Abstract
Subarachnoid hemorrhage (SAH) is a major cause of high morbidity, disability, and mortality in the field of neurovascular disease. Most previous SAH studies have focused on improving cerebral blood flow, reducing cerebral vasospasm, reducing neuronal calcium overload, and other treatments. While these studies showed exciting findings in basic science, therapeutic strategies based on the findings have not significantly improved neurological outcomes in patients with SAH. Currently, the only drug proven to effectively reduce the neurological defects of SAH patients is nimodipine. Current advances in imaging technologies in the field of stroke have confirmed that white matter injury (WMI) plays an important role in the prognosis of types of stroke, and suggests that WMI protection is essential for functional recovery and poststroke rehabilitation. However, WMI injury in relation to SAH has remained obscure until recently. An increasing number of studies suggest that the current limitations for SAH treatment are probably linked to overlooked WMI in previous studies that focused only on neurons and gray matter. In this review, we discuss the biology and functions of white matter in the normal brain, and discuss the potential pathophysiology and mechanisms of early brain injury after SAH. Our review demonstrates that WMI encompasses multiple substrates, and, therefore, more than one pharmacological approach is necessary to preserve WMI and prevent neurobehavioral impairment after SAH. Strategies targeting both neuronal injury and WMI may potentially provide a novel future for SAH knowledge and treatment.
Keywords: subarachnoid hemorrhage, early brain injury, white matter injury, neuroinflammation
Introduction
Subarachnoid hemorrhage (SAH)—one of the most common and critical cases of cerebrovascular disease—is caused mainly by sudden rupture of an intracranial aneurysm1. Thanks to the development of neuroimaging, microsurgical treatments, and endovascular interventional techniques, the overall mortality of SAH patients has greatly decreased2–4. However, there is still a very high morbidity and mortality in SAH patients; approximately 12% of patients die before receiving any medical attention, up to 33% of patients die within 48 h, and only approximately 40% of survivors can return to a prehemorrhage functional status after treatment5. Therefore, although aneurysmal SAH accounts for only 5–7% of all strokes, its burden on society and families cannot be ignored6. Moreover, the drug treatment choice for SAH is still very narrow, although more and more physiopathologic mechanisms have been identified by basic studies. Nowadays, the only drug proven to effectively reduce the neurological defects of SAH patients is nimodipine7.
Recent studies have found that, within 72 h of SAH, pathophysiological changes, including an increase in intracranial pressure (ICP), cerebral edema, neuroinflammation, oxidative stress, and destruction of the blood–brain barrier are closely related to neurological dysfunction in SAH patients8. Early brain injury (EBI) refers to the secondary brain injuries of patients after SAH9. In recent years, the idea that EBI is the main prognosticator of SAH patients has been widely accepted. Although increasing basic and clinical research has focused on EBI after SAH and demonstrated promising findings10–12, therapeutic options that adequately address this disease are still limited.
Reviewing previous studies of EBI after SAH, it was surprising to find that studies on secondary brain injury after SAH were concentrated mainly on gray matter and neuronal cells. Although white matter accounts for more than 50% of the human brain volume, and an autopsy study reported that there were changes in the white matter after SAH13, the pathophysiological changes in the EBI post-SAH have been underreported14,15. A limited understanding of the white matter injury pathophysiological process in EBI after SAH may be one of the reasons for the current high mortality and morbidity of SAH patients. Thus, understanding the extent to which WMI contributes to neurological impairment after SAH is extremely urgent. Fortunately, some studies have focused on the role of WMI in EBI after SAH16,17, and highlighted the need for therapeutic interventions aimed at ameliorating WMI or promoting white matter recovery, as well as the need to dissect the molecular mechanisms involved in the pathophysiology of this injury.
However, current studies describing WMI in SAH remain limited. We reviewed the pertinent literature, focusing on the biology and functions of white matter in the normal brain, and discuss the related pathophysiological mechanisms underlying WMI and potential therapeutic targets of WMI in EBI after SAH.
General Description of Cerebral WMI
The human brain is comprised of both gray matter and white matter; white matter constitutes over 50% of the total brain volume18. Gray matter mainly consists of neuronal cell bodies, glial cells, and blood vessels, whereas white matter mainly consists of myelinated and unmyelinated axons, white matter fiber tracts and supporting glial cells including oligodendrocytes19–21. White matter is named for its relatively lighter appearance resulting from the lipid content of myelin and fewer capillaries than the gray matter. Myelin is found in almost all long nerve fibers and acts as electrical insulation, allowing signals to pass quickly from one brain region to another22.
Though it was thought to be passive tissue, white matter actually affects multiple brain functions. The role of white matter in the central nervous system includes supporting and connecting different parts of brain gray matter together, modulating the distribution of action potentials, acting as a relay and coordinating essential neuronal communications23, as well as information conveyance among the network of efferent and afferent axonal fibers between the brain and spine24,25. In the brain, white matter is mainly located in the deep brain, such as periventricular area and subcortical area, where the blood supply is usually poor26,27. Due to its unusual shape and location, white matter tends to be more sensitive to injury factors such as mechanical forces, hypoperfusion and hypoxia, and neuroinflammation. Notably, even in the normal aging process, there is a decline in the total length of white matter at a speed of approximately 10% each decade28,29. Therefore, once a brain insult causes WMI, it may cause subsequent changes in the neurosignal conduction pathway, leading to significant sensorimotor disturbances and cognitive abnormalities. Moreover, it was reported that WMI is related to a more rapid and greater global cognitive performance decline in later cognition30,31.
WMI includes oligodendrocytes injury-induced demyelination, axonotmesis, and parenchymal abnormalities changes. In clinical settings, there are many kinds of WMI classification according to different criteria. WMI can be roughly divided into two distinct parts: global WMI and focal WMI32. For focal WMI, the white matter is injured at a specific locus, such as periventricular WMI33. On the other hand, global WMI is diffuse axonal injury over the entire brain34. It is usually a result of whole brain hypoxic/ischemic injury, which commonly occurs in stroke, cardiac arrest, persistent intense neuroinflammation such as autoimmune encephalomyelitis and pathogenic infection, and demyelination due to drug use.
WMI in relation to demyelination and axonotmesis may be reversible35, while gray matter injury, such as neuronal apoptosis, is less likely to regenerate. Therefore, WMI may have more importance in brain recovery than it is currently known. Despite the fact that several types of pathological damage, such as neuroinflammation, oxidative stress, and blood–brain barrier (BBB) disruption, have been identified as potential factors of WMI, the exact molecular mechanisms remain unclear.
