclophosphamide (a CYP2B6 substrate), warfarin, midazolam, dextromethorphan, omeprazole or paclitaxel did not result in any significant changes in AUC of these substrates. |
NA | ketoconazole | 26% increase | 11% increase | Sorafenib administration with strong inhibitors or inducers of CYP3A4 is considered safe. For UGT1A1 and UGT1A9 substrate specific side effects should be closely monitored. The use of strong UGT1A9 inhibitors or inducers should be avoided. | Minor | EMA;14 US FDA15 | |
| Rifampicin | no significant difference | 37% reduction | |||||||||
| Sunitinib | CYP3A4 | CYP1A2 | NA | NA | ketoconazole | 49% increase | 51% increase | Dose reduction is advised when co-administered with strong CYP3A4 inhibitors to a minimum of 37.5 mg for GIST and metastatic renal cell carcinoma or 25 mg for neuro-endocrine tumors based on monitoring of tolerability. For strong CYP3A4 inducers an increase in 12.5 mg increments may be applied with monitoring of tolerability. | Minor | EMA;14 US FDA15 | |
| Rifampicin | 23% decrease | 46% decrease | |||||||||
| Tivozanib | CYP3A4 | UGT1A, CYP1A1 | CYP2B6, CYP2C8 | NA | Ketoconazole | 3% decrease | 5% increase | Administration with strong inhibitors of CYP3A4 is considered safe. The use of strong CYP3A4 inducers must be minimized. Also, close monitoring of side effects is recommended when administered with CYP2B6 or CYP2C8 substrates. | Moderate | EMA;14 US FDA;15 Cotreau and colleagues91 | |
| Rifampicin | 9% increase | 52% decrease | |||||||||
| Trametinib | Deacetylation and glucuronidation | CYP3A4 | CYP2C8, CYP2C9, CYP2C19 (in vitro) | CYP3A4 (in vitro) | No studies available | NA | NA | Administration with strong inhibitors or inducers of CYP enzymes is considered safe since primary metabolism is not due to metabolism. DDI potential is likely to be low. | Minor | EMA;14 US FDA15 | |
| no studies available | NA | NA | |||||||||
| Vandetanib | CYP3A4 | FMO1, FMO3 | CYP2D6 | CYP1A2, CYP2C9, CYP3A4 Midazolam AUC did not change |
Itraconazole | 4% decrease | 9% increase | Administration with strong inhibitors of CYP3A4 is considered safe. Concomitant administration with strong inducers must be avoided or dose may be gradually increased. When administered with substrates for CYP2D6, CYP1A2, CYP2C9 and CYP3A4 close monitoring of side effects is recommended. | Minor | EMA;14 US FDA;15 Martin and colleagues92 | |
| rifampicin | 3% increase | 40% decrease | |||||||||
| Vemurafenib | CYP3A4 | UGT |
in vitro: CYP1A2, CYP2C8, CYP2C9 150% increase in caffeine (CYP1A2 substrate) exposure was seen Warfarin (CYP2C9 substrate) exposure increased with 18% |
CYP3A4, CYP2B6 Midazolam AUC decreased with 32% |
no completed clinical study | NA | NA | The influence of CYP3A4 or UGT inhibitors or inducers is considered minimal. When administered with CYP1A2, CYP2C8, CYP2C9, CYP3A4 or CYP2B6 substrates close monitoring of side effects is recommended. | Minor | EMA;14 US FDA15 | |
| rifampicin | unknown | 40% decrease |
Clinical relevance is scored by means of the US FDA Clinical Drug Interaction Studies, Study Design, Data Analysis, and Clinical Implications Guidance for Industry, for inducers as major (AUC decrease ⩾80%), moderate (AUC decrease ⩾50 to 80%), minor (AUC decrease ⩾20 to <50%) or unknown and for inhibitors as major (AUC increase ⩾400%), moderate (AUC increase ⩾100 to 400%), minor (AUC increase ⩾25 to <100%) or unknown as on the basis of the available evidence regarding inhibitory concentrations and the assessment report. Clinical relevance was scored on the basis of the highest score. Major CYP inhibitors: CYP1A2: Ciprofloxacin, enoxacin, fluvoxamine, zafirlukast CYP2C8: clopidogrel, gemfibrozil CYP2C9: fluconazole CYP2C19: fluconazole, fluoxetine, fluvoxamine, ticlopidine CYP2D6: bupropion, fluoxetine, paroxetine, quinidine, terbinafine, cinacalcet CYP3A4: boceprevir, cobicistat, conivaptan, danoprevir, elvitegravir, ritonavir, grapefruit juice, indinavir, itraconazole, ketoconazole, lopinavir, paritaprevir, posaconazole, ritonavir, saquinavir, telaprevir, tipranavir, troleandomycin, voriconazole, clarithromycin, diltiazem, idelalisib, nefazodone, nelfinavir, itraconazole, ketoconazole Major CYP inducers: CYP2B6: carbamazepine CYP2C9: carbamazepine, enzalutamide CYP2C19: enzalutamide, rifampicin, ritonavir CYP3A4: carbamazepine, enzalutamide, mitotane, phenytoin, rifampin, St. John’s wort.10,41,58,93
AUC, area under the curve; CYP, cytochrome P450 iso-enzyme; DDI, drug–drug interaction; EMA, European Medicines Agency; FMO, flavin-containing monooxygenase; GIST, gastrointestinal stromal tumor; MKI, multikinase inhibitor; NA, not applicable/not available; PBPK, physiologically based pharmacokinetic; UGT, UDP-glucuronosyltransferase; US FDA, United States Food and Drug Administration.
Interactions with novel MKIs
In the last decade there has been a significant increase in the development of and treatment with MKIs resulting in more than a doubling of registered MKIs in the past 5 years. Earlier, we described the DDIs with MKIs which were approved before 1 August 2013.6 Here, we give an extensive overview of the DDI potential and management of the novel MKIs, which have been approved after August 2013. A complete overview including all (new and older) MKIs is presented in Tables 1–3.
