Abstract
Background:
Hormonal contraception (HC) is widely used throughout the world and has been associated with venous thrombosis (VT) such as deep vein thrombosis, pulmonary emboli, and cerebral VT.
Objectives:
To provide a current comprehensive overview of the risk of objectively confirmed VT with HC in healthy women compared to nonusers.
Search methods:
PubMed was searched from inception to April 2018 for eligible studies in the English language, with hand searching from past systematic reviews.
Selection criteria:
We selected original research evaluating risk of objectively confirmed VT in healthy women taking oral or nonoral HC compared with nonusers.
Data collection:
The primary outcome of interest was a fatal or nonfatal VT in users of HC compared to nonusers or past users. Studies with at least twenty events were eligible. Adjusted relative risks with 95 percent confidence intervals were reported. Three independent reviewers extracted data from selected studies.
Results:
1,962 publications were retrieved through the search strategy, with 15 publications included. Users of oral contraception with levonorgesterol had increased risk of VT by a range of 2.79–4.07, while other oral hormonal preparations increased risk by 4.0–48.6. Levonorgestrel intrauterine devices did not increase risk. Etonogestrel/ethinyl estradiol vaginal rings increased the risk of VT by 6.5. Norelgestromin/ethinyl estradiol patches increased risk of VT by 7.9. Etonogestrel subcutaneous implants by 1.4 and depot-medroxyprogesterone by 3.6. The risk of fatal VT was increased in women aged fifteen to twenty-four by 18.8-fold.
Conclusion:
Users of HC have a significant increased risk of VT compared to nonusers. Current risks would project at least 300–400 healthy young women dying yearly in the United States due to HC. Women should be informed of these risks and offered education in fertility-awareness-based methods with comparable efficacy for family planning.
Summary:
HC is widely used throughout the world and has been associated with blood clots in the legs and lungs. We searched the literature and found the risks of currently used forms of birth control increased between three- and ninefold for blood clots for healthy women. The risks found would project 300–400 women dying from using HC each year in the United States.
Keywords: Hormonal contraception, Oral contraception, Pulmonary emboli, Systematic review, Venous thrombosis
Introduction
Hormonal contraception (HC) has widespread use, with estimates of 100 million women worldwide (World Health Organization [WHO] 1998). In the United States, about 21 percent of women of reproductive years, which equates to about 13 million women, are using pills, injections, implants, rings, or patches (Daniels 2015). Over 80 percent of women in the United States have taken HC at some point in the reproductive years. Venous thrombosis (VT), including deep vein thrombosis (DVT), pulmonary emboli (PE), or central venous thrombosis (CVT), is the most common serious side effect of HC. The 2013 Cochrane review showed a relative risk (RR) of VT of 3.5 for women using oral contraception compared to nonusers (de Bastos et al. 2014).
Despite the high prevalence of use of HC, randomized controlled trials for more than a few months have not been conducted due to the concern over the ethics of having women on placebo when trying to avoid pregnancy. Thus, the safety of HC is based mostly on observational and case control trials. Hormone replacement therapy (HRT) for menopause was similarly recommended based on health benefits seen in observational studies. When the women’s health initiative, a double-blinded, randomized controlled trial contradicted previous results and showed increased risks with HRT, practice patterns changed rapidly (Rossouw et al. 2002). HRT was associated with a small absolute risk to the individual, but looking at data across large populations, the benefits did not outweigh the harms. The drugs and doses used for HC have five to ten times the pharmacological effect of regimens used for HRT.
Continually monitoring the available safety data of HC is important for women to make informed choices for family planning. There has not been a recent systematic review comparing women using any form of HC to nonusers and evaluating for DVT, PE, and CVT. There have been large studies recently performed that have not been included in systematic reviews. Of note, the Cochrane review found that all risk estimates were higher in studies with objectively confirmed VT, of which none were industry sponsored (de Bastos et al. 2014). Our goal was to provide a comprehensive review of the risk of any type of VT with any form of HC in a healthy population of women.
