Abstract
BACKGROUND
Younger children in a school grade cohort may be more likely to receive a diagnosis of attention deficit–hyperactivity disorder (ADHD) than their older peers because of age-based variation in behavior that may be attributed to ADHD rather than to the younger age of the children. Most U.S. states have arbitrary age cutoffs for entry into public school. Therefore, within the same grade, children with birthdays close to the cutoff date can differ in age by nearly 1 year.
METHODS
We used data from 2007 through 2015 from a large insurance database to compare the rate of ADHD diagnosis among children born in August with that among children born in September in states with and states without the requirement that children be 5 years old by September 1 for enrollment in kindergarten. ADHD diagnosis was determined on the basis of diagnosis codes from the International Classification of Diseases, 9th Revision. We also used prescription records to compare ADHD treatment between children born in August and children born in September in states with and states without the cutoff date of September 1.
RESULTS
The study population included 407,846 children in all U.S. states who were born in the period from 2007 through 2009 and were followed through December 2015. The rate of claims-based ADHD diagnosis among children in states with a September 1 cutoff was 85.1 per 10,000 children (309 cases among 36,319 children; 95% confidence interval [CI], 75.6 to 94.2) among those born in August and 63.6 per 10,000 children (225 cases among 35,353 children; 95% CI, 55.4 to 71.9) among those born in September, an absolute difference of 21.5 per 10,000 children (95% CI, 8.8 to 34.0); the corresponding difference in states without the September 1 cutoff was 8.9 per 10,000 children (95% CI, −14.9 to 20.8). The rate of ADHD treatment was 52.9 per 10,000 children (192 of 36,319 children; 95% CI, 45.4 to 60.3) among those born in August and 40.4 per 10,000 children (143 of 35,353 children; 95% CI, 33.8 to 47.1) among those born in September, an absolute difference of 12.5 per 10,000 children (95% CI, 2.43 to 22.4). These differences were not observed for other month-to-month comparisons, nor were they observed in states with non-September cutoff dates for starting kindergarten. In addition, in states with a September 1 cutoff, no significant differences between August-born and September-born children were observed in rates of asthma, diabetes, or obesity.
CONCLUSIONS
Rates of diagnosis and treatment of ADHD are higher among children born in August than among children born in September in states with a September 1 cutoff for kindergarten entry. (Funded by the National Institutes of Health.)
Variable rates of diagnosis and treatment of attention deficit–hyperactivity disorder (ADHD) across the United States have aroused concern about overdiagnosis, under-diagnosis, and appropriate treatment.1–6 Over the past two decades, rates of diagnosis and treatment of ADHD have increased. Among children 2 to 5 years of age, the rate of diagnosis increased by more than 50% from 2007 to 2012.7 In 2016, 5.2% of all children 2 to 17 years of age in the United States were taking medication to treat ADHD.8
The increasing rates and the geographic variation in the prevalence of ADHD have generated controversy about the basis of clinical diagnosis and treatment of the disorder.2–6 The main factor that may contribute to the probability that a child will receive a diagnosis of ADHD is the child’s behavior, as observed by teachers or parents, relative to the behavior of other children in the same school grade. Some evidence suggests that teachers or school personnel are more likely than parents or physicians to first suggest a diagnosis of ADHD.9 In the classroom setting, teachers and parents may presume that all children should behave in a similar manner; therefore, behavior that appears to be anomalous relative to the behavior of other children in the same peer group stands out. In any classroom, there is likely to be a range of normal behavior among the children, given the 12-month span of ages within a grade. It is possible that younger children within a grade cohort may be more likely to receive a diagnosis of ADHD than older children in the same grade because inattentive behavior that is developmentally determined may be attributed to ADHD rather than to younger age.
