Abstract
Female pattern hair loss (FPHL) is the most common form of alopecia in women. Affected women may experience psychological distress and impaired social functioning. Early diagnosis and initiation of treatment are desirable because treatments are more effective to avoid the progression of hair loss than stimulating regrowth. Typically, a diagnosis of FPHL can be confirmed by review of a patient's medical history and a physical examination alone. Testing a scalp biopsy is diagnostic but usually not required. In women with signs of hyperandrogenism, an investigation for ovarian or adrenal disorders should be performed. Treatment for FPHL is obscured by myths. The aim of FPHL treatment could be two-fold: Reverse or stabilize the process of hair follicle miniaturization. Mild-to-moderate FPHL in women can be treated with oral antiandrogen therapies (cyproterone acetate and spironolactone) and/or topical minoxidil with good results in many cases. If used correctly, available medical treatments arrest the progression of the disease and reverse miniaturization in most patients with mild-to-moderate FPHL. Hair systems and surgery may be considered for selected cases of severe FPHL.
Keywords: Androgenetic alopecia, female pattern hair loss, finasteride, minoxidil, platelet-rich plasma
Introduction
Female pattern hair loss (FPHL) has emerged as the preferred term for androgenetic alopecia (AGA) in women due to the uncertain relationship between androgens and this entity (Olsen, 2001). FPHL is the most common hair loss disorder in women. Initial symptoms may develop during the teenage years and lead to progressive hair loss with a characteristic pattern distribution (Vujovic and Del Marmol, 2014). FPHL is characterized as a nonscarring diffuse alopecia that evolves from the progressive miniaturization of hair follicles and subsequent reduction in the number of hairs, especially in the central, frontal, and parietal scalp regions (Olsen, 2002; Fig. 1).
Fig. 1.
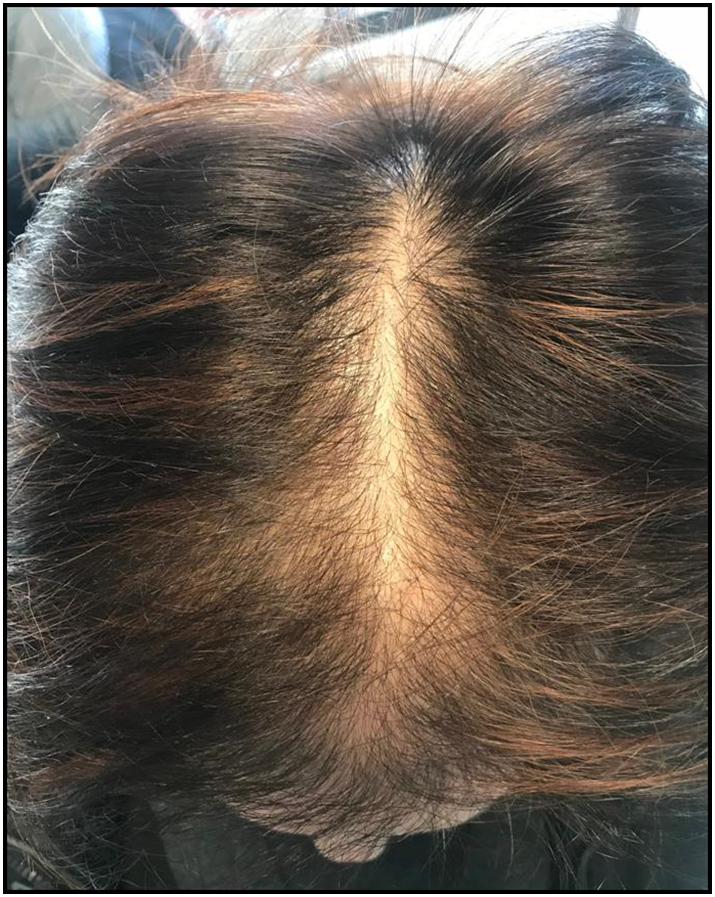
Clinical example of female pattern hair loss
FPHL has three main clinical manifestations. The first manifestation is the diffuse thinning of the upper biparietal and vertex regions and preservation of the anterior hair implantation line. There are several hair loss scales that attempt to categorize FPHL and each has advantages and disadvantages (Ludwig, 1977, Ramos and Miot, 2015, Savin, 1994, Yip and Sinclair, 2006; Fig. 2, Fig. 3). A wide discussion of each scale is not the primary scope of the paper.
Fig. 2.
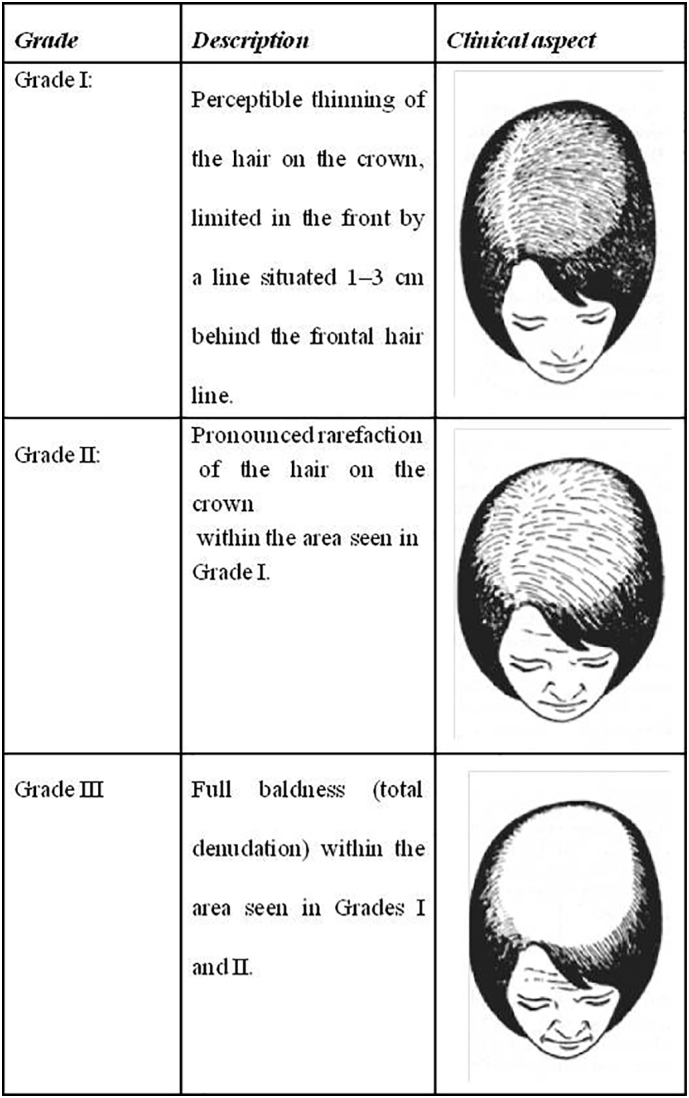
Ludwig scale representation
Fig. 3.
Sinclair Scale Sinclair’s classification. MPA is divided into four levels of intensity on the basis of normal scalp to the left (Sinclair et al., 2005).
Another manifestation is the thinning of the upper bitemporal region and vertex with frontal accentuation that configures as a triangular or Christmas tree form with hair loss in a triangular shape in the frontal-vertical area (Olsen, 1999; Fig. 4). A third manifestation is a deep recession of the frontal-temporal hairline and true vertex balding, which is typically seen in men but occasionally occurs in women although uncommon (Redler et al., 2017).
Fig. 4.
Olsen´s classification. Olsen patterns incorporate the accentuation of the front-overtical alopecia, which has a triangular or Christmas tree form with hair loss in a triangular form in the front-overtical area (Olsen, 2002).
Several management options are available to treat FPHL but every treatment usually requires a long period of time to get a significative improvement. Therefore, a considerable amount of time should always be dedicated to doctor-patient counseling to improve compliance.
