Abstract
Vascular cognitive impairment (VCI) defines an entire spectrum of neurologic disorders from mild cognitive impairment to dementia caused by cerebral vascular disease. The pathogenesis of VCI includes ischemic factors (e.g., large vessel occlusion and small vessel dysfunction); hemorrhagic factors (e.g., intracerebral hemorrhage and subarachnoid hemorrhage); and other factors (combined with Alzheimer’s disease). Clinical evaluations of VCI mainly refer to neuropsychological testing and imaging assessments, including structural and functional neuroimaging, with different advantages. At present, the main treatment for VCI focuses on neurological protection, cerebral blood flow reconstruction, and neurological rehabilitation, such as pharmacological treatment, revascularization, and cognitive training. In this review, we discuss the pathogenesis, neuroimaging evaluation, and treatment of VCI.
Keywords: vascular cognitive impairment, pathogenesis, neuroimaging, treatment, progression
Vascular cognitive impairment (VCI) is a syndrome that includes all cognitive disorders attributable to various kinds of cerebral vascular disease and relative risk factors, and it is generally used to capture the entire spectrum of neurologic disorders, ranging from mild to severe1. It usually affects advanced brain functions, especially executive function and memory2. Although the definition and diagnostic criteria of VCI remain disputed, VCI can be classified by its clinical characteristics as vascular mild cognitive impairment, vascular dementia, and mixed dementia (MD) associated with vascular dysfunction, whose risk factors include age, hypertension, hyperlipidemia, hyperuricemia, diabetes, cardiopathy, stroke, carotid plaque, smoking, and low educational level3,4. Recent studies of VCI have mainly focused on its pathogenesis, evaluation, and treatment, and the present study aimed to summarize these advances.
Pathogenesis
The pathogenesis of VCI can be attributed to ischemic factors, hemorrhagic factors, and other factors affecting functional brain regions5,6. On this basis, atrophy of the gray matter and hemispheric white matter lesions caused by cerebral vascular diseases (CVD) becomes the main structural change of VCI7–9. Relating to these pathophysiological changes, many studies have provided new insight10,11.
Ischemic Factors
Large Vessel Occlusion
The occlusion of large vessels, such as ischemic stroke caused by cardio embolic and atherosclerotic diseases, constitutes a large brain infarct5. Many findings and the TABASCO study have confirmed that inflammatory mediators play an important role, together with amyloid deposition, in the development of VCI after stroke12. Back et al.13 reported that the occlusion of a large vessel may interfere with amyloid clearance through the glymphatic pathway and concomitant neuroinflammation to form VCI. Relevant results have suggested that cognitive decline appears, on average, after 2 years because of long-lasting effects on remote white matter integrity8. In particular, Mandzia et al.14 found that changes in executive function and psychomotor processing speed appear within 90 d after stroke.
Small Vessel Dysfunction
The dysfunction of small vessels supplying important brain regions, such as arteriosclerosis and arteritis, can cause cortical and subcortical micro-infarcts15, which result in long-time hypoperfusion because of decompensation of collateral circulation, and which appear to be the most robust substrates of cognitive dysfunction16,17. In particular, some findings18 on hereditary diseases, such as cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), have provided new insight into the mechanisms of dementia associated with cerebral small vessel disease. Rosenberg19 found that cerebral hypoperfusion leads to fibrosis of the extracellular matrix and activates neuroinflammation, which is most damaging to the deep white matter. These types of multiple infarctions and diffuse white matter lesions often appear in the lateral ventricle and subcortex, resulting in multicognitive domain impairment.
Hemorrhagic Factors
Intracerebral Hemorrhage
Patients have significant cognitive impairment after intracranial hemorrhage (ICH)20,21. Visual-processing functions decline obviously in this type22,23. Many studies22,24–27 on intracranial microbleeds have confirmed that cerebrovascular amyloidosis contributes to dementia and cognitive impairment by means of worsening vascular amyloid-β accumulation, activation of vascular injury pathways, and impaired vascular physiology6, while others28 indicate that the disturbance of brain iron metabolism after ICH caused by inflammation can also enhance brain injury and contribute to VCI.
Subarachnoid Hemorrhage
Cognitive dysfunction commonly appears with subarachnoid hemorrhage (SAH)29. The anterior cingulate gyrus and frontobasal regions are often involved in SAH, resulting in neurocognitive deficits including visuospatial memory and language30. A recent study31 showed that the left parahippocampal gyrus, left inferior temporal gyrus, and left thalamus are also involved in the formation of VCI in patients with SAH. New findings32 established that the pathogenesis of VCI may be attributed to the impact of the subdural membrane on dural lymphatics. This mechanism, however, is still controversial, and relevant research is needed.
Other Factors
VCI can appear in the condition of CVD combined with Alzheimer’s disease (AD), which often results from both vascular disorders and structural changes in protein in brain tissue1. Apart from the increased levels of tau protein in cerebrospinal fluid among patients with VCI, Kalaria33 believes that there is a vascular basis for neuronal atrophy in MD entirely independent of AD pathology, which often appears with memory decline34.
