Abstract
Background
Recent data have demonstrated the potential immunosuppressive roles of interleukin-37 (IL-37) in several diseases, but whether it is involved in the pathogenesis of inflammatory myopathy has not been elucidated.
Material/Methods
An experimental autoimmune myositis (EAM) model was built by subcutaneous injections of pertussis toxin (PTX) and purified rabbit myosin (10mg/kg) emulsified with an equal volume of conventional complete Freund’s adjuvant (CFA) in a Lewis model. Autoimmune myositis Lewis model rats were divided into 3 groups: group A rats (control group) were injected with CFA in saline weekly; group B (IL-37 group) rats were injected with saline with IL-37 and CFA in saline weekly; and group C (IL-37 + SIS3 group) rats were injected with IL-37, CFA, and SIS3. ELISA was also used to assess the expressions of TNF-α, IL-6, IL-1β, TGF-β1, and CK. HE staining was performed to assess pathological changes in lung and muscle tissues.
Results
The expressions of TNF-α, IL-6, IL-1β, TGF-β1, and CK significantly increased in autoimmune myositis Lewis model rats. After IL-37 treatment, the expression of TNF-α, IL-6, IL-1β, TGF-β1, and CK was significantly reduced, as were the inflammatory responses of lung and muscle. However, SIS3 reduced the effects of IL-37 on the autoimmune myositis Lewis model rats.
Conclusioans
These findings indicate that IL-37 protects against inflammatory response via regulating Smad3 in autoimmune myositis Lewis model rats.
MeSH Keywords: Interleukins, Myositis, Pertussis Toxin, Smad3 Protein
Background
Inflammatory myopathies are a group of systemic autoimmune diseases that involve chronic muscle inflammation with muscle weakness [1,2]. Based on clinical features, muscle histopathology, and autoantibody profiles, myositis can be classified into dermatomyositis (DM), polymyositis (PM), sporadic inclusion body myositis (IBM), and necrotizing autoimmune myopathy (NAM) [2,3]. Previous studies have shown that inflammatory myopathies are characterized by acute or subacute progressive muscle weakness and endomysia inflammatory cell infiltration, but the etiology and pathogenesis of the disease remain unclear [4,5]. Recent research indicates that muscle-specific autoantibodies and immunosuppressive drugs can help to alleviate symptoms [6–8]. However, these types of treatments have many adverse effects. Identification of novel targets involved in immune-mediated processes would shed further light on the pathophysiology and treatment options for inflammatory myopathies.
Cytokines are small proteins involved in the development and pathogenesis of inflammatory and/or autoimmune diseases through their secretion [9–11]. Interleukin-37 (IL-37) is a recently identified member of the interleukin-1 (IL-1) family and is a pivotal anti-inflammatory cytokine involved in regulating inflammation [12–15]. IL-37 has been reported to play a crucial role in gouty arthritis by regulating the MSU crystal-induced inflammatory process, partly in a MerTK-dependent manner [16]. Previous studies have shown that Smad3 is involved in the immunosuppressive and anti-inflammatory properties of IL-37 both in vitro and in vivo; blocking Smad3 activation or knockdown of Smad3 decreased the anti-inflammatory activity of IL-37 [12,15]. However, the effects of the IL-37-Smad3 signaling pathway and sequential inflammatory factors during the development of inflammatory myopathies remain largely unknown.
In the present study, we investigated the effect of recombinant human IL-37 (rhIL-37) in autoimmune myositis Lewis model rats, finding that rhIL-37 attenuates the inflammatory process of inflammatory myopathies, which could be used as a novel molecular targeted therapy.
Material and Methods
Animals
Six-week-old female Lewis rats were purchased from Shanghai SLAC Laboratory Animal Company (Shanghai, China) and housed in a specific pathogen-free facility in our hospital. All experiments were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (revised 1996) and the protocol was approved by the Ethics Committee of Qilu Hospital of Shandong University.
Experimental autoimmune myositis (EAM) model
The EAM model was performed as previously described [17,18] with several modifications. In brief, myosin from rabbit (10 mg/kg) combined with complete Freund’s adjuvant (CFA, Sigma) in saline were multi-injected into back muscle tissue for 5 weeks. Then, the rats were divided into 3 groups: in group A (PTX group), intraperitoneal injection of pertussis toxin (PTX, Sigma) was performed once a week for the first 2 weeks; in group B (PTX + IL-37 group), after injection of PTX, 3 μg recombinant human IL-37 was also intraperitoneally injected at the sixth weeks once per week for 5 weeks; and in group C (PTX + IL-37 + SIS3 group), after the same treatment as in group B, 10 μmol SIS3 was injected via the tail vein.
