Abstract
Identifying the return of fertility with cervical mucus observations is challenging during the postpartum period. Use of urinary measurements of estrogen and progesterone can assist in understanding the return to fertility during this period. The purposes of this study were to describe the postpartum return of fertility by an analysis of total estrogen (TE) and pregnanediol glucuronide (PDG) profiles and to correlate these profiles with cervical mucus observations. Twenty-six participants collected urine samples during the postpartum period and recorded mucus scores. TE and PDG hormones were analyzed and compared with mucus scores. During amenorrhea, mucus reflected TE changes in only 35 percent of women; after amenorrhea, typical mucus patterns were seen in 33 percent of cycles. We concluded that postpartum mucus and hormone profiles are significantly dissociated but that monitoring urinary hormones may assist in identifying the return of fertility. We also identified different hormonal patterns in the return to fertility.
The postpartum period is a challenging time for identifying the return of fertility. The purposes of this study were to describe the hormonal patterns during the return of fertility and to correlate these patterns with cervical mucus observations. Twenty-six postpartum women collected urine samples and recorded mucus scores. Urinary estrogen and progesterone hormones were analyzed and compared with mucus scores. Before the return of menses, mucus reflected hormonal changes in only 35 percent women and after first menses in 33 percent of cycles. We found that hormone profiles do not correlate well with mucus observations during the postpartum return of fertility.
Keywords: Breastfeeding, Estrone-3-glucuronide, Natural family planning, Postpartum, Pregnanediol
Although natural family planning (NFP) is recommended as a healthy option for avoiding pregnancy among postpartum, breastfeeding women, use of NFP postpartum can be difficult with lower rates of effectiveness (Labbok, Nichols-Johnson, and Valdes-Anderson 2006; Academy Breastfeeding Medicine 2006). One of the major reasons that NFP can be difficult for use during the postpartum period is because of the great variability and the unpredictability of the return to fertility (Li and Qiu 2007). Another reason is that natural indicators of fertility, such as cervical mucus observations and basal body temperature are often difficult to interpret during the postpartum transition to fertility (Tommaselli et al. 2000). Finally, some researchers have found a dissociation between cervical mucus observations and hormonal indicators of ovarian activity (Brown, Harrison, and Smith 1985; Blackwell et al. 2016) and a dissociation between follicular growth and hormone activity (Velasquez et al. 2006a).
The time from the birth of a baby until the resumption of regular ovulatory menstrual cycles is known as the postpartum/breastfeeding transition to fertility. In general, the postpartum transition includes (1) the immediate postpartum anovulatory amenorrhea with ovarian quiescence, (2) a phase of ovarian activity (follicular development), (3) possible ovulation prior to first menses, (4) the first menses and irregular menstrual cycle phase (with long follicular phases and short luteal phases that may last three to six months after the resumption of menses), and, finally, (5) the resumption of regular menstrual cycles.
Physiology of the Breastfeeding Transition
The return of fertility is summarized in detail from an endocrine point of view by McNeilly (2001), who was involved in many studies evaluating hormonal changes with lactation. Initially, high levels of estrogen and progesterone from the placenta inhibit ovulation during pregnancy and the immediate postpartum period. In addition, these high levels of hormones from the placenta suppress the release of follicle-stimulating hormone (FSH) and the development of follicles, so that minimal follicles are present at the time of delivery. During pregnancy, luteinizing hormone (LH) synthesis is chronically inhibited, and LH content in the pituitary is minimal. For women who breastfeed, suckling continues to inhibit the normal pulsatility of gonadotropin-releasing hormone (GnRH) and consequently LH pulsatility as well. This inhibition is correlated with prolactin levels, but the precise action of prolactin is unclear since some women do not always have increased prolactin with suckling, and there is variability in the actual levels of prolactin when ovulation resumes, suggesting that different women have different sensitivity to prolactin concentrations. Suckling enhances the feedback mechanism of estrogen on the pituitary, so that even small amounts of estrogen from immature follicles have a strong effect. These small amounts of estrogen, which in a normally menstruating woman would not affect the pituitary, lead to suppression (negative feedback) of FSH in breastfeeding women. In addition, suckling dampens estrogen’s positive feedback which usually stimulates the GnRH/LH pulse generator. The dampened stimulation leads to GnRH/LH pulses that are erratic and which are not sufficient to lead to ovulation (GnRH/LH requires a regular pulse interval of sixty to ninety minutes to drive normal follicle secretion). Since suckling increases the sensitivity of the pituitary to small amounts of estrogen, there are cycles of follicle growth and regression which do not lead to ovulation for several months (McNeilly 2001). It is only later when suckling is decreased and other forms of nutrition are introduced (often after six months when most guidelines suggest solids should be introduced) that ovulation then resumes.
Interestingly, a group of researchers recently found that developing follicles were larger during the amenorrhea phase than during the follicular phase in the first menstrual cycle postpartum (Velasquez et al. 2006a), and in a separate study, they reported that there were different isoforms of FSH present during amenorrhea compared to the first menstrual cycle and that the isoform present during amenorrhea was less potent/bioactive (Velasquez 2006b). In the first study, both the number and diameter of the follicles were significantly greater during breastfeeding amenorrhea compared to the early and mid-follicular phases of regular menstrual cycles. However, these larger follicles had low levels of inhibin B (produced in early stages of follicular development) and overall absence of inhibin A (secreted by more differentiated and luteinized cells). These authors concluded that during lactational amenorrhea, follicular response to FSH is altered due to the reduced biopotency of FSH isoforms leading to follicles that are larger but immature, producing minimal amounts of estrogen. Interpreting McNeilly’s model and Velasquez’s findings together, it is possible that suckling and prolactin levels affect the estrogen-FSH feedback loop by favoring less potent FSH isoforms. Eventually with less suckling, the more bioactive FSH becomes upregulated instead, leading to the development of more mature (albeit smaller) follicles. In these more mature follicles, sufficient amounts of estrogen can be produced to trigger the GnRH/LH pulse generator that becomes more regular (less erratic), thus leading to ovulation.