Evaluations
Clinical Evaluation
The diagnosis of VCI relies on wide-ranging clinical evaluations, including pathological confirmation, neuropsychological tests, and multi-modal neuroimaging measures35. When lacking the appropriate brain samples for pathological diagnosis, Skrobot et al.36 suggest using neuropsychological and imaging assessments for the diagnosis of VCI. However, there are no specific neuropsychological tests for patients with VCI37. Neuropsychological testing is easy to do in the clinic, although, due to self-limitations such as ceiling effects, floor effects, and subjective influence38–40, only neuroimaging has been relevant for clinical practice regarding the differential diagnosis of dementia41.
Neuroimaging
Structural Neuroimaging
Structural changes in the brain have tight connections with VCI, which can be detected on structural magnetic resonance imaging (sMRI)42. Some studies7,43 suggest that different sequences of MRI can make contributions to the objective evidence of latent VCI, such as cortical lesions and significant gray matter atrophy. By means of using dynamic contrast enhanced MRI, Raja et al.44 found a new mechanism of VCI as dysfunction of the blood–brain barrier. However, routine MRI has low sensitivity to brain microstructural changes, which are common in VCI. Suri et al.45 determined the association of intracranial atherosclerotic stenosis and cognitive dysfunction with the evidence of white matter hyperintensity and vascular change using 3.0 T time-of-flight MRI. In addition, the application of high-field MRI can provide high resolution to the evidence of VCI, such as changes in the cerebral perivascular spaces46.
Other sequences of MRI, such as diffusion tensor imaging (DTI) in animals, suggest the structural damage of white matter can become a biomarker of VCI47, which is also confirmed with the evidence of white matter connective dysfunction. Fragata et al.48 suggested that DTI parameters can make contributions to the monitoring of delayed cerebral ischemia at early stages of post-SAH, which is independently associated with functional outcome and can be a prognosis in SAH-related VCI. Williams et al.49 used a segmentation technique to predict white matter microstructural damage as a surrogate marker of VCI.
Hemorrhagic factors related to VCI, however, may produce obvious structural changes, while ischemic factors, such as hypoperfusion, may result in VCI with functional changes at first50. Such differences should be differentiated and clarified.
Functional Neuroimaging
Cerebral hemodynamic perfusion, such as single photon emission computed tomography (SPECT) and positron emission computed tomography (PET), can evaluate the level of brain metabolism and blood perfusion to reflect brain function and provide evidence of VCI6. Ishikawa et al.51 reported that cognitive dysfunction has a correlation with cerebral perfusion. Although it has been confirmed that mild cognitive impairment is tightly related to cerebral glucose hypometabolism, the relevance of cerebral metabolism to VCI is still not clear52.
As a burgeoning, noninvasive examination with high spatial sensibility, functional MRI (fMRI) has become an efficient method to assess neural function based on the principle of contrast enhancement of blood oxygen level dependence (BOLD), which measures the neuron activity related to hemodynamic changes in various brain regions53. fMRI provides us a chance to understand the pathogenesis of VCI from neural, regional, and network levels54. At the regional level, Diciotti et al.55 noted that high regional homogeneity in the left posterior cerebellum and middle cingulate cortex indicates global cognitive impairment and worse executive functions. At the network level, Lei et al.56 found that the default mode network and executive-control network can influence executive performance for patients with VCI by means of analyzing the amplitude of low-frequency fluctuations in the dorsolateral prefrontal cortex and posterior cingulate cortex. fMRI is a measurement of high spatial resolution but low temporal resolution, thus other measurements with high temporal resolution are needed.
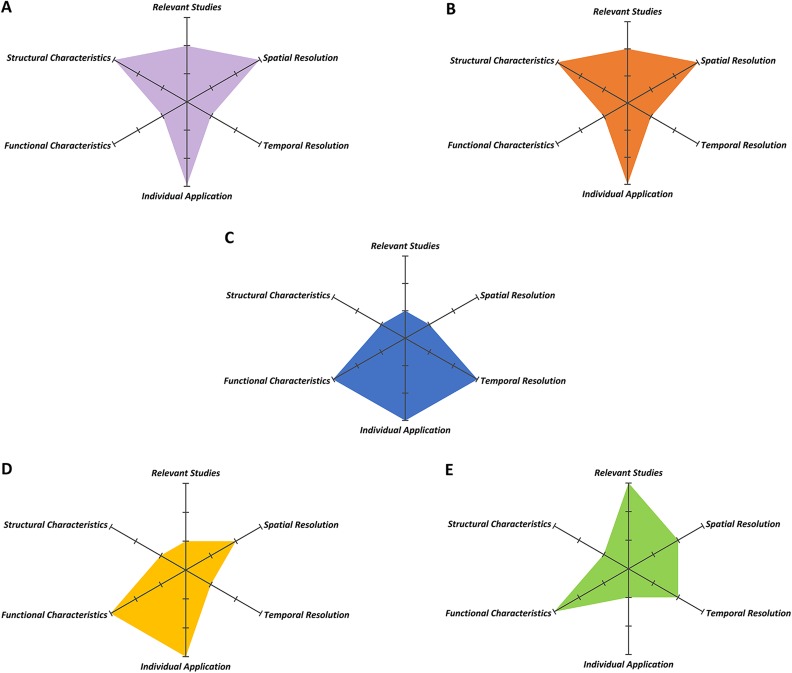
Electroencephalogram (EEG), which can reflect the overall electrophysiological effect and the function of the brain network, is a noninvasive, time-focused information transmission and processing method with high temporal resolution that uses nonlinear dynamic analysis and time frequency analysis to reflect the dynamic time processing of information transmission accurately57. Moretti et al.52 found that patients with cognitive impairment show abnormal activation in the H-alpha/L-alpha power ratio compared with normal persons, as a clinical biomarker, indicating the adaptation in these brain region changes. With the progression of cognitive dysfunction, the degree of abnormal EEG is also aggravated, especially in event-related potentials, which indicates that EEG can be used as a reliable objective index for evaluating the severity of cognitive impairment58. The application of EEG in brain default networks provides new insight into the mechanism of cognitive impairment, while related research on VCI by means of EEG is still poor. Some limitations of EEG also restrict the use of this technique to only detect the neuronal activity of the cortex, and it is easily affected by the skull. Some deep neuronal activity can hardly be observed with existing devices, which suggests that new methods need to be developed59,60 (Fig. 2).
