Abstract
Chronic anogenital pruritus can significantly impair affected patients’ quality of life by disrupting their sleep, mood, sexual function, and personal relationships. Although a significant portion of these patients can be managed with hygiene measures, topical therapy, oral anti-pruritics, and allergen avoidance after patch testing, guidelines to treat patients who do not respond to standard therapy have yet to be established. We describe the therapeutic response of a case of anogenital pruritus recalcitrant to multiple topical and systemic therapies. Treatment of this patient with dupilumab, an interleukin-4 receptor alpha blocker, resulted in clinical remission at 1 year from the initiation of the therapy, without significant adverse effects.
Keywords: Atopic dermatitis, allergic contact dermatitis, dupilumab, pruritus ani, itch, genital pruritus
Introduction
Anogenital dermatoses can cause significant itching, burning, and stinging that can greatly impair patients’ quality of life (Cather et al., 2017). Patients who are affected by anogenital skin disease experience even greater disruptions in sleep, mood, sexual function, and personal relationships than patients with dermatologic conditions elsewhere on their body (Cather et al., 2017, Malakouti et al., 2017, Ryan et al., 2015). Despite this, a large portion of these patients remain undertreated (Meeuwis et al., 2012). Many patients with anogenital symptoms respond to management with improved hygiene and topical treatments (Guerrero and Venkatesan, 2015), but guidelines for systemic treatment of patients who are resistant to these first-line measures have yet to be established.
We describe the therapeutic response of a case of anogenital itch recalcitrant to multiple topical and systemic therapies. The patient was eventually treated with dupilumab, a biologic agent that binds to the alpha subunit of the interleukin-4 (IL-4) receptor to modulate the signal of IL-4 and IL-13 (primary interleukins involved in type 2 helper T-cell response), which resulted in the clinical remission of the disease. The patient has maintained adequate clinical response at 1 year after the initiation of dupilumab without significant adverse effects.
Case
A 62-year-old Caucasian man with a history of asthma and allergic rhinitis presented with a 2-month history of itching in the anal area. The patient described constant itching throughout the day that would also wake him from sleep. The patient had previously applied topical hydrocortisone 2.5% cream and over-the-counter antifungal cream to the affected area, which slightly reduced the itch but did not prevent recurrence or resolve the itch. During a physical examination, pink erythema of the perianal skin was observed, but no scale or papules were noted.
The patient’s medical history was also significant for attention deficit hyperactivity disorder and depression, which were well controlled on stable doses of bupropion and methylphenidate. His asthma was also well controlled on a stable regimen of albuterol, budesonide/formoterol, and montelukast. The patient also showed mild grade 1 anterolisthesis of L5 on S1 on pelvic magnetic resonance imaging that was related to bilateral L5 spondylolyses.
Over the next 2 years, the patient was treated with various topical and systemic therapies in combination, without significant resolution of his symptoms. Topical therapies applied included corticosteroid treatments (triamcinolone 1% cream, desonide 0.1% cream, fluocinonide 0.05% ointment, and hydrocortisone 2.5% cream), tacrolimus ointment, propylene-glycol free lidocaine 5% ointment, doxepin 5% cream, capsaicin 0.006% cream, and antifungal treatments (clotrimazole cream, miconazole, nystatin powder, and econazole cream). Systemic therapies used by the patient included anti-histamine (cetirizine, hydroxyzine, and doxepin), antibiotic (cephalexin, fluconazole, and rifampin), intramuscular triamcinolone, budesonide, and gabapentin treatments. Although many of these therapies provided transient relief of the patient’s symptoms, he experienced breakthrough itching despite strict adherence to the treatment regimens.
During this time, the patient’s itch worsened and spread to the genital area. Repeat physical examinations revealed pink papules on the base of the patient’s penis, shaft, glans, and scrotum and demonstrated intense perianal erythema with overlying edematous papules (Fig. 1A-C). The patient underwent thorough diagnostic testing to identify the etiology of the itch. Biopsy tests of the perianal area and head of the penis both revealed subacute spongiotic dermatitis, which ruled out lichen planus.
Fig. 1.