Method
Literature Search
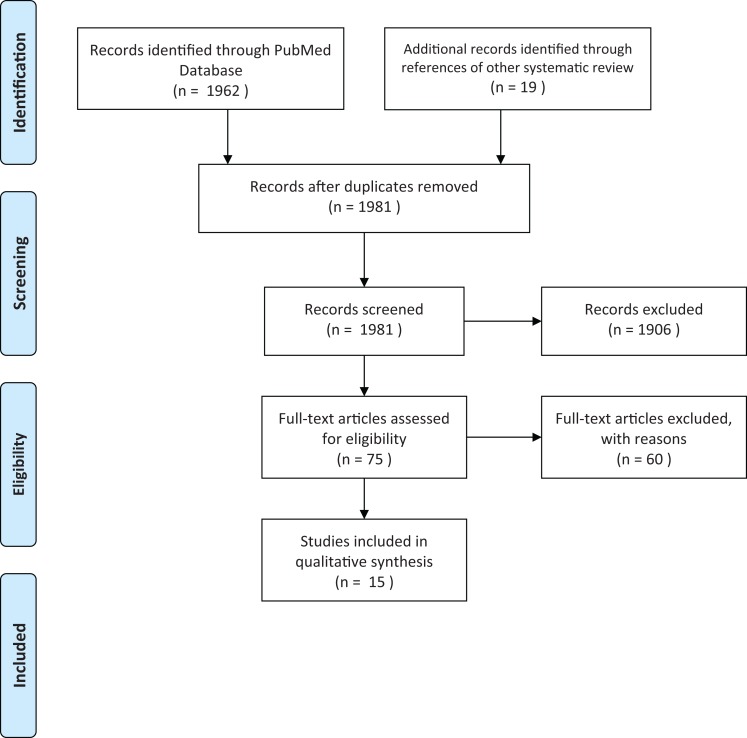
The PubMed database was searched for all relevant articles published from inception to April 2018 using the search terms ((((“Venous Thromboembolism”[Mesh]) OR “Pulmonary Embolism”[Mesh]) OR “Venous Thrombosis”[Mesh] OR ((vein[All Fields] OR venous[All Fields]) AND (thrombosis[All Fields] OR thromboemboism[All Fields])) OR pulmonary embolus[All Fields])) AND ((“Contraceptive Agents, Female”[Mesh]) OR “Contraceptive Devices, Female”[Mesh]). Two reviewers (L.K. and T.K.) searched these titles and abstracts to find original research studies in English. References from systematic reviews were also hand searched for relevant articles. We did not consider unpublished studies or abstracts of conference presentations. Each included article was then reviewed in detail (L.K., T.K., and K.V.G.) to extract main study characteristics and RRs with 95 percent confidence intervals. In cases of discrepancy, consensus was achieved through discussion (see Figure 1).
Figure 1.
Study flow diagram.
Selection criteria
Inclusion criteria were articles evaluating risk of HC for first VT in healthy women. Although most prior systematic reviews have included many articles comparing different forms of HC to each other, this systematic review is to provide data of risk of any form of HC to the natural healthy state. Articles were excluded if there was not a nonuser comparison group or if VT were not confirmed by either imaging study or at least strong clinical diagnosis with a minimum of four weeks of anticoagulation therapy. Studies were evaluated to avoid duplication of data from same women in two studies, taking the most recent or comprehensive study to be included in the review. Studies needed to report RRs or odds ratios (ORs) with 95 percent confidence intervals: RR in the cohort studies is the percentage of HC users with VT divided by the percentage of nonusers with VT. OR in the case control is the proportion of HC users who had a VT versus did not have a VT divided by the proportion of nonusers who had a VT versus did not have a VT.
Type of HC and generation of oral contraceptives had to be identified in the study as there is a significant difference in the risk of VT with the type of oral contraceptive. All oral contraceptives use ethinyl estradiol, which was originally at 150 µg, whereas current preparations mostly use 20–30 µg, though sometimes up to 50 µg. The generations vary mostly by the type of progestogen. First-generation oral contraceptives contain lynestrenol and are rarely used now. Second-generation pills contain levonorgestrel, or less often norgestrel. Third-generation ones have either desogestrel or gestodene as the progestogen. Another type combines ethinyl estradiol with drospirenone, an antimineralocorticoid which inhibits ovulation.