Efficacy of NFP during the Breastfeeding Transition
There are few studies that have investigated the efficacy of NFP methods during the breastfeeding transition. Over thirty years ago, an Australian study followed fifty-five postpartum, breastfeeding women for a mean of 7.8 months to discern patterns of returning fertility by comparing both mucus changes and urinary hormone measurements of TE and PDG (Brown, Harrisson, and Smith 1985). They discovered that mucus symptoms overestimated urinary hormone changes in 21 percent of women, underestimated hormone changes in 21 percent of women, with the remaining 58 percent of women with mucus score accurately reflecting their ovarian activity determined by urinary hormones. In their small study, fourteen of the fifty-five participants (25 percent) had an unintended pregnancy. Hatherley (1985) investigated the efficacy of NFP among 251 postpartum women who were using NFP methods to avoid pregnancy and found, after one full year postpartum, there were thirty-four (16 percent) unintended pregnancies among the breastfeeding group and two (5 percent) among women who did not breastfeed at all. Hatherley concluded that the variability of the menstrual cycles and the dissociation with the hormonal fluctuations during the postpartum period were not conducive to the use of NFP. Other researchers found that unintended pregnancy rates were higher among breastfeeding women following the ovulation method when back in cycles compared to nonbreastfeeding women (Labbok et al. 1991). This may be because observing mucus signs while breastfeeding may lead to increased confusion in the interpretation of mucus. They concluded that special emphasis needs to be made among breastfeeding women using the ovulation method postpartum, including this increased potential for method failure while breastfeeding. With an experienced NFP instructor, many women can navigate this challenging time with more discerning attention to mucus, although this is not always the case and often increases required abstinence (Kennedy et al. 1995).
Howard and Stanford (1999) investigated a subset of breastfeeding women who were using the Creighton Model ovulation method of NFP and discovered a net pregnancy rate of 24 percent and gross rate of 32 percent. They concluded that the higher pregnancy rate (compared to regularly cycling women using the Creighton Model) might be due to a higher rate of “achieving related behaviors” (having intercourse when mucus is identified as fertile) among the breastfeeding group of women. Although they did not comment on this, it is possible that some of the method failure could be related to mucus not reflecting fertility status. In another study, researchers from Georgetown applied the rules of two simpler methods of NFP retrospectively on a data set of breastfeeding women and found that they may be effective, but this requires a more robust follow-up trial to evaluate effectiveness prospectively (Aravelo et al. 2003).
A more recent NFP efficacy study during the postpartum period (which included both breastfeeding and nonbreastfeeding women), using an electronic hormonal fertility monitor that measured daily urinary levels of E1G and a threshold level of LH found a typical use pregnancy rate of eight per 100 women over twelve months (Bouchard, Schneider, and Fehring 2013). Ahead of their time, Brown, Harrison, and Smith’s (1985) study remarkably suggested that simple methods for measuring urinary hormones (estrogen and PDG) are required to assist women whose mucus observations do not reflect underlying hormone changes.
Variability during the Breastfeeding Transition
Understanding the dynamics and complexity of the breastfeeding transition will help with designing protocols for use with NFP methods since the breastfeeding transition is complex and extremely variable (Fehring, Schneider, and Barron 2005). For example, studies on the postpartum transition to fertility in breastfeeding women found a mean range of the first ovulation from 193 to 322 days (6.3–10.6 months, and actual range of 24–750 days) and a mean range of the first menses from 179 to 298 days (5.9–9.8 months, actual range 35–698 days; Hatherley 1985; Lewis et al. 1991; Li and Qui 2007). About 33 percent of breastfeeding women will have their first ovulation before their first menses if it occurs in the first three months postpartum, but this figure goes up to 87 percent if first menses occurs after the first twelve months (ovulation was based on salivary progesterone rise or urinary pregnanediol rise above the established threshold for ovulation; Lewis et al. 1991). In the Lewis et al. (1991) study, they found that only 20 percent had ovulated and more than 25 percent had menstruated by six months postpartum, but by twelve months 64 percent had ovulated and 70 percent had menstruated. Based on several studies, it has also been demonstrated that after the first ovulation, the luteal phases are often short (i.e., less than six days) with a gradual lengthening over the course of the transition to regular cycles (Lewis et al. 1991; Zinaman and Stevenson 1991; Diaz et al. 1992; Li and Qui 2007). Mothers who ovulate early during full breastfeeding will tend to do the same for future babies. Research has shown that mothers tend to have a return to fertility at about the same time postpartum from one breastfeeding experience to the other when breastfeeding intensity is similar (Ecochard et al. 1988).
In 1995, a study was published which involved a sample of seventy-one postpartum, breastfeeding women who were users of the symptothermal method (STM) of NFP from Sydney, Montreal, and Birmingham (England) (Kennedy et al. 1995). The purpose of this study was to correlate the estimated days of fertility (i.e., required abstinence for avoiding pregnancy) with daily urinary measures of TE and PDG to estimate the day of ovulation and the actual fertile days during the postpartum transition to fertility. The result was that the STM overestimated the actual days of fertility by 50 percent or more. However, the hormonal data from this study have not been extensively analyzed in describing the hormonal return to fertility.
Another study evaluating urinary PDG and the return of fertility postpartum addressed breastfeeding patterns in relation to return of fertility (Taylor et al. 2003). This study (N = 144) found that more “ecological breastfeeding” or breastfeeding on demand with frequent suckling was associated with a median postpartum delay of fifty-five weeks until the first PDG rise.
The purpose of the current study was to identify the return of fertility postpartum with a descriptive analysis of TE and PDG profiles as measured in the urine as well as to correlate these hormonal profiles to cervical mucus observations. The results will serve to assist in the development of postpartum transition protocols for NFP.
Method
Participants
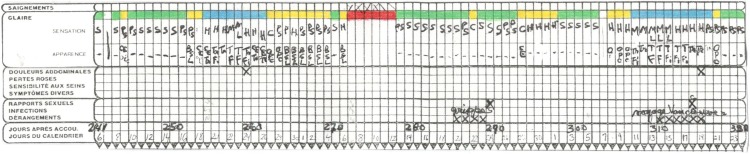
Of the seventy-one participants in the original study (Kennedy et al. 1995), only twenty-six had demographics available for the current study (Montreal data). The mother’s age, parity, gravity, and breastfeeding characteristics were collected. As described in detail in the 1995 study, data were collected from 1986 to 1990 and included daily records regarding breastfeeding, STM signs, coital frequency (see partial STM chart in Figure 1, excluding temperature and cervix changes, which were not analyzed in this study), and a daily sample of urine. Data collection began in the seventh-week postpartum. The protocol that women followed is included in Appendix A, Brown Ovarian Monitor Guidelines.