Previous studies in the United States and elsewhere have suggested that younger children within a grade cohort are more likely to receive a diagnosis of and treatment for ADHD than older children in the same cohort.10–14 These studies were based on data that are more than a decade old and relied on survey responses, which may be vulnerable to recall bias. To study whether the rates of ADHD diagnosis and treatment differ among otherwise similar children who have been arbitrarily assigned to different grade cohorts, we made use of the fact that many state public school systems require that children be 5 years old before September 1 to be eligible to start kindergarten during that year. As a consequence, children born shortly after September 1 are arbitrarily assigned to grade cohorts in which they are the oldest children in their class, and children born shortly before September 1 are assigned to grade cohorts in which they are the youngest children in their class. This technique of comparison has been used in other studies of ADHD.10–14 Using data from 2007 through 2015 from a large insurance database, we compared rates of ADHD diagnosis and pharmacologic treatment among children born in August with those of children born in September.
Methods
Data Sources
We analyzed data from the Truven Health MarketScan Research Database, a large health insurance claims database that contains deidentified, individual-level information on more than 80 million enrollees in all U.S. states from approximately 100 commercial payers and self-insured corporations, not including Medicaid claims. The database has been described previously.15,16 Cutoff dates for kindergarten entry for each state were obtained from the Education Commission of the States17 and the National Center for Education Statistics.18 The study was approved by the institutional review board at Harvard Medical School.
Study Population
The study population was restricted to children born from 2007 through 2009, which meant that all children completed at least 1 year of elementary school by 2015, the last year of data collection. Children were linked to parents within the database, and the location of the family was recorded as the last state in which the family was insured. Children were linked by state to cutoff dates for kindergarten entry in the respective state. Children and their parents were matched with all outpatient, inpatient, and drug claims for all years included in the analyses.
Study Measures
We sought to determine whether, in the 18 states that use the September 1 cutoff for school entry, the rate of ADHD diagnosis among children born in August (who are the youngest in their grade cohort) was higher than the rate among those born in September (who are the oldest in their grade cohort). Data from children in other states, which either use different cutoffs statewide or allow local jurisdictions to decide cutoff dates for school entry, were used in sensitivity analyses. The primary outcome was a diagnosis of ADHD, defined on the basis of International Classification of Diseases, 9th Revision (ICD-9), code 314.01 (attention deficit disorder with hyperactivity) or 314.00 (attention deficit disorder without hyperactivity) or on the basis of any prescription filled for a stimulant (either an amphetamine or another type) in any insurance claim for a health care encounter between the child’s date of birth and the end of the study period. The second criterion was added to account for potential undercoding of ICD-9 ADHD codes in claims data. Details regarding diagnosis and classification are provided in the Supplementary Appendix, available with the full text of this article at NEJM.org. A secondary outcome was treatment for ADHD. We also assessed the intensity of treatment among children who received ADHD medication by determining the total number of days of ADHD medication supplied to each child throughout all years of the study period.
For children in a birth-year cohort who were born before September 1, we excluded diagnoses from 2015 so that children with August birthdays, who entered kindergarten at a younger age than their peers, would have the same number of school years to potentially receive a diagnosis of ADHD as those with September birthdays, who entered kindergarten at an older age than their peers. This ensured that any difference in diagnosis rates between children born in August and children born in September was due to relative age within the child’s grade rather than to August-born children having spent more years in school, which would have provided a longer period during which the diagnosis could be given.
Statistical Analysis
For our primary analysis, we used multivariable linear regression to compare the rate of ADHD diagnosis among children born in August with the rate among children born in September, and we evaluated statistical differences with t-tests. This analysis relied on the assumption that children born in August and September were similar both in known demographic and clinical characteristics and in unobserved factors that could influence rates of ADHD diagnosis, given that the September 1 cutoff is arbitrary, and parents most likely do not systematically plan births to occur in a particular month in preference to another. To validate this assumption, we compared rates of childhood health conditions other than ADHD among August-born and September-born children and assessed the characteristics of the parents of children born in August and the parents of children born in September with chi-square and t-tests where appropriate; all these variables should have been similar in these two groups. We also plotted birth months to ensure an even distribution of children in each month; an uneven distribution would suggest unobserved differences between groups.
In addition to comparing rates of ADHD diagnosis between children born in August and those born in September, we investigated the age at which the difference in ADHD diagnosis between August-born and September-born children appeared, using outcome variables indicating ADHD diagnosis in 1-year age groups from ages 4 through 7. We compared these age-specific rates of ADHD diagnosis and treatment with the use of methods similar to those used in our analyses of overall rates of diagnosis and treatment; we hypothesized that there would be no difference in ADHD diagnosis between August-born and September-born children by age 4 (before the start of kindergarten) but that there would be a difference by age 7 (after school entry).
