Abstract
Pheromone (PHB)-receptor (RCB) interaction in the mating pheromone response pathway of Lentinula edodes was investigated using synthetic PHBs. Functionality of the C-terminally carboxymethylated synthetic PHBs was demonstrated by concentration-dependent induction of a mating-related gene (znf2) expression and by pseudoclamp formation in a monokaryotic strain S1-11 of L. edodes. Treatment with synthetic PHBs activated the expression of homeodomain genes (HDs) residing in the A mating type locus, and of A-regulated genes, including znf2, clp1, and priA, as well as genes in the B mating type locus, including pheromone (phb) and receptor (rcb) genes. The synthetic PHBs failed to discriminate self from non-self RCBs. PHBs of the B4 mating type (B4 PHBs) were able to activate the mating pheromone response pathway in both monokaryotic S1-11 and S1-13 strains, whose B mating types were B4 (self) and B12 (non-self), respectively. The same was true for B12 PHBs in the B4 (non-self) and B12 (self) mating types. The synthetic PHBs also promoted the mating of two monokaryotic strains carrying B4-common incompatible mating types (A5B4 × A1B4). However, the dikaryon generated by this process exhibited abnormally high content of hyphal branching and frequent clamp connections and, more importantly, was found to be genetically unstable due to overexpression of mating-related genes such as clp1. Although synthetic PHBs were unable to discriminate self from non-self RCBs, they showed a higher affinity for non-self RCBs, through which the mating pheromone response pathway in non-self cells may be preferentially activated.
KEYWORDS: B mating type, lentinula, pheromone, receptor, synthetic peptide
1. Introduction
In filamentous basidiomycetes, mating occurs through hyphal fusion of two compatible monokaryotic strains. Mating compatibility is determined by mating type-specific genes in genetic loci A and B. Particularly, pheromone and pheromone receptor at the B locus are involved in the initial stage of mating through the activation of the pheromone response pathway. The pheromone response pathway of basidiomycetes has been suggested to share strong genetic similarities with the most well-studied model fungus, Saccharomyces cerevisiae [1,2]. In S. cerevisiae, pheromone-receptor interaction activates the transcription of genes related to the mating process through the MAP kinase-mediated signaling cascade [3]. The pheromone response pathway of MATa haploid cells is activated by the binding of α-factor from MATα cells to pheromone receptor Ste2, whereas that of MATα cells is activated by the binding of a-factor from MATa cells to receptor Ste3 [3,4]. Unlike the freely diffusible α-factor, a-factor is a membrane-incorporated pheromone. The precursor of a-factor (36 residues) from MATa cells is converted into a 12-amino acid mature peptide through proteolytic cleavage catalyzed by membrane-bound metalloprotease Ste24 [5–7]. The C-terminus of a-factor is prenylated and carboxymethylated at the cysteine residue in a CAAX (C, cysteine; A, aliphatic residue; X, any amino acid) motif by the activities of Ram1/Ram2 and Ste14, respectively [8,9]. Mature a-factor is translocated across the membrane by exporter protein Ste6 [10].
Filamentous basidiomycetes have only Ste3-type pheromone receptors, which are activated by a-factor-like pheromones [11]. Similar to a-factor, pheromones in this class are incorporated into cell membrane through acylation at the cysteine residue in the C-terminal CAAX motif [11]. Therefore, it is highly possible that activation of the mating pathway occurs only after membrane fusion between two compatible cells, since there are no diffusible pheromones in basidiomycetes. The B mating locus of basidiomycetes is much more complex than the single receptor-pheromone pair in haploid cells of S. cerevisiae. In Schizophyllum commune, 81 B mating types have been suggested to occur through recombination between nine allelic Bα and nine allelic Bβ subloci [2,12]. The B locus of Coprinopsis cinerea consists of three subloci, with two alleles in sublocus 1, five alleles in sublocus 2, and seven alleles in sublocus 3, constituting a total of 70 theoretical B mating types [13]. In these basidiomycetes, the pheromone response pathway is activated only when non-self pheromones are bound to compatible pheromone receptors at the same sublocus [14–16]. The specificity of a pheromone to a pheromone receptor relies on subtle variations in their amino acid sequences [2,13,17]. One example of this was shown in Ustilago maydis using synthetic peptides that mimic mature pheromones. Synthetic pheromone a1 was able to activate only a2 receptor, while synthetic pheromone a2 was found to activate a1 receptor [18]. Alanine scanning of a1 pheromone showed that Gly5, Gly9, and Tyr10 are highly important amino acid residues in the activation of pheromone pathways, and these residues have been suggested to play a role in the binding of pheromone to target receptor.
