Abstract
Purpose of review
The discovery of Kaposi sarcoma herpesvirus (KSHV) led to recognition of KSHV-associated multicentric Castleman disease (MCD) as a distinct lymphoproliferative disorder. The pathogenesis of KSHV-MCD is attributed to proliferation of KSHV-infected B cells, production of KSHV-encoded viral interleukin 6 by these cells, and dysregulation of human interleukin 6 and interleukin 10. This article reviews advances in the field of disease pathogenesis and targeted therapies.
Recent findings
Our understanding of the pathogenesis of KSHV-MCD has increased in recent years and improved therapies have been developed. Recent studies demonstrate that the anti-CD20 monoclonal antibody, rituximab, as well as virus-activated cytotoxic therapy using high-dose zidovudine and valganciclovir, can control symptoms and decrease adenopathy. With treatment, 1-year survival now exceeds 85%. Interestingly, even in the absence of pathologic findings of MCD, KSHV-infected patients may have inflammatory symptoms, excess cytokine production, and elevated KSHV viral load similar to KSHV-associated MCD. The term KSHV-associated inflammatory cytokine syndrome has been proposed to describe such patients.
Summary
Recent advances in targeted therapy have improved outcomes in KSHV-MCD, and decreased need for cytotoxic chemotherapy. Improved understanding of the pathogenesis of KSHV-MCD and KSHV-associated inflammatory cytokine syndrome is needed, and will likely lead to additional advances in therapy for these disorders.
Keywords: human herpesvirus-8, Kaposi sarcoma herpesvirus, KSHV-associated inflammatory cytokine syndrome, multi-centric Castleman disease, viral interleukin-6
INTRODUCTION
Kaposi sarcoma herpesvirus (KSHV) associated multicentric Castleman disease (KSHV-MCD) is a lymphoproliferative disorder characterized by inflammatory symptoms, cytopenias, lymphadenopathy, splenomegaly and a waxing and waning course that is eventually lethal if untreated [1,2]. It is caused by KSHV, also called human herpesvirus 8 (HHV-8) [1], which is also the etiologic agent of Kaposi sarcoma [3], primary effusion lymphoma (PEL) [4,5] and a recently described interleukin 6 (IL-6) related disease called KSHV-inflammatory cytokine syndrome (KICS) [6,7▪].
KSHV-MCD is one form of a cluster of diseases called Castleman disease. Benjamin Castleman first described angiofollicular lymph node hyperplasia in localized mediastinal masses in a disease now considered a hyaline vascular variant of Castleman disease (HV-CD) [8,9]. Variants with expansion of plasmacytic [10] or plasmablastic cells [11] were subsequently described and also called Castleman disease. Most plasmacytic or plasmablastic Castle-man disease cases are multicentric and remarkable for IL-6-associated inflammatory symptoms [2,12,13]. However, some cases of plasmacytic or plasmablastic Castleman disease are unicentric, and HV-CD may occasionally be multicentric [14,15]. After discovery of KSHV [3], it was appreciated that most plasmablastic MCD arising in HIV-infected individuals was KSHV-associated [1], and that this was a distinct form of MCD.
KSHV-negative Castleman disease may be associated with or difficult to distinguish from other malignancies including dendritic cell tumors [16–18] and lymphoma [19,20]. KSHV-negative MCD also has clinical overlap with other plasma cell disorders, including polyneuropathy, organomegaly, endocrinopathy, edema, M-protein and skin syndrome [21–24] and IgG4-related sclerosing disease [25]. The clinicopathologic spectrum of Castleman disease may be due to variable driving factors that lead to dysregulation of IL-6 and/or vascular endothelial growth factor (VEGF) [26,27], accounting for overlapping clinical and histopatho-logic findings. The remainder of this review focuses on KSHV-MCD.
CLINICAL FEATURES OF KAPOSI SARCOMA HERPESVIRUS-ASSOCIATED MULTICENTRIC CASTLEMAN DISEASE
KSHV-MCD most often presents in HIV-infected individuals; symptoms include progressive fatigue, fever, night sweats, weight loss and adenopathy. Many patients have concurrent Kaposi sarcoma. Nonspecific respiratory and gastrointestinal symptoms, rashes and neuropathy are also common; edema and effusions are observed less frequently. Patients can become critically ill, either due to sepsis-like manifestations or hemophagocytic syndrome [28,29]. Patients with symptomatic disease generally have elevated C-reactive protein (CRP) and KSHV viral load [30], usually with other laboratory abnormalities such as anemia, thrombocytopenia, hypoalbuminemia, hyponatremia and elevated g-globulin [2,31,32▪▪,33▪,34]. Computerized tomography typically shows diffuse adenopathy and splenomegaly (Fig. 1). Diagnosis requires pathologic confirmation, usually from a lymph node biopsy (Fig. 2). Patients with KSHV-MCD are at high risk of developing KSHV-associated lymphomas, including PEL and large cell-lymphoma arising in KSHV-MCD, the latter which may represent clonal expansion of the KSHV-infected plasmablasts. Diffuse large B-cell lymphoma is also seen in this patient population [11,35,36▪,37]. In patients with effusions, PEL should be excluded through evaluation of cytopathology, Epstein–Barr virus and KSHV, flow cytometry and B-cell clonality [38,39].
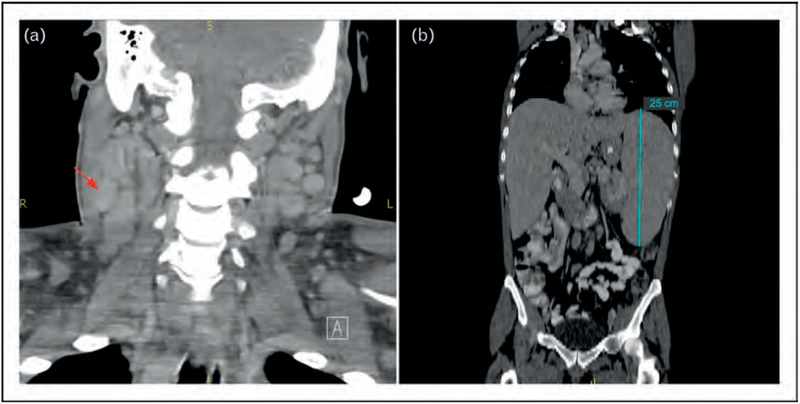
FIGURE 1.
Computerized tomography in Kaposi sarcoma herpesvirus-multicentric Castleman disease. (a) Coronal image of neck showing bilateral diffuse cervical adenopathy, red arrow points to example of enlarged lymph node on right. (b) Coronal image of torso, demonstrating dramatic splenomegaly, measuring 25 cm (upper limit of normal = 12 cm).
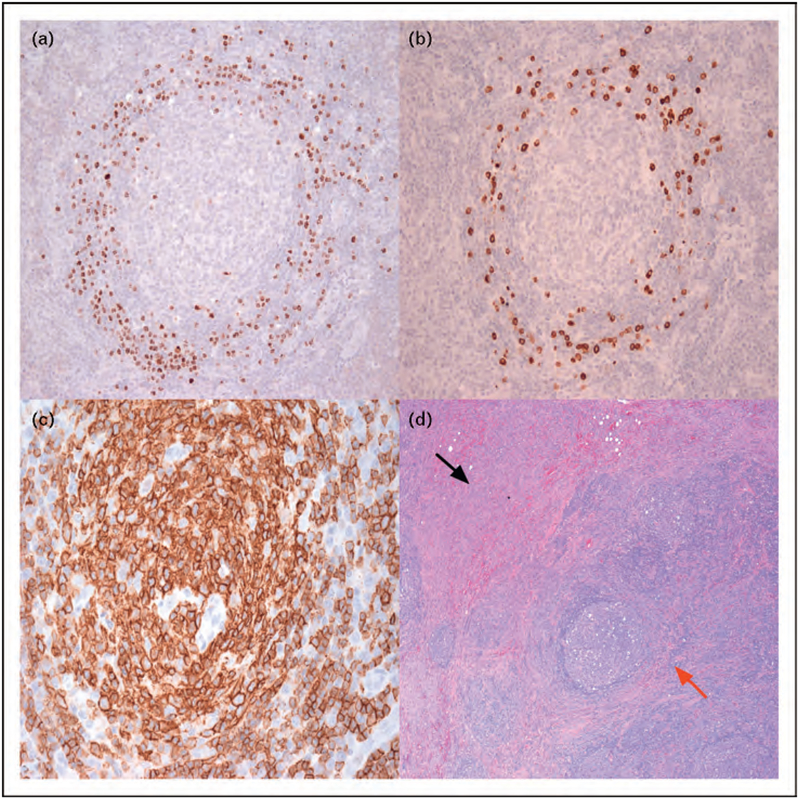
FIGURE 2.