Evidence of WMI After SAH
WMI can occur in many situations, such as traumatic brain injury (TBI), spinal cord injury, hypoxia-ischemic injury, cardiac arrest, and stroke. Although stroke has an acute onset, it might have long-lasting effects on remote white matter integrity, and, thereby, increases the risk of long-term cognitive impairment36. It was reported that cognitive impairment is very common after SAH37, which could also be due to WMI. However, in both clinical and basic studies involving SAH, the role of WMI is usually overlooked, even though white matter plays an at least as important, if not more important, role as gray matter in maintaining normal brain functions. Fortunately, the importance of SAH-induced WMI has been increasingly recognized, and WMI has been recommended as a priority for basic and clinical SAH research15–17.
Post-SAH WMI was first studied systematically in basic research by Egashira and colleagues in an experimental SAH model15. The authors established a mouse SAH model by endovascular perforation and performed MRI scanning 24 h after SAH. They found that, compared with sham-injured animals, the volumes of T2-hyperintensity in white matter increased significantly after SAH. This result was supported by immunohistochemistry staining using NG2, β-amyloid precursor protein (β-APP) and degraded myelin basic protein (DMBP) antibodies. Later, Kummer and colleagues used more immunohistochemistry staining techniques and found obvious white matter damage after SAH in a mouse model38. They reported that SAH can induce multifocal axonal injury in the early stage after SAH, and that it lasts for a long time, suggesting that WMI is long-lasting following SAH. Weiner et al. found that WMI following SAH is closely related to the extent of EBI, thus providing a potential mechanistic explanation39. Guo and colleagues demonstrated that WMI could be considered as a criterion to class SAH severity into different grades40.
Although the above-mentioned histological studies were all performed in rodents, which have much lower levels of white matter volume than humans, both studies demonstrated remarkable WMI during the acute stage, and it persisted for a long period of time after SAH. These results are probably associated with the residual disability and cognitive impairment observed in most clinical SAH patients. Further studies in animals with abundant white matter may provide more effective information of widespread WMI following SAH. However, to date, almost no attention has been given to WMI after SAH in clinical studies. The neglect of white matter damage after SAH in previous studies may be a major reason for the lack of significant improvement in SAH treatment. To truly overcome EBI and improve patient outcomes after SAH, a concept of whole brain protection that takes both gray matter and white matter into consideration may be a breakthrough.
Potential Mechanisms of WMI After SAH
Although a growing number of studies have focused on white matter damage after SAH, there is not enough information about the underlying mechanisms of WMI after SAH. It was reported that some pathological mechanisms overlap in SAH, TBI, ischemic stroke, and other neurological diseases. Therefore, we tried to explain the mechanisms of WMI after SAH from the aspect of some commonly shared pathophysiologic changes in SAH, TBI, and ischemic stroke.
Blood-brain Barrier Disruptions
WM cells incorporating astrocytes, oligodendrocytes, pericytes, and microglia are recognized as key cellular components of the gliovascular unit, playing a prominent role in maintaining homeostasis of the blood–brainBBB41,42. After BBB disruption, plasma toxicants enter the brain parenchyma, which can cause oligodendrocyte apoptosis and axonal demyelination43. Studies have shown that BBB destruction is commonly involved in the pathophysiological processes of white matter damage after numerous central nervous system injuries44–46. Furthermore, due to developmental factors, BBB integrity is incomplete in some special white matter areas of brain tissue, even under normal circumstances. For example, there are usually inherent defects of the BBB integrity in the periventricular white matter47. Therefore, a brain injury that causes BBB disruption may theoretically result in WMI, which subsequently causes further BBB disruption, forming a vicious cycle.
In TBI, BBB damage can significantly aggravate axonal injury, thus aggravating sensorimotor dysfunctions48. In stroke studies involving both cerebral hemorrhage and ischemic stroke, BBB disruption was also demonstrated as a core element for WMI49,50. After SAH, activation of matrix enzymes such as MMP-9 can degrade tight junction proteins and vascular basement, causing endothelial cells apoptosis, thereby promoting EBI51. The idea that BBB disruption is one of the most important reasons for poor neurological outcomes in SAH patients is widely accepted. Therefore, it can be speculated that damage of BBB is an important cause of WMI in EBI after SAH.
In a recent study, Egashira et al. used magnetic resonance imaging (MRI) with T2-weighted scanning and found significantly higher hyperintensity signals in the white area 24 h after SAH17,52. The transmission electron microscopy test showed that the ultrastructure of the BBB in the affected white matter region was significantly destroyed, as shown by tight junction destruction, astrocyte swelling, and microvascular basement membrane damage. To further confirm that BBB disruption contributes to WMI after SAH, the author measured WMI again in MMP-9–/– mice. The results showed that after SAH, MMP-9–/– mice showed less BBB disruption and subsequently fewer white matter abnormalities. In another study, Qu et al. demonstrated that inhibiting SAH-induced BBB disruption can reduce white matter after SAH53. We previously found that the white matter area hyperintensity signal increased after SAH as early as 6 h, and peaked at 48 h after the initial bleeding. Furthermore, after knockout of the apolipoprotein E gene, a key regulator of BBB integrity, white matter abnormalities increased to a more severe level due to more serious BBB disruption51. While an apolipoprotein E mimetic peptide reduced BBB disruption, the white matter area hyperintensity was reserved, while the BBB tight junction proteins were maintained11. Collectively, BBB disruption could be an important cause of WMI in EBI after SAH.
Physical and Mechanical Damage
Due to the location of white matter and the structural particularity of white matter fibers, white matter is more sensitive to mechanical injury. In patients with TBI, the brain is usually directly subjected to a mechanical force that is often accompanied by white matter damage54. In addition, the indirect mechanical force can also result in WMI. Friess et al. found that elevated ICP after TBI is closely related to white matter damage55. Early reduction of ICP by decompressive craniectomy can significantly reduce traumatic axonal injury.
After SAH, ICP elevation is also tightly related to adverse neurological outcomes. Kummer et al. found that the white matter in a perforating SAH mouse model showed multifocal lesions, and white matter damage opposite to the bleeding point was more obvious38. After analyzing this phenomenon with a biomechanical model, the authors found that WMI following SAH was closely related to the direction of biomechanical force at the moment of bleeding. Based on this study, we further studied the biomechanical force change after SAH in both an autogenous blood injection and an endovascular perforation SAH model. Our results showed that, after SAH, the biomechanical force affected the extent of WMI, but the existence of the cerebral ventricles may act as a pressure snubber, thereby reducing WMI after SAH56.
