Abstract
Radioactive dermatitis is caused by the exposure of skin and mucous membranes to radiation fields. The pathogenesis of radioactive dermatitis is complex and difficult to cure. Wharton’s jelly-derived mesenchymal stem cells (WJ-MSCs) may serve as a promising candidate for the therapy of cutaneous wounds. The aim of this study was to investigate whether a WJ-MSC-derived conditioned medium (MSC-CM) could be used to treat radiation-induced skin wounds in rats using a radiation-induced cutaneous injury model. The present study was designed to examine MSC-CM therapy in the recovery of radiation-induced skin wounds in vitro and in vivo. Firstly, we prepared the MSC-CM and tested the effects of the MSC-CM on human umbilical vein endothelial cell proliferation in vitro. After that, we used a β-ray beam to make skin wounds in rats and tested the effects of MSC-CM on cutaneous wound healing in vivo. Our results indicated that MSC-CM secreted factors that promoted HUVEC proliferation, regeneration of sebaceous glands, and angiogenesis. Importantly, MSC-CM promoted wound healing in excess of the positive control (epidermal growth factor), with no, or smaller, scar formation. In conclusion, MSC-CM significantly accelerated wound closure and enhanced the wound healing quality. MSC-CM has a beneficial therapeutic effect on radiation-induced cutaneous injury skin in rats and in this way MSC-CM may serve as a basis of a novel cell-free therapeutic approach for radiation dermatitis.
Keywords: Mesenchymal stem cells (MSCs), conditioned medium, radiation dermatitis, wound healing
Introduction
Radiation-induced cutaneous injury (radiation dermatitis) is mainly caused by radiation exposure to the skin’s mucous membrane1. Radiotherapy is the one of the most common causes in the clinic, where it is used for anticancer therapy. Approximately 50% of patients with cancers receive radiotherapy at some point during the course of their treatment2. However, the main side effect of radiotherapy is the damage of normal tissue, especially radiation-induced cutaneous injury, which is a serious concern during radiotherapy3, and may limit the duration of radiation and the dose to be delivered4. Deep radiation ulcers may involve subcutaneous tissue, muscle and even bone and deep organs, accompanied by chronic infection, tissue necrosis and severe fibrosis5. This serious injury from such side effects cannot heal itself and requires surgical intervention1. Unfortunately, currently used therapeutic strategies are inconsistent and unsatisfactory.
Increasing evidence supports that mesenchymal stem cells (MSCs) and their secretions provide the therapeutic potential to restore the functionality of irradiated skin tissue6–8. There is a great interest in the therapeutic potential of MSCs because of their immunosuppressive properties, and ability to repair and regenerate damaged tissues9,10. It has been reported that MSCs have an important role in skin repair and regeneration, not only to promote cell proliferation but also for the secretion of soluble factors via the paracrine system10–12. Indeed, previous work has shown that Wharton’s jelly-derived MSCs (WJ-MSCs) have a higher wound-healing capability in treating skin injuries in vivo13,14. Other studies have shown that WJ-MSCs can improve the microcirculation and microenvironment significantly through therapeutic paracrine effects in a radiation rat model13,15.
In this study we investigated whether WJ-MSC-derived conditioned medium (MSC-CM) can be used to treat radiation-induced skin in rats using a radiation-induced cutaneous injury model. To achieve this aim, we prepared the MSC-CM, and tested the treatment effects of MSC-CM on cutaneous wound healing in vitro and in vivo. The results show that concentrated MSC-CM can modulate wound repair, with a combination of protective mechanisms that might jointly promote radiation-induced cutaneous wound healing. The results of this work may provide a new strategy to therapy radiation dermatitis.
Materials and Methods
Conditioned Medium Preparation
WJ-MSCs were generously provided by Professor M. Li (Jilin University, China). The method of preparing the MSC-CM was performed as described previously16. Briefly, passage 3 cells were cultured to 60% confluence in normal culture media then replaced with serum-free medium (SFM) composed of an Ultra Cuture SFM (Lonza, Basel, Switzerland) with 2% supplements. The cells were maintained at 37°C with saturated humidity and 5% CO2. After 48 hours, the conditioned medium was harvested as MSC-CM. The negative control (NC) and positive control (epidermal growth factor (EGF) 5 ng/ml; Sigma, San Francisco, CA, USA) was obtained under the same culture conditions, but in absence of cells. After that, supernatants were collected, pooled and centrifuged at 1000 g and filtered with a 0.22-µm filter. Finally, the CM was lyophilized, stored at −80°C, and dissolved in SFM when being used.
MSC-CM Coating Plate
The collected MSC-CM (5 ng/ml) was coated in a 96-well plate with 100 μl/well, then dried in the super-clean bench. After 2 hours, human umbilical vein endothelial cells (HUVECs) were plated into the coating plate at a density of 3000 cells/well, maintained at 37°C with saturated humidity and 5% CO2, and incubated for 48 hours. Then, cell viability was examined by Cell Counting Kit-8 (CCK-8; Beyotime, Nantong, China) and corresponding optical density (OD) value measured at the 490 nm wavelength. The experiments were repeated three times.