We investigated differences in the intensity of ADHD treatment between children born in August and children born in September to determine whether the August-born children who received a diagnosis of ADHD but who may not have received this diagnosis if they had been born in September (and therefore would have started school later) received less intensive treatment, which would have occurred if the diagnosis had been less certain for these children. A finding that August-born children received fewer days of medication than September-born children would indicate that these children at the margin for ADHD diagnosis and treatment may receive treatment of lesser duration than those born in September; this might occur if children born in August have symptoms that resolve with age, which would lead to earlier discontinuation of therapy.
We conducted several additional analyses to ensure that differences in ADHD diagnosis rates between August-born and September-born children were not spurious. First, we compared differences in diagnosis rates between other consecutive birth months (e.g., between January and February and between February and March), expecting no between-month differences in diagnosis rates.19 Second, we assessed ADHD diagnosis rates among children who lived in states without a September 1 cutoff, expecting no difference in diagnosis rates between August-born and September-born children. Third, we compared rates of asthma, diabetes, and obesity among August-born and September-born children in an additional sensitivity analysis; these rates should not have been affected by birth in August as compared with birth in September because the diagnosis of those conditions is not influenced by the relative behavior of a child within a class.20 Fourth, we modified our baseline analysis with the use of a multivariable linear-regression model, with adjustment for a detailed set of covariates, including the sex of the child, the age of each insured parent, and the Charlson comorbidity index for each parent. (The Charlson comorbidity index includes 19 conditions and is used to predict 10-year mortality; values range from 0 to 37, with higher values indicating more coexisting conditions.21) We also adjusted for indicator variables for status with respect to asthma, anxiety, depression, obsessive–compulsive disorder, bipolar disorder, obesity, and diabetes in children and parents. Fixed effects for the year of birth and state of residence of each child were also included. Finally, we performed a regression-discontinuity analysis (see the Supplementary Appendix). Analyses were performed with Stata software, version 15 (StataCorp). The 95% confidence interval around the reported estimates reflects an alpha level of 0.025 in each tail (or P≤0.05). P values were calculated with the use of t-tests of the coefficients from linear-regression models.
Results
Study Population
The study population included 407,846 children who were born in the period from 2007 through 2009 and were entering kindergarten from 2012 through 2014. There were no significant differences between August-born children and September-born children in identifiable characteristics such as sex or status with respect to chronic diseases or in the parents’ ages or status with respect to chronic diseases (Table 1). The distribution of births across months was even, and no discontinuous cutoffs in births occurred around September 1 (Fig. S1 in the Supplementary Appendix).
Table 1.
Characteristics of the Study Population.*
| Cohort and Characteristic | Children Born in August (N = 36,319) | Children Born in September (N = 35,353) | P Value for Difference |
|---|---|---|---|
| Children | |||
| Year of birth — no. (%) | |||
| 2007 | 11,136 (30.7) | 10,244 (29.0) | |