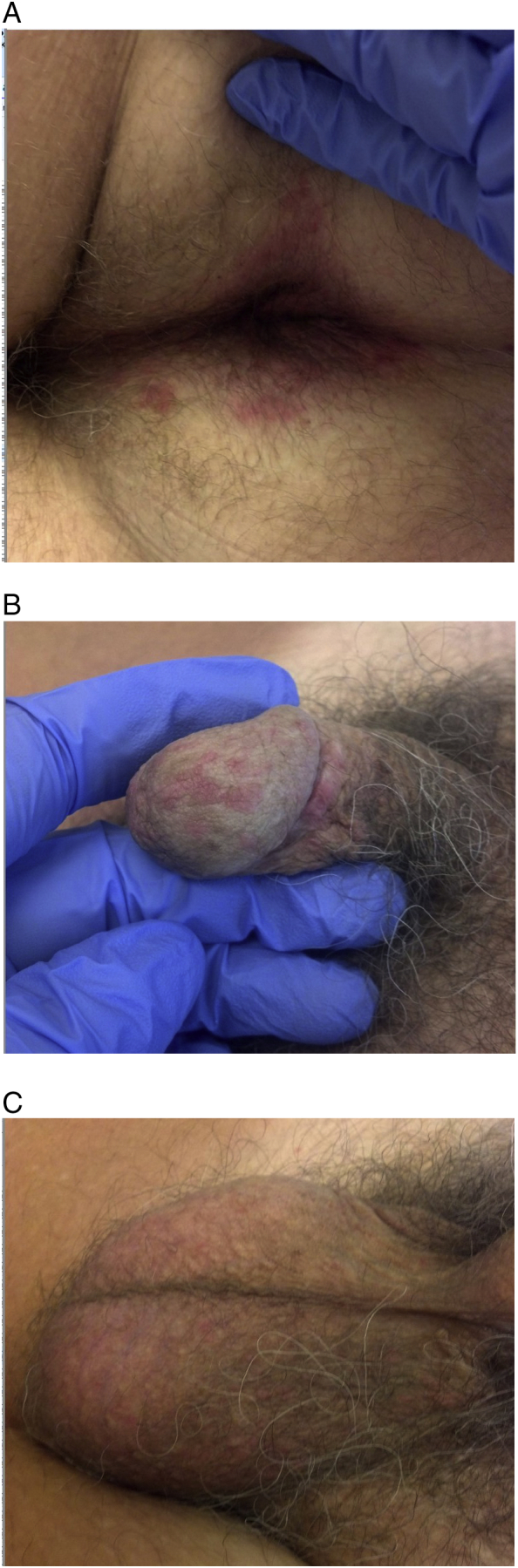
(A) Perianal, (B) penile, and (C) scrotal inflammatory papules prior to patch testing and initiation of dupilumab.
Patch testing using the North American Contact Dermatitis Group tray (75 allergens, SmartPractice, Calgary, Alberta, Canada) and the patient’s personal products (20 allergens) was positive for propylene glycol, neomycin, desoximetasone, fragrance mix 1, methyldibromo glutaronitrile/phenoxyethanol, and glutaraldehyde, as well as the patient’s toothpaste, deodorant, and shaving cream. Repeat patch testing 1 year later using trays for metals (47 allergens, Metals Series, Dormer Laboratories, Rexdate, Ontario, Candida), emulsifiers (35 allergens, External Agents/Emulsifier Series, SmartPractice), fragrances (45 allergens, F-1000 Fragrance Series, Dormer Laboratories, Rexdate), anesthetic products (13 allergens, Local Anesthetics, SmartPractice), and corticosteroid treatments (9 allergens, Corticosteroid Series, SmartPractice) was positive for propylene glycol, various metals (iridium chloride trihydrate, zinc chloride, titanium oxalate decahydrate, vanadium chloride, zirconium chloride, manganese chloride, and potassium dichromate), coumarin, anise alcohol, linalool, and limonene.
Food allergy testing revealed allergies to legumes, soy, peas, lentils, chickpeas, cruciferous vegetables, lettuce, avocado, onion, egg, and aged foods. The patient also followed a fragrance- and propylene glycol-free diet and used products only on the Contact Allergen Management Program list with strict avoidance of known allergens for several months, but with minimal improvement in his symptoms.