Histological analysis of inflammatory lesions
The lung and muscle tissue from rats were removed, fixed in 10% formaldehyde, embedded in paraffin, and stained with hematoxylin and eosin (H&E) and Masson’s trichrome staining. The presence of mononuclear cell infiltration and degeneration of the muscle fibers were graded as previously described [19]. The extent of lung inflammation and injury was assessed as previously described [20]. All slides were evaluated with a light microscope independently by 2 evaluators who were blinded to the immunization protocol (Olympus Corporation, Japan).
Enzyme-linked immunosorbent assay (ELISA)
The concentrations of serum of IL-6, tumor necrosis factor (TNF)-α, transforming growth factor (TGF)-β1, IL-1β, and creatine kinase (CK) were measured using an ELISA kit according to the manufacturer’s instructions. The Rat IL-1β Mini ELISA Development Kit and Rat IL-6 ELISA Development Kit were purchased from PeproTech, the Rat TGF-β1 ELISA Kit was obtained from Sciencell, the TNF (Rat) ELISA Kit was obtained from Abnova, and the Creatine Kinase Activity Assay Kit was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Statistical analysis
All values are expressed as means ± standard deviation (SD) and statistical analyses were performed with SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). The t test was used to evaluate the differences between groups, and one-way analysis of variance (ANOVA) was used to evaluate the differences when more than 2 groups were compared. P<0.05 was considered as statistically significant.
Results
PTX promotes development of inflammatory myopathies
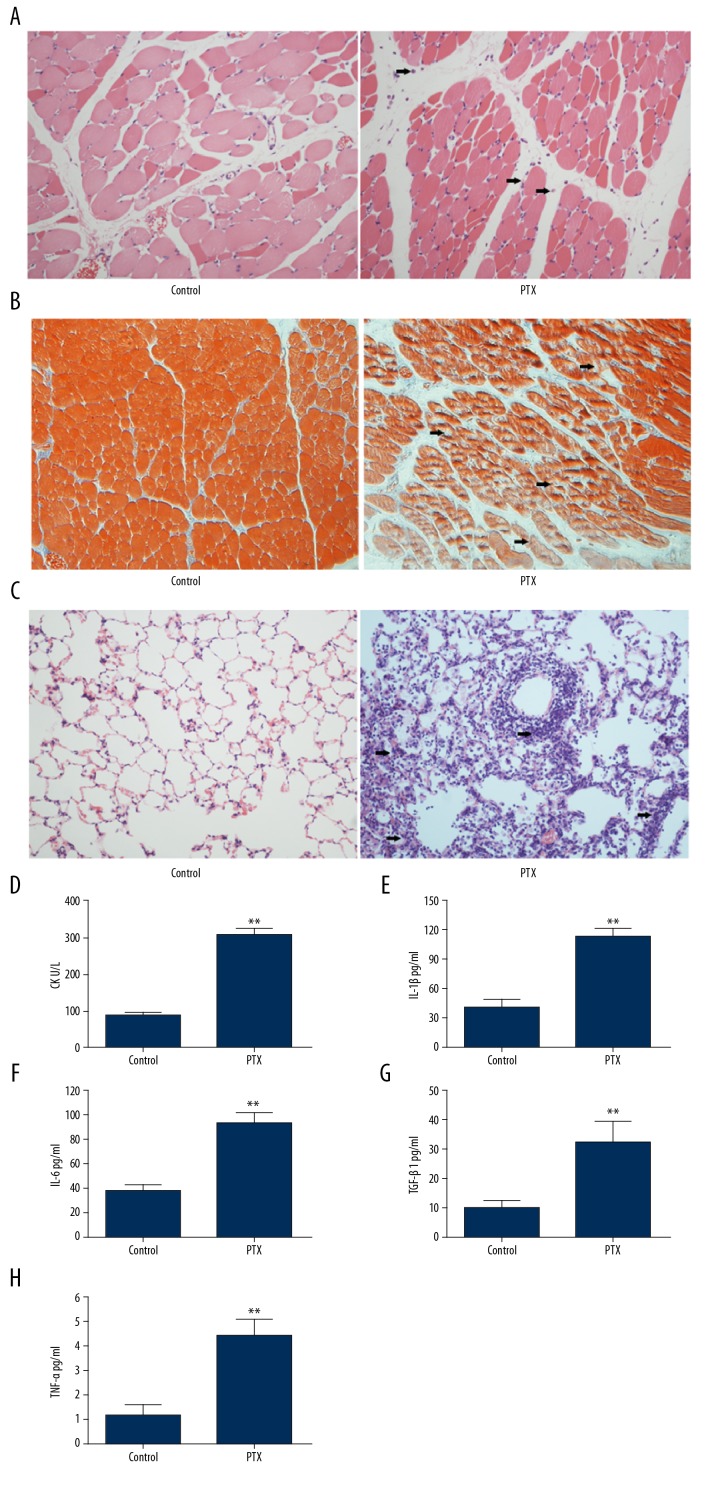
The effect of PTX on myosin combined with the complete Freund’s adjuvant-induced EAM rat model was examined using light microscopy. In PTX-stimulated EAM rats, hematoxylin and Eosin (H&E) staining in tendon showed that mononuclear cell infiltrated into the muscle tissues in EAM group as compared to normal control group (Figure 1A). Masson’s