Some studies had subgroups with definite diagnosis of VT with imaging or anticoagulation and those with only clinical diagnosis. Those studies were included if risks were calculated separately for the confirmed VTs. Of the studies excluded, the most common reason was no nonuser comparison group, followed by data duplicated in other studies, lack of confirmation of VT, or of specification of type of HC.
Results
Of the 1,962 papers initially retrieved, 1,906 studies were excluded by reading title and abstract. Nineteen were added by reviewing reference lists of pertinent articles, leaving seventy-five studies potentially appropriate for the systematic review. Six studies were excluded due to repeated data. Fifty-three studies were excluded for not satisfying inclusion criteria. The remaining fifteen articles fit the criteria for evaluating the risk of first, idiopathic VT in a healthy population, with users of a specified type of hormonal contraceptive compared to nonusers, and confirmed diagnosis of VT by imaging or at least four weeks anticoagulation treatment. There were twelve case control studies (Andersen et al. 1998; Bloemenkamp et al. 1995, 1999; Lewis 1996; Lidegaard, Edstrom, and Kreiner 2002; Martinelli et al. 2016; Parkin et al. 2000; Sidney et al. 2004; van Hylckama Vlieg, Helmerhorst, and Rosendaal 2010; van Hylckama Vlieg et al. 2009; WHO 1995a, 1995b) and three cohort studies (Lidegaard et al. 2011; Lidegaard, Nielsen, et al. 2012; Samuelsson, Hedenmalm, and Persson 2005). The studies were published from 1995 to 2017, with study periods covering 1977–2014. The case control studies ranged in size from 36 cases to 1,524. The cohort studies ranged in size from 8 to 17.2 million-women-years of observation (see Table 1).
Table 1.
Comparison of Users versus Nonusers of Hormonal Contraception.
| Author, Year | Size | Users versus Nonusers | Pharm. Funding | Quality | ||||
|---|---|---|---|---|---|---|---|---|
| First Generation OC | Second Generation OC | Third Generation OC | Other | Type | ||||
| Case control studies | ||||||||
| Anderson, 1998 | 67/134 | 48.6 (5.6–423) | 5.2 (1.6–16.4) | Gen 1+2 | No | II-2, fair | ||
| Bloemenkamp, 1995 | 126/159 | 3.8 (1.2–12.5) | 8.7 (3.9–19.3) | No | II-2, fair | |||
| Bloemenkamp, 1999 | 185/591 | 8.7 (2.9–25.8) | 3.7 (1.9–7.2) | 4.9 (2.5–9.4)a | No | II-2, good | ||
| 5.2 (1.3–20.6)b | ||||||||
| Lewis, 1996 | 505/1,877 | 3.6 (2.6–4.8) | 5.3 (3.8–7.3) | Yes | II-2, good | |||
| Lidegaard, 2002 | 987/4,054 | 2.9 (2.2–3.8) | 4.0 (3.2–4.9) | Yes | II-2, good | |||