| 2008 | 12,421 (34.2) | 12,562 (35.5) | |
| 2009 | 12,762 (35.1) | 12,547 (35.5) | 0.32 |
| Female sex — no. (%) | 17,755 (48.9) | 16,970 (48.0) | 0.02 |
| Chronic conditions — no. (%) | |||
| ADHD | 268 (0.7) | 176 (0.5) | <0.001 |
| Asthma | 3169 (8.7) | 3048 (8.6) | 0.62 |
| Anxiety | 105 (0.3) | 121 (0.3) | 0.20 |
| Depression | 7 (<0.1) | 5 (<0.1) | 0.60 |
| Obsessive-compulsive disorder | 11 (<0.1) | 10 (<0.1) | 0.88 |
| Bipolar disorder | 4 (<0.1) | 3 (<0.1) | 0.73 |
| Diabetes | 37 (0.1) | 47 (0.1) | 0.22 |
| Charlson comorbidity index† | 0.5 | 0.5 | 0.98 |
| Parents | |||
| Female sex — no./total no. (%) | 12,707/24,774 (51.3) | 12,447/24,200 (51.4) | 0.75 |
| Chronic conditions — no./total no. (%) | |||
| ADHD | 708/24,774 (2.9) | 697/24,200 (2.9) | 0.88 |
| Asthma | 1,547/24,774 (6.2) | 1,461/24,200 (6.0) | 0.34 |
| Anxiety | 3,434/24,774 (13.9) | 3,317/24,200 (13.7) | 0.62 |
| Depression | 1,143/24,774 (4.6) | 1,068/24,200 (4.4) | 0.29 |
| Obsessive-compulsive disorder | 133/24,774 (0.5) | 130/24,200 (0.5) | 0.99 |
| Bipolar disorder | 158/24,774 (0.6) | 144/24,200 (0.6) | 0.55 |
| Diabetes | 1,842/24,774 (7.4) | 1,798/24,200 (7.4) | 0.98 |
| Mean age — yr | 36.3 | 36.2 | 0.11 |
| Charlson comorbidity index† | 1.03 | 1.02 | 0.21 |
P values reflect t-tests for continuous variables and chi-square comparisons for categorical variables. ADHD denotes attention deficit–hyperactivity disorder.
The Charlson comorbidity index includes 19 conditions and is used to predict 10-year mortality; scores range from 0 to 37, with higher scores indicating more coexisting conditions.
ADHD Diagnosis and Treatment
The rate of ADHD diagnosis was 85.1 per 10,000 children born in August (309 cases among 36,319 children; 95% confidence interval [CI], 75.6 to 94.2) and 63.6 per 10,000 children born in September (225 cases among 35,353 children; 95% CI, 55.4 to 71.9) — an absolute difference of 21.5 per 10,000 children (95% CI, 8.8 to 34.0) and a 34% higher rate among children born in August than among children born in September (Fig. 1). Month-to-month differences in diagnosis rates were not significant for all other consecutive pairs of months. Rates of ADHD treatment were 52.9 per 10,000 children born in August (192 of 36,319 children; 95% CI, 45.4 to 60.3) and 40.4 per 10,000 children born in September (143 of 35,353 children; 95% CI, 33.8 to 47.1) — an absolute difference of 12.5 per 10,000 children (95% CI, 2.43 to 22.4) and a 32% higher rate among children born in August. Adjustment of the main analyses for child and parental characteristics did not affect these findings. Similar findings were also observed in an analysis that compared trends in ADHD diagnosis within 7-day birth cohorts from 3 weeks before to 3 weeks after the September 1 cutoff. (For more information, see Figs. S2 through S4 in the Supplementary Appendix.)
Figure 1. Differences in Diagnosis Rates of Attention Deficit–Hyperactivity Disorder (ADHD) According to Month of Birth.
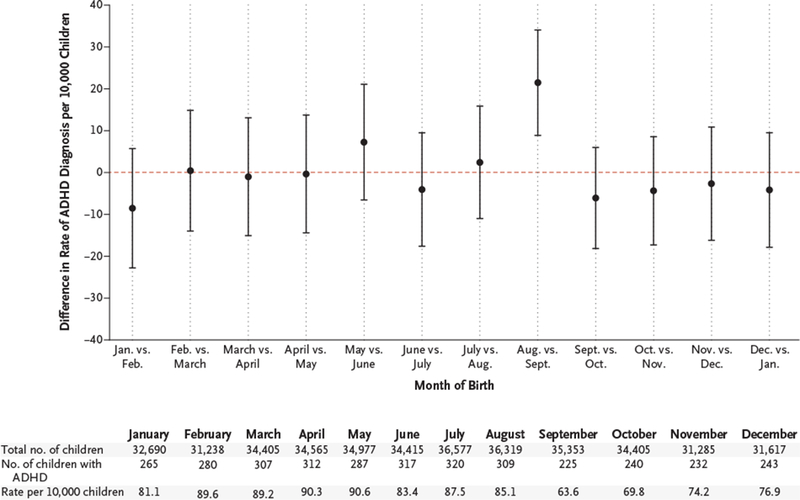
Each point represents the absolute difference in the rate of ADHD diagnosis per 10,000 children between children born in a given month and children born in the following month. For example, the “Aug. vs. Sept.” marker indicates that the absolute difference in the diagnosis rate between children born in August and children born in September was 21.5 per 10,000 children (95% CI, 8.8 to 34.0). The dashed line indicates no difference. I bars indicate 95% confidence intervals. ADHD diagnosis was defined on the basis of a diagnosis code for ADHD according to the International Classification of Diseases, 9th Revision (ICD-9), on billing claims or on the basis of a prescription filled for an ADHD medication.
