Abstract
Background
Rheumatoid arthritis is an autoimmune disease that causes chronic joint inflammation and there is no cure. Baicalin, as an ingredient in the roots of Scutellaria baicalensis, is supposed to possess an anti-inflammatory effect. However, the protective effect of baicalin on collagen-induced arthritis requires further investigation.
Material/Methods
A model of rheumatoid arthritis was established in 20 mice (8- to 10-weeks old). The mice were randomly divided into 2 groups after modeling and then injected with saline or baicalin, respectively. The synovial fluids and tissues were collected, and the pressure pain threshold and clinical arthritis score were measured. The levels of tumor necrosis factor (TNF)-α, interlukin-1β (IL-1β), IL-6, matrix metalloproteinases (MMP)-2, MMP-9, nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2) and their downstream inflammatory mediators Janus kinase 1 (JAK1)/signal transducer and activator of transcription 3 (STAT3), extracellular signal-regulated kinases 1/2 (ERK1/2), p38, Jun N-terminal kinases (JNK) activation were detected using enzyme-linked immunosorbent assay (ELISA), and western blotting analyses. The mononuclear cells apoptosis ratio was calculated by flowcytometry analyses.
Results
Baicalin significantly reduced disease activities in a rheumatoid arthritis mouse model, which were reflected by pressure pain thresholds and clinical arthritis scores. Relevant proinflammatory cytokines such as TNF-α, IL-1β, IL-6, gelatinases (MMP-2, MMP-9) and inducible enzymes (iNOS, COX-2) were generally suppressed. Moreover, baicalin treatment induced cells apoptosis in synovial fluid monocytes and markedly down regulated JAK1/STAT3 but not mitogen-activated protein kinases (MAPKs) expressions in synovium of arthritis.
Conclusions
These observations confirm the relief of rheumatoid arthritis by baicalin. Our results indicate the effect is related with the modulation of decreased proinflammatory cytokines and inflammatory markers. And the apoptosis promotion of monocytes in synovial fluid were also inhibited. Moreover, the molecular mechanism implies suppressed JAK1/STAT3 signaling with baicalin treatment.
MeSH Keywords: Arthritis, Rheumatoid; Drugs, Chinese Herbal; Janus Kinase 1; STAT3 Transcription Factor
Background
Rheumatoid arthritis is a systemic autoimmune disease which is characterized by synovial hypertrophy, joint inflammation, and tenderness of the small joints. It is estimated that around 0.5–1.0% of adults in developed country suffer from this disease and it is also an important cause of loss of productivity and limb-related disabilities especially in women [1]. The pathophysiology of this disease has been briefly identified as the following process: initially, some unknown genetic or environmental factor activates abnormal immune responses and produces a lot of autoantibodies, such as rheumatoid factor, and antibodies to citrullinated peptides. Then activated inflammatory cell infiltration occurs resulting in edema, vasodilation, destruction of cartilage, and granulation tissue formation. In the late stage, all these changes results in ankylosis and joint deformities [2,3].
During the disease progression, activated inflammatory cells persistently secrete a large number of inflammatory mediators [e.g., IL-1α, IL-1β, IL-6, and tumor necrosis factor (TNF)-α], chemokines, matrix metalloproteinases (MMPs) and so on [4]. All of these factors can cause further inflammation represented by the expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) in tissues. These 2 inducible enzymatic pathways would produce mediators mainly as prostaglandins and nitric oxide (NO) to cause clinical symptoms [5]. All these chronic processes play a major role in the synovial and joint damage. To suppress the chronic immune overreaction of rheumatoid arthritis, the non-steroidal anti-inflammatory drugs, disease-modifying anti-rheumatic drugs, and biological agents are widely used for the disease management [6]. However, the application of these treatments has been limited due to the high incidence of side effects like abnormal liver function, birth defects, and relatively poor cost-effectiveness in developing countries [7,8]. Thus, there is an urgent need to conduct further studies for novel therapies.
Baicalin is a flavonoids compound mainly from the dry root of Scutellaria baicalensis (called Huang-Qin in Chinese traditional medicine). It was widely used in the past as a Chinese herb in treatment of febrile diseases, memory loss, and hepatic disorders [9]. Previous studies showed that it had anti-oxidative, anti-inflammatory, anti-tumor, and anti-apoptotic activities but the molecular mechanisms involved were complicated and required further investigation [10–13]. It has been suggested that baicalin’s relief of joint inflammation is partially related to the suppression of synovial nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) p65 protein expression in rats with rheumatoid arthritis [14]. However, other inflammatory mediators and chemokines have not been identified and some novel, related downstream pathways also need identification. Therefore, in this study, we used a collagen-induced rheumatoid arthritis mouse model to evaluate the potential anti-inflammatory effect of baicalin and its possible molecular mechanisms.
Material and Methods
Rheumatoid arthritis model establishment and treatment in mice
The 30 BALB/c male mice, between 8- to 10-weeks old, were purchased and maintained in an environment at 21°C to 24°C and 55% to 65% humidity with a 12-hour light-dark cycle. Food and water were provided ad libitum. This study was conducted in accordance with the National Institutes of Health Laboratory Animal Care and Use Guidelines 8th edition. The animal experiments were performed with protocols approved by the Institutional Animal Care and Use Committee at Shandong University (KYLL-2017(KS)-266). Rheumatoid arthritis mice were efficiently induced by a collagen II monoclonal antibody cocktail (MD Biosciences, USA) injection intraperitoneally at a dose of 5 mg/kg dissolved in phosphate buffered saline (PBS) for consecutive 10 days. The total amount of collagen II monoclonal antibody used was around 1.5 mg to 2 mg and the establishment of collagen antibody-induced arthritis (CAIA) was confirmed and stable in about 2 weeks, which were described in the company’s instruction and some previous reports [15–17]. The mice were also intraperitoneally injected with lipopolysaccharide (LPS; Sigma, USA) at 100 μg per mouse in 200 μL sterile PBS on day 1 or day 4.