Immunofluorescence Staining
HUVECs were incubated in 24-well plates at a density of 5000 cells/well. After 24 hours, the medium was replaced with CM and incubated for 48 hours. Cells were further incubated with 4% paraformaldehyde for 10 minutes at room temperature, and 1% bovine serum albumin (Boster, Wuhan, China) for 30 minutes. After that, cells were incubated with anti-Ki-67 antibody (Abcam, Cambridge, UK), 1:200 dilution, and goat anti-rabbit immunoglobulin (Ig)G, (Abcam), 1:1000 dilution. 4’,6-diamidino-2-phenylindole (DAPI) was used to label the cell nuclei (Thermal Scientific, Waltham, MA, USA) and finally, fluorescence microscopy was used to examine positive cells.
Cell Cycle Analysis
The collected MSC-CM was added to the HUVEC cultured system and incubated for 24 hours. Briefly, CM cultured cells were collected, washed, and suspended in cold 75% ethanol overnight at 4°C. Cells were further centrifuged, washed and stained with 50 μg/ml propidium iodide (PI) and 50 μg/ml RNase-A (Sigma) dissolved in 500 μl phosphate-buffered saline . The suspension was incubated for another 30 min. and analyzed using flow cytometry. The experiments were repeated three times.
Animals and Treatments
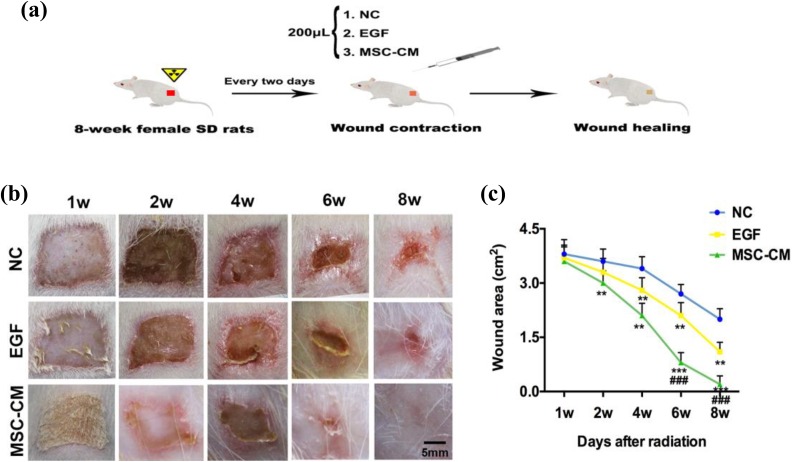
Female Sprague–Dawley (SD) rats of 180–200 g were used in this study. All the protocols and procedures were approved by the Animal Experiment Ethics Committee of the Jilin University, China (approval No. XYSK2017-0125). The radiation-induced skin injury rat model was created as described in previously published methods1. Briefly, the rats were anesthetized with an intraperitoneal injection of 10% chloral hydrate (500 μl/100 g) and the buttock hairs were shaved off. Rats were shielded with a 2-cm thick piece for localizing the radiation field. A β-ray beam was applied to irradiate the skin of the rat’s buttock. The intensity of radiation used was 40 Gy in an area of 2 cm × 2 cm for 25 min. A recombinant human EGF gel (Pavay, Guilin, China) was used as a positive control. The rats were divided into three groups with 12 rats per group: NC, EGF, MSC-CM. A total of 200 μl of CM-hydrogel or EGF-hydrogel was pipetted onto the radiation wound every 2 days. The concentrations of EGF and MSC-CM were all 5 μg/ml. The experiment lasted for 8 weeks, and skin damage was photographically recorded every week. The wounds were traced on clear autoclaved plastic, and the images were scanned and used to calculate the wound area (Scion image analysis software, National Institutes of Health, USA).
Skin Histological Analysis
The healed tissue was extracted on the eighth week, fixed in 4% formaldehyde and embedded in paraffin. Tissue cross-sections were stained with hematoxylin and eosin following the manufacturer’s (Sigma) standardized protocols and immunohistochemistry (IHC) was measured using the kit (Maixin KIT-9710, Fuzhou, China) in accordance with the manufacturer’s instructions. Briefly, the sections were deparaffinized, rehydrated, incubated in a 99°C water bath for 15 min, and then in 3% H2O2 for 15 min. Afterwards, sections were blocked with 10% normal goat serum for 1 h at 37°C and incubated with anti-Ki-67 antibody (Abcam), 1:500 dilution, and α-smooth muscle actin (α-SMA; Abcam), 1:500 dilution, and overnight at 4°C. Next, the sections were incubated with biotinylated goat-anti-rabbit IgG antibody (Abcam). Diaminobenzidine solution was used as the chromogenic agent for 15 min at 37°C and sequentially incubated with avidin peroxidase reagent. Hematoxylin was used for counterstaining. Finally, the sections were photographed using an optical microscope (Olympus, Tokyo Metropolitan, Japan).