In clinics, approximately 80% of SAHs are caused by a cerebral aneurysm rupture. When an aneurysm ruptures, blood enters the subarachnoid space at high velocity from the ruptured site, creating a strong biomechanical impact on the corresponding white matter region, which may result in myelination and axon damage. When SAH occurs, blood entering the subarachnoid space can result in a sharp increase in ICP, which may also form a mechanical compression of deep white matter, also leading to WMI. Early decompressive craniectomy has been shown to play an important role in improving both the motorial and cognitive prognosis of patients with SAH57. This may be due to the reduction of white matter damage caused by the biomechanics of ICP after SAH, but further research is needed to confirm this hypothesis.
Neuroinflammation
The principal components of the white matter are fibers and glial cells, including a large number of oligodendrocytes, astrocytes, and microglia. When the brain is injured, a large number of proinflammatory glial cells are activated. Activated glial cells then secrete a variety of inflammatory cytokines58. In turn, the released inflammatory cytokines can further activate glial cells through their specific receptors, forming a vicious cycle, which aggravates brain damage. Studies found that in TBI, glial activation is closely related to oligodendrocyte apoptosis, axonal demyelination, and axonal fragmentation. Inhibition of glial cell activation can significantly reduce this damage59,60. In the process of intracerebral hemorrhage, astrocytes and microglia activation and the release of inflammatory cytokines are also essential factors of white matter damage61. It was also found that there is an obvious correlation between glial-cell-activation-mediated inflammatory response and white matter damage in ischemic stroke studies62.
Neuroinflammation was reported to cause both EBI and delayed cerebral ischemia after SAH, which are currently the most significant two issues in secondary brain injury after SAH63,64. After SAH, peripheral immunocyte infiltration and local glial cells activation can lead to severe neuroinflammatory responses. In addition, ischemic and oxidative stress after SAH can lead to neuronal cell death and raise endogenous upregulation of Toll-like receptors and other inflammatory signaling pathways, which will also cause widespread neuroinflammation65. However, whether neuroinflammation is associated with WMI after SAH is unclear.
According to Kummer and colleagues, microglia activation was markedly increased in the white matter area when looking into the mechanism of white matter damage after SAH38. They found that, in the corpus callosum, outer capsule, and internal capsule, microglial activation was closely related to the severity of an axonal injury. Our previous study also found that in the corpus callosum and periventricular area, microglia, and astrocytes were significantly increased, and this was tightly related to WMI after SAH56. In summary, the neuroinflammatory response is another important factor of WMI in EBI after SAH.
Ischemia and Oxidative Stress
The brain accounts for only 2–3% of body weight, but the blood supply required is approximately 20% of the heart output, and the brain has virtually no excess oxygen and glucose reserves66. Therefore, brain tissue is very susceptible to ischemic injuries. Unlike gray matter, which has an abundant blood supply, white matter exists predominately in the deep brain. Its blood supply relies mainly on a few long perforating branches of the pia artery, which shares a common feature of terminal artery branches with poor collateral communications67. White matter, with its axons and oligodendrocytes, is therefore even more sensitive to ischemia and oxidative stress injury. Moreover, ischemia is often accompanied by the increase of reactive oxygen species (ROS) and other oxidative stress responses. On the one hand, the increase in free radicals can lead directly to the destruction of white matter components such as oligodendrocytes and axons68. On the other hand, the increase in oxidative stress can activate MMP-9 and lead to increased BBB disruption69. Increased oxidative stress can also increase the neuroinflammatory reaction70, thereby aggravating white matter damage. Therefore, ischemia and oxidative stress are also very important factors for white matter injury.
After TBI, posttraumatic hypoxia and chronic cerebral blood flow reduction usually exacerbate brain axonal injury71,72. In the middle cerebral artery occlusion (MACO) model, Irving et al. found that oligodendrocyte swelling was more obvious and severe and lasted for a longer time in the ischemic white matter area73. Shindo and colleagues also found that bilateral common carotid artery ligation in rats can result in significant cerebral ischemia and white matter injury74. Although ischemic-stroke-caused cerebral ischemia is usually due to a long-lasting complete blood flow disruption, while ischemia caused by SAH is often local small-vessel-based, other factors such as ICP increase, vascular constriction, and microcirculation thrombosis can also lead to extensive cerebral ischemia after SAH. Hence, cerebral ischemia is probably another mechanism of white matter injury after SAH. In fact, a previous study demonstrated that delayed cerebral infarction was associated with patients’ cognitive impairment after SAH75. Muroi et al. found that cerebral blood flow in the white matter region was significantly reduced when MRI scanning detected a significant T2 hyperintensity signal in these areas after SAH76. Our previous study also found that, even in good-grade SAH patients, the incidence of elevated ICP was associated with a lower blood perfusion level in the white matter region after SAH77.
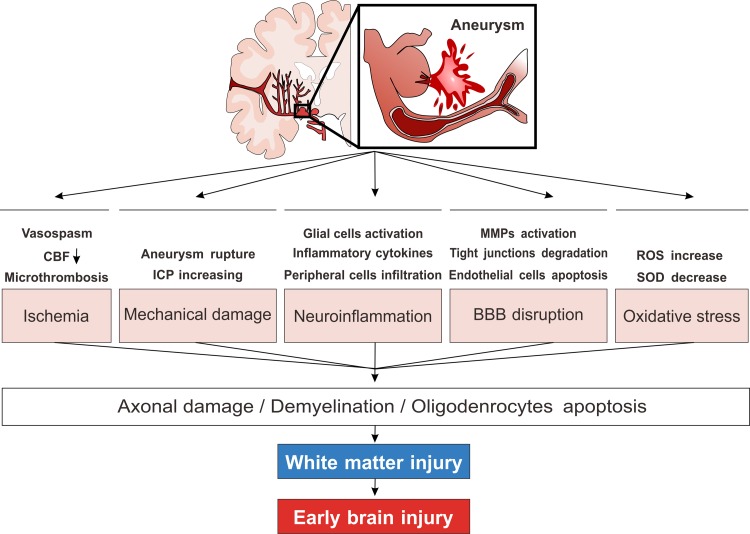
Collectively, the abovementioned mechanisms are associated with WMI in EBI after SAH, which are summarized in Fig. 1. Other mechanisms, such as excitotoxic amino acids, which contribute to EBI after SAH and take part in WMI in other cerebral injuries, may also be an explanation of WMI after SAH. However, because WMI after SAH has only been emphasized recently, further understanding of the exact mechanism of WMI after SAH is required and more clinical and basic studies are needed.