Baicalin or saline treatment in the mice
The mice used in the experiment were randomly divided into 3 groups: the control group (n=10), the rheumatoid arthritis model + saline treatment group (n=10), and the rheumatoid arthritis model + baicalin treatment group (n=10). Mice of the rheumatoid arthritis model + saline treatment group were intraperitoneally injected with 0.9% sodium chloride (Nanjing Chemical Reagent, China) once per day for 5 consecutive days following the establishment of the collagen-induced rheumatoid arthritis model. However, mice from rheumatoid arthritis model + baicalin treatment group were injected with 30 mg/kg baicalin (Jinsui Bio-Technology, China) for 5 consecutive days. Mice in the control group were normal mice and only treated once daily with 0.9% NaCl injection during the study period.
Measurement of pain threshold
The mean value of the pressure pain thresholds was measured before and after modeling and after the 5 days of treatment. It was measured using an electronic pressure pain detector following the company’s instructions (Shanghai United Press, China).
Measurement of clinical arthritis score
On each day of the 10 days establishment and 5 days treatment, the mice were evaluated for arthritis score using a macroscopic scoring system as followings: 11–15, severe arthritis of the entire paw and digits; 6–10, more than 2 joints involved; 1–5, 2 joints involved and 0, no signs of arthritis. And the scores from each day were calculated in one after another.
Enzyme-linked immunosorbent assay (ELISA) assays
Following the treatment with baicalin or saline in the mice, blood samples were collected from retro-orbital bleeds using heparin-coated glass capillaries and stored at −80°C for analysis. TNF-α, IL-1β, and IL-6 ELISA assay kits were purchased from Ryobi Biotech in Guangzhou, China. The standards and mice serum were prepared and measured according to the manufacturer’s instructions. Optical reagents density (OD) values were measured at 450 nm.
Western blotting
The synovium tissues and enriched synovial fluids from joints were homogenized in RIPA buffer (Beyotime, China). A pierce BCA protein assay kit (Thermo Fisher Scientific, USA) were used to determine the protein concentration in lysates. Equal amounts of protein (20 μg) were loaded into 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred on a polyvinylidene difluoride (PVDF) membrane (Bio-Rad Laboratories, Germany). The PVDF membrane was blocked with 5% skimmed milk in tris-buffered saline and Tween 20 (TBST) buffer on a shaker for 1 hour at room temperature. The membrane was then incubated with anti-MMP-2 (87809), anti-MMP-9 (13667), anti-iNOS (13120), anti-COX-2 (12282), anti-JAK1 (Janus kinase 1) (3344), anti-phospho-JAK1 (Tyr1022/1023) (3331), anti-STAT3 (signal transducer and activator of transcription 3) (4904), anti-phospho-STAT3 (Tyr705) (9131), anti-phospho-p38 (Thr180/Tyr182) (4511), anti-p38 (8690), anti-phospho-JNK (Thr183/Tyr185) (4668), anti-JNK (9252), anti-phospho-ERK1/2 (Thr202/Tyr204) (4370), anti- ERK1/2 (4695) from Cell Signaling Technology, USA, and anti-β-actin (sc-130656, Santa Cruz Biotechnology, USA) or anti-GAPDH (14c10) primary antibodies overnight. After washing, the membrane was incubated with secondary antibody (7074, Cell Signaling Technology, USA) for 1 hour on the shaker at room temperature. The membrane was then incubated with chemiluminescence reagent (GE Healthcare Life Sciences, UK). The relative quantity of the protein was measured using ImageJ software (National Institutes of Health, USA).
Hematoxylin-eosin staining
Following the 5 days treatment, the joint tissues were harvested and partially fixed in formalin overnight. Briefly, after paraffin embedding, slicing, slide preparation, deparaffinization and rehydration, hematoxylin solution was used for staining with for 5 min followed by 5 dips in 1% acid ethanol (1% HCl in 70% ethanol) and immediately rinsed in distilled water. Then the sections were stained with eosin solution for 3 min and followed by dehydration with graded alcohol and clearing in xylene. The mounted slides were then examined by a pathologist and photographed using a microscope.
Cell nucleus morphological detection
Synovial fluids were directly aspirated from the joints. Mononuclear cells in synovial fluids were Mouse Monocyte Isolation kit (Miltenyi Biotec, China). Target cells washed and the cytospins were made within 30 minutes. Slides were blocked with 10% bovine serum albumin (BSA) for 30 minutes in room temperature and followed by DAPI (4′,6-diamidino-2-phenylindole) staining (Cell Signaling Technology, USA). Fluorescence microscope was used to detect the morphological changes of nucleus.
Cell apoptosis analysis
The APOAF Annexin V apoptosis kit (Sigma, USA) was used for staining annexin V on the outside of the cells according to the manufacturer’s protocol. All samples were quantified using a Canto II flow cytometer (BD Biosciences, USA) and analyzed by FlowJo software 7.6 software (TreeStar, USA). Annexin V-FITC with high affinity cells were recognized as apoptosis.
Statistical analysis
Summarized data are shown as mean ± standard error of the mean. SPSS for windows (version 17.0, SPSS, Chicago, IL, USA) was used to perform the statistical analyses. Statistical significance between 2 groups of interest was analyzed using the unpaired Student’s t-test. One-way analysis of variance (ANOVA) and the least significant difference (LSD) test were used to analyze more than 2 subgroups. The significance level was P value <0.05. Each group of experiments were repeated at least 3 times in parallel.