In an analysis that assessed children younger than 4 years of age (before school entry) and younger than 7 years of age (after all children are in school), there was no significant difference in the ADHD diagnosis rate between August-born and September-born children by age 4 (the diagnosis rate for August-born children was higher than that for September-born children by 0.2 per 10,000 children; 95% CI, −5.6 to 5.9), but there was a significant difference by age 7 (the diagnosis rate for August-born children was higher than that for September-born children by 17.8 per 10,000 children; 95% CI, 6.8 to 28.7) (Figs. S5 and S6 in the Supplementary Appendix).
We replicated the main analysis in states that do not use September 1 as the cutoff for entry into kindergarten. The difference in diagnosis rates between August-born children and September-born children that was present in states with a September 1 cutoff was not present in states that do not use this cutoff; the absolute difference in ADHD diagnosis rates between August-born and September-born children in states that do not use the September 1 cutoff was 8.9 diagnoses per 10,000 children (95% CI, −14.9 to 20.8) (Fig. 2). This suggests that unobservable factors that differed between August-born and September-born children that could have correlated with ADHD diagnosis were probably not responsible for the main finding of differences in observed rates in states with a September 1 cutoff. Rates of three prespecified childhood conditions (asthma, obesity, and diabetes), the diagnosis and treatment of which are unlikely to be affected by school performance and relative age within a school cohort, did not differ between August-born and September-born children (Fig. S7 in the Supplementary Appendix).
Figure 2. Differences in ADHD Diagnosis Rates According to Month of Birth in States with and States without a September 1 Cutoff.
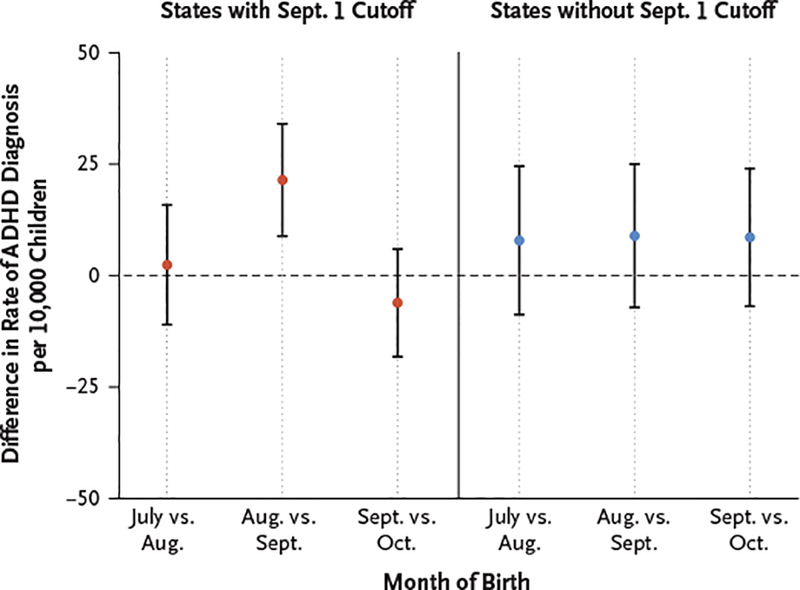
Shown are the differences in ADHD diagnosis rates between children in the 18 states with a September 1 cutoff for kindergarten entry and children in all states without a September 1 cutoff. The dashed line indicates no difference. I bars indicate 95% confidence intervals. ADHD diagnosis was defined on the basis of an ICD-9 diagnosis code for ADHD on billing claims or a prescription filled for an ADHD medication.