Fig. 1.
Simplified schematic diagram of the possible pathophysiological mechanisms of WMI following SAH. Vasoconstriction induced CBF decrease, aneurysm rupture, and ICP increase induced mechanical barotrauma are both responsible for the WMI in EBI after SAH. Later on, BBB disruption characterized by MMPs activation and endothelial cells apoptosis, neuroinflammation characterized by glial cells activation, peripheral inflammatory cell infiltration and cytokines release, oxidative stress will further deteriorate WMI. Although the detailed molecular mechanisms remain unclear, these mechanisms will finally lead the consequence of oligodendrocytes apoptosis, white matter demyelination and axonal damage. SAH: subarachnoid hemorrhage; CBF: cerebral blood flow; WMI: white matter injury; MMPs: matrix metalloproteinase; ICP: intracranial pressure.
WMI Diagnosis after SAH
Since white matter has an important influence on both motorial and cognitive neurological performance, once SAH patients have shown neurological dysfunction, the possibility of WMI should be considered. In clinical work, it is of great importance to evaluate patients’ language, cognitive performance, and memory functions with special score scales in the diagnosis of WMI. The age-related white matter changes scale (ARWMC), Fazekas scale, modified Scheltens scale and Ylikoshi scale are all commonly used to assess WMI. The Montreal cognitive assessment scale (MoCA) is commonly used in both stroke and other types of brain injury-induced cognitive impairment. Therefore, using these exact score scales in SAH diagnosis may help early identification of WMI.
Advances in neuroimaging techniques have enabled greater understanding of the progression of cerebral WMI54. MRI with T2-weighted scanning after SAH can easily detect increases in the white matter area signal51. A previous study reported that hyperintensity T2 signal areas are highly consistent with the histological detection of myelin degradation, axonal edema, and other white matter damage markers15,17, suggesting that MRI with T2-weighted imaging is a useful way to detect WMI after SAH. In addition, MRI diffusion tensor imaging (DTI) has good sensitivity for the diffusion of water molecules, and has significant advantages for white matter imaging78,79. By calculating a certain technical value, such as the fractional anisotropy (FA), mean diffusivity (MD), and other parameters, DTI can assess the integrity and connectivity of WM tracts and reconstruct the 3D distribution of white matter. It was reported that the white matter ADC values of DTI scanning were significantly increased in SAH patients80. Therefore, MRI scanning is very useful for determining the microstructural pathophysiology of WMI after SAH.
MRI perfusion imaging and CT perfusion imaging can detect abnormal blood perfusion in the brain white matter area at an early stage26,81, which has critical implications for the diagnosis of white matter injury after SAH. Transcranial Doppler can rapidly and noninvasively detect the blood flow changes in specific brain regions; therefore, this technique has certain significance in the diagnosis of WMI after SAH82. However, due to its lower resolution and requirement for operator experience, its use is limited to clinics.
An additional method that may help detect WMI after SAH is serum or cerebrospinal fluid (CSF) biomarker assessment. WMI can cause degradation of axons and myelin and facilitate the outflow of intracellular contents into the extracellular space, followed by diffusion of the contents into the CSF and even into the blood circulating through a disrupted BBB. Neurofilament, tau, amyloid beta, amyloid precursor protein (App), and myelin basic protein (MBP) are usually used as biomarkers in studies related to WMI83–85. In SAH, it was reported that the CSF concentration of tau and amyloid beta were increased significantly and correlated tightly with patients’ cognitive performance at both early and later stages86. In another study, Levis et al. reported that the levels of the phosphorylated axonal form neurofilament subunit NF-H (pNF-H) in both the CSF and blood were significantly increased, which presumably reflected axonal degeneration secondary to the original insult87. Therefore, detection of these WMI biomarkers may also help diagnose WMI after SAH.
WMI Treatment after SAH
Based on the important effects of WMI on the prognosis of SAH, reduction in WMI after SAH is of great significance in promoting patients’ recovery. As SAH is mostly induced by a ruptured intracerebral aneurysm, the first step for reducing SAH-induced WMI is to prevent aneurysm rupture by either microsurgical or endovascular interventions. Due to the fact that WMIs frequently induce cognitive impairments, once severe physical and cognitive dysfunction appears, referral to rehabilitation centers appears to be a good way to help the patient recover88.
Although nimodipine administration was reported to improve SAH patients’ cognition, other drugs that are currently under clinical trials have also been reported to improve SAH patients’ cognition; it is not clear whether these benefits are associated with reduced WMI. As WMI is usually characterized by disruption of axons, failure of axonal regeneration and demyelination, strategies that reduce these changes may help promote WMI recovery after SAH. It was reported that minocycline treatment can reduce both TBI and ischemic injury-induced WMI89. Erythropoietin is another widely studied therapy for WMI89,90. Other treatments such as cell implantation can also reduce WMI. It has been reported that intranasal stem cell treatment can significantly reduce white matter loss and decrease SAH-induced cognitive impairments91.
Notably, current research about WMI after SAH is still rare. Its mechanism is not yet fully understood. Therefore, effective treatment strategies for WMI after SAH are still lacking. Based on the fact that multiple mechanisms, including neuroinflammation and BBB disruption, may affect WMI after SAH, future studies should develop an effective therapeutic strategy that covers both neuroinflammation, ischemic and oxidative stress, and BBB disruption.
Summary
Consequential changes result in both gray matter injury and WMI in EBI after SAH. It is probable that the lack of attention to WMI in past studies is partially responsible for the current lack of SAH treatment options. With increasing knowledge of WMI after SAH, a strategy targeting WMI may provide new hope for SAH patients. However, both gray and white matter are very important for proper brain function; thus, concentrating only on WMI after SAH may be as fruitless as targeting only gray matter injury. Owing to the complex pathophysiological process of EBI, developing therapeutic strategies that target both gray matter injury and WMI may alleviate EBI and improve patients’ neurological function after SAH.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by grants from the National Natural Science Foundation of China (81771278, 81801176), Sichuan Provincial Health and Family Planning Commission research project (17PJ076), Luzhou Government-Southwest Medical University Strategic Cooperation Project (2016LZXNYD-J12, 2016LZXNYD-Z02) and the Youth Innovation Project of Sichuan Medical Scientific Research (Q17082).