trichrome staining was performed to evaluate histological changes during the phases of lung injury and our data showed that tissues from mice administrated with PTX exhibited more fibrosis and muscle fiber atrophy (Figure 1B). Similar inflammatory infiltrates in the lung tissues was observed in the EAM group of mice (Figure 1C). The level of CK in PTX mice serum was significantly increased compared with control mice (Figure 1D). Overexpression of inflammatory cytokines have been reported to increase in inflammatory myopathies. Then we measured cytokine levels related to EAM via ELISA in mice serum. Levels of inflammatory cytokines such as IL-1β, IL-6, TGF-β and TNF-α were increased overall in mice administrated with PTX (Figure 1E–1H). These results indicated that the model of experimental autoimmune myositis was constructed in vivo.
Figure 1.
Histological analysis of the muscle and lung tissues. (A) Representative results of H&E staining of muscle tissue from mice treated with PBS or PTX. (B) Representative images of Masson’s trichrome staining of muscle tissue from mice in the PTX-induced inflammatory myopathies model. (C) Representative results of H&E staining of lung tissue from mice treated with PBS or PTX. (D) Serum levels of CK in mice treated with PBS or PTX. The level of IL-1β (E), IL-6 (F), TGF-β (G) and TNF-α (H) in mice treated with PBS or PTX. ** P<0.01.
IL-37 suppressed the inflammatory responses induced by PTX stimulation in the autoimmune myositis Lewis model
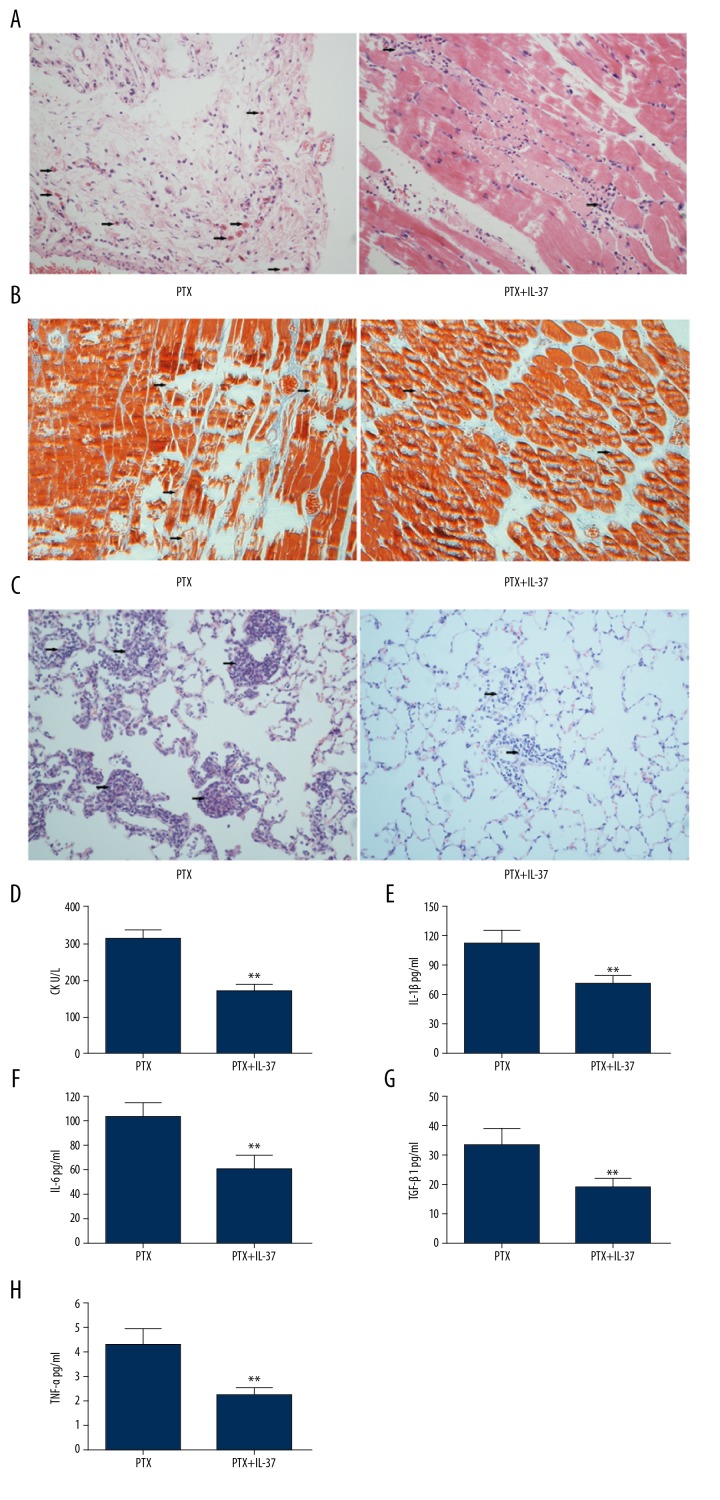
Emerging evidences have shown that IL-37 played a crucial role in the regulation of inflammation, cellular differentiation, and proliferation in many inflammatory diseases. Then we assess the effects of IL-37 on the muscle lesions induced by PTX. As shown in Figure 2A–2C, IL-37 significantly decreased the histological lesions of muscle and lung induced by PTX stimulation in the autoimmune myositis Lewis model rats. Commensurately, the CK level also decreased in rats administrated PTX and IL-37 compared to that in rats administrated only PTX (Figure 2D), as did the other 4 cytokines (IL-1β, IL-6, TGF-β1, and TNF-α) (Figure 2E–2H).