Epidemiology
The frequency of FPHL varies among population groups and ordinarily increases with age. However, a comparison of prevalence between different studies is hampered by the lack of universally accepted criteria for the disease (Ramos and Miot, 2015). Among healthy women, approximately 6% to 38% experience some degree of frontal and/or frontal-parietal hair loss (Birch et al., 2001).
The age of onset for FPHL is during the reproductive years, which is later than in men. Twelve percent of women first develop clinically detectable FPHL by age 29 years, 25% by age 49 years, 41% by 69 years, and > 50% have some element of FPHL by 79 years (Birch et al., 2002). More severe cases of the disease during puberty are more rarely described. Nevertheless, there is a greater demand for treatment among patients ages 25 to 40 years (Tosti and Piraccini, 2006). In the United Kingdom, 6% of women younger than age 30 years have FPHL for women older than 70 years, FPHL reaches a rate of 42% (Birch et al., 2002). Only 43% of women age > 80 years show no evidence of FPHL (Sinclair and Dawber, 2001).
Pathophysiology
FPHL and male AGA share a final common pathway that causes follicular regression but current knowledge suggests that the etiology is not necessarily the same in both sexes. Although the role of androgens in the pathogenesis of male hair loss has been clearly established, the role of androgens in FPHL is less clear. In fact, FPHL may develop even in the absence of androgens (Herskovitz and Tosti, 2013). However, it is likely that other nonandrogenic factors that are currently unidentified may play a role in the pathogenesis of FPHL (Redler et al., 2017). Therefore, the involvement of these genes in the etiopathogenesis of FPHL cannot be completely excluded.
In women with FPHL who do not have elevated androgen levels, a genetic predisposition may be involved. This genetic disposition permits normal levels of circulating androgen to act on follicular target cells, which are specially sensitized by binding to specific intracellular androgen receptors. In other cases, an androgen-independent mechanism may be involved in the development of FPHL (Orme et al., 1999). Two recent studies by Heilmann-Heimbach et al. (2017) and Pickrell et al. (2016) have substantially found an increased number of gene loci (> 60) associated with male AGA.
Hair loss in women is polygenic and multifactorial with the additional influence of environmental factors. Several studies focused on the importance of several genes related to alopecia (Carey et al., 1993, Hillmer et al., 2008, Randall, 2008). FPHL involves progressive hair follicle miniaturization and subsequently the conversion of terminal follicles into vellus-like follicles. These vellus-like follicles have a shortened hair cycle because of a reduction in the anagen phase, which leads to the production of short and fine hair shafts. Unlike in men, the miniaturization is not uniform and intense in women; therefore, there are no complete areas of baldness except in very rare cases (Birch et al., 2001). Moreover, the miniaturization process may be accompanied by a mild-to-moderate lympho-histiocytic inflammatory infiltrate in the peri-infundibular region. The term “microinflamation” has been used to differentiate this infiltrate from the inflammation that occurs in scarring alopecia (Stefanato, 2010).
FPHL and male balding share a final common pathway of follicular regression but current knowledge suggests that the etiology is not necessarily the same in both sexes. Androgens are a key driver of male balding and also involved in the etiology of pattern hair loss in some women. However, other nonandrogenic factors that are still unidentified likely play a role in causing FPHL (Herskovitz and Tosti, 2013).
Comorbidities
The most common endocrinologic comorbidity that is associated with FPHL is polycystic ovarian syndrome (El Sayed et al., 2016). Metabolic syndrome, which is characterized by obesity, insulin resistance, hypertension, hyperprolactinemia, and raised aldosterone levels, also appears to be frequently associated with FPHL (El Sayed et al., 2016). An increased risk of carotid and coronary artery diseases have also been reported (Arias-Santiago et al., 2010). To further clarify the comorbidity profile of FPHL, systematic studies in larger population-based samples are needed.
An association between ferritin levels and FPHL is controversial. Some studies have demonstrated lower ferritin levels in patients with FPHL compared with controls and antiandrogen therapy seem to work better in patients with ferritin levels > 40 μg/l (Ramos and Miot, 2015).
Diagnosis
Women with increased hair shedding but little or no reduction in hair volume over the mid-frontal scalp could be suffering from several diseases and acute and chronic telogen effluvium (TE) should be considered in particular. Anamnesis and a physical examination are needed to get the right diagnosis. Anamnesis should focus on when the hair loss started, whether the loss was gradual or involved handfuls of hair as well as any physical, mental, or emotional stressors that may have occurred within the previous 3 to 6 months. A history and physical examination should aim at detecting signs of hyperandrogenism such as hirsutism, ovarian abnormalities, menstrual irregularities, acne, and infertility. Laboratory test results are rarely evaluated in women who suffer from FPHL with no signs of hyperandrogenism.
Moreover, hair loss may occur in patients who are treated with oral contraceptive medications that contain progesterone with a high androgenic potential such as norethindrone or who recently discontinued an estrogenic oral contraceptive medication that was taken for a long period of time. A physical examination should include all aspects of the scalp and especially evaluate the involvement of the occipital area, which will show a widening of the central part with a diffuse reduction in hair density over the frontal scalp rather than baldness per se. Although these areas show the most marked reduction in hair density, evidence of global reduction in hair density usually exists throughout the all scalp.
Pull test
The pull test is an evaluation of the number of hairs that are shed after a slight traction on the scalp hair. This test helps to roughly estimate the severity of hair loss in daily practice with a high interobserver variability. A bundle of approximately 50 to 60 hairs is grasped between the thumb, index finger, and middle finger from the base close to the scalp. The hair is firmly but not forcibly tugged away from the scalp as the fingers slide along the hair shaft. Afterward, the number of extracted hairs is counted. If more than 10% of the grasped hair (six hairs) are pulled away from the scalp, the pull test is positive and implies active hair shedding. Fewer than six hairs that are easily pulled out is considered normal physiologic shedding.
Nonetheless, McDonald et al. (2017) recently tried to quantify normal hair pull test values by elucidating the effect of pretest hair washing and brushing and performing the pull test on 181 participants. The study showed that normal values for the hair pull test should be reduced to 2 hairs or fewer. In addition, neither hair brushing nor washing altered the hair pull test results. Hair washing and brushing may now occur at any time before the hair pull test instead of 4 to 5 days prior. The pull test is only a rough approach to diagnosis and more reliable tests and objective measurements should be added.
Standardized wash test
With the standardized wash test, women refrain from shampooing for 5 days and then shampoo and rinse their hair in a basin with the hole covered with gauze. All the hairs that remain in the water and gauze are collected and sent for examination. A total of 34 hairs must be counted and divided into ≤ 3 cm and ≥ 5 cm in length. This is an important technique to differentiate TE from FPHL.
Modified wash test
The wash test was modified by Rebora et al. (2005) and called the AGA/TE wash test to distinguish between AGA and TE. For the AGA/TE modified wash test, the hairs are counted and divided into three groups by hair length: 1) Long hair > 5 cm, 2) intermediate-length hair (> 3 to < 5 cm), and 3) short vellus hair (< 3 cm). Hairs shorter than 3 cm are counted as telogen vellus hairs. The final results of the AGA/TE modified wash test are given as the total number of telogen hairs and the percentage of telogen vellus hairs.
Trichogram
The trichogram is a semi-invasive (plucking) microscopic method for hair root and cycle evaluation. The trichogram is based on the hair cycle and quantifies hair follicles in their different growth phases. With a rubber-armed forceps, 60 to 80 hairs are plucked at two specific scalp locations depending on the hair disorder. Hairs are removed with one, quick, forceful pull perpendicular to the scalp and always along the direction of hair growth. Hair bulbs are immediately embedded with their roots on a glass slide and evaluated under a magnifying lens or low-power microscope to determine the number of hairs in the different phases of the hair cycle. The results are given as a percentage of the total number of plucked hairs (Blume-Peytavi and Orfanos, 2006).