Afatinib
Afatinib is used in the treatment of non-small cell lung cancer (NSCLC). It is a substrate of P-gp and BCRP and is mainly metabolized through enzyme-catalyzed Michael adduct formation (phase II) and only in a minor extent to phase I enzymes like CYP3A4 and FMO (2%).14,15 Concomitant administration with ritonavir (a P-gp inhibitor) showed a 48% increase in AUC and 39% increase in Cmax.43 Treatment with a potent P-gp inducer (rifampicin) prior to single-dose afatinib showed a moderate effect on both afatinib AUC and Cmax (34% and 22% decrease respectively).43 When afatinib is administered with strong P-gp and BCRP inhibitors, staggered dosing may be used, preferably 6 h or 12 h apart from afatinib intake. When afatinib is administered with strong P-gp inducers the dose may be increased with 10 mg with close monitoring of side effects. Administration with strong CYP inducers or inhibitors is considered safe, since no CYP enzymes are involved in afatinib metabolism. Furthermore in vitro studies showed afatinib itself to be an inhibitor of P-gp and BCRP, so close monitoring of side effects when administered with substrates for these transporters with a narrow therapeutic window is recommended.14,15
Alectinib
The anaplastic lymphoma kinase (ALK) inhibitor alectinib is used in the treatment of metastatic lung cancer. Alectinib as well as its M4 metabolite are considered equally active. Alectinib is primary metabolized by CYP3A4.14,15 Co-administration with the strong CYP3A4 inhibitor posaconazole resulted in a 75% increase of AUC, while co-administration with rifampicin led to a 73% decrease in alectinib AUC.44 Since alectinib and M4 are equally active, a dose modification is not necessary (unless patients experience a significant increase in toxicity) when alectinib is administered with strong inhibitors or inducers of CYP3A4. Since alectinib is a P-gp and BCRP inhibitor, close monitoring of side effects of these substrates is recommended, especially for drugs with a narrow therapeutic window (e.g. digoxin).
Bosutinib
Bosutinib is used in the treatment of chronic myeloid leukemia (CML). Although bosutinib is a P-gp substrate and inhibitor, DDIs are not likely to appear, since clinical studies demonstrated no significant effect on dabigatran (P-gp substrate) or bosutinib (when administered with the P-gp inhibitor lansoprazole) pharmacokinetics.18,45 Therefore no bosutinib dose reductions are necessary, when administered with strong P-gp inducers or inhibitors. Bosutinib is mainly metabolized through CYP3A4 and co-administration with the strong inhibitor ketoconazole resulted in 420% increase in Cmax and 760% increase in AUC.74 Administration with rifampicin showed a significant 86% reduction in Cmax and a 92% decrease in AUC of bosutinib. Administration with the moderate inhibitor aprepitant also showed an increase in AUC and Cmax.73 In conclusion; strong inhibitors or inducers of CYP3A4 must be avoided or a gradual 20% dose reduction should be applied, when co-administered with strong inhibitors of CYP3A4. Increasing the bosutinib dose is not useful, when co-administered with strong CYP3A4 inducers, since a maximal tolerated bosutinib dose of 600 mg is often not sufficient to compensate for the relatively large loss of exposure.14,15
Cabozantinib
Cabozantinib is used in the treatment of medullary thyroid carcinoma and renal cell carcinoma (RCC). Since cabozantinib is a P-gp and BCRP inhibitor, close monitoring of side effects of substrates with a narrow therapeutic window is recommended when co-administered with cabozantinib.14,15 A study with ketoconazole and rifampicin showed a significant change in AUC (38% increase and 77% decrease, respectively).75 There was no significant effect of cabozantinib on rosiglitazone (a CYP2C8 substrate) plasma pharmacokinetics, indicating no inhibitory effect on CYP2C8 in contrast to the in vitro data.75 The product label recommends minimizing the risk of a DDI by avoiding co-administration with strong inducers or inhibitors of CYP3A4. If necessary, a dose adjustment (decrease or increase) of 20 mg following a step-by-step approach may be warranted.
Ceritinib
Ceritinib is used in the treatment of ALK-positive NSCLC. Ceritinib is a substrate and inhibitor for P-gp. Furthermore, ceritinib is mainly metabolized by CYP3A4. Treatment with ketoconazole resulted in 190% and 20% increase in ceritinib AUC and Cmax, respectively.14,15 Co-administration with rifampicin showed a 70% and 44% decrease in AUC and Cmax, respectively.14,15 If concomitant administration with strong inhibitors of CYP3A4 is unavoidable a dose reduction by one third of the initial dose is necessary (rounded to units of 150 mg). For strong CYP3A4 inducers gradual dose escalation is possible with close monitoring of MKI-specific side effects.
Cobimetinib
Cobimetinib is a BRAF inhibitor used in the treatment of melanoma. It is a substrate for P-gp and inhibits BCRP, OATP1B1, OATP1B3, and OCT1.14,15 Therefore, close monitoring of side effects is warranted when cobimetinib is administered with BCRP (e.g. rosuvastatin), OATP1B1, OATP1B3 (e.g. atorvastatin) or OCT1 substrates (metformin) with a narrow therapeutic window. Cobimetinib is primarily metabolized by CYP3A4 and UGT2B7. When co-administered with itraconazole 570% and 220% increase in AUC and Cmax was seen, respectively.14,15 A physiologically based pharmacokinetic (PBPK) model demonstrated rifampicin to decrease cobimetinib AUC by 83% and Cmax by 63%.76 So, the co-administration with strong inhibitors or inducers of CYP3A4 and P-gp must be avoided. However, rabeprazole (a P-gp inhibitor) showed no effects on the pharmacokinetics of cobimetinib.21 If concomitant use of cobimetinib and strong CYP3A4 inhibitors is unavoidable, the cobimetinib dose should be decreased with 20 mg (33%) following a step-by-step approach. Furthermore, since cobimetinib is a CYP1A2 inhibitor, concomitant use with CYP1A2 substrates (e.g. haloperidol) may lead to altered plasma concentrations of these substrates.14,15
Dabrafenib
Dabrafenib is a BRAF inhibitor used in the treatment of advanced melanoma and NSCLC. Dabrafenib was shown to be a substrate for P-gp and BCRP. Since the bioavailability of dabrafenib is high (95%), only limited pharmacokinetic effects can be expected with inhibitors and inducers of these drug transporters. Dabrafenib is metabolized by both CYP3A4 (24%) and CYP2C8 (67%). Administration of dabrafenib with ketoconazole, gemfibrozil (a CYP2C8 inhibitor), and rifampicin showed significant changes in AUC, however these effects were mostly relatively small.14,15 Furthermore, dabrafenib is known to auto-induce CYP3A4 mediated metabolism.14,15 In conclusion, concomitant administration with strong CYP3A4 and CYP2C8 inhibitors or inducers must be avoided. Furthermore, a study with warfarin showed a 37% and 33% decrease in AUC and an 18% and 19% decrease in Cmax for S-warfarin (a CYP2C9 substrate) and R-warfarin (a CYP3A4/CYP1A2 substrate), respectively.78 Therefore, dabrafenib is characterized as a moderate CYP3A4 inducer and a weak CYP2C9 inducer and as a result concomitant use of substrates for these enzymes must be avoided.78
Ibrutinib
Ibrutinib is used as treatment for chronic lymphatic leukemia (CLL) and mantle cell lymphoma. Ibrutinib is an inhibitor of P-gp and BCRP.14,15 Ibrutinib is mainly metabolized by CYP3A4. Ketoconazole gave 2800% and 2300% increase in Cmax and AUC respectively.14,15,51 Furthermore concomitant administration with rifampicin showed 92% and 90% decrease in Cmax and AUC respectively.14,15 Administration with a moderate inhibitor of CYP3A4 (e.g. erythromycin) led to 240% and 200% increase in Cmax and AUC respectively.14,15,82 Overall concomitant administration with strong CYP3A4 inhibitors or inducers must be avoided. If ibrutinib is administered with moderate and strong CYP3A4 inhibitors the ibrutinib dose should be reduced to 280 mg and 140 mg respectively. When ibrutinib is administered with substrates of P-gp and BCRP monitoring of side effects of these substrates is warranted. When toxicity appears the dose of these substrates may be decreased.