Results
Protective effect of baicalin against disease activity in a rheumatoid arthritis mouse model
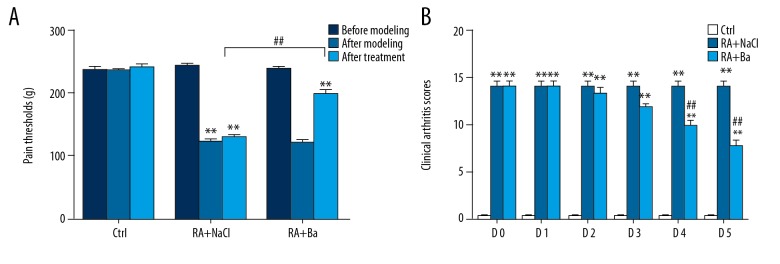
After injection of collagen II antibody, a mouse model of rheumatoid arthritis was well established. The disease onset (about day 8) and its peak (day 10) can be easily evaluated from clinical arthritis score. Furthermore, the clinical arthritis score system was also used to verify the protective effect of baicalin in a mouse model. The dosage and period of baicalin treatment from preliminary test was determined as 30 mg/kg for 5 days because this schedule showed a remarkable decrease of disease activity during the whole steady and controlled disease progression period (Supplementary Figure 1). Meanwhile, an electronic pressure pain detector was used to access the disease activity after different treatments. Compared with normal mice (n=10), a similar decrease of pain threshold was detected among the saline group (n=10) and the baicalin treatment group (n=10). However, a significant increase of pain threshold was recorded in the baicalin group just after 5 days treatment (Figure 1A). Similarly, there was a significant decrement of clinical arthritis score in the baicalin treatment group compared with the saline treatment group during 5 consecutive days (Figure 1B).
Figure 1.
Relief of rheumatoid arthritis by baicalin evaluated by pain thresholds (A) and clinical arthritic scores (B) in a mouse model. ** Compared with control group P<0.01, ## compared with saline treatment group P <0.01.
Reduction of the inflammatory mediators by baicalin in rheumatoid arthritis mouse model
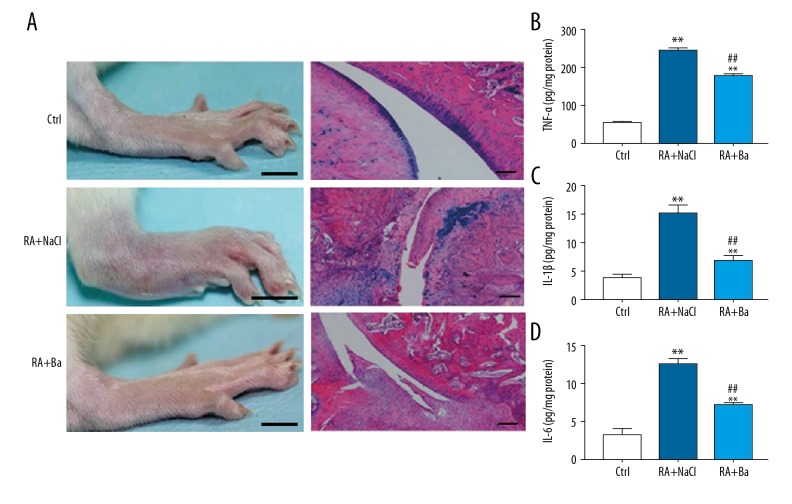
To further determine the effect of baicalin on the inflammation in mice with rheumatoid arthritis, the local histopathological changes of the ankle joint and systemic TNF-α, IL-1β, and IL-6 levels were investigated. An obvious reduction of swelling on gross and less infiltration of inflammatory cells were observed in articular cartilages. Furthermore, the limitation of cartilage degradation and joint destruction were also confirmed from the tissues samples of the rheumatoid arthritis mouse model with baicalin treatment (Figure 2A). At the same time, a large increase of TNF-α, IL-1β, and IL-6 levels in circulation were recorded after modeling (53.4±2.6 pg/mg vs. 243.6±6.74 pg/mg, 3.9±1.8 pg/mg vs. 15.2±1.4 pg/mg and 3.3±0.8 pg/mg vs. 12.5±0.9 pg/mg). However, these elevated inflammatory factors were significantly reversed in baicalin treatment group when compared with the saline group (TNF-α: 177.2±5.7 pg/mg, IL-1β: 6.9±0.8 pg/mg, and IL-6: 7.1±0.4 pg/mg) (Figure 2B–2D).
Figure 2.
Gross paw appearance and limited effect of cartilage degradation after baicalin treatment by immunohistochemical assays (A). Reduction of the inflammation against tumor necrosis factor-α (B), interlukin-1β (C), and interlukin-6 (D) levels with baicalin in a rheumatoid arthritis mouse model. ** Compared with control group P<0.01, ## compared with saline treatment group P<0.01.