Lentinula edodes is one of the most cultivated mushrooms worldwide, and particularly in East Asia. Much effort has been made to develop new strains suitable for changing climates and alternative substrates. One such example is our classical mating experiment in monokaryotic strains from various origins [19]. During this experiment, we got to know that the mating type of L. edodes is multiallelic both in the A and B loci [20,21]. The B mating locus has been shown to consist of two subloci, Bα and Bβ, similar to S. commune [22]. Moreover, we have found 15 B mating types through a combinatorial assortment of five Bα and three Bβ subloci [21]. The Bα and Bβ subloci are respectively composed of five alleles of pheromone receptors (rcbs) with nine associated pheromones (phbs), and of three alleles of rcbs with five phbs [21]. Each sublocus contains an rcb and two phb genes. For example, the Bα subloci of the B4 and B12 mating types, two frequently occurring B mating types, are constituted by phb5-phb6-rcb1-2 and phb11- phb12-rcb1-4, respectively, while the Bβ subloci of the B4 and B12 mating types are consisted of rcb2-1-phb7-phb4 and rcb2-3-phb9-phb10, respectively (Figure 1(A)). The complexity of the B mating type in L. edodes has led us to investigate the pheromone-receptor interactions. Hence, we investigated the effect of synthetic pheromones on the expression of mating genes as a measure of the specificity of pheromone-receptor interactions. In the present study, eight synthetic peptides corresponding to the mature forms of B4 and B12 PHBs were employed to determine the specificity of RCB proteins using two monokaryotic strains, S1-11 and S1-13, which have the B4 and B12 mating types, respectively.
Figure 1.
Pheromone and pheromone receptor structures in the B mating type locus of L. edodes. (A) Structures of the B4 and B12 mating types. The B mating type loci consist of distinct Bα and Bβ subloci which contain different pheromones (phbs) and receptor genes (rcbs). Arrow boxes indicate the direction of transcription. (B) Amino acid sequence of mature pheromones. (C) Amino acid sequence comparison of pheromone receptors. Loop regions are shown within boxes and the amino acids predicted to be located in transmembrane domains are colored in red. RCB1-2 and RCB1-4 represent pheromone receptors found in the Bα sublocus of B4 and B12 mating types, respectively, whereas RCB2-1 and RCB2-3 are the receptors found in the Bβ sublocus of B4 and B12, respectively.
2. Materials and methods
2.1. Strains and growth conditions
The monokaryotic strains S1-11 and S1-13 of L. edodes, whose B mating types were determined to be B4 and B12, respectively [19], were employed to investigate the self- and nonself-PHB responses. Their A mating types were A5 and A1 for S1-11 and S1-13, respectively [19]. The monokaryotic strain S1-10, whose mating types were A1 in the A mating type locus and B4 in the B mating type locus, was used to induce forced mating between B-common monokaryotic strains. These strains had been generated from basidiospores of the dikaryotic SJ701 strain [19]. All strains were grown on potato dextrose agar (PDA; Oxoid, Hampshire, England) at 25 °C.
2.2. Pheromone response assay using synthetic PHBs
PHB peptides were commercially synthesized with carboxymethylation at the C-teminal cysteine residue (Genscript, Piscataway, New Jersey). The C-terminal carboxymethyl group is suggested to be important for the binding of pheromone to receptor [23,24]. In order to investigate the pheromone response in L. edodes, mycelia grown for two weeks in potato dextrose broth (PDB; Oxoid, Hampshire, England) at 25 °C were harvested and then washed twice with distilled water. Collected mycelia were suspended in fresh PDB containing synthetic PHBs (20 μg/ml) for 12 h at 25 °C. Pheromone response was determined by measuring the expression of downstream genes in the mating pheromone pathway. For this, total RNA from PHB-treated mycelia was extracted using RNeasy Plant Mini Kit (Qiagen, Hinden, Germany) and was then transcribed into cDNA using a cDNA synthesis kit (TOPscript; Enzynomics, Daejeon, Korea) with an oligo-dT primer. Real-time qRT-RCR was performed using Lightcycler Nano system (Roche; Manheim, Germany) with the synthesized cDNA (100 ng/μl) and FastStart Universal SYBR Green Master (Roche; Manheim, Germany). Primer sets used for this analysis are described in Supplementary Table S1. PCR was carried out under the following conditions: hold for 10 min at 95 °C; 45 cycles of 95 °C for 15 s and 60 °C for 60 s. The relative expression of the target gene was calibrated on the basis of the cycle threshold value of the L. edodes β-tubulin gene (GenBank accession number AF106239). All assays were performed in triplicate.