Fig. 2.
Various kinds of neuroimaging in the evaluation of VCI. Different kinds of neuroimaging (A. Gray matter volume, B. DTI, C. EEG, D. PET/SPECT, E. fMRI) in the contributions of VCI are summarized from six dimensionalities including the evidence from relevant studies and individual application in diagnosis for VCI (Relevant Studies, Individual Application), spatial and temporal resolution (Spatial Resolution, Temporal Resolution), and functional and structural characteristics of each neuroimaging (Functional Characteristics, Structural Characteristics). Each dimensionality is divided into three levels of excellent, medium, or lacking. The superior and inferior kinds of neuroimaging are shown below.
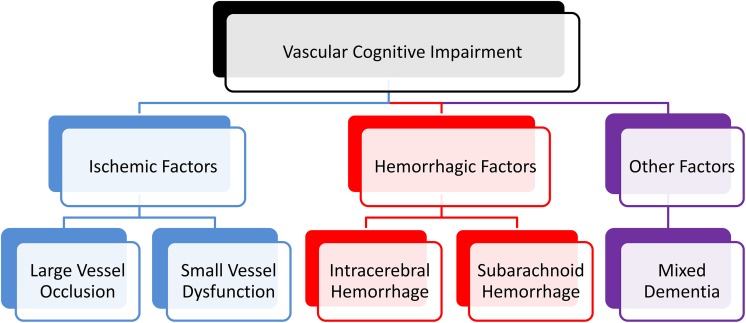
Fig. 1.
Various pathogenic factors of VCI. From the angle of pathogenic factors, VCI can be classified into three subtypes. The ischemic type includes large vessel occlusion and small vessel dysfunction. The hemorrhagic type often refers to all kinds of intracranial hemorrhage as well as subarachnoid hemorrhage. In addition, AD can often appear along with vascular disorder to form mixed dementia.
Functional near-infrared spectroscopy (fNIRS) has been used as a new monitoring method to reflect the level of advanced cognition. It has the advantage to reflect neural mechanisms in natural situation from special tasks in the cognitive evaluation61. Beishon et al.62 used fNIRS to evaluate brain hemodynamics and oxygen metabolism to predict cognitive decline at an early stage. Similar to EEG, magnetoencephalography (MEG) is another new method to detect deeper comprehension of brain dynamics with fewer conduction effects and higher temporal resolution compared with EEG63. Using MEG, Baillet64 concluded the mechanisms of functional connectivity between regions and the emergence of modes of network communication in brain systems.
Treatment
Medical Treatment
VCI and AD often coexist and share clinical features and multiple neurotransmission involvement. According to the mechanism of cognitive impairment, acetylcholinesterase inhibitors, such as donepezil and rivastigmine, have been proven to decrease the amyloid beta deposition in the development of AD in mouse models65. Excitatory amino acid receptor antagonists, such as memantine, are another pharmacotherapy for cognitive impairment that have been confirmed in the clinic. Recently, various new drugs have been explored for the treatment of cognitive impairment. Guekht et al.66 reported that actovegin has been tested for post-stroke cognitive impairment in clinical trials. Some drugs used in the treatment of cognitive dysfunction, such as donepezil and galantamine, have been generally accepted to treat AD and approved for modest cognitive benefits for VCI in the clinic67. More evidence and related research are still needed to confirm efficiency.
Revascularization
According to the pathogenesis of VCI, increasing regional cerebral blood flow by means of revascularization is hypothesized to improve cognition68,69. Lattanzi et al.70 showed that cognitive performance can be improved with the development of cerebral vasomotor reactivity after carotid endarterectomy. Carotid artery stenting is another method used to improve cerebral perfusion that is also reported, using fMRI, to partly improve global cognition and memory, resulting from the increased perfusion in the left frontal gyrus and amplitude of low-frequency fluctuation in the right precentral gyrus connectivity to the posterior cingulate cortex in the right supra frontal gyrus71. Noshiro et al.72 confirmed the improvement of brain networks by means of neuroimaging after bypass surgery in moyamoya disease. With the accumulating evidence, it is generally accepted that the cerebrovascular reserve may be related to cognition51, which provides a potential method for the surgical treatment of VCI.