Figure 1.
Representative symptoms chart from participant 111, from April to June 1986 (in French). Mucus coding is represented by a green color for “dry” mucus (Colombo score of 1, Marquette “Low” mucus), a yellow color for “moist, damp” mucus (Colombo score of 2 or 3, Marquette “High” mucus), and a blue color for “clear, slippery, stretchy” (Colombo score of 4, Marquette “Peak” mucus). Other signs, used in the symptothermal method, including temperature and cervix changes, are not shown here because they have not been analyzed in this study. (French terms: saignements = bleeding, glaire = mucus, douleurs abdominales = abdominal pain, pertes roses = spotting, sensibilité aux seins = breast tenderness, symptômes divers = miscellaneous symptoms, rapports sexuels = intercourse, infections = infections, dérangements = disturbances, jours après accou. = days after parturition, jours du calendrier = calendar days.)
Mucus Observations
As shown in Figure 1, a variety of mucus characteristics can be observed readily by women and are generally classified according to sensation and appearance. Sensation descriptions include dry, damp, moist, sticky, wet, and slippery. Appearance descriptions include thick, white, yellowish, stretchy, and clear. In order to simplify these descriptions, one of the researchers gave a three-tiered code to the mucus based on these descriptions: (1) a green color for “dry” mucus, (2) a yellow color for “moist, damp, sticky” mucus, and (3) a blue color for “clear, wet, slippery, stretchy” mucus. Of interest, this coding is analogous to other groups’ simplified mucus coding schemes including Colombo’s score of 1 for dry mucus (“green”), a score of 2–3 for damp/thick/white/yellow/sticky mucus (“yellow”), and a score of 4 for slippery/transparent/stretchy mucus (“blue”; Bigelow et al. 2004). The Marquette Method’s coding of “Low,” “High,” and “Peak” mucus also corresponds to the three color levels used in these charts (Bouchard et al. 2013).
Urine Collection
Daily timed urine samples were collected until two ovulations were confirmed by the laboratory. The women collected urine daily using the following procedure. The women collected urine passed for a period of at least three hours, in the morning or at a convenient time of the day. The minimum collection period of three hours was specified to reduce error caused by unpassed urine. The urine sample was collected into a container, the number of hours of collection and the volume was calculated, and a small aliquot transferred into a tube that was labeled with the participant number, center number, date, hours, and volume and kept frozen until the batch was sent in dry ice to the laboratory in Melbourne by air. The samples were thawed, diluted to 100 ml/h, and analyzed by homogeneous enzyme immunoassay (Rubenstein, Schneider, and Ullman 1972).
TE Assays
The estrogen assay used was a “total” estrogen spectrophotofluorometric method using purified extracts of acid hydrolyzed urine as described previously that measured the sum of estrone, estradiol, and estriol, using the Kober–Ittrich procedure (Brown et al. 1968). The sum (or “total” estrogens) excreted into the urine in twenty-four hours (the excretion rate) is important since it is directly related to the ovarian estradiol production rate (Brown 1957, 2011), whereas simple concentration (amount per volume) is not. The data from the total estrogen method were the most accurate available as the total estrogen method was of proven reliability and specificity (Brown 2011), had no urine blank or matrix effects, and had a coefficient of variation of 8 percent determined from quality controls included with each assay run.
PDG Assay
The PDG assay was carried out with the laboratory version of the homogeneous enzyme immunoassay used in the Ovarian Monitor system employing a specially designed rate colorimeter thermostated at 40°C with timer settings for the warm-up and enzyme reactions (Brown 1985). For the multiple assays in this study, a thermostated heating block was used which accommodates up to forty-eight assay tubes; the timings were performed manually using a stop watch. In this way up to twenty PDG assays could be performed in rapid sequence since the time for the PDG assay is five minutes. The PDG assay utilizes the principle of homogeneous enzyme immunoassay (Rubenstein, Schneider, and Ullman 1972; Blackwell et al. 2003) in which an antibody to a ligand specifically inhibits the enzyme activity of a conjugate of the ligand chemically bound to the enzyme. It is important to appreciate that the assay has been designed to operate over a limited but clinically significant concentration range. The sensitivities of the assays using 10 µl of a time-diluted urine sample for PDG were set to include the most important ranges of values required to identify the thresholds of fertility and infertility during the human ovulatory cycle (Blackwell 2003; Brown 2011; Blackwell et al. 2011). The homogeneous enzyme immunoassay has an upper ceiling reached when the entire antibody is neutralized by the hormone metabolite in the urine and a lower limit largely determined by nonspecific components of the urine. This latter effect is termed the urine bias; it is more limiting for the estrogen tubes than for the PDG tubes because of the larger urine volumes and longer incubation times used. For quantitative results, all values approaching the upper ceiling were repeated using less urine in the assay tubes. Quality control urine samples were added to each run, and the coefficient of variation was 5–10 percent.
TE Thresholds and Ovarian Activity
Both TE and PDG are measured as excretion rates, expressed in µg/24 h for TE and in µmol/24 h for PDG. For the TE excretion rates, values between 0 and 10 µg/24 h were reflective of minimal ovarian activity, values between 10 and 20 µg/24 h were reflective of follicular development, and values >20 µg/24 h were reflective of peak changes and impending ovulation (based on previous experience using the assay used in Brown, Harrison, and Smith 1985).
PDG Thresholds and the Retrospective Definition of Ovulation with PDG Levels
Based on previous studies using the same assays (Brown 2011), PDG excretion rates have been defined as (see Table 1): (1) the start of the postovulatory infertile period (7 µmol/24 h), (2) a luteinized unruptured follicle (7 to <9 µmol/24 h), (3) biochemical evidence that ovulation has occurred (≥9 µmol/24 h), (4) a deficient luteal phase (9–13.5 µmol/24 h), and (5) a fertile cycle (>13.5 μmol/24 h within six days of a TE peak and a luteal phase of eleven to seventeen days).
Table 1.
PDG Thresholds and Definitions.
| PDG Threshold | Physiologic Definition |
|---|---|
| 7 µmol/24 h | Threshold for the onset of the postovulatory infertile phase |
| >7 but <9 µmol/24 h | Luteinized unruptured follicle |
| ≥9 µmol/24 h | Biochemical proof of ovulation |
| ≥9 but <13.5 µmol/24 h | Deficient luteal phase |
| >13.5 µmol/24 h | Fertile cycle if this followed within six days of a TE peak and the luteal phase is eleven to seventeen days long |
Note: PDG = pregnanediol glucuronide; TE = total estrogen.