| Martinelli, 2016 | 1,020/887 | 7.5 (6.1–9.3) | Any type | Yes | II-2, fair | |||
| 8.8 (6.4–12.1) | <1 year | |||||||
| 6.9 (5.0–9.4) | 1–5 years | |||||||
| 6.1 (4.5–8.5) | <5 years | |||||||
| Sidney, 2004 | 196/746 | 4.07 (2.77–6.00) | Any type | no | II-2, good | |||
| 3.34 (2.04–5.46) | BMI < 30 | |||||||
| 6.04 (3.11–11.72) | BMI > 30 | |||||||
| van Hylckama Vlieg, 2009 | 1,524/1,760 | 3.7 (2.9–4.6) | 7.3 (5.3–10)a
|
6.3 (2.9–13.7) | Fourth generation | no | II-2, good | |
| 5.6 (3.7–8.4)c | 12.6 (7.1–22.4) | <3 months | ||||||
| van Hylckama Vlieg, 2010 | 446/1,146 | 3.6 (1.8–7.1) | DMPA | no | II-2, fair | |||
| 0.3 (0.1–1.1) | IUD | |||||||
| WHO, 1995a | 11,43/2,998 | 3.37 (1.44–7.93)c | 3.61 (2.53–5.13)c | 7.36 (4.2–12.9)c | no | II-2, fair | ||
| 2.79 (2.08–3.75)d | 12.23 (4.76–31.43)d | |||||||
| WHO, 1995a | 769/2,225 | 3.5 (2.6–4.7) | 9.1 (4.9–17.0)a | no | II-2, fair | |||
| 9.1 (4.9–16.7)b | ||||||||
| Cohort studies | ||||||||
| Lidegaard, 2011 | 8,010,290 w-y, 4,246 events | 2.9 (2.2–3.8) | 6.6 (5.6–7.8)a | partial | II-2, good | |||
| 6.2 (5.6–7.0)b | ||||||||
| Lidegaard, 2012 | 9,429,128 w-y, 3,434 events | 0.6 (0.4–0.8) | IUD | no | II-2, good | |||
| 7.9 (3.5–17.7) | Patch | |||||||
| 6.5 (4.7–8.9) | Ring | |||||||
| 1.4 (0.4–3.4) | Implant | |||||||
| Mortality studies | ||||||||
| Parkin, 2000 | 26/111 | 10.5 (6.2–16.6) | Gen 2+3 | no | II-2, fair | |||
| Samuelsson, 2005 | 17,202,000 w-y, 28 deaths | 7.5 (4.7–10.3) | Gen 2+3 | no | II-2, fair | |||
a Desogestrel.
b Gestodene.
c Europe.
d Developing countries.
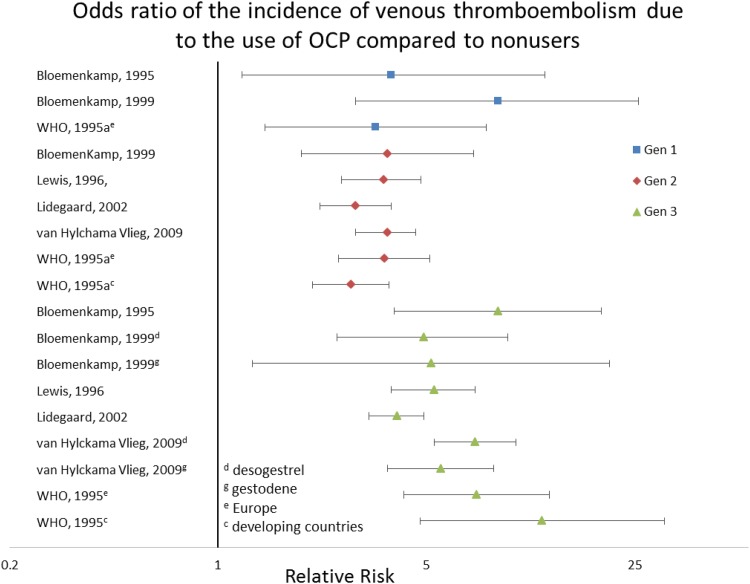
All studies showed an increased risk of hormonal contraceptives to nonusers except the levonorgestrel releasing intrauterine device (IUD). Of the case control trials, the OR varied by generation of oral contraceptives. The first-generation contraceptives had an increased risk of VT of 3.37–8.7. The second-generation oral contraceptives had an OR range of 2.79–4.07. Third-generation had a range of 4.0–48.6, though most studies were in the range of 4–9. Of those that separated gestodene from desogestrel, the gestodene range was 5.2–9.1, and the desogestrel was 4.9–9.1. Oral contraceptives with drospirinone had a risk of 6.3 (2.9–13.7). The nonoral forms showed that depot-medroxyprogesterone carried an RR of 3.6 (1.8–7.1). The case control evaluating levonorgestrel-releasing IUD had an OR of 0.3, though the confidence interval was 0.1–1.1, so lacked significance (see Figure 2).
Figure 2.
Risk of venous thromboembolism among users of oral contraceptives compared to nonusers.