Western Blot Analysis
Proteins were extracted from the healed skin tissue in a lysis buffer (Roche, Basel, Switzerland) following the manufacturer’s standardized protocols. The primary antibody was anti-Ki-67 antibody (Abcam), 1:100 dilution, and α-SMA (Abcam), 1:100 dilution and the secondary antibody was goat anti-rabbit IgG (Abcam), 1:1000 dilution. The Lab Works version 4.0 software was used for relative expressions and calculated according to the reference glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Quantitative Real-Time Polymerase Chain Analysis
Total RNA was extracted using Trizol (Invitrogen Shanghai, China) from the HUVECs after culturing in CM for 48 h. SYBR Green dye (Roche) was used for amplification of cDNA. The levels of interferon (IFN), tumor necrosis factor (TNF), interleukin (IL)-1, IL-6, vascular endothelial growth factor (VEGF), EGF, b-fibroblast growth factor (bFGF), kinase insert domain receptor (KDR), Col1A2, Ki-67, α-SMA, and the internal standard GAPDH mRNA were measured by quantitative real-time polymerase chain reaction (qRT-PCR) in triplicate. The primer sequences are listed in Table 1. The expression levels of mRNA were quantified by qRT-PCR according to the manufacturer’s protocols (Gene Pharma, Shanghai, China). The data were analyzed using the 2-ΔΔCt methods.
Table 1.
Primers Used for qRT-PCR.
| Gene name | Primers | Sequences | Product size (bp) |
|---|---|---|---|
| GAPDH | Forward | AAGAAGGTGGTGAAGCAGG | 151 |
| Reverse | CAGCATCAAAGGTGGAAGA | ||
| IFN | Forward | GAGGGTCAACAGAGGAGC | 156 |
| Reverse | GTGGGAATCAGAGGTAGAAG | ||
| TNF | Forward | ATGGTGGACCGCAACAAC | 182 |
| Reverse | TGAGCACTGAAGCGAAAGC | ||
| IL-1 | Forward | TGAAGGGCAGGGAACAAC | 143 |
| Reverse | GGTGAAGCAGGGTGAGAA | ||
| IL-6 | Forward | CAAGTCAACTGTGGAGCAA | 193 |
| Reverse | TGAGGAGCAGGAAGGGTC | ||
| VEGF | Forward | CCCAGAAGTTGGACGAAAA | 180 |
| Reverse | TGAGTTGGGAGGAGGATG | ||
| EGF | Forward | ACACGGAGGGAGGCTACA | 198 |
| Reverse | GTAGCCTCCCTCCGTGTT | ||
| bFGF | Forward | CGCACCCTATCCCTTCACA | 114 |
| Reverse | CAACGACCAGCCTTCCAC | ||
| KDR | Forward | ACTCCTCCTCATTCAGCG | 174 |
| Reverse | GGGTCCCACAACTTCTCA | ||
| Col1A2 | Forward | AATCCCATCCAGCCAACA | 158 |
| Reverse | ACAAACGGCAGCGTCAAT | ||
| Ki-67 | Forward | CGCACCCTATCCCTTCACA | 189 |
| Reverse | CAACGACCAGCCTTCCAC | ||
| α-SMA | Forward | CAGCGGTGAAGAAGGAAAG | 183 |
| Reverse | CCAGTTGAACCACGATTGC |
α-SMA: α-smooth muscle actin; bFGF: b-fibroblast growth factor; bp: base pairs; EGF: epidermal growth factor; GAPDH: glyceraldehyde 3-phosphate dehydrogenase; IFN: interferon; IL: interleukin; KDR: kinase insert domain receptor; qRT-PCR: quantitative real-time polymerase chain reaction; TNF: tumor necrosis factor; VEGF: vascular endothelial growth factor.
Statistical Analysis
Statistical analysis was performed using Prism 6 (GraphPad Software, San Diego, CA, USA). Multiple comparisons were analyzed by one-way analysis of variance. All quantitative data were given as the mean ± standard deviation (SD), and a p-value < 0.05 was considered statistically significant. Additional statistical details pertaining to each experiment are provided within the relevant results and legend sections.
Results
Effects of the MSC-CM on Cell Proliferation
In order to investigate the effect of MSC-CM on cell proliferation, HUVEC proliferation was measured by CCK-8 (Beyotime) for 5 days. The results showed that in the MSC-CM group the growth rate was significantly increased compared with the NC group (p < 0.001, Fig. 1a). Furthermore, the results of the MSC-CM coating experiment (Fig. 1b), showed that the proliferation rate of the MSC-CM group was significantly increased compared with the NC group (p < 0.001), as well as the EGF group (p < 0.01).
Fig. 1.
Effects of MSC-CM on HUVEC proliferation by CCK-8 assay. (a) The proliferation curves of HUVEC using MSC-CM. **p < 0.01, ***p < 0.001 when compared with NC. (b) MSC-CM coating plates promote HUVEC proliferation. **p < 0.01, ***p < 0.001 when compared with NC; ##p < 0.01 when compared with EGF. Data are reported as mean ± SEM. (c–e) Flow cytometry analysis the HUVEC cycle. The three groups were NC, EGF, and MSC-CM in order. Data are reported as mean ± SD, n=3.
CCK-8: Cell Counting Kit-8; EGF: epidermal growth factor; HUVEC: human umbilical vein endothelial cell; MSC-CM: WJ-MSC-derived conditioned medium; NC: negative control; SD: standard deviation; SEM: standard error of the mean; WJ-MSC: Wharton’s jelly-derived mesenchymal stem cell.