Lenvatinib
Lenvatinib is used in the treatment of RCC and advanced thyroid carcinoma. It was shown to be a MDR1 substrate, a P-gp and BCRP substrate and inhibitor and an OATP1B3 inhibitor in vitro.14,15 When lenvatinib is administered with ketoconazole or rifampicin, only marginal changes in AUC and Cmax were observed.54,55 Since lenvatinib is mainly metabolized through several phase II mechanisms (e.g. aldehyde oxidase and glutathione conjugation) into less active metabolites and only for a small part by CYP3A4, these changes were most likely due to an interaction with P-gp.14,15 Lenvatinib has an overall low DDI potential and dose modifications are currently not considered necessary.
Nintedanib
Nintedanib is used in the treatment of NSCLC. It is a substrate and weak inhibitor of P-gp.14,15,94 When nintedanib is administered with a strong P-gp inhibitor, a 100 mg (25%) step-wise daily dose reduction must be considered with close monitoring of side effects. Use of strong P-gp inducers must be avoided, since nintedanib plasma concentrations may decrease. Nintedanib is mainly metabolized due to hydrolysis by esterases and glucuronidated by UGT with only a minor involvement of CYP enzymes (CYP3A4; 5%).14,15 Administration with ketoconazole resulted in 61% and 83% increase in AUC and Cmax respectively and administration with rifampicin demonstrated a decrease in AUC of 50% and 60% of Cmax respectively.42 These differences were probably due to a DDI with P-gp. Therefore, concomitant administration with strong inhibitors or inducers of CYP3A4 is considered safe.
Osimertinib
Osimertinib is used in the treatment of NSCLC.14,15 Osimertinib is a substrate and inhibitor for P-gp and BCRP.14,15 A study with rosuvastatin (a sensitive BCRP substrate) showed an increase in AUC and Cmax of 35% and 72% of rosuvastatin respectively.87 Osimertinib is mainly metabolized by CYP3A4 and CYP3A5, but only rifampicin resulted in a significant change in both AUC and Cmax in contrast to itraconazole.86 A study with simvastatin (a CYP3A4 substrate) resulted in a slight decrease in AUC and Cmax of simvastatin of 9% and 23%, but these changes are not considered to be of clinical significance.87 In conclusion only strong CYP3A4 inducers must be used with caution and close monitoring of side effects of osimertinib is warranted.
Ponatinib
Ponatinib is used in the treatment of CML and Acute lymphatic leukemia (ALL). Ponatinib is a substrate and inhibitor of P-gp and BCRP.14,15 Therefore, concomitant use of ponatinib with strong inhibitors or inducers of these transporters should be avoided. Ponatinib is mainly metabolized into nonactive metabolites by CYP3A4 and to a lesser extent by CYP2D6, CYP2C8 and CYP3A5.14,15 A study with concomitant ketoconazole administration showed an increase in Cmax of 47% and 78% in AUC of ponatinib.88 Multiple dosing of rifampicin demonstrated a decrease in AUC and Cmax of 42% and 62% respectively.89 As a consequence, concomitant administration with inhibitors of CYP3A4 and P-gp should be avoided or a dose reduction to 30 mg should be applied when administered concomitantly. Moreover, the use of strong CYP3A4 or P-gp inducers must be avoided or duration must be minimized, since ponatinib exposure may change.
Tivozanib
Tivozanib is used in the treatment of RCC. Tivozanib is an inhibitor of BCRP and is metabolized by multiple liver enzymes, including CYP3A4, CYP1A1 and several UGT1A enzymes (e.g. UGT1A1, UGT1A3 and UGT1A7).14,15 A study with rifampicin showed a 52% decrease in tivozanib AUC. Therefore, the administration with strong CYP3A4 inducers should be avoided. A dose escalation is not necessary since the effect on tivozanib exposure is relatively small. Ketoconazole did not result in significant changes in tivozanib exposure.14,15,91 Administration with strong CYP3A4 inhibitors is therefore considered safe. Furthermore, the concomitant administration with strong UGT inhibitors or inducers (e.g. probenecid or ibuprofen) should be avoided since tivozanib plasma concentrations potentially may change.
Trametinib
Trametinib is used in the treatment of melanoma and NSCLC. It is a known inhibitor of P-gp, BCRP, OAT1, OAT3, OATP1B1, OATP1B3, OAT2B1, OCT2 and MATE1 and a substrate for P-gp.14,15 As a result, the use of strong inhibitors or inducers of P-gp (e.g. ketoconazole) must be avoided. Trametinib is metabolized through deacetylation, oxidation and glucuronidation pathways.14,15 No drug interaction studies are available to date, however since trametinib is not dependent on CYP isoenzymes, no DDIs with CYPs are to be expected.
DDI studies with longer available MKIs
In recent years several new studies have been published that investigated DDIs with longer available MKIs. Most of these studies are listed in Tables 1–3. There are only a few clinical DDI studies concerning drug transporters, since most studies mainly focus on CYP interactions. A phase I study investigated the combination of gefitinib and irinotecan and found an increase in SN-38 (the active irinotecan metabolite) and irinotecan plasma exposure, attributed to an enhanced BCRP activity in the gut.50 Moreover, in patients using sorafenib with rifampicin, the concentration of the metabolite sorafenib-glucuronide increased, suggesting inhibition of OATP1B1 by rifampicin and confirms sorafenib as an OATP1B1 substrate.57
Several new studies investigated possible DDIs regarding drug metabolism. For a complete overview see Table 3. For example: imatinib co-administration caused a 26% increase in cyclosporine (CYP3A4 and CYP2C8 substrate) plasma levels, explained by CYP3A4 inhibition by imatinib.69 In addition, lapatinib and pazopanib demonstrated an increase of 23% and 26% in paclitaxel AUC respectively, suggesting inhibition of CYP2C8 by these MKIs.83,95 Furthermore, regorafenib significantly increased the exposure to irinotecan and its active metabolite SN-38 due to UGT1A1 inhibition.96,97
Although most MKIs are metabolized through CYP enzymes it becomes more apparent that MKI metabolism is multifactorial and the inhibition and induction of other pathways (such as drug transporters) may also significantly influence MKI exposure. More research is needed to fully assess the DDI potential of these new pathways and their clinical relevance.
