Abstract
Myelocytomatosis viral oncogene homolog (MYC) plays an important role in the regulation of many cellular processes, and its expression is tightly regulated at the level of transcription, translation, protein stability, and activity. Despite this tight regulation, MYC is overexpressed in many cancers and contributes to multiple hallmarks of cancer. In recent years, it has become clear that noncoding RNAs add a crucial additional layer to the regulation of MYC and its downstream effects. So far, twenty‐five microRNAs and eighteen long noncoding RNAs that regulate MYC have been identified. Thirty‐three miRNAs and nineteen lncRNAs are downstream effectors of MYC that contribute to the broad oncogenic role of MYC, including its effects on diverse hallmarks of cancer. In this review, we give an overview of this extensive, multilayered noncoding RNA network that exists around MYC. Current data clearly show explicit roles of crosstalk between MYC and ncRNAs to allow tumorigenesis.
Keywords: lncRNA, miRNA, MYC, ncRNA
Abbreviations
- AGO2
Argonaute 2
- Akt
AKT serine/threonine kinase
- AMBRA1
activating molecule in Beclin‐1‐regulated autophagy
- AMPK
AMP‐activated kinase
- ARE
A+U‐rich element
- ATP
adenosine triphosphate
- AUF1
A + U‐rich element RNA‐binding protein
- BAD
BCL2‐associated agonist of cell death
- BCYRN1
brain cytoplasmic RNA 1
- CASC11
cancer susceptibility candidate 11
- CCAT
colon cancer‐associated transcript
- CCN
cyclin
- CDC
cell division cycle
- CDK
cyclin‐dependent kinase
- CDKN
cyclin‐dependent kinase inhibitor
- ceRNA
competing endogenous RNA
- CIP2A
cancerous inhibitor of protein phosphatase 2A
- CNBP
cellular nucleic acid‐binding protein
- CONCR
cohesion regulator noncoding RNA
- CTCF
CCCTC‐binding factor
- CTGF
connective tissue growth factor
- DANCR
differentiation antagonizing non‐protein‐coding RNA
- DDX11
DEAD/H‐box helicase 11
- DNMT3a
DNA methyltransferase 3a
- EMT
epithelial–mesenchymal transition
- EPIC1
epigenetically induced lncRNA 1
- EZH2
enhancer of zeste 2 polycomb repressive complex 2
- Fbxw7
F‐box and WD repeat‐containing protein 7
- FILNC1
FoxO‐induced lncRNA 1
- G6PD
glucose‐6‐phosphate dehydrogenase
- GADD45A
growth arrest and DNA damage‐inducible alpha
- GHET1
gastric carcinoma high expressed transcript 1
- GIT2
G‐protein‐coupled receptor kinase interactor 2
- GLS
glutaminase
- GLUT
glucose transporter member
- GSK3β
glycogen synthase kinase 3 beta
- HDAC3
histone deacetylase 3
- HIF1α
hypoxia‐inducible factor 1α
- HK2
hexokinase 2
- HMGA2
high‐mobility group AT‐hook 2
- hnRNP
heterogeneous nuclear ribonucleoprotein
- HOTAIR
homeobox transcript antisense intergenic RNA
- HuR
RNA‐binding protein human antigen R
- IDH1
isocitrate dehydrogenase 1
- IGF2BP
insulin‐like growth factor 2 mRNA‐binding protein
- IL‐6
interleukin 6
- IRES
internal ribosome entry segment
- JAK
Janus kinase
- LAST
lncRNA‐assisted stabilization of transcripts
- LDHA
lactate dehydrogenase A
- LIFR
leukemia inhibitory factor receptor
- Lin28B
Lin28 homolog B
- Linc‐RoR
lincRNA regulator of reprogramming
- lncRNA
long noncoding RNA
- MAX
MYC‐associated protein X
- MIF
MYC inhibitory factor
- MINCR
MYC‐induced long noncoding RNA
- miRNA
microRNA
- MMP
metalloproteinase
- mTOR
mechanistic target of rapamycin kinase
- MYCBP
MYC‐binding protein
- MYC
myelocytomatosis viral oncogene homolog
- MYU
MYC‐induced lncRNA
- ncRNA
noncoding RNA
- NEAT1
nuclear‐enriched abundant transcript 1
- NFκB
nuclear factor kappa B
- OGT
O‐GlcNAc transferase
- PCAT‐1
prostate cancer‐associated ncRNA transcript 1
- PCGEM1
prostate cancer gene expression marker 1
- PDH
pyruvate dehydrogenase
- PDIA3P
protein disulfide isomerase family A member 3 pseudogene 1
- PDK
pyruvate dehydrogenase kinase
- PFK
phosphofructokinase
- PI3K
phosphatidylinositol‐4,5‐biphosphate 3 kinase
- PKM2
pyruvate kinase M2
- PP2A
protein phosphatase 2A
- PPP
pentose phosphate pathway
- PTEN
phosphatase and tensin homolog
- Puma
p53 upregulated modulator of apoptosis
- PVT1
plasmacytoma variant translocation 1
- RB1
RB transcriptional corepressor 1
- RISC
RNA‐induced silencing complex
- ROCK
Rho‐associated coiled‐coil‐containing protein kinase
- SMAD
mothers against decapentaplegic homolog
- SNAI
snail family transcriptional repressor 1/2
- SNHG
small nucleolar RNA host gene
- SOCS5
suppressor of cytokine signaling 5
- SPOP
speckle‐type POZ
- STAT3
signal transducer and activator of transcription 3
- SUFU
suppressor of fused homolog
- TCA
tricarboxylic acid cycle
- TCF7L2
transcription factor 7 like 2
- TFAP4
transcription factor AP‐4
- TFDP2
transcription factor Dp‐2
- TGFBR2
TGF‐β receptor type II
- TGF‐β
transforming growth factor‐β
- THBS
thrombospondin‐1
- THOR
testis‐associated highly conserved oncogenic long noncoding RNA
- TOB2
transducer of ERBBS
- TUSC2
tumor suppressor candidate 2
- UBE3C
ubiquitin protein ligase E3C
- USP28
ubiquitin‐specific peptidase 28
- VASH2
vasohibin‐2
- VEGF
vascular endothelial growth factor
- Wif1
Wnt inhibitory factor 1
- YAP
yes‐associated protein
- ZEB
zinc finger E‐box‐binding homeobox
- ZNF281
zinc finger protein 281
1. Introduction
The MYC gene family consist of three members, that is, c‐MYC, n‐MYC, and l‐MYC. c‐MYC forms a central hub in all cells by regulating many cellular processes, while n‐MYC and l‐MYC are more tissue‐specific regulators. MYC proteins are overexpressed in more than half of all human cancers, including lung, breast, and colon cancers (Albihn et al., 2010). This overexpression is caused by diverse mechanisms including amplifications, translocations, and epigenetic alterations (Kalkat et al., 2017). In this review, we will focus on c‐MYC, hereafter referred to as MYC.
MYC belongs to the basic helix–loop–helix superfamily and functions as a transcription factor. Upon dimerization with its binding partner MAX, the MYC‐MAX dimer binds to E‐box sequences in the promoter region of its targets genes, thereby activating transcription of these genes (Tu et al., 2015). In addition to interacting with MAX, MYC can also interact with other transcription factors, histone‐modifying enzymes, and DNA methyltransferases to repress transcription. MYC regulates the transcription of many different genes, which include protein‐coding as well as noncoding genes (Dang, 2012; Hart et al., 2014; Winkle et al., 2015). These noncoding genes can include various RNA molecules, for example, miRNAs and lncRNAs.
miRNAs are noncoding, regulatory RNA molecules of about 22 nucleotides in length. A miRNA is transcribed as a longer primary transcript, which is processed in two steps into a mature single‐stranded miRNA and subsequently incorporated into the RISC. The miRNA guides the RISC complex to its target mRNA by recognition of a complementary sequence, most often in the 3′ UTR. Usually, conserved Watson–Crick pairing with nucleotides 2–7 of the miRNA, the so‐called seed region, is essential for target recognition (Bartel, 2009). Binding to the target mRNA will subsequently result in mRNA cleavage by AGO2 in case the miRNA has high complementarity with the binding site region on the mRNA. In case of a low level of complementarity, binding will lead to translational repression.
LncRNAs are defined as noncoding RNA molecules of more than 200 nucleotides in length. Their expression is often tissue specific or cell type specific, and their transcripts can have subcellular compartment‐specific localizations. Together, this restricts their function to specific cell types and locations. LncRNAs can regulate gene expression at the transcriptional and post‐transcriptional level, as well as by modulating protein stability, localization, and functionality via diverse mechanisms. In the nucleus, lncRNAs can regulate transcription of nearby genes in cis or of more distant genes in trans, for example, by recruiting transcription factors, chromatin‐modifying complexes, or heterogeneous nuclear ribonucleoprotein (hnRNP) complexes. LncRNAs residing in the cytoplasm can modulate mRNA stability, translation efficiency, or protein stability, localization, or activity. Cytoplasmic lncRNAs can act as decoys to sequester RNA binding proteins or miRNAs (sponges or ceRNAs) or interfere with post‐translational modifier proteins (Chen, 2016; Schmitt and Chang, 2016).
Over the last decades, it has become clear that MYC is not only regulated by and regulates many protein‐coding genes, but this extensive network also includes the family of ncRNAs. The overall aim of this review was to present an overview of the intricate crosstalk between ncRNAs and MYC. We first focus on ncRNAs acting upstream of MYC by regulating its transcription, translation, and activity. In addition, we focus on ncRNAs acting downstream of MYC and pinpoint their contributions to crucial hallmarks of cancer.
2. ncRNAs regulating MYC
2.1. miRNAs regulating MYC
In total, twenty‐five miRNAs belonging to twenty different seed families have been described to directly regulate MYC (Fig. 1). Most of the miRNAs bind to the MYC transcript in a canonical fashion, that is, with so‐called seed‐containing binding sites in the 3′UTR. Binding of let‐7b/c‐5p is enhanced by adjacent binding of the RNA‐binding protein HuR, which makes the miRNA binding site accessible (Kim et al., 2009). One of the two miR‐24‐3p binding sites is seed‐containing, while the other less‐efficient site is ‘seedless’ and has extensive complementarity at the 3′‐end of the miRNA (Lal et al., 2009). MiR‐17‐5p was shown to bind to the 5′ UTR of the MYC mRNA (Liu et al., 2016), while miR‐184‐3p (Zhen et al., 2013), miR‐185‐3p (Liao and Liu, 2011), miR‐320b‐3p (Wang et al., 2015), and miR‐744‐5p (Lin et al., 2014) bind to the MYC ORF.
Figure 1.
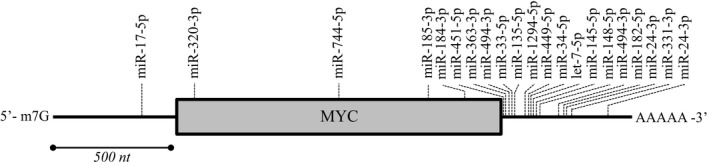
miRNA‐binding sites on the MYC mRNA. Schematic representation of the MYC mRNA with the binding sites of the MYC‐regulating miRNAs indicated. Only miRNAs for which binding to the mRNA was proven at least by reporter assay have been implemented in the figure. The miRNAs let‐7a/b/c/e/f‐5p and miR‐98‐5p of the let‐7 family (Bueno et al., 2011; Kim et al., 2009), miR‐24‐3p (Lal et al., 2009), miR‐33b‐5p (Takwi et al., 2012), miR‐34a/c‐5p (Christoffersen et al., 2010; Kong et al., 2008), miR‐145‐5p (Sachdeva et al., 2009), miR‐135b‐5p (Liu et al., 2014), miR‐148a‐5p (Han et al., 2013), miR‐182a‐5p (Huang et al., 2017), miR‐331‐3p (Bueno et al., 2011), miR‐363‐3p (Bueno et al., 2011), miR‐449c‐5p (Miao et al., 2013), miR‐451‐5p (Li et al., 2011), miR‐494‐3p (Zhang et al., 2012b), and miR‐1294‐5p (Liu et al., 2015a) target the MYC mRNA by binding to its 3′ UTR, while miR‐17‐5p binds to the 5′ UTR (Liu et al., 2016) and miR‐184‐3p (Zhen et al., 2013), miR‐185‐3p (Liao and Liu, 2011), miR‐320b‐3p (Wang et al., 2015), and miR‐744‐5p (Lin et al., 2014) bind to the MYC ORF.
Next to regulating MYC in a direct fashion, miR‐24‐3p can also influence MYC protein levels indirectly by targeting OGT. OGT can O‐GlcNAcylate the MYC protein and thereby increase its stability (Liu et al., 2017). A second miRNA that can act indirectly on MYC is miR‐375‐3p, which targets CIP2A. CIP2A prevents phosphorylation of Ser62 on MYC by PP2A and thereby prevents degradation of MYC (Jung et al., 2013). So, miR‐24‐3p and miR‐375‐3p can downregulate MYC protein levels indirectly by targeting OGT and CIP2A, respectively.
Many of the miRNAs that can directly downregulate MYC by binding to the MYC mRNA, show reduced levels in cancer. The decreased expression of these miRNAs can thus contribute to the high levels of MYC as commonly observed in cancer. Examples are the let‐7‐5p family, miR‐148a‐5p, miR‐331‐3p, and miR‐363‐3p, which are downregulated in Burkitt lymphoma compared to normal lymph nodes (Bueno et al., 2011). A well‐known exception is miR‐17‐5p, which is part of the oncogenic miR‐17~92 cluster that is often upregulated in MYC‐driven cancers. As too high MYC levels are potentially dangerous for cancer cells, targeting of MYC by miR‐17‐5p may be a means to maintain optimal MYC levels and sustain continuous tumor growth (Liu et al., 2016).