References
- 1. Etminan N. Aneurysmal subarachnoid hemorrhage—status quo and perspective. Transl Stroke Res. 2015;6(3):167–170. [DOI] [PubMed] [Google Scholar]
- 2. Gritti P, Akeju O, Lorini FL, Lanterna LA, Brembilla C, Bilotta F. A narrative review of adherence to subarachnoid hemorrhage guidelines. J Neurosurg Anesthesiol. 2018;30(3):203–216. [DOI] [PubMed] [Google Scholar]
- 3. Anokwute MC, Braca JA, Bohnstedt B, DeNardo A, Scott J, Cohen-Gadol A, Sahlein DH. Endovascular treatment of ruptured tiny (3 mm) intracranial aneurysms in the setting of subarachnoid hemorrhage: a case series of 20 patients and literature review. J Clin Neurosci. 2017;40:52–56. [DOI] [PubMed] [Google Scholar]
- 4. Nelson SE, Sair HI, Stevens RD. Magnetic resonance imaging in aneurysmal subarachnoid hemorrhage: current evidence and future directions. Neurocrit Care. 2018;29(2):241–252. [DOI] [PubMed] [Google Scholar]
- 5. Dabus G, Nogueira RG. Current options for the management of aneurysmal subarachnoid hemorrhage-induced cerebral vasospasm: a comprehensive review of the literature. Interv Neurol. 2013;2(1):30–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ishihara H, Sugimoto K, Shirao S, Suzuki M. Required knowledge for stroke specialists (7): current trend of subarachnoid hemorrhage [in Japanese]. No Shinkei Geka. 2015;43(2):159–166. [DOI] [PubMed] [Google Scholar]
- 7. Steiger HJ, Beez T, Beseoglu K, Hanggi D, Kamp MA. Perioperative measures to improve outcome after subarachnoid hemorrhage-revisiting the concept of secondary brain injury. Acta Neurochir Suppl. 2015;120:211–216. [DOI] [PubMed] [Google Scholar]
- 8. Serrone JC, Maekawa H, Tjahjadi M, Hernesniemi J. Aneurysmal subarachnoid hemorrhage: pathobiology, current treatment and future directions. Expert Rev Neurother. 2015;15(4):367–380. [DOI] [PubMed] [Google Scholar]
- 9. Fujii M, Yan J, Rolland WB, Soejima Y, Caner B, Zhang JH. Early brain injury, an evolving frontier in subarachnoid hemorrhage research. Transl Stroke Res. 2013;4(4):432–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tso MK, Macdonald RL. Subarachnoid hemorrhage: a review of experimental studies on the microcirculation and the neurovascular unit. Transl Stroke Res. 2014;5(2):174–189. [DOI] [PubMed] [Google Scholar]
- 11. Pang J, Chen Y, Kuai L, Yang P, Peng J, Wu Y, Chen Y, Vitek MP, Chen L, Sun X, Jiang Y. Inhibition of blood–brain barrier disruption by an apolipoprotein E-mimetic peptide ameliorates early brain injury in experimental subarachnoid hemorrhage. Transl Stroke Res. 2017;8(3):257–272. [DOI] [PubMed] [Google Scholar]
- 12. Shi L, Al-Baadani A, Zhou K, Shao A, Xu S, Chen S, Zhang J. PCMT1 ameliorates neuronal apoptosis by inhibiting the activation of MST1 after subarachnoid hemorrhage in rats. Transl Stroke Res. 2017;8(5):474–483. [DOI] [PubMed] [Google Scholar]
- 13. Satomi J, Hadeishi H, Yoshida Y, Suzuki A, Nagahiro S. Histopathological findings in brains of patients who died in the acute stage of poor-grade subarachnoid hemorrhage. Neurol Med Chir (Tokyo). 2016;56(12):766–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Egashira Y, Xi G, Chaudhary N, Hua Y, Pandey AS. Acute brain injury after subarachnoid hemorrhage. World Neurosurg. 2015;84(1):22–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Egashira Y, Hua Y, Keep RF, Xi G. Acute white matter injury after experimental subarachnoid hemorrhage: potential role of lipocalin 2. Stroke. 2014;45(7):2141–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Egashira Y, Hua Y, Keep RF, Iwama T, Xi G. Lipocalin 2 and blood–brain barrier disruption in white matter after experimental subarachnoid hemorrhage. Acta Neurochir Suppl. 2016;121:131–134. [DOI] [PubMed] [Google Scholar]
- 17. Egashira Y, Zhao H, Hua Y, Keep RF, Xi G. White matter injury after subarachnoid hemorrhage: role of blood–brain barrier disruption and matrix metalloproteinase-9. Stroke. 2015;46(10):2909–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gaynor JW, Nicolson SC, Spray DM, Burnham NB, Chittams JL, Sammarco T, Walsh KW, Spray TL, Licht DJ. Remote ischemic preconditioning does not prevent white matter injury in neonates. Ann Thorac Surg. 2018;106(1):151–155. [DOI] [PubMed] [Google Scholar]
- 19. Lee YA. White matter injury of prematurity: its mechanisms and clinical features. J Pathol Transl Med. 2017;51(5):449–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mierzwa AJ, Marion CM, Sullivan GM, McDaniel DP, Armstrong RC. Components of myelin damage and repair in the progression of white matter pathology after mild traumatic brain injury. J Neuropathol Exp Neurol. 2015;74(3):218–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vanezis P, Chan KK, Scholtz CL. White matter damage following acute head injury. Forensic Sci Int. 1987;35(1):1–10. [DOI] [PubMed] [Google Scholar]
- 22. Fields RD. White matter matters. Sci Am. 2008;298(3):42–49. [PubMed] [Google Scholar]
- 23. Stetler RA, Gao Y, Leak RK, Weng Z, Shi Y, Zhang L, Pu H, Zhang F, Hu X, Hassan S, Ferguson C, Homanics GE, Cao G, Bennett MV, Chen J. APE1/Ref-1 facilitates recovery of gray and white matter and neurological function after mild stroke injury. Proc Natl Acad Sci U S A. 2016;113(25):E3558–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yuan W, Meller A, Shimony JS, Nash T, Jones BV, Holland SK, Altaye M, Barnard H, Phillips J, Powell S, McKinstry RC, Limbrick DD, Rajagopal A, Mangano FT. Left hemisphere structural connectivity abnormality in pediatric hydrocephalus patients following surgery. Neuroimage Clin. 2016;12:631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fozouni N, Chopp M, Nejad-Davarani SP, Zhang ZG, Lehman NL, Gu S, Ueno Y, Lu M, Ding G, Li L, Hu J, Bagher-Ebadian H, Hearshen D, Jiang Q. Characterizing brain structures and remodeling after TBI based on information content, diffusion entropy. PLoS One. 2013;8(10):e76343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tortora D, Mattei PA, Navarra R, Panara V, Salomone R, Rossi A, Detre JA, Caulo M. Prematurity and brain perfusion: arterial spin labeling MRI. Neuroimage Clin. 2017;15:401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grysiewicz R, Gorelick PB. Key neuroanatomical structures for post-stroke cognitive impairment. Curr Neurol Neurosci Rep. 2012;12(6):703–708. [DOI] [PubMed] [Google Scholar]