Decreased MMP-2, MMP-9 expression in synovium but increased induction of monocytes apoptosis by baicalin in rheumatoid arthritis mouse model
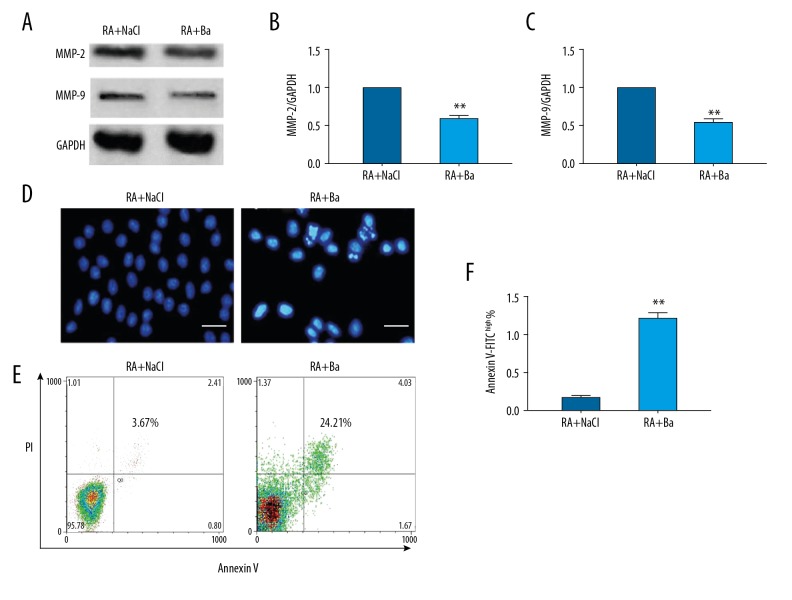
Interestingly, MMP-2 and MMP-9 expressions in synovium which are related with the dynamics of inflammatory cells, were also decreased after baicalin therapy from western blotting (Figure 3A–3C). The nuclear condensation change was a precursor sign of apoptosis. After baicalin therapy, the nuclei of infiltrated monocytes from rheumatoid arthritis mice showed more condensations when compared with saline treatment group (Figure 3D). Then the ratio of late apoptotic cells with Annexin V-FITC with high affinity was quantified by flow cytometry analysis. This confirmed that there were significantly more apoptotic cells in the baicalin treatment group compared to the saline control group (3.57±0.54% vs. 24.04±1.64%). Once representative data was plotted (Figure 3E, 3F).
Figure 3.
Impaired matrix metalloproteinase (MMP)-2, MMP-9 expression and induction of monocytes apoptosis in synovium by baicalin treatment in a rheumatoid arthritis mouse model. The MMP-2 and MMP-9 expression by western blotting (A, B) and their quantification analyses (C). The nuclear morphological changes of monocytes detected by immunofluorescence (bar=40 um) (D). Once typical apoptosis detection by flow cytometry (E) and quantification analysis (F). ** Compared with saline treatment group P<0.01.
Decreased inflammatory markers expression in synovium by baicalin in rheumatoid arthritis mouse model
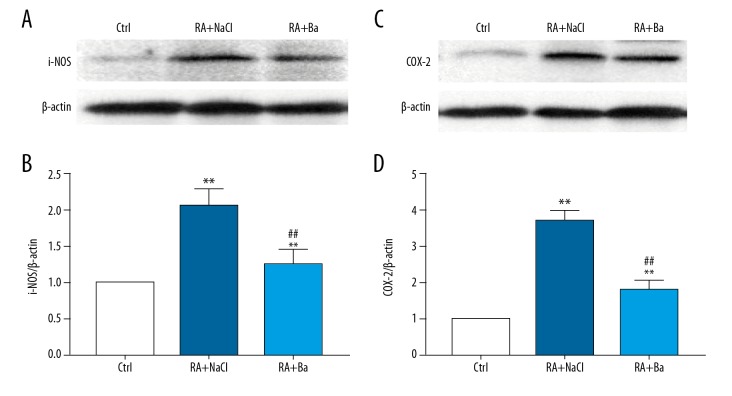
Consistent with the development of inflammation in mice with rheumatoid arthritis modeling, the inflammatory markers such as iNOS and COX-2 protein levels were elevated in synovium respectively. And baicalin therapy also significantly decreased the production of iNOS and COX-2 compared with the saline group (Figure 4A, 4B).
Figure 4.
Inhibition of inflammatory markers expression including nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2) in synovium by baicalin treatment in a rheumatoid arthritis mouse model. The iNOS and COX-2 expression by western blotting (A, C) and their quantification analyses (B, D). ** Compared with saline treatment group P<0.01, ## compared with saline treatment group P<0.01.
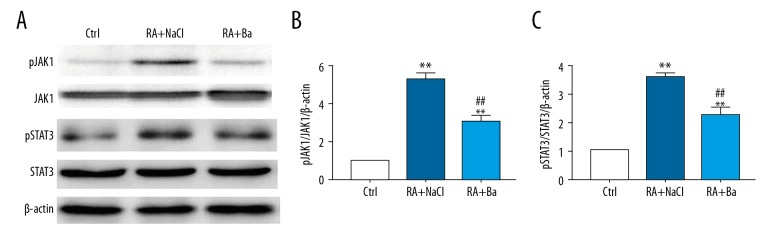
Downregulation of JAK1/STAT3 signaling by baicalin in rheumatoid arthritis mouse model
The JAK1/STAT3 signaling was one of the most important downstream inflammatory mediators in rheumatoid arthritis. In order to define the protective effect of baicalin against arthritis progression on molecular levels, the phosphorylation of JAK1 and STAT3 expression were examined. We found that the baicalin treatment significantly attenuated JAK1/STAT3 signaling in the rheumatoid arthritis mouse model with baicalin therapy compared to the saline treatment group (Figure 5A). A quantitative calculation also showed a significantly reduction of over-activated JAK1/STAT3 signaling from western blotting analysis (Figure 5B, 5C).
Figure 5.