Videodermoscopy
Videodermoscopy is a noninvasive technique that was initially used for the in vivo evaluation of pigmented lesions but has proven to be a useful tool to study in vivo scalp and hair disorders. This technique allows physicians to distinguish FPHL from acute and chronic TE, especially in the early stages of the disease (Ramos and Miot, 2015). A videomicroscope equipped with various objective lenses (from × 20 through × 1000) is used. The magnification enhances the images of the scalp and hair and detects the hair shaft in the follicle (if present) and its length, diameter, and possible anomalies. All digital images may be stored for further controls.
FPHL results from the progressive miniaturization of hair follicles; thus, the earliest diagnostic feature is a hair shaft diameter variation of > 20% hair shafts. A central parting to compare hair density at the top with hair density at the occipital region can easily demonstrate this condition. The dermoscopic features include (Lacarrubba et al., 2015) hair diameter diversity (diversity of > 20% is diagnostic for AGA), short vellus hair (< 0.03 mm; sign of severe miniaturization and their presence on the frontal scalp is a very useful clue for diagnosis with FPHL diagnosis > 7 vellus hairs on the frontal scalp), yellow dots (sign of severe miniaturization and more numerous in patients with severe FPHL), pinpoint white dots and scalp pigmentation (honeycombed-like pattern on sun-exposed scalp), peripilar sign (subtle brown halo, which is a specific finding in early stages of the disease and reflects perifollicular inflammation; focal areas of baldness [atrichia] could be recognized in postmenopausal women), and scalp biopsy (best way to distinguish between chronic TE and FPHL [Sinclair et al., 2004] through the calculation of the terminal-to-vellus hair ratio; ratio of < 4:1 is considered diagnostic of FPHL; ratio of > 8:1 is considered diagnostic of chornic TE). Scalp biopsy is an invasive technique and the indication for a scalp biopsy should be carefully provided but, when necessary, the intervention should not be delayed.
Differential diagnosis
A differential diagnosis of FPHL includes TE, postpartum hair loss, cicatricial alopecia in pattern distribution, and alopecia areata (diffuse or incognita; Asz-Sigall et al., 2016). As discussed, dermoscopy is a very useful complementary tool to get the right diagnosis, especially in the early stages of the disease.
Treatment
Since FPHL can mimic and often runs concurrently with other diagnoses, a detailed medical history overview and physical examination should be performed on the patient. With the help of other diagnostic tools and techniques, other concurrent conditions should be investigated and treated if applicable.
Treatment options available for FPHL can be classified in two categories: Topical and systemic drugs. Because FPHL is a biological process determined by a sensitivity to androgens that are genetically mediated, most of these drugs act on the androgen activity by altering the production, transport, or metabolism of androgens or preventing the binding to androgenic receptors. Moreover, androgen-dependent medications may cause abnormalities in the genitalia of the male fetus; thus, these drugs are contraindicated in pregnant women. This finding leads many physicians to recommend an oral contraceptive therapy throughout the entire course of treatment.
Topical therapy
Minoxidil
Minoxidil is a piperidinopyrimidine derivative and potent vasodilator that is effective orally for severe hypertension. The drug was approved in 1979 by the U.S. Food and Drug Administration (FDA) for the treatment of hypertension. Minoxidil was first noticed to improve hair loss in male AGA in 1980 when used topically (Varothai and Bergfeld, 2014). Minoxidil solutions of 2% and 5% were approved for the treatment of male AGA in 1988 and 1991, respectively. In FPHL, 2% minoxidil was approved by the FDA in 1991 and a 5% minoxidil foam with once daily application was approved in 2014 (Varothai and Bergfeld, 2014).
Minoxidil is effective in both sexes (Blumeyer et al., 2011, van Zuuren et al., 2012a, van Zuuren et al., 2012b) and statistically significantly increases nonvellus and total hair count at 24 weeks of treatment. In male AGA, the 5% solution shows better results than the 2% solution (Tsuboi et al., 2009). For FPHL, no study has compared the effectiveness of the 2% minoxidil solution twice daily with the 5% minoxidil solution once daily but a randomized trial revealed that the 5% minoxidil foam once daily was similar in effectiveness to the 2% minoxidil solution twice daily for the treatment of female AGA (Blume-Peytavi et al., 2011).
Minoxidil is a potassium channel opener and stimulates hair growth by increasing the anagen phase of the hair cycle. Minoxidil enhances angiogenesis around the follicle but the exact mechanisms are currently unknown (Blumeyer et al., 2011, Gupta and Foley, 2014). An activation of cyto-protective prostaglandin synthase-1 may exist that increases hair count and weight. The topical minoxidil 2% solution should be applied only to the affected area of the scalp at 1 ml twice daily (once daily for the minoxidil 5% foam) for a minimum period of 12 months before determining the efficacy. The clinical response to 5% topical minoxidil for the treatment of AGA is typically observed after 3 to 6 months and approximately 40% of patients show a significative improvement. For this reason, minoxidil response testing to rule out nonresponders has significant clinical utility.
When effective, treatment should be continued indefinitely as with a chronic disease because discontinuation may induce TE in the minoxidil-dependent hair within 4 to 6 months (Banka et al., 2013). Patients should also be warned that during the first months of treatment, a transient increase shedding may occur. Treatment side effects are uncommon and include allergic or irritative contact dermatitis, which is more commonly related to the solution vehicle propylene glycol. This can be overcome with use of the 5% foam that does not contain this ingredient. Another possible side effect is hypertrichosis of the forehead or face, which is usually caused by accidental contamination or improper application (Herskovitz and Tosti, 2013). Additionally, the 5% minoxidil foam provides an alternative option for women who do not wish or are unable to use oral anti-androgen or hormonal contraceptive medications (Gupta and Foley, 2014).
Minoxidil is a pro-drug that is converted to its active form, minoxidil sulfate, by sulfotransferase enzymes in the outer root sheath of hair. Minoxidil sulfate is the active form that is required for both the promotion of hair regrowth and the vasodilatory effects of minoxidil. Several studies have demonstrated that sulfotransferase enzyme activity in plucked hair follicles predicts topical minoxidil response in patients with FPHL. Moreover, different studies have been conducted to confirm the clinical utility of a sulfotransferase activity assay to guide treatment (Goren et al., 2015). These analysis show that the sulfotransferase enzyme test can successfully rule out 95.9% of nonresponders to topical minoxidil for the treatment of AGA (McCoy et al., 2016).
Prostaglandin analog treatments
Latanoprost and bimatoprost were initially developed for eye glaucoma when the growth of eye lashes was noticed as a side effect. In fact, among the prostaglandins (PG), the PG-F2 analog treatments latanoprost and bimatoprost are known to stimulate hair growth by prolonging the anagen phase (Valente Duarte de Sousa and Tosti, 2013). A small placebo-controlled trial in men with mild AGA showed that 0.1 % latanoprost significantly increased hair density and pigmentation at 24 weeks compared with baseline and compared with the placebo-treated site. Nevertheless, the study included only 16 male patients and the medication was applied to a very small area of the scalp (Blume-Peytavi et al., 2012, Herskovitz and Tosti, 2013). However, a case report of postmenopausal patients with FPHL failed to demonstrate the efficacy of locally injected 0.03 % bimatoprost for 16 weeks (Emer et al., 2011).
Others studies have revealed that an increased PG-D2 level is correlated with the miniaturization of hair follicles and, moreover, the topical application of PG-D2 also inhibited hair growth (Garza et al., 2012). Recent research studies are looking for other drugs that are able to block the PG-D2 receptor (GPR44), which has an inhibitory effect on hair growth and is known to be elevated in the scalp of patients with AGA (Nieves and Garza, 2014). Setipiprant (KITH-105) is an orally administered GPR44 receptor inhibitor in a clinical trial for asthma and could have a potential application for patients with AGA (Keaney, 2015). A phase 2 clinical trial is evaluating the use of oral setipiprant in comparison with placebo and finasteride 1 mg/d in men ages 18 to 41 years with AGA (Study NCT02781311).