2.3. Mating assay and microscopic examination
For mating, two monokaryotic mycelia were placed on PDA at a distance of 0.5 cm from each other. The generation of the dikaryon was determined by observation of clamp connections under the light microscope after two weeks of incubation at 25 °C. Mating between common B strains was induced by treatment with 10 μl of a mixture of synthetic PHBs (1 mg/ml) in the middle of two monokaryotic strains. Mycelia were stained with calcofluor white (Sigma-Aldrich, St. Louis, MO) so as to enhance their visualization. The stained mycelia were examined under a fluorescence microscope (AX-80; Olympus, Tokyo, Japan) at 400× magnification with excitation at 355 nm.
3. Results
3.1. Structures of pheromones and pheromone receptors
Mature PHBs were predicted to be polypeptides with a length of 11–16 amino acids (Figure 1(B)), similar to a-factor of S. cerevisiae. PHB5 and PHB11 at the Bα sublocus of B4 and B12, respectively, shared high sequence identity. Only two amino acids were different at the positions 2 (H/R) and 5 (D/N), whereas PHB6 in B4 was remotely related to these two. PHB12 was different in length and sequence composition to the three PHBs. PHBs at the Bβ sublocus were shorter and contained different sequence motifs, such as EA and A(G)FC. Similar to PHB5 and PHB11, PHB7 in B4 and PHB10 in B12 shared high sequence homology.
Pheromone receptor (RCB) was predicted to be a membrane protein with seven transmembrane domains. RCBs were highly homologous regardless of the subloci to which they belong. However, even in this case, RCBs at Bα differed from those at Bβ in sequence compositions. The loop domain L7, which conceivably allows RCBs to recognize their specific ligands (PHBs), was the most variable sequence region predicted to be located in the outer membrane (Figure 1(C)).
3.2. Synthetic pheromones are functional in the activation of the mating pathway
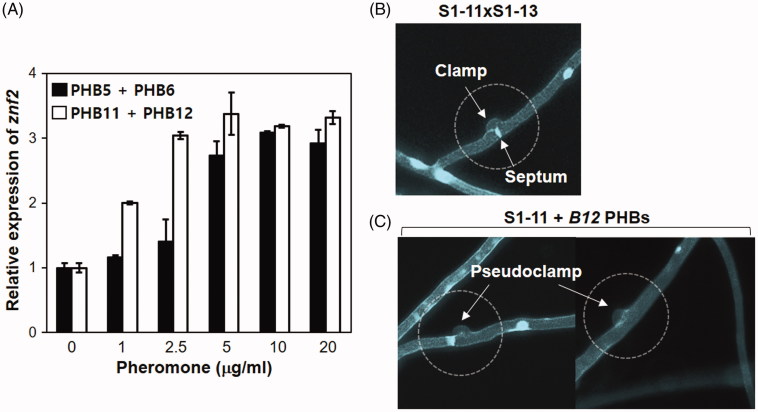
In order to better understand the pheromone-receptor interaction, we synthesized PHB polypeptides with a C-terminal carboxymethylation that can mimic mature PHBs in L. edodes, as it also does in C. cinerea [25]. Since the mature PHB in basidiomycetes is a polypeptide known to bind to the membrane through farnesylation at the C-terminus [10], we tested the functionality of synthetic pheromones on the expression of genes related to the mating pathway of L. edodes. First, the expression of znf2, a downstream transcription factor of the mating pathway [26,27], was investigated through RT-PCR analysis, upon addition of a mixture of Bα pheromones from either the B4 or the B12 mating type to the actively growing monokarytoic strain S1-11, whose B mating type is B4. As shown in Figure 2(A), Znf2 expression was induced by both pheromone mixtures in a concentration-dependent manner. However, the B12-Bα pheromones, PHB11 and PHB12, were more effective at inducing znf2 than the B4-Bα pheromones, PHB5 and PHB6. The expression levels were saturated at concentrations of 2.5 μg/ml and 5 μg/ml for B12-Bα pheromones and B4-Bα pheromones, respectively.