A few studies (Martinelli et al. 2016; van Hylckama Vlieg et al. 2009; Suissa et al. 1997) stratified the risk according to years of use, with risk generally decreasing with longer use time. In the first three months, the risk is greatest at 12.6 (van Hylckama Vlieg et al. 2009). In the first year, the risks ranged from 6.6 to 8.8 for second generation to 14.6 for third generation. Second year for second generation is 7.2, third generation 4.3, and in three to five years for second generation is 2.5 and third is 4.2. Obesity is also associated with higher risk of VT with HC. Most studies adjusted the risk according to BMI. The data from the Kaiser study (Sidney et al. 2004) showed risk of second generation is 3.34 if BMI is less than 30, but increases to 6.04 if body mass index (BMI) is greater than 30.
The New Zealand case control study looking at fatal PE (Parkin et al. 2000) showed second and third generation have a risk fatal VT of 9.6 (3.1–29.1). Only 35 percent of deaths while on oral contraceptives were reported to the Centre for Adverse Reactions. The Samuelsson study looking at the Swedish death registry showed an RR of 18.8 for women aged fifteen to twenty-four who used oral contraceptives, with no statistical differences between second and third generation.
The cohort studies showed less risk than the case control, similar to other systematic reviews. The risk of second generation was 2.9; for the third generation, desogestrel risk was 6.6, and gestodene was 6.2; and fourth generation, drospirenone was 6.4. For nonoral forms, the transdermal contraceptive patch with norelgestromin and ethinylestradiol had an RR of 7.9 (3.5–17.7). Combined hormonal vaginal rings with etonogestrel and ethinylestradiol had a risk of 6.5 (4.7–8.9). The progestogen only implants (etonogestrel) had a nonsignificant increase at 1.4 with confidence intervals of 0.6–3.4, and the levonorgestrel IUD showed decreased risk at 0.6 (0.4–0.8).
Discussion
HC significantly increases the risk of venous thrombotic events in women. Focusing only on studies that compared users of HC to nonusers, with adequately diagnosed VT, there is an increased risk of three- to ninefold. Even a threefold increase has led to estimates of an extra 100 VT events due to HC per 100,000 women years of use (Manzoli 2012). This would equate to 13,000 extra venous thrombotic events per year. Mortality risks have been estimated at 2 percent of VT (Anderson et al. 1991), whereas Parkin et al.’s study (2000) estimated 10.5 fatalities per million user years. Lidegaard’s cohort study evaluating risks of stroke and myocardial infarction estimated that 21 strokes/100,000 user years would be added by HC, with a case fatality of 1 percent, and 10/100,000 myocardial infarctions, with a case fatality of 10.8 percent (Lidegaard, Løkkegaard, et al. 2012). Given the 13 million hormonal contraceptive users per year in the United States, one could anticipate every year 136–260 deaths from VT, 140 deaths from myocardial infarction, and 27 deaths from stroke in previously healthy young women. Using the minimal risk range of only a threefold increased risk, it is estimated that 300–400 healthy women die each year in the United States due to their choice of using HC for family planning.
Many of the articles justify the risk of HC by comparing it to the safety of pregnancy. The study in New Zealand of fatal VT showed no statistically significant difference between the death rate of oral contraceptives and pregnancy. About two-thirds of the fatal VT in young women were due to contraception since there were far greater user women years than pregnancy women years (Parkin et al. 2000). However, not being on contraception does not equal being pregnant. The real question is comparing the risk of HC to other forms of family planning. Several of the fertility-awareness-based methods (FABMs), which have no medical side effects, have effectiveness rates comparable to the typical use rate of the pill, making the projected death rate of HC avoidable (Manhart et al. 2013).
Many women receive counseling of the increased risk of VT with HC, but personalizing it is important. With the growing rate of obesity in the United States, many would fall in the category of a sixfold increased risk due to a BMI above thirty (Sidney et al. 2004). Genetic testing is not advised prior to starting contraception due to cost effectiveness, but if a woman is found to have thrombophilia, her risk is increased to sixty-two-fold in the first year, and twenty-five-fold after the first year (Martinelli et al. 2016).