2.2. lncRNAs regulating MYC
Expression of MYC is controlled at the level of transcription, translation, and protein stability. Several lncRNAs have been demonstrated to play a role in these regulatory processes. Here, we describe the lncRNAs with a well‐characterized role in MYC regulation (Fig. 2).
Figure 2.
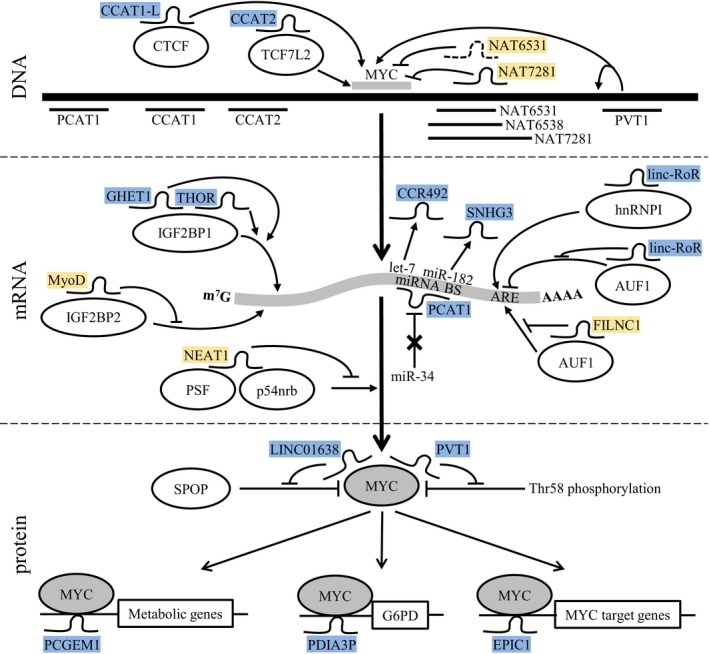
lncRNAs regulating MYC at the DNA, mRNA, or protein levels. LncRNAs and their interaction partners involved in regulation of MYC transcription, translation, stability, and functionality at protein level are indicated. The genomic region around MYC and the MYC mRNA (central thick and curved gray line) are not drawn to scale. LncRNAs are indicated by curved lines and proteins by ellipses. LncRNAs highlighted in blue indicate that they stimulate and lncRNA highlighted in yellow indicate that they repress MYC transcription, translation, stability, or functionality, and the arrows indicate stimulating or repressing effects on MYC.
2.2.1. LncRNAs regulating MYC transcription in cis
Besides the MYC gene, the 8q24 region harbors several noncoding genes that can regulate MYC transcription. CCAT1‐L transcript variant of the CCAT1 gene and CCAT2 are specifically expressed in colorectal cancer (Ling et al., 2013; Xiang et al., 2014). CCAT1‐L is a nuclear lncRNA that accumulates in distinct nuclear foci near its site of transcription. Knockdown of CCAT1‐L reduced, while overexpression enhanced transcription of MYC in cis. This regulatory effect on MYC was attributed to the spatial proximity of the CCAT1‐L locus with the MYC promoter. Indeed, reduced chromatin loop formation between the CCAT1‐L and MYC loci was observed upon knockdown of CCAT1‐L transcription. The loop formation was dependent on interaction of CCAT1‐L with CTCF, which enhanced binding of CTCF to the MYC locus (Xiang et al., 2014). CCAT2 regulates MYC by enhancing the activity of TCF7L2, a transcription factor for MYC (Ling et al., 2013). Thus, both CCAT1‐L and CCAT2 positively regulate MYC transcription.
Interaction between an enhancer region downstream the first transcriptional start site of PVT1 and the PVT1 promoter itself has tumor suppressor activity by reducing MYC transcription (Cho et al., 2018). Silencing of the PVT1 promoter increased MYC expression independent of the PVT1 transcript itself. The underlying mechanism has been identified as a competition between the PVT1 promoter and the MYC promoter for interaction with the intragenic enhancer region in the PVT1 locus. Under normal conditions, these enhancers preferentially bind to the PVT1 promoter. Silencing of the PVT1 promoter allowed interaction of enhancers with the MYC promoter, leading to increased MYC transcription. Importantly, this effect is restricted to cells where MYC forms chromatin loops with PVT1, for example, breast cancer, as opposed to colorectal cancer or cervical carcinoma cells where MYC loops to the CCAT1 enhancer.
The levels of three partially overlapping lncRNA transcripts antisense to the 3′ distal region of MYC, NAT6531, NAT6538, and NAT7281, are regulated by histone H3 acetylation in prostate cancer cells. Under normal conditions, NAT6531 is expressed and processed by DICER into several short RNAs, which have a repressive effect on MYC transcription, possibly by binding to the MYC promoter and intron 1 through partial sequence complementarity. Partial inhibition of histone deacetylation shifts transcription from NAT6531 to NAT6538, and this releases the block on MYC transcription. Strong inhibition of histone deacetylation results in transcription of the longer NAT7281, which strongly represses MYC transcription (Napoli et al., 2017).
2.2.2. LncRNAs controlling MYC mRNA stability and translation
IGF2BPs enhance mRNA stability and promote translation by binding to the MYC mRNA (Huang et al., 2018). A number of cell type‐specific lncRNAs have been identified that modulate this interaction. Interaction of IGF2BP1 with lncRNA GHET1 in gastric cancer and THOR in renal and skin cancer increased MYC mRNA and protein levels (Liu et al., 2018; Yang et al., 2014; Ye et al., 2018). In contrast, binding of the skeletal muscle‐specific lncRNA lncMyoD to IGF2BP2 decreased MYC mRNA levels by preventing binding of IGF2BP2 to MYC mRNA (Gong et al., 2015).
Binding of AUF1 to an ARE site in the 3′UTR of the MYC transcript can both positively and negatively affect MYC levels, depending on the cell‐type. In normal kidney cells, FILNC1 acts as a decoy for AUF1 preventing binding of AUF1 to the MYC mRNA, thereby resulting in low MYC protein levels. In renal cancer, FILNC1 is downregulated, resulting in an AUF1‐dependent increase in MYC protein levels (Xiao et al., 2017). In breast and colon cancers, binding of linc‐RoR to AUF1 inhibits binding of AUF1 to MYC mRNA and thereby increases MYC levels (Huang et al., 2015). It is currently unclear why sequestering of AUF1 has opposite effects on MYC levels in these different cell types. In addition, linc‐RoR facilitates binding of RNA binding protein hnRNP‐I to MYC mRNA and this also enhances MYC protein levels.
MYC can be translated using an IRES in case the regular cap‐dependent translation is compromised. This requires binding of the IRES trans‐acting factors PSF and p54nrb (Cobbold et al., 2008). These factors are sequestered by lncRNA NEAT1 to the paraspeckles. In HeLa cells, depletion of NEAT1 during nucleolar stress released PSF and p54nrb from paraspeckles and allowed IRES‐dependent translation of MYC (Shen et al., 2017).
LncRNAs can also stimulate MYC mRNA translation by competing with MYC‐regulating miRNAs. This has been shown for PCAT‐1, which competes with miR‐34a‐5p for interaction with its binding site in the 3′ UTR of the MYC mRNA (Prensner et al., 2011, 2014). The effect of PCAT‐1 can be antagonized by miR‐3667‐3p, which targets PCAT‐1.
2.2.3. LncRNAs affecting MYC protein stability and activity
The stability of MYC protein can be increased by two lncRNAs that both prevent its degradation, but via distinct mechanisms. In contrast to the tumor‐suppressive role of the PVT1 promoter, the PVT1 transcript can act as an oncogene. PVT1 stabilizes the MYC protein by preventing phosphorylation of threonine 58, which is a signal for its degradation (Tseng et al., 2014). LINC01638 prevents MYC protein degradation by preventing binding of E3 ubiquitin ligase adapter SPOP to MYC (Luo et al., 2018).
Three lncRNAs modulate interaction of MYC with (subsets of) its target genes by directly binding to MYC. PCGEM1 is a prostate‐specific lncRNA, which together with MYC co‐occupies the promoter regions of several metabolic genes documented to be MYC targets. Knockdown of PCGEM1 reduced recruitment of MYC to the promoters of these PCGEM1‐dependent metabolic genes without affecting MYC protein levels (Hung et al., 2014). Thus, PCGEM1 affects the metabolic state of cancer cells by enhancing MYC occupancy at the promoters of several metabolic genes. LncRNA PDIA3P regulates the metabolic state of multiple myeloma cells via induction of G6PD, an enzyme crucial for promoting the PPP flux (Yang et al., 2018). This effect is achieved by interaction of PDIA3P with MYC and promoting MYC binding to the G6PD promoter. Together with MYC, lncRNA EPIC1 co‐occupies the promoters of > 97% of EPIC1‐regulated genes involved in cell cycle progression, and thereby regulates transcriptional activity of these genes in breast cancer cells (Wang et al., 2018).
From the studies presented here, lncRNAs emerge as important regulators of MYC expression and activity, either directly or indirectly by interacting with proteins. Often, these lncRNAs are deregulated in cancer and promote high MYC levels and activity. Since expression of lncRNAs is highly cell type specific, many of the lncRNA‐MYC interactions are restricted to certain tissues. Future studies will likely broaden the repertoire of lncRNAs regulating MYC and improve the understanding of the underlying mechanisms in normal and cancer cells.
2.3. Feedback loops on MYC
Next to the more straightforward regulation of MYC by ncRNAs as described above, more complex feedback loops between MYC and MYC‐regulating ncRNAs have been identified. These include feedback loops that involve MYC‐regulated miRNAs, as well as MYC‐regulated lncRNAs that act as sponges for MYC‐regulating miRNAs.
2.3.1. Feedback loops involving MYC‐regulated miRNAs
Several miRNAs that regulate MYC can be induced or repressed by MYC as well, resulting in the formation of feedback loops. Examples of this are the feedback loops between MYC and MYC‐induced miR‐7‐5p (Capizzi et al., 2017; Chou et al., 2010), miR‐17‐5p (Liu et al., 2016), and miR‐185‐3p (Liao and Liu, 2011). For miR‐7‐5p, a positive feedback loop is formed via the miR‐7‐5p target AMBRA1, which promotes dephosphorylation of Ser62 on MYC upon binding to PP2A. This leads to stimulation of proteosomal degradation of MYC (Capizzi et al., 2017; Cianfanelli et al., 2015). In this way, miR‐7‐5p indirectly enhances MYC protein stability and promotes its own MYC‐mediated transcription. MiR‐17‐5p and miR‐185‐3p were shown to directly target MYC mRNA resulting in a negative feedback loop (Liao and Liu, 2011; Liu et al., 2016).
Positive feedback loops that result in sustaining high MYC expression also involve MYC‐repressed miRNAs. Let‐7a‐5p, miR‐34a‐5p, miR‐148a‐5p, miR‐363‐3p, and miR‐451‐5p are examples of MYC‐repressed miRNAs that can directly repress MYC translation (Bommer et al., 2007; Bueno et al., 2011; Christoffersen et al., 2010; Ding et al., 2018; Han et al., 2013; Sampson et al., 2007). Besides MYC, miR‐363‐3p also targets USP28, a de‐ubiquitinase involved in MYC stabilization (Han et al., 2013). MiR‐22 forms a feedback loop with MYC by targeting the MYCBP transcript, which encodes a positive regulator of MYC transcriptional activity (Xiong et al., 2010). In hepatocellular carcinoma, repression of liver‐specific miR‐122‐5p results in derepression of the miR‐122 targets E2F1 and its interaction partner TFDP2 (Wang et al., 2014). Both targets are involved in the induction of MYC transcription, creating another feedback loop. MiR‐200b‐3p participates in a feedback loop that involves MYC protein stability by targeting Akt2 mRNA (Lv et al., 2017). Akt2 represses the activity of GSK3β, an enzyme that destabilizes the MYC protein by phosphorylation of threonine residue 58. Thus, by repressing miR‐200b‐3p, MYC ensures inhibition of GSK3β, thereby stimulating its own stability. In contrast, MYC‐repressed miR‐30a‐5p is involved in a negative feedback loop by targeting UBE3C mRNA, a protein that can ubiquitinate MYC for proteosomal degradation (Chang et al., 2008; Xiong et al., 2016).
2.3.2. Feedback loops involving MYC‐regulated lncRNAs acting as miRNA sponges
The functions of several MYC‐regulating miRNAs can be antagonized by MYC‐regulated lncRNAs, which act as sponges. By sequestering those miRNAs, the following MYC‐induced lncRNAs ensure high MYC levels and create a positive feedback loop on MYC: CCAT1‐S, the short isoform of CCAT1‐L (let‐7a/b/c/e‐5p) (Deng et al., 2015), DANCR (miR‐33b‐5p) (Ma et al., 2018), H19 (let‐7a/b‐5p) (Peng et al., 2017; Zhou et al., 2017), linc00176 (miR‐185‐5p) (Tran et al., 2017), and SNHG3 (miR‐182a‐5p) (Huang et al., 2017). Another lncRNA that ensures high MYC levels by sequestering miRNAs of the let‐7‐5p family without being regulated by MYC is lincRNA CCR492 (Maldotti et al., 2016). In contrast, the MYC‐induced lncRNA‐MIF reduces MYC levels and creates a negative feedback loop by sequestering miR‐586 (Zhang et al., 2016a). This miRNA targets the mRNA encoding E3 ubiquitin ligase Fbxw7, which stimulates MYC degradation. Although this does not seem beneficial for cancer cells, it might be that with the overall broad effects of MYC, lncRNA‐MIF is an additional factor in fine‐tuning the most optimal MYC levels.