In an analysis that included stratification according to sex, the rate of ADHD diagnosis among boys who were born in August was higher by 32.5 per 10,000 children (95% CI, 11.9 to 53.2) than the rate among boys born in September. The rate among girls born in August was higher by 10.7 per 10,000 children (95% CI, −3.2 to 24.6) than among girls born in September, but this difference was not significant (Fig. 3).
Figure 3. Differences in ADHD Diagnosis Rates According to Sex and Month of Birth.
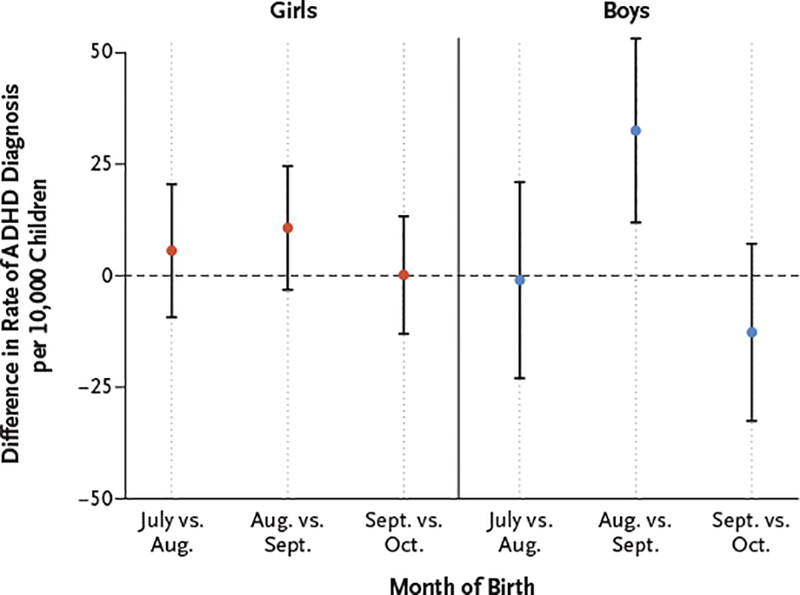
The dashed line indicates no difference. I bars indicate 95% confidence intervals. ADHD diagnosis was defined on the basis of an ICD-9 diagnosis code for ADHD on billing claims or a prescription filled for an ADHD medication.
Among children receiving medication for ADHD, children born in August received an average of 120 more days of medication (95% CI, 40 to 200) than children born in September, which suggests that among children who received a diagnosis of ADHD and were treated for it, the higher rate of ADHD diagnosis among August-born children was not accompanied by less intensive treatment (Fig. S8 in the Supplementary Appendix).
Discussion
Using data through 2015 from a large national insurance database, we found that in states with an age cutoff at September 1 for kindergarten entry, children born in August were significantly more likely to receive a diagnosis of ADHD than children born in September. Furthermore, children in these states who were born in August were more likely than children born in September to receive medical treatment for ADHD. The demographic and clinical characteristics were similar in August-born and September-born children and in their parents. There were no significant differences in the rates of ADHD diagnosis in any other pairs of birth months or in the rates of various medical conditions for which diagnoses are not expected to be affected by child-to-child comparisons of behavior in a school setting. Finally, we found no significant differences in rates of ADHD diagnosis between August-born and September-born children who were living in states without a September 1 cutoff for kindergarten entry, nor did we find, in states with a September 1 cutoff, differences between August-born and September-born children who had not yet started school.
The results of this study suggest that a child’s age relative to the ages of classmates could affect the likelihood that the child will receive a diagnosis of and treatment for ADHD. We found that among children who were taking ADHD medications, the average duration of treatment among children born in August was significantly longer than that for children born in September, which suggests that the children born in August who received a diagnosis of ADHD because of their August birthdays received more intensive treatment than the average child with ADHD who was born in September.