Ketoconazole
The pathobiology of AGA is not completely clear. The genetic predisposition and influence of androgens have shown to play a role in AGA. However, these factors do not explain the presence of a substantial lymphoid infiltrate that abuts on the infra-infundibulum and isthmus of transitional hair follicles (Piérard et al., 1996). The negative influence of inflammation on AGA hair status is confirmed by AGA exacerbation after intercurrent episodes of other inflammatory dermatoses and especially seborrheic dermatitis.
Ketoconazole (KCZ) is considered highly effective to treat dandruff and seborrheic dermatitis. KCZ has an anti-inflammatory property and also acts as androgen-receptor antagonist. These issues may explain the efficacy of topical KCZ (Piérard-Franchimont et al., 1998). The inflammation that abuts on the AGA hair follicles might also be related to the presence of some members of that microflora that normally marks seborrheic dermatitis. Hence, KCZ, by reducing AGA inflammation, improves the hair status (Piérard et al., 1996). In FPHL with hyperandrogenism, 2% ketoconazole shampoo has shown a benefit in treatment (Sonino et al., 1990).
Melatonin
Melatonin is a pineal gland neurohormone that is released with a circadian rhythm and regulates different physiological processes such as seasonal biorhythms and daily sleep-wake cycles that influence the aging process. Melatonin is notable for its protective and anti-apoptotic effects due to its strong anti-oxidant properties and ability to actively capture free radicals (Fischer et al., 2001).
Hair follicle is a target organ for numerous neurohormones, neuropeptides, neurotrophins, and neurotransmitters but also produces many of these molecules (Arck et al., 2006). Therefore, the pilosebaceous unit is best recognized as a neuroendocrine organ. In this context, melatonin modulates hair growth, pigmentation, and molting in many species including humans (Fischer et al., 2008, Singh and Jadhav, 2014). The topical application of the melatonin 0.1 % solution was shown to significantly increase anagen hair in male and female AGA with a good compliance in a controlled study (Fischer et al., 2012).
Platelet-rich plasma
Platelet-rich plasma (PRP) is an autologous concentration of human platelets contained in a small volume of plasma. Platelets can be likened to cell reservoirs that produce, store, and release numerous growth factors capable of stimulating the proliferation of stem cells and the replication of mesenchymal cells, fibroblasts, osteoblasts, and endothelial cells. PRP is composed by several different growth factors: Platelet-derived growth factor, transforming growth factor a, vascular endothelial growth factor, insulin-like growth factor 1, epidermal growth factor, basic fibroblast growth factor, transforming growth factor-b1, and platelet-activating factor that are released through degranulation and stimulate bone and soft tissue healing.
The secretion of these growth factors begins within 10 minutes after clotting and > 95% of the presynthesized growth factors are secreted within 1 hour. The addition of thrombin and calcium chloride actives platelets in PRP and induces the release of factors from alpha granules. In dermatology and aesthetic medicine, indications range from hair restoration such as nonsurgical therapeutic options for patients with hair loss to chronic ulcers (Dhurat and Sukesh, 2014, Fabi and Sundaram, 2014).
Some hair transplant surgeons have used PRP in hair transplantation procedures, either by storing the grafts in PRP until they are placed on the scalp or by injecting PRP into the scalp prior to the placement of grafts (Rose, 2011). Although studies may differ in methodology, patient selection, and treatment technique, some authors have reported regrowth rates after five local treatments of 3 mL of PRP at 2- to 3-week intervals and histologic examinations showed thickened epithelium, proliferation of collagen fibers and fibroblasts, and increased vessels around follicles (Cervantes et al., 2018, Lee et al., 2015, Leo et al., 2015, Miao et al., 2013, Takikawa et al., 2011)-63).
Microneedling
Microneedling is a minimally invasive dermatologic procedure in which fine needles are rolled over the skin to puncture the stratum corneum. Through the physical trauma from needle penetration, microneedling induces a wound healing cascade with minimal damage to the epidermis that induces collagen formation, neovascularization, and growth factor production of the treated areas. Microneedling has shown promising results as an adjuvant therapy for enhanced drug delivery in the treatment of atrophic scars, AGA, alopecia areata, and pigmentation disorders such as melasma.
Although here are only a limited number of studies that have examined this therapy in the use of hair loss, microneedling has been successfully paired with other hair-growth promoting therapies such as minoxidil, platelet-rich plasma, and topical steroidal medications. Microneedling penetration of such first-line medications may be facilitated and is one mechanism that promotes hair growth (Fertig et al., 2018). To date, microneedling treatment has achieved the best results in AGA. Indeed, microneedling shows some promise in improving hair growth and especially in combination with existing techniques (Shah et al., 2017).
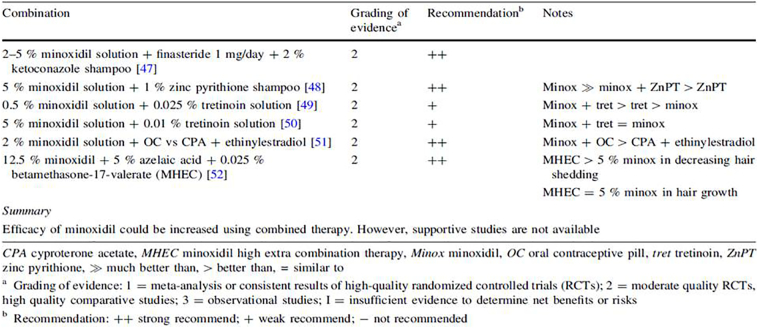
Combination therapies
Examples of combination therapies in patients with AGA (male and female) are listed in Figure 5.
Fig. 5.
Summary of evidence for the use of combination treatment of topical minoxidil in male and female androgenetic alopecia (Varothai and Bergfeld, 2014)
Light treatments
Low-level light therapy (LLLT) is a relatively new technique in the treatment of AGA. The biochemical mechanisms are not completely understood but the cellular respiratory chain of mitochondria probably absorb the light energy, which results in increased electron transport and the promotion of cellular signaling and in turn allows for hair regrowth (Rangwala and Rashid, 2012). Currently, several LLLT devices are available for the treatment of alopecia including a comb, hood, and helmet.
Many research studies started investigations into the efficacy of LLLT for AGA in men and women but the power of the device together with no standardization of study methods resulted in a high risk of bias. Therefore, the efficacy of LLLT devices remains unclear. The devices that were studied the most include the Hair Max LaserComb (Lexington International, LLC, Boca Raton, FL), which a hand-held, noninvasive device with a wavelength of 655 nm that was approved by the FDA for the safe treatment of male and female AGA. Its effectiveness has been shown to statistically significantly increase terminal hair density in comparison with a control group at 6.5 months (Jimenez et al., 2014, Leavitt et al., 2009). Therefore, LLLT and particularly a 650 to 900 nm wavelength at 5 mW may be a therapeutic option for patients with AGA.
Systemic treatment
Finasteride
Finasteride works by inhibiting the 5α-reductase II enzyme, which is responsible to catalyze the conversion of testosterone to the much more active chemical 5 dihydrotestosterone. Finasteride is not FDA-approved for use in women and contraindicated in pregnant women and during lactation because of the risk of feminization of the male fetus. Large scale studies on its efficacy are currently limited. Price et al. (2000) proved the ineffectiveness of finasteride 1 mg/day taken for 12 months in postmenopausal women with AGA. However, case reports and series have demonstrated the efficacy of low-dosage finasteride in some cases of FPHL in both pre- and postmenopausal women. Shum et al. (2002) found that finasteride 1.25 mg/day improves pattern hair loss in women with hyperandrogenism but not in postmenopausal women with FPHL without hyperandrogenism. Thai and Sinclair (2002) reported on the improvement with finasteride 5 mg weekly in a woman who was intolerant to other antiandrogen therapies.
Higher doses (2.5-5 mg/day) appear to be necessary to treat FPHL effectively (Iorizzo et al., 2006). In addition, Finasteride 0.05% in a gel formulation has been used for the treatment of pattern hair loss and shown promising results (Hajheydari et al., 2009). Further randomized controlled trials with finasteride (different dosages) are needed to determine optimal dosing regimens and its efficacy in FPHL.