The studies that evaluated effect of duration of use on risk showed an increased risk in the first year ranging between 7.0 and 12.6, but if a woman is also younger than thirty, her risk increases further to thirteen (Martinelli 1999; Lidegaard, Edstrom, and Kreiner 2002).
There is significant underreporting of thromboembolic events related to HC. A study looking at the reporting of hospitalized cases of VT in France found only 7.5 percent were reported, similar to other studies (Gourbil et al. 2014). One of the common reasons cited for the underreporting was expectedness of the events. Underreporting may be minimizing the understanding of the impact that HC has on the health of women.
A randomized controlled trial was very powerful for making the risks of hormone replacement known in menopausal women and changing practice. In the case of HC, though the synthetic hormones and doses used provide greater pharmacological effects, it is not feasible to have a prolonged randomized controlled trial to evaluate for adverse events. Side-effect risk estimates of an intervention may be just as valid from a meta-analysis of observational studies as from randomized controlled trials in many situations (Golder, Loke, and Bland 2011). In the future, following a large cohort of women choosing FABM compared to a cohort on HC may make the difference in health outcomes more clear. A nonuser of HC encompasses but does not equal the health status of those who use FABM, as FABM users are empowered by knowledge of their body to detect signs of health or disorder and so be able to be proactive in correcting problems.
The current status of our medical choices as a country has been acceptance of widespread use of agents that suppress the normal function of a woman’s reproductive-health system and which concomitantly are responsible for the deaths of 300–400 healthy young women per year. This is equivalent to a jumbo jet crashing yearly resulting in the deaths of women for treating something that is not a disease. Women should be informed of these risks and improved access to training in FABMs should be provided.
Conclusion
Current hormonal contraceptives carry a three- to ninefold risk of VT compared to nonusers. Third-generation contraceptives are about twice the risk of the other generations. Combined HC vaginal rings and transdermal patches carry a six- to eightfold risk. Obesity can double the risk compared to normal weight. Use during the first year carries the greatest risk, especially in women under age thirty, where the risk increases thirteenfold. Women should be made aware of these risks when choosing family planning, and the FABMs should be made more accessible.
Biographical Notes
Lynn Keenan, MD, is a clinical professor of medicine at University of California, San Francisco Fresno and is board certified in Sleep and Internal Medicine. She is the vice president of the International Institute of Restorative Reproductive Medicine.
Tyson Kerr is a research assistant of the Department of Psychology, University of California, Los Angeles.
Marguerite Duane, MD, MHA, FAAFP, is an adjunct associate professor at Georgetown University and an executive director of FACTS, Fertility Awareness Collaborative to Teach the Science.
Karl Van Gundy, MD, FCCP, is a clinical professor of medicine at University of California, San Francisco Fresno and is board-certified in sleep, pulmonary, and critical care medicine.