- 28. Brown WR, Thore CR, Moody DM, Robbins ME, Wheeler KT. Vascular damage after fractionated whole-brain irradiation in rats. Radiat Res. 2005;164(5):662–668. [DOI] [PubMed] [Google Scholar]
- 29. Marner L, Nyengaard JR, Tang Y, Pakkenberg B. Marked loss of myelinated nerve fibers in the human brain with age. J Comp Neurol. 2003;462(2):144–152. [DOI] [PubMed] [Google Scholar]
- 30. Debette S, Seshadri S, Beiser A, Au R, Himali JJ, Palumbo C, Wolf PA, DeCarli C. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77(5):461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jolly TA, Cooper PS, Rennie JL, Levi CR, Lenroot R, Parsons MW, Michie PT, Karayanidis F. Age-related decline in task switching is linked to both global and tract-specific changes in white matter microstructure. Hum Brain Mapp. 2017;38(3):1588–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tasker RC, Westland AG, White DK, Williams GB. Corpus callosum and inferior forebrain white matter microstructure are related to functional outcome from raised intracranial pressure in child traumatic brain injury. Dev Neurosci. 2010;32(5-6):374–384. [DOI] [PubMed] [Google Scholar]
- 33. Selip DB, Jantzie LL, Chang M, Jackson MC, Fitzgerald EC, Boll G, Murphy A, Jensen FE. Regional differences in susceptibility to hypoxic-ischemic injury in the preterm brain: exploring the spectrum from white matter loss to selective grey matter injury in a rat model. Neurol Res Int. 2012;2012:725184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Riddle A, Maire J, Gong X, Chen KX, Kroenke CD, Hohimer AR, Back SA. Differential susceptibility to axonopathy in necrotic and non-necrotic perinatal white matter injury. Stroke. 2012;43(1):178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Adina MT, Patricia R, Peter S. Chapter 11-Stroke and head injury The Nervous System. 2nd ed Edinburgh: Churchill Livingstone, 2010;199–226, ISBN 9780702033735. [Google Scholar]
- 36. Schaapsmeerders P, Tuladhar AM, Arntz RM, Franssen S, Maaijwee NA, Rutten-Jacobs LC, Schoonderwaldt HC, Dorresteijn LD, van Dijk EJ, Kessels RP, de Leeuw FE. Remote lower white matter integrity increases the risk of long-term cognitive impairment after ischemic stroke in young adults. Stroke. 2016;47(10):2517–2525. [DOI] [PubMed] [Google Scholar]
- 37. Wong GK, Lam SW, Wong A, Mok V, Siu D, Ngai K, Poon WS. Early MOCA-assessed cognitive impairment after aneurysmal subarachnoid hemorrhage and relationship to 1-year functional outcome. Transl Stroke Res. 2014;5(2):286–291. [DOI] [PubMed] [Google Scholar]
- 38. Kummer TT, Magnoni S, MacDonald CL, Dikranian K, Milner E, Sorrell J, Conte V, Benetatos JJ, Zipfel GJ, Brody DL. Experimental subarachnoid haemorrhage results in multifocal axonal injury. Brain. 2015;138(Pt 9):2608–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Weiner GM, Ozpinar A, Ducruet AF. The role of matrix metalloproteinase-9 in subarachnoid hemorrhage-induced white matter injury. Neurosurgery. 2016;78(2):N11–N12. [DOI] [PubMed] [Google Scholar]
- 40. Guo D, Wilkinson DA, Thompson BG, Pandey AS, Keep RF, Xi G, Hua Y. MRI characterization in the acute phase of experimental subarachnoid hemorrhage. Transl Stroke Res. 2017;8(3):234–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cai W, Liu H, Zhao J, Chen LY, Chen J, Lu Z, Hu X. Pericytes in brain injury and repair after ischemic stroke. Transl Stroke Res. 2017;8(2):107–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Alhadidi Q, Bin Sayeed MS, Shah ZA. Cofilin as a promising therapeutic target for ischemic and hemorrhagic stroke. Transl Stroke Res. 2016;7(1):33–41. [DOI] [PubMed] [Google Scholar]
- 43. Wang LW, Tu YF, Huang CC, Ho CJ. JNK signaling is the shared pathway linking neuroinflammation, blood–brain barrier disruption, and oligodendroglial apoptosis in the white matter injury of the immature brain. J Neuroinflammation. 2012;9:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Badaut J, Bix GJ. Vascular neural network phenotypic transformation after traumatic injury: potential role in long-term sequelae. Transl Stroke Res. 2014;5(3):394–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hamanaka G, Ohtomo R, Takase H, Lok J, Arai K. Role of oligodendrocyte-neurovascular unit in white matter repair. Neurosci Lett. 2018;684:175–180. [DOI] [PubMed] [Google Scholar]
- 46. Yang Y, Kimura-Ohba S, Thompson JF, Salayandia VM, Cosse M, Raz L, Jalal FY, Rosenberg GA. Vascular tight junction disruption and angiogenesis in spontaneously hypertensive rat with neuroinflammatory white matter injury. Neurobiol Dis. 2018;114:95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ueno M, Akiguchi I, Hosokawa M, Kotani H, Kanenishi K, Sakamoto H. Blood–brain barrier permeability in the periventricular areas of the normal mouse brain. Acta Neuropathol. 2000;99(4):385–392. [DOI] [PubMed] [Google Scholar]
- 48. Glushakova OY, Johnson D, Hayes RL. Delayed increases in microvascular pathology after experimental traumatic brain injury are associated with prolonged inflammation, blood–brain barrier disruption, and progressive white matter damage. J Neurotrauma. 2014;31(13):1180–1193. [DOI] [PubMed] [Google Scholar]