Dutasteride
Dutasteride is a 5α-reductase type I and II inhibitor that has not currently been approved in men and women for the treatment of hair loss. However, dutasteride has been approved for the treatment of benign prostatic hyperplasia at the dose of 0.5 mg daily. Dutasteride can reduce serum dihydrotestosterone levels by > 90% and has been used with success at a dose of 0.5 mg daily in male AGA (Jung et al., 2014). Dutasteride has been reported to treat FPHL successfully with no side effects at doses that range from 0.25 to 0.5 mg/day (Olsen et al., 2006).
Dutasteride should not be given to women of childbearing age unless they are using birth control measures because of the potential feminizing effects on the male fetus or to female patients who have test results that show impaired liver function. In another study, 25 postmenopausal women with female AGA of a male pattern were treated with dutasteride 0.25 mg/d. The results demonstrated a wide improvement that started in the frontotemporal region, followed by the vertex and frontal areas in 60% of cases at 1 year of treatment and in 80% of cases at 2 years (Camacho and Tosti, 2005).
Cyproterone acetate
Cyproterone acetate inhibits gonadotropin-releasing hormones and blocks androgen receptors but is not available in the United States. The best study to investigate cyproterone actetate was a 12-month randomized trial that compared the use of topical minoxidil 2% and cyproterone acetate in 66 women with FPHL. The authors found that minoxidil 2% was more effective in women without evidence of hyperandrogenism but cyproterone acetate was more effective in women with multiple symptoms (Vexiau et al., 2002).
In contrast, there is only one significant randomized study that has shown no benefit for cyproterone acetate (Carmina and Lobo, 2003). The treatment doses that were utilized vary but one of the most effective doses appears to be 100 mg/day on days 5 to 15 of the menstrual cycle and supplemented by 50 μg ethinyl estradiol on days 5 to 25 (Dawber et al., 1982). However, there is insufficient evidence to date that oral hormonal treatment prevents progression or improves AGA in female patients. Nevertheless, a subgroup analysis suggests that oral cyproterone acetate may improve AGA in female patients with hyperandrogenism (Blumeyer et al., 2011).
Spironolactone
Spironolactone is the most commonly used, off-label anti-androgen for the treatment of female AGA and hirsutism. Spironolactone is a potassium-sparing diuretic and structural antagonist of aldosterone (van Zuuren et al., 2012a) and acts as an androgen antagonist by competitively blocking androgen receptors as well as inhibiting ovarian androgen production. The usual daily dose is 100 to 200 mg. The side effects of spironolactone are due partly to its additional actions as it may act as an aldosterone antagonist and cause postural hypotension, electrolyte disturbances, menstrual irregularities, fatigue, urticaria, breast tenderness, and hematologic disturbances. Because of these known side effects, blood pressure and electrolyte balance, especially in patients with comorbid conditions or concurrent potentially interactive medications, should be checked during the first few months of treatment.
Published studies that support the efficacy of spironolactone are limited. An open intervention study concluded that spironolactone 200 mg/day was equally effective in either restoring hair growth or preventing further progression of hair loss compared with cyproterone acetate at a dose of either 50 mg/day or 100 mg/day for 10 days every menstrual cycle (Sinclair et al., 2005).
Oral minoxidil
Oral minoxidil is not often used in the treatment of AGA and FPHL, mainly because of the side-effect profile at standard doses. Off-label use of oral minoxidil is known to improve hair density in the treated patients but could be complicated by postural hypotension, fluid retention, and hypertrichosis. Fuid retention can often be managed by the addition of spironolactone but has the potential to increase postural hypotension. As minoxidil side effects are all dose-related, the dose could be 0.25 mg every day (Sinclair, 2018). Low-dose oral minoxidil is usually well tolerated in the majority of patients with FPHL and a reasonable alternative in women who are intolerant of or unwilling to use topical minoxidil (Sinclair, 2018).
Nutritional supplementation
The benefit of oral supplementation with amino acids, biotin, zinc, and other micronutrients in hair loss of any origin is controversial. For patients with TE, oral supplementation with L-cystine and B-complex vitamins showed a normalization of the anagen hair rate in a TrichoScan analysis but the effectiveness for FPHL is doubtful (Singal et al., 2013).
Rasheed et al. (2013) examined the relation between insufficiency/deficiency of serum ferritin and vitamin D and between TE or FPHL and demonstrated relation in both conditions. There are several mechanisms by which both iron and vitamin D have possible effects on hair growth. As the role of iron and ferritin levels increase in nondividing cells, rapidly proliferating cells such as hair follicle matrix cells have lower levels of ferritin and higher levels of free iron. This balance of ferritin and iron is partially controlled by the transcription factor c-Myc (Rasheed et al., 2013). Overexpression of c-Myc in the cutaneous epithelium results in a loss of follicular differentiation and decrease in stem cells but whether this phenotype is related to an abnormal iron metabolism remains to be determined.
Furthermore, iron acts as a metabolic cofactor for ribonucleotide reductase, which is the rate-limiting enzyme for DNA synthesis of hair growth stems. Therefore, the depletion of iron results in the inhibition of proliferation. Vitamin D has been suggested as an optimal concentration of this micronutrient that is necessary to delay aging phenomena including hair loss. Data from animal models show that vitamin D receptor activation plays an important role in anagen initiation and recent data suggested that vitamin D receptors regulate the expression of genes that are required for hair follicle cycling (Amor et al., 2010, Demay, 2012). Also, the definition of iron deficiency in hair loss remains an important question because some studies suggest that with a serum ferritin level of ≤ 30 μg/l and active hair loss, patients should be treated with iron therapy. However, other studies suggest higher cutoff limits such as 40 μg/l and 70 μg/l or lower cutoff limits such as 10 μg/l to 15 μg/l (Bregy and Trueb, 2008, Deloche et al., 2007, Rushton and Ramsay, 1992).
Other ingredients such as saw palmetto (Nutrafol) or marine protein complexes (Viviscal) may have anti-androgenic and anti-inflammatory properties but their efficacy alone as a monotherapy needs more investigation.
Hair transplantation
Hair transplantation is an important option for patients over 25 years of age with FPHL who do not have success with medical therapies when the hair loss has been stabilized (Atanaskova Mesinkovska and Bergfeld, 2013). Hair transplantation involves the relocation or transfer of hairs from the occipital to the bald area. Ideal surgical candidates for hair transplantation are women with high hair density in the donor site over the occipital scalp and extensive hair loss or thinning of the frontal scalp.
Follicular unit hair transplantation is a surgical treatment of baldness in which follicular units of hair (consisting of naturally occurring bundles of hairs) are dissected under a stereomicroscope and transplanted in the bald area to give a natural look. The procedure is performed under local anesthesia whereby one session involves the transplantation of 800 to 1200 grafts. Thanks to the use of follicular unit transplantation, the technique has become much less invasive and produces natural, undetectable, and reproducible results.
The most common problems that are encountered in hair transplantation in women are related to insufficient hair donor areas, the need for magnification to insert the grafts between the existing hair follicles in the recipient area, and temporary worsening of global aspect after the transplant. To achieve a good result, the correct selection of ideal candidates is important for this procedure. A new trend in hair transplantations is the adjuvant use of PRP. The growth factors and plasma components can be injected directly into the scalp before placement of the grafts or the hair grafts may be stored in PRP until placed on the scalp (Carter et al., 2011, Rose, 2011). Moreover, robotic systems can select and remove individual hair follicles from the donor area with great precision and without fatigue (Pereira et al., 2016).
Conclusions
Despite the high prevalence of FPHL, its management still imposes several difficulties to dermatologists' clinical practice. The investigation must be improved to identify the potential elements (other than genetic and hormonal) that are involved in the pathogenesis of FPHL. These findings are needed to develop new and more effective therapies to prevent and reverse the disease’s course. Since the response to treatment of FPHL is a challenge for dermatologists, new data with regard to the epidemiology, genetics, and pathophysiology of FPHL may help to improve the quality of life of patients affected by this disease.