Figure 2.
Role of IL-37 in EAM. (A) Endomysial inflammation was determined in muscle tissue by H&E staining in EAM group treated with recombinant human IL-37 or without recombinant human IL-37. (B) Scattered fibers undergoing necrosis and phagocytosis of muscle tissue in EAM group treated with recombinant human IL-37 or without recombinant human IL-37 by Masson’s trichrome staining. (C) H&E staining of representative lung sections of EAM group mice with or without recombinant human IL-37. (D) Serum levels of CK in mice treated with or without recombinant human IL-37. Production of IL-1β (E), IL-6 (F), TGF-β (G) and TNF-α (H) in PTX treated mice administrated with 20 ng/mL of recombinant human IL-37 for the indicated time. ** P<0.01.
Inhibition of Smad3 partly attenuated IL-37-mediated anti-inflammatory effects in vivo
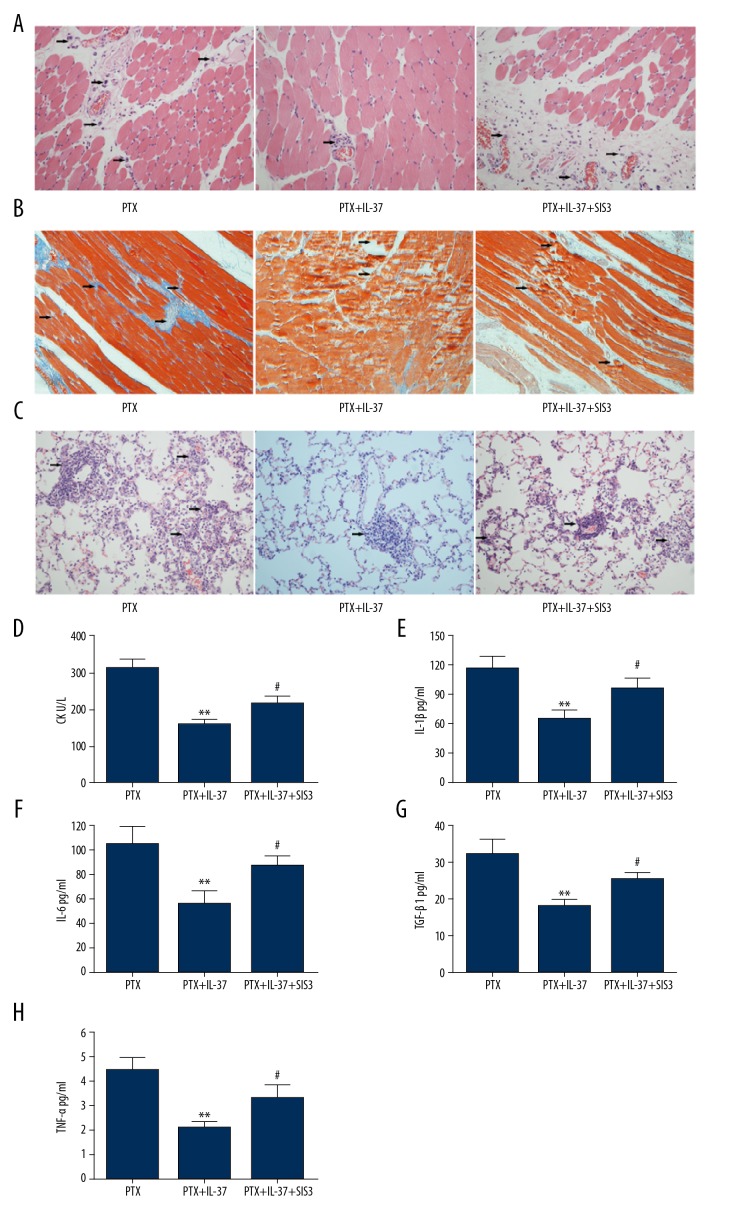
SIS3 is an inhibitor of Smad3, which interact with IL-37. When the EAM rats were administrated both IL-37 and SIS3, histological analysis showed that the role of IL-37 in decreasing the lesions of muscle and lung induced by PTX stimulation was significantly attenuated (Figure 3A, 3B and 3C). Synchronized with the change of histological lesions, the serum levels of CK (Figure 3D), IL-1β (Figure 3E), IL-6 (Figure 3F), TGF-β1 (Figure 3G), and TNF-α (Figure 3H) were all significantly higher in EAM rats administered PTX+IL-37+SIS3 than in EAM rats administered PTX+IL-37.
Figure 3.
Involvement of Smad3 in EAM mice. (A) Representative H&E-stained images of muscle tissue in PTX injection mice as indicated. (B) Representative Masson’s trichrome-stained images of muscle tissue in PTX injection mice as indicated. (C) Representative H&E-stained images of lung tissue in PTX injection mice as indicated. (D) Serum levels of CK in PTX injection mice as indicated. Quantification of IL-1β (E), IL-6 (F), TGF-β (G) and TNF-α (H) by ELISA in PTX injection mice as indicated. ** P<0.01 vs. the PTX group. # P<0.05 indicated PTX+IL-37 vs. PTX+IL-37+SIS group.