Discussion
Many MKIs have a narrow therapeutic window, with a clear relation between exposure and response on one hand and toxicity on the other.98 For example, sunitinib and pazopanib show increasing severe toxicity with raising plasma concentration, leading to dose reductions and discontinuation of treatment in many patients.99,100 Meanwhile, a threshold for efficacy for these drugs is seen.98–100 Therefore, it is important to provide the right dose for the individual patient, in order to optimize treatment efficacy and minimize toxicity. To accomplish this, there is a shifting paradigm towards personalized dosing in oncology practice.5 Along with other factors, DDIs are key factors influencing MKI exposure and subsequent clinical outcome. In addition, cancer patients are at greater risk for DDIs.7 Therefore, a structured medication review for clinically relevant DDIs should take place on a regular basis.
To create a solid base for medication review, more DDI studies are strongly needed and results should be weighed on their clinical relevance. Specific and practical guidelines must be developed to guide clinicians and pharmacists in the management of DDIs in clinical practice. A practical way to reach this goal is by establishing clinical expert groups for consensus-based evaluation of clinical significance and management of the DDIs.101
ASAs may strongly decrease MKI bioavailability. Since there is no clear general consensus on the management of this DDI we presented a practical advice for all ASAs. However, another problem is that there is no standard design for clinical DDI research with ASAs. Ideally, drug exposure should be compared in a crossover design between MKI monotherapy and during co-administration of the strongest ASA [e.g. the PPI esomeprazole (40 mg)] 3 h prior to MKI administration, since maximum intragastric pH elevating effect of this PPI is reached after this time period.38 In that case, when no effects are seen, a DDI between MKIs and PPIs can be ruled out. When a significant DDI with H2-antagonists and antacids is expected, a corresponding treatment arm may be added. A more standardized study design of these ASA-DDI studies may provide a solid basis for practical management of this DDI, since study results could more easily be interpreted and compared between different MKIs.
Drug transporters are located throughout the body and thus potentially influence pharmacokinetics on multiple levels.39 To date, insufficient attention has been given to the clinical relevance of these DDIs concerning drug transporters. Unfortunately, there is a lack of clinical studies investigating this type of DDI. Furthermore, many registration studies use ketoconazole or rifampicin as an inhibitor or inducer of CYP3A4, but these drugs are also strong inhibitors or inducers of P-gp. As a result, the P-gp effect may be underestimated or overestimated in the assessment reports. More research is needed to fully assess the DDI potential concerning drug transporters.
In contrast, DDIs with drug transporters may also be used for beneficial purposes. For instance, inhibition of certain drug transporters (e.g. P-gp) in the blood–brain barrier might theoretically lead to altered blood–brain barrier penetration, which may result in better brain (metastasis) penetration of a MKI, for example, osimertinib.102 In addition, Zimmerman and colleagues demonstrated a protective effect on hand-foot skin reaction in mice, a frequently seen side effect of sorafenib, when sorafenib was concomitantly taken with the OAT6 inhibitor probenecid.103 Furthermore erlotinib may reduce cisplatin toxicity (e.g. nephrotoxicity and ototoxicity) through OCT2 inhibition.48 Such potentially useful applications of DDIs between MKIs and drug transporters need to be further explored, and may in the future result in more effective MKI therapy.
In current DDI research there is a trend towards a model-based DDI prediction, like the PBPK-models.104,105 PBPK-models are multi-compartmental (often represented as single organs or tissues) models which use (in vitro) pharmacokinetic data and human physiologically-dependent system parameters to predict DDIs with a mathematical model.106 A disadvantage of PBPK modeling is the lack of sufficient in vivo data that adds to the uncertainty in the predictions of the PBPK model. Also, the lack of knowledge regarding multifactorial physiologic changes in, for instance, enzyme and transporter expression and activity might be a possible confounding factor. Despite the evident benefits of PBPK modeling in current DDI research, confirmatory evidence from clinical trials in humans is needed to assess a good predicting model.105
Another novel approach in oncology in managing DDIs is therapeutic drug monitoring (TDM). For many MKIs there is a clear relationship between exposure, toxicity and treatment efficacy (e.g. imatinib, pazopanib and sunitinib).98,100,107 For some MKIs TDM could be an alternative way to manage DDIs in MKI therapy, where dose adjustments can be made if plasma levels are outside the therapeutic range. Furthermore, TDM has the advantage of monitoring MKI treatment, continuously over a longer time period which may result in better therapy efficacy. However, further research is needed to confirm the clinical relevance of TDM as a tool in DDI management.
In conclusion, most MKIs are highly prone to cause DDIs. Drugs that elevate intragastric pH, strong inhibitors or inducers of CYP enzymes and drug transporters can result in clinically relevant changes in MKI exposure. For many DDIs the only evidence for a potential DDI comes from in vitro data or is predicted based on PBPK modeling. Without clinical data it is difficult to determine the exact clinical relevance of these possible DDIs. In this review, we present practical recommendations for management of MKI interactions in clinical practice. Acknowledging these DDIs by clinicians may eventually result in a more personalized and effective treatment with MKIs.
Acknowledgments
We thank Egied C.C.M. Simons (Erasmus Medical Centere, The Netherlands) for the graphic design of the figures.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Koen G. A. M. Hussaarts, Department of Medical Oncology, Erasmus MC Cancer Institute, Erasmus University Medical Center, Dr. Molewaterplein 40, 3015 GD Rotterdam, The Netherlands.
G. D. Marijn Veerman, Department of Medical Oncology, Erasmus MC Cancer Institute, Rotterdam, The Netherlands.
Frank G. A. Jansman, Department of Clinical Pharmacy, Deventer Hospital, Deventer, The Netherlands Groningen Research Institute of Pharmacy, Pharmacotherapy, Epidemiology & Economics, University of Groningen, Groningen, The Netherlands.
Teun van Gelder, Department of Hospital Pharmacy, Erasmus MC, Rotterdam, The Netherlands.
Ron H. J. Mathijssen, Department of Medical Oncology, Erasmus MC Cancer Institute, Rotterdam, The Netherlands
Roelof W. F. van Leeuwen, Department of Medical Oncology, Erasmus MC Cancer Institute, Rotterdam, The Netherlands Department of Hospital Pharmacy, Erasmus MC, Rotterdam, The Netherlands.