Downregulation of Janus kinase 1 (JAK1)/signal transducer and activator of transcription 3 (STAT3), signaling by baicalin in a rheumatoid arthritis mouse model. The changes of JAK1/STAT3 activation detected by western blotting (A) and their quantification analyses (B, C). ** Compared with control group P<0.01, ## compared with saline treatment group P<0.01.
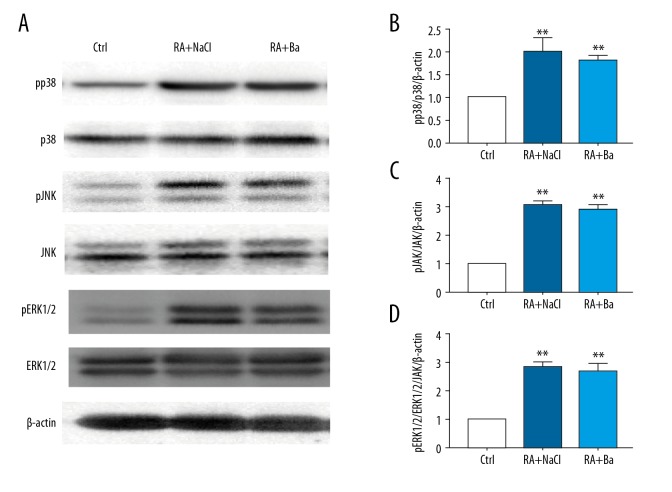
Poor inhibitory effect on activation of p38, JNK and ERK1/2 pathways by baicalin in rheumatoid arthritis mouse model
Besides the JAK/STAT pathway, the role of the mitogen-activated protein kinases (MAPKs) expressions pathway was also explored. We found that the MAPKs pathway, including ERK1/2, p38 and JNK activation, was confirmed from western-blot analyzes after modeling. But no significant reduction of MAPKs activation was observed after baicalin therapy (Figure 6A–6D).
Figure 6.
Activated mitogen-activated protein kinases (MAPKs) signaling including Jun N-terminal kinases (JNK), extracellular signal-regulated kinases 1/2 (ERK1/2), were not regulated by baicalin in a rheumatoid arthritis mouse model. The changes of phosphorylated p38, JNK, ERK1/2 expression detected by western blotting (A) and their quantification analyses (B–D). ** Compared with control group P<0.01, ## compared with saline treatment group P<0.01.
Discussion
The results of this study suggested that baicalin treatment in a collagen-induced arthritis model significantly decreased pain thresholds and reduced clinical arthritic scores. These effects were related to the suppressed levels of inflammatory factors including TNF-α, IL-1β, and IL-6 in circulation, decreased inflammatory markers such as iNOS and COX-2, and impaired induction of inflammatory cells apoptosis. These therapeutic effects were accompanied with downregulation of JAK1/STAT3 signaling but not MAPKs like p38, JNK, and ERK1/2 activations in pathogenic sites.
It has been widely accepted that inflammatory cell infiltration in the joint synovium is one of the prominent characteristics of progression of rheumatoid arthritis. These activated inflammatory cells continuously secrete proinflammatory factors like IL-1β, IL-2, IL-6, TNF-α, IFN-γ, and GM-CSF, and express MMPs such as MMP-1, MMP-2, MMP-3, and MMP-9. These overexpressed inflammatory mediators could induce aggravation of inflammation and cause the destruction of cartilage and bones [18,19]. Among the inflammatory responses, IL-1β is an important cytokine expressed by activated macrophages, and plays an important role in many cellular activities such as cell proliferation, differentiation, and even apoptosis [20]. IL-6 is regarded as an endogenous pyrogen especially prevalent in fever and in initial immune reactions induced by LPS [21]. TNF-α has been identified as a key cytokine in the dysregulated immune response, and its production is found to be crucially required for the synergistic induction of NO synthesis in LPS-treated macrophages [22]. These proinflammatory mediators stimulate biological synthesis of NO and prostaglandins to activate more immune cells and cause pain and destruction. Thus, with proinflammatory stimuli, iNOS expression is highly induced to produce NO. Therefore, NO is thought to be one of the core secondary mediators that acts as an important activator of the inflammatory response and in the cascade of proinflammatory cytokines such as TNF-α, IL-1β, and IL-6 [23]. Consistent with our findings in an arthritis mouse model, it has been reported that short-term feeding of baicalin in older rats induced the reduced production of iNOS to a greater extent in renal tissues [24]. Similarly, induced COX-2 appears to be the dominant source of prostaglandins formation in inflammation. It is known that in macrophages, LPS triggers induction and activation of signal transduction pathways and leads to transcriptional activation of the COX-2 gene [25]. But in previous experiments, it was confirmed that LPS-induced activation of macrophages would be attenuated by baicalin treatment, however, this might be an indirect effect because as a main metabolite of baicalin, baicalein has been shown to maintained higher activities [26,27]. Furthermore, other upstream inflammatory mediators have been found in rheumatoid arthritis such as IL-17. Yang et al. found that baicalin downregulated the joint inflammation caused by IL-17, which is thought to likely be produced by an expanded population of splenic Th17 cells in experimental arthritis. IL-17 has been found to suppress synovial expression of the cytokines IL-6 and TNF-α [28]. Indeed, following excessive inflammatory markers production in synovial fluid and serum, it can be detected at the same time as inflammatory signals amplification,and occurrences of edema, pain, and impaired joint mobility. Our current study results suggest that baicalin treatment improved rheumatoid arthritis through inhibition of most inflammatory mediators and blocked those critical transformations represented by iNOS and COX-2 expression in the disease progression. Thus, baicalin treatment can have a wide and deep suppressive effect on proinflammatory factors and its cascades in chronic inflammatory diseases.