After 2 years of persistent itching in the anogenital area, the patient initiated mycophenolate mofetil 2 g daily for suspected atopic dermatitis around the anal area, given his history of mild atopy. After 4 months, the dose was increased to 3 g daily, and the patient reported some improvement in itching with mild breakthrough pruritus. However, the patient experienced an increased number of upper respiratory infections while on the medication and was concerned about its immunosuppressive effects.
The patient was switched to dupilumab with a 600-mg loading dose and 300 mg every 2 weeks for his symptoms. During reevaluation 1 month later, the patient reported a 95% resolution of the itching, and a physical examination revealed a complete resolution of his perianal dermatitis (Fig. 2). At the time of follow-up 12 months after initiating dupilumab therapy, the patient continued to report that the itching was well controlled, with occasional breakthrough itching managed by propylene glycol–free topical lidocaine cream up to two times per month. The patient denied experiencing any significant adverse effects since initiating dupilumab treatment.
Fig. 2.
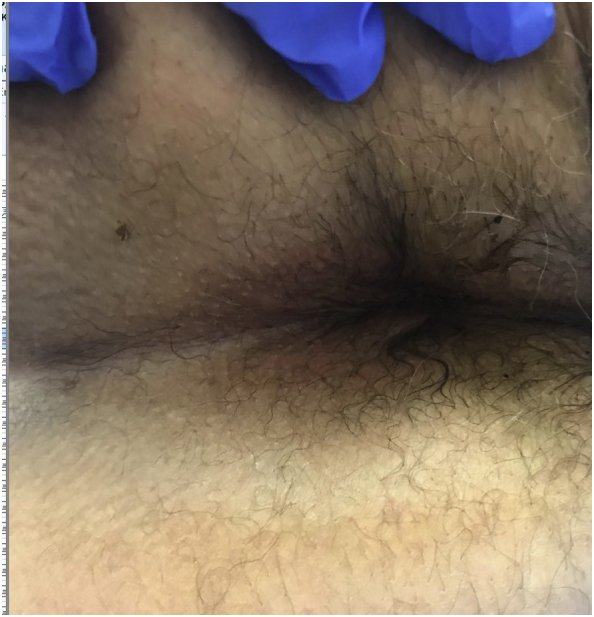
Resolution of perianal dermatitis after initiation of perianal dermatitis (week 4 from initiation of therapy).
Discussion
A wide range of conditions affect the genital skin, including lichen sclerosus, lichen simplex chronicus, atopic dermatitis, psoriasis, allergic contact dermatitis, and irritant contact dermatitis, but diagnosis can be difficult (Chan and Zimarowski, 2015). These common skin conditions often have a different morphology in the genital area compared with other areas of the body due to friction, heat, and occlusion in the genital area (Drummond, 2011). Thus, dermatologic conditions of the genitalia may present similarly both clinically and histologically and often require extensive testing and empiric treatment (Chan and Zimarowski, 2015).
Most cases of anogenital pruritus arise from fecal contamination of the perianal area and are exacerbated by trauma such as repeated scratching (Markell and Billingham, 2010, Siddiqi et al., 2008). However, a close physical examination may reveal secondary causes of anogenital pruritus, such as hemorrhoids and fissures, inflammatory skin disease, or infection.
Patients who are suspected to have allergic triggers for anogenital pruritus or itch that is recalcitrant to topical therapies should undergo patch testing to evaluate allergic contact dermatitis. Approximately half of patients with anogenital pruritus have at least one positive reaction with patch testing, with 20% of these clinical reactions clinically relevant for their itch (Bauer et al., 2000, Warshaw et al., 2008). Patients with allergic contact dermatitis of the anogenital area can often be managed with a withdrawal from allergic triggers and symptomatic treatment (Siddiqi et al., 2008). However, these patients often have other concomitant anogenital diseases that may require further intervention (Trivedi et al., 2018).
One often overlooked cause of idiopathic anogenital pruritus, particularly in older patients, is lumbosacral radiculopathy (Berger et al., 2013, Cohen et al., 2005). Spinal trauma or degenerative disc disease can cause severe itching in the genital area in the absence of a primary rash. The papular rash that was observed in the genital area of our patient suggested that his anogenital itch was not solely due to a neuropathic cause but more likely was caused by an inflammatory disease process.