References
- 1. Krause DS, Van Etten RA. Tyrosine kinases as targets for cancer therapy. N Engl J Med 2005; 353: 172–187. [DOI] [PubMed] [Google Scholar]
- 2. Yu H, Steeghs N, Nijenhuis CM, et al. Practical guidelines for therapeutic drug monitoring of anticancer tyrosine kinase inhibitors: focus on the pharmacokinetic targets. Clin Pharmacokinet 2014; 53: 305–325. [DOI] [PubMed] [Google Scholar]
- 3. Shah DR, Shah RR, Morganroth J. Tyrosine kinase inhibitors: their on-target toxicities as potential indicators of efficacy. Drug Saf 2013; 36: 413–426. [DOI] [PubMed] [Google Scholar]
- 4. Chatelut E, Bruno R, Ratain MJ. Intraindividual pharmacokinetic variability: focus on small-molecule kinase inhibitors. Clin Pharmacol Ther 2018; 103: 956–958. [DOI] [PubMed] [Google Scholar]
- 5. Mathijssen RH, Sparreboom A, Verweij J. Determining the optimal dose in the development of anticancer agents. Nat Rev Clin Oncol 2014; 11: 272–281. [DOI] [PubMed] [Google Scholar]
- 6. van Leeuwen RW, van Gelder T, Mathijssen RH, et al. Drug–drug interactions with tyrosine-kinase inhibitors: a clinical perspective. Lancet Oncol 2014; 15: e315–e326. [DOI] [PubMed] [Google Scholar]
- 7. van Leeuwen RW, Brundel DH, Neef C, et al. Prevalence of potential drug–drug interactions in cancer patients treated with oral anticancer drugs. Br J Cancer 2013; 108: 1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scripture CD, Figg WD. Drug interactions in cancer therapy. Nat Rev Cancer 2006; 6: 546–558. [DOI] [PubMed] [Google Scholar]
- 9. Visentin M, Biason P, Toffoli G. Drug interactions among the epidermal growth factor receptor inhibitors, other biologics and cytotoxic agents. Pharmacol Ther 2010; 128: 82–90. [DOI] [PubMed] [Google Scholar]
- 10. Food and Drug Administration. Clinical drug interaction studies — study design, data analysis, and clinical implications. https://www.fda.gov/downloads/drugs/guidances/ucm292362.pdf (Published: 24 october 2017) (accessed 28 September 2018).
- 11. Budha NR, Frymoyer A, Smelick GS, et al. Drug absorption interactions between oral targeted anticancer agents and PPIs: is pH-dependent solubility the Achilles heel of targeted therapy? Clin Pharmacol Ther 2012; 92: 203–213. [DOI] [PubMed] [Google Scholar]
- 12. Tang W, Tomkinson H, Masson E. Effect of sustained elevated gastric pH levels on gefitinib exposure. Clin Pharmacol Drug Dev 2017; 6: 517–523. [DOI] [PubMed] [Google Scholar]
- 13. van Leeuwen RWF, Jansman FGA, Hunfeld NG, et al. Tyrosine kinase inhibitors and proton pump inhibitors: an evaluation of treatment options. Clin Pharmacokinet 2017; 56: 683–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. European Medicines Agency. European public assessment reports assessment history and product information. https://www.ema.europa.eu/ (accessed September 2017–July 2018).
- 15. U.S. Food and Drug Administration. Product reviews and labels. https://www.fda.gov/Drugs/default.htm (accessed September 2017–July 2018).
- 16. Morcos PN, Guerini E, Parrott N, et al. Effect of food and esomeprazole on the pharmacokinetics of alectinib, a highly selective ALK inhibitor, in healthy subjects. Clin Pharmacol Drug Dev 2017; 6: 388–397. [DOI] [PubMed] [Google Scholar]
- 17. Rugo HS, Herbst RS, Liu G, et al. Phase I trial of the oral antiangiogenesis agent AG-013736 in patients with advanced solid tumors: pharmacokinetic and clinical results. J Clin Oncol 2005; 23: 5474–5483. [DOI] [PubMed] [Google Scholar]
- 18. Abbas R, Leister C, Sonnichsen D. A clinical study to examine the potential effect of lansoprazole on the pharmacokinetics of bosutinib when administered concomitantly to healthy subjects. Clin Drug Investig 2013; 33: 589–595. [DOI] [PubMed] [Google Scholar]
- 19. Nguyen L, Holland J, Mamelok R, et al. Evaluation of the effect of food and gastric pH on the single-dose pharmacokinetics of cabozantinib in healthy adult subjects. J Clin Pharmacol 2015; 55: 1293–1302. [DOI] [PubMed] [Google Scholar]
- 20. Lau YY, Gu W, Lin T, et al. Assessment of drug–drug interaction potential between ceritinib and proton pump inhibitors in healthy subjects and in patients with ALK-positive non-small cell lung cancer. Cancer Chemother Pharmacol 2017; 79: 1119–1128. [DOI] [PubMed] [Google Scholar]
- 21. Musib L, Choo E, Deng Y, et al. Absolute bioavailability and effect of formulation change, food, or elevated pH with rabeprazole on cobimetinib absorption in healthy subjects. Mol Pharm 2013; 10: 4046–4054. [DOI] [PubMed] [Google Scholar]
- 22. Eley T, Luo FR, Agrawal S, et al. Phase I study of the effect of gastric acid pH modulators on the bioavailability of oral dasatinib in healthy subjects. J Clin Pharmacol 2009; 49: 700–709. [DOI] [PubMed] [Google Scholar]
- 23. van Leeuwen RWF, Peric R, Hussaarts KGAM, et al. Influence of the acidic beverage cola on the absorption of erlotinib in patients with non-small-cell lung cancer. J Clin Oncol 2016; 34: 1309–1314. [DOI] [PubMed] [Google Scholar]
- 24. Kletzl H, Giraudon M, Ducray PS, et al. Effect of gastric pH on erlotinib pharmacokinetics in healthy individuals: omeprazole and ranitidine. Anticancer Drugs 2015; 26: 565–572. [DOI] [PubMed] [Google Scholar]
- 25. Yokota H, Sato K, Okuda Y, et al. Effects of histamine 2-receptor antagonists and proton pump inhibitors on the pharmacokinetics of gefitinib in patients with non-small-cell lung cancer. Clin Lung Cancer 2017; 18: e433–e439. [DOI] [PubMed] [Google Scholar]
- 26. de Jong J, Haddish-Berhane N, Hellemans P, et al. The pH-altering agent omeprazole affects rate but not the extent of ibrutinib exposure. Cancer Chemother Pharmacol. Epub ahead of print 7 June 2018. DOI: 10.1007/s00280-018-3613-9. [DOI] [PubMed] [Google Scholar]
- 27. Sparano BA, Egorin MJ, Parise RA, et al. Effect of antacid on imatinib absorption. Cancer Chemother Pharmacol 2009; 63: 525–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Egorin MJ, Shah DD, Christner SM, et al. Effect of a proton pump inhibitor on the pharmacokinetics of imatinib. Br J Clin Pharmacol 2009; 68: 370–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yin OQ, Bedoucha V, McCulloch T, et al. Effects of famotidine or an antacid preparation on the pharmacokinetics of nilotinib in healthy volunteers. Cancer Chemother Pharmacol 2013; 71: 219–226. [DOI] [PubMed] [Google Scholar]