- 49. Moxon-Emre I, Schlichter LC. Neutrophil depletion reduces blood–brain barrier breakdown, axon injury, and inflammation after intracerebral hemorrhage. J Neuropathol Exp Neurol. 2011;70(3):218–235. [DOI] [PubMed] [Google Scholar]
- 50. Yang Y, Rosenberg GA. Blood–brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke. 2011;42(11):3323–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pang J, Wu Y, Peng J, Yang P, Kuai L, Qin X, Cao F, Sun X, Chen L, Vitek MP, Jiang Y. Potential implications of apolipoprotein E in early brain injury after experimental subarachnoid hemorrhage: involvement in the modulation of blood–brain barrier integrity. Oncotarget. 2016;7(35):56030–56044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Choi JY, Cui Y, Kim BG. Interaction between hypertension and cerebral hypoperfusion in the development of cognitive dysfunction and white matter pathology in rats. Neuroscience. 2015;303:115–125. [DOI] [PubMed] [Google Scholar]
- 53. Qu J, Zhao H, Li Q, Pan P, Ma K, Liu X, Feng H, Chen Y. MST1 suppression reduces early brain injury by inhibiting the NF-κB/MMP-9 pathway after subarachnoid hemorrhage in mice. Behav Neurol. 2018;2018:6470957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sharp DJ, Ham TE. Investigating white matter injury after mild traumatic brain injury. Curr Opin Neurol. 2011;24(6):558–563. [DOI] [PubMed] [Google Scholar]
- 55. Friess SH, Lapidus JB, Brody DL. Decompressive craniectomy reduces white matter injury after controlled cortical impact in mice. J Neurotrauma. 2015;32(11):791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wu Y, Peng J, Pang J, Sun X, Jiang Y. Potential mechanisms of white matter injury in the acute phase of experimental subarachnoid haemorrhage. Brain. 2017;140(6):e36. [DOI] [PubMed] [Google Scholar]
- 57. Jabbarli R, Oppong MD, Dammann P, Wrede KH, El Hindy N, Ozkan N, Muller O, Forsting M, Sure U. Time is brain! Analysis of 245 cases with decompressive craniectomy due to subarachnoid hemorrhage. World Neurosurg. 2017;98:689–694.e2. [DOI] [PubMed] [Google Scholar]
- 58. Karve IP, Taylor JM, Crack PJ. The contribution of astrocytes and microglia to traumatic brain injury. Br J Pharmacol. 2016;173(4):692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wen L, You W, Wang H, Meng Y, Feng J, Yang X. Polarization of microglia to the M2 phenotype in a peroxisome proliferator-activated receptor gamma-dependent manner attenuates axonal injury induced by traumatic brain injury in mice. J Neurotrauma. 2018;35(19):2330–2340. [DOI] [PubMed] [Google Scholar]
- 60. Menzel L, Kleber L, Friedrich C, Hummel R, Dangel L, Winter J, Schmitz K, Tegeder I, Schafer MK. Progranulin protects against exaggerated axonal injury and astrogliosis following traumatic brain injury. Glia. 2017;65(2):278–292. [DOI] [PubMed] [Google Scholar]
- 61. Zhao H, Qu J, Li Q, Cui M, Wang J, Zhang K, Liu X, Feng H, Chen Y. Taurine supplementation reduces neuroinflammation and protects against white matter injury after intracerebral hemorrhage in rats. Amino Acids. 2018;50(3–4):439–451. [DOI] [PubMed] [Google Scholar]
- 62. Wang Y, Liu G, Hong D, Chen F, Ji X, Cao G. White matter injury in ischemic stroke. Prog Neurobiol. 2016;141:45–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lucke-Wold BP, Logsdon AF, Manoranjan B, Turner RC, McConnell E, Vates GE, Huber JD, Rosen CL, Simard JM. Aneurysmal subarachnoid hemorrhage and neuroinflammation: a comprehensive review. Int J Mol Sci. 2016;17(4):497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. de Oliveira Manoel AL, Macdonald RL. Neuroinflammation as a target for intervention in subarachnoid hemorrhage. Front Neurol. 2018;9:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Liu L, Fujimoto M, Nakano F, Nishikawa H, Okada T, Kawakita F, Imanaka-Yoshida K, Yoshida T, Suzuki H. Deficiency of tenascin-C alleviates neuronal apoptosis and neuroinflammation after experimental subarachnoid hemorrhage in mice. Mol Neurobiol. 2018;55(11):8346–8354. [DOI] [PubMed] [Google Scholar]
- 66. Brooks GA, Martin NA. Cerebral metabolism following traumatic brain injury: new discoveries with implications for treatment. Front Neurosci. 2014;8:408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Martinez Sosa S, Smith KJ. Understanding a role for hypoxia in lesion formation and location in the deep and periventricular white matter in small vessel disease and multiple sclerosis. Clin Sci (Lond). 2017;131(20):2503–2524. [DOI] [PubMed] [Google Scholar]
- 68. Frati A, Cerretani D, Fiaschi AI, Frati P, Gatto V, La Russa R, Pesce A, Pinchi E, Santurro A, Fraschetti F, Fineschi V. Diffuse axonal injury and oxidative stress: a comprehensive review. Int J Mol Sci. 2017;18(12): pii: E2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Janyou A, Wicha P, Jittiwat J, Suksamrarn A, Tocharus C, Tocharus J. Dihydrocapsaicin attenuates blood brain barrier and cerebral damage in focal cerebral ischemia/reperfusion via oxidative stress and inflammatory. Sci Rep. 2017;7(1):10556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shah SA, Amin FU, Khan M, Abid MN, Rehman SU, Kim TH, Kim MW, Kim MO. Anthocyanins abrogate glutamate-induced ampk activation, oxidative stress, neuroinflammation, and neurodegeneration in postnatal rat brain. J Neuroinflammation. 2016;13(1):286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ojo JO, Mouzon B, Algamal M, Leary P, Lynch C, Abdullah L, Evans J, Mullan M, Bachmeier C, Stewart W, Crawford F. Chronic repetitive mild traumatic brain injury results in reduced cerebral blood flow, axonal injury, gliosis, and increased T-Tau and Tau oligomers. J Neuropathol Exp Neurol. 2016;75(7):636–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hellewell SC, Yan EB, Agyapomaa DA, Bye N, Morganti-Kossmann MC. Post-traumatic hypoxia exacerbates brain tissue damage: analysis of axonal injury and glial responses. J Neurotrauma. 2010;27(11):1997–2010. [DOI] [PubMed] [Google Scholar]