3. MYC‐regulated ncRNAs involved in five important hallmarks of cancer
The C13ORF25 RNA also known as the primary transcript of the oncogenic miR‐17~92 cluster was identified as being MYC‐induced in 2005 (He et al., 2005; O'Donnell et al., 2005). The induction of this cluster is achieved by binding of MYC together with E2F1‐3 transcription factors to its promoter (Sylvestre et al., 2007; Woods et al., 2007). The miR‐17~92 cluster has two paralogs: the miR‐106a~363 cluster and the miR‐106b~25 cluster (Tanzer and Stadler, 2004). The miR‐106b~25 cluster is also regulated by E2F1 in combination with MYC (Petrocca et al., 2008). In 2008, multiple MYC‐repressed miRNAs were identified using a human and a mouse B‐cell lymphoma model (Chang et al., 2008). MYC represses expression of specific pri‐miRNAs by binding to their promoter regions and recruitment of HDAC3 (miR‐15a/16 cluster) (Zhang et al., 2012a), HDAC3 and EZH2 (miR‐26a, miR‐19, and miR‐129) (Han et al., 2016; Zhang et al., 2012b; Zhao et al., 2013), or DNMT3a (miR‐34a) (Craig et al., 2011). Repression of the members of the let‐7 family by MYC is regulated post‐transcriptionally by the MYC‐induced RNA binding protein Lin28B (Chang et al., 2009).
One of the first identified MYC‐regulated lncRNAs is CCAT1. While the CCAT1‐L transcript variant is specifically overexpressed in colorectal cancer, the CCAT1‐S variant is upregulated in many other cancers, including gastric carcinoma and colon cancer (He et al., 2014; Yang et al., 2013a). By binding to the E‐box element in the promoter region of CCAT1, MYC induces expression of CCAT1‐S. As the short transcript variant is most likely formed by 3′ processing of the long variant, MYC probably induces expression of CCAT1‐L, but this has not been proven. Besides CCAT1 and CCAT2, six other colorectal cancer‐associated MYC‐regulated lncRNAs (MYCLos/CCAT3‐8) have been identified (Kim et al., 2015a,b). Three of them are MYC‐induced, and the other three are MYC‐repressed. In the last five years, many more MYC‐regulated lncRNAs have been identified although for many their function has not yet been identified (Hart et al., 2014; Winkle et al., 2015).
Below, we describe in more detail the MYC‐regulated miRNAs (Table 1 and Fig. 3) and lncRNAs (Table 2 and Fig. 4) with a clear role in five main hallmarks of cancer, that is, cell cycle progression, apoptosis, metabolism, angiogenesis, and metastasis.
Table 1.
MYC‐regulated miRNAs with a function related to important hallmarks of cancer
| Proven target gene(s)a | Cellular processesb | |
|---|---|---|
| MYC‐induced | ||
| miR‐9‐5p | CDH1, LIFR, SOCS5 | Angiogenesis, metastasis |
| miR‐17‐5p | BIM, CCND1/2, E2F1‐3, CDKN1A, PTEN, TGFBR, VEGF | Cell cycle progression, angiogenesis, apoptosis, metastasis |
| miR‐18‐5p | CTGF, SMAD4 | Angiogenesis, metastasis |
| miR‐19‐3p | AMPK, BIM, PP2A, PTEN, THBS1 | Apoptosis, angiogenesis, metabolism |
| miR‐25‐3p | BIM, USP28 | Cell cycle progression |
| miR‐378‐3p | TOB2 | Cell cycle progression |
| miR‐378a‐5p | SUFU, TUSC1 | Angiogenesis |
| MYC‐repressed | ||
| let‐7‐5p | CCND2, CDC25, CDC34, CDK6, HMGA2 | Cell cycle progression, metastasis |
| miR‐15‐5p | AP4, BCL2, CCND1/E1, CDK6, E2F3, GLUT3, SMAD3, VEGF | Cell cycle progression, apoptosis, metabolism, angiogenesis |
| miR‐23‐3p | GLS, LDHA/B, SMAD3‐5 | Metabolism, metastasis |
| miR‐26‐5p | CCND2/E1‐2, CDK6, E2F3, EZH2, IL‐6, MCL1, PDHX, PTEN, RB1 | Cell cycle progression, apoptosis, metabolism, metastasis |
| miR‐29‐3p | AKT3, CDK6, MCL1, MMPP2, VASH2, VEGF | Cell cycle progression, apoptosis, angiogenesis, metastasis |
| miR‐30‐5p | LDHA, UBE3C | Metabolism, MYC regulation |
| miR‐34‐5p | BCL2, CCND1/E2, CDK4, CDK6, SNAI1, ZNF281 | Cell cycle progression, apoptosis, metastasis |
| miR‐122‐5p | BCL2L2, E2F1, TFDP2 | Apoptosis |
| miR‐129‐5p | PDK4 | Metabolism |
| miR‐200‐3p | AKT2, CDKN1B, CTNNB1, GIT2, ROCK2, VEGF, ZEB‐1, ZEB‐2 | Cell cycle progression, angiogenesis, metastasis |
aNot all members of the seed families target the proven target genes. bNot all target genes mentioned in column two are involved in the cellular processes mentioned here.
Figure 3.
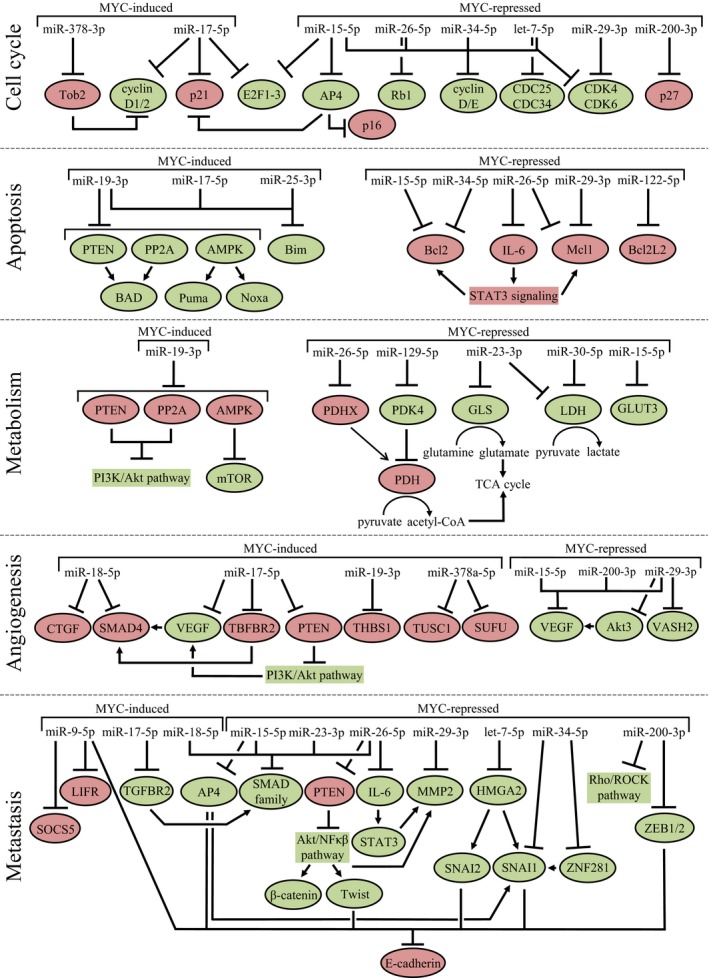
MYC‐regulated miRNAs involved in five important hallmarks of cancer. For each hallmark, the MYC‐regulated miRNAs and their protein targets involved in stimulation (green) or repression (red) of the respective hallmark are indicated.
Table 2.
MYC‐regulated lncRNAs with a function related to important hallmarks of cancer
| Proven target gene(s) | Cellular processesa | |
|---|---|---|
| MYC‐induced | ||
| BCYRN1 | ↑ MMP2/9/13, VEGF | Angiogenesis, metastasis |
| CASC11 | ↑ HNRNPK | Cell cycle progression |
| ↓ WIF1 | Metastasis | |
| CCAT1‐S | ↑ BIRC7 | Apoptosis |
| ↓ CDKN1A, let‐7a/b/c/e‐5p, miR‐148a‐3p | Cell cycle progression, metastasis | |
| CCAT6 | ↓ CDKN2B | Cell cycle progression |
| CONCR | ↑ DDX11 | Cell cycle progression |
| DANCR | ↓ CDKN1A, miR‐33b‐5p | Cell cycle progression |
| H19 | ↓ CDH1, let‐7a/b‐5p, miR‐29a‐3p, miR‐106a‐5p, miR‐200a‐c‐3p | Cell cycle progression, metabolism, angiogenesis, metastasis |
| HOTAIR | ↓ CDKN1A, WIF1, miR‐34a‐5p | Cell cycle progression, metastasis, |
| LAST | ↑ CCND1 | Cell cycle progression |
| Linc00176 | ↓ miR‐9‐5p, miR‐185‐5p | Cell cycle progression |
| LncRNA‐MIF | ↓ miR‐586‐5p | Metabolism |
| MINCR | ↓ miR‐26a‐5p | Cell cycle progression, apoptosis, metastasis |
| MYCLo‐1 | ↓ CDKN1A | Cell cycle progression |
| MYU | ↑ CDK6 | Cell cycle progression |
| SINGH12 | ↑ MMP13 | Metastasis |
| MYC‐repressed | ||
| IDH1‐AS1 | ↑ IDH1 | Metabolism |
| MYCLo‐4 | ↑ GADD45A | Cell cycle progression |
| MYCLo‐5 | Unknown | Cell cycle progression |
| MYCLo‐6 | ↑ GADD45A | Cell cycle progression |
↑ indicates induced/stabilized/activated by the lncRNA, and ↓ indicates being repressed by the lncRNA. aNot all proven target genes mentioned in column two are involved in the cellular processes mentioned here.
Figure 4.
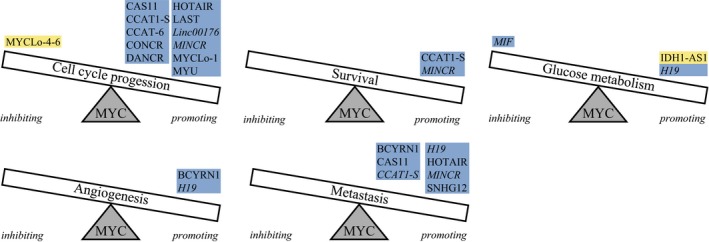
MYC‐regulated lncRNAs involved in five important hallmarks of cancer. For each hallmark, the lncRNAs that are promoting or inhibiting are indicated. Cell survival represents the opposite of apoptosis in this figure. LncRNAs highlighted in blue are MYC‐induced, lncRNAs highlighted in yellow are MYC‐repressed, and lncRNAs in italic function as sponges for miRNAs.
3.1. Cell cycle progression
Nineteen MYC‐induced ncRNAs have a role in cell cycle progression. LncRNA‐assisted stabilization of transcripts (LAST) stimulates CCND1 expression by stabilizing CCND1 mRNA together with CNBP (Cao et al., 2017). MiR‐378a‐3p ensures CCND1 expression by targeting mRNA encoding TOB2, which is a repressor of CCND1 expression (Feng et al., 2011). CASC11 (CARLo‐7) promotes CCND1 transcription by stabilizing the hnRNP‐K mRNA, which leads to an hnRNP‐K‐dependent enhanced nuclear accumulation of β‐catenin (Zhang et al., 2016b). This leads to activation of WNT/β‐catenin signaling, and the subsequent induction of CCND1 transcription. The MYC‐induced lncRNA MY (VSP9D1‐AS1) associates with hnRNP‐K and stimulates CDK6 mRNA translation by competing with miR‐16‐5p for binding to CDK6 mRNA (Kawasaki et al., 2016). CDKN2B transcription is repressed by lncRNA CCAT‐6 upon binding of this lncRNA to hnRNP‐K (Kim et al., 2015b). All three lncRNAs interacting with hnRNP‐K (CASC11, MYU, and CCAT‐6) have been shown to stimulate cell cycle progression in colon cancer. The four lncRNAs HOTAIR, MYCLo‐1, CCAT1‐S, and DANCR all repress CDKN1A transcription (Kim et al., 2014, 2015b; Liu et al., 2013; Lu et al., 2018; Ma et al., 2014). HOTAIR represses CDKN1A transcription by recruiting EZH2 and inducing epigenetic changes, while MYCLo‐1 is assisted by HuR to repress the transcription of CDKN1A. The mechanisms by which CCAT1‐S and DANCR repress CDKN1A transcription are not yet known. Members of the miR‐17‐5p seed family have been strongly implicated in stimulation of cell cycle progression by targeting CDKN1A (Ivanovska et al., 2008; Kim et al., 2009; Trompeter et al., 2011). Conversely, the same seed family represses cell cycle progression by targeting CCND1/2 transcripts (Trompeter et al., 2011; Yu et al., 2008) and E2F1‐3 transcripts (He et al., 2005; Luan et al., 2018; O'Donnell et al., 2005; Trompeter et al., 2011). This is consistent with the cell type‐specific roles as oncomiR as well as tumor suppressor miR that have been observed for individual members of the miR‐17‐5p seed family (He et al., 2005; O'Donnell et al., 2005). The MYC‐induced lncRNA CONCR plays a role during S‐phase and is required for cell division by regulating the activity of helicase DDX11, which is involved in DNA replication and sister chromatid cohesion (Marchese et al., 2016). The MYC‐induced lncRNA MINCR promotes MYC‐mediated transcription of a selected set of cell cycle genes (Doose et al., 2015), although there is some debate about whether this lncRNA is a direct MYC‐induced lncRNA or not (Doose et al., 2015, 2016; Hart et al., 2016). Besides, MINCR functions as a sponge for miR‐26a‐5p to stimulate cell cycle progression (Wang et al., 2016).