The analysis of the cell cycle distribution of HUVECs treated with MSC-CM (Fig. 1c–e) showed that, in the MSC-CM group, the percentage of G2/M-phase cells (26.51% ± 0.62) were increased compared with the NC group (15.41% ± 0.83). Accordingly, cells in the G0/G1-phase decreased in the MSC-CM group (61.34% ± 1.47), compared with the NC group (81.35%±1.57). The G0/G1-phase and G2/M-phase cells in the EGF group were between the other two groups. The cell cycle results suggested that MSC-CM effectively enhanced the cell proliferation induced by a G0/G1-phase cell cycle arrest.
To study the effect of MSC-CM on HUVEC proliferation, immunofluorescence (IF) staining of Ki-67 was performed. As shown in Fig. 2, the intensity of Ki-67 in the MSC-CM group (76% ± 6.11) was significantly higher than the NC group (58% ± 5.24, p < 0.05). Next, we tested the inflammatory and angiogenesis gene expression of the HUVECs after MSC-CM culture for 48 h and we observed that the inflammatory genes of IFN, TNF, IL-1, and IL-6 expression were significantly down-regulated (Fig. 2c), while the key genes involved with angiogenesis of VEGF, EGF, bFGF, and KDR expressions were up-regulated (Fig. 2d; p < 0.05, p < 0.01).
Fig. 2.
Effects of MSC-CM on HUVEC proliferation, inflammation and angiogenesis gene expression. (a) Immunofluorescence staining of HUVECs labeled with Ki-67. (b) The percentages of Ki-67-positive cells in HUVECs. Scale bar = 100 μm, *p < 0.05 compared with the NC group. (c) The inflammatory gene of IFN, TNF, IL-1, and IL-6 expressions. (d) The key genes of VEGF, EGF, bFGF, and KDR expressions involved with angiogenesis. *p < 0.05, **p < 0.01 compared with the control (SFM). Data are reported as mean ± SD, n = 3.
bFGF: b-fibroblast growth factor; EGF: epidermal growth factor; HUVEC: human umbilical vein endothelial cell; IFN: interferon; IL: interleukin; KDR: kinase insert domain receptor; MSC-CM: WJ-MSC-derived conditioned medium; NC: negative control; SD: standard deviation; SFM: serum-free medium; TNF: tumor necrosis factor; VEGF: vascular endothelial growth factor; WJ-MSC: Wharton’s jelly-derived mesenchymal stem cell.
MSC-CM Promotes the Healing of Radiation-Induced Cutaneous Injury in Rats
A β-ray beam radiation skin wound in rats was treated with 200 μl of MSC-CM-hydrogel every 2 days after irradiation (Fig. 3a). Different degrees of injury began to appear on each group after the same dose of 40 Gy radiation in the first week. Our results showed that an obvious erosion and ulcer appeared on the fourth week, followed by dry desquamation, crusting, superficial minimal scabbing, then healing (Fig. 3b). The great difference between the NC and MSC-CM group was observed on the sixth week (Fig. 3c), with a relative wound size of the MSC-CM group 3.38-fold smaller than the NC group (0.8 ± 0.29 mm2 to 2.7 ± 0.34 mm2, p < 0.001), and 2.63-fold smaller than the EGF group (0.8 ± 0.29 mm2 to 2.1 ± 0.37 mm2, p < 0.001). Our data showed that the wounds in the MSC-CM group had complete healing on the eighth week. However, the NC group wounds did not fully heal during our experimental observations.
Fig. 3.
Effects of MSC-CM on cutaneous wound healing. (a) Experimental procedure. (b) Overall observed wound morphological changes after radiation. (c) The wound area changes during the wound healing course. **p < 0.01, ***p < 0.001 when compared with the NC, ###p < 0.001 compared with EGF, data are reported as mean ± SD, n = 12.
EGF: epidermal growth factor; MSC-CM: WJ-MSC-derived conditioned medium; NC: negative control; SD: standard deviation; SFM: serum-free medium.
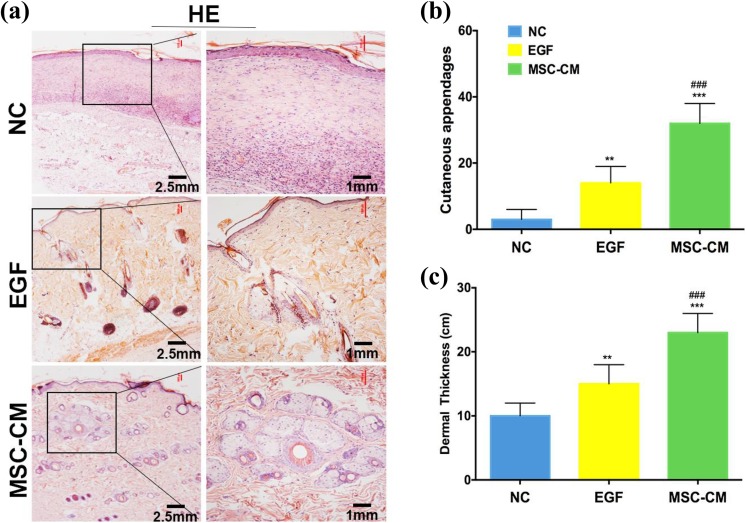
Histological analysis was used to evaluate the quality of the wound healing. Hematoxylin and eosin staining results indicated that cutaneous appendages in the MSC-CM group (32.4 ± 6.2/high-power field (HPF)) were increased significantly compared with the NC group (3.5 ± 3.4/HPF, p < 0.001) and the EGF group (14.7 ± 3.6/HPF, p < 0.001; Fig. 4a, b). In addition, the MSC-CM group showed a thicker dermis than the NC or EGF groups (p < 0.001; Fig. 4c).