These results emphasize the importance of the context of other children in a child’s grade in the diagnosis and treatment of ADHD, and the results complement previous studies that have shown a relationship between ADHD and birth-month cohort.10–14 Our study used more recent data than other published studies and focused on young children (4 to 7 years of age), for whom the possibility of a diagnosis of ADHD on the basis of child-to-child comparisons may be most relevant. Our analyses were based on data from insurance claims rather than on survey reports, which are vulnerable to recall bias and possible overestimation of the prevalence of ADHD.
Given the potential adverse effects associated with ADHD medications,22 the possibility that a child might receive medication as a result of an arbitrary cutoff date for school entry may be of interest to physicians, teachers, and parents. Because of the limitations of our method of data collection and study design, we could not assess the independent roles of teachers, parents, and physicians in ADHD diagnoses. However, given that teachers and parents observe children in the context of their school cohort and physicians do not, it is possible that teachers and parents may be the first to determine that a child’s behavior appears to be anomalous relative to peers. Previous studies have shown that there is a significant difference in teachers’ assessments of the presence of ADHD symptoms in children born in August as compared with children born in September and that the difference is much smaller when parents make these assessments, which suggests that teachers have a stronger role in ADHD diagnosis than parents.12,23 The age of the child relative to peers may be useful to physicians in assessing whether behaviors reported by teachers and parents are indeed indicative of ADHD.
There are several limitations of our study. First, we were unable to assess the appropriateness of an ADHD diagnosis in any child or the outcomes related to treatment. Because of this, we cannot conclude that ADHD is overdiagnosed in children born in August relative to children born in September. It is possible that the additional August-born children who receive ADHD diagnoses are receiving the appropriate diagnosis, and that there are September-born children who have ADHD that remains undiagnosed. In addition, children born in August who are among the youngest in their class may benefit from the additional attention that is associated with an ADHD diagnosis, especially given evidence that younger children in a school cohort do not perform as well as older children in academic and athletic measures, that fewer of them attend college, and that they are more likely to engage in juvenile criminal behavior.19,24,25 We are able to conclude only that a child’s age relative to peers has an association with diagnosis and treatment rates of ADHD, not whether this association is harmful or helpful.
Second, data from insurance claims do not allow us to determine when a child starts school. Parents may delay school entry for children born in August, which would mean that those children start school at 6 years of age rather than 5 years of age. Because we did not directly observe children’s ages when they entered school, we cannot know how often this occurred. However, this behavior on the part of parents should mean that our results underestimate the true effects on children of being among the youngest members of a grade cohort as compared with the oldest members of a grade cohort in the probability of receiving a diagnosis of ADHD, since some of the children with August birthdays actually start school at the same age as the children with September birthdays. Finally, our claims data included only children who had employer-provided insurance coverage and specifically excluded Medicaid and uninsured patients, which yielded a selected group with a lower rate of ADHD diagnosis than the national average.1
In conclusion, using recent data and several analytic approaches, we confirmed findings from previous studies that in states with September 1 cutoffs for kindergarten entry, children born in August are significantly more likely to receive a diagnosis of and treatment for ADHD than children born in September. Our findings are consistent with the hypothesis that the context of behaviors within a grade or school class influences the likelihood of a diagnosis of ADHD.
Supplementary Material
Acknowledgments
Supported by a National Institutes of Health Director’s Early Independence Award (1DP5OD017897–01 [to Dr. Jena]).