- 30. Yin OQP, Gallagher N, Fischer D, et al. Effect of the proton pump inhibitor esomeprazole on the oral absorption and pharmacokinetics of nilotinib. J Clin Pharmacol 2010; 50: 960–967. [DOI] [PubMed] [Google Scholar]
- 31. Yin OQ, Giles FJ, Baccarani M, et al. Concurrent use of proton pump inhibitors or H2 blockers did not adversely affect nilotinib efficacy in patients with chronic myeloid leukemia. Cancer Chemother Pharmacol 2012; 70: 345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tan AR, Gibbon DG, Stein MN, et al. Effects of ketoconazole and esomeprazole on the pharmacokinetics of pazopanib in patients with solid tumors. Cancer Chemother Pharmacol 2013; 71: 1635–1643. [DOI] [PubMed] [Google Scholar]
- 33. Narasimhan NI, Dorer DJ, Davis J, et al. Evaluation of the effect of multiple doses of lansoprazole on the pharmacokinetics and safety of ponatinib in healthy subjects. Clin Drug Investig 2014; 34: 723–729. [DOI] [PubMed] [Google Scholar]
- 34. Olivier M, Romain C, Angelo P, et al. Impact of proton pump inhibitors (PPIs) on sunitinib (SU) pharmacokinetics (PK) and activity in GIST patients (pts). J Clin Oncol 2018; 36(Suppl.15): 11538. [Google Scholar]
- 35. Johansson S, Read J, Oliver S, et al. Pharmacokinetic evaluations of the co-administrations of vandetanib and metformin, digoxin, midazolam, omeprazole or ranitidine. Clin Pharmacokinet 2014; 53: 837–847. [DOI] [PubMed] [Google Scholar]
- 36. Smelick GS, Heffron TP, Chu L, et al. Prevalence of acid-reducing agents (ARA) in cancer populations and ARA drug–drug interaction potential for molecular targeted agents in clinical development. Mol Pharm 2013; 10: 4055–4062. [DOI] [PubMed] [Google Scholar]
- 37. Malfertheiner P, Kandulski A, Venerito M. Proton-pump inhibitors: understanding the complications and risks. Nat Rev Gastroenterol Hepatol 2017; 14: 697–710. [DOI] [PubMed] [Google Scholar]
- 38. Hunfeld NG, Touw DJ, Mathot RA, et al. A comparison of the acid-inhibitory effects of esomeprazole and pantoprazole in relation to pharmacokinetics and CYP2C19 polymorphism. Aliment Pharmacol Ther 2010; 31: 150–159. [DOI] [PubMed] [Google Scholar]
- 39. Nigam SK. What do drug transporters really do? Nat Rev Drug Discov 2015; 14: 29–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Roberts MS, Magnusson BM, Burczynski FJ, et al. Enterohepatic circulation: physiological, pharmacokinetic and clinical implications. Clin Pharmacokinet 2002; 41: 751–790. [DOI] [PubMed] [Google Scholar]
- 41. U.S. Food and Drug Administration. Drug development and drug interactions. U.S. Food and Drug Administration, 2018. https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/DrugInteractionsLabeling/ucm080499.htm (accessed January–June 2018). [Google Scholar]
- 42. Luedtke D, Marzin K, Jungnik A, et al. Effects of ketoconazole and rifampicin on the pharmacokinetics of nintedanib in healthy subjects. Eur J Drug Metab Pharmacokinet 2018; 43: 533–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wind S, Giessmann T, Jungnik A, et al. Pharmacokinetic drug interactions of afatinib with rifampicin and ritonavir. Clin Drug Investig 2014; 34: 173–182. [DOI] [PubMed] [Google Scholar]
- 44. Morcos PN, Cleary Y, Guerini E, et al. Clinical drug–drug interactions through cytochrome P450 3A (CYP3A) for the selective ALK inhibitor alectinib. Clin Pharmacol Drug Dev 2017; 6: 280–291. [DOI] [PubMed] [Google Scholar]
- 45. Hsyu P-H, Pignataro DS, Matschke K. Effect of bosutinib on the absorption of dabigatran etexilate mesylate, a P-glycoprotein substrate, in healthy subjects. Eur J Clin Pharmacol 2017; 73: 57–63. [DOI] [PubMed] [Google Scholar]
- 46. Haouala A, Widmer N, Duchosal MA, et al. Drug interactions with the tyrosine kinase inhibitors imatinib, dasatinib, and nilotinib. Blood 2011; 117: e75–e87. [DOI] [PubMed] [Google Scholar]
- 47. Marchetti S, de Vries NA, Buckle T, et al. Effect of the ATP-binding cassette drug transporters ABCB1, ABCG2, and ABCC2 on erlotinib hydrochloride (Tarceva) disposition in in vitro and in vivo pharmacokinetic studies employing Bcrp1-/-/Mdr1a/1b-/- (triple-knockout) and wild-type mice. Mol Cancer Ther 2008; 7: 2280–2287. [DOI] [PubMed] [Google Scholar]
- 48. Sprowl JA, Mathijssen RH, Sparreboom A. Can erlotinib ameliorate cisplatin-induced toxicities? J Clin Oncol 2013; 31: 3442–3443. [DOI] [PubMed] [Google Scholar]
- 49. Elmeliegy MA, Carcaboso AM, Tagen M, et al. Role of ATP-binding cassette and solute carrier transporters in erlotinib CNS penetration and intracellular accumulation. Clin Cancer Res 2011; 17: 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stewart CF, Leggas M, Schuetz JD, et al. Gefitinib enhances the antitumor activity and oral bioavailability of irinotecan in mice. Cancer Res 2004; 64: 7491–7499. [DOI] [PubMed] [Google Scholar]
- 51. de Jong J, Skee D, Murphy J, et al. Effect of CYP3A perpetrators on ibrutinib exposure in healthy participants. Pharmacol Res Perspect 2015; 3: e00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Eechoute K, Sparreboom A, Burger H, et al. Drug transporters and imatinib treatment: implications for clinical practice. Clin Cancer Res 2011; 17: 406–415. [DOI] [PubMed] [Google Scholar]
- 53. Koch KM, Smith DA, Botbyl J, et al. Effect of lapatinib on oral digoxin absorption in patients. Clin Pharmacol Drug Dev 2015; 4: 449–453. [DOI] [PubMed] [Google Scholar]