In contrast, some studies have shown that there is no significant improvement of cognitive level after revascularization73. Turan et al.74 argued that angioplasty and stenting showed no improvement in cognitive impairment compared with medical treatment alone during follow-up. The RECON trial75 showed cognitive improvement following bypass surgery was not superior to medical therapy. The inconsistent results across different studies may be attributed to the different evaluating standards and methods, and more studies and randomized clinical trials are needed to confirm the efficiency of surgical treatment.
Neurological Rehabilitation
Although neuroprotection and neurorecovery enhancement have become important methods for treating VCI, studies regarding neurological rehabilitation are also faced with the difficulty of establishing a standard protocol that can embrace a holistic approach in cognitively impaired patients76. Perng et al.77 performed a meta-analysis and found that symptomatic cognitive training is an effective intervention for VCI. Ahn et al.78 also suggested that long-term treadmill exercise can restore memory function through replacement of multiple damaged structures in the ischemic aged hippocampus, and indicated that long-term exercise begun after ischemic neuronal death as a chronic neurorestorative strategy is efficient.
Transcranial magnetic stimulation (TMS) was first used in cerebrovascular disease to identify a pattern of cortical hyperexcitability, which is caused by a disruption in the integrity of white matter79; however, in VCI, the application of TMS points to enhancing brain cortical excitability and synaptic plasticity, indicating its potential to become an innovative rehabilitative tool to restore impaired neural plasticity and provide further understanding of neurotransmission pathways and plastic remodeling in the pathogenesis of VCI80. More relative research is needed to confirm the efficiency and safety of TMS to find more alternative methods for the neurological rehabilitation of VCI.
Conclusion and Future Direction
In conclusion, the pathogenesis, neuroimaging evaluation, and treatment of VCI have made a lot of progress. However, there is still scope for further exploration of the mechanism, especially in the field of correlation between molecular biology and multi-model neuroimaging, and there is great potential for the early identification and diagnosis of VCI. Meanwhile, more evidence about medical treatment in VCI is needed. The safety and efficiency of neurological rehabilitation should also be confirmed. The combination of pharmacotherapy, revascularization, and rehabilitation may become a main therapeutic method for VCI in the future.
Author Contribution: Xin Zhang and Jiabin Su, equal contribution on this work as the first authors.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Natural Science Foundation of China (No. 81771237, 81501120, 81870917 & 81500987); the Natural Science Foundation and Major Basic Research Program of Shanghai (No. 16JC1420100); the “Dawn” Program of Shanghai Education Commission (No. 16SG02); the Scientific Research Project of Huashan Hospital, Fudan University (No. 2016QD082); Shanghai Rising-Star Program (No.16QA1400900); and the Shanghai Municipal Commission of Health and Family Planning (No.2017BR003).
References
- 1. Dichgans M, Leys D. Vascular cognitive impairment. Circ Res. 2017;120(3):573–591. [DOI] [PubMed] [Google Scholar]
- 2. Smith EE. Clinical presentations and epidemiology of vascular dementia. Clin Sci (Lond). 2017;131(11):1059–1068. [DOI] [PubMed] [Google Scholar]
- 3. Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42(9):2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Iadecola C, Yaffe K, Biller J, Faraci FM, Gorelick PB, Gulati M, Kamel H, Knopman DS, Launer LJ, Saczynski JS, et al. Impact of hypertension on cognitive function: A scientific statement from the american heart association. Hypertension. 2016;68(6): e67–e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ernst M, Boers AMM, Aigner A, Berkhemer OA, Yoo AJ, Roos YB, Dippel DWJ, van der Lugt A, van Oostenbrugge RJ, van Zwam WH, et al. Association of computed tomography ischemic lesion location with functional outcome in acute large vessel occlusion ischemic stroke. Stroke. 2017;48(9):2426–2433. [DOI] [PubMed] [Google Scholar]
- 6. Charidimou A, Boulouis G, Gurol ME, Ayata C, Bacskai BJ, Frosch MP, Viswanathan A, Greenberg SM. Emerging concepts in sporadic cerebral amyloid angiopathy. Brain. 2017;140(7):1829–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lei Y, Su J, Guo Q, Yang H, Gu Y, Mao Y. Regional gray matter atrophy in vascular mild cognitive impairment. J Stroke Cerebrovasc Dis. 2016;25(1):95–101. [DOI] [PubMed] [Google Scholar]