Footnotes
Funding sources: None.
Conflicts of interest: The authors declare that they have no competing interests.
References
- Amor K.T., Rashid R.M., Mirmirani P. Does D matter? The role of vitamin D in hair disorders and hair follicle cycling. Dermatol Online J. 2010;16:3. [PubMed] [Google Scholar]
- Arck P.C., Slominski A., Theoharides T.C., Peters E.M.J., Paus R. Neuroimmunology of stress: Skin takes center stage. J Invest Dermatol. 2006;126:1697–1704. doi: 10.1038/sj.jid.5700104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias-Santiago S., Gutiérrez-Salmerón M.T., Castellote-Caballero L., Buendía-Eisman A., Naranjo-Sintes R. Androgenetic alopecia and cardiovascular risk factors in men and women: A comparative study. J Am Acad Dermatol. 2010;63:420–429. doi: 10.1016/j.jaad.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Asz-Sigall D., González-de-Cossio-Hernández A.C., Rodríguez-Lobato E., Ortega-Springall M.F., Vega-Memije M.E., GuzmánSkin R.A. Differential diagnosis of female-pattern hair loss. Skin Appendage Disord. 2016;2:18–21. doi: 10.1159/000445806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atanaskova Mesinkovska N., Bergfeld W.F. Hair: What is new in diagnosis and management? Female pattern hair loss update: diagnosis and treatment. Dermatol Clin. 2013;31:119–127. doi: 10.1016/j.det.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Banka N., Bunagan M.J., Shapiro J. Pattern hair loss in men: Diagnosis and medical treatment. Dermatol Clin. 2013;31:129–140. doi: 10.1016/j.det.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Birch M.P., Messenger J.F., Messenger A.G. Hair density, hair diameter and the prevalence of female pattern hair loss. Br J Dermatol. 2001;144:297–304. doi: 10.1046/j.1365-2133.2001.04018.x. [DOI] [PubMed] [Google Scholar]
- Birch M.P., Lalla S.C., Messenger A.G. Female pattern hair loss. Clin Exp Dermatol. 2002;27:383–388. doi: 10.1046/j.1365-2230.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- Blume-Peytavi U., Orfanos C.E. Microscopy of the hair – the trichogram. In: Serup J., Jemec G.B.E., Grove G.L., editors. Handbook of non-invasive methods and the skin. 2nd ed. CRC Press; Boca Raton, FL: 2006. [Google Scholar]
- Blume-Peytavi U., Hillmann K., Dietz E., Canfield D., Garcia Bartels N. A randomized, single-blind trial of 5% minoxidil foam once daily versus 2% minoxidil solution twice daily in the treatment of androgenetic alopecia in women. J Am Acad Dermatol. 2011;65:1126–1134. doi: 10.1016/j.jaad.2010.09.724. [DOI] [PubMed] [Google Scholar]
- Blume-Peytavi U., Lonnfors S., Hillmann K., Garcia Bartels N. A randomized double-blind placebo-controlled pilot study to assess the efficacy of a 24-week topical treatment by latanoprost 0.1% on hair growth and pigmentation in healthy volunteers with androgenetic alopecia. J Am Acad Dermatol. 2012;66:794–800. doi: 10.1016/j.jaad.2011.05.026. [DOI] [PubMed] [Google Scholar]
- Blumeyer A., Tosti A., Messenger A., Reygagne P., Del Marmol V., Spuls P.I. Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men. J Dtsch Dermatol Ges. 2011;(Suppl. 6):S1–57. doi: 10.1111/j.1610-0379.2011.07802.x. [DOI] [PubMed] [Google Scholar]
- Bregy A., Trueb R.M. No association between serum ferritin levels > 10 microg/l and hair loss activity in women. Dermatology. 2008;217:1–6. doi: 10.1159/000118505. [DOI] [PubMed] [Google Scholar]
- Camacho F., Tosti A. Tratamiento médico de las alopecias femeninas. Monogr Dermatol. 2005;18:92–117. [Google Scholar]
- Carey A.H., Chan K.L., Short F., White D., Williamson R., Franks S. Evidence for a single gene effect causing polycystic ovaries and male pattern baldness. Clin Endocrinol. 1993;38:653–658. doi: 10.1111/j.1365-2265.1993.tb02150.x. [DOI] [PubMed] [Google Scholar]
- Carmina E., Lobo R.A. Treatment of hyperandrogenic alopecia in women. Fertil Steril. 2003;79:91–95. doi: 10.1016/s0015-0282(02)04551-x. [DOI] [PubMed] [Google Scholar]
- Carter M.J., Fylling C.P., Parnell L.K. Use of platelet rich plasma gel on wound healing: A systematic review and meta-analysis. Eplasty. 2011;11:38. [PMC free article] [PubMed] [Google Scholar]
- Cervantes J., Perper M., Wong L.L., Eber A.E., Villasante Fricke A.C., Wikramanayake T.C. Effectiveness of platelet-rich plasma for androgenetic alopecia: A review of the literature. Skin Appendage Disord. 2018;4:1–11. doi: 10.1159/000477671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawber R.P., Sonnex T., Ralfs I. Oral antiandrogen treatment of common baldness in women. Br J Dermatol. 1982;107:20–21. [Google Scholar]
- Deloche C., Bastien P., Chadoutaud S., Galan P., Bertrais S., Hercberg S. Low iron stores: A risk factor for excessive hair loss in non menopausal women. Eur J Dermatol. 2007;17:507–512. doi: 10.1684/ejd.2007.0265. [DOI] [PubMed] [Google Scholar]
- Demay M.B. The hair cycle and vitamin D receptor. Arch Biochem Biophys. 2012;523:19–21. doi: 10.1016/j.abb.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Dhurat R., Sukesh M. Principles and methods of preparation of platelet-rich plasma: A review and author's perspective. J Cutan Aesthet Surg. 2014;7:189–197. doi: 10.4103/0974-2077.150734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Sayed M.H., Abdallah M.A., Aly D.G., Khater N.H. Association of metabolic syndrome with female pattern hair loss in women: A case-control study. Int J Dermatol. 2016;55:1131–1137. doi: 10.1111/ijd.13303. [DOI] [PubMed] [Google Scholar]
- Emer J.J., Stevenson M.L., Markowitz O. Novel treatment of female-pattern androgenetic alopecia with injected bimatoprost 0.03% solution. J Drugs Dermatol. 2011;10:795–798. [PubMed] [Google Scholar]
- Fabi S., Sundaram H. The potential of topical and injectable growth factors and cytokines for skin rejuvenation. Facial Plast Surg. 2014;30:157–171. doi: 10.1055/s-0034-1372423. [DOI] [PubMed] [Google Scholar]
- Fertig R.M., Gamret A.C., Cervantes J., Tosti A. Microneedling for the treatment of hair loss? J Eur Acad Dermatol Venereol. 2018;32:564–569. doi: 10.1111/jdv.14722. [DOI] [PubMed] [Google Scholar]
- Fischer T.W., Scholz G., Knoll B. Melatonin reduces UV-induced reactive oxygen species in a dose-dependent manner in IL-3-stimulated leukocytes. J Pineal Res. 2001;31:39–45. doi: 10.1034/j.1600-079x.2001.310106.x. [DOI] [PubMed] [Google Scholar]
- Fischer T.W., Slominski A., Tobin D.J., Paus R. Melatonin and the hair follicle. J Pineal Res. 2008;44:1–15. doi: 10.1111/j.1600-079X.2007.00512.x. [DOI] [PubMed] [Google Scholar]