- 54. Shumaker R, Aluri J, Fan J, et al. Effects of ketoconazole on the pharmacokinetics of lenvatinib (E7080) in healthy participants. Clin Pharmacol Drug Dev 2015; 4: 155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shumaker RC, Aluri J, Fan J, et al. Effect of rifampicin on the pharmacokinetics of lenvatinib in healthy adults. Clin Drug Investig 2014; 34: 651–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lemos C, Jansen G, Peters GJ. Drug transporters: recent advances concerning BCRP and tyrosine kinase inhibitors. Br J Cancer 2008; 98: 857–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bins S, van Doorn L, Phelps MA, et al. Influence of OATP1B1 function on the disposition of sorafenib-beta-D-glucuronide. Clin Transl Sci 2017; 10: 271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Konig J, Muller F, Fromm MF. Transporters and drug–drug interactions: important determinants of drug disposition and effects. Pharmacol Rev 2013; 65: 944–966. [DOI] [PubMed] [Google Scholar]
- 59. Zhang W, McIntyre C, Kuhn M, et al. Effect of vemurafenib on the pharmacokinetics of a single dose of digoxin in patients with BRAF(V600) mutation-positive metastatic malignancy. J Clin Pharmacol. Epub ahead of print 12 April 2018. DOI: 10.1002/jcph.1111. [DOI] [PubMed] [Google Scholar]
- 60. Tiwari AK, Sodani K, Dai CL, et al. Nilotinib potentiates anticancer drug sensitivity in murine ABCB1-, ABCG2-, and ABCC10-multidrug resistance xenograft models. Cancer Lett 2013; 328: 307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. White DL, Saunders VA, Quinn SR, et al. Imatinib increases the intracellular concentration of nilotinib, which may explain the observed synergy between these drugs. Blood 2007; 109: 3609–3610. [DOI] [PubMed] [Google Scholar]
- 62. Veronese ML, Gillen LP, Burke JP, et al. Exposure-dependent inhibition of intestinal and hepatic CYP3A4 in vivo by grapefruit juice. J Clin Pharmacol 2003; 43: 831–839. [DOI] [PubMed] [Google Scholar]
- 63. van Erp NP, Baker SD, Zandvliet AS, et al. Marginal increase of sunitinib exposure by grapefruit juice. Cancer Chemother Pharmacol 2011; 67: 695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jancova P, Anzenbacher P, Anzenbacherova E. Phase II drug metabolizing enzymes. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2010; 154: 103–116. [DOI] [PubMed] [Google Scholar]
- 65. Teo YL, Ho HK, Chan A. Metabolism-related pharmacokinetic drug−drug interactions with tyrosine kinase inhibitors: current understanding, challenges and recommendations. Br J Clin Pharmacol 2015; 79: 241–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. European Medicines Agency. Clinical efficacy and safety: clinical pharmacology and pharmacokinetics, http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_000370.jspmid=WC0b01ac0580032ec5 Published: 30 june 2017 (accessed January–June 2018).
- 67. Wang Y, Zhou L, Dutreix C, et al. Effects of imatinib (Glivec) on the pharmacokinetics of metoprolol, a CYP2D6 substrate, in Chinese patients with chronic myelogenous leukaemia. Br J Clin Pharmacol 2008; 65: 885–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. O’Brien SG, Meinhardt P, Bond E, et al. Effects of imatinib mesylate (STI571, Glivec) on the pharmacokinetics of simvastatin, a cytochrome p450 3A4 substrate, in patients with chronic myeloid leukaemia. Br J Cancer 2003; 89: 1855–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Atiq F, Broers AEC, Andrews LM, et al. A clinically relevant pharmacokinetic interaction between cyclosporine and imatinib. Eur J Clin Pharmacol 2016; 72: 719–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hamberg P, Mathijssen RH, de Bruijn P, et al. Impact of pazopanib on docetaxel exposure: results of a phase I combination study with two different docetaxel schedules. Cancer Chemother Pharmacol 2015; 75: 365–371. [DOI] [PubMed] [Google Scholar]
- 71. Pithavala YK, Tortorici M, Toh M, et al. Effect of rifampin on the pharmacokinetics of Axitinib (AG-013736) in Japanese and Caucasian healthy volunteers. Cancer Chemother Pharmacol 2010; 65: 563–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pithavala YK, Tong W, Mount J, et al. Effect of ketoconazole on the pharmacokinetics of axitinib in healthy volunteers. Invest New Drugs 2012; 30: 273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hsyu P-H, Pignataro DS, Matschke K. Effect of aprepitant, a moderate CYP3A4 inhibitor, on bosutinib exposure in healthy subjects. Eur J Clin Pharmacol 2017; 73: 49–56. [DOI] [PubMed] [Google Scholar]
- 74. Abbas R, Hug BA, Leister C, et al. Effect of ketoconazole on the pharmacokinetics of oral bosutinib in healthy subjects. J Clin Pharmacol 2011; 51: 1721–1727. [DOI] [PubMed] [Google Scholar]
- 75. Nguyen L, Holland J, Miles D, et al. Pharmacokinetic (PK) drug interaction studies of cabozantinib: effect of CYP3A inducer rifampin and inhibitor ketoconazole on cabozantinib plasma PK and effect of cabozantinib on CYP2C8 probe substrate rosiglitazone plasma PK. J Clin Pharmacol 2015; 55: 1012–1023. [DOI] [PubMed] [Google Scholar]
- 76. Budha NR, Ji T, Musib L, et al. Evaluation of cytochrome P450 3A4-mediated drug–drug interaction potential for cobimetinib using physiologically based pharmacokinetic modeling and simulation. Clin Pharmacokinet 2016; 55: 1435–1445. [DOI] [PubMed] [Google Scholar]
- 77. Xu H, O’Gorman M, Tan W, et al. The effects of ketoconazole and rifampin on the single-dose pharmacokinetics of crizotinib in healthy subjects. Eur J Clin Pharmacol 2015; 71: 1441–1449. [DOI] [PubMed] [Google Scholar]
- 78. Suttle AB, Grossmann KF, Ouellet D, et al. Assessment of the drug interaction potential and single- and repeat-dose pharmacokinetics of the BRAF inhibitor dabrafenib. J Clin Pharmacol 2015; 55: 392–400. [DOI] [PubMed] [Google Scholar]
- 79. Johnson FM, Agrawal S, Burris H, et al. Phase 1 pharmacokinetic and drug interaction study of dasatinib in patients with advanced solid tumors. Cancer 2010; 116: 1582–1591. [DOI] [PubMed] [Google Scholar]
- 80. Hamilton M, Wolf JL, Drolet DW, et al. The effect of rifampicin, a prototypical CYP3A4 inducer, on erlotinib pharmacokinetics in healthy subjects. Cancer Chemother Pharmacol 2014; 73: 613–621. [DOI] [PubMed] [Google Scholar]
- 81. Swaisland HC, Ranson M, Smith RP, et al. Pharmacokinetic drug interactions of gefitinib with rifampicin, itraconazole and metoprolol. Clin Pharmacokinet 2005; 44: 1067–1081. [DOI] [PubMed] [Google Scholar]