Lymph node histopathology in Kaposi sarcoma herpesvirus-multicentric Castleman disease. (a) Regressed germinal center with expansion of plasmacytic cells in the mantle zone. Immunohistochemical staining for KSHV latency associated nuclear antigen (LANA) shows a proportion of the cells are KSHV infected. 20X magnification. (b) Immunohistochemical staining for viral interleukin-6 (vIL-6) shows a proportion of cells in the mantle zone express vIL-6. 20X magnification. (c) Immunohistochemistry demonstrating a high proportion of cells expressing CD20 (stained brown), with strongest expression in the germinal center, and a mixture of CD20 positive and negative cells in the mantle zone. 40X magnification. (d) Lymph node biopsy showing spindle cell expansion with leaky vascularity representing Kaposi sarcoma (black arrow), as well as typical finding of KSHV-MCD (red arrow) in the same lymph node. 10X magnification Images and pathology samples from patients on National Cancer Institute Institutional Review Board approved KSHV-MCD Natural History Protocol (NCT00099073). All patients gave written informed consent in accordance with the Declaration of Helsinki.
EPIDEMIOLOGY OF KAPOSI SARCOMA HERPESVIRUS-MULTICENTRIC CASTLEMAN DISEASE
Although KSHV-MCD is considered to be extremely rare, its incidence and prevalence are uncertain. It is not tracked in cancer registries. Given its nonspecific symptoms and a waxing and waning course, KSHV-MCD is almost certainly underrecognized. Unlike Kaposi sarcoma, whose incidence decreased with broad availability of highly active antiretroviral therapy (HAART) [40▪], the incidence of KSHV-MCD appears to be increasing in the HAART era [41]. Biologically, this is plausible. Unlike Kaposi sarcoma that is associated with degree of immunosuppression [42] and lack of KSHV specific T-cell response[43], KSHV-MCD frequently occurs despite suppressed HIV, relatively preserved CD4 counts [32▪▪,33▪,36▪], evidence of KSHV-specific T-cell response [44] and elevated levels of antibodies directed against KSHV capsid protein K8.1 [45].
There are almost no reports of KSHV-MCD in sub-Saharan Africa, despite high prevalence of both HIV and KSHV [46,47,48▪▪]. This likely reflects underdiagnosis and underreporting of KSHV-MCD; indeed, of 32 cases of KSHV-MCD followed at the NIH Clinical Center in Bethesda, Maryland, USA, five were in recent African immigrants (Uldrick, Polizzotto, and Yarchoan, unpublished observation). Additional studies are needed to define the incidence and prevalence of KSHV-MCD in different populations.
PATHOGENESIS OF KAPOSI SARCOMA HERPESVIRUS-MULTICENTRIC CASTLEMAN DISEASE
Like other herpesviruses, KSHV has two principal phases of gene expression: a latent phase, and a lytic phase in which production of new virions occurs. Latency-associated nuclear antigen (LANA) and other latent genes, as well as 12 KSHV encoded microRNA (miRNA) are expressed in both phases, whereas lytic genes are generally expressed only during the lytic phase. Certain genes, such as KSHV-encoded viral IL-6 (vIL-6), are predominantly released during lytic replication but can also be produced in small amounts during latent infection [49]. In all MCD, the main pathological process is B-cell hyperproliferation, caused in part by auto-crine and paracrine signaling and symptoms and laboratory abnormalities are related to increased levels of IL-6 [2,6]. In KSHV-MCD, there is also production of KSHV-encoded vIL-6 with detectable levels in the serum, and this is believed to be an important cause of disease manifestations [6,50]. Unlike human IL-6, which first binds a coreceptor, gp80, and then to gp130, vIL-6 signals directly through gp130 [51–58], and thus potentially affects a wider range of cells than human IL-6. In addition, KSHV-MCD pathogenesis may involve upregulation of NF-kB by latently expressed viral-FLICE inhibitory protein (vFLIP) [59,60] or viral miRNA-K1 [61], and upregulation of VEGF and other factors by a viral G-protein coupled receptor [59–62]. Patients with KSHV-MCD also frequently have elevated serum IL-10 and other cytokine abnormalities. The relative contribution of these various factors to KSHV-MCD (Table 1) [13,50,61,63,64,65▪▪,66–68,69▪▪] is poorly understood.
Table 1.
Human and KSHV-encoded genes and KSHV-encoded microRNA implicated in KSHV-associated multicentric Castleman disease pathogenesis
| Gene/miRNA | Model | Findings | Reference |
|---|---|---|---|
| Human Genes | |||
| IL-6 | Retroviral transfer of IL-6 gene into mouse hematopoietic stem cells, transplanted into congenitally anemic W/Wv mice | Anemia, leukocytosis, thrombocytopenia, polyclonal hypergammaglobinemia, massive splenomegaly, lymphadenopathy. Lesser infiltrates of kidney, lung and liver. Glomerular mesangial cell hyperplasia | Brandt, et al. [13] |
| IL-10 | Placebo-controlled clinical study of recombinant human IL-10 in patients with Crohn’s disease | Toxicity evaluation demonstrated dose-dependent anemia with markedly elevated ferritin, and thrombocytopenia | Fedorak, et al. [63] Tilg, et al. [64] |
| KSHV-encoded Genes/miRNA | |||
| Viral IL-6 | Murine fibroblasts transfected with vlL-6, inoculated into flank of nude mice | Leukocytosis, moderate splenomegaly, plasmacytosis in spleen and lymph nodes, polyclonal hypergammaglobinemia. Spindle cell tumors. Increased VEGF production in tumors, lymph nodes and spleen | Aoki, et al. [50] |
| vFLIP | Conditional knock-in transgenic mice that express vFLIP in all CD19+ B cells or lgG1+ germinal center B cells | Splenomegaly. Certain histologic features similar to those seen in KSHV-MCD: expansion of marginal zone B cells, lack of CD1 38 staining, impaired germinal center formation and increased λ-light chain production. Elevated IgE with immunization. Mice developed transgenic derived tumors with histologic features of histiocytic/dendritic cell sarcoma | Ballon, et al. [65▪▪] |
| miRNA-K1 | Transfection of 293 T cells with mutated KSHV miRNA | Cells transfected with KSHV with mutated miRNA had decreased NF-κB activity due to downregulation of the NF-κB regulating protein IκBα by KSHV miRNA-K1, whereas miRNA-K1 transfection restored NF-κB activity | Lei, ef al. [61] |
| miRNA K12–3 and miRNA K12–7 | Transfection of KSHV miRNA in murine macrophage and human myelomonocytic leukemia cell lines | Transfection lead to upregulation of human IL-6, IL-6 receptor, and IL-10. Mechanism of action is KSHV miRNA downregulation of C/EBP-β, a transcription factor that negatively regulates IL-6 [66,67] | Qin, et al. [68] |
| miRNAK12–11 | Viral transfer of KSHV-encoded miRNA K12–1 1 in human hematopoietic progenitors and immune reconstitutions in NOD/LtSz-scid IL2Rγ(null) mice | Expansion of splenic CD19+ cells. 3′ UTR of C/EBP-β contains a putative miRNA K1 2–11 binding site. Splenic B cells in miRNA Kl 2–11 transplanted animals had decreased C/EBP-β miRNA compared to controls | Boss, et al. [69▪▪] |
vFLIP, viral-FLICE inhibitory protein; KSHV-MCD, Kaposi sarcoma herpesvirus-associated multicentric Castleman disease; miRNA, microRNA.
It is also unclear why only occasional KSHV-infected patients develop MCD. In asymptomatic KSHV-infected individuals, KSHV can often be detected in saliva [70–72,73▪▪,74], but relatively infrequently in peripheral blood mononuclear cells (PBMC) [47]. By contrast, patients with KSHV-MCD have a markedly increased KSHV viral load [6,33▪,75–81] and expansion of KSHV-infected plasmablasts in lymph nodes and spleen. Affected lymphoid tissues have regressed germinal centers, increased vascularization and mantle zone expansion notable for KSHV-infected plasmablastoid cells, usually IgM+ [82], that are monotypic (λ-restricted) but polyclonal, and a larger population of KSHV-uninfected B cells [11,83]. All KSHV-infected cells express LANA, whereas a portion also express vIL-6 or other lytic genes [84–86].