Eleven MYC‐repressed ncRNAs inhibit cell cycle progression, while one MYC‐repressed miRNA stimulates cell cycle progression. The CCND1‐3 and CCNE1‐2 transcripts are targeted by let‐7b‐5p (Johnson et al., 2007), the miR‐15‐5p seed family (Bonci et al., 2008; Wang et al., 2009; Xu et al., 2009), miR‐26a/b‐5p (Kota et al., 2009; Zhu et al., 2012), and miR‐34a‐5p (He et al., 2007; Pok et al., 2013; Sun et al., 2008). In addition, these miRNAs and miR‐29a‐c‐3p target CDK4/6 transcripts (He et al., 2007; Johnson et al., 2007; Kawasaki et al., 2016; Sun et al., 2008; Xu et al., 2009; Zhao et al., 2010; Zhu et al., 2012). The RB1 transcript is targeted by miR‐26a‐5p (López‐Urrutia et al., 2017), and the E2F3 transcript is targeted by miR‐195‐5p, a member of the miR‐15‐5p seed family (Xu et al., 2009). Let‐7b‐5p targets the CDC25 transcript, which results in reactivation of CDKs to enable cell cycle progression (Hoffmann, 2000). Let‐7b‐5p also targets CDC34, which is an ubiquitin‐conjugating enzyme that is involved in the degradation of Wee1, an inhibitor of CDK1 (Legesse‐Miller et al., 2009). The miR‐15‐5p seed family members target TFAP4, which results in repression of CDKN1A and CDKN2A transcription and reduced p21 and p16 levels (Jackstadt et al., 2013a). MiR‐200b‐3p targets the CDKN1B transcript, leading to reduced p27 levels and stimulation of cell cycle progression (Fu et al., 2014). So, it seems not beneficial for cancer cells that MYC represses miR‐200b‐3p. MYCLo‐4 and MYCLo‐6 both block G2 to M phase progression by stimulating growth arrest and GADD45A expression, a critical regulator of G2 arrest (Kim et al., 2015a). MYCLo‐5 is involved in controlling S to G2 phase progression, but the exact mechanism is not yet known.
3.2. Apoptosis
Seven MYC‐induced and eight MYC‐repressed ncRNAs influence the balance between pro‐ and anti‐apoptotic factors. The MYC‐induced miR‐19a/b‐3p, miR‐20a‐5p, miR‐25‐3p, and miR‐92a‐3p prevent apoptosis by targeting the BIM transcript (Mogilyansky and Rigoutsos, 2013; Petrocca et al., 2008; Xiao et al., 2008). In addition, miR‐19a/b‐3p target transcripts of the PTEN, PP2A, and AMPK genes, resulting in decreased levels of the downstream pro‐apoptotic proteins BAD, Puma, and Noxa (Mavrakis et al., 2010; Mu et al., 2009; Olive et al., 2009). CCAT1‐S was shown to upregulate the expression of Livin, which is a member of the inhibitor of apoptosis protein family that can interact with caspases to prevent apoptosis (Chen et al., 2017).
Many of the MYC‐repressed miRNAs directly target anti‐apoptotic factors; for example, miR‐15a/16‐5p and miR‐34a‐5p target the BCL2 transcript (Bommer et al., 2007; Bonci et al., 2008; Cimmino et al., 2005), miR‐122‐5p targets the BCL2L2 transcript (Lin et al., 2008; Wang et al., 2014), and miR‐26b‐5p and miR‐29b‐3p target the MCL1 transcript (Jiang et al., 2015; Mott et al., 2007). Moreover, by targeting the IL‐6 transcript, miR‐26a‐5p represses STAT3 signaling, which results in reduced Bcl2 and Mcl1 expression levels (Yang et al., 2013b). The effects of miR‐26a‐5p can be antagonized by MYC‐induced MINCR, which functions as a sponge for this miRNA and prevent apoptosis (Wang et al., 2016).
3.3. Metabolism
Three MYC‐induced and eight MYC‐repressed ncRNAs are involved in the regulation of aerobic glycolysis, a feature of cancer cells. By targeting PTEN and PP2K transcripts, miR‐19a/b‐3p enhances PI3K activity (Mavrakis et al., 2010; Mu et al., 2009; Olive et al., 2009). This results in phosphorylation of Akt by PDK1, which stimulates glycolysis through multiple mechanisms, such as increased expression of several glucose transporters, activation of PFK1/2 (important regulatory enzymes of glycolysis), and mTOR. To further ensure high mTOR activity, miR‐19a/b‐3p also targets AMPK, an inhibitor of mTOR activity (Bolster et al., 2002; Mavrakis et al., 2010). MiR‐106a‐5p targets the E2F3 transcript, which results in repression of the glucose metabolism (Luan et al., 2018). This is antagonized by H19, which has been proposed to promote glucose metabolism by acting as a sponge for miR‐106a‐5p. MIF influences the glycolytic activity by sequestering miR‐586, thereby preventing expression of MYC target genes involved in glycolysis, that is, GLUT1, LDHA, PKM2, and HK2 (Zhang et al., 2016a).
miRNAs repressed by MYC typically inhibit high metabolic activity. The initial uptake of glucose is regulated by miR‐195‐5p, which targets GLUT3 (Fei et al., 2012). MiR‐23a/b‐3p targets the mRNA encoding GLS, which converts glutamine to glutamate and thereby contributes to production of ATP (Gao et al., 2009). In addition, miR‐23a‐3p targets LDH subunits A and B (LDHA/LDHB), which convert the glycolytic end product pyruvate to lactate (Poyyakkara et al., 2018). Moreover, LDHA is also targeted by miR‐30a‐5p (Chang et al., 2008; Li et al., 2017a). MiR‐26a‐5p inhibits PDH activity by targeting PDHX and therefore inhibits the conversion of pyruvate to coenzyme A, an important component of the TCA cycle (Chen et al., 2014a). Instead, pyruvate is converted to lactate, showing an oncogenic role for miR‐26a‐5p in metabolism. In contrast, miR‐129 targets PDK4 mRNA, thereby stimulating PDH activity (Han et al., 2016). MYC‐repressed lncRNA IDH1‐AS1 stimulates homodimerization of IDH1 by forming a ternary structure with the enzyme, thereby enhancing its activity (Xiang et al., 2018). IDH1 converts isocitrate to α‐ketoglutarate, which is an intermediate in the TCA cycle and can inhibit glycolysis via degradation of HIF1α under normoxic condition (MacKenzie et al., 2007). By repressing IDH1‐AS1, MYC downregulates IDH1 activity and ensures glycolysis.
3.4. Angiogenesis
Stimulation of angiogenesis by different mechanisms has been reported for eight MYC‐induced ncRNAs, while five MYC‐induced and four MYC‐repressed miRNAs inhibit angiogenesis by targeting pro‐angiogenetic factors. Angiogenesis is enhanced by repression of the TGF‐β signaling pathway. MiR‐17‐5p and miR‐20a‐5p target the TGFBR2 transcript, while miR‐18a‐5p targets the downstream effector SMAD4 (Dews et al., 2010). Besides, several inhibitors of angiogenesis are targeted; miR‐19a‐3p targets THBS1 (Dews et al., 2010), miR‐18a‐5p targets CTGF (Ernst et al., 2010; Fox et al., 2013), and miR‐378‐5p targets TUSC2 and SUFU (Lee et al., 2007). VEGF expression is stimulated directly by lncRNA BCYRN1 (Hu and Lu, 2015; Peng et al., 2018) and indirectly by miR‐20a‐5p (Wang et al., 2017a). MiR‐20a‐5p targets PTEN, which leads to increased VEGF levels via activation of the PI3K/Akt pathway. In contrast, VEGF is inhibited by miR‐16‐5p, miR‐17‐5p, miR‐20a/b‐5p, miR‐29a‐3p, miR‐106a/b‐5p, and miR‐200b‐3p (Chen et al., 2014b; Choi et al., 2011; Hua et al., 2006). In this context, miR‐200b‐3p acts a tumor suppressor in contrast to its oncogenic role in cell cycle regulation. MiR‐29b‐5p indirectly lowers VEGF levels by targeting the Akt3 transcript (Li et al., 2017b). In melanoma cells, the effect of miR‐106a‐5p on VEGF expression can be counteracted by H19, which acts as a sponge for this miRNA (Luan et al., 2018). At first sight, it seems conflictive that both MYC‐induced and MYC‐repressed miRNAs target VEGF mRNA. However, as angiogenesis is crucial for a wide variety of physiological and pathological processes, VEGF expression has to be tightly regulated. This can be achieved by a combination of several regulatory factors including MYC‐induced and MYC‐repressed miRNAs, as well as other ncRNAs ensuring optimal VEGF levels under various conditions. MiR‐29a‐3p also targets the mRNA encoding a second pro‐angiogenetic factor, VASH2 (Jia et al., 2016). VASH2 inhibition by miR‐29a‐3p can also be antagonized by H19, which acts as a sponge for miR‐29a‐3p in glioma microvessels and epithelial cells (Jia et al., 2016).
3.5. Metastasis
Ten MYC‐induced ncRNAs target metastasis‐associated genes. H19 promotes metastasis by recruitment of EZH2 and the subsequent epigenetic suppression of E‐cadherin expression (Luo et al., 2013). Loss of E‐cadherin allows EMT, an early step in metastasis. MiR‐9‐5p promotes metastasis by targeting E‐cadherin, LIFR, and SOCS5 (Chen et al., 2012; Ma et al., 2010; Zhuang et al., 2012). LIFR inhibits metastasis through the Hippo/YAP pathway, and SOCS5 inhibits endothelial cell migration by inhibiting the JAK/STAT pathway. By interacting with EZH2, CASC11 and HOTAIR epigenetically suppress Wif1 expression and ensure stimulation of metastasis by the Wnt/β‐catenin pathway (Ge et al., 2013; Zhang et al., 2016b). As described in the paragraph above, three members of the miR‐17‐5p seed family target genes involved in the TGFβ signaling pathway, a crucial pathway also for the induction of metastasis. BCYRN1 stimulates metastasis by inducing the expression of MMP2, MMP9, and MMP13 (Hu and Lu, 2015; Peng et al., 2018). SNHG12 is a second lncRNA that induces the expression of MMP13 (Wang et al., 2017b). In contrast to BCYRN1 that induces MMP13 transcription, SNHG12 enhances MMP13 expression at the post‐transcriptional level.
Ten MYC‐repressed miRNAs prevent metastasis, while one MYC‐repressed miRNA can both induce and prevent metastasis, depending on the cell type. The transcription factors SNAI1/2, ZEB1/2, Twist, and AP4 all repress E‐cadherin expression at the transcriptional level (Tania et al., 2014). MiR‐34a‐5p targets the SNAI1 transcript directly and indirectly by targeting the Krüppel‐type transcription factor ZNF281 transcript (Hahn et al., 2013). In addition to being repressed by MYC, miR‐34a is also repressed by HOTAIR upon interaction with EZH2, thereby promoting metastasis in gastric cancer cells (Liu et al., 2015b). Let‐7a/b/e‐5p repress SNAI1 and SNAI2 expression indirectly by targeting the chromatin remodeling HMGA2 transcript (Lee and Dutta, 2007; Mayr et al., 2007). This is counteracted by CCAT1‐S functioning as a sponge for let‐7 family members let‐7a/b/c/e‐5p (Deng et al., 2015). CCAT1‐S can also sequester miR‐148a‐3p in osteosarcoma cells, thereby stimulating invasion and migration via unknown mechanisms (Zhao and Cheng, 2017). ZEB1/2 transcripts are targeted by miR‐200a‐c‐3p (Korpal et al., 2008; Park et al., 2008). The miR‐15a‐5p seed family targets mRNA encoding AP4, which induces SNAI1 expression (Jackstadt et al., 2013b). The role of miR‐26a‐5p with respect to metastasis seems to be contradictory. By targeting PTEN mRNA, miR‐26a‐5p stimulates the Akt/NFκB pathway and thereby induces expression of Twist, β‐catenin, and MMP2 in lung cancer (Liu et al., 2012). Increased levels of β‐catenin will initiate Wnt signaling, which stimulates metastasis. MMP2 is an essential protease involved in adhesion, invasion, and migration by proteolytic degradation of type IV collagen. In contrast, by targeting IL‐6 in hepatocellular carcinoma, miR‐26a‐5p represses STAT3 signaling and this results in lower MMP2 levels (Yang et al., 2013b). Furthermore, MMP2 is also targeted by miR‐29b‐3p (Fang et al., 2011). miRNAs that repress metastasis by repressing the downstream SMAD proteins of the TGFβ signaling pathway are miR‐23b‐3p (SMAD3‐5) (Rogler et al., 2009) and miR‐195‐5p (SMAD3) (Zhou et al., 2016). MiR‐200a‐3p targets the mRNA encoding β‐catenin in colorectal cancer, thereby repressing metastasis (Yang et al., 2017). Another pathway involved in metastasis by influencing cell motility is the Rho/ROCK signaling pathway, which is repressed by targeting of ROCK2 and GIT2 transcripts by miR‐200b/c‐3p (Peng et al., 2013; Wong et al., 2015; Zhou et al., 2017). All repressing effects of the miR‐200 seed family can be antagonized by H19, which functions as a sponge for these miRNAs (Li et al., 2016; Liang et al., 2015; Yang et al., 2017; Zhou et al., 2017). Besides, MINCR stimulates metastasis by sequestering miR‐26a‐5p (Wang et al., 2016).