Figure 2.
Effects of synthetic pheromones on the monokaryotic S1-11 strain of L. edodes. (A) Expression of znf2 in a monokaryotic strain S1-11 at different concentrations of synthetic PHBs. Actively growing mycelia were treated with mixtures (1:1) of self PHBs or non-self PHBs for 12 h at 25 °C. (B) Clamp connections on the dikaryotic mycelia generated by the mating of S1-11 (B4 mating type) and S1-13 (B12 mating type). (C) Occurrence of pseudoclamp structures on the monokaryotic S1-11 strain upon treatment with B12 PHBs (PHB11, PHB12, PHB9, and PHB10) at a concentration of 20 μg/ml.
Next, we examined the microscopic structure of mycelia using calcoflour staining after treatment with a mixture of B12 PHBs (PHB11, PHB12, PHB9, and PHB10) to the actively growing mycelia of the S1-11 strain. The dikaryotic strain generated through the mating of S1-11 and S1-13 showed typical clamp connections with septal structure (Figure 2(B)). Interestingly, the monokaryotic S1-11 strain also formed a clamp-like structure (pseudoclamp) without septum upon treatment with B12 PHBs (Figure 2(C)). These data indicate that (1) the synthetic PHBs in soluble forms are functionally active in the activation of the mating pathway even without C-terminal farnesylation; (2) B4 pheromones can activate their own mating pheromone pathway (self-activation); and (3) cross-activation by B12-Bα pheromones appeared to be favored over self-activation by B4-Bα pheromones in the B4 mating pheromone pathway (Figure 2).
3.3. Synthetic PHBs induce genes in the mating pathway
We next explored the expression of genes in the mating pheromone pathway, including A mating type locus genes (HD1 and HD2), clp1, znf2, and pri-A, upon treatment with mixtures of B4 or B12 pheromones at a fixed total concentration of 20 μg/ml. Treatment with PHBs from individual subloci, i.e. B4-Bα PHBs (PHB5 + PHB6, 10 μg/ml each), B4-Bβ PHBs (PHB4 + PHB7, 10 μg/ml each), B12-Bα PHBs (PHB11 + PHB12, 10 μg/ml each), or B12-Bβ PHBs (PHB9 + PHB10, 10 μg/ml each), was able to induce the expression of the five mating genes in the S1-11 strain (Supplementary Figure S1). The combined treatment with B4 pheromones (PHB5 + PHB6 + PHB4 + PHB7, 5 μg/ml each) or B12 pheromones (PHB11 + PHB12 + PHB9 + PHB10, 5 μg/ml each) was also able to induce the mating genes (Figure 3). In the S1-11 strain (B4 mating type), both B4 and B12 pheromone mixtures induced the expression of mating genes with different degrees of activation (Figure 3). Similarly, both pheromone mixtures were able to activate mating genes in the S1-13 strain (B12 mating type), except for HD1 and HD2 (Figure 3). There is currently no explanation for the unresponsiveness of these two genes in the A mating type locus to the mating pheromones in the S1-13 strain. However, these data suggest that PHBs can induce self-activation as well as cross-activation of the mating pheromone pathway.
Figure 3.
Expression of A mating type genes and A-related genes in the presence of synthetic PHBs. Monokaryotic strains (S1-11 and S1-13) were treated with a mixture of B4 PHBs (PHB5, PHB6, PHB7, and PHB4) or B12 PHBs (PHB11, PHB12, PHB9, and PHB10) at a concentration of 20 μg/ml. The gene expression level was compared with that of untreated mycelia (Control).