Discussion
Further exploration of the pathogenesis of inflammatory myopathy will not only allow clinical practitioners to increase understanding of the disease, but will also help to find the potential therapeutic target by focusing on the key point of pathogenesis. Currently, the optimal pharmacologic treatment in PM and DM is unclear. However, corticosteroids and cytotoxic drugs remain the most common therapies for inflammatory muscle disease. In the present study, IL-37 showed a significant anti-inflammatory effect in the IIM rat model, which indicates it is a potential therapeutic target.
Several studies have demonstrated that pro-inflammatory cytokines such as IL-1α, IL-1β, TNF, and IFN-α in muscle tissue from patients with IIMs play a role in the pathogenesis of myositis [21–24]. Bilgic reported that serum IL-6 production and the type I IFN gene signature in the peripheral blood are correlated with disease activity in patients with DM [25].
IL-37 is involved in the pathogenesis of multiple autoimmune diseases [26,27]. The serum concentration of IL-37 is usually elevated in multiple autoimmune diseases. Increased levels of IL-37 have been detected in serum and synovial fluid of patients with RA, and IL-37 levels are significantly correlated with the levels of IL-4, IL-7, IL-10, IL-12, and IL-13 [28–30]. Similar results were also reported in systemic lupus erythematosus [27,31]. On the contrary, IL-37 production of human PBMCs was significantly lower in allergic bronchial asthma [32,33] and Behcet’s disease (BD).[34] In the present study, IL-37, IL-1β, IL-6, TGF-β1, and TNF-α levels were significantly higher in EAM rats stimulated by PTX compared to the rats without PTX stimulation. Thus, increased IL-37 and TGF-β1 is likely to be the compensatory result due to increased pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α.