KSHV has tropism for B cells, monocytes, dendritic cells, epitehelial, keratinocytes and endothelial cells [87–90]. Infection of target cells is complex [91]; xCT, integrin α3β1 [92–94] and DC-SIGN [95,96] can function as receptors for KSHV entry. B-cell infection by KSHV is enhanced by activation signals such as IL-4 and CD40-ligand [95–97]. Stable B-cell infection in vitro predominantly occurs in IgM+, l-restricted B cells with a ‘blasting’ morphology that is promoted by IL-6, recapitulating lymph node findings [98▪▪]. There is evidence that vFLIP plays a role in the bias toward l-restriction [65▪▪]. Additional efforts to dissect interactions between specific KSHV genes and human genes associated with plasmablast differentiation [82,99,100] may provide insights into disease pathogenesis. KSHV-MCD patients also tend to have certain KSHV miRNA polymorphisms [101▪▪], and further assessment of these findings as well as assessment of polymorphisms in other KSHV genes or the host genome affecting IL-6 signaling is warranted.
More recently, we described a group of patients with MCD-like fever and inflammatory symptoms, elevated serum vIL-6, and elevated KSHV viral load, but without pathological findings of KSHV-MCD[6]; this syndrome has been tentatively named KSHV-associated inflammatory cytokine syndrome (KICS) [7▪]. Studies to evaluate its natural history, pathogenesis and relation to KSHV-MCD are underway (NCT01419561).
THERAPY FOR KAPOSI SARCOMA HERPESVIRUS-MULTICENTRIC CASTLEMAN DISEASE
There is currently no established therapy for KSHVMCD. Until recently, most published literature consisted of case reports or small case series. Although HAART has not been formally studied, a strong case can be made for its use concurrent with specific therapy in HIV-infected patients with KSHV-MCD [30,102]. Additional specific treatment of KSHVMCD is generally reserved for patients with symptomatic disease. Such patients can become critically ill, and may require urgent treatment and supportive care, in an ICU if needed. Successful treatment has been reported with various chemotherapeutic agents, including etoposide, vincristine, vinblastine, cyclophosphamide or doxorubicin, either as singly or in combination. Likewise, temporary remission of symptoms after splenectomy has been described, although splenectomy in HIV-infected patients carries a high risk of infectious complications [31,103,104▪▪]. Steroids may reduce inflammation in symptomatic patients; however, their utility is limited, and prolonged use commonly exacerbates Kaposi sarcoma [105]. Immunomodulation with interferon-α [31,106,107] and thalidomide [108] has also been noted to have activity. With most of these modalities, relapse is common, and until recently, overall survival has been poor; a pooled evaluation of 86 cases between 1985 and 2006 reported a median survival of about 12 months, although somewhat better in patients on HAART [102].
In the past several years, targeted approaches have been prospectively evaluated in KSHV-MCD. Results suggest improved outcomes over most modalities listed above. In clinical studies, several criteria have been employed for initiating therapy (Table 2), generally including elevated CRP and at least two to four clinical and laboratory abnormalities. Importantly, patients were not treated based on abnormal radiographic findings alone. Also, there are no commonly used response criteria. Investigators have primarily graded responses in relation to resolution of clinical symptoms and laboratory abnormalities. In an attempt to establish response criteria for KSHV-MCD, we prospectively evaluated KSHV-MCD response criteria that integrate clinical, common biochemical and radiographic findings [32▪▪]. Further efforts to harmonize response criteria among clinical trials are needed.
Table 2.
Criteria for patient selection for treatment of KSHV-associated multicentric Castleman disease utilized in two prospective studies
| Revised NCI Criteria for Treatmenta | MCD Attack Criteria (CastlemaB Trial) Criteria for Treatmentb |
|---|---|
| At least one clinical symptom (commonest examples listed) and one laboratory abnormality probably or definitely attributed to KSHV-MCD and elevated serum CRP | Fever |
| Clinical symptoms | At least 3 of the following symptoms |
| Fatigue (CTCAE equivalent ≥ Grade 2) | Peripheral lymphadenopathy |
| Fever, night sweats | Enlarged spleen |
| Weight loss | Edema |
| Respiratory symptoms | Pleural effusion |
| Gastrointestinal symptoms | Ascites |
| Neurologic symptoms | Cough |
| Edema or effusions | Xerostomia |
| Rash | Rash |
| Laboratory abnormalities | Central neurologic symptom |
| Anemia | Jaundice |
| Thrombocytopenia | Autoimmune hemolytic anemia |
| Hypoalbuminemia | |
| Elevated serum CRP (>3 mg/l) | Elevated serum CRP (>20mg/l) in the absence of any other etiology |
| If HIV+, on effective combination antiretroviral therapy or willing to initiate therapy | If HIV+, on combination antiretroviral therapy for at least 3 months |
CRP, C-reactive protein; CTCAE, Common Terminology Criteria for Adverse Events; MCD, multicentric Castleman disease; NCI, National Cancer Institute; KSHVMCD, Kaposi sarcoma herpesvirus-associated multicentric Castleman disease.
Original criteria proposed in 2004 [32▪▪] included additional liver function abnormalities, leukopenia, hyponatremia, and elevated creatinine as potential laboratory abnormalities. From clinical trial NCT00099073.
Adapted with permission from [109].
Radiographic responses and KSHV viral load dynamics have generally been evaluated as secondary outcomes [32▪▪,109,110]. Importantly, residual adenopathy, splenomegaly and detectable KSHV viral load have been noted even after resolution of symptoms. Such patients may represent a group with reservoirs of KSHV-infected B cells that are inadequately treated, and who may be at higher risk of recurrent KSHV-MCD symptoms [111▪]. Nonetheless, optimal duration of therapy and approach to such patients remains an area of uncertainty.
Rituximab
The best-studied agent in KSHV-MCD is rituximab, a humanized monoclonal antibody against the B-cell antigen CD20. Rituximab has been evaluated in two prospective phase 2 studies. In the CastlemaB Study, 24 patients with HIV and KSHV-MCD received rituximab 375 mg/m2 weekly for 4 weeks after completion of chemotherapy [109]. Ninety-two percent of patients met the primary outcome of sustained resolution of their MCD attack (Table 2) 60 days after completion of chemotherapy. In eight of 10 evaluated patients, splenomegaly resolved, and at 1 year, 71% were alive and disease-free [109]. In a separate study, 21 patients with symptomatic KSHVMCD were treated with rituximab 375 mg/m2 weekly for 4 weeks without chemotherapy [110]. Ninety-five percent had resolution of symptoms and fever, with an estimated 79% of patients relapse-free at 2 years. In the latter study, none had a complete radiographic response by Response Evaluation Criteria in Solid Tumors criteria. However, KSHV viral load and CRP decreased significantly 1 month after completion of therapy [110].
Infusion reactions occur in most KSHV-MCD patients administered rituximab [109]. Additionally, rituximab is associated with worsening Kaposi sarcoma in 35–67% of patients [109,110]. The pathophysiology of Kaposi sarcoma relapses is unknown, but likely due to adverse immunologic effects of B-cell depletion [45,112–115]. Risk of additional rare but serious infectious complications persist beyond dosing of rituximab [116,117]. Importantly, combination of rituximab with cytotoxic chemotherapy may be required for some patients with concurrent Kaposi sarcoma or severe manifestations of KSHV-MCD [118–120]. To address the issue of Kaposi sarcoma relapse, and to potentially also target Kaposi sarcoma spindle cells or monocytes that may be secreting vIL-6, we are currently evaluating rituximab in combination with liposomal doxorubicin in patients with concurrent KSHV-MCD and Kaposi sarcoma or severe KSHVMCD (NCT00099073).
Interestingly, the mechanism of action of rituximab in KSHV-MCD is unclear. KSHV-infected, vIL-6 expressing plasmablasts in KSHV-MCD [85,86] are generally CD20 negative and unlikely to be directly targeted by rituximab [82,121]. However, CD20+ cells are noted in KSHV-uninfected lymphocytes within lymph node specimens [11] (Fig. 2), and rituximab activity likely results in part from diminished autocrine and paracrine signaling in the tumor microenvironment [122–127].
Ganciclovir and other inhibitors of Kaposi sarcoma herpesvirus replication
Ganciclovir is a 2’-deoxyguanosine nucleoside analogue phosphorylated by several herpesvirus thymidine kinases, including KSHV ORF36 [128–130] and ORF21 [128,129]. Ganciclovir triphosphate inhibits viral replication through incorporation into viral DNA and subsequent chain termination. Valganciclovir (VGC), an oral prodrug, decreases KSHV oral shedding [72]. In a case series of three patients with KSHV-MCD, ganciclovir administration led to at least short-term improvement in symptoms and decreased KSHV viral load, suggesting that control of KSHV replication may have a role in therapy [78]. However, cidofovir, a viral DNA polymerase inhibitor with greater in-vitro activity against KSHV replication [131] failed to demonstrate comparable activity in five patients with chemotherapy-dependent KSHV-MCD [132]. In a retrospective study of 52 patients with KSHV-MCD, 12 were treated with variable antiherpesvirus therapy with or without cytotoxic therapy (but no rituximab), and only 33% of these patients obtained a sustained clinical response [133▪]. Of eight patients who received valganciclovir, only three responded. This may be due to the fact that for B cells already infected with KSHV, ganciclovir blocks a late step in the KSHV lytic cycle and would not be expected to suppress vIL-6, an early lytic gene [134–136].