3.4. PHBs selectively induce genes in the B mating type locus
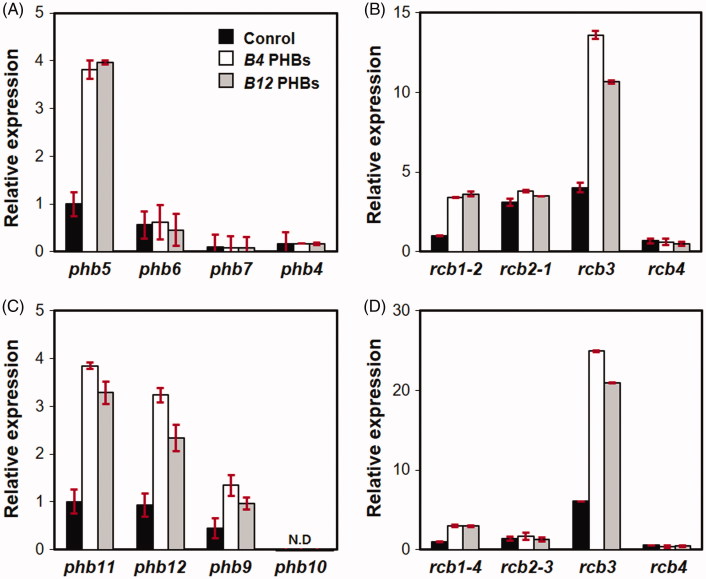
We further investigated the effect of PHBs on the expression of genes in the B mating type locus by treating the S1-11 strain with self PHBs (B4-Bα PHBs, PHB5 and PHB6; B4-Bβ PHBs, PHB4 and PHB7) and with non-self PHBs (B12-Bα PHBs, PHB11 and PHB12; B12-Bβ PHBs, PHB9 and PHB10). Unlike the genes of the mating pheromone pathway, PHBs from a single sublocus, regardless of whether self or non-self PHBs were used for treatment, failed to activate any phb or rcb genes in the B4 mating type locus (Figure 4). However, a combination of Bα PHBs and Bβ PHBs from B4 (self) or B12 (non-self) was able to selectively activate the expression of two mating type genes (phb5 and rcb1-2) and a non-mating type rcb gene (rcb3) (Figures 4 and 5). Knowing that the genes in the B mating type locus are induced only in the presence of a mixture of Bα and Bβ PHBs, we also examined the expression of B mating type genes in the monokaryotic S1-13 strain after treatment with B4 PHBs (non-self) or B12 PHBs (self). Unlike S1-11, the three phbs (i.e. phb11 and phb12 at the Bα sublocus and phb9 at the Bβ sublocus) were induced by treatment with either self or non-self PHBs (Figure 5(C)), whereas rcbs, including rcb1-4 from the Bα sublocus and a nonmating type rcb3, were overexpressed, similar to observations in the S1-11 strain (Figure 5(D)).
Figure 4.
Expression of genes in the B mating type locus of the monokaryotic S1-11 strain in the presence of synthetic PHBs. Mycelia of S1-11 strain (B4 mating type) were treated with B4-Bα PHBs (PHB5 + PHB6) and B4-Bβ PHBs (PHB7 + PHB4) for the B4 mating type, and B12-Bα PHBs (PHB11 + PHB12) and B12-Bβ PHBs (PHB9 + PHB10) for the B12 mating type at the indicated concentrations.
Figure 5.
Expression of B genes in two compatible monokaryotic strains in the presence of synthetic self or non-self PHBs. The expression of phbs and rcbs in S1-11 (A, B) and S1-13 (C, D) was investigated upon treatment with B4 PHBs or B12 PHBs at a concentration of 20 μg/ml. B4 PHBs are self PHBs for S1-11 and non-self PHBs for S1-13 whereas B12 PHBs are non-self PHBs for S1-11 and self PHBs for S1-13. The expression levels of genes in the treated mycelia were compared with the expression levels of phb5, rcb1-2, phb11, and rcb1-4 in the untreated control mycelia.