Fig. 4.
Hematoxylin and eosin analysis on healed wound skin. (a) Hematoxylin and eosin staining evaluation of full-thickness skin wounds (left scale bar = 2.5 mm, right scale bar = 1 mm). (b) The number of cutaneous appendages/HPF in healed wound tissue. (c) The dermal thickness in healed wound tissue. **p < 0.01, ***p < 0.001 compared with NC, ###p < 0.001 compared with PC, data are reported as mean ± SD, n = 12)
HPF: high-power field; NC: negative control; PC: positive control; SD: standard deviation.
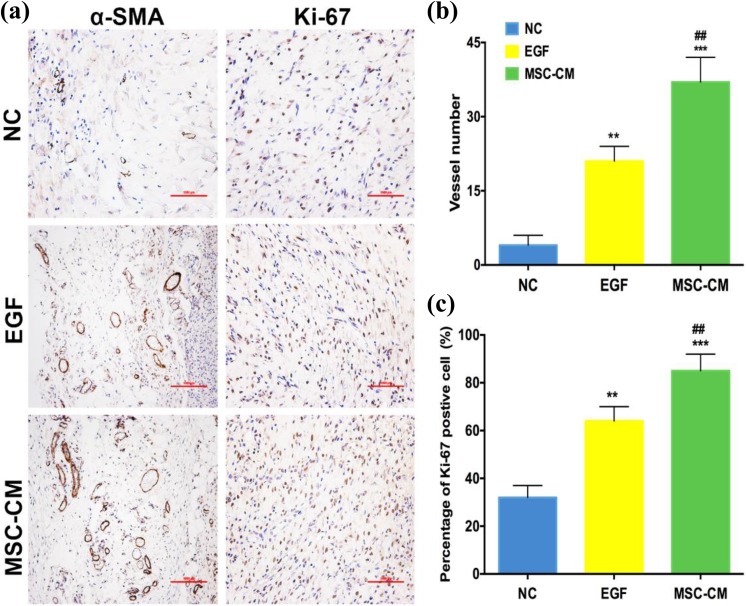
IHC results showed that α-SMA expression and the number of vessels/HPF in wound-healed tissue was increased significantly in the MSC-CM group (34.6 ± 5.3/HPF) when compared with the NC group (3.9 ± 2.8/HPF, p < 0.001), as well as the EGF group (8.3 ± 4.0, p < 0.001; Fig. 5a, b). The percentage of Ki-67 positivity in the MSC-CM group (85.2% ± 7.1) was higher than the NC group (64.1% ± 5.8, p < 0.001) and EGF group (32.4% ± 4.3, p < 0.01; Fig. 5a, c).
Fig. 5.
IHC analysis on healed wound skin. (a) IHC evaluation of the full-thickness skin wounds (scale bar = 1 mm). (b) The number of vessels/HPF in healed wound tissue. (c) The number of Ki-67 brown-positive proliferating cells/HPF in healed wound tissue.
**p < 0.01, ***p < 0.001 compared with NC, ##p < 0.01, ###p < 0.001 compared with EGF, data are reported as mean ± SD, n = 12.
α-SMA: α-smooth muscle actin; EGF: epidermal growth factor; HPF: high-power field; IHC: immunohistochemistry; NC: negative control; SD: standard deviation.
The results of protein expression showed that the expression of Ki-67 and α-SMA in the MSC-CM group was significantly increased compared with the NC group (p < 0.01; Fig. 5a–c). Consistent with the above results, the gene expression of Ki-67 and α-SMA was also significantly increased compared with the NC group (p < 0.01; Fig. 5e, f). In addition, expression level of Col1A2 was significantly enhanced with a relative gene expression of 20.1-fold higher than the NC group, and 3.2-fold higher than the EGF group.
Discussion
To the best of our knowledge, this study was the first to use MSC-CM to treat radiation dermatitis in rats. Overall, our results demonstrated that MSC-CM significantly increased the wound-healing rate and quality. MSC-CM not only stimulated HUVEC proliferation significantly in vitro, but also effectively promoted tissue repair and regeneration of radiation-damaged skin in rats. We used β-rays (Sr-90) for skin injury modeling, which was serious and complicated. If it has a beneficial therapeutic effect on radioactive skin damage, it may also have a repairing effect on a relatively simple mechanical damage model. Therefore, CM harvested from WJ-MSCs may enhance the beneficial effect of cellular-based skin wound therapy.