- Fischer T.W., Trueb R.M., Hanggi G., Innocenti M., Elsner P. Topical melatonin for treatment of androgenetic alopecia. Int J Trichology. 2012;4:236–245. doi: 10.4103/0974-7753.111199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza L.A., Liu Y., Yang Z., Alagesan B., Lawson J.A., Norberg S.M. Prostaglandin D2 inhibits hair growth and is elevated in bald scalp of men with androgenetic alopecia. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3003122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goren A., Shapiro J., Roberts J., McCoy J., Desai N., Zarrab Z. Clinical utility and validity of minoxidil response testing in androgenetic alopecia. Dermatol Ther. 2015;28:13–16. doi: 10.1111/dth.12164. [DOI] [PubMed] [Google Scholar]
- Gupta A.K., Foley K.A. 5% minoxidil: Treatment for female pattern hair loss. Skin Therapy Lett. 2014;19:5–7. [PubMed] [Google Scholar]
- Hajheydari Z., Akbari J., Saeedi M., Shokoohi L. Comparing the therapeutic effects of finasteride gel and tablet in treatment of the androgenetic alopecia. Indian J Dermatol Venereol Leprol. 2009;75:47–51. doi: 10.4103/0378-6323.45220. [DOI] [PubMed] [Google Scholar]
- Heilmann-Heimbach S., Herold C., Hochfeld L.M., Hillmer A.M., Nyholt D.R., Hecker J. Meta-analysis identifies novel risk loci and yields systematic insights into the biology of male-pattern baldness. Nat Commun. 2017;8 doi: 10.1038/ncomms14694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskovitz I., Tosti A. Female pattern hair loss. Int J Endocrinol Metab. 2013;11(4) doi: 10.5812/ijem.9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillmer A.M., Flaquer A., Hanneken S., Eigelshoven S., Kortüm A.K., Brockschmidt F.F. Genome-wide scan and fine-mapping linkage study of androgenetic alopecia reveals a locus on chromosome 3q26. Am J Hum Genet. 2008;82:737–743. doi: 10.1016/j.ajhg.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iorizzo M., Vincenzi C., Voudouris S., Piraccini B.M., Tosti A. Finasteride treatment of female pattern hair loss. Arch Dermatol. 2006;142:298–302. doi: 10.1001/archderm.142.3.298. [DOI] [PubMed] [Google Scholar]
- Jimenez J.J., Wikramanayake T.C., Bergfeld W., Hordinsky M., Hickman J.G., Hamblin M.R. Efficacy and safety of a low-level laser device in the treatment of male and female pattern hair loss: A multicenter, randomized, sham device-controlled, double-blind Study. Am J Clin Dermatol. 2014;15:115–127. doi: 10.1007/s40257-013-0060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J.Y., Yeon J.H., Choi J.W., Kwon S.H., Kim B.J., Youn S.W. Effect of dutasteride 0.5 mg/d in men with androgenetic alopecia recalcitrant to finasteride. Int J Dermatol. 2014;53:1351–1357. doi: 10.1111/ijd.12060. [DOI] [PubMed] [Google Scholar]
- Keaney T. Emerging therapies for androgenetic alopecia. J Drugs Dermatol. 2015;14:1036–1040. [PubMed] [Google Scholar]
- Lacarrubba F., Micali G., Tosti A. Scalp dermoscopy or trichoscopy. Curr Probl Dermatol. 2015;47:21–32. doi: 10.1159/000369402. [DOI] [PubMed] [Google Scholar]
- Leavitt M., Charles G., Heyman E., Michaels D. HairMax LaserComb laser phototherapy device in the treatment of male androgenetic alopecia: A randomized, double-blind, sham device-controlled, multicentre trial. Clin Drug Investig. 2009;29:283–292. doi: 10.2165/00044011-200929050-00001. [DOI] [PubMed] [Google Scholar]
- Lee S.H., Zheng Z., Kang J.S., Kim D.Y., Oh S.H., Cho S.B. Therapeutic efficacy of autologous platelet-rich plasma and polydeoxyribonucleotide on female pattern hair loss. Wound Repair Regen. 2015;23:30–36. doi: 10.1111/wrr.12250. [DOI] [PubMed] [Google Scholar]
- Leo M.S., Kumar A.S., Kirit R., Konathan R., Sivamani R.K. Systematic review of the use of platelet-rich plasma in aesthetic dermatology. J Cosmet Dermatol. 2015;14(4):315–323. doi: 10.1111/jocd.12167. [DOI] [PubMed] [Google Scholar]
- Ludwig E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br J Dermatol. 1977;97:247–254. doi: 10.1111/j.1365-2133.1977.tb15179.x. [DOI] [PubMed] [Google Scholar]
- McCoy J., Kovacevic M., Situm M., Stanimirovic A., Bolanca Z., Goren A. Doppler laser imaging predicts response to topical minoxidil in the treatment of female pattern hair loss. J Biol Regul Homeost Agents. 2016;30:131–134. [PubMed] [Google Scholar]
- McDonald K.A., Shelley A.J., Colantonio S., Beecker J. Hair pull test: Evidence-based update and revision of guidelines. J Am Acad Dermatol. 2017;76:472–477. doi: 10.1016/j.jaad.2016.10.002. [DOI] [PubMed] [Google Scholar]
- Miao Y., Sun Y.B., Sun X.J., Du B.J., Jiang J.D., Hu Z.Q. Promotional effect of platelet-rich plasma on hair follicle reconstitution in vivo. Dermatol Surg. 2013;39:1868–1876. doi: 10.1111/dsu.12292. [DOI] [PubMed] [Google Scholar]
- Nieves A., Garza L.A. Does prostaglandin D2 hold the cure to male pattern baldness? Exp Dermatol. 2014;23:224–227. doi: 10.1111/exd.12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen E.A. The midline part: An important physical clue to the clinical diagnosis of androgenetic alopecia in women. J Am Acad Dermatol. 1999;40:106–109. doi: 10.1016/s0190-9622(99)70539-6. [DOI] [PubMed] [Google Scholar]
- Olsen E.A. Female pattern hair loss. J Am Acad Dermatol. 2001;45:S70–S80. doi: 10.1067/mjd.2001.117426. [DOI] [PubMed] [Google Scholar]
- Olsen E.A. Female pattern hair loss. J Am Acad Dermatol. 2002;45:S70–S80. doi: 10.1067/mjd.2001.117426. [DOI] [PubMed] [Google Scholar]
- Olsen E.A., Hordinsky M., Whiting D., Stough D., Hobbs S., Ellis M.L., Wilson T., Rittmaster R.S., Dutasteride Alopecia Research Team The importance of dual 5alpha-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finsteride. J Am Acad Dermatol. 2006;55:1014–1023. doi: 10.1016/j.jaad.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Orme S., Cullen D.R., Messenger A.G. Diffuse female hair loss: Are androgens necessary? Br J Dermatol. 1999;141:521–523. doi: 10.1046/j.1365-2133.1999.03049.x. [DOI] [PubMed] [Google Scholar]
- Pereira J.O., Pereira Filho J.O., Cabrera Pereira J.O. Mega-sessions for robotic hair restoration. J Drugs Dermatol. 2016;15:1407–1412. [PubMed] [Google Scholar]
- Pickrell J.K., Berisa T., Liu J.Z., Segureal L., Tung J.Y., Hinds D.A. Detection and interpretation of shared genetic influences on 42 human traits. Nat Genet. 2016;48:709–717. doi: 10.1038/ng.3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piérard G.E., Piérard-Franchimont C., NikkelsTassoudji N., Nikkels A.F., Saint Leger D. Improvement in the inflammatory aspect of androgenetic alopecia: A pilot study with an antimicrobial lotion. J Dermatol Treat. 1996;7:153–157. [Google Scholar]
- Piérard-Franchimont C., De Doncker P., Cauwenbergh G., Piérard G.E. Ketoconazole shampoo: Effect of long-term use in androgenic alopecia. Dermatology. 1998;196:474–477. doi: 10.1159/000017954. [DOI] [PubMed] [Google Scholar]