IL-37 is an anti-inflammatory cytokine that inhibits the expression, production, and function of pro-inflammatory cytokines [15]. As shown in the present study, IL-37 significantly decreases the histological lesions of muscle and lung tissue induced by PTX stimulation in the autoimmune myositis Lewis rat model, as do the CK and other cytokines. This means IL-37 plays an anti-inflammatory role in IIMs.
SIS3 is a specific inhibitor of Smad3 and can block the activation of Smad3 [35]. IL-37 plays its role by interacting with Smad3 [36]. In the present study, the anti-inflammatory role of IL-37 significantly decreased when the IIM model rats were administered IL-37 followed by SIS3. Moreover, IL-37 can inhibit IL-18-dependent pro-inflammatory cytokine production by binding to IL-18 receptors and IL-18 binding protein [37]. Several studies have reported that the anti-inflammatory effects of IL-37 were completely abolished in mice deficient in IL-18Rα or SIGIRR/IL-1R8 [32,38,39]. Thus, we conclude that IL-37 functions, at least in part, with Smad3 in IIMs.
Muscle biopsy is the criterion standard for diagnosis of inflammatory myopathies and is a critical component of the definitive diagnosis of IIMs [40]. The general features include necrosis, degeneration, fiber diameter variation, regeneration, increase in connective tissue, and inflammation. Lung involvement is frequent in IIM diseases and is a major risk factor for morbidity and mortality. Moretti et al. reported that IL-37 reduced neutrophil infiltration into the lung [41]. Lunding et al. reported that intranasal administration of IL-37 dampened allergic airway inflammation as well as pro-inflammatory cytokine production, mucus hyperproduction, and airway hyperresponsiveness [32,38,39]. In the present study, we found necrosis, degeneration, fiber diameter variation, regeneration, increased connective tissue, and inflammation in muscle tissues of EAM rats stimulated by PTX. However, after administration of IL-37, the muscle disorders were significantly reversed, as were changes in lung tissue. This means IL-37 can suppress the inflammation reaction in muscle and lung tissue induced by PTX, and these results are consistent with previous studies [32,38,39].
Conclusions
IL-37 and other pro-inflammatory cytokines are involved in the pathogenesis of IIM. IL-37 suppresses the inflammatory responses induced by PTX stimulation in the autoimmune myositis Lewis rat model by the Smad3 signal transduction pathway. The relative importance of IL-37 in patients with myositis remains uncertain, but the molecule is a potential biomarker candidate in IIM disease.
Footnotes
Source of support: This work was supported by grants from the Natural Science Foundation of Shandong Province (ZR2013HQ044)
References
- 1.Dalakas MC. Immunotherapy of myositis: Issues, concerns and future prospects. Nat Rev Rheumatol. 2010;6:129–37. doi: 10.1038/nrrheum.2010.2. [DOI] [PubMed] [Google Scholar]
- 2.Ernste FC, Reed AM. Idiopathic inflammatory myopathies: Current trends in pathogenesis, clinical features, and up-to-date treatment recommendations. Mayo Clin Proc. 2013;88:83–105. doi: 10.1016/j.mayocp.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 3.Vattemi G, Mirabella M, Guglielmi V, et al. Muscle biopsy features of idiopathic inflammatory myopathies and differential diagnosis. Auto Immun Highlights. 2014;5:77–85. doi: 10.1007/s13317-014-0062-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Findlay AR, Goyal NA, Mozaffar T. An overview of polymyositis and dermatomyositis. Muscle Nerve. 2015;51:638–56. doi: 10.1002/mus.24566. [DOI] [PubMed] [Google Scholar]