- 82. de Jong J, Hellemans P, De Wilde S, et al. A drug–drug interaction study of ibrutinib with moderate/strong CYP3A inhibitors in patients with B-cell malignancies. Leuk Lymphoma. Epub ahead of print 30 May 2018. DOI: 10.1080/10428194.2018.1460474. [DOI] [PubMed] [Google Scholar]
- 83. Tan AR, Dowlati A, Stein MN, et al. Phase I study of weekly paclitaxel in combination with pazopanib and lapatinib in advanced solid malignancies. Br J Cancer 2014; 110: 2647–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Koch KM, Dees EC, Coker SA, et al. The effects of lapatinib on CYP3A metabolism of midazolam in patients with advanced cancer. Cancer Chemother Pharmacol 2017; 80: 1141–1146. [DOI] [PubMed] [Google Scholar]
- 85. Zhang H, Sheng J, Ko JH, et al. Inhibitory effect of single and repeated doses of nilotinib on the pharmacokinetics of CYP3A substrate midazolam. J Clin Pharmacol 2015; 55: 401–408. [DOI] [PubMed] [Google Scholar]
- 86. Vishwanathan K, Dickinson PA, So K, et al. The effect of itraconazole and rifampicin on the pharmacokinetics of osimertinib. Br J Clin Pharmacol 2018; 84: 1156–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Harvey RD, Isambert N, Rafii S, et al. Effect of multiple-dose osimertinib (AZD9291) on the pharmacokinetics (PK) of simvastatin and rosuvastatin. J Clin Oncol 2016; 34(15 Suppl.): e14098. [Google Scholar]
- 88. Narasimhan NI, Dorer DJ, Niland K, et al. Effects of ketoconazole on the pharmacokinetics of ponatinib in healthy subjects. J Clin Pharmacol 2013; 53: 974–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Narasimhan NI, Dorer DJ, Davis J, et al. Evaluation of the effect of multiple doses of rifampin on the pharmacokinetics and safety of ponatinib in healthy subjects. Clin Pharmacol Drug Dev 2015; 4: 354–360. [DOI] [PubMed] [Google Scholar]
- 90. Shi JG, Chen X, Emm T, et al. The effect of CYP3A4 inhibition or induction on the pharmacokinetics and pharmacodynamics of orally administered ruxolitinib (INCB018424 phosphate) in healthy volunteers. J Clin Pharmacol 2012; 52: 809–818. [DOI] [PubMed] [Google Scholar]
- 91. Cotreau MM, Siebers NM, Miller J, et al. Effects of ketoconazole or rifampin on the pharmacokinetics of tivozanib hydrochloride, a vascular endothelial growth factor receptor tyrosine kinase inhibitor. Clin Pharmacol Drug Dev 2015; 4: 137–142. [DOI] [PubMed] [Google Scholar]
- 92. Martin P, Oliver S, Robertson J, et al. Pharmacokinetic drug interactions with vandetanib during coadministration with rifampicin or itraconazole. Drugs R D 2011; 11: 37–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. The trustees of the Indiana University. Flockhart TableTM. Drug interactions. Indiana University, School of Medicine, Department of Medicine, https://drug-interactions.medicine.iu.edu/Main-Table.aspx Publication date latest version: 12 April 2018 (accessed January–June 2018). [Google Scholar]
- 94. Xiang Q-F, Wang F, Su X-D, et al. Effect of BIBF 1120 on reversal of ABCB1-mediated multidrug resistance. Cell Oncol (Dordr) 2011; 34: 33–44. [DOI] [PubMed] [Google Scholar]
- 95. Inoue K, Kuroi K, Shimizu S, et al. Safety, pharmacokinetics and efficacy findings in an open-label, single-arm study of weekly paclitaxel plus lapatinib as first-line therapy for Japanese women with HER2-positive metastatic breast cancer. Int J Clin Oncol 2015; 20: 1102–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Schultheis B, Folprecht G, Kuhlmann J, et al. Regorafenib in combination with FOLFOX or FOLFIRI as first- or second-line treatment of colorectal cancer: results of a multicenter, phase Ib study. Ann Oncol 2013; 24: 1560–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. de Man FM, Goey AKL, van Schaik RHN, et al. Individualization of irinotecan treatment: a review of pharmacokinetics, pharmacodynamics, and pharmacogenetics. Clin Pharmacokinet 2018; 57: 1229–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Verheijen RB, Yu H, Schellens JHM, et al. Practical recommendations for therapeutic drug monitoring of kinase inhibitors in oncology. Clin Pharmacol Ther 2017; 102: 765–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Teo YL, Chue XP, Chau NM, et al. Association of drug exposure with toxicity and clinical response in metastatic renal cell carcinoma patients receiving an attenuated dosing regimen of sunitinib. Target Oncol 2015; 10: 429–437. [DOI] [PubMed] [Google Scholar]
- 100. Suttle AB, Ball HA, Molimard M, et al. Relationships between pazopanib exposure and clinical safety and efficacy in patients with advanced renal cell carcinoma. Br J Cancer 2014; 111: 1909–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Jansman FG, Reyners AK, van Roon EN, et al. Consensus-based evaluation of clinical significance and management of anticancer drug interactions. Clin Ther 2011; 33: 305–314. [DOI] [PubMed] [Google Scholar]
- 102. Deeken JF, Loscher W. The blood–brain barrier and cancer: transporters, treatment, and Trojan horses. Clin Cancer Res 2007; 13: 1663–1674. [DOI] [PubMed] [Google Scholar]
- 103. Zimmerman EI, Gibson AA, Hu S, et al. Multikinase inhibitors induce cutaneous toxicity through OAT6-mediated uptake and MAP3K7-driven cell death. Cancer Res 2016; 76: 117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Sager JE, Yu J, Ragueneau-Majlessi I, et al. Physiologically Based Pharmacokinetic (PBPK) modeling and simulation approaches: a systematic review of published models, applications, and model verification. Drug Metab Dispos 2015; 43: 1823–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zhuang X, Lu C. PBPK modeling and simulation in drug research and development. Acta Pharm Sin B 2016; 6: 430–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Espie P, Tytgat D, Sargentini-Maier ML, et al. Physiologically based pharmacokinetics (PBPK). Drug Metab Rev 2009; 41: 391–407. [DOI] [PubMed] [Google Scholar]
- 107. Lankheet NA, Kloth JS, Gadellaa-van Hooijdonk CG, et al. Pharmacokinetically guided sunitinib dosing: a feasibility study in patients with advanced solid tumours. Br J Cancer 2014; 110: 2441–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]