- Price V.H., Roberts J.L., Hordinsky M., Olsen E.A., Savin R., Bergfeld W. Lack of efficacy of finasteride in post-menopausal women with androgenetic alopecia. J Am Acad Dermatol. 2000;43:768–776. doi: 10.1067/mjd.2000.107953. [DOI] [PubMed] [Google Scholar]
- Ramos P.M., Miot H.A. Female pattern hair loss: A clinical and pathophysiological review. An Bras Dermatol. 2015;90(4):529–543. doi: 10.1590/abd1806-4841.20153370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall V.A. Androgens and hair growth. Dermatol Ther. 2008;21:314–328. doi: 10.1111/j.1529-8019.2008.00214.x. [DOI] [PubMed] [Google Scholar]
- Rangwala S., Rashid R.M. Alopecia: A review of laser and light therapies. Dermatol Online J. 2012;18:3. [PubMed] [Google Scholar]
- Rasheed H., Mahgoub D., Hegazy R., El-Komy M., Abdel Hay R., Hamid M.A. Serum ferritin and vitamin d in female hair loss: Do they play a role? Skin Pharmacol Physiol. 2013;26:101–107. doi: 10.1159/000346698. [DOI] [PubMed] [Google Scholar]
- Rebora A., Guarrera M., Baldari M., Vecchio F. Distinguishing androgenetic alopecia from chronic telone effluvium when associated in the same patient. Arch Dermatol. 2005;143:1243–1245. doi: 10.1001/archderm.141.10.1243. [DOI] [PubMed] [Google Scholar]
- Redler S., Messenger A.G., Betz R.C. Genetics and other factors in the aetiology of female pattern hair loss. Exp Dermatol. 2017;26:510–517. doi: 10.1111/exd.13373. [DOI] [PubMed] [Google Scholar]
- Rose P.T. The latest innovations in hair transplantation. Facial Plast Surg. 2011;27:366–377. doi: 10.1055/s-0031-1283055. [DOI] [PubMed] [Google Scholar]
- Rushton D.H., Ramsay I.D. The importance of adequate serum ferritin levels during oral cyproterone acetate and ethinyl oestradiol treatment of diffuse androgen-dependent alopecia in women. Clin Endocrinol. 1992;36:421–427. doi: 10.1111/j.1365-2265.1992.tb01470.x. [DOI] [PubMed] [Google Scholar]
- Savin R.C. The Upjohn Company; Kalamazoo, MI: 1994. Evaluating androgenetic alopecia in male and female patients. [Google Scholar]
- Shah K.B., Shah A.N., Solanki R.B., Raval R.C. A comparative study of microneedling with platelet-rich plasma plus topical minoxidil (5%) and topical minoxidil (5%) alone in androgenetic alopecia. Int J Trichology. 2017;9:14–18. doi: 10.4103/ijt.ijt_75_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum K.W., Cullen D.R., Messenger A.G. Hair loss in women with hyperandrogenism: Four cases responding to finasteride. J Am Acad Dermatol. 2002;47:733–739. doi: 10.1067/mjd.2002.124608. [DOI] [PubMed] [Google Scholar]
- Sinclair R.D., Dawber R.P. Androgenetic alopecia in men and women. Clin Dermatol. 2001;19:167–178. doi: 10.1016/s0738-081x(00)00128-0. [DOI] [PubMed] [Google Scholar]
- Sinclair R., Wewerinke M., Jolley D. Treatment of female pattern hair loss with oral antiandrogens. Br J Dermatol. 2005;152:466–473. doi: 10.1111/j.1365-2133.2005.06218.x. [DOI] [PubMed] [Google Scholar]
- Sinclair R., Jolley D., Mallari R., Magee J. The reliability of horizontally sectioned scalp biopsies in the diagnosis of chronic diffuse telogen hair loss in women. J Am Acad Dermatol. 2004;51:189–199. doi: 10.1016/s0190-9622(03)00045-8. [DOI] [PubMed] [Google Scholar]
- Sinclair R.D. Female pattern hair loss: A pilot study investigating combination therapy with low-dose oral minoxidil and spironolactone. Int J Dermatol. 2018;57:104–109. doi: 10.1111/ijd.13838. [DOI] [PubMed] [Google Scholar]
- Singal A., Sonthalia S., Verma P. Female pattern hair loss. Indian J Dermatol Venereol Leprol. 2013;79:626–640. doi: 10.4103/0378-6323.116732. [DOI] [PubMed] [Google Scholar]
- Singh M., Jadhav H.R. Melatonin: Functions and ligands. Drug Discov Today. 2014;19:1410–1418. doi: 10.1016/j.drudis.2014.04.014. [DOI] [PubMed] [Google Scholar]
- Sonino N., Scaroni C., Biason A., Boscaro M., Mantero F. Low-dose ketoconazole treatment in hirsute women. J Endocrinol Investig. 1990;13:35–40. doi: 10.1007/BF03348578. [DOI] [PubMed] [Google Scholar]
- Stefanato C.M. Histopathology of alopecia: A clinicopathological approach to diagnosis. Histopathology. 2010;56:24–38. doi: 10.1111/j.1365-2559.2009.03439.x. [DOI] [PubMed] [Google Scholar]
- Takikawa M., Nakamura S., Nakamura S., Ishirara M., Kishimoto S., Sasaki K. Enhanced effect of platelet-rich plasma containing a new carrier on hair growth. Dermatol Surg. 2011;37:1721–1729. doi: 10.1111/j.1524-4725.2011.02123.x. [DOI] [PubMed] [Google Scholar]
- Thai K.E., Sinclair R.D. Finasteride for female androgenetic alopecia. Br J Dermatol. 2002;147:812–813. doi: 10.1046/j.1365-2133.2002.49084.x. [DOI] [PubMed] [Google Scholar]
- Tosti A., Piraccini B.M. Androgenetic alopecia. In: Tosti A., Piraccini B.M., editors. Diagnosis and treatment of hair disorders: An evidence based atlas. Taylor and Francis; London, UK: 2006. [Google Scholar]
- Tsuboi R., Arano O., Nishikawa T., Yamada H., Katsuoka K. Randomized clinical trial comparing 5% and 1% topical minoxidil for the treatment of androgenetic alopecia in Japanese men. J Dermatol. 2009;36:437–446. doi: 10.1111/j.1346-8138.2009.00673.x. [DOI] [PubMed] [Google Scholar]
- Valente Duarte de Sousa I.C., Tosti A. New investigational drugs for androgenetic alopecia. Expert Opin Investig Drugs. 2013;22:573–589. doi: 10.1517/13543784.2013.784743. [DOI] [PubMed] [Google Scholar]
- Varothai S., Bergfeld W.F. Androgenetic alopecia: An evidence-based treatment update. Am J Clin Dermatol. 2014;15:217–230. doi: 10.1007/s40257-014-0077-5. [DOI] [PubMed] [Google Scholar]
- Vexiau P., Chaspoux C., Boudou P., Fiet J., Jouanique C., Hardy N. Effects of minoxidil 2% vs. cyproterone acetate treatment on female androgenetic alopecia: A controlled, 12-month randomized trial. Br J Dermatol. 2002;146:992–999. doi: 10.1046/j.1365-2133.2002.04798.x. [DOI] [PubMed] [Google Scholar]
- Vujovic A., Del Marmol V. The female pattern hair loss: Review of etiopathogenesis and diagnosis. Biomed Res Int. 2014;2014 doi: 10.1155/2014/767628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip Y., Sinclair R.D. Antiandrogen therapy for androgenetic alopecia. Expert Rev Dermatol. 2006;1:261–269. [Google Scholar]
- van Zuuren E.J., Fedorowicz Z., Carter B. Evidence-based treatments for female pattern hair loss: A summary of a Cochrane systematic review. Br J Dermatol. 2012;167:995–1010. doi: 10.1111/j.1365-2133.2012.11166.x. [DOI] [PubMed] [Google Scholar]
- van Zuuren E.J., Fedorowicz Z., Carter B., Andriolo R.B., Schoones J. Interventions for female pattern hair loss. Cochrane Database Syst Rev. 2012;5 doi: 10.1002/14651858.CD007628.pub3. [DOI] [PubMed] [Google Scholar]