- 5.van de Vlekkert J, Hoogendijk JE, de Visser M. Myositis with endomysial cell invasion indicates inclusion body myositis even if other criteria are not fulfilled. Neuromuscul Disord. 2015;25:451–56. doi: 10.1016/j.nmd.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 6.Albrecht I, Wick C, Hallgren A, et al. Development of autoantibodies against muscle-specific FHL1 in severe inflammatory myopathies. J Clin Invest. 2015;125:4612–24. doi: 10.1172/JCI81031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang PL, Hou MS, Wang SW, et al. Skeletal muscle interleukin 15 promotes CD8(+) T-cell function and autoimmune myositis. Skelet Muscle. 2015;5:33. doi: 10.1186/s13395-015-0058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simon JP, Marie I, Jouen F, et al. Autoimmune myopathies: Where do we stand? Front Immunol. 2016;7:234. doi: 10.3389/fimmu.2016.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai Y, Dong C. Therapeutic antibodies that target inflammatory cytokines in autoimmune diseases. Int Immunol. 2016;28:181–88. doi: 10.1093/intimm/dxv063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dutra WO, Menezes CA, Magalhaes LM, Gollob KJ. Immunoregulatory networks in human Chagas disease. Parasite Immunol. 2014;36:377–87. doi: 10.1111/pim.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Paepe B, Zschuntzsch J. Scanning for therapeutic targets within the cytokine network of idiopathic inflammatory myopathies. Int J Mol Sci. 2015;16:18683–713. doi: 10.3390/ijms160818683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dinarello CA, Nold-Petry C, Nold M, et al. Suppression of innate inflammation and immunity by interleukin-37. Eur J Immunol. 2016;46:1067–81. doi: 10.1002/eji.201545828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu WD, Zhao Y, Liu Y. Insights into IL-37, the role in autoimmune diseases. Autoimmun Rev. 2015;14:1170–75. doi: 10.1016/j.autrev.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Quirk S, Agrawal DK. Immunobiology of IL-37: Mechanism of action and clinical perspectives. Expert Rev Clin Immunol. 2014;10:1703–9. doi: 10.1586/1744666X.2014.971014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nold MF, Nold-Petry CA, Zepp JA, et al. IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol. 2010;11:1014–22. doi: 10.1038/ni.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu L, Xue Y, Zhu Y, et al. Interleukin 37 limits monosodium urate crystal-induced innate immune responses in human and murine models of gout. Arthritis Res Ther. 2016;18:268. doi: 10.1186/s13075-016-1167-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wan Z, Zhang X, Peng A, et al. TLR4-HMGB1 signaling pathway affects the inflammatory reaction of autoimmune myositis by regulating MHC-I. Int Immunopharmacol. 2016;41:74–81. doi: 10.1016/j.intimp.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Kang J, Zhang HY, Feng GD, et al. Development of an improved animal model of experimental autoimmune myositis. Int J Clin Exp Pathol. 2015;8:14457–64. [PMC free article] [PubMed] [Google Scholar]
- 19.Allenbach Y, Solly S, Gregoire S, et al. Role of regulatory T cells in a new mouse model of experimental autoimmune myositis. Am J Pathol. 2009;174:989–98. doi: 10.2353/ajpath.2009.080422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szapiel SV, Elson NA, Fulmer JD, et al. Bleomycin-induced interstitial pulmonary disease in the nude, athymic mouse. Am Rev Respir Dis. 1979;120:893–99. doi: 10.1164/arrd.1979.120.4.893. [DOI] [PubMed] [Google Scholar]
- 21.Lundberg I, Ulfgren AK, Nyberg P, et al. Cytokine production in muscle tissue of patients with idiopathic inflammatory myopathies. Arthritis Rheum. 1997;40:865–74. doi: 10.1002/art.1780400514. [DOI] [PubMed] [Google Scholar]
- 22.Nagaraju K, Raben N, Merritt G, et al. A variety of cytokines and immunologically relevant surface molecules are expressed by normal human skeletal muscle cells under proinflammatory stimuli. Clin Exp Immunol. 1998;113:407–14. doi: 10.1046/j.1365-2249.1998.00664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reid MB, Lannergren J, Westerblad H. Respiratory and limb muscle weakness induced by tumor necrosis factor-alpha: Involvement of muscle myofilaments. Am J Respir Crit Care Med. 2002;166:479–84. doi: 10.1164/rccm.2202005. [DOI] [PubMed] [Google Scholar]