Footnotes
Authors’ Note: The views expressed in this article are our own and not an official position of University of California or Georgetown University.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- Andersen B. S., Olsen J., Nielsen G. L., Steffensen F. H., Sorensen H. T., Baech J., Gergersen H. 1998. “Third Generation Oral Contraceptives and Heritable Thrombophilia as Risk Factors of Non-fatal Venous Thromboembolism.” Thrombosis and Haemostasis 79:28–31. [PubMed] [Google Scholar]
- Anderson F. A., Jr., Wheeler H. B., Goldberg R. J., Hosmer D. W., Patwardhan N. A., Jovanovic B., Forcier A., Dalen J. E. 1991. “A Population-based Perspective of the Hospital Incidence and Case-fatality Rates of Deep Vein Thrombosis and Pulmonary Embolism: The Worcester Dvt Study.” Archives of Internal Medicine 151, no. 5: 933–38. doi: 10.1001/archinte.1991.00400050081016. [PubMed] [Google Scholar]
- Bloemenkamp K. W., Rosendaal F. R., Helmerhorst F. M., Buller H. R., Vandenbroucke J. P. 1995. “Enhancement by Factor V Leiden Mutation of Risk of Deep-vein Thrombosis Associated with Oral Contraceptives Containing a Third-generation Progestagen.” Lancet 346, no. 8990: 1593–96. [DOI] [PubMed] [Google Scholar]
- Bloemenkamp K. W., Rosendall F. R., Buller H. R., Helmerhorst F. M., Colly L. P., Vandenbrouke J. P. 1999. “Risk of Venous Thrombosis with Use of Current Low Dose Oral Contraceptives Is Not Explained by Diagnostic Suspicioun and Referral Bias.” Archives of Internal Medicine 159: 65–70. [DOI] [PubMed] [Google Scholar]
- Daniels K. 2015, November 10 “Current Contraceptive Use and Variation by Selected Characteristics Among Women Aged 15–44: United States, 2011–2013.” National Health Statistics Reports 86: 1–15. [PubMed] [Google Scholar]
- de Bastos M., Stegeman B. H., Rosendaal F. R., Van Hylckama Vlieg A., Helmerhorst F. M., Stijnen T., Dekkers O. M. 2014. “Combined Oral Contraceptives: Venous Thrombosis.” Cochrane Database of Systematic Reviews, no. 3: CD010813 doi: 10.1002/14651858.CD010813.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder Su, Loke Yoon K., Bland Martin. 2011. “Meta-analyses of Adverse Effects Data Derived from Randomised Controlled Trials as Compared to Observational Studies: Methodological Overview.” PLoS Medicine 8, no. 5: e1001026 doi: 10.1371/journal.pmed.1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourbil M., Grandvuillemin A., Beyens M. N., Massy N., Gras V., D’Amico A., Miremont-Salame G., Petitpain N. 2014. “Thromboembolic Events in Women Exposed to Hormonal Contraception or Cyproterone Acetate in 2012: A Cross-sectional Observational Study in 30 French Public Hospitals.” Drug Safety 37, no. 4: 269–82. doi: 10.1007/s40264-014-0149-8. [DOI] [PubMed] [Google Scholar]
- Lewis M. A. 1996. “The Increased Risk of Venous Thromboembolism and the Use of Third Generation Progestagens: Role of Bias in Observational Research.” Contraception 54:5–13. [DOI] [PubMed] [Google Scholar]
- Lidegaard O., Edstrom B., Kreiner S. 2002. “Oral Contraceptives and Venous Thromboembolism: A Five-year National Case-control Study.” Contraception 65, no. 3: 187–96. [DOI] [PubMed] [Google Scholar]
- Lidegaard Øjvind, Løkkegaard Ellen, Jensen Aksel, Skovlund Charlotte Wessel, Keiding Niels. 2012. “Thrombotic Stroke and Myocardial Infarction with Hormonal Contraception.” New England Journal of Medicine 366, no. 24: 2257–66. doi: 10.1056/NEJMoa1111840. [DOI] [PubMed] [Google Scholar]
- Lidegaard O., Nielsen L. H., Skovlund C. W., Lokkegaard E. 2012. “Venous Thrombosis in Users of Non-oral Hormonal Contraception: Follow-up Study, Denmark 2001–10.” British Medical Journal 344:e2990 doi: 10.1136/bmj.e2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidegaard O., Nielsen L. H., Skovlund C. W., Skjeldestad F. E., Lokkegaard E. 2011. “Risk of Venous Thromboembolism from Use of Oral Contraceptives Containing Different Progestogens and Oestrogen Doses: Danish Cohort Study, 2001–9.” British Medical Journal 343:d6423 doi: 10.1136/bmj.d6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manhart Michael D., Duane Marguerite, Lind April, Sinai Irit, Golden-Tevald Jean. 2013. “Fertility Awareness-based Methods of Family Planning: A Review of Effectiveness for Avoiding Pregnancy Using SORT.” Osteopathic Family Physician 5, no. 1: 2–8. [Google Scholar]