Genital dermatoses are often difficult to treat due to the unique nature of this environment. Genital skin is thin, sensitive, and often occluded and may have increased absorption of topical treatments (Farage and Maibach, 2004). Additionally, genital skin demonstrates an exaggerated response to irritants compared with other areas of the body (Britz and Maibach, 1979). Localized atopic dermatitis is commonly managed with topical corticosteroid treatments, but application of higher-potency steroid ointments to the genital area may result in treatment-related adverse effects, such as skin atrophy and increased risk of a secondary infection (Johnson et al., 2012).
Systemic therapy should be considered for atopic dermatitis recalcitrant to topical therapy or with significant quality of life impairment (Simpson et al., 2017). Systemic immunosuppressant treatments, such as azathioprine, cyclosporine, methotrexate, and mycophenolate mofetil, have traditionally been used off-label to treat these patients, but they often require frequent laboratory monitoring for potentially serious side effects. Due to the immunosuppressive nature of these systemic medications, patients such as the man described in this case are at an increased risk of infection.
Dupilumab is a human monoclonal antibody that targets IL-4 receptor and is approved for the treatment of moderate-to-severe atopic dermatitis in adults. To our knowledge, dupilumab has not previously been described in the medical literature as an effective treatment for anogenital pruritus. This biologic treatment inhibits IL-4– and IL-13–mediated inflammatory responses and has been shown in phase 3 trials to significantly decrease disease severity and risk of skin infections in patients affected with atopic dermatitis when given subcutaneously 300 mg every 2 weeks (Fleming and Drucker, 2018, Simpson et al., 2016). No laboratory monitoring is required for this medication.
Side effects reported with dupilumab to date are minor, including slightly elevated rates of conjunctivitis (4%-5%) and injection-site reactions (8%-14%) over placebo (Simpson et al., 2016). Dupilumab is a relatively safe alternative for patients who experience significant pruritus that is thought to be inflammatory in origin. Because of our patient’s history of atopic disease and visible skin inflammation despite allergen avoidance, his anogenital pruritus likely had a component of underlying atopic dermatitis, which was treated with dupilumab.
Conclusions
Anogenital itching is a burdensome condition that significantly impairs affected patients' sleep, sexual function, personal relationships, and overall quality of life. However, guidelines for the treatment of these conditions are lacking. Pruritus in the anogenital area requires testing for systemic, gynecologic, neurologic, and dermatologic causes and can often be difficult to treat. Patients with recalcitrant anogenital itching or significant quality of life impairment should be considered for systemic therapy. This case demonstrates the efficacy of dupilumab in a patient with recalcitrant anogenital pruritus.
Footnotes
Meeting information: There have been no prior presentations of this material.
Funding sources: None.
Institutional review board approval status: Not required.
Conflicts of interest: Dr. J. E. Murase has participated in advisory boards for Genzyme/Sanofi, Dermira, and UCB.
References
- Bauer A., Geier J., Elsner P. Allergic contact dermatitis in patients with anogenital complaints. J Reprod Med. 2000;45:649–654. [PubMed] [Google Scholar]
- Berger T.G., Shive M., Harper G.M. Pruritus in the older patient: A clinical review. JAMA. 2013;310:2443–2450. doi: 10.1001/jama.2013.282023. [DOI] [PubMed] [Google Scholar]
- Britz M.B., Maibach H.I. Human cutaneous vulvar reactivity to irritants. Contact Dermatitis. 1979;5:375–377. doi: 10.1111/j.1600-0536.1979.tb04908.x. [DOI] [PubMed] [Google Scholar]