Virus activated cytotoxic therapy
Unlike most herpesvirus-induced tumors, many KSHV-infected plasmablasts in KSHV-MCD express lytic viral genes that can provide targets for selective cytotoxicity. In addition to ganciclovir, KSHV ORF21 phosphorylates zidovudine (AZT) [128,130]. Although antiviral activity of these drugs is through inhibition of viral DNA replication, their triphosphate moieties have cytotoxic effects at relatively high concentrations in PEL cells in which KSHV lytic genes are activated; these effects are at least additive [128]. This virus-induced cytotoxic activity is distinct from the antiviral activity of these drugs. On the basis of these findings, we performed a pilot study of high-dose AZT and VGC in 14 patients with symptomatic KSHVMCD. Patients received AZT 600 mg every 6 h combined with VGC 900 mg every 12 h. Eighty-six percent of the treated patients obtained a clinical partial response (PR) or better, and 50% obtained a biochemical PR or better. Most patients had decreased adenopathy and splenomegaly, with 36% meeting criteria for PR or better. From baseline to time of best response, KSHV viral load, IL-6, IL-10 and CRP all decreased significantly. All five patients with detectable serum vIL-6 at baseline had decreases with therapy. These results support the activity of virus-activated cytotoxic therapy in KSHV-MCD. However, high-dose AZT combined with VGC has limitations. Toxicities are mainly hematologic, prohibiting continuous administration. Therapy was generally administered on days 1–7 of a 21-day cycle. Also, whereas long-term efficacy was observed in three of 14 patients (21%), additional therapies were required in the other patients because of persistent or recurrent KSHVMCD symptoms [32▪▪]. This regimen may be best utilized in patients with mild disease or in combination with other modalities. Future studies will be needed to define the best use of this approach.
Experimental approaches
Outcomes in therapeutic trials of rituximab and AZT and VCG have been considerably better than historical controls [31,102]. Nonetheless, KSHV-MCD remains a challenging disease. Relapses are common, current approaches have toxicities and have not been standardized, and new effective therapies are urgently needed. With improved understanding of KSHV biology and KSHV-MCD pathogenesis, several approaches merit exploration. One is targeting IL-6 using monoclonal antibodies. Tocilizumab is a humanized anti-IL-6 receptor (gp80) antibody [137–139], whereas siltuximab (formerly CNTO 328) [140] and sirukumab (CNTO 136) [141] are human monoclonal antibodies to IL-6. Tocilizumab and siltuximab have demonstrated activity in KSHV-negative MCD [140,142,143]. Although targeting human IL-6 does not directly affect vIL-6 signaling, it may still be sufficient in KSHV-MCD. Indeed, two patients with KSHV-MCD have been reported to respond to tocilizumab [143]. Tocilizumab is currently being evaluated alone and in combination with AZT and VGC (NCT01441063). Other agents worth considering for future studies include mTOR [144] inhibitors or newer immune modulators derivatives of thalidomide (IMiDs).
CONCLUSION
KSHV-MCD can be difficult to recognize and diagnose. Clinicians should be alert for this condition in HIV-infected patients with persistent, intermittent fever, especially if they have Kaposi sarcoma or are in a risk group for KSHV infection. Targeted therapy, combined with HAART, has led to substantial improvements in patient survival. Unlike early series in which median survival was about 12 months [31], overall survival with current targeted therapies exceeds 85% at 1 year and sustained responses are often seen [32▪▪,33▪,109,133▪]. Effective therapy for KSHV-MCD, especially rituximab, may also be associated with decreased risk of subsequent development of lymphoma [145], although this observation requires confirmation. Future studies evaluating novel therapies or rational combinations may further improve outcomes, limit toxicities and allow for personalized approaches in patients with KSHV-MCD, with concurrent KSHV-associated malignancies or with relapsed KSHV-MCD. Additional studies of biomarkers of KSHV viral reservoirs, such as KSHV viral load, or novel imaging modalities [146] may lead to improved risk stratification and allow for evaluation of consolidation therapy beyond resolution of symptoms. With improved recognition of KSHV-MCD and ongoing therapeutic advances, KSHV-MCD should continue to move from being a highly fatal disorder to a manageable manifestation of chronic KSHV infection.
KEY POINTS.
KSHV-MCD is a rare lymphoproliferative disorder diagnosed by biopsy, which shows characteristic findings of MCD with evidence of KSHV infection and KSHV-encoded viral IL-6.
KSHV-MCD most often arises in patients with HIV, and can occur despite suppressed HIV and a relatively preserved CD4 count.
KSHV-MCD presents with waxing and waning inflammatory symptoms that may include fevers, fatigue, nonspecific respiratory and gastrointestinal symptoms, adenopathy, splenomegaly, anemia, thrombocytopenia, low albumin and elevated inflammatory markers such as C-reactive protein.
KSHV-MCD is likely underdiagnosed, especially in Africa, where HIV and KSHV coinfection is common.
Targeted therapies for KSHV-MCD, including virus-activated cytotoxic therapy and rituximab, control symptoms and improve survival in patients with KSHV-MCD.
Acknowledgements
We thank Stefania Pittaluga for review of pathology and providing pathology images; Yoshi Aoki, Giovanna Tosato, and Victoria Wang for work with the vIL-6 assay; Denise Whitby and Vickie Marshall for KSHV virologic testing; and Karen Aleman and Kathleen Wyvill for patient care.
This research was supported in part by the Intramural Research Program, National Cancer Institute (NCI), NIH. Additional funding comes from the NCI, NIH, under Contract No. HHSN261200800001E.
Footnotes
Conflicts of interest
The spouse of one of the authors (RY) is a co-inventor on a patent describing the measurement of KSHV vIL-6. This invention was made when the inventor was an employee of the US Government under 45 Code of Federal Regulations Part 7. All rights, title, and interest to this patent have been assigned to the US Department of Health and Human Services. The government conveys a portion of the royalties it received to its employee inventors under the Federal Technology Transfer Act of 1986 (P.L. 99–502). The authors have a Cooperative Research and Development Agreement with Celegene Corporation to develop pomalidomide for KS.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
- 1.Soulier J, Grollet L, Oksenhendler E, et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood 1995; 86:1276–1280. [PubMed] [Google Scholar]
- 2.Oksenhendler E, Carcelain G, Aoki Y, et al. High levels of human herpesvirus 8 viral load, human interleukin-6, interleukin-10, and C reactive protein correlate with exacerbation of multicentric castleman disease in HIV-infected patients. Blood 2000; 96:2069–2073. [PubMed] [Google Scholar]
- 3.Chang Y, Cesarman E, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science 1994; 266:1865–1869. [DOI] [PubMed] [Google Scholar]
- 4.Cesarman E, Chang Y, Moore PS, et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med 1995; 332:1186–1191. [DOI] [PubMed] [Google Scholar]
- 5.Nador RG, Cesarman E, Chadburn A, et al. Primary effusion lymphoma: a distinct clinicopathologic entity associated with the Kaposi’s sarcoma-associated herpes virus. Blood 1996; 88:645–656. [PubMed] [Google Scholar]
- 6.Uldrick TS, Wang V, O’Mahony D, et al. An Interleukin-6-Related Systemic Inflammatory Syndrome in Patients Co-Infected with Kaposi Sarcoma-Associated Herpesvirus and HIV but without Multicentric Castleman Disease. Clin Infect Dis 2010; 51:350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.▪.Polizzotto MN, Uldrick TS, Hu D, Yarchoan R. Clinical Manifestations of Kaposi Sarcoma Herpesvirus Lytic Activation: Multicentric Castleman Disease (KSHV-MCD) and the KSHV Inflammatory Cytokine Syndrome. Front Microbiol 2012; 3:73.Review of manifestations of KICS with proposed working case definition.