- Manzoli L. 2012. “Oral Contraceptives and Venous Thromboembolism a Systematic Review and Meta-analysis.” Drug Safety 35, no. 3: 191–205. [DOI] [PubMed] [Google Scholar]
- Martinelli I. 1999. “Interaction between the G20210A Mutation of the Prothrombin Gene and Oral Contraceptive Use in Deep Vein Thrombosis.” Arteriosclerosis, Thrombosis, and Vascular Biology 19: 700–703. [DOI] [PubMed] [Google Scholar]
- Martinelli I., Maino A., Abbattista M., Bucciarelli P., Passamonti S. M., Artoni A., Gianniello F., Peyvandi F. 2016. “Duration of Oral Contraceptive Use and the Risk of Venous Thromboembolism. A Case-control Study.” Thrombosis Research 141:153–57. doi: 10.1016/j.thromres.2016.03.025. [DOI] [PubMed] [Google Scholar]
- Parkin L., Skegg D. C., Wilson M., Herbison G. P., Paul C. 2000. “Oral Contraceptives and Fatal Pulmonary Embolism.” Lancet 355, no. 9221: 2133–34. doi: 10.1016/s0140-6736(00)02382-5. [DOI] [PubMed] [Google Scholar]
- Rossouw J. E., Anderson G. L., Prentice R. L., LaCroix A. Z., Kooperberg C., Stefanick M. L., Jackson R. D., Beresford S. A., Howard B. V., Johnson K. C., Kotchen J. M., Ockene J. 2002. “Risks and Benefits of Estrogen Plus Progestin in Healthy Postmenopausal Women: Principal Results from the Women’s Health Initiative Randomized Controlled Trial.” Journal of the American Medical Association 288, no. 3: 321–33. [DOI] [PubMed] [Google Scholar]
- Samuelsson E., Hedenmalm K., Persson I. 2005. “Mortality from Venous Thromboembolism in Young Swedish Women and Its Relation to Pregnancy and Use of Oral Contraceptives—An Approach to Specifying Rates.” European Journal of Epidemiology 20, no. 6: 509–16. [DOI] [PubMed] [Google Scholar]
- Sidney S., Petitti D. B., Soff G. A., Cundiff D. L., Tolan K. K., Quesenberry C. P., Jr. 2004. “Venous Thromboembolic Disease in Users of Low-estrogen Combined Estrogen-progestin Oral Contraceptives.” Contraception 70, no. 1: 3–10. doi: 10.1016/j.contraception.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Suissa S., Blais L., Spitzer W. O., Cusson J., Lewis M., Heinemann L. 1997. “First-time Use of Newer Oral Contraceptives and the Risk of Venous Thromboembolism.” Contraception 56, no. 3: 141–46. [DOI] [PubMed] [Google Scholar]
- van Hylckama Vlieg A., Helmerhorst F. M., Rosendaal F. R. 2010. “The Risk of Deep Venous Thrombosis Associated with Injectable Depot-medroxyprogesterone Acetate Contraceptives or a Levonorgestrel Intrauterine Device.” Arteriosclerosis, Thrombosis, and Vascular Biology 30, no. 11: 2297–300. doi: 10.1161/atvbaha.110.211482. [DOI] [PubMed] [Google Scholar]
- van Hylckama Vlieg A., Helmerhorst F. M., Vandenbroucke J. P., Doggen C. J., Rosendaal F. R. 2009. “The Venous Thrombotic Risk of Oral Contraceptives, Effects of Oestrogen Dose and Progestogen Type: Results of the MEGA Case-control Study.” British Medical Journal 339:b2921 doi: 10.1136/bmj.b2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (World Health Organization). 1995. a. “Effect of Different Progestagens in Low Oestrogen Oral Contraceptives on Venous Thromboembolic Disease.” Lancet 346:1582–88. [PubMed] [Google Scholar]
- WHO (World Health Organization). 1995. b. “World Health Organization Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Venous Thromboembolic Disease and Combined Oral Contraception; Results of International Multicentre Case-control Study.” Lancet 346:1575–82. [PubMed] [Google Scholar]
- WHO (World Health Organization). 1998. “Cardiovascular Disease and Steroid Hormone Contraception. Report of a WHO Scientific Group.” World Health Organization Technical Report Series 877: i–vii, 1–89. [PubMed] [Google Scholar]