Previously, it has been reported that MSCs secrete various growth factors and chemokines for promoting wound healing17,18. In in-vivo experiments, Nauta et al. treated a mouse skin excisional model using adipose-derived MSCs, which accelerated wound healing by overexpressing VEGF19. WJ-MSCs secrete growth factors, including angiogenin, monocyte chemotactic protein 1 (MCP-1), and IL-8, and can stimulate neovascularization and perfusion20. MSCs might secrete several factors including insulin-like growth factors (IGF), transforming growth factor beta 1 (TGF-β1), and hepatocyte growth factor (HGF) to promote angiogenesis and skin wound healing21. In the present study, our results demonstrated that MSC-CM promoted HUVEC proliferation using in-vitro experiments (Figs. 1 and 2). HUVEC proliferation was beneficial to angiogenesis with the healing of skin wounds10,22–24. In addition, MSC-CM enhanced α-SMA expression and increased the total number of vessels significantly on healed wound skin (Figs. 5b and 6c). Furthermore, we observed that MSC-CM secreted some factors to reduce inflammation and to promote angiogenesis in vitro (Fig. 2). Therefore, MSC-CM has a great potential in promoting wound healing.
Fig. 6.
Protein and gene expressions on healed skin tissue. (a–c) Ki-67 and α-SMA protein expression. (d–f) Col1A2, Ki-67 and α-SMA gene expression.
*p < 0.05, **p < 0.01 compared with NC, ##p < 0.01, ###p < 0.001 compared with EGF, data are reported as mean ± SD, n = 12.
α-SMA: α-smooth muscle actin; EGF: epidermal growth factor; NC: negative control; SD: standard deviation.
It has been reported that MSCs and their secretions could effectively increase the quality of wound healing25,26. The optimal healing of skin injury is by restoring the physiology and anatomy of the skin25,27. Sabapathy et al. used human WJ-MSCs to prometed scar-free skin wound healing with hair growth13. Another study also demonstrated that WJ-MSCs had more capacity on sweat gland restoration and cutaneous regeneration after skin injury28. Our results showed that the MSC-CM group exhibited the smallest wound size and highest Col1A2 expression (Fig. 3b and 6d). In addition, MSC-CM had more sebaceous gland cell-like differentiative capacity in our study (Fig. 5). MSC-CM had more therapeutic potential in the recovery of skin function and enhanced the wound-healing quality in comparison with the control group.
Increasing evidence supports that MSCs contribute to skin repair and regeneration through paracrine effects29,30. Recent studies demonstrated that conditioned medium from human umbilical-derived MSCs promoted primary wound healing and regeneration31. In accordance with the above studies, Fong et al. used the same conditioned medium containing key molecules to increase cell density and promote skin regeneration14. Our results indicated that expression of Ki-67 significantly increased on healed skin tissue (Figs. 5c, 6b, and 6e). This suggests that MSC-CM may secrete various factors to stimulate fibroblasts and keratinocyte proliferation, migration and differentiation, to enhance injured skin regeneration and functional recovery32–34.
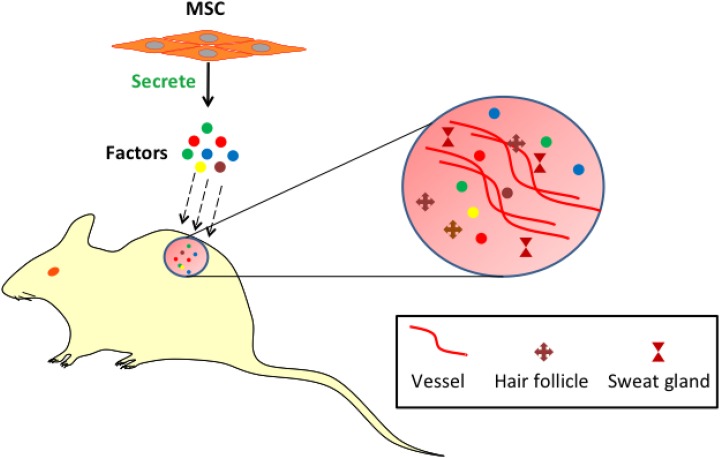
In summary, MSC-CM secretes factors that have greater therapeutic potential on radiation-induced cutaneous skin injury in rats. Our results indicate that MSC-CM promoted cell proliferation, sebaceous gland cell-like regeneration and angiogenesis. Furthermore, MSC-CM accelerates wound closure and enhances the quality of wound healing (Fig. 7). Using MSC-CM as a novel cell-free therapeutic approach for cutaneous wound healing avoids the ethical issues of cell transplantation and the risk of tumor genesis, and it possesses the similar function, and even better therapeutic effect than their parent cells. In addition, it is convenient for clinical applications. We will apply it to clinical practice in our further research. We believe that MSC-CM may serve as a basis of a novel cell-free therapeutic approach for radiation dermatitis and propose to use it in the clinic in the future.
Fig. 7.
MSCs secrete several factors including IGF, HGF, angiogenin, and MCP-1 to promote the angiogenesis and cutaneous appendages (hair follicles and sweat glands) regeneration in damaged skin.
HGF: hepatocyte growth factor; IGF: insulin-like growth factor; MCP-1: monocyte chemotactic protein 1; MSC: mesenchymal stem cell.
Acknowledgments
The authors thank Professor M. Li, Jilin University, for his generously provided WJ-MSCs, and Professor Hai Zhang, Jilin University, for his assistance in histology.
Footnotes
Ethical Approval: This study was approved by the Animal Experiment Ethics Committee of Jilin University, China.