- Cather J.C., Ryan C., Meeuwis K., Potts Bleakman A.J., Naegeli A.N., Edson-Heredia E. Patients' perspectives on the impact of genital psoriasis: A qualitative study. Dermatol Ther (Heidelb) 2017;7:447–461. doi: 10.1007/s13555-017-0204-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan M.P., Zimarowski M.J. Vulvar dermatoses: A histopathologic review and classification of 183 cases. J Cutan Pathol. 2015;42:510–518. doi: 10.1111/cup.12541. [DOI] [PubMed] [Google Scholar]
- Cohen A.D., Vander T., Medvendovsky E., Biton A., Naimer S., Shalev R. Neuropathic scrotal pruritus: Anogenital pruritus is a symptom of lumbosacral radiculopathy. J Am Acad Dermatol. 2005;52:61–66. doi: 10.1016/j.jaad.2004.04.039. [DOI] [PubMed] [Google Scholar]
- Drummond C. Common vulval dermatoses. Aust Fam Physician. 2011;40:490–496. [PubMed] [Google Scholar]
- Farage M., Maibach H.I. The vulvar epithelium differs from the skin: Implications for cutaneous testing to address topical vulvar exposures. Contact Dermatitis. 2004;51:201–209. doi: 10.1111/j.0105-1873.2004.00444.x. [DOI] [PubMed] [Google Scholar]
- Fleming P., Drucker A.M. Risk of infection in patients with atopic dermatitis treated with dupilumab: A meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2018;78:62–69.e1. doi: 10.1016/j.jaad.2017.09.052. [DOI] [PubMed] [Google Scholar]
- Guerrero A., Venkatesan A. Inflammatory vulvar dermatoses. Clin Obstet Gynecol. 2015;58:464–475. doi: 10.1097/GRF.0000000000000125. [DOI] [PubMed] [Google Scholar]
- Johnson E., Groben P., Eanes A., Iyer P., Ugoeke J., Zolnoun D. Vulvar skin atrophy induced by topical glucocorticoids. J Midwifery Womens Health. 2012;57:296–299. doi: 10.1111/j.1542-2011.2012.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malakouti M., Brown G.E., Leon A., Wang E., Naegeli A.N., Edson-Heredia E. The dermatologic intimacy scale: Quantitatively measuring the impact of skin disease on intimacy. J Dermatolog Treat. 2017;28:347–352. doi: 10.1080/09546634.2016.1252032. [DOI] [PubMed] [Google Scholar]
- Markell K.W., Billingham R.P. Pruritus ani: Etiology and management. Surg Clin North Am. 2010;90:125–135. doi: 10.1016/j.suc.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Meeuwis K.A., van de Kerkhof P.C., Massuger L.F., de Hullu J.A., van Rossum M.M. Patients' experience of psoriasis in the genital area. Dermatology. 2012;224:271–276. doi: 10.1159/000338858. [DOI] [PubMed] [Google Scholar]
- Ryan C., Sadlier M., De Vol E., Patel M., Lloyd A.A., Day A. Genital psoriasis is associated with significant impairment in quality of life and sexual functioning. J Am Acad Dermatol. 2015;72:978–983. doi: 10.1016/j.jaad.2015.02.1127. [DOI] [PubMed] [Google Scholar]
- Siddiqi S., Vijay V., Ward M., Mahendran R., Warren S. Pruritus ani. Ann R Coll Surg Engl. 2008;90:457–463. doi: 10.1308/003588408X317940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson E.L., Bieber T., Guttman-Yassky E., Beck L.A., Blauvelt A., Cork M.J. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335–2348. doi: 10.1056/NEJMoa1610020. [DOI] [PubMed] [Google Scholar]
- Simpson E.L., Bruin-Weller M., Flohr C., Ardern-Jones M.R., Barbarot S., Deleuran M. When does atopic dermatitis warrant systemic therapy? Recommendations from an expert panel of the International Eczema Council. J Am Acad Dermatol. 2017;77:623–633. doi: 10.1016/j.jaad.2017.06.042. [DOI] [PubMed] [Google Scholar]
- Trivedi M.K., Woodruff C.M., Kornik R., Botto N. Patch testing in vulvar allergic contact dermatitis. Dermatitis. 2018;29:95–96. doi: 10.1097/DER.0000000000000345. [DOI] [PubMed] [Google Scholar]
- Warshaw E.M., Furda L.M., Maibach H.I., Rietschel R.L., Fowler J.F., Jr., Belsito D.V. Anogenital dermatitis in patients referred for patch testing: retrospective analysis of cross-sectional data from the North American Contact Dermatitis Group, 1994-2004. Arch Dermatol. 2008;144:749–755. doi: 10.1001/archderm.144.6.749. [DOI] [PubMed] [Google Scholar]