Statistical Analyses
Demographics were analyzed using means and standard deviations. Time to biochemically proven ovulation (in months) was calculated from the time of the baby’s birth until urinary PDG levels were at least 9 µmol/24 h, which is a biochemical proof of ovulation based on previous analyses (Brown 2011). First menses was determined by identifying a bleeding pattern that spanned three days or more and included at least one day of moderate or heavy bleeding. Time to menses and time to ovulation were also compared to the time of weaning from full breastfeeding. Time to fertility was based on the first ovulation that met the criteria for a fertile cycle (PDG > 13.5).
Hormone values for individual women were graphically displayed using R software (Version 0.16) (Ihaka and Gentleman 1996) and interpreted qualitatively. Patterns of ovarian activity were classified based on presumed follicular activity inferred from TE changes, and ovulation based on PDG changes. These patterns were then organized into broad categories for the sake of describing the postpartum transition.
Daily mucus ratings of “green,” “yellow,” and “blue” were correlated with the daily TE levels. On any given day, a mucus rating was identified as accurately reflecting the TE level if mucus was rated “green” when TE levels were <10 µg/24 h, if mucus was rated “yellow” when TE levels were 10–20 µg/24 h, and if mucus was rated “blue” when TE levels were >20 µg/24 h. In addition, a mucus rating was identified as either underestimating the TE level (if mucus was rated “green” when the TE levels were >10 µg/24 h, or was rated “yellow” when TE levels were >20 µg/24 h) or overestimating the TE level (if mucus was rated “yellow” or “blue” when TE levels were <10 µg/24 h, or if mucus was rated “blue” when TE levels were between 10 and 20 µg/24 h). These levels were based on field experience using the monitor developed based on the methods in Brown, Harrison, and Smith (1985).
Mucus ratings were also compared to progesterone activity and were evaluated for each cycle after the first ovulation. Since rising progesterone levels are believed to be responsible for the drying effect on the mucus after ovulation, each cycle was identified as having a typical mucus pattern if mucus changed to a basic infertile pattern (not necessarily dry mucus) after the progesterone rise. And conversely, cycles were identified as not reflecting the underlying progesterone changes if mucus did not return to the basic infertile pattern. Also, cycles were identified as not reflecting the underlying progesterone changes when the mucus pattern showed an apparent drying effect when the progesterone rise had not yet started. The progesterone changes were identified from the urinary PDG excretion rates that closely reflect the serum progesterone changes (Munro et al. 1991). The PDG values on the day following the apparent mucus peak days (a change from blue to yellow or green) were assessed for each patch of mucus.
Results
Demographics
The mean age of women in our sample (at the time of their baby’s birth) was 29 (±3.4, range 23–35). The average number of living children (including the one just delivered) was 2.3 (±1.3, range: 1–5).
Time to Menses and Time to Ovulation (Table 2)
Table 2.
Average Time to Menses and Ovulation in Relation to Breastfeeding Status.
| Overall | Return of Menses and Ovulation during Full Breastfeeding | Return of Menses and Ovulation during Partial Breastfeeding | Return of Menses and Ovulation after Full Weaning | |
|---|---|---|---|---|
| Time to menses | 7.4 months (±3.0, range: 1.3–11.7 months; N = 24) | 4.1 months (±2.2, range: 1.3–6.0 months; N = 5) | 9.8 months (±2.7, range: 4.2–14.4 months; N = 12) | 6.4 months (±3.0, range: 3.1–10.5 months; N = 5) |
| Time to ovulation | 8.4 months (±3.1, range: 3.3–14.3 months; N = 22) | 5.6 months (±1.7, range: 3.0–7.0 months; N = 5) | 9.0 months (±2.2, range: 4.4–11.7 months; N = 12) | 7.9 months (±3.5, range: 3.8–12.3 months; N = 5) |
Time to first menses could be calculated for twenty-four women and was on average 7.4 months (±3.0, range: 1.3–11.7 months). Time to first ovulation (biochemical proof of ovulation: PDG ≥9 µmol/24 h) could be calculated for twenty-two women and was on average 8.4 months (±3.1, range: 3.3–14.4 months).
The time of full breastfeeding was an average of 4.9 months (±1.4, range: 2.8–8.7 months), and the time to full weaning was an average of 16.1 months (±11.9, range: 2.4–44.7). Thus, the interval between full breastfeeding and complete wean was on average another 11.1 months ± 10.9, and one woman breastfed up to 44.7 months. Time from the end of full breastfeeding to menses was 2.4 months (±3.2, range: −5.2 to 6.7 months) and from the end of full breastfeeding to ovulation was on 3.3 months (±3.3, range: −1.6 to 9.1 months).
Time from first menses to ovulation was on average 1.16 months (±1.5, range: −0.3 to 4.8 months). In nine women (41 percent), ovulation occurred before menses, and in thirteen women (59 percent) ovulation occurred after menses. In the nine women who ovulated before menses, ovulation occurred on average at 7.9 months (±2.45, range: 4.2–11.6 months), and had a luteal phase of three to eight days which would be considered too short for fertility. Of these nine women, seven of them (77 percent) had ended full breastfeeding (0.25–5.0 months prior). Of the thirteen women who had ovulation after menses, nine of the thirteen (69 percent) had ended full breastfeeding (0.75–9.0 months prior). Of the four women who ovulated prior to six months, two of these women ovulated before their first menses (and had four- and six-day luteal phases), and two ovulated after their first menses (both had five-day luteal phases). In these ovulations prior to six months, three of four women ended full breastfeeding one, two, and three weeks prior, and the fourth woman ended full breastfeeding two months after first ovulation.
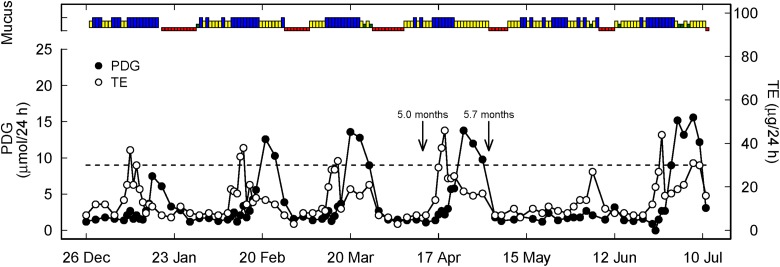
In the five women (22 percent) who ovulated while still fully breastfeeding (in two women ovulation occurred seven weeks before the end of full breastfeeding—see Figure 4, in two women two weeks before, and in one woman one week before), their luteal phases were only three to five days long. These women were part of the category of women who ovulate early as described qualitatively below and shown in Figure 4.