- 8. Schaapsmeerders P, Tuladhar AM, Arntz RM, Franssen S, Maaijwee NA, Rutten-Jacobs LC, Schoonderwaldt HC, Dorresteijn LD, van Dijk EJ, Kessels RP, et al. Remote lower white matter integrity increases the risk of long-term cognitive impairment after ischemic stroke in young adults. Stroke. 2016;47(10):2517–2525. [DOI] [PubMed] [Google Scholar]
- 9. Love S, Miners JS. Small vessel disease, neurovascular regulation and cognitive impairment: post-mortem studies reveal a complex relationship, still poorly understood. Clin Sci (Lond). 2017;131(14):1579–1589. [DOI] [PubMed] [Google Scholar]
- 10. Hainsworth AH, Allan SM, Boltze J, Cunningham C, Farris C, Head E, Ihara M, Isaacs JD, Kalaria RN, Lesnik Oberstein SA, et al. Translational models for vascular cognitive impairment: a review including larger species. BMC Med. 2017;15(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Perneczky R, Tene O, Attems J, Giannakopoulos P, Ikram MA, Federico A, Sarazin M, Middleton LT. Is the time ripe for new diagnostic criteria of cognitive impairment due to cerebrovascular disease? Consensus report of the International Congress on Vascular Dementia working group. BMC Med. 2016;14(1):162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mijajlović MD, Pavlović A, Brainin M, Heiss WD, Quinn TJ, Ihle-Hansen HB, Hermann DM, Assayag EB, Richard E, Thiel A, et al. Post-stroke dementia - a comprehensive review. BMC Med. 2017;15(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Back DB, Kwon KJ, Choi DH, Shin CY, Lee J, Han SH, Kim HY. Chronic cerebral hypoperfusion induces post-stroke dementia following acute ischemic stroke in rats. J Neuroinflammation. 2017;14(1):216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mandzia JL, Smith EE, Horton M, Hanly P, Barber PA, Godzwon C, Donaldson E, Asdaghi N, Patel S, Coutts SB. Imaging and baseline predictors of cognitive performance in minor ischemic stroke and patients with transient ischemic attack at 90 days. Stroke. 2016;47(3):726–731. [DOI] [PubMed] [Google Scholar]
- 15. Rosenberg GA, Wallin A, Wardlaw JM, Markus HS, Montaner J, Wolfson L, Iadecola C, Zlokovic BV, Joutel A, Dichgans M, et al. Consensus statement for diagnosis of subcortical small vessel disease. J Cereb Blood Flow Metab. 2016;36(1):6–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shindo A, Liang AC, Maki T, Miyamoto N, Tomimoto H, Lo EH, Arai K. Subcortical ischemic vascular disease: Roles of oligodendrocyte function in experimental models of subcortical white-matter injury. J Cereb Blood Flow Metab. 2016;36(1):187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Choi BR, Kim DH, Back DB. Characterization of white matter injury in a rat model of chronic cerebral hypoperfusion. Stroke. 2016;47(2):542–547. [DOI] [PubMed] [Google Scholar]
- 18. Ping S, Qiu X, Gonzalez-Toledo ME, Liu X, Zhao LR. Stem cell factor in combination with granulocyte colony-stimulating factor reduces cerebral capillary thrombosis in a mouse model of CADASIL. Cell Transplant. 2018;27(4):637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rosenberg GA. Extracellular matrix inflammation in vascular cognitive impairment and dementia. Clin Sci (Lond). 2017;131(6):425–437. [DOI] [PubMed] [Google Scholar]
- 20. Moulin S, Labreuche J, Bombois S, Rossi C, Boulouis G, Hénon H, Duhamel A, Leys D, Cordonnier C. Dementia risk after spontaneous intracerebral haemorrhage: a prospective cohort study. Lancet Neurol. 2016;15(8):820–829. [DOI] [PubMed] [Google Scholar]
- 21. You S, Wang X, Lindley RI, Robinson T, Anderson CS, Cao Y, Chalmer J. Early cognitive impairment after intracerebral hemorrhage in the INTERACT1 study. Cerebrovasc Dis. 2017;44(5-6):320–324. [DOI] [PubMed] [Google Scholar]
- 22. Chung CP, Chou KH, Chen WT, Liu LK, Lee WJ, Chen LK, Lin CP, Wang PN. Strictly lobar cerebral microbleeds are associated with cognitive impairment. Stroke. 2016;(10):2497–2502. [DOI] [PubMed] [Google Scholar]
- 23. Valenti R, Charidimou A, Xiong L, Boulouis G, Fotiadis P, Ayres A, Riley G, Kuijf HJ, Reijmer YD, Pantoni L, et al. Visuospatial functioning in cerebral amyloid angiopathy: A pilot study. J Alzheimers Dis. 2017;56(4):1223–1227. [DOI] [PubMed] [Google Scholar]