Statement of Human and Animal Rights: All the protocols and procedures were approved by the Animal Experiment Ethics Committee of the Jilin University, China (approval No. XYSK2017-0125).
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (No. 1500792167890).
References
- 1. Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54(1):28–46. [DOI] [PubMed] [Google Scholar]
- 2. Moding EJ, Kastan MB, Kirsch DG. Strategies for optimizing the response of cancer and normal tissues to radiation. Nat Rev Drug Discov. 2013;12(7):526–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coppes RP, van der Goot A, Lombaert IM. Stem cell therapy to reduce radiation-induced normal tissue damage. Semin Radiat Oncol. 2009;19(2):112–121. [DOI] [PubMed] [Google Scholar]
- 4. Kohl RR, Kolozsvary A, Brown SL, Zhu G, Kim JH. Differential radiation effect in tumor and normal tissue after treatment with ramipril, an angiotensin-converting enzyme inhibitor. Radiat Res. 2007;168(4):440–445. [DOI] [PubMed] [Google Scholar]
- 5. Bray FN, Simmons BJ, Wolfson AH, Nouri K. Acute and chronic cutaneous reactions to ionizing radiation therapy. Dermatol Ther (Heidelb). 2016;6(2):185–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Benderitter M, Caviggioli F, Chapel A, Coppes RP, Guha C, Klinger M, Malard O, Stewart F, Tamarat R, Luijk PV, Limoli CL. Stem cell therapies for the treatment of radiation-induced normal tissue side effects. Antioxid Redox Signal. 2014;21(2):338–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moroz BB, Onizhshenko NA, Lebedev VG, Deshevoi Iu B, Sidorovich GI, Lyrshchikova AV, Rasulov MF, Krasheninnikov ME, Sevast’ianov VI. The influence of multipotent mesenchymal stromal cells of bone marrow on process of local radiation injury in rats after local beta-irradiation [in Russian]. Radiats Biol Radioecol. 2009;49(6):688–693. [PubMed] [Google Scholar]
- 8. Portas M, Mansilla E, Drago H, Dubner D, Radl A, Coppola A, Di Giorgio M. Use of human cadaveric mesenchymal stem cells for cell therapy of a chronic radiation-induced skin lesion: a case report. Radiat Prot Dosimetry. 2016;171(1):99–106. [DOI] [PubMed] [Google Scholar]
- 9. Nuschke A. Activity of mesenchymal stem cells in therapies for chronic skin wound healing. Organogenesis. 2013;10(1):29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aynardi M, Zahoor T, Mitchell R, Loube J, Feltham T, Manandhar L, Paudel S, Schon L, Zhang Z. Orthotopic transplantation of achilles tendon allograft in rats: with or without incorporation of autologous mesenchymal stem cells. Cell Transplant. 2018;27(2):245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ong HT, Redmond SL, Marano RJ, Atlas MD, von Unge M, Aabel P, Dilley RJ. Paracrine activity from adipose-derived stem cells on in-vitro wound healing in human tympanic membrane keratinocytes. Stem Cells Dev. 2017;26(6):405–418. [DOI] [PubMed] [Google Scholar]
- 12. Sassoli C, Frati A, Tani A, Anderloni G, Pierucci F, Matteini F, Chellini F, Zecchi Orlandini S, Formigli L, Meacci E. Mesenchymal stromal cell secreted sphingosine 1-phosphate (s1p) exerts a stimulatory effect on skeletal myoblast proliferation. PLoS One. 2014;9(9):e108662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sabapathy V, Sundaram B, S VM, Mankuzhy P, Kumar S. Human wharton’s jelly mesenchymal stem cells plasticity augments scar-free skin wound healing with hair growth. PLoS One. 2014;9(4):e93726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fong CY, Tam K, Cheyyatraivendran S, Gan SU, Gauthaman K, Armugam A, Jeyaseelan K, Choolani M, Biswas A, Bongso A. Human wharton’s jelly stem cells and its conditioned medium enhance healing of excisional and diabetic wounds. J Cell Biochem. 2014;115(2):290–302. [DOI] [PubMed] [Google Scholar]
- 15. Wei L, Zhang J, Xiao XB, Mai HX, Zheng K, Sun WL, Wang L, Liang F, Yang ZL, Liu Y, Wang YQ, Li ZF, Wang JN, Zhang WJ, You H. Multiple injections of human umbilical cord-derived mesenchymal stromal cells through the tail vein improve microcirculation and the microenvironment in a rat model of radiation myelopathy. J Transl Med. 2014;12:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dong L, Hao H, Liu J, Ti D, Tong C, Hou Q, Li M, Zheng J, Liu G, Fu X, Han W. A conditioned medium of umbilical cord mesenchymal stem cells overexpressing wnt7a promotes wound repair and regeneration of hair follicles in mice. Stem Cells Int. 2017;2017:3738071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee SC, Jeong HJ, Lee SK, Kim SJ. Hypoxic conditioned medium from human adipose-derived stem cells promotes mouse liver regeneration through JAK/STAT3 signaling. Stem Cells Transl Med. 2016;5(6):816–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Menendez-Menendez Y, Otero-Hernandez J, Vega JA, Perez-Basterrechea M, Perez-Lopez