- 8.Castleman B, Towne VW. CASE records of the Massachusetts General Hospital Weekly Clinicopathological Exercises: Case 40011. N Engl J Med 1954; 250:26–30. [DOI] [PubMed] [Google Scholar]
- 9.Castleman B, Iverson L, Menendez VP. Localized mediastinal lymphnode hyperplasia resembling thymoma. Cancer 1956; 9:822–830. [DOI] [PubMed] [Google Scholar]
- 10.Keller AR, Hochholzer L, Castleman B. Hyaline-vascular and plasma-cell types of giant lymph node hyperplasia of the mediastinum and other locations. Cancer 1972; 29:670–683. [DOI] [PubMed] [Google Scholar]
- 11.Dupin N, Diss TL, Kellam P, et al. HHV-8 is associated with a plasmablastic variant of Castleman disease that is linked to HHV-8-positive plasmablastic lymphoma. Blood 2000; 95:1406–1412. [PubMed] [Google Scholar]
- 12.Yoshizaki K, Matsuda T, Nishimoto N, et al. Pathogenic significance of interleukin-6 (IL-6/BSF-2) in Castleman’s disease. Blood 1989; 74:1360–1367. [PubMed] [Google Scholar]
- 13.Brandt SJ, Bodine DM, Dunbar CE, Nienhuis AW. Dysregulated interleukin 6 expression produces a syndrome resembling Castleman’s disease in mice. J Clin Invest 1990; 86:592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McClain KL, Natkunam Y, Swerdlow SH. Atypical cellular disorders. Hematol Am Soc Hematol Educ Program 2004; 2004:283–296. [DOI] [PubMed] [Google Scholar]
- 15.Cronin DM, Warnke RA. Castleman disease: an update on classification and the spectrum of associated lesions. Adv Anat Pathol 2009; 16:236–246. [DOI] [PubMed] [Google Scholar]
- 16.Lin O, Frizzera G. Angiomyoid and follicular dendritic cell proliferative lesions in Castleman’s disease of hyaline-vascular type: a study of 10 cases. Am J Surg Pathol 1997; 21:1295–1306. [DOI] [PubMed] [Google Scholar]
- 17.Lorenzi L, Lonardi S, Petrilli G, et al. Folliculocentric B-cell-rich follicular dendritic cells sarcoma: a hitherto unreported morphological variant mimicking lymphoproliferative disorders. Hum Pathol 2011; 43:209–215. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen DT, Diamond LW, Hansmann ML, et al. Castleman’s disease. Differences in follicular dendritic network in the hyaline vascular and plasma cell variants. Histopathology 1994; 24:437–443. [DOI] [PubMed] [Google Scholar]
- 19.Larroche C, Cacoub P, Soulier J, et al. Castleman’s disease and lymphoma: report of eight cases in HIV-negative patients and literature review. Am J Hematol 2002; 69:119–126. [DOI] [PubMed] [Google Scholar]
- 20.Molinie V, Perie G, Melo I, et al. [Association of Castleman’s disease and Hodgkin’s disease. Eight cases and review of the literature]. Ann Pathol 1994; 14:384–391. [PubMed] [Google Scholar]
- 21.Belec L, Mohamed AS, Authier FJ, et al. Human herpesvirus 8 infection in patients with POEMS syndrome-associated multicentric Castleman’s disease. Blood 1999; 93:3643–3653. [PubMed] [Google Scholar]
- 22.Belec L, Authier FJ, Mohamed AS, et al. Antibodies to human herpesvirus 8 in POEMS (polyneuropathy, organomegaly, endocrinopathy, M protein, skin changes) syndrome with multicentric Castleman’s disease. Clin Infect Dis 1999; 28:678–679. [DOI] [PubMed] [Google Scholar]
- 23.Dispenzieri A POEMS syndrome: 2011 update on diagnosis, risk-stratification, and management. Am J Hematol 2011; 86:591–601. [DOI] [PubMed] [Google Scholar]
- 24.Dispenzieri A POEMS syndrome. Blood Rev 2007; 21:285–299. [DOI] [PubMed] [Google Scholar]
- 25.Grimm KE, Barry TS, Chizhevsky V, et al. Histopathological findings in 29 lymph node biopsies with increased IgG4 plasma cells. Mod Pathol 2011; 25:480–491. [DOI] [PubMed] [Google Scholar]
- 26.Foss HD, Araujo I, Demel G, et al. Expression of vascular endothelial growth factor in lymphomas and Castleman’s disease. J Pathol 1997; 183:44–50. [DOI] [PubMed] [Google Scholar]
- 27.Nishi J, Maruyama I. Increased expression of vascular endothelial growth factor (VEGF) in Castleman’s disease: proposed pathomechanism of vascular proliferation in the affected lymph node. Leuk Lymphoma 2000; 38:387–394. [DOI] [PubMed] [Google Scholar]
- 28.Fardet L, Blum L, Kerob D, et al. Human herpesvirus 8-associated hemophagocytic lymphohistiocytosis in human immunodeficiency virus-infected patients. Clin Infect Dis 2003; 37:285–291. [DOI] [PubMed] [Google Scholar]
- 29.Stebbing J, Ngan S, Ibrahim H, et al. The successful treatment of haemophagocytic syndrome in patients with human immunodeficiency virus-associated multicentric Castleman’s disease. Clin Exp Immunol 2008; 154:399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bower M How I treat HIV-associated multicentric Castleman’s disease. Blood 2010; 116:4415–4421. [DOI] [PubMed] [Google Scholar]
- 31.Oksenhendler E, Duarte M, Soulier J, et al. Multicentric Castleman’s disease in HIV infection: a clinical and pathological study of 20 patients. Aids 1996; 10:61–67. [PubMed] [Google Scholar]
- 32.▪▪.Uldrick TS, Polizzotto MN, Aleman K, et al. High-dose zidovudine plus valganciclovir for Kaposi sarcoma herpesvirus-associated multicentric Castleman disease: a pilot study of virus-activated cytotoxic therapy. Blood 2011; 117:6977–6986.First clinical study of virus-activated cytotoxic therapy for KSHV-MCD.
- 33.▪.Bower M, Newsom-Davis T, Naresh K, et al. Clinical Features and Outcome in HIV-Associated Multicentric Castleman’s Disease. J Clin Oncol 2011;29:2481–2486.Recent cohort data decribing clinical characteristics of KSHV-MCD, confirms improved overall survival compared with historical controls.
- 34.Hoffmann C, Tabrizian S, Wolf E, et al. Survival of AIDS patients with primary central nervous system lymphoma is dramatically improved by HAART-induced immune recovery. Aids 2001; 15:2119–2127. [DOI] [PubMed] [Google Scholar]
- 35.Oksenhendler E, Boulanger E, Galicier L, et al. High incidence of Kaposi sarcoma-associated herpesvirus-related non-Hodgkin lymphoma in patients with HIV infection and multicentric Castleman disease. Blood 2002; 99:2331–2336. [DOI] [PubMed] [Google Scholar]
- 36.▪.Gerard L, Michot JM, Burcheri S, et al. Rituximab decreases the risk of lymphoma in patients with HIV-associated multicentric Castleman disease.Blood 2012; 119:2228–2233.Retrospective cohort data showing improved survival and fewer diagnoses of lymphoma in patients receiving rituximab as compared with other therapies for KSHV-MCD.
- 37.Carbone A, Cesarman E, Spina M, et al. HIV-associated lymphomas and gamma-herpesviruses. Blood 2009; 113:1213–1224. [DOI] [PubMed] [Google Scholar]
- 38.Ramasamy I, Brisco M, Morley A. Improved PCR method for detecting monoclonal immunoglobulin heavy chain rearrangement in B cell neoplasms. J Clin Pathol 1992; 45:770–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Dongen JJ, Langerak AW, Bruggemann M, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98–3936. Leukemia 2003; 17:2257–2317. [DOI] [PubMed] [Google Scholar]
- 40.▪.Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst 2011; 103:753–762.Recent data estimating burden of AIDS-defining and non AIDS-defining malignancies in the United States.
- 41.Powles T, Stebbing J, Bazeos A, et al. The role of immune suppression and HHV-8 in the increasing incidence of HIV-associated multicentric Castle-man’s disease. Ann Oncol 2009; 20:775–779. [DOI] [PubMed] [Google Scholar]
- 42.Biggar RJ, Chaturvedi AK, Goedert JJ, Engels EA. AIDS-related cancer and severity of immunosuppression in persons with AIDS. J Natl Cancer Inst 2007; 99:962–972. [DOI] [PubMed] [Google Scholar]
- 43.Guihot A, Dupin N, Marcelin AG, et al. Low T cell responses to human herpesvirus 8 in patients with AIDS-related and classic Kaposi sarcoma. J Infect Dis 2006; 194:1078–1088. [DOI] [PubMed] [Google Scholar]
- 44.Guihot A, Oksenhendler E, Galicier L, et al. Multicentric Castleman disease is associated with polyfunctional effector memory HHV-8-specific CD8 T cells. Blood 2008; 111:1387–1395. [DOI] [PubMed] [Google Scholar]
- 45.Burbelo PD, Issa AT, Ching KH, et al. Distinct profiles of antibodies to Kaposi sarcoma-associated herpesvirus antigens in patients with Kaposi sarcoma, multicentric Castleman disease, and primary effusion lymphoma. J Infect Dis 2010; 201:1919–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao S-J, Kingsley L, Zheng ML, et al. KSHV antibodies among Americans, Italians, and Ugandans with and without Kaposi’s sarcoma. Nat Med 1996; 2:925–928. [DOI] [PubMed] [Google Scholar]
- 47.Maskew M, Macphail AP, Whitby D, et al. Prevalence and predictors of kaposi sarcoma herpes virus seropositivity: a cross-sectional analysis of HIV-infected adults initiating ART in Johannesburg, South Africa. Infect Agent Cancer 2011; 6:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.▪▪.Shebl FM, Dollard SC, Pfeiffer RM, et al. Human herpesvirus 8 seropositivity among sexually active adults in Uganda. PLoS One 2011; 6:e21286.References [47,48▪▪] provide evidence of a very high prevalence of HIV and KSHV coinfection in sub-Saharan Africa.