Figure 4.
Representative hormone, mucus, and temperature profile of a woman with relatively early ovulation prior to the end of full breastfeeding (green, yellow, blue as described in Figure 1, red represents menses). Two arrows represent the end of full breastfeeding with the introduction of formula or solids (5.0 months) and complete weaning (5.7 months). The dotted line is the PDG threshold of 9 µmol/24 h as the biochemical criterion for ovulation.
As shown in the last column of Table 2, the five women who had ovulation after ending full breastfeeding did not fully breastfeed for very long (2.5–4.5 months) and completely weaned earlier than the overall group (6.3 vs. 16.1 months).
Time to Fertility
Return to fertility could be identified by pregnancies that occurred in three of the twenty-six women, at six (still fully breastfeeding), twelve, and eighteen months postpartum (both completely weaned from breastfeeding). Among the other women, none of their cycles met criteria for fertility as described in Table 1. Of the remaining eighteen women who had transition cycles to be analyzed, fourteen of them had fertile range PDG levels (>13.5 µmol/24 h) in their transition cycles, but none of these had luteal phase lengths that were adequate (range: two to ten days).
Qualitative Description of the Postpartum Transition
After visually inspecting each woman’s postpartum transition, the patterns were divided into three broad categories (Table 3): ovarian quiescence with delayed ovulation (Figure 2, N = 11 or 42 percent), follicular activity with delayed ovulation (Figure 3, N = 4 or 15 percent), and relatively early ovulation (Figure 4, N = 11 or 42 percent).
Table 3.
Qualitative Categories of Postpartum Transitions in the Twenty-Six Participants.
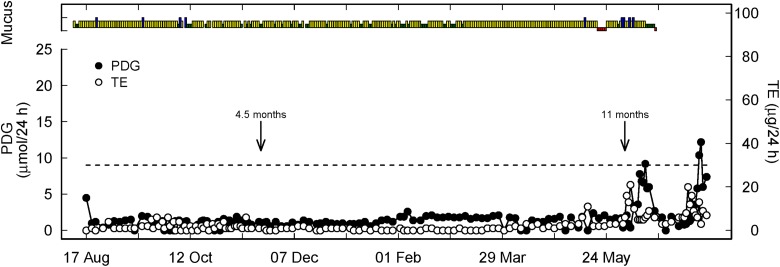
Figure 2.
Representative hormone, mucus, and menses profile of a woman with prolonged ovarian quiescence and delayed ovulation (green, yellow, blue as described in Figure 1, red represents menses). Two arrows represent the end of full breastfeeding with the introduction of formula or solids (4.5 months) and complete weaning (11 months). The fact that full weaning occurred 6.5 months after the introduction of supplements indicates that the latter was very gradual and that breastfeeding was maintained at a rather intense level. This is recognized as a factor of delay in the return to fertility. The dotted line is the PDG threshold of 9 µmol/24 h as the biochemical criterion of ovulation. PDG = pregnanediol glucuronide.
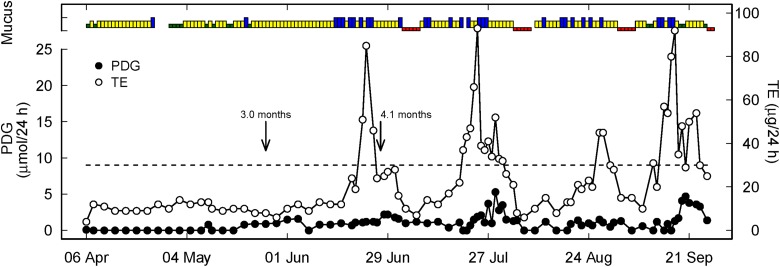
Figure 3.
Representative hormone, mucus, and menses profile of a woman with follicular activity and delayed ovulation (green, yellow, blue as described in Figure 1, red represents menses). Two arrows represent the end of full breastfeeding with the introduction of formula or solids (3.0 months) and complete weaning (4.1 months). The short time span between the two landmarks indicates that breastfeeding intensity decreased rapidly, thereby probably stimulating reactivation of the hypothalamic–pituitary–ovarian axis. The dotted line is the PDG threshold of 9 µmol/24 h as the biochemical criterion of ovulation. PDG = pregnanediol glucuronide.
Although the sample sizes were not large enough for statistical power to compare these three groups, it is interesting to note that first menses occurred on average at 7.5, 7.5, and 6.5 months in the three categories, respectively (ovarian quiescence, follicular activity and delayed ovulation, and early ovulation). First ovulation occurred on average at 9.0 months for those with ovarian quiescence, at 8.8 months for those with follicular activity and delayed ovulation, and at 7.9 months for those with early ovulation (approximately one month earlier). All of the women who ovulated before weaning from full breastfeeding were in the early ovulation category. Also, as can be seen in Table 2, the earlier that menses and ovulation occurred, menses usually occurred before ovulation.
Correlation between Mucus and Total Estrogen (Follicular Activity)
During amenorrhea and before first ovulation, the daily mucus observations were compared with underlying hormone activity; it was found that 53.6 percent of the total daily mucus observations overestimated the underlying hormone changes, 39.1 percent corresponded to the underlying hormone changes, and 7.3 percent underestimated the underlying hormone changes.
Correlation between Mucus and Progesterone (Postovulation)
In the transition cycles after first menses, cycles were analyzed for mucus changes reflecting the postovulatory progesterone rise; it was found that thirty-three of seventy-eight cycles (42 percent) had typical mucus changes expected (where mucus changes from peak mucus to the basic infertile pattern), and forty-five of seventy-eight cycles (58 percent) did not have these mucus changes with the progesterone rise. Another three cycles did not have enough data to analyze.