4. Discussion
It is evident that an extensive, multilayered ncRNA network exists around MYC with critical roles for multiple lncRNAs and miRNAs in crucial cellular processes and in tumorigenesis. The picture that we present here is most likely still far from complete, as functions of most of the MYC‐regulated ncRNAs are not known yet (Hart et al., 2014; Robertus et al., 2010; Winkle et al., 2015). It is clear that many miRNAs and lncRNAs regulate MYC and that they can do this via diverse mechanisms at the level of transcription, translation, protein stability, and functionality. This suggests that redundancy is important to ensure optimal MYC levels and thereby cell viability under various conditions, as well as in different cell types. As MYC is involved in many cellular processes in redundant ways, it is remarkable that repression or reintroduction of a single MYC‐regulated ncRNA can already show strong effects on MYC‐associated phenotypes, as has been shown for many ncRNAs described in this review.
Expression of lncRNAs was shown to be more cell type specific than that of protein‐coding genes (Derrien et al., 2012). Also compared to miRNAs, lncRNAs appear to be more cell type‐specific. However, this might be biased as there are many more lncRNAs than miRNAs, which increases the chance to find cell type‐specific lncRNAs. Based on current knowledge, it seems that the cell type‐specific expression of certain lncRNAs can influence the output of MYC in two ways. First, cell type‐specific lncRNAs can influence important cellular processes downstream of MYC (Fig. 4). Second, other cell type‐specific lncRNAs, like PCGEM1 and PDIA3P, can modulate binding efficiency of MYC to promoters of a specific set of genes. So, these lncRNAs may direct the cell type‐specific target gene repertoire of MYC, rather than MYC acting as a general amplifier of expression. Altogether, a picture is emerging that lncRNAs guide cell type‐specific effects of MYC.
Although MYC has a central role in tumorigenesis, no effective MYC‐specific drugs are being employed in the clinic to date. Given the crucial functions of multiple lncRNAs and miRNAs in the oncogenic MYC network, it is tempting to speculate that targeting of ncRNAs within the MYC network might be an alternative to explore novel anticancer therapies. These ncRNAs can have profound impacts on MYC levels and activity and can also act downstream of MYC enabling cancer cells to gain the crucial hallmarks of cancer. To allow selection of the most optimal ncRNA targets, a more systematic analysis of their functional networks in normal cells as well as in cancer cells needs to be performed to oversee the consequences of targeting them.
Currently, more and more institutes and companies investigate how to specifically target miRNAs and lncRNAs, using both antisense and small molecule‐based strategies (Chakraborty et al., 2017; Warner et al., 2018). Inhibitors for miR‐92 and miR‐122, as well as mimics of miR‐16, miR‐29 and miR‐34, have been developed and tested or are currently tested in clinical trials (NIH U.S. National Library of Medicine, https://clinicaltrails.gov/ (accessed 06.08.2018)). As miR‐34a‐5p has tumor suppressor activity by both targeting MYC and stimulating apoptosis, while repressing cell cycle progression and metastasis, it is an attractive target for novel anticancer therapies. MiR‐16‐5p and miR‐29‐3p too have tumor‐suppressive roles in four of the five hallmarks discussed and form attractive targets as well. The cell type‐specific expression of lncRNAs adds to their attractiveness as targets for therapy (Derrien et al., 2012). The choice for an attractive target will therefore depend on the type of cancer. For example, CCAT1‐L and CCAT2 form attractive targets to specifically inhibit MYC transcription in colorectal cancer. A drug against CCAT1‐L, which will also target CCAT1‐S, would be very interesting as it will inhibit cell cycle progression and metastasis, while promoting apoptosis. However, a main problem for testing effectivity of lncRNA‐based drugs is the limited conservation for many of the lncRNAs, which prevents pre‐clinical experiments in relevant mouse models. Patient‐derived xenotransplantation models or organoid cultures might represent an alternative approach to test effectiveness of targeting human‐specific lncRNAs.
Thus, although MYC is described as one of the most important oncogenes, it is important to realize that there is an extensive, multilayered ncRNA network around MYC, in which intricate crosstalk contributes to hallmarks of cancer.
Author contributions
LJYMS, AD‐K, AvdB, and JK conceived the outline, and LJYMS, AD‐K, MW, AvdB, and JK wrote the manuscript. LJYMS, AD‐K, and JK made the figures. LJYMS, AD‐K, MW, AvdB, and JK critically read the manuscript and LJYMS, AvdB, and JK finalized the manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgements
We would like to thank Emily Ploeg for her help with gathering literature and background information. This work was supported by grants from Lymph & Co (to AvdB and JK), the Dutch Cancer Society (KWF #10478/2016‐1 to AvdB and JK) and the National Science Centre, Poland (2016/23/D/NZ1/01611 to AD‐K).
Lotteke J. Y. M. Swier and Agnieszka Dzikiewicz‐Krawczyk should be considered as cofirst authors
References
- Albihn A, Johnsen JI and Henriksson MA (2010) MYC in oncogenesis and as a target for cancer therapies. Adv Cancer Res 107, 163–224. [DOI] [PubMed] [Google Scholar]
- Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136, 215–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolster DR, Crozier SJ, Kimball SR and Jefferson LS (2002) AMP‐activated protein kinase suppresses protein synthesis in rat skeletal muscle through down‐regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem 277, 23977–23980. [DOI] [PubMed] [Google Scholar]
- Bommer GT, Gerin I, Feng Y, Kaczorowski AJ, Kuick R, Love RE, Zhai Y, Giordano TJ, Qin ZS, Moore BB et al (2007) p53‐mediated activation of miRNA34 candidate tumor‐suppressor genes. Curr Biol 17, 1298–1307. [DOI] [PubMed] [Google Scholar]
- Bonci D, Coppola V, Musumeci M, Addario A, Giuffrida R, Memeo L, D'Urso L, Pagliuca A, Biffoni M, Labbaye C et al (2008) The miR‐15a–miR‐16‐1 cluster controls prostate cancer by targeting multiple oncogenic activities. Nat Med 14, 1271–1277. [DOI] [PubMed] [Google Scholar]
- Bueno MJ, Gómez de Cedrón M, Gómez‐López G, Pérez de Castro I, Di Lisio L, Montes‐Moreno S, Martínez N, Guerrero M, Sánchez‐Martínez R, Santos J et al (2011) Combinatorial effects of microRNAs to suppress the Myc oncogenic pathway. Blood 117, 6255–6266. [DOI] [PubMed] [Google Scholar]
- Cao L, Zhang P, Li J and Wu M (2017) LAST, a c‐Myc‐inducible long noncoding RNA, cooperates with CNBP to promote CCND mRNA stability in human cells. Elife 6, e30433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capizzi M, Strappazzon F, Cianfanelli V, Papaleo E, Cecconi F (2017) MIR7–3HG, a MYC‐dependent modulator of cell proliferation, inhibits autophagy by a regulatory loop involving AMBRA1. Autophagy 13, 554–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty C, Sharma AR, Sharma G, Doss CGP and Lee SS (2017) Therapeutic miRNA and siRNA: moving from bench to clinic as next generation medicine. Mol Ther Nucleic Acids 8, 132–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang TC, Yu D, Lee YS, Wentzel EA, Arking DE, West KM, Dang CV, Thomas‐Tikhonenko A, Mendell JT (2008) Widespread microRNA repression by Myc contributes to tumorigenesis. Nat Genet 40, 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang TC, Zeitels LR, Hwang HW, Chivukula RR, Wentzel EA, Dews M, Jung J, Gao P, Dang CV, Beer MA et al (2009) Lin‐28B transactivation is necessary for Myc‐mediated let‐7 repression and proliferation. Proc Natl Acad Sci U S A 106, 3384–3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L (2016) Linking long noncoding RNA localization and function. Trends Biochem Sci 41, 761–772. [DOI] [PubMed] [Google Scholar]
- Chen B, Liu Y, Jin X, Lu W, Liu J, Xia Z, Yuan Q, Zhao X, Xu N, Liang S (2014a) MicroRNA‐26a regulates glucose metabolism by direct targeting PDHX in colorectal cancer cells. BMC Cancer 14, 443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Ma P, Li B, Zhu D, Chen X, Xiang Y, Wang T, Ren X, Liu C, Jin X (2017) LncRNA CCAT1 inhibits cell apoptosis of renal cell carcinoma through up‐regulation of Livin protein. Mol Cell Biochem, 434, 135–142. [DOI] [PubMed] [Google Scholar]
- Chen D, Sun Y, Wei Y, Zhang P, Rezaeian AH, Teruya‐Feldstein J, Gupta S, Liang H, Lin HK, Hung MC et al (2012) LIFR is a breast cancer metastasis suppressor upstream of the Hippo‐YAP pathway and a prognostic marker. Nat Med 18, 1511–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Xiao H, Wang Z‐H, Huang Y, Liu Z‐P, Ren H, Song H (2014b) MiR‐29a suppresses growth and invasion of gastric cancer cells in vitro by targeting VEGF‐A. BMB Rep 47, 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SW, Xu J, Sun R, Mumbach MR, Carter AC, Chen YG, Yost KE, Kim J, He J, Nevins SA et al (2018) Promoter of lncRNA Gene PVT1 is a tumor‐suppressor DNA boundary element. Cell 173, 1398–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y, Yoon S, Jeong Y, Yoon J, Baek K (2011) Regulation of vascular endothelial growth factor signaling by miR‐200b. Mol Cells 32, 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou Y‐T, Lin H‐H, Lien Y‐C, Wang Y‐H, Hong C‐F, Kao Y‐R, Lin S‐C, Chang Y‐C, Lin S‐Y, Chen S‐J et al (2010) EGFR promotes lung tumorigenesis by activating miR‐7 through a Ras/ERK/Myc pathway that targets the Ets2 transcriptional repressor ERF. Cancer Res 70, 8822–8831. [DOI] [PubMed] [Google Scholar]
- Christoffersen NR, Shalgi R, Frankel LB, Leucci E, Lees M, Klausen M, Pilpel Y, Nielsen FC, Oren M, Lund AH (2010) p53‐independent upregulation of miR‐34a during oncogene‐induced senescence represses MYC. Cell Death Differ 17, 236–245. [DOI] [PubMed] [Google Scholar]
- Cianfanelli V, Fuoco C, Lorente M, Salazar M, Quondamatteo F, Gherardini PF, De Zio D, Nazio F, Antonioli M, D'Orazio M et al (2015) AMBRA1 links autophagy to cell proliferation and tumorigenesis by promoting c‐Myc dephosphorylation and degradation. Nat Cell Biol 17, 20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M, Wojcik SE, Aqeilan RI, Zupo S, Dono M et al (2005) miR‐15 and miR‐16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci U S A 102, 13944–13949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold LC, Spriggs KA, Haines SJ, Dobbyn HC, Hayes C, de MoorCH, Lilley KS, Bushell M, Willis AE (2008) Identification of internal ribosome entry segment (IRES)‐trans‐acting factors for the Myc family of IRESs. Mol Cell Biol 28, 40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig VJ, Cogliatti SB, Imig J, Renner C, Neuenschwander S, Rehrauer H, Schlapbach R, Dirnhofer S, Tzankov A, Müller A (2011) Myc‐mediated repression of microRNA‐34a promotes high‐grade transformation of B‐cell lymphoma by dysregulation of FoxP1. Blood 117, 6227–6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang CV (2012) MYC on the path to cancer. Cell 149, 22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Yang S, Xu F, Zhang J (2015) Long noncoding RNA CCAT1 promotes hepatocellular carcinoma progression by functioning as let‐7 sponge. J Exp Clin Cancer Res 34, 18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S, Tilgner H, Guernec G, Martin D, Merkel A, Knowles DG et al (2012) The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res 22, 1775–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dews M, Fox JL, Hultine S, Sundaram P, Wang W, Liu YY, Furth E, Enders GH, El‐Deiry W, Schelter JM et al (2010) Myc ‐ miR‐17~92 axis blunts TGFβ signaling and production of multiple TGFβ‐dependent anti‐angiogenic factors. Cancer Res 70, 8233–8246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Zhang Y, Han L, Fu L, Mei X, Wang J, Itkow J, Elabid AEI, Pang L, Yu D (2018) Activating and sustaining c‐Myc by depletion of miR‐144/451 gene locus contributes to B‐lymphomagenesis. Oncogene 37, 1293–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doose G, Haake A, Bernhart SH, López C, Duggimpudi S, Wojciech F, Bergmann AK, Borkhardt A, Burkhardt B, Claviez A et al (2015) MINCR is a MYC‐induced lncRNA able to modulate MYC's transcriptional network in Burkitt lymphoma cells. Proc Natl Acad Sci U S A 112, 5261–5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doose G, Hoffmann S, Iaccarino I (2016) Reply to Hart et al.: MINCR and MYC: more than expression correlation. Proc Natl Acad Sci U S A 113, E498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst A, Campos B, Meier J, Devens F, Liesenberg F, Wolter M, Reifenberger G, Herold‐Mende C, Lichter P, Radlwimmer B (2010) De‐repression of CTGF via the miR‐17‐92 cluster upon differentiation of human glioblastoma spheroid cultures. Oncogene 29, 3411–3422. [DOI] [PubMed] [Google Scholar]