- 24. Ding J, Sigurðsson S, Jónsson PV, Eiriksdottir G, Meirelles O, Kjartansson O, Lopez OL, van Buchem MA, Gudnason V, Launer LJ. Space and location of cerebral microbleeds, cognitive decline, and dementia in the community. Neurology. 2017;88(22):2089–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reijmer YD, Fotiadis P, Riley GA, Xiong L, Charidimou A, Boulouis G, Ayres AM, Schwab K, Rosand J, Gurol ME. Progression of brain network alterations in cerebral amyloid angiopathy. Stroke. 2016;47(10):2470–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Barnaure I, Montandon ML, Rodriguez C, Herrmann F, Lövblad KO, Giannakopoulos P, Haller S. Clinicoradiologic correlations of cerebral microbleeds in advanced age. AJNR Am J Neuroradiol. 2017;38(1):39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Banerjee G, Wilson D, Ambler G, Osei-Bonsu Appiah K, Shakeshaft C, Lunawat S, Cohen H, Dr Yousry T, Lip GYH, Muir KW, et al. Cognitive impairment before intracerebral hemorrhage is associated with cerebral amyloid angiopathy. Stroke. 2018;49(1):40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xiong XY, Liu L, Wang FX, Yang YR, Hao JW, Wang PF, Zhong Q, Zhou K, Xiong A, Zhu WY, et al. Toll-like receptor 4/MyD88-mediated signaling of hepcidin expression causing brain iron accumulation, oxidative injury, and cognitive impairment after intracerebral hemorrhage. Circulation. 2016;134(14):1025–1038. [DOI] [PubMed] [Google Scholar]
- 29. Wong GK, Lam SW, Wong A, Ngai K, Mok V, Poon WS. Early cognitive domain deficits in patients with aneurysmal subarachnoid hemorrhage correlate with functional status. Acta Neurochir Suppl. 2016;122:129–132. [DOI] [PubMed] [Google Scholar]
- 30. da Costa L, Dunkley BT, Bethune A, Robertson A, Keller A, Pang EW. Increased frontal lobe activation after aneurysmal subarachnoid hemorrhage. Stroke. 2016;47(10):2503–2510. [DOI] [PubMed] [Google Scholar]
- 31. Su J, E T, Guo Q, Lei Y, Gu Y. Memory deficits after aneurysmal subarachnoid hemorrhage: A functional magnetic resonance imaging study. World Neurosurg. 2018;111:e500–e506. [DOI] [PubMed] [Google Scholar]
- 32. Sahyouni R, Goshtasbi K, Mahmoodi A, Tran DK, Chen JW. Chronic subdural hematoma: A perspective on subdural membranes and dementia. World Neurosurg. 2017;108:954–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kalaria RN. Neuropathological diagnosis of vascular cognitive impairment and vascular dementia with implications for Alzheimer’s disease. Acta Neuropathol. 2016;131(5):659–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Counts SE, Mufson EJ. Regulator of Cell Cycle (RGCC) Expression during the progression of Alzheimer’s Disease. Cell Transplant. 2017;26(4):693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kalaria RN. The pathology and pathophysiology of vascular dementia. Neuropharmacology. 2018;134(Pt B):226–239. [DOI] [PubMed] [Google Scholar]
- 36. Skrobot OA, Black SE, Chen C, DeCarli C, Erkinjuntti T, Ford GA, Kalaria RN, O’Brien J, Pantoni L, Pasquier F, et al. Progress toward standardized diagnosis of vascular cognitive impairment: Guidelines from the vascular impairment of cognition classification consensus study. Alzheimers Dement. 2018;14(3):280–292. [DOI] [PubMed] [Google Scholar]
- 37. Behrman S, Valkanova V, Allan CL. Diagnosing and managing mild cognitive impairment. Practitioner. 2017;261(1804):17–20. [PubMed] [Google Scholar]
- 38. Barbay M, Taillia H, Nedelec-Ciceri C, Arnoux A, Puy L, Wiener E, Canaple S, Lamy C, Godefroy O, Roussel M; GRECOGVASC Study Group. Vascular cognitive impairment: Advances and trends. Rev Neurol (Paris). 2017;173(7-8):473–480. [DOI] [PubMed] [Google Scholar]
- 39. Keefe RSE, Davis VG, Harvey PD, Atkins AS, Haig GM, Hagino O, Marder S, Hilt DC, Umbricht D. Placebo response and practice effects in schizophrenia cognition trials. JAMA Psychiatry. 2017;74(8):807–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Scherr M, Kunz A, Doll A, Mutzenbach JS, Broussalis E, Bergmann HJ, Kirschner M, Trinka E, Killer-Oberpfalzer M. Ignoring floor and ceiling effects may underestimate the effect of carotid artery stenting on cognitive performance. J Neurointerv Surg. 2016;8(7):747–751. [DOI] [PubMed] [Google Scholar]
- 41. Obrig H. NIRS in clinical neurology - a ‘promising’ tool? Neuroimage. 2014, 85(Pt 1):535–546. [DOI] [PubMed] [Google Scholar]
- 42. Narayanan L, Murray AD. What can imaging tell us about cognitive impairment and dementia? World J Radiol. 2016;8(3):240–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wollenweber FA, Baykara E, Zedde M, Gesierich B, Achmüller M, Jouvent E, Viswanathan A, Ropele S, Chabriat H, Schmidt R, et al. Cortical superficial siderosis in different types of cerebral small vessel disease. Stroke. 2017;48(5):1404–1407. [DOI] [PubMed] [Google Scholar]