Moreover, it has been suggested that activated pro-inflammatory cytokines can also increase expression of MMPs and further stimulate the development of inflammation. IL-1β is one of the crucial mediators in the initiation and progression of collagen-induced arthritis by accelerating MMPs expression to stimulate destruction of articular cartilage and reduction in the synthesis of collagen [29]. Meanwhile, the IL-6 inflammatory signal is involved in the regulation of MMP-2 and MMP-9 dependent cell migration and invasion abilities [30]. MMP-2 and MMP-9 are mainly produced by mesenchymal cells, macrophages, and peripheral blood mononuclear cells. They are identified as gelatinases and degrade fibrillar collagens, basement membrane components, and stromal ECM molecules in rheumatoid arthritis [31]. Their overexpression is closely related to pathophysiological changes of joints. Our results show that obvious suppressive effects in an arthritis mouse model induced by baicalin is encouraging for patients suffering from osteoarthritis.
Baicalin has been shown to have anti-inflammatory effects on not only systemic pro-inflammatory cytokines secretion mainly related with NF-κB signaling [14], but also survival signaling of involved inflammatory cells. Baicalin was previously suggested to exert its dual-directional effect in different conditions such as induction of malignant cell apoptosis in high proliferative cancer cells [32] and preservation of endothelial cells viability during ischemia reperfusion injury [33]. In our study, for the first time, we found pathogenic mononuclear cells in synovial fluid showed increased apoptosis after baicalin treatment and this was accompanied with decreased JAK1/STAT3 from relevant synovial tissues. The JAK/STAT signaling pathway plays a significant role in various physiological processes, including immune function and cell growth [34]. Although there is a large amount of evidence for JAK/STAT pathway activation in synovial tissues of rheumatoid arthritis, there is limited knowledge regarding the protective role of baicalin treatment through JAK/STAT signaling [35,36]. Consistent with previous reports that the activity of the JAK/STAT pathway is negatively regulated by suppressors of cytokine signaling proteins [37], our results showed that among the various subtypes molecules of JAK and STAT, the JAK1/STAT3 signaling was dramatically downregulated. The JAK1/STAT3 activation reportedly occurs when IL-6 binds to cytokine receptors, inducing a conformational change in the receptor. This recruits JAK into apposition and result in transphosphorylation and subsequent STAT activation which would bind with certain DNA sequence [34,38]. Others also discovered that IL-1β and TNF-α can directly or indirectly activate STAT3 activity in arthritis mice [39]. Diverse research suggests that STAT3 may play a significant role in inflammatory arthritis. It has been confirmed that its activation is related to developing joint pathology with synovial hyperplasia, chronic inflammation, and secondary cartilaginous metaplasia [40]. Thus, the corresponding modulation of JAK1/STAT3 signaling pathway makes baicalin treatment become a useful agent for treating rheumatoid arthritis based on molecular mechanisms. Besides that, MAPKs such as p38, JNK, and ERK1/2 are widely believed to be involved in the pathogenesis of rheumatoid arthritis. Their expressions have been discovered in the synovial tissue of rheumatoid arthritis patients and their activation would further enhanced cell infiltration, inflammation and cartilage destruction [41]. But our results showed no obvious reduction in these MAPKs activation after baicalin treatment in the current models and implied that baicalin effect maybe independent of MAPKs pathway, but it required more evaluation in other conditions.
In conclusion, the present study showed that baicalin limited the joint destruction and reversed the pathophysiological alterations including proinflammatory cytokines reduction, MMPs inhibition, inflammatory markers attenuation, inflammatory cells apoptosis with downregulated JAK1/STAT3 signaling in rheumatoid arthritis. Because this study lacked long-time treatment in a mouse model and consecutive records during baicalin input, it remains to be determined whether there is a curative effect of baicalin treatment. We need to do more investigations, especially self-control designed ones, in the future. In a word, our results revealed that baicalin might be a novel supplement for rheumatoid arthritis treatment in the near future. The potential and promising applications of baicalin will arise more interests and require more intensive researches in the future.
Conclusions
Baicalin is a novel supplement for rheumatoid arthritis treatment as it shows proinflammatory cytokines reduction, MMPs inhibition, inflammatory markers attenuation, and inflammatory cells apoptosis with downregulated JAK1/STAT3 signaling.
Supplementary Figure
The trial of a rheumatoid arthritis mouse model establishment and baicalin treatment regime determination by clinical arthritic scores. Baicalin in phosphate buffered saline was intraperitoneally injected at 10, 30, or 90 mg/kg daily, starting at day 10 (disease peak) for 5 days and 30 mg/kg lasting for consecutive 3 days respectively (each n=5). * Compared with control group P<0.01.
Footnotes
Source of support: This work was supported by grants from the Natural Science Foundation of Shandong Province (ZR2013HQ044)
Conflict of interest
None.