- 24.Lundberg IE. New possibilities to achieve increased understanding of disease mechanisms in idiopathic inflammatory myopathies. Curr Opin Rheumatol. 2002;14:639–42. doi: 10.1097/00002281-200211000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Bilgic H, Ytterberg SR, Amin S, et al. Interleukin-6 and type I interferon-regulated genes and chemokines mark disease activity in dermatomyositis. Arthritis Rheum. 2009;60:3436–46. doi: 10.1002/art.24936. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Shu Q, Gao L, et al. Increased Tim-3 expression on peripheral lymphocytes from patients with rheumatoid arthritis negatively correlates with disease activity. Clin Immunol. 2010;137:288–95. doi: 10.1016/j.clim.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 27.Song L, Qiu F, Fan Y, et al. Glucocorticoid regulates interleukin-37 in systemic lupus erythematosus. J Clin Immunol. 2013;33:111–17. doi: 10.1007/s10875-012-9791-z. [DOI] [PubMed] [Google Scholar]
- 28.Zhao PW, Jiang WG, Wang L, et al. Plasma levels of IL-37 and correlation with TNF-alpha, IL-17A, and disease activity during DMARD treatment of rheumatoid arthritis. PLoS One. 2014;9:e95346. doi: 10.1371/journal.pone.0095346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xia L, Shen H, Lu J. Elevated serum and synovial fluid levels of interleukin-37 in patients with rheumatoid arthritis: Attenuated the production of inflammatory cytokines. Cytokine. 2015;76:553–57. doi: 10.1016/j.cyto.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Xia T, Zheng XF, Qian BH, et al. Plasma interleukin-37 is elevated in patients with rheumatoid arthritis: its correlation with disease activity and Th1/Th2/Th17-related cytokines. Disease Markers. 2015;2015 doi: 10.1155/2015/795043. 795043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu GC, Li HM, Wang JB, et al. Elevated plasma interleukin-37 levels in systemic lupus erythematosus patients. Lupus. 2016;25:1377–80. doi: 10.1177/0961203316646462. [DOI] [PubMed] [Google Scholar]
- 32.Lunding L, Webering S, Vock C, et al. IL-37 requires IL-18Ralpha and SIGIRR/IL-1R8 to diminish allergic airway inflammation in mice. Allergy. 2015;70:366–73. doi: 10.1111/all.12566. [DOI] [PubMed] [Google Scholar]
- 33.Raedler D, Ballenberger N, Klucker E, et al. Identification of novel immune phenotypes for allergic and nonallergic childhood asthma. J Allergy Clin Immunol. 2015;135:81–91. doi: 10.1016/j.jaci.2014.07.046. [DOI] [PubMed] [Google Scholar]
- 34.Ye Z, Wang C, Kijlstra A, et al. A possible role for interleukin 37 in the pathogenesis of Behcet’s disease. Curr Mol Med. 2014;14:535–42. doi: 10.2174/1566524014666140414210831. [DOI] [PubMed] [Google Scholar]
- 35.Jinnin M, Ihn H, Tamaki K. Characterization of SIS3, a novel specific inhibitor of Smad3, and its effect on transforming growth factor-beta1-induced extracellular matrix expression. Mol Pharmacol. 2006;69:597–607. doi: 10.1124/mol.105.017483. [DOI] [PubMed] [Google Scholar]
- 36.Grimsby S, Jaensson H, Dubrovska A, et al. Proteomics-based identification of proteins interacting with Smad3: SREBP-2 forms a complex with Smad3 and inhibits its transcriptional activity. FEBS Lett. 2004;577:93–100. doi: 10.1016/j.febslet.2004.09.069. [DOI] [PubMed] [Google Scholar]
- 37.Bufler P, Azam T, Gamboni-Robertson F, et al. A complex of the IL-1 homologue IL-1F7b and IL-18-binding protein reduces IL-18 activity. Proc Natl Acad Sci USA. 2002;99:13723–28. doi: 10.1073/pnas.212519099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lunding L, Schroder A, Wegmann M. Allergic airway inflammation: Unravelling the relationship between IL-37, IL-18Ralpha and Tir8/SIGIRR. Expert Rev Respir Med. 2015;9:739–50. doi: 10.1586/17476348.2015.1109452. [DOI] [PubMed] [Google Scholar]
- 39.Lunding L, Webering S, Vock C, et al. Effect of IL-37 on allergic airway inflammation. Ann Am Thorac Soc. 2016;13(Suppl 1):S95–96. doi: 10.1513/AnnalsATS.201506-380MG. [DOI] [PubMed] [Google Scholar]
- 40.Dalakas MC. Muscle biopsy findings in inflammatory myopathies. Rheum Dis Clin North Am. 2002;28:779–98. vi. doi: 10.1016/s0889-857x(02)00030-3. [DOI] [PubMed] [Google Scholar]
- 41.Moretti S, Bozza S, Oikonomou V, et al. IL-37 inhibits inflammasome activation and disease severity in murine aspergillosis. PLoS Pathogens. 2014;10:e1004462. doi: 10.1371/journal.ppat.1004462. [DOI] [PMC free article] [PubMed] [Google Scholar]