- 73. Irving EA, Bentley DL, Parsons AA. Assessment of white matter injury following prolonged focal cerebral ischaemia in the rat. Acta Neuropathol. 2001;102(6):627–635. [DOI] [PubMed] [Google Scholar]
- 74. Shindo A, Liang AC, Maki T, Miyamoto N, Tomimoto H, Lo EH, Arai K. Subcortical ischemic vascular disease: roles of oligodendrocyte function in experimental models of subcortical white-matter injury. J Cereb Blood Flow Metab. 2016;36(1):187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chu AC, Wong GK, Lam SW, Wong A, Ngai K, Poon WS, Mok V. Cognitive impairment in aneurysmal subarachnoid hemorrhage patients with delayed cerebral infarction: prevalence and pattern. Acta Neurochir Suppl. 2015;120:303–306. [DOI] [PubMed] [Google Scholar]
- 76. Muroi C, Kashiwagi Y, Rokugawa T, Tonomura M, Obata A, Nevzati E, Tsuboi A, Okuchi K, Mishima K, Abe K, Fujioka M. Evaluation of a filament perforation model for mouse subarachnoid hemorrhage using 7.0 tesla MRI. J Clin Neurosci. 2016;28:141–147. [DOI] [PubMed] [Google Scholar]
- 77. Peng JH, Qin XH, Pang JW, Wu Y, Dong JH, Huang CR, Wan WF, Yang XB, Sun XC, Chen LG, Jiang Y. Apolipoprotein E epsilon4: a possible risk factor of intracranial pressure and white matter perfusion in good-grade aneurysmal subarachnoid hemorrhage patients at early stage. Front Neurol. 2017;8:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Castano Leon AM, Cicuendez M, Navarro B, Munarriz PM, Cepeda S, Paredes I, Hilario A, Ramos A, Gomez PA, Lagares A. What can be learned from diffusion tensor imaging from a large traumatic brain injury cohort?: white matter integrity and its relationship with outcome. J Neurotrauma. 2018;35(20):2365–2376. [DOI] [PubMed] [Google Scholar]
- 79. Lepage C, de Pierrefeu A, Koerte IK, Coleman MJ, Pasternak O, Grant G, Marx CE, Morey RA, Flashman LA, George MS, McAllister TW, Andaluz N, Shutter L, Coimbra R, Zafonte RD, Stein MB, Shenton ME, Bouix S. White matter abnormalities in mild traumatic brain injury with and without post-traumatic stress disorder: a subject-specific diffusion tensor imaging study. Brain Imaging Behav. 2018;12(3):870–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Weimer JM, Jones SE, Frontera JA. Acute cytotoxic and vasogenic edema after subarachnoid hemorrhage: a quantitative MRI study. AJNR Am J Neuroradiol. 2017;38(5):928–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Metting Z, Cerliani L, Rodiger LA, van der Naalt J. Pathophysiological concepts in mild traumatic brain injury: diffusion tensor imaging related to acute perfusion CT imaging. PLoS One. 2013;8(5):e64461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Berman SE, Wang X, Mitchell CC, Kundu B, Jackson DC, Wilbrand SM, Varghese T, Hermann BP, Rowley HA, Johnson SC, Dempsey RJ. The relationship between carotid artery plaque stability and white matter ischemic injury. Neuroimage Clin. 2015;9:216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bergman J, Dring A, Zetterberg H, Blennow K, Norgren N, Gilthorpe J, Bergenheim T, Svenningsson A. Neurofilament light in CSF and serum is a sensitive marker for axonal white matter injury in MS. Neurol Neuroimmunol Neuroinflamm. 2016;3(5):e271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Cheung YT, Khan RB, Liu W, Brinkman TM, Edelmann MN, Reddick WE, Pei D, Panoskaltsis-Mortari A, Srivastava D, Cheng C, Robison LL, Hudson MM, Pui CH, Krull KR. Association of cerebrospinal fluid biomarkers of central nervous system injury with neurocognitive and brain imaging outcomes in children receiving chemotherapy for acute lymphoblastic leukemia. JAMA Oncol. 2018;4(7):e180089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Canuet L, Pusil S, Lopez ME, Bajo R, Pineda-Pardo JA, Cuesta P, Galvez G, Gaztelu JM, Lourido D, Garcia-Ribas G, Maestu F. Network disruption and cerebrospinal fluid amyloid-beta and phospho-tau levels in mild cognitive impairment. J Neurosci. 2015;35(28):10325–10330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Joswig H, Korte W, Fruh S, Epprecht L, Hildebrandt G, Fournier JY, Stienen MN. Neurodegenerative cerebrospinal fluid biomarkers tau and amyloid beta predict functional, quality of life, and neuropsychological outcomes after aneurysmal subarachnoid hemorrhage. Neurosurg Rev. 2018;41(2):605–614. [DOI] [PubMed] [Google Scholar]
- 87. Lewis SB, Wolper RA, Miralia L, Yang C, Shaw G. Detection of phosphorylated NF-H in the cerebrospinal fluid and blood of aneurysmal subarachnoid hemorrhage patients. J Cereb Blood Flow Metab. 2008;28(6):1261–1271. [DOI] [PubMed] [Google Scholar]
- 88. Fertl E, Killer M, Eder H, Linzmayer L, Richling B, Auff E. Long-term functional effects of aneurysmal subarachnoid haemorrhage with special emphasis on the patient’s view. Acta Neurochir (Wien). 1999;141(6):571–577. [DOI] [PubMed] [Google Scholar]
- 89. Genc S, Genc K, Kumral A, Ozkan H. White matter protection by erythropoietin: an emerging matter in the treatment of neonatal hypoxic-ischemic brain injury. Stroke. 2010;41(11):e595; author reply e596. [DOI] [PubMed] [Google Scholar]
- 90. Vitellaro-Zuccarello L, Mazzetti S, Madaschi L, Bosisio P, Gorio A, De Biasi S. Erythropoietin-mediated preservation of the white matter in rat spinal cord injury. Neuroscience. 2007;144(3):865–877. [DOI] [PubMed] [Google Scholar]
- 91. Nijboer CH, Kooijman E, van Velthoven CT, van Tilborg E, Tiebosch IA, Eijkelkamp N, Dijkhuizen RM, Kesecioglu J, Heijnen CJ. Intranasal stem cell treatment as a novel therapy for subarachnoid hemorrhage. Stem Cells Dev. 2018;27(5):313–325. [DOI] [PubMed] [Google Scholar]