- 49.Chatterjee M, Osborne J, Bestetti G, et al. Viral IL-6-induced cell proliferation and immune evasion of interferon activity. Science 2002; 298:1432–1435. [DOI] [PubMed] [Google Scholar]
- 50.Aoki Y, Jaffe ES, Chang Y, et al. Angiogenesis and hematopoiesis induced by Kaposi’s sarcoma-associated herpesvirus-encoded interleukin-6. Blood 1999; 93:4034–4043. [PubMed] [Google Scholar]
- 51.Molden J, Chang Y, You Y, et al. A Kaposi’s sarcoma-associated herpesvirus-encoded cytokine homolog (vIL-6) activates signaling through the shared gp130 receptor subunit. J Biol Chem 1997; 272:19625–19631. [DOI] [PubMed] [Google Scholar]
- 52.Aoki Y, Narazaki M, Kishimoto T, Tosato G. Receptor engagement by viral interleukin-6 encoded by Kaposi sarcoma-associated herpesvirus. Blood 2001; 98:3042–3049. [DOI] [PubMed] [Google Scholar]
- 53.Chow D, He X, Snow AL, et al. Structure of an extracellular gp130 cytokine receptor signaling complex. Science 2001; 291:2150–2155. [DOI] [PubMed] [Google Scholar]
- 54.Osborne J, Moore PS, Chang Y. KSHV-encoded viral IL-6 activates multiple human IL-6 signaling pathways. Hum Immunol 1999; 60:921–927. [DOI] [PubMed] [Google Scholar]
- 55.Chen D, Sandford G, Nicholas J. Intracellular signaling mechanisms and activities of human herpesvirus 8 interleukin-6. J Virol 2009; 83:722–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hideshima T, Chauhan D, Teoh G, et al. Characterization of signaling cascades triggered by human interleukin-6 versus Kaposi’s sarcoma-associated herpes virus-encoded viral interleukin 6. Clin Cancer Res 2000; 6:1180–1189. [PubMed] [Google Scholar]
- 57.Meads M Kaposi’s sarcoma-associated herpesvirus-encoded viral interleukin-6 is secreted and modified differently than human interleukin-6 - Evidence for a unique autocrine signaling mechanism. J Biol Chem 2004; 279:51793–51803. [DOI] [PubMed] [Google Scholar]
- 58.Breen EC, Gage JR, Guo B, et al. Viral interleukin 6 stimulates human peripheral blood B cells that are unresponsive to human interleukin 6. Cell Immunol 2001; 212:118–125. [DOI] [PubMed] [Google Scholar]
- 59.Chaudhary PM, Jasmin A, Eby MT, Hood L. Modulation of the NF-kappa B pathway by virally encoded death effector domains-containing proteins. Oncogene 1999; 18:5738–5746. [DOI] [PubMed] [Google Scholar]
- 60.Punj V, Matta H, Schamus S, Chaudhary PM. Integrated microarray and multiplex cytokine analyses of Kaposi’s Sarcoma Associated Herpesvirus viral FLICE Inhibitory Protein K13 affected genes and cytokines in human blood vascular endothelial cells. BMC Med Genomics 2009; 2:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lei X, Bai Z, Ye F, et al. Regulation of NF-kappaB inhibitor IkappaBalpha and viral replication by a KSHV microRNA. Nat Cell Biol 2010; 12:193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pati S, Cavrois M, Guo HG, et al. Activation of NF-kappaB by the human herpesvirus 8 chemokine receptor ORF74: evidence for a paracrine model of Kaposi’s sarcoma pathogenesis. J Virol 2001; 75:8660–8673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fedorak RN, Gangl A, Elson CO, et al. Recombinant human interleukin 10 in the treatment of patients with mild to moderately active Crohn’s disease. The Interleukin 10 Inflammatory Bowel Disease Cooperative Study Group. Gastroenterology 2000; 119:1473–1482. [DOI] [PubMed] [Google Scholar]
- 64.Tilg H, Ulmer H, Kaser A, Weiss G. Role of IL-10 for induction of anemia during inflammation. J Immunol 2002; 169:2204–2209. [DOI] [PubMed] [Google Scholar]
- 65.▪▪.Ballon G, Chen K, Perez R, et al. Kaposi sarcoma herpesvirus (KSHV) vFLIP oncoprotein induces B cell transdifferentiation and tumorigenesis in mice.J Clin Invest 2011; 121:1141–1153.Evidence for a role of the latently expressed KSHV protein vFLIP in B-cell proliferation.
- 66.Screpanti I, Romani L, Musiani P, et al. Lymphoproliferative disorder and imbalanced T-helper response in C/EBP beta-deficient mice. EMBO J 1995; 14:1932–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Screpanti I, Musiani P, Bellavia D, et al. Inactivation of the IL-6 gene prevents development of multicentric Castleman’s disease in C/EBP beta-deficient mice. J Exp Med 1996; 184:1561–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qin Z, Kearney P, Plaisance K, Parsons CH. Pivotal advance: Kaposi’s sarcoma-associated herpesvirus (KSHV)-encoded microRNA specifically induce IL-6 and IL-10 secretion by macrophages and monocytes. J Leukoc Biol 2010; 87:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.▪▪.Boss IW, Nadeau PE, Abbott JR, et al. A Kaposi’s sarcoma-associated herpesvirus-encoded ortholog of microRNA miR-155 induces human splenic B-cell expansion in NOD/LtSz-scid IL2Rgammanull mice. J Virol 2011; 85:9877–9886.References [61,68,69▪▪] are advances in the field of miRNA biology, demonstrating interactions between KSHV-encoded miRNA and human genes that modulate immune responses and may contribute to KSHV-MCD pathogenesis.
- 70.Pauk J, Huang ML, Brodie SJ, et al. Mucosal shedding of human herpesvirus 8 in men. N Engl J Med 2000; 343:1369–1377. [DOI] [PubMed] [Google Scholar]
- 71.Casper C, Krantz E, Selke S, et al. Frequent and Asymptomatic Oropharyngeal Shedding of Human Herpesvirus 8 among Immunocompetent Men. J Infect Dis 2007; 195:30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Casper C, Krantz EM, Corey L, et al. Valganciclovir for suppression of human herpesvirus-8 replication: a randomized, double-blind, placebo-controlled, crossover trial. J Infect Dis 2008; 198:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.▪▪.Cattamanchi A, Saracino M, Selke S, et al. Treatment with valacyclovir, famciclovir, or antiretrovirals reduces human herpesvirus-8 replication in HIV-1 seropositive men. J Med Virol 2011; 83:1696–1703.Demonstration that both antiherpesvirus therapy and antiretroviral therapy effect oral KSHV replication in HIV infected individuals.