Discussion
Findings
Time to first menses and first ovulation were similar to other studies in postpartum women (Taylor et al. 2003; Wei and Qiu 2007) at 7.0 and 8.0 months, respectively, and representing the usual introduction of solids and end of full breastfeeding which leads to the removal of the inhibitory mechanism of ovulation. Taylor et al. (2003) found that trained, breastfeeding women had a median return of fertility (i.e., first menses) at 12.75 months versus untrained breastfeeding women at four months. In another study, Li and Qui (2007) discovered among 101 breastfeeding mothers, 52.5 percent had a resumption of ovulation on average within 5.1 months after delivery. However, there was a lot of variability in the Li and Qui study due to total versus partial breastfeeding and the initiation of supplements.
We recognized three broad categories of urinary hormonal profiles for the return of fertility postpartum: (1) ovarian quiescence and delayed ovulation (low TE and PDG excretion rates for many months as in Figure 2), (2) follicular activity without luteinization and delayed ovulation (initial constant low TE and PDG excretion rates followed by cyclical rises and falls in the TE excretion rates but little response from the PDG excretion rates as shown in Figure 3), and (3) relatively early ovulation (cyclical changes in both TE and PDG excretion rates with the absolute values of the PDG excretion rates increasing to exceed 9 µmol/24 h). Of interest, women who ovulated (with normal PDG profiles) during full breastfeeding were in the category of women with early ovulation, and it is possible that these women represent a group of women whose hypothalamic–pituitary–ovarian axis is less impacted by the inhibitory effect of suckling and/or prolactin on ovulation. Women with a history of early ovulation (and perhaps early unintended pregnancy in the postpartum period) should be more cautious in future postpartum periods regarding the possibility of early ovulation.
As discussed in the Kennedy et al. (1995) paper using the same data, mucus changes were found to overestimate hormone changes over half the time during amenorrhea, which means that the mucus was not always related to the underlying TE excretion rates. This leads to more required abstinence during the postpartum period since if it is believed that the mucus is reflecting the underlying physiological events fertility has to be assumed (albeit incorrectly in these cases). In addition, when the mucus scores underestimated the urinary hormone measurements (on 7.3 percent of days during amenorrhea), this means that despite underlying potential fertility the mucus symptom is not reflecting it and infertility may be assumed incorrectly. Comparing our findings to Brown, Harrison, and Smith’s data from 1985 in a separate study but using similar urinary hormone measurements, they found that 21 percent (seven of thirty-three) had mucus scores that overestimated hormone values indicating fertility when there was none, 21 percent (seven of thirty-three) had mucus that underestimated hormone values indicating infertility when fertility was actually present, and 58 percent (nineteen of thirty-three) had mucus scores that reflected ovarian activity. Our findings were less positive, showing 54 percent had mucus scores that overestimated hormone values, 7 percent had mucus scores that underestimated hormone values, and only 39 percent had mucus scores that reflected correctly the underlying ovarian activity.
In the transition cycles, couples using a mucus-only approach would be waiting for mucus to dry up or return to a basic infertile pattern to indicate the beginning of the luteal infertile phase. Unfortunately, this occurred in only 42 percent of transition cycles postpartum. This would lead to extended periods of abstinence, just like the overestimate of estrogen changes in the amenorrheic period. The overestimation of fertility with cervical mucus observation and ratings would make a postpartum NFP method more conservative and result in very low correct use unintended pregnancies. However, the extended abstinence may also lead couples to knowingly taking a chance with intercourse on estimated fertile days, potentially increasing total unintended pregnancies. This phenomenon is exhibited in the high pregnancy rates in postpartum NFP studies.
Limitations
The most significant limitation of this study is its small sample size. Unfortunately, it has not been possible to retrieve and analyze demographic and cycle data for the Sydney and Birmingham participants.
The intention in the present study was to follow-up this older data set with an evaluation of a current population of postpartum women with a urinary hormone measurement device, but up to this point we have not had enough urinary hormone monitors to measure E1G and PDG together in a pilot group of postpartum women. Thus, the ability to replicate the data in a new population is another limitation that we did not foresee.
Implications and Future Directions
Future studies examining the trends in hormone patterns using estrogen (with current devices this would be estrone-3-glucuronide rather than total estrogens as was done in this study), LH, and PDG in the postpartum period with larger numbers of participants would be useful.
At present, the Marquette Method Postpartum Protocol is the most robust method of identifying the return of fertility postpartum, and its efficacy for avoiding pregnancy has previously been demonstrated in two studies (Bouchard, Schneider, and Fehring 2013; Fehring, Schneider, and Bouchard 2017). A study is underway to evaluate the parameters of the return of fertility in a population of women who are presently using the Marquette Method Postpartum Protocol. The addition of urinary PDG measurement could assist in determining whether a rise in LH (as shown by the Clearblue Fertility Monitor used in the Marquette Method Postpartum Protocol) was actually ovulatory. A lower-cost protocol might include the combination of mucus rating with use of LH test strips to help clarify whether peak mucus is potentially ovulatory (Leiva et al. 2014), and this could be combined with subsequent measurements of PDG (Blackwell et al. 2016) to guide a woman through her postpartum fertility.
Conclusions
Recognizing specific patterns of ovarian activity in postpartum women is helpful in the future development of postpartum protocols for NFP. Using a home-based urinary PDG measurement would provide further detail to confirm when ovulation occurs, giving a more precise definition for the luteal infertile phase for women seeking to avoid pregnancy postpartum.
Acknowledgments
The authors are grateful to the Linacre Quarterly Research and Education Fund which awarded the Edmund D. Pellegrino Research Award to the coauthors. The authors wish to thank Dr. Bill Taylor, PhD, for his helpful comments on this manuscript and Dr. Carlos Lara for his kind data entry efforts.
Biographical Notes
Thomas Bouchard is a family medicine physician and a medical consultant for the Institute for Natural Family Planning at Marquette University and medical advisor to Serena Canada.
Len Blackwell is a pioneer in the monitoring of hormones excreted in the urine and helped developed the Ovarian Monitor used in the present study.
Simon Brown has a special interest in monitoring fertility with urinary hormones and the nuances of accurate quantification of hormones.
Richard Fehring is the founder of the Marquette Method of Natural Family Planning and the director of the Institute for Natural Family Planning at Marquette University and a prolific author of scholarly articles on NFP.
Suzanne Parenteau-Carreau was one of the first pioneers of the Sympto-Thermal method of Natural Family Planning in Canada and has promoted research in this area for several decades.
Appendix A
Brown Ovarian Monitor Guidelines
Monitor use during breastfeeding
Establish E1G baseline
This is done by testing daily, consecutive urine samples for E1G over seven days. Notify your contact person of the results who will then establish your baseline level.