- Fang JH, Zhou HC, Zeng C, Yang J, Liu Y, Huang X, Zhang JP, Guan XY, Zhuang SM (2011) MicroRNA‐29b suppresses tumor angiogenesis, invasion, and metastasis by regulating matrix metalloproteinase 2 expression. Hepatology 54, 1729–1740. [DOI] [PubMed] [Google Scholar]
- Fei X, Qi M, Wu B, Song Y, Wang Y and Li T (2012) MicroRNA‐195‐5p suppresses glucose uptake and proliferation of human bladder cancer T24 cells by regulating GLUT3 expression. FEBS Lett 586, 392–397. [DOI] [PubMed] [Google Scholar]
- Feng M, Li Z, Aau M, Wong CH, Yang X and Yu Q (2011) Myc/miR‐378/TOB2/cyclin D1 functional module regulates oncogenic transformation. Oncogene 30, 2242–2251. [DOI] [PubMed] [Google Scholar]
- Fox JL, Dews M, Minn AJ and Thomas‐Tikhonenko A (2013) Targeting of TGFβ signature and its essential component CTGF by miR‐18 correlates with improved survival in glioblastoma. RNA 19, 177–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Liu X, Zhou N, Du L, Sun Y, Zhang X, Ge Y (2014) MicroRNA‐200b stimulates tumour growth in TGFBR2‐null colorectal cancers by negatively regulating p27/kip1. J Cell Physiol 229, 772–782. [DOI] [PubMed] [Google Scholar]
- Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T, Zeller KI, De Marzo AM, Van Eyk JE, Mendell JT et al (2009) c‐Myc suppression of miR‐23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature 458, 762–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X‐S, Ma H‐J, Zheng X‐H, Ruan H‐L, Liao X‐Y, Xue W‐Q, Chen Y‐B, Zhang Y, Jia W‐H (2013) HOTAIR, a prognostic factor in esophageal squamous cell carcinoma, inhibits WIF‐1 expression and activates Wnt pathway. Cancer Sci 104, 1675–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong C, Li Z, Ramanujan K, Clay I, Zhang Y, Lemire‐Brachat S, Glass DJ (2015) A long non‐coding RNA, LncMyoD, regulates skeletal muscle differentiation by blocking IMP2‐mediated mRNA translation. Dev Cell 34, 181–191. [DOI] [PubMed] [Google Scholar]
- Hahn S, Jackstadt R, Siemens H, Hünten S and Hermeking H (2013) SNAI1 and miR‐34a feed‐forward regulation of ZNF281/ZBP99 promotes epithelial–mesenchymal transition. EMBO J 32, 3079–3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H, Li W, Shen H, Zhang J, Zhu Y and Li Y (2016) microRNA‐129‐5p, a c‐Myc negative target, affects hepatocellular carcinoma progression by blocking the Warburg effect. J Mol Cell Biol 8, 400–410. [DOI] [PubMed] [Google Scholar]
- Han H, Sun D, Li W, Shen H, Zhu Y, Li C, Chen Y, Lu L, Li W, Zhang J et al (2013) A c‐Myc‐MicroRNA functional feedback loop affects hepatocarcinogenesis. Hepatology 57, 2378–2389. [DOI] [PubMed] [Google Scholar]
- Hart JR, Roberts TC, Weinberg MS, Morris KV and Vogt PK (2014) MYC regulates the non‐coding transcriptome. Oncotarget 5, 12543–12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart JR, Weinberg MS, Morris KV, Roberts TC, Janda KD, Garner AL, Vogt PK (2016) MINCR is not a MYC‐induced lncRNA. Proc Natl Acad Sci U S A 113, E496–E497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, He X, Lim LP, de Stanchina E, Xuan Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D et al (2007) A microRNA component of the p53 tumour suppressor network. Nature 447, 1130–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Tan X, Wang X, Jin H, Liu L, Ma L, Yu H, Fan Z (2014) C‐Myc‐activated long noncoding RNA CCAT1 promotes colon cancer cell proliferation and invasion. Tumor Biol 35, 12181–12188. [DOI] [PubMed] [Google Scholar]
- He L, Thomson JM, Hemann MT, Hernando‐Monge E, Mu D, Goodson S, Powers S, Cordon‐Cardo C, Lowe SW, Hannon GJ et al (2005) A microRNA polycistron as a potential human oncogene. Nature 435, 828–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann I (2000) The role of Cdc25 phosphatases in cell cycle checkpoints. Protoplasma 211, 8–11. [Google Scholar]
- Hu T and Lu YR (2015) BCYRN1, a c‐MYC‐activated long non‐coding RNA, regulates cell metastasis of non‐small‐cell lung cancer. Cancer Cell Int 15, 36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua Z, Lv Q, Ye W, Wong C‐KA, Cai G, Gu D, Ji Y, Zhao C, Wang J, Yang BB et al (2006) MiRNA‐directed regulation of VEGF and other angiogenic factors under hypoxia. PLoS One 1, e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Tian Y, Dong S, Cha Y, Li J, Guo X, Yuan X (2017) The long non‐coding RNA SNHG3 functions as a competing endogenous RNA to promote malignant development of colorectal cancer. Oncol Rep 38, 1402–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Weng H, Sun W, Qin X, Shi H, Wu H, Zhao BS, Mesquita A, Liu C, Yuan CL et al (2018) Recognition of RNA N6‐methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat Cell Biol 20, 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Zhang A, Ho TT, Zhang Z, Zhou N, Ding X, Zhang X, Xu M, Mo YY (2015) Linc‐RoR promotes c‐Myc expression through hnRNP I and AUF1. Nucleic Acid Res 44, 3059–3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung CL, Wang LY, Yu YL, Chen HW, Srivastava S, Petrovics G, Kung HJ (2014) A long noncoding RNA connects c‐Myc to tumor metabolism. Proc Natl Acad Sci U S A 111, 18697–18702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanovska I, Ball AS, Diaz RL, Magnus JF, Kibukawa M, Schelter JM, Kobayashi SV, Lim L, Burchard J, Jackson AL et al (2008) MicroRNAs in the miR‐106b family regulate p21/CDKN1A and promote cell cycle progression. Mol Cell Biol 28, 2167–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackstadt R, Jung P and Hermeking H (2013a) AP4 directly downregulates p16 and p21 to suppress senescence and mediate transformation. Cell Death Dis 15, e775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackstadt R, Röh S, Neumann J, Jung P, Hoffmann R, Horst D, Berens C, Bornkamm GW, Kirchner T, Menssen A et al (2013b) AP4 is a mediator of epithelial‐mesenchymal transition and metastasis in colorectal cancer. J Exp Med 210, 1331–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia P, Cai H, Liu X, Chen J, Ma J, Wang P, Liu Y, Zheng J, Xue Y (2016) Long non‐coding RNA H19 regulates glioma angiogenesis and the biological behavior of glioma‐associated endothelial cells by inhibiting microRNA‐29a. Cancer Lett 381, 359–369. [DOI] [PubMed] [Google Scholar]
- Jiang C, Long J, Liu B, Xie X, Kuang M (2015) Mcl‐1 is a novel target of miR‐26b that is associated with the apoptosis induced by TRAIL in HCC cells. Biomed Res Int 2015, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CD, Esquela‐Kerscher A, Stefani G, Byrom M, Kelnar K, Ovcharenko D, Wilson M, Wang X, Shelton J, Shingara J et al (2007) The let‐7 microRNA represses cell proliferation pathways in human cells. Cancer Res 67, 7713–7722. [DOI] [PubMed] [Google Scholar]
- Jung HM, Patel RS, Phillips BL, Wang H, Cohen DM, Reinhold WC, Chang LJ, Yang LJ, Chan EK (2013) Tumor suppressor miR‐375 regulates MYC expression via repression of CIP2A coding sequence through multiple miRNA–mRNA interactions. Mol Biol Cell 24, 1638–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkat M, De Melo J, Hickman K, Lourenco C, Redel C, Resetca D, Tamachi A, Tu W, Penn L (2017) MYC deregulation in primary human cancers. Genes 8, 151–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki Y, Komiya M, Matsumura K, Negishi L, Suda S, Okuno M, Yokota N, Osada T, Nagashima T, Hiyoshi M et al (2016) MYU, a target lncRNA for Wnt/c‐Myc signaling, mediates induction of CDK6 to promote cell cycle progression. Cell Rep 16, 2554–2564. [DOI] [PubMed] [Google Scholar]
- Kim T, Cui R, Jeon Y‐, Fadda P, Alder H, Croce CM (2015a) MYC‐repressed long noncoding RNAs antagonize MYC‐induced cell proliferation and cell cycle progression. Oncotarget 6, 18780–18789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T, Cui R, Jeon Y‐J, Lee J‐H, Lee JH, Sim H, Park JK, Fadda P, Tili E, Nakanishi H et al (2014) Long‐range interaction and correlation between MYC enhancer and oncogenic long noncoding RNA CARLo‐5. Proc Natl Acad Sci U S A 111, 4173–4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T, Jeon Y‐J, Cui R, Lee J‐H, Peng Y, Kim S‐H, Tili E, Alder H, Croce CM (2015b) Role of MYC‐regulated long noncoding RNAs in cell cycle regulation and tumorigenesis. J Natl Cancer Inst 107, dju505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HH, Kuwano Y, Srikantan S, Lee EK, Martindale JL and Gorospe M (2009) HuR recruits let‐7/RISC to repress c‐Myc expression. Genes Dev 23, 1743–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong YW, Cannell IG, de Moor CH, Hill K, Garside PG, Hamilton TL, Meijer HA, Dobbyn HC, Stoneley M, Spriggs KA et al (2008) The mechanism of micro‐RNA‐mediated translation repression is determined by the promoter of the target gene. Proc Natl Acad Sci U S A 105, 8866–8871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpal M, Lee ES, Hu G and Kang Y (2008) The miR‐200 family inhibits epithelial‐mesenchymal transition and cancer cell migration by direct targeting of E‐cadherin Transcriptional Repressors ZEB1 and ZEB2 . J Biol Chem 3, 14910–14914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota J, Chivukula RR, O'Donnell KA, Wentzel EA, Montgomery CL, Hwang HW, Chang TC, Vivekanandan P, Torbenson M, Clark KR et al (2009) Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell 137, 1005–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal A, Navarro F, Maher CA, Maliszewski LE, Yan N, O'Day E, Chowdhury D, Dykxhoorn DM, Tsai P, Hofmann O et al (2009) miR‐24 inhibits cell proliferation by targeting E2F2, MYC, and other cell‐cycle genes via binding to “seedless” 3′UTR microRNA recognition elements. Mol Cell 35, 610–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DY, Deng Z, Wang C and Yang BB (2007) MicroRNA‐378 promotes cell survival, tumor growth, and angiogenesis by targeting SuFu and Fus‐1 expression. Proc Natl Acad Sci U S A 104, 20350–20355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS and Dutta A (2007) The tumor suppressor microRNA let‐7 represses the HMGA2 oncogene. Genes Dev 21, 1025–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legesse‐Miller A, Elemento O, Pfau SJ, Forman JJ, Tavazoie S, Coller HA (2009) let‐7 Overexpression leads to an increased fraction of cells in G2/M, direct down‐regulation of Cdc34, and stabilization of Wee1 kinase in primary fibroblasts. J Biol Chem 284, 6605–6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Cai B, Shen L, Dong Y, Lu Q, Sun S, Liu S, Ma S, Ma PX, Chen J (2017b) MiRNA‐29b suppresses tumor growth through simultaneously inhibiting angiogenesis and tumorigenesis by targeting Akt3. Cancer Lett 397, 111–119. [DOI] [PubMed] [Google Scholar]
- Li M, Chen H, Zhao Y, Gao S and Cheng C (2016) H19 functions as a ceRNA in promoting metastasis through decreasing miR‐200s activity in osteosarcoma. DNA Cell Biol 35, 235–240. [DOI] [PubMed] [Google Scholar]
- Li L, Kang L, Zhao W, Feng Y, Liu W, Wang T, Mai H, Huang J, Chen S, Liang Y et al (2017a) miR‐30a‐5p suppresses breast tumor growth and metastasis through inhibition of LDHA‐mediated Warburg effect. Cancer Lett 400, 89–98. [DOI] [PubMed] [Google Scholar]