References
- 1.Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376(9746):1094–108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 2.Vogel WV, van Riel PL, Oyen WJ. FDG-PET/CT can visualise the extent of inflammation in rheumatoid arthritis of the tarsus. Eur J Nucl Med Mol Imaging. 2007;34(3):439. doi: 10.1007/s00259-006-0246-8. [DOI] [PubMed] [Google Scholar]
- 3.Pitzalis C, Kelly S, Humby F. New learnings on the pathophysiology of RA from synovial biopsies. Curr Opin Rheumatol. 2013;25(3):334–44. doi: 10.1097/BOR.0b013e32835fd8eb. [DOI] [PubMed] [Google Scholar]
- 4.Rho YH, Chung CP, Oeser A, et al. Inflammatory mediators and premature coronary atherosclerosis in rheumatoid arthritis. Arthritis Rheumatol. 2009;61(11):1580–85. doi: 10.1002/art.25009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amin AR, Attur M, Abramson SB. Nitric oxide synthase and cyclooxygenases: Distribution, regulation, and intervention in arthritis. Curr Opin Rheumatol. 1999;11(3):202–9. doi: 10.1097/00002281-199905000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Emery P. Treatment of rheumatoid arthritis. BMJ. 2006;332(7534):152–55. doi: 10.1136/bmj.332.7534.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldmann M, Maini RN. Perspectives from masters in rheumatology and autoimmunity: Can we get closer to a cure for rheumatoid arthritis? Arthritis Rheumatol. 2015;67(9):2283–91. doi: 10.1002/art.39269. [DOI] [PubMed] [Google Scholar]
- 8.Bendtzen K. Anti-TNF-alpha biotherapies: Perspectives for evidence-based personalized medicine. Immunotherapy. 2012;4(11):1167–79. doi: 10.2217/imt.12.114. [DOI] [PubMed] [Google Scholar]
- 9.Foster S, Chongxi Y. Herbal emissaries: bringing Chinese herbs to the West: A guide to gardening, herbal wisdom, and well-being. Inner Traditions/Bear & Co.; 1992. [Google Scholar]
- 10.Lee W, Ku S-K, Bae J-S. Anti-inflammatory effects of Baicalin, Baicalein, and Wogonin in vitro and in vivo. Inflammation. 2015;38(1):110–25. doi: 10.1007/s10753-014-0013-0. [DOI] [PubMed] [Google Scholar]
- 11.Ding Y, Dou J, Teng Z, et al. Antiviral activity of baicalin against influenza A (H1N1/H3N2) virus in cell culture and in mice and its inhibition of neuraminidase. Arch Virol. 2014;159(12):3269–78. doi: 10.1007/s00705-014-2192-2. [DOI] [PubMed] [Google Scholar]
- 12.Gao C, Zhou Y, Li H, et al. Antitumor effects of baicalin on ovarian cancer cells through induction of cell apoptosis and inhibition of cell migration in vitro. Mol Med Rep. 2017;16(6):8729–34. doi: 10.3892/mmr.2017.7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang H, Lv P, Li J, et al. Baicalin inhibits colistin sulfate-induced apoptosis of PC12 cells. Neural Regen Res. 2013;8(28):2597–604. doi: 10.3969/j.issn.1673-5374.2013.28.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H-Z, Wang H-H, Huang S-S, et al. Inhibitory effect of baicalin on collagen-induced arthritis in rats through the nuclear factor-κB pathway. J Pharmacol Exp Ther. 2014;350(2):435–43. doi: 10.1124/jpet.114.215145. [DOI] [PubMed] [Google Scholar]
- 15.Terato K, Hasty K, Reife R, et al. Induction of arthritis with monoclonal antibodies to collagen. J Immunol. 1992;148(7):2103–8. [PubMed] [Google Scholar]
- 16.Nandakumar K, Holmdahl R. Efficient promotion of collagen antibody induced arthritis (CAIA) using four monoclonal antibodies specific for the major epitopes recognized in both collagen-induced arthritis and rheumatoid arthritis. J Immunol Methods. 2005;304(1–2):126–36. doi: 10.1016/j.jim.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 17.Nandakumar K, Svensson L, Holmdahl R. Collagen type II-specific monoclonal antibody-induced arthritis in mice: Description of the disease and the influence of age, sex, and genes. Am J Pathol. 2003;163(5):1827–37. doi: 10.1016/S0002-9440(10)63542-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zangerle PF, De Groote D, Lopez M, et al. Direct stimulation of cytokines (IL-1 beta, TNF-alpha, IL-6, IL-2, IFN-gamma and GM-CSF) in whole blood: II. Application to rheumatoid arthritis and osteoarthritis. Cytokine. 1992;4(6):568–75. doi: 10.1016/1043-4666(92)90021-i. [DOI] [PubMed] [Google Scholar]
- 19.Pap T, Shigeyama Y, Kuchen S, et al. Differential expression pattern of membrane-type matrix metalloproteinases in rheumatoid arthritis. Arthritis Rheum. 2000;43(6):1226–32. doi: 10.1002/1529-0131(200006)43:6<1226::AID-ANR5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 20.Xie Q, Shen W-W, Zhong J, et al. Lipopolysaccharide/adenosine triphosphate induces IL-1β and IL-18 secretion through the NLRP3 inflammasome in RAW264. 7 murine macrophage cells. Int J Mol Med. 2014;34(1):341–49. doi: 10.3892/ijmm.2014.1755. [DOI] [PubMed] [Google Scholar]
- 21.Kagari T, Doi H, Shimozato T. The importance of IL-1 beta and TNF-alpha, and the noninvolvement of IL-6, in the development of monoclonal antibody-induced arthritis. J Immunol. 2002;169(3):1459–66. doi: 10.4049/jimmunol.169.3.1459. [DOI] [PubMed] [Google Scholar]
- 22.Zelová H, Hošek J. TNF-α signalling and inflammation: Interactions between old acquaintances. Inflamm Res. 2013;62(7):641–51. doi: 10.1007/s00011-013-0633-0. [DOI] [PubMed] [Google Scholar]