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Visser SN, Danielson ML, Bitsko RH, et al. Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003–2011. J Am Acad Child Adolesc Psychiatry 2014; 53(1): 34–46. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayes R, Bagwell C, Erkulwater J. ADHD and the rise in stimulant use among children. Harv Rev Psychiatry 2008; 16: 151–66. [DOI] [PubMed] [Google Scholar]
- 3.Hinshaw S, Scheffler RM. The ADHD explosion: myths, medication, money, and today’s push for performance. New York: Oxford University Press, 2014. [Google Scholar]
- 4.Schwarz A ADHD nation: children, doctors, big pharma, and the making of an American epidemic. New York: Simon and Schuster, 2016. [Google Scholar]
- 5.Schwarz A The selling of attention deficit disorder. New York Times; December 15, 2013: A1. [Google Scholar]
- 6.Schwarz A, Cohen S. A.D.H.D. seen in 11% of U.S. children as diagnoses rise. New York Times; April 1, 2013: A1. [Google Scholar]
- 7.Danielson ML, Visser SN, Gleason MM, Peacock G, Claussen AH, Blumberg SJ. A national profile of attention-deficit hyperactivity disorder diagnosis and treatment among US children aged 2 to 5 years. J Dev Behav Pediatr 2017; 38: 455–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danielson ML, Bitsko RH, Ghandour RM, Holbrook JR, Kogan MD, Blumberg SJ. Prevalence of parent-reported ADHD diagnosis and associated treatment among U.S. children and adolescents, 2016. J Clin Child Adolesc Psychol 2018; 47: 199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sax L, Kautz KJ. Who first suggests the diagnosis of attention-deficit/hyper-activity disorder? Ann Fam Med 2003; 1: 171–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans WN, Morrill MS, Parente ST. Measuring inappropriate medical diagnosis and treatment in survey data: the case of ADHD among school-age children. J Health Econ 2010; 29: 657–73. [DOI] [PubMed] [Google Scholar]
- 11.Morrow RL, Garland EJ, Wright JM, Maclure M, Taylor S, Dormuth CR. Influence of relative age on diagnosis and treatment of attention-deficit/hyperactivity disorder in children. CMAJ 2012; 184: 755–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elder TE. The importance of relative standards in ADHD diagnoses: evidence based on exact birth dates. J Health Econ 2010; 29: 641–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krabbe EE, Thoutenhoofd ED, Conradi M, Pijl SJ, Batstra L. Birth month as predictor of ADHD medication use in Dutch school classes. Eur J Spec Needs Educ 2014; 29: 571–8. [Google Scholar]
- 14.Schwandt H, Wuppermann A. The youngest get the pill: ADHD misdiagnosis in Germany, its regional correlates and international comparison. Labour Econ 2016;43:72–86. [Google Scholar]
- 15.Chernew ME, Sabik LM, Chandra A, Gibson TB, Newhouse JP. Geographic correlation between large-firm commercial spending and Medicare spending. Am J Manag Care 2010; 16: 131–8. [PMC free article] [PubMed] [Google Scholar]
- 16.Desai S, Hatfield LA, Hicks AL, Chernew ME, Mehrotra A. Association between availability of a price transparency tool and outpatient spending. JAMA 2016; 315: 1874–81. [DOI] [PubMed] [Google Scholar]
- 17.Kindergarten entrance ages: a 35 year trend analysis. Denver: Education Commission of the States, 2007. (http://www.ecs.org/clearinghouse/73/67/7367.pdf). [Google Scholar]
- 18.Types of state and district requirements for kindergarten entrance and attendance, waivers and exemptions for kindergarten entrance, by state: 2014. National Center for Education Statistics; (https://nces.ed.gov/programs/statereform/tab5_3.asp). [Google Scholar]
- 19.Dhuey E, Figlio D, Karbownik K, Roth J. School starting age and cognitive development NBER working paper no. 23660. Cambridge, MA: National Bureau of Economic Research, August 2017. [Google Scholar]
- 20.Prasad V, Jena AB. Prespecified falsification end points: can they validate true observational associations? JAMA 2013; 309: 241–2. [DOI] [PubMed] [Google Scholar]
- 21.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992; 45: 613–9. [DOI] [PubMed] [Google Scholar]
- 22.Cooper WO, Habel LA, Sox CM, et al. ADHD drugs and serious cardiovascular events in children and young adults. N Engl J Med 2011; 365: 1896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fulton BD, Scheffler RM, Hinshaw SP. State variation in increased ADHD prevalence: links to NCLB school accountability and state medication laws. Psychiatr Serv 2015; 66: 1074–82. [DOI] [PubMed] [Google Scholar]
- 24.Bedard K, Dhuey E. The persistence of early childhood maturity: international evidence of long-run age effects. Q J Econ 2006; 121: 1437–72. [Google Scholar]
- 25.Deaner RO, Lowen A, Cobley S. Born at the wrong time: selection bias in the NHL draft. PLoS One 2013; 8(2): e57753. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