Zero to six months postpartum
Check E1G level twice a week. If below the baseline, you are in an infertile phase. If the E1G level is above the baseline or there is a change in your Basic Infertile Pattern (BIP)—Billings Ovulation Method (BOM), continue to do daily E1G tests. Notify your contact person who will advise you of further testing. If you have a previous history of returning to fertility earlier than six months then E1G should be checked twice a week for zero to two months increasing to three times a week after that.
Six to nine months postpartum
Check E1G level every third day. If at or below your baseline, you are in an infertile phase. If the E1G level is above the baseline or there is a change in your BIP, continue daily E1G tests. Notify your contact person who will advise you of further testing.
Nine months to weaning
Check E1G level every second day. If at or below your baseline, you are in an infertile phase. If the E1G level is above the baseline or there is a change in your BIP, continue daily E1G tests. Notify your contact person who will advise you of further testing.
Interpreting the results
Days of infertility: 1. E1G values at or below the baseline and 2. Pregnanediol glucuronide (PDG) values above the ΔT cutoff.
Days of fertility: All days with raised E1G values above your baseline (associated with low PDG values).
A rise in E1G above the baseline indicates the beginning of the fertile phase. The E1G values continue to rise for three to seven days to reach a peak. They then fall abruptly. On the day of the fall, PDG measurements should be commenced. The PDG value on the day of the E1G fall will be low, but it will continue to rise and on the third day be approaching or will have passed the PdG “ΔT cutoff.” Once the ΔT cutoff has been reached, ovulation has already occurred, and the fertile phase has ended. No further testing is required for the remainder of the cycle.
The E1G level may go above the baseline for one day only with no change in the BIP. While the day of raised E1G level is unavailable for intercourse, the following day with a baseline E1G level and continued BIP (BOM) indicates a return to the infertile phase.
If the E1G level remains above the baseline for two or more days, look for the E1G fall and commence PDG measurements on this day. If the PDG value rises to the ΔT cutoff, ovulation has occurred, and the late infertile days have commenced. However, if the PDG value remains low two to three days after the E1G fall, recommence or continue testing the E1G levels. If the E1G value returns to baseline and remains at baseline for three days with an associated low PDG level, then you have returned to infertile phase. Intercourse can be resumed applying the E1G baseline in conjunction with the BIP.
When monitoring ovarian activity, do not expect stereotyped patterns and do not ignore the hormone values no matter what you expect; they are very unlikely to be wrong.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- Academy of Breastfeeding Medicine Protocol Committee. 2006. ABM clinical protocol# 13: contraception during breastfeeding. Breastfeeding Medicine 1(1): 43–51. [DOI] [PubMed] [Google Scholar]
- Arevalo M., Jennings V., Sinai I. 2003. “Application of Simple Fertility Awareness-based Methods of Family Planning to Breastfeeding Women.” Fertility and Sterility 80:1241–48. [DOI] [PubMed] [Google Scholar]
- Bigelow J. L., Dunson D. B., Stanford J. B., Ecochard R., Gnoth C., Colombo B. 2004. “Mucus Observations in the Fertile Window: A Better Predictor of Conception than Timing of Intercourse.” Human Reproduction 19:889–92. doi: 10.1093/humrep/deh173. [DOI] [PubMed] [Google Scholar]
- Blackwell L. F., Brown J. B., Vigil P., Gross B., Sufi S., d’Arcangues C. 2003. “Hormonal Monitoring of Ovarian Activity Using the Ovarian Monitor, Part I. Validation of Home and Laboratory Results Obtained During Ovulatory Cycles by Comparison with Radioimmunoassay.” Steroids, 68, no. 5: 465–76. [DOI] [PubMed] [Google Scholar]
- Blackwell L. F., Vigil P., Gross B., d’Arcangues C., Cooke D. G., Brown J. B. 2011. “Monitoring of Ovarian Activity by Measurement of Urinary Excretion Rates of Estrone Glucuronide and Pregnanediol Glucuronide Using the Ovarian Monitor, Part II: Reliability of Home Testing.” Human Reproduction 27: 550–557. doi: 10.1093/humrep/der409. [DOI] [PubMed] [Google Scholar]
- Blackwell L. F., Vigil P., Alliende M. E., Brown S., Festin M., Cooke D. G. 2016. “Monitoring of Ovarian Activity by Measurement of Urinary Excretion Rates Using the Ovarian Monitor, Part IV: The Relationship of the Pregnanediol Glucuronide Threshold to Basal Body Temperature and Cervical Mucus as Markers for the Beginning of the Postovulatory Infertile Period.” Human Reproduction 31, no. 2: 445–53. 10.1093/humrep/dev303. [DOI] [PubMed] [Google Scholar]
- Bouchard T., Schneider M., Fehring R. 2013. “Efficacy of a New Postpartum Transition Protocol for Avoiding Pregnancy.” Journal of the American Board of Family Medicine 26:35–44. [DOI] [PubMed] [Google Scholar]
- Brown J. B. 1957. “The Relationship between Urinary Oestrogens and Oestrogens Produced in the Body.” Journal of Endocrinology 16, no. 2: 202–12. [DOI] [PubMed] [Google Scholar]
- Brown J. B. 2011. “Types of Ovarian Activity in Women and Their Significance: The Continuum (a Reinterpretation of Early Findings).” Human Reproduction Update 17, no. 2: 141–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. B., Harrisson P., Smith M. A. 1985. “A Study of Returning Fertility after Childbirth and During Lactation by Measurement of Urinary Oestrogen and Pregnanediol Excretion and Cervical Mucus Production.” Journal of Biosocial Science 9:5–23. [DOI] [PubMed] [Google Scholar]