- 23.Blaser H, Dostert C, Mak TW, Brenner D. TNF and ROS crosstalk in inflammation. Trends Cell Biol. 2016;26(4):249–61. doi: 10.1016/j.tcb.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Kim DH, Kim HK, Park S, et al. Short-term feeding of baicalin inhibits age-associated NF-kappaB activation. Mech Ageing Dev. 2006;127(9):719–25. doi: 10.1016/j.mad.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Murakami A, Ohigashi H. Targeting NOX, INOS and COX-2 in inflammatory cells: Chemoprevention using food phytochemicals. Int J Cancer. 2007;121(11):2357–63. doi: 10.1002/ijc.23161. [DOI] [PubMed] [Google Scholar]
- 26.Liu LL, Gong LK, Wang H, et al. Baicalin inhibits macrophage activation by lipopolysaccharide and protects mice from endotoxin shock. Biochem Pharmacol. 2008;75(4):914–22. doi: 10.1016/j.bcp.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 27.Fan GW, Zhang Y, Jiang X, et al. Anti-inflammatory activity of baicalein in LPS-stimulated RAW264.7 macrophages via estrogen receptor and NF-kappaB-dependent pathways. Inflammation. 2013;36(6):1584–91. doi: 10.1007/s10753-013-9703-2. [DOI] [PubMed] [Google Scholar]
- 28.Yang X, Yang J, Zou H. Baicalin inhibits IL-17-mediated joint inflammation in murine adjuvant-induced arthritis. Clin Dev Immunol. 2013;2013 doi: 10.1155/2013/268065. 268065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aida Y, Maeno M, Suzuki N, et al. The effect of IL-1beta on the expression of matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases in human chondrocytes. Life Sci. 2005;77(25):3210–21. doi: 10.1016/j.lfs.2005.05.052. [DOI] [PubMed] [Google Scholar]
- 30.Zergoun AA, Zebboudj A, Sellam SL, et al. IL-6/NOS2 inflammatory signals regulate MMP-9 and MMP-2 activity and disease outcome in nasopharyngeal carcinoma patients. Tumour Biol. 2016;37(3):3505–14. doi: 10.1007/s13277-015-4186-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li G, Zhang Y, Qian Y, et al. Interleukin-17A promotes rheumatoid arthritis synoviocytes migration and invasion under hypoxia by increasing MMP2 and MMP9 expression through NF-kappaB/HIF-1alpha pathway. Mol Immunol. 2013;53(3):227–36. doi: 10.1016/j.molimm.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 32.Shu YJ, Bao RF, Wu XS, et al. Baicalin induces apoptosis of gallbladder carcinoma cells in vitro via a mitochondrial-mediated pathway and suppresses tumor growth in vivo. Anticancer Agents Med Chem. 2014;14(8):1136–45. doi: 10.2174/1871520614666140223191626. [DOI] [PubMed] [Google Scholar]
- 33.Shou X, Wang B, Zhou R, et al. Baicalin suppresses hypoxia-reoxygenation-induced arterial endothelial cell apoptosis via suppressing PKCdelta/p53 signaling. Med Sci Monit. 2017;23:6057–63. doi: 10.12659/MSM.907989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walker JG, Smith MD. The Jak-STAT pathway in rheumatoid arthritis. J Rheumatol. 2005;32(9):1650–53. [PubMed] [Google Scholar]
- 35.Isomaki P, Junttila I, Vidqvist KL, et al. The activity of JAK-STAT pathways in rheumatoid arthritis: Constitutive activation of STAT3 correlates with interleukin 6 levels. Rheumatology. 2015;54(6):1103–13. doi: 10.1093/rheumatology/keu430. [DOI] [PubMed] [Google Scholar]
- 36.Gao W, McCormick J, Connolly M, et al. Hypoxia and STAT3 signalling interactions regulate pro-inflammatory pathways in rheumatoid arthritis. Ann Rheum Dis. 2015;74(6):1275–83. doi: 10.1136/annrheumdis-2013-204105. [DOI] [PubMed] [Google Scholar]
- 37.Malemud CJ. Negative regulators of JAK/STAT signaling in rheumatoid arthritis and osteoarthritis. Int J Mol Sci. 2017;18(3) doi: 10.3390/ijms18030484. pii: E484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murray PJ. The JAK-STAT signaling pathway: Input and output integration. J Immunol. 2007;178(5):2623–29. doi: 10.4049/jimmunol.178.5.2623. [DOI] [PubMed] [Google Scholar]
- 39.Mori T, Miyamoto T, Yoshida H, et al. IL-1beta and TNFalpha-initiated IL-6-STAT3 pathway is critical in mediating inflammatory cytokines and RANKL expression in inflammatory arthritis. Int Immunol. 2011;23(11):701–12. doi: 10.1093/intimm/dxr077. [DOI] [PubMed] [Google Scholar]
- 40.Ernst M, Inglese M, Waring P, et al. Defective gp130-mediated signal transducer and activator of transcription (STAT) signaling results in degenerative joint disease, gastrointestinal ulceration, and failure of uterine implantation. J Exp Med. 2001;194(2):189–203. doi: 10.1084/jem.194.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schett G, Tohidast-Akrad M, Smolen JS, et al. Activation, differential localization, and regulation of the stress-activated protein kinases, extracellular signal-regulated kinase, c-JUN N-terminal kinase, and p38 mitogen-activated protein kinase, in synovial tissue and cells in rheumatoid arthritis. Arthritis Rheum. 2000;43(11):2501–12. doi: 10.1002/1529-0131(200011)43:11<2501::AID-ANR18>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The trial of a rheumatoid arthritis mouse model establishment and baicalin treatment regime determination by clinical arthritic scores. Baicalin in phosphate buffered saline was intraperitoneally injected at 10, 30, or 90 mg/kg daily, starting at day 10 (disease peak) for 5 days and 30 mg/kg lasting for consecutive 3 days respectively (each n=5). * Compared with control group P<0.01.