- 74.Marcelin AG, Gorin I, Morand P, et al. Quantification of Kaposi’s sarcoma-associated herpesvirus in blood, oral mucosa, and saliva in patients with Kaposi’s sarcoma. AIDS Res Hum Retroviruses 2004; 20:704–708. [DOI] [PubMed] [Google Scholar]
- 75.Ambroziak JA, Blackbourn DJ, Herndier BG, et al. Herpes-like sequences in HIV-infected and uninfected Kaposi’s sarcoma patients. Science 1995; 268:582–583. [DOI] [PubMed] [Google Scholar]
- 76.Mesri EA, Cesarman E, Arvanitakis L, et al. Human herpesvirus-8/Kaposi’s sarcoma-associated herpesvirus is a new transmissible virus that infects B cells. J Exp Med 1996; 183:2385–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grandadam M, Dupin N, Calvez V, et al. Exacerbations of clinical symptoms in human immunodeficiency virus type 1-infected patients with multicentric Castleman’s disease are associated with a high increase in Kaposi’s sarcoma herpesvirus DNA load in peripheral blood mononuclear cells. J Infect Dis 1997; 175:1198–1201. [DOI] [PubMed] [Google Scholar]
- 78.Casper C, Nichols WG, Huang M-L, et al. Remission of HHV-8 and HIV-associated multicentric Castleman disease with ganciclovir treatment. Blood 2004; 103:1632–1634. [DOI] [PubMed] [Google Scholar]
- 79.Marcelin AG, Motol J, Guihot A, et al. Relationship between the quantity of Kaposi sarcoma-associated herpesvirus (KSHV) in peripheral blood and effusion fluid samples and KSHV-associated disease. J Infect Dis 2007; 196:1163–1166. [DOI] [PubMed] [Google Scholar]
- 80.Tedeschi R, Enbom M, Bidoli E, et al. Viral load of human herpesvirus 8 in peripheral blood of human immunodeficiency virus-infected patients with Kaposi’s sarcoma. J Clin Microbiol 2001; 39:4269–4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Harrington WJ Jr, Bagasra O, Sosa CE, et al. Human herpesvirus type 8 DNA sequences in cell-free plasma and mononuclear cells of Kaposi’s sarcoma patients. J Infect Dis 1996; 174:1101–1105. [DOI] [PubMed] [Google Scholar]
- 82.Chadburn A, Hyjek EM, Tam W, et al. Immunophenotypic analysis of the Kaposi sarcoma herpesvirus (KSHV; HHV-8)-infected B cells in HIV+ multicentric Castleman disease (MCD). Histopathology 2008; 53:513–524. [DOI] [PubMed] [Google Scholar]
- 83.Abe Y, Matsubara D, Gatanaga H, et al. Distinct expression of Kaposi’s sarcoma-associated herpesvirus-encoded proteins in Kaposi’s sarcoma and multicentric Castleman’s disease. Pathol Int 2006; 56:617–624. [DOI] [PubMed] [Google Scholar]
- 84.Parravicini C, Corbellino M, Paulli M, et al. Expression of a virus-derived cytokine, KSHV vIL-6, in HIV-seronegative Castleman’s disease. Am J Pathol 1997; 151:1517–1522. [PMC free article] [PubMed] [Google Scholar]
- 85.Staskus KA, Sun R, Miller G, et al. Cellular tropism and viral interleukin-6 expression distinguish human herpesvirus 8 involvement in Kaposi’s sarcoma, primary effusion lymphoma, and multicentric Castleman’s disease. J Virol 1999; 73:4181–4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Du MQ, Liu H, Diss TC, et al. Kaposi sarcoma-associated herpesvirus infects monotypic (IgM lambda) but polyclonal naive B cells in Castleman disease and associated lymphoproliferative disorders. Blood 2001; 97:2130–2136. [DOI] [PubMed] [Google Scholar]
- 87.Blasig C, Zietz C, Haar B, et al. Monocytes in Kaposi’s sarcoma lesions are productively infected by human herpesvirus 8. J Virol 1997; 71:7963–7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Renne R, Blackbourn D, Whitby D, et al. Limited transmission of Kaposi’s sarcoma-associated herpesvirus in cultured cells. J Virol 1998; 72:5182–5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Blackbourn DJ, Lennette E, Klencke B, et al. The restricted cellular host range of human herpesvirus 8. AIDS 2000; 14:1123–1133. [DOI] [PubMed] [Google Scholar]
- 90.Webster-Cyriaque J, Duus K, Cooper C, Duncan M. Oral EBV and KSHV infection in HIV. Adv Dent Res 2006; 19:91–95. [DOI] [PubMed] [Google Scholar]
- 91.Chakraborty S, Veettil MV, Chandran B. Kaposi’s Sarcoma Associated Herpesvirus Entry into Target Cells. Front Microbiol 2012; 3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Akula SM, Pramod NP, Wang FZ, Chandran B. Integrin alpha3beta1 (CD 49c/29) is a cellular receptor for Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 2002; 108:407–419. [DOI] [PubMed] [Google Scholar]
- 93.Veettil MV, Sadagopan S, Sharma-Walia N, et al. Kaposi’s sarcoma-associated herpesvirus forms a multimolecular complex of integrins (alphaVbeta5, alphaVbeta3, and alpha3beta1) and CD98-xCT during infection of human dermal microvascular endothelial cells, and CD98-xCT is essential for the postentry stage of infection. J Virol 2008; 82:12126–12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kerur N, Veettil MV, Sharma-Walia N, et al. Characterization of entry and infection of monocytic THP-1 cells by Kaposi’s sarcoma associated herpes-virus (KSHV): role of heparan sulfate, DC-SIGN, integrins and signaling. Virology 2010; 406:103–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rappocciolo G, Hensler HR, Jais M, et al. Human herpesvirus 8 infects and replicates in primary cultures of activated B lymphocytes through DC-SIGN. J Virol 2008; 82:4793–4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rappocciolo G, Jenkins FJ, Hensler HR, et al. DC-SIGN Is a Receptor for Human Herpesvirus 8 on Dendritic Cells and Macrophages. J Immunol 2006; 176:1741–1749. [DOI] [PubMed] [Google Scholar]
- 97.Lozach PY, Burleigh L, Staropoli I, Amara A. The C type lectins DC-SIGN and L-SIGN: receptors for viral glycoproteins. Methods Mol Biol 2007; 379:51–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.▪▪.Hassman LM, Ellison TJ, Kedes DH. KSHV infects a subset of human tonsillar B cells, driving proliferation and plasmablast differentiation. J Clin Invest 2011; 121:752–768.Recent advances in the biology of B-cell infection by KSHV, with relevance to KSHV-MCD.
- 99.Jourdan M, Caraux A, De Vos J, et al. An in vitro model of differentiation of memory B cells into plasmablasts and plasma cells including detailed phenotypic and molecular characterization. Blood 2009; 114:5173–5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dalton-Griffin L, Wilson SJ, Kellam P. X-box binding protein 1 contributes to induction of the Kaposi’s sarcoma-associated herpesvirus lytic cycle under hypoxic conditions. J Virol 2009; 83:7202–7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.▪▪.Ray A, Marshall V, Uldrick T, et al. Sequence Analysis of KSHV microRNAs in patients with Multicentric Castleman Disease and KSHV-associated Inflammatory Cytokine Syndrome. J Infect Dis 2012; 205:1665–1676.Evidence that patients with KSHV-MCD or KICS tend to have ceratin polymorphisms in KSHV-encoded miRNA.
- 102.Mylona EE, Baraboutis IG, Lekakis LJ, et al. Multicentric Castleman’s disease in HIV infection: a systematic review of the literature. AIDS Rev 2008; 10:25–35. [PubMed] [Google Scholar]
- 103.Scott D, Cabral L, Harrington WJ Jr. Treatment of HIV-associated multi-centric Castleman’s disease with oral etoposide. Am J Hematol 2001; 66:148–150. [DOI] [PubMed] [Google Scholar]
- 104.▪▪.Polizzotto MN, Jones P, Cameron PU, et al. The influence of splenectomy on the infectious complications and outcomes of people with HIV: marked, sustained elevation in risk of severe infection with bacteria including Streptococcus pneumoniae. J Acquir Immune Defic Syndr 2010; 55:e24–e26.Strong evidence against splenectomy in patients with HIV.
- 105.Gill PS, Loureiro C, Bernstein-Singer M, et al. Clinical effect of glucocorticoids on Kaposi sarcoma related to the acquired immunodeficiency syndrome (AIDS). Ann Intern Med 1989; 110:937–940. [DOI] [PubMed] [Google Scholar]
- 106.Nord JA, Karter D. Low dose interferon-alpha therapy for HIV-associated multicentric Castleman’s disease. Int J STD AIDS 2003; 14:61–62. [DOI] [PubMed] [Google Scholar]
- 107.Kumari P, Schechter GP, Saini N, Benator DA. Successful treatment of human immunodeficiency virus-related Castleman’s disease with interferon-alpha. Clin Infect Dis 2000; 31:602–604. [DOI] [PubMed] [Google Scholar]
- 108.Lee FC, Merchant SH. Alleviation of systemic manifestations of multicentric Castleman’s disease by thalidomide. Am J Hematol 2003; 73:48–53. [DOI] [PubMed] [Google Scholar]
- 109.Gerard L, Berezne A, Galicier L, et al. Prospective study of rituximab in chemotherapy-dependent human immunodeficiency virus associated multi-centric Castleman’s disease: ANRS 117 CastlemaB Trial. J Clin Oncol 2007; 25:3350–3356. [DOI] [PubMed] [Google Scholar]
- 110.Bower M, Powles T, Williams S, et al. Brief communication: rituximab in HIV-associated multicentric Castleman disease. Ann Intern Med 2007; 147:836–839. [DOI] [PubMed] [Google Scholar]
- 111.▪.Stebbing J, Adams C, Sanitt A, et al. Plasma HHV8 DNA predicts relapse in individuals with HIV-associated multicentric Castleman disease. Blood 2011; 118:271–275.Demonstration that KSHV viremia may predict recurrent symptoms in KSHV-MCD.