- Li X, Sanda T, Look AT, Novina CD and von Boehmer H (2011) Repression of tumor suppressor miR‐451 is essential for NOTCH1‐induced oncogenesis in T‐ALL. J Exp Med 208, 663–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang WC, Fu WM, Wong CW, Wang Y, Wang WM, Hu GX, Zhang L, Xiao LJ, Wan DC, Zhang JF et al (2015) The lncRNA H19 promotes epithelial to mesenchymal transition by functioning as miRNA sponges in colorectal cancer. Oncotarget 6, 22513–22525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao J, Liu H (2011) Autoregulatory suppression of c‐Myc by miR‐185‐3p. J Biol Chem 286, 33901–33909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Ding R, Zheng S, Xing D, Hong W, Zhou Z, Shen J (2014) Decrease expression of microRNA‐744 promotes cell proliferation by targeting c‐Myc in human hepatocellular carcinoma. Cancer Cell Int 14, 58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CJ, Gong H, Tseng H, Wang W, Wu J (2008) miR‐122 targets an anti‐apoptotic gene, Bcl‐w, in human hepatocellular carcinoma cell lines. Biochem Biophys Res Comm 375, 315–320. [DOI] [PubMed] [Google Scholar]
- Ling H, Spizzo R, Atlasi Y, Nicoloso M, Shimizu M, Redis RS, Nishida N, Gafà R, Song J, Guo Z et al (2013) CCAT2, a novel noncoding RNA mapping to 8q24, underlies metastatic progression and chromosomal instability in colon cancer. Genome Res 23, 1446–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Dong L, Liu Y, Wen D, Gao D, Sun H, Fan J, Wu W (2016) A c‐Myc/miR‐17‐5p feedback loop regulates metastasis and invasion of hepatocellular carcinoma. Tumor Biol 37, 5039–5047. [DOI] [PubMed] [Google Scholar]
- Liu Y, Huang H, Liu M, Wu Q, Li W and Zhang J (2017) MicroRNA‐24‐1 suppresses mouse hepatoma cell invasion and metastasis via directly targeting O‐GlcNAc transferase. Biomed Pharmacother 91, 731–738. [DOI] [PubMed] [Google Scholar]
- Liu K, Li L, Rusidanmu A, Wang Y and Lv X (2015a) Down‐regulation of MiR‐1294 is related to dismal prognosis of patients with esophageal squamous cell carcinoma through elevating C‐MYC expression. Cell Physiol Biochem 36, 100–110. [DOI] [PubMed] [Google Scholar]
- Liu Z, Sun M, Lu K, Liu J, Zhang M, Wu W, De W, Wang Z, Wang R (2013) The long noncoding RNA HOTAIR contributes to cisplatin resistance of human lung adenocarcinoma cells via downregulation of p21WAF1/CIP1 expression. PLoS One 8, e77293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Sun M, Xia R, Zhang E, Liu X, Zhang Z, Xu T, De W, Liu B, Wang Z (2015b) LincHOTAIR epigenetically silences miR34a by binding to PRC2 to promote the epithelial‐to‐mesenchymal transition in human gastric cancer. Cell Death Dis 6, e1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Wu G, Lin C, Guo H, Xu J and Zhao T (2018) IGF2BP1 over‐expression in skin squamous cell carcinoma cells is essential for cell growth. Biochem Biophys Res Comm 501, 731–738. [DOI] [PubMed] [Google Scholar]
- Liu B, Wu X, Liu B, Wang C, Liu Y, Zhou Q, Xu K (2012) MiR‐26a enhances metastasis potential of lung cancer cells via AKT pathway by targeting PTEN. Biochem Biophys Acta 1822, 1692–1704. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zhang G, Li J, Liu J and Lv P (2014) The tumor‐suppressive microRNA‐135b targets c‐Myc in osteosarcoma. PLoS One 9, e102621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- López‐Urrutia E, Coronel‐Hernández J, García‐Castillo V, Contreras‐Romero C, Martínez‐Gutierrez A, Estrada‐Galicia D, Terrazas LI, López‐Camarillo C, Maldonado‐Martínez H, Jacobo‐Herrera N et al (2017) MiR‐26a downregulates retinoblastoma in colorectal cancer. Tumor Biol 39, 1010428317695945. [DOI] [PubMed] [Google Scholar]
- Lu Y, Hu Z, Mangala LS, Stine ZE, Hu X, Jiang D, Xiang Y, Zhang Y, Pradeep S, Rodriguez‐Aguayo C et al (2018) MYC targeted long noncoding RNA DANCR promotes cancer in part by reducing p21 levels. Cancer Res 78, 64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan W, Zhou Z, Ni X, Xia Y, Wang J, Yan Y, Xu B (2018) Long non‐coding RNA H19 promotes glucose metabolism and cell growth in malignant melanoma via miR‐106a‐5p/E2F3 axis. J Cancer Res Clin Oncol 144, 531–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Li Z, Wang W, Zeng Y, Liu Z and Qiu J (2013) Long non‐coding RNA H19 increases bladder cancer metastasis by associating with EZH2 and inhibiting E‐cadherin expression. Cancer Lett 333, 213–221. [DOI] [PubMed] [Google Scholar]
- Luo L, Tang H, Ling L, Li N, Jia X, Zhang Z, Wang X, Shi L, Yin J, Qiu N et al (2018) LINC01638 lncRNA activates MTDH‐Twist1 signaling by preventing SPOP‐mediated c‐Myc degradation in triple‐negative breast cancer. Oncogene 37, 6166–6179. [DOI] [PubMed] [Google Scholar]
- Lv Z, Wei J, You W, Wang R, Shang J, Xiong Y, Yang H, Yang X, Fu Z (2017) Disruption of the c–Myc/miR–200b–3p/PRDX2 regulatory loop enhances tumor metastasis and chemotherapeutic resistance in colorectal cancer. J Transl Med 15, 257–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Li C, Zhang Y, Weng M, Zhang M, Qin Y, Gong W, Quan Z (2014) Long non‐coding RNA HOTAIR, a c‐Myc activated driver of malignancy, negatively regulates miRNA‐130a in gallbladder cancer. Mol Cancer 13, 156–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Young J, Prabhala H, Pan E, Mestdagh P, Muth D, Teruya‐Feldstein J, Reinhardt F, Onder TT, Valastyan S et al (2010) miR‐9, a MYC/MYCN‐activated microRNA, regulates E‐cadherin and cancer metastasis. Nat Cell Biol 12, 247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Zhou G, Li M, Hu D, Zhang L, Liu P, Lin K (2018) Long noncoding RNA DANCR mediates cisplatin resistance in glioma cells via activating AXL/PI3K/Akt/NF‐κB signaling pathway. Neurochem Int 118, 233–241. [DOI] [PubMed] [Google Scholar]
- MacKenzie ED, Selak MA, Tennant DA, Payne LJ, Crosby S, Frederiksen CM, Watson DG, Gottlieb E (2007) Cell‐permeating alpha‐ketoglutarate derivatives alleviate pseudohypoxia in succinate dehydrogenase‐deficient cells. Mol Cell Biol 27, 3282–3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldotti M, Incarnato D, Neri F, Krepelova A, Rapelli S, Anselmi F, Parlato C, Basile G, Dettori D, Calogero R et al (2016) The long intergenic non‐coding RNA CCR492 functions as a let‐7 competitive endogenous RNA to regulate c‐Myc expression. Biochem Biophys Acta 1859, 1322–1332. [DOI] [PubMed] [Google Scholar]
- Marchese FP, Grossi E, Marín‐Béjar O, Bharti SK, Raimondi I, González J, Martínez‐Herrera DJ, Athie A, Amadoz A, Brosh RM Jr et al (2016) A long noncoding RNA regulates sister chromatid cohesion. Mol Cell 63, 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavrakis KJ, Wolfe AL, Oricchio E, Palomero T, de Keersmaecker K, McJunkin K, Zuber J, James T, Khan AA, Leslie CS et al (2010) Genome‐wide RNAi screen identifies miR‐19 targets in Notch‐induced acute T‐cell leukaemia (T‐ALL). Nat Cell Biol 12, 372–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr C, Hemann MT and Bartel DP (2007) Disrupting the pairing between let‐7 and Hmga2 enhances oncogenic transformation. Science 315, 1576–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao L‐J, Huang S‐F, Sun Z‐T, Gao Z‐Y, Zhang R, Liu Y, Wang J (2013) MiR‐449c targets c‐Myc and inhibits NSCLC cell progression. FEBS Lett 587, 1359–1365. [DOI] [PubMed] [Google Scholar]
- Mogilyansky E and Rigoutsos I (2013) The miR‐17/92 cluster: a comprehensive update on its genomics, genetics, functions and increasingly important and numerous roles in health and disease. Cell Death Differ 20, 1603–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott JL, Kobayashi S, Bronk SF and Gores GJ (2007) mir‐29 regulates Mcl‐1 protein expression and apoptosis. Oncogene 26, 6133–6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu P, Han Y‐C, Betel D, Yao E, Squatrito M, Ogrodowski P, de Stanchina E, D'Andrea A, Sander C, Ventura A (2009) Genetic dissection of the miR‐17∼92 cluster of microRNAs in Myc‐induced B‐cell lymphomas. Genes Dev 23, 2806–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli S, Piccinelli V, Mapelli SN, Pisignano G and Catapano CV (2017) Natural antisense transcripts drive a regulatory cascade controlling c‐MYC transcription. RNA Biol 14, 1742–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell KA, Wentzel EA, Zeller KI, Dang CV and Mendell JT (2005) c‐Myc‐regulated microRNAs modulate E2F1 expression. Nature 435, 839–843. [DOI] [PubMed] [Google Scholar]
- Olive V, Bennett MJ, Walker JC, Ma C, Jiang I, Cordon‐Cardo C, Li QJ, Lowe SW, Hannon GJ, He L (2009) miR‐19 is a key oncogenic component of mir‐17‐92 . Genes Dev 23, 2839–2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Gaur AB, Lengyel E, Peter ME (2008) The miR‐200 family determines the epithelial phenotype of cancer cells by targeting the E‐cadherin repressors ZEB1 and ZEB2. Genes Dev 22, 894–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Hou F, Feng J, Xu S and Meng X (2018) Long non‐coding RNA BCYRN1 promotes the proliferation and metastasis of cervical cancer via targeting microRNA‐138 in vitro and in vivo . Oncol Lett 15, 5809–5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng F, Jiang J, Yu Y, Tian R, Guo X, Li X, Shen M, Xu M, Zhu F, Shi C et al (2013) Direct targeting of SUZ12/ROCK2 by miR‐200b/c inhibits cholangiocarcinoma tumourigenesis and metastasis. Br J Cancer 109, 3092–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng F, Li T‐T, Wang K‐L, Xiao G‐Q, Wang J‐H, Zhao H‐D, Kang Z‐J, Fan W‐J, Zhu L‐L, Li M et al (2017) H19/let‐7/LIN28 reciprocal negative regulatory circuit promotes breast cancer stem cell maintenance. Cell Death Dis 8, e2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrocca F, Vecchione A and Croce CM (2008) Emerging role of miR‐106b‐25/miR‐17‐92 clusters in the control of transforming growth factor B signaling. Cancer Res 68, 8191–8194. [DOI] [PubMed] [Google Scholar]
- Pok S, Wen V, Shackel N, Alsop A, Pyakurel P, Fahrer A, Farrell GC, Teoh NC (2013) Cyclin E facilitates dysplastic hepatocytes to bypass G1/S checkpoint in hepatocarcinogenesis. J Gastroenterol Hepatol 28, 1545–1554. [DOI] [PubMed] [Google Scholar]
- Poyyakkara A, Raji GR, Kunhiraman H, Edatt L and Kumar SVB (2018) ER stress mediated regulation of miR23a confer Hela cells better adaptability to utilize glycolytic pathway. J Cell Biochem 119, 4907–4917. [DOI] [PubMed] [Google Scholar]
- Prensner JR, Chen W, Han S, Iyer MK, Cao Q, Kothari V, Evans JR, Knudsen KE, Paulsen MT, Ljungman M et al (2014) The long non‐coding RNA PCAT‐1 promotes prostate cancer cell proliferation through cMyc. Neoplasia 16, 900–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prensner JR, Iyer MK, Balbin OA, Dhanasekaran SM, Cao Q, Brenner JC, Laxman B, Asangani IA, Grasso CS, Kominsky HD et al (2011) Transcriptome sequencing across a prostate cancer cohort identifies PCAT‐1, an unannotated lincRNA implicated in disease progression. Nat Biotechnol 29, 742–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertus J‐L, Kluiver J, Weggemans C, Harms G, Reijmers RM, Swart Y, Kok K, Rosati S, Schuuring E, Van Imhoff G et al (2010) MiRNA profiling in B non‐Hodgkin lymphoma: a MYC‐related miRNA profile characterizes Burkitt lymphoma. Br J Haematol 149, 896–918. [DOI] [PubMed] [Google Scholar]
- Rogler CE, Levoci L, Ader T, Massimi A, Tchaikovskaya T, Norel R, Rogler LE (2009) MicroRNA‐23b cluster microRNAs regulate transforming growth factor‐beta/bone morphogenetic protein signaling and liver stem cell differentiation by targeting Smads. Hepatology 50, 575–584. [DOI] [PubMed] [Google Scholar]
- Sachdeva M, Zhu S, Wu F, Wu H, Walia V, Kumar S, Elble R, Watabe K, Mo YY (2009) p53 represses c‐Myc through induction of the tumor suppressor miR‐145. Proc Natl Acad Sci U S A 106, 3207–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson VB, Rong NH, Han J, Yang Q, Aris V, Soteropoulos P, Petrelli NJ, Dunn SP, Krueger LJ (2007) MicroRNA let‐7a down‐regulates MYC and reverts MYC‐induced growth in Burkitt lymphoma cells. Cancer Res 67, 9762–9770. [DOI] [PubMed] [Google Scholar]