- 44. Raja R, Rosenberg GA, Caprihan A. MRI measurements of blood-brain barrier function in dementia: A review of recent studies. Neuropharmacology. 2018;134(Pt B):259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Suri MFK, Zhou J, Qiao Y, Chu H, Qureshi AI, Mosley T, Gottesman RF, Wruck L, Sharrett AR, Alonso A, et al. Cognitive impairment and intracranial atherosclerotic stenosis in general population. Neurology. 2018;90(14): e1240–e1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bouvy WH, Zwanenburg JJ, Reinink R, Wisse LEM, Luijten PR, Kappelle LJ, Geerlings MI, Biessels GJ; Utrecht Vascular Cognitive Impairment (VCI) Study group. Perivascular spaces on 7 Tesla brain MRI are related to markers of small vessel disease but not to age or cardiovascular risk factors. J Cereb Blood Flow Metab. 2016;36(10):1708–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Boehm-Sturm P, Füchtemeier M, Foddis M, Mueller S, Trueman RC, Zille M, Rinnenthal JL, Kypraios T, Shaw L, Dirnagl U, et al. Neuroimaging biomarkers predict brain structural connectivity change in a mouse model of vascular cognitive impairment. Stroke. 2017;48(2):468–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fragata I, Alves M, Papoila AL, Nunes AP, Ferreira P, Canto-Moreira N, Canhão P. Early prediction of delayed ischemia and functional outcome in acute subarachnoid hemorrhage: Role of diffusion tensor imaging. Stroke. 2017;48(8):2091–2097. [DOI] [PubMed] [Google Scholar]
- 49. Williams OA, Zeestraten EA, Benjamin P, Lambert C, Lawrence AJ, Mackinnon AD, Morris RG, Markus HS, Charlton RA, Barrick TR. Diffusion tensor image segmentation of the cerebrum provides a single measure of cerebral small vessel disease severity related to cognitive change. Neuroimage Clin. 2017;16:330–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Heiss WD, Rosenberg GA, Thiel A, Berlot R, de Reuck J. Neuroimaging in vascular cognitive impairment: A state-of-the-art review. BMC Med. 2016;14(1):174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ishikawa M, Kusaka G, Terao S, Nagai M, Tanaka Y, Naritaka H. Improvement of neurovascular function and cognitive impairment after STA-MCA anastomosis. J Neurol Sci. 2017;373:201–207. [DOI] [PubMed] [Google Scholar]
- 52. Moretti DV, Pievani M, Pini L. Cerebral PET glucose hypometabolism in subjects with mild cognitive impairment and higher EEG high-alpha/low-alpha frequency power ratio. Neurobiol Aging. 2017;58:213–224. [DOI] [PubMed] [Google Scholar]
- 53. Cheema I, Switzer AR, McCreary CR, Hill MD, Frayne R, Goodyear BG, Smith EE. Functional magnetic resonance imaging responses in CADASIL. J Neurol Sci. 2017;375:248–254. [DOI] [PubMed] [Google Scholar]
- 54. Sakamoto Y, Okamoto S, Maesawa S, Bagarinao E, Araki Y, Izumi T, Watanabe H, Sobue G, Wakabayashi T. Default mode network changes in moyamoya disease before and after bypass surgery: Preliminary report. World Neurosurg. 2018;112: e652–e661. [DOI] [PubMed] [Google Scholar]
- 55. Diciotti S, Orsolini S, Salvadori E, Giorgio A, Toschi N, Ciulli S, Ginestroni A, Poggesi A, De Stefano N, Pantoni L, et al. Resting state fMRI regional homogeneity correlates with cognition measures in subcortical vascular cognitive impairment. J Neurol Sci. 2017;373:1–6. [DOI] [PubMed] [Google Scholar]
- 56. Lei Y, Li YJ, Guo QH, Liu XD, Liu Z, Ni W, Su JB, Yang H, Jiang HQ, Xu B, et al. Postoperative executive function in adult moyamoya disease: a preliminary study of its functional anatomy and behavioral correlates. J Neurosurg. 2017;126(2):527–536. [DOI] [PubMed] [Google Scholar]
- 57. Al-Qazzaz NK, Ali SHBM, Ahmad SA. Discrimination of stroke-related mild cognitive impairment and vascular dementia using EEG signal analysis. Med Biol Eng Comput. 2018;56(1):137–157. [DOI] [PubMed] [Google Scholar]
- 58. Swatridge K, Regan K, Staines WR. The acute effects of aerobic exercise on cognitive control among people with chronic stroke. J Stroke Cerebrovasc Dis. 2017;26(12):2742–2748. [DOI] [PubMed] [Google Scholar]
- 59. Chaudhuri R, He BJ, Wang XJ. Random recurrent networks near criticality capture the broadband power distribution of human ECoG dynamics. Cereb Cortex. 2017;13:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fox KCR, Foster BL, Kucyi A, Daitch AL, Parvizi J. Intracranial electrophysiology of the human default network. Trends Cogn Sci. 2018;22(4):307–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. de Moraes FM, Bertolucci PF. The contribution of supplementary tests in the differential diagnosis of dementia. Am J Alzheimers Dis Other Demen. 2018;33(2):131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Beishon L, Haunton VJ, Panerai RB. Cerebral hemodynamics in mild cognitive impairment: A systematic review. J Alzheimers Dis. 2017;59(1):369–385. [DOI] [PubMed] [Google Scholar]