- Brown J. B., MacNaughtan C., Smith M. A., Smyth B. 1968. “Further Observations on the Kober Colour and Ittrich Fluorescence Reactions in the Measurement of Oestriol, Oestrone and Oestradiol.” Journal of Endocrinology 40:175–88. [DOI] [PubMed] [Google Scholar]
- Diaz S., Cárdenas H., Brandeis A., Miranda P., Salvatierra A. M., Croxatto H. B. 1992. “Relative Contributions of Anovulation and Luteal Phase Defect to the Reduced Pregnancy Rate of Breastfeeding Women.” Fertility and Sterility 58:498–503. [PubMed] [Google Scholar]
- Ecochard R., Frey R., Blanche N., Ecochard I., Pinguet F., Coste M. O., Guy M., Guy F. 1988. “Can Analysis of Previous Postpartum Periods Provide Prognostic Indicators on the Return of Fertility after Delivery?” Revue Française de Gynécologie et d’Obstétrique 83, no. 6: 415–19. [PubMed] [Google Scholar]
- Fehring R., Schneider M., Barron M. L. 2005. “Protocol for Determining Fertility while Breastfeeding.” Fertility and Sterility 84:805–7. [DOI] [PubMed] [Google Scholar]
- Fehring R. J., Schneider M., Bouchard T. 2017. “Effectiveness of an Online Natural Family Planning Program for Breastfeeding Women.” Journal of Obstetric, Gynecologic, and Neonatal Nursing: JOGNN/NAACOG 46, no. 4: e129–37. [DOI] [PubMed] [Google Scholar]
- Hatherley L. I. 1985. “Lactation and Postpartum Infertility: The Use-effectiveness of Natural Family Planning (NFP) after Term Pregnancy.” Clinical Reproduction and Fertility 3:319–34. [PubMed] [Google Scholar]
- Howard M. P., Stanford J. B. 1999. “Pregnancy Probabilities during Use of the Creighton Model Fertility Care System.” Archives of Family Medicine 8:391–402. [DOI] [PubMed] [Google Scholar]
- Ihaka R., Gentleman R. 1996. “R: A Language for Data Analysis and Graphics.” Journal of Computational and Graphical Statistics 5:299–314. [Google Scholar]
- Kennedy K. I., Gross B. A., Parenteau-Carreau S., Flynn A., Brown J., Visness C. 1995. “Breastfeeding and the Symptothermal Method.” Studies in Family Planning 26:107–15. [PubMed] [Google Scholar]
- Labbok M. H., Nichols-Johnson V., Valdes-Anderson V. 2006. “ABM Clinical Protocol#13: Contraception during breastfeeding: The Academy of Breastfeeding Medicine Protocol Committee.” Breastfeeding Medicine 1 (2006): 43–80. [DOI] [PubMed] [Google Scholar]
- Labbok M. H., Stallings R. Y., Shah F., Perez A., Klaus H., Jacobson M., Muruthi T. 1991. “Ovulation Method Use during Breastfeeding: Is There Increased Risk of Unplanned Pregnancy?” American Journal of Obstetrics and Gynecology 165 (Suppl): 2031–36. [DOI] [PubMed] [Google Scholar]
- Leiva R., Burhan U., Kyrillos E., Fehring R., McLaren R., Dalzell C., Tanguay E. 2014. “Use of ‘Ovulation Predictor Kits’ as Adjuncts When Using Fertility Awareness-based Methods: A Pilot Study.” Journal of the American Board of Family Medicine 27, no. 3: 427–29. [DOI] [PubMed] [Google Scholar]
- Lewis P. R., Brown J. B., Renfree M. B., Short R. V. 1991. “The Resumption of Ovulation and Menstruation in a Well-nourished Population of Women Breastfeeding for an Extended Period of Time.” Fertility and Sterility 55:529–36. [PubMed] [Google Scholar]
- Li W., Qiu Y. 2007. “Relation of Supplementary Feeding to Resumption of Menstruation and Ovulation in Lactating Postpartum Women.” Chinese Medical Journal 120:868–70. [PubMed] [Google Scholar]
- McNeilly A. S. 2001. “Lactational Control of Reproduction.” Reproduction, Fertility, and Development 13, no. 7–8: 583–90. [DOI] [PubMed] [Google Scholar]
- Munro C. J., Stabenfeldt G. H., Cragun J. R., Addiego L. A., Overstreet J. W., Lasley B. L. 1991. “Relationship of Serum Estradiol and Progesterone Concentrations to the Excretion Profiles of Their Major Urinary Metabolites as Measured by Enzyme Immunoassay and Radioimmunoassay.” Clinical Chemistry 37, no. 6: 838–44. [PubMed] [Google Scholar]
- Rubenstein K. E., Schneider R. S., Ullman E. F. 1972. “‘Homogeneous’ Enzyme Immunoassay. A New Immunochemical Technique.” Biochemical and Biophysical Research Communications 47, no. 4: 846–51. [DOI] [PubMed] [Google Scholar]
- Taylor H. W., Shideler S., Samuels S. J., Lasley L. 2003. “Survival-time Analysis of the Postpartum Anovulatory Interval as Measured by Rise in Urinary Pregnanediol-3-glucuronide in Lactating Women.” Conference Proceedings—IEEE Engineering in Medicine and Biology Society 2995–97. [Google Scholar]
- Tommaselli G. A., Guida M., Palomba S., Barbato M., Nappi C. 2000. “Using Complete Breastfeeding and Lactational Amenorrhea as Birth Spacing Methods.” Contraception 6:253–57. [DOI] [PubMed] [Google Scholar]
- Velasquez E. V., Trigo R. V., Creu S., Campo S., Croxatto H. B. 2006. a. “Pituitary-ovarian Axis during Lactational Amenorrhoea. I. Longitudinal Assessment of Follicular Growth, Gonadotrophins, Sex Steroid and Inhibin Levels before and after Recovery of Menstrual Cyclicity.” Human Reproduction 4:909–15. [DOI] [PubMed] [Google Scholar]
- Velasquez E. V., Creus S., Trigo R. V., Cigorraga S. B., Pellizzari E. H., Croxatto H. B., Campo S. 2006. b. “Pituitary-ovarian Axis During Lactational Amenorrhoea. II. Longitudinal Assessment of Serum FSH Polymorphism Before and After Recovery of Menstrual Cycles”. Human Reproduction (Oxford, England) 21(4): 916–23. 10.1093/humrep/dei411 [DOI] [PubMed] [Google Scholar]
- Wei L., Qiu Y. 2007. “Relation of Supplementary Feeding to Resumptions of Menstruation and Ovulation in Lactating Postpartum Women.” Chinese Medical Journal 120: 868–70. [PubMed] [Google Scholar]
- Zinaman M., Stevenson W. 1991. “Efficacy of the Symptothermal Method of Natural Family Planning in Lactating Women after the Return of Menses.” American Journal of Obstetrics and Gynecology 165 (Suppl): 2037–39. [DOI] [PubMed] [Google Scholar]