- Schmitt AM and Chang HY (2016) Long noncoding RNAs in cancer pathways. Cancer Cell 29, 452–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Liang X, Sun H, De Hoyos CL and Crooke ST (2017) Depletion of NEAT1 lncRNA attenuates nucleolar stress by releasing sequestered P54nrb and PSF to facilitate c‐Myc translation. PLoS One 12, e0173494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F, Fu H, Liu Q, Tie Y, Zhu J, Xing R, Sun Z, Zheng X (2008) Downregulation of CCND1 and CDK6 by miR‐34a induces cell cycle arrest. FEBS Lett 582, 1564–1568. [DOI] [PubMed] [Google Scholar]
- Sylvestre Y, De Guire V, Querido E, Mukhopadhyay UK, Bourdeau V, Major F, Ferbeyre G, Chartrand P (2007) An E2F/miR‐20a autoregulatory feedback loop. J Biol Chem 282, 2135–2143. [DOI] [PubMed] [Google Scholar]
- Takwi AA, Li Y, Becker Buscaglia LE, Zhang J, Choudhury S, Park AK, Liu M, Young KH, Park WY, Martin RC et al (2012) A statin‐regulated microRNA represses human c‐Myc expression and function. EMBO Mol Med 4, 896–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tania M, Khan MA and Fu J (2014) Epithelial to mesenchymal transition inducing transcription factors and metastatic cancer. Tumor Biol 35, 7335–7342. [DOI] [PubMed] [Google Scholar]
- Tanzer A and Stadler PF (2004) Molecular evolution of a microRNA cluster. J Mol Biol 339, 327–335. [DOI] [PubMed] [Google Scholar]
- Tran DDH, Kessler C, Niehus SE, Mahnkopf M, Koch A and Tamura T (2017) Myc target gene, long intergenic noncoding RNA, Linc00176 in hepatocellular carcinoma regulates cell cycle and cell survival by titrating tumor suppressor microRNAs. Oncogene 37, 75–85. [DOI] [PubMed] [Google Scholar]
- Trompeter HI, Abbad H, Iwaniuk KM, Hafner M, Renwick N, Tuschl T, Schira J, Müller HW, Wernet P (2011) MicroRNAs MiR‐17, MiR‐20a, and MiR‐106b Act in concert to modulate E2F activity on cell cycle arrest during neuronal lineage differentiation of USSC. PLoS One 6, e16138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng YY, Moriarity BS, Gong W, Akiyama R, Tiwari A, Kawakami H, Ronning P, Reuland B, Guenther K, Beadnell TC et al (2014) PVT1 dependence in cancer with MYC copy‐number increase. Nature 512, 82–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu WB, Helander S, Pilstål R, Hickman KA, Lourenco C, Jurisica I, Raught B, Wallner B, Sunnerhagen M, Penn LZ (2015) Myc and its interactors take shape. Biochem Biophys Acta 1849, 469–483. [DOI] [PubMed] [Google Scholar]
- Wang H, Cao F, Li X, Miao H, E J, Xing J, Fu C (2015) miR‐320b suppresses cell proliferation by targeting c‐Myc in human colorectal cancer cells. BMC Cancer 15, 748–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Fu X, Zhou Y, Zhang Y (2009) Down‐regulation of the cyclin E1 oncogene expression by microRNA‐16‐1 induces cell cycle arrest in human cancer cells. BMB Rep 42, 725–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Hsu SH, Wang X, Kutay H, Bid HK, Yu J, Ganju RK, Jacob ST, Yuneva M, Ghoshal K (2014) Reciprocal regulation of microRNA‐122 and c‐Myc in hepatocellular cancer: role of E2F1 and transcription factor dimerization partner 2. Hepatology 59, 555–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Wang Y, Ma J, Wang W, Sun B, Zheng T, Wei M, Sun Y (2017a) MicroRNA‐20a participates in the aerobic exercise‐based prevention of coronary artery disease by targeting PTEN. Biomed Pharmacother 95, 756–763. [DOI] [PubMed] [Google Scholar]
- Wang O, Yang F, Liu Y, Lv L, Ma R, Chen C, Wang J, Tan Q, Cheng Y, Xia E et al (2017b) C‐MYC‐induced upregulation of lncRNA SNHG12 regulates cell proliferation, apoptosis and migration in triple‐negative breast cancer. Am J Transl Res 9, 533–545. [PMC free article] [PubMed] [Google Scholar]
- Wang S‐H, Yang Y, Wu X‐C, Zhang M‐D, Weng M‐Z, Zhou D, Wang J‐D, Quan Z‐W (2016) Long non‐coding RNA MINCR promotes gallbladder cancer progression through stimulating EZH2 expression. Cancer Lett 380, 122–133. [DOI] [PubMed] [Google Scholar]
- Wang Z, Yang B, Zhang M, Guo W, Wu Z, Wang Y, Jia L, Li S; Cancer Genome Atlas Research Network , Xie W et al (2018) lncRNA epigenetic landscape analysis identifies EPIC1 an oncogenic lncRNA that interacts with MYC and promotes cell‐cycle progression in cancer. Cancer Cell 33, 706–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner KD, Hajdin CE, Weeks KM (2018) Principles for targeting RNA with drug‐like small molecules. Nat Rev Drug Discovery 17, 547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkle M, van den Berg A, Tayari M, Sietzema J, Terpstra M, Kortman G, de Jong D, Visser L, Diepstra A, Kok K et al (2015) Long noncoding RNAs as a novel component of the Myc transcriptional network. FASEB J 29, 2338–2346. [DOI] [PubMed] [Google Scholar]
- Wong C‐M, Wei L, Au L‐KS, Fan N‐YD, Zhou Y, Tsang H‐CF, Law C‐T, Lee M‐FJ, He X, Shi J et al (2015) MiR‐200b/200c/429 subfamily negatively regulates Rho/ROCK signaling pathway to suppress hepatocellular carcinoma metastasis. Oncotarget 6, 13658–13670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods K, Thomson JM and Hammond SM (2007) Direct regulation of an oncogenic Micro‐RNA cluster by E2F transcription factors. J Biol Chem 282, 2130–2134. [DOI] [PubMed] [Google Scholar]
- Xiang S, Gua H, Lei Jin L, Thorne RF, Zhang XD and Wua M (2018) LncRNA IDH1‐AS1 links the functions of c‐Myc and HIF1α via IDH1 to regulate the Warburg effect. Proc Natl Acad Sci U S A 115, 1465–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang J‐F, Yin Q‐F, Chen T, Zhang Y, Zhang X‐O, Wu Z, Zhang S, Wang H‐B, Ge J, Lu X et al (2014) Human colorectal cancer‐specific CCAT1‐L lncRNA regulates long‐range chromatin interactions at the MYC locus. Cell Res 24, 513–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z‐D, Han L, Lee H, Zhuang L, Zhang Y, Baddour J, Nagrath D, Wood CG, Gu J, Wu X et al (2017) Energy stress‐induced lncRNA FILNC1 represses c‐Myc‐mediated energy metabolism and inhibits renal tumor development. Nat Commun 8, 783–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Srinivasan L, Calado DP, Patterson HC, Zhang B, Wang J, Henderson JM, Kutok JL, Rajewsky K (2008) Lymphoproliferative disease and autoimmunity in mice with elevated miR‐17–92 expression in lymphocytes. Nat Immunol 9, 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J, Du Q and Liang Z (2010) Tumor‐suppressive microRNA‐22 inhibits the transcription of E‐box‐containing c‐Myc target genes by silencing c‐Myc binding protein. Oncogene 29, 4980–4988. [DOI] [PubMed] [Google Scholar]
- Xiong J, Wei B, Ye Q, Liu W (2016) MiR‐30a‐5p/UBE3C axis regulates breast cancer cell proliferation and migration. Biochem Biophys Res Comm pii: S0006‐291X(16)30381‐3. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Xu T, Zhu Y, Xiong Y, Ge YY, Yun JP and Zhuang SM (2009) MicroRNA‐195 suppresses tumorigenicity and regulates G1/S transition of human hepatocellular carcinoma cells. Hepatology 50, 113–121. [DOI] [PubMed] [Google Scholar]
- Yang X, Liang L, Zhang X‐F, Jia H‐L, Qin Y, Zhu X‐C, Gao X‐M, Qiao P, Zheng Y, Sheng Y‐Y et al (2013b) MicroRNA‐26a suppresses tumor growth and metastasis of human hepatocellular carcinoma by targeting interleukin‐6‐Stat3 pathway. Hepatology 58, 158–170. [DOI] [PubMed] [Google Scholar]
- Yang W, Ning N and Jin X (2017) The lncRNA H19 promotes cell proliferation by competitively binding to miR‐200a and Derepressing β‐catenin expression in colorectal cancer. Biomed Res Int 2017, 2767484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Xue X, Bi J, Zheng L, Zhi K, Gu Y, Fang G (2013a) Long noncoding RNA CCAT1, which could be activated by c‐Myc, promotes the progression of gastric carcinoma. J Cancer Res Clin Oncol 139, 437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Xue X, Zheng L, Bi J, Zhou Y, Zhi K, Gu Y, Fang G (2014) Long non‐coding RNA GHET1 promotes gastric carcinoma cell proliferation by increasing c‐Myc mRNA stability. FEBS J 281, 802–813. [DOI] [PubMed] [Google Scholar]
- Yang X, Ye H, He M, Zhou X, Sun N, Guo W, Lin X, Huang H, Lin Y, Yao R et al (2018) LncRNA PDIA3P interacts with c‐Myc to regulate cell proliferation via induction of pentose phosphate pathway in multiple myeloma. Biochem Biophys Res Comm 498, 207–213. [DOI] [PubMed] [Google Scholar]
- Ye X, Huang H, Huang W and Hu W (2018) LncRNA THOR promotes human renal cell carcinoma cell growth. Biochem Biophys Res Comm 501, 661–667. [DOI] [PubMed] [Google Scholar]
- Yu Z, Wang C, Wang M, Li Z, Casimiro MC, Liu M, Wu K, Whittle J, Ju X, Hyslop T et al (2008) A cyclin D1/microRNA 17/20 regulatory feedback loop in control of breast cancer cell proliferation. J Cell Biol 182, 509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Cao L, Fan P, Mei Y and Wu M (2016a) LncRNA‐MIF, a c‐Myc‐activated long non‐coding RNA, suppresses glycolysis by promoting Fbxw7‐mediated c‐Myc degradation. EMBO Rep 17, 1204–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Chen X, Lin J, Lwin T, Wright G, Moscinski LC, Dalton WS, Seto E, Wright K, Sotomayor E et al (2012a) Myc represses miR‐15a/miR‐16‐1 expression through recruitment of HDAC3 in mantle cell and other non‐Hodgkin B‐cell lymphomas. Oncogene 31, 3002–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhao X, Fiskus W, Lin J, Lwin T, Rao R, Zhang Y, Chan JC, Fu K, Marquez VE et al (2012b) Coordinated silencing of MYC‐mediated miR‐29 by HDAC3 and EZH2 as a therapeutic target of histone modification in aggressive B‐cell lymphomas. Cancer Cell 22, 506–523. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhang Z, Zhou C, Chang Y, Zhang Z, Hu Y, Zhang F, Lu Y, Zheng L, Zhang W, Li X et al (2016b) Long non‐coding RNA CASC11 interacts with hnRNP‐K and activates the WNT/β‐catenin pathway to promote growth and metastasis in colorectal cancer. Cancer Lett 376, 62–73. [DOI] [PubMed] [Google Scholar]
- Zhao J and Cheng L (2017) Long non‐coding RNA CCAT1/miR‐148a axis promotes osteosarcoma proliferation and migration through regulating PIK3IP1. Acta Biochim Biophys Sin 49, 503–512. [DOI] [PubMed] [Google Scholar]
- Zhao J‐J, Lin J, Lwin T, Yang H, Guo J, Kong W, Dessureault S, Moscinski LC, Rezania D, Dalton WS et al (2010) MicroRNA expression profile and identification of miR‐29 as a prognostic marker and pathogenetic factor by targeting CDK6 in mantle cell lymphoma. Blood 115, 2630–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Lwin T, Zhang X, Huang A, Wang J, Marquez VE, Chen‐Kiang S, Dalton WS, Sotomayor E, Tao J (2013) Disruption of the MYC‐miRNA‐EZH2 loop to suppress aggressive B‐cell lymphoma survival and clonogenicity. Leukemia 27, 2341–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen Y, Liu Z, Yang H, Yu X, Wu Q, Hua S, Long X, Jiang Q, Song Y, Cheng C et al (2013) Tumor suppressor PDCD4 modulates miR‐184‐mediated direct suppression of C‐MYC and BCL2 blocking cell growth and survival in nasopharyngeal carcinoma. Cell Death Dis 4, e872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Han LR, Zhou YX and Li Y (2016) MiR‐195 suppresses cervical cancer migration and invasion through targeting Smad3. Int J Gynecol Cancer 26, 817–824. [DOI] [PubMed] [Google Scholar]
- Zhou W, Ye X, Xu J, Cao M‐G, Fang Z‐Y, Li L‐Y, Guan G‐H, Liu Q, Qian Y‐H, Xie D (2017) The lncRNA H19 mediates breast cancer cell plasticity during EMT and MET plasticity by differentially sponging miR‐200b/c and let‐7b. Sci Signal 10, eaak9557. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Lu Y, Zhang Q, Liu J‐J, Li T‐J, Yang J‐R, Zeng C, Zhuang S‐M (2012) MicroRNA‐26a/b and their host genes cooperate to inhibit the G1/S transition by activating the pRb protein. Nucleic Acid Res 40, 4615–4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang G, Wu X, Jiang Z, Kasman I, Yao J, Guan Y, Oeh J, Modrusan Z, Bais C, Sampath D et al (2012) Tumour‐secreted miR‐9 promotes endothelial cell migration and angiogenesis by activating the JAK‐STAT pathway. EMBO J 31, 3513–3523. [DOI] [PMC free article] [PubMed] [Google Scholar]