- 63. Amezquita-Sanchez JP, Adeli A, Adeli H. A new methodology for automated diagnosis of mild cognitive impairment (MCI) using magnetoencephalography (MEG). Behav Brain Res. 2016;305: 174–180. [DOI] [PubMed] [Google Scholar]
- 64. Baillet S. Magnetoencephalography for brain electrophysiology and imaging. Nat Neurosci. 2017;20(3):327–339. [DOI] [PubMed] [Google Scholar]
- 65. Izumi H, Shinoda Y, Saito T, Saido TC, Sato K, Yabuki Y, Matsumoto Y, Kanemitsu Y, Tomioka Y, Abolhassani N. The disease-modifying drug candidate, SAK3 improves cognitive impairment and inhibits amyloid beta deposition in app knock-in mice. Neuroscience. 2018;377:87–97. [DOI] [PubMed] [Google Scholar]
- 66. Guekht A, Skoog I, Edmundson S, Zakharov V, Korczyn AD. ARTEMIDA Trial (A Randomized Trial of Efficacy, 12 Months International Double-Blind Actovegin): A randomized controlled trial to assess the efficacy of actovegin in poststroke cognitive impairment. Stroke. 2017;48(5):1262–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Farooq MU, Min J, Goshgarian C, Gorelick PB. Pharmacotherapy for vascular cognitive impairment. CNS Drugs. 2017;31(9):759–776. [DOI] [PubMed] [Google Scholar]
- 68. Soman S, Prasad G, Hitchner E, Massaband P, Moseley ME, Zhou W, Rosen AC. Brain structural connectivity distinguishes patients at risk for cognitive decline after carotid interventions. Hum Brain Mapp. 2016;37(6):2185–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zuniga MC, Tran TB, Baughman BD, Raghuraman G, Hitchner E, Rosen A, Zhou W. A prospective evaluation of systemic biomarkers and cognitive function associated with carotid revascularization. Ann Surg. 2016;264(4):659–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lattanzi S, Carbonari L, Pagliariccio G, Bartolini M, Cagnetti C, Viticchi G, Buratti L, Provinciali L, Silvestrini M. Neurocognitive functioning and cerebrovascular reactivity after carotid endarterectomy. Neurology. 2018;90(4): e307–e315. [DOI] [PubMed] [Google Scholar]
- 71. Wang T, Sun D, Liu Y, Mei B, Li H, Zhang S, Zhang J. The impact of carotid artery stenting on cerebral perfusion, functional connectivity, and cognition in severe asymptomatic carotid stenosis patients. Front Neurol. 2017;8:403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Noshiro S, Mikami T, Komatsu K, Kanno A, Enatsu R, Yazawa S, Nagamine T, Matsuhashi M, Mikuni N. Neuromodulatory role of revascularization surgery in moyamoya disease. World Neurosurg. 2016;91:473–482. [DOI] [PubMed] [Google Scholar]
- 73. Zeifert PD, Karzmark P, Bell-Stephens TE, Steinberg GK, Dorfman LJ. Neurocognitive performance after cerebral revascularization in adult moyamoya disease. Stroke. 2017;48(6):1514–1517. [DOI] [PubMed] [Google Scholar]
- 74. Turan TN, Smock A, Cotsonis G, Bachman D, Al Kasab S, Lynn MJ, Nizham A, Derdeyn CP, Fiorella D, Janis S, et al. Is there benefit from stenting on cognitive function in intracranial atherosclerosis? Cerebrovasc Dis. 2017;43(1-2):31–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Marshall RS, Festa JR, Cheung YK, Pavol MA, Derdeyn CP, Clarke WR, Videen TO, Grubb RL, Slane K, Powers WJ, et al. Randomized evaluation of carotid occlusion and neurocognition (RECON) trial: Main results. Neurology. 2014;82(9):744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Balea M, Muresanu D, Alvarez A, Homberg V, Bajenaru O, Guekht A, Heiss WD, Popa L, Vester J, Muresanu I, et al. VaD - An integrated framework for cognitive rehabilitation. CNS Neurol Disord Drug Targets. 2018;17(1):22–33. [DOI] [PubMed] [Google Scholar]
- 77. Perng CH, Chang YC, Tzang RF. The treatment of cognitive dysfunction in dementia: a multiple treatments meta-analysis. Psychopharmacology (Berl). 2018;235(5):1571–1580. [DOI] [PubMed] [Google Scholar]
- 78. Ahn JH, Choi JH, Park JH, Kim IH, Cho JH, Lee JC, Koo HM, Hwangbo G, Yoo KY, Lee CH. Long-term exercise improves memory deficits via restoration of myelin and microvessel damage, and enhancement of neurogenesis in the aged gerbil hippocampus after ischemic stroke. Neurorehabil Neural Repair. 2016;30(9):894–905. [DOI] [PubMed] [Google Scholar]
- 79. Allart E, Delval A, Caux-Dedeystere A, Labreuche J, Viard R, Lopes R, Devanne H. Parietomotor connectivity in the contralesional hemisphere after stroke: A paired-pulse TMS study. Clin Neurophysiol. 2017;128(5):707–715. [DOI] [PubMed] [Google Scholar]
- 80. Lanza G, Bramanti P, Cantone M, Pennisi M, Pennisi G, Bella R. Vascular cognitive impairment through the looking glass of transcranial magnetic stimulation. Behav Neurol. 2017:1421326. [DOI] [PMC free article] [PubMed] [Google Scholar]