- 112.Shortt J, Spencer A. Adjuvant rituximab causes prolonged hypogammaglobulinaemia following autologous stem cell transplant for non-Hodgkin’s lymphoma. Bone Marrow Transplant 2006; 38:433–436. [DOI] [PubMed] [Google Scholar]
- 113.Stebbing J, Gazzard B, Newsom-Davis T, et al. Nadir B cell counts are significantly correlated with the risk of Kaposi’s sarcoma. Int J Cancer 2004; 108:473–474. [DOI] [PubMed] [Google Scholar]
- 114.Huang KH, Bonsall D, Katzourakis A, et al. B-cell depletion reveals a role for antibodies in the control of chronic HIV-1 infection. Nat Commun 2010; 1:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kimball LE, Casper C, Koelle DM, et al. Reduced levels of neutralizing antibodies to Kaposi sarcoma-associated herpesvirus in persons with a history of Kaposi sarcoma. J Infect Dis 2004; 189:2016–2022. [DOI] [PubMed] [Google Scholar]
- 116.Kaplan LD, Lee JY, Ambinder RF, et al. Rituximab does not improve clinical outcome in a randomized phase III trial of CHOP with or without rituximab in patients with HIV-associated non-Hodgkin’s lymphoma: AIDS-malignancies consortium trial 010. Blood 2005; 106:1538–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Carson KR, Evens AM, Richey EA, et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood 2009; 113:4834–4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Neuville S, Agbalika F, Rabian C, et al. Failure of rituximab in human immunodeficiency virus-associated multicentric Castleman disease. Am J Hematol 2005; 79:337–339. [DOI] [PubMed] [Google Scholar]
- 119.Buchler T, Dubash S, Lee V, et al. Rituximab failure in fulminant multicentric HIV/human herpesvirus 8-associated Castleman’s disease with multiorgan failure: report of two cases. AIDS 2008; 22:1685–1687. [DOI] [PubMed] [Google Scholar]
- 120.Schmidt SM, Raible A, Kortum F, et al. Successful treatment of multicentric Castleman’s disease with combined immunochemotherapy in an AIDS patient with multiorgan failure. Leukemia 2008; 22:1782–1785. [DOI] [PubMed] [Google Scholar]
- 121.Naresh KN, Trivedi P, Horncastle D, Bower M. CD20 expression in the HHV-8-infected lymphoid cells in multicentric Castleman disease. Histopathology 2009; 55:358–359. [DOI] [PubMed] [Google Scholar]
- 122.Tosato G, Seamon KB, Goldman ND, et al. Monocyte-derived human B-cell growth factor identified as interferon-beta 2 (BSF-2, IL-6). Science 1988; 239:502–504. [DOI] [PubMed] [Google Scholar]
- 123.Tosato G, Tanner J, Jones KD, et al. Identification of interleukin-6 as an autocrine growth factor for Epstein-Barr virus-immortalized B cells. J Virol 1990; 64:3033–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kehrl JH, Rieckmann P, Kozlow E, Fauci AS. Lymphokine production by B cells from normal and HIV-infected individuals. Ann N Y Acad Sci 1992; 651:220–227. [DOI] [PubMed] [Google Scholar]
- 125.Kitani A, Hara M, Hirose T, et al. Autostimulatory effects of IL-6 on excessive B cell differentiation in patients with systemic lupus erythematosus: analysis of IL-6 production and IL-6R expression. Clin Exp Immunol 1992; 88:75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Burdin N, Rousset F, Banchereau J. B-cell-derived IL-10: production and function. Methods 1997; 11:98–111. [DOI] [PubMed] [Google Scholar]
- 127.Song J, Ohkura T, Sugimoto M, et al. Human interleukin-6 induces human herpesvirus-8 replication in a body cavity-based lymphoma cell line. J Med Virol 2002; 68:404–411. [DOI] [PubMed] [Google Scholar]
- 128.Davis DA, Singer KE, Reynolds IP, et al. Hypoxia enhances the phosphor-ylation and cytotoxicity of ganciclovir and zidovudine in Kaposi’s sarcoma-associated herpesvirus infected cells. Cancer Res 2007; 67:7003–7010. [DOI] [PubMed] [Google Scholar]
- 129.Cannon JS, Hamzeh F, Moore S, et al. Human herpesvirus 8-encoded thymidine kinase and phosphotransferase homologues confer sensitivity to ganciclovir. J Virol 1999; 73:4786–4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gustafson EA, Schinazi RF, Fingeroth JD. Human herpesvirus 8 open reading frame 21 is a thymidine and thymidylate kinase of narrow substrate specificity that efficiently phosphorylates zidovudine but not ganciclovir. J Virol 2000; 74:684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kedes DH, Ganem D. Sensitivity of Kaposi’s sarcoma-associated herpes-virus replication to antiviral drugs. Implications for potential therapy. J Clin Invest 1997; 99:2082–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Berezne A, Agbalika F, Oksenhendler E, et al. Failure of cidofovir in HIV-associated multicentric Castleman disease. Blood 2004; 103:4368–4369. [DOI] [PubMed] [Google Scholar]
- 133.▪.Hoffmann C, Schmid H, Muller M, et al. Improved outcome with rituximab in patients with HIV-associated multicentric Castleman disease. Blood 2011;118:3499–3503.Confirmation of improved outcomes in a large cohort of patients with KSHV-MCD treated with rituximab.
- 134.Jenner RG, Alba MM, Boshoff C, Kellam P. Kaposi’s sarcoma-associated herpesvirus latent and lytic gene expression as revealed by DNA arrays. J Virol 2001; 75:891–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lu M Dissection of the Kaposi’s sarcoma-associated herpesvirus gene expression program by using the viral DNA replication inhibitor cidofovir. J Virol 2004; 78:13637–13652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Little RF, Merced-Galindez F, Staskus K, et al. A pilot study of cidofovir in patients with Kaposi’s sarcoma. J Infect Dis 2003; 187:149–153. [DOI] [PubMed] [Google Scholar]
- 137.Mihara M, Kasutani K, Okazaki M, et al. Tocilizumab inhibits signal transduction mediated by both mIL-6R and sIL-6R, but not by the receptors of other members of IL-6 cytokine family. Int Immunopharmacol 2005; 5:1731–1740. [DOI] [PubMed] [Google Scholar]
- 138.Igawa T, Ishii S, Tachibana T, et al. Antibody recycling by engineered pH-dependent antigen binding improves the duration of antigen neutralization. Nat Biotechnol 2010; 28:1203–1207. [DOI] [PubMed] [Google Scholar]
- 139.Hashizume M, Mihara M. Influence of humanized anti-IL-6R antibody, tocilizumab on the activity of soluble gp130, natural inhibitor of IL-6 signaling. Rheumatol Int 2009; 29:397–401. [DOI] [PubMed] [Google Scholar]
- 140.van Rhee F, Fayad L, Voorhees P, et al. Siltuximab, a novel antiinterleukin-6 monoclonal antibody, for Castleman’s disease. J Clin Oncol 2010; 28:3701–3708. [DOI] [PubMed] [Google Scholar]
- 141.Xu Z, Bouman-Thio E, Comisar C, et al. Pharmacokinetics, pharmacodynamics and safety of a human anti-IL-6 monoclonal antibody (sirukumab) in healthy subjects in a first-in-human study. Br J Clin Pharmacol 2011; 72:270–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nishimoto N, Kanakura Y, Aozasa K, et al. Humanized antiinterleukin-6 receptor antibody treatment of multicentric Castleman’s disease. Blood 2005:106:2627–2632. [DOI] [PubMed] [Google Scholar]
- 143.Song SN, Tomosugi N, Kawabata H, et al. Down-regulation of hepcidin resulting from long-term treatment with an anti-IL-6 receptor antibody (tocilizumab) improves anemia of inflammation in multicentric Castleman disease. Blood 2010; 116:3627–3634. [DOI] [PubMed] [Google Scholar]
- 144.He J, Chen Y, Farzan M, et al. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature 1997; 385:645–649. [DOI] [PubMed] [Google Scholar]
- 145.Gerard L, Galicier L, Maillard A, et al. Systemic non-Hodgkin lymphoma in HIV-infected patients with effective suppression of HIV replication: persistent occurrence but improved survival. J Acquir Immune Defic Syndr 2002; 30:478–484. [DOI] [PubMed] [Google Scholar]
- 146.Fu DX, Tanhehco YC, Chen J, et al. Virus-associated tumor imaging by induction of viral gene expression. Clin Cancer Res 2007; 13:1453–1458. [DOI] [PubMed] [Google Scholar]