S, Alvarez-Viejo M, Ferrero-Gutierrez A. The role of bone marrow mononuclear cell-conditioned medium in the proliferation and migration of human dermal fibroblasts. Cell Mol Biol Lett. 2017;22:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nauta A, Seidel C, Deveza L, Montoro D, Grova M, Ko SH, Hyun J, Gurtner GC, Longaker MT, Yang F. Adipose-derived stromal cells overexpressing vascular endothelial growth factor accelerate mouse excisional wound healing. Mol Ther. 2013;21(2):445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Choi M, Lee HS, Naidansaren P, Kim HK, E O, Cha JH, Ahn HY, Yang PI, Shin JC, Joe YA. Proangiogenic features of Wharton’s jelly-derived mesenchymal stromal/stem cells and their ability to form functional vessels. Int J Biochem Cell Biol. 2013;45(3):560–570. [DOI] [PubMed] [Google Scholar]
- 21. Johnson TV, DeKorver NW, Levasseur VA, Osborne A, Tassoni A, Lorber B, Heller JP, Villasmil R, Bull ND, Martin KR, Tomarev SI. Identification of retinal ganglion cell neuroprotection conferred by platelet-derived growth factor through analysis of the mesenchymal stem cell secretome. Brain. 2014;137(Pt 2):503–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tang J, Xie Q, Pan G, Wang J, Wang M. Mesenchymal stem cells participate in angiogenesis and improve heart function in rat model of myocardial ischemia with reperfusion. Eur J Cardiothorac Surg. 2006;30(2):353–361. [DOI] [PubMed] [Google Scholar]
- 23. Del Papa N, Caviggioli F, Sambataro D, Zaccara E, Vinci V, Di Luca G, Parafioriti A, Armiraglio E, Maglione W, Polosa R, Klinger F, Klinger M. Autologous fat grafting in the treatment of fibrotic perioral changes in patients with systemic sclerosis. Cell Transplant. 2015;24(1):63–72. [DOI] [PubMed] [Google Scholar]
- 24. Angelopoulos I, Brizuela C, Khoury M. Gingival mesenchymal stem cells outperform haploidentical dental pulp-derived mesenchymal stem cells in proliferation rate, migration ability, and angiogenic potential. Cell Transplant. 2018;27(6):967–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee DE, Ayoub N, Agrawal DK. Mesenchymal stem cells and cutaneous wound healing: novel methods to increase cell delivery and therapeutic efficacy. Stem Cell Res Ther. 2016;7:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Milan PB, Lotfibakhshaiesh N, Joghataie MT, Ai J, Pazouki A, Kaplan DL, Kargozar S, Amini N, Hamblin MR, Mozafari M, Samadikuchaksaraei A. Accelerated wound healing in a diabetic rat model using decellularized dermal matrix and human umbilical cord perivascular cells. Acta Biomater. 2016;45;234–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moore AL, Marshall CD, Barnes LA, Murphy MP, Ransom RC, Longaker MT. Scarless wound healing: transitioning from fetal research to regenerative healing. Wiley Interdiscip Rev Dev Biol. 2018;7(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu Y, Huang S, Ma K, Fu X, Han W, Sheng Z. Promising new potential for mesenchymal stem cells derived from human umbilical cord Wharton’s jelly: sweat gland cell-like differentiative capacity. J Tissue Eng Regen Med. 2012;6(8):645–654. [DOI] [PubMed] [Google Scholar]
- 29. Zhang Y, Niu X, Dong X, Wang Y, Li H. Bioglass enhanced wound healing ability of urine-derived stem cells through*h promoting paracrine effects between stem cells and recipient cells. J Tissue Eng Regen Med. 2018;12(3):e1609–e1622. [DOI] [PubMed] [Google Scholar]
- 30. Costa MHG, McDevitt TC, Cabral JMS, da Silva CL, Ferreira FC. Tridimensional configurations of human mesenchymal stem/stromal cells to enhance cell paracrine potential towards wound healing processes. J Biotechnol. 2017;262:28–39. [DOI] [PubMed] [Google Scholar]
- 31. Kusindarta DL, Wihadmadyatami H, Fibrianto YH, Nugroho WS, Susetya H, Musana DK, Wijayanto H, Prihatna SA, Wahyuni AE. Human umbilical mesenchymal stem cells conditioned medium promote primary wound healing regeneration. Vet World. 2016;9(6):605–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shabbir A, Cox A, Rodriguez-Menocal L, Salgado M, Van Badiavas E. Mesenchymal stem cell exosomes induce proliferation and migration of normal and chronic wound fibroblasts, and enhance angiogenesis in vitro. Stem Cells Dev. 2015;24(14):1635–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liang X, Huang X, Zhou Y, Jin R, Li Q. Mechanical stretching promotes skin tissue regeneration via enhancing mesenchymal stem cell homing and transdifferentiation. Stem Cells Transl Med. 2016;5(7):960–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee EY, Xia Y, Kim WS, Kim MH, Kim TH, Kim KJ, Park BS, Sung JH. Hypoxia-enhanced wound-healing function of adipose-derived stem cells: increase in stem cell proliferation and up-regulation of VEGF and BFGF. Wound Repair Regen. 2009;17(4):540–547. [DOI] [PubMed] [Google Scholar]