3.5. Synthetic PHBs can induce mating in an incompatible mating pair
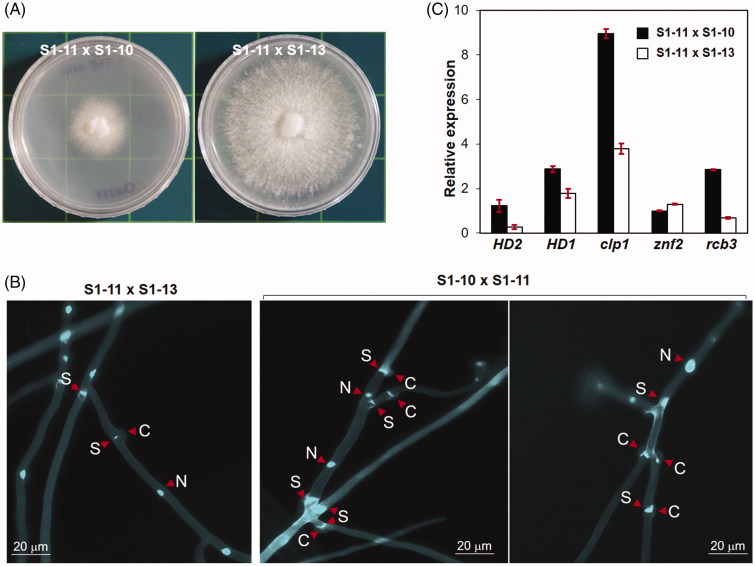
Mating between two monokaryotic strains occurs only when the two strains have different A and B mating types. Since expression of genes in the A mating type locus is also controlled by the mating pheromone pathway as described above, the mating process in L. edodes is essentially governed by pheromone-receptor interactions. This suggests that a monokaryon can mate with a monokaryon of an incompatible mating type, provided that the mating pheromone pathway is activated. To prove this hypothesis, we employed two monokaryotic strains, S1-11 and S1-10, whose mating types are A5B4 and A1B4, respectively. These two strains are basically incompatible for mating because of the B mating type they have in common. However, we were able to generate a dikaryotic strain (S1-11 × S1-10) through a classical mating experiment on PDA medium containing a mixture of B12 PHBs. The strain S1-11 × S1-10 exhibited a slower growth rate than its monokaryotic strains, whereas the dikaryotic strain S1-11 × S1-13, which was generated by compatible mating between S1-11 and S1-13 (A1B12), exhibited a better growth than the monokaryotic strains (Figure 6(A)). Mycelia of S1-11 × S1-10 showed more frequent and irregular branching than mycelia of S1-11 × S1-13 (Figure 6(B) and Supplementary Figure S2). S1-11 × S1-10 mycelia also presented frequent septations and multiple clamp connections that resulted in short cells without nuclei (Figure 6(B)).
Figure 6.
Forced mating between strains of incompatible mating type by synthetic PHBs. (A) Dikaryotic strains generated by the mating of two incompatible monokaryons [S1-11 (A5B4) × S1-10 (A1B4), left panel] and two compatible monokaryons [S1-11 (A5B4) × S1-13 (A1B12), right panel]. The A and B mating types of each strain are provided in parentheses. For the forced mating between S1-11 and S1-10, a mixture (10 μl) of B12 PHBs (non-self PHBs, 1 mg/ml) was dropped on PDA between the two monokaryotic strains. The resultant dikaryon was isolated at the place where hyphal fusion occurred. (B) Difference in the mycelial structure between the compatible mate (S1-11 × S1-13) and the B-common incompatible mate (S1-10 × S1-11) upon calcofluor staining. Clamp connections (C), septa (S), and nuclei (N) are indicated by arrowheads. (C) Expression of some representative mating-related genes in S1-11 × S1-13 or S1-10 × S1-11.
To account for this irregular development, we examined the expression of genes in the mating pheromone pathway. Most notably, clp1, which has been known to be involved in clamp formation [28], was found to be highly expressed in S1-11 × S1-10 (Figure 6(C)). Expression of the HD2 gene, unresponsive to PHBs in monokaryotic strains (Figure 3), was 4.5-fold higher in S1-11 × S1-10 than in S1-13 × S1-10 (Figure 6(C)). The nonmating type receptor rcb3 was also 4-fold overexpressed in S1-11 × S1-10. Detailed functions of the latter two are yet to be clarified. The dikaryon generated by this process was very poor in growth on PDA.
4. Discussion
Self/non-self recognition in the mating process of basidiomycetes is governed by interactions between mating pheromones and pheromone receptors. Furthermore, pheromones and pheromone receptors are essentially multiallelic, and therefore, the molecular mechanism underlying the correct matching of pheromone and receptor is one of the main aspects in the mating process of basidiomycete. C. cinerea is known to have more than 79 B mating types [12,13,29]. Selective and non-self interaction of pheromone and pheromone receptor, and thus the selective activation of the mating pathway, in this mushroom has been shown by mating analysis of tester strains and transformants, harboring plasmids expressing pheromones and receptors [29–31]. Strains of S. cerevisiae that express mushroom pheromone receptor proteins also exhibit a selective interaction with plasmid-borne pheromones or synthetic peptides of C. cinerea [17,25] and S. commune [14,15].
L. edodes, a popular edible mushroom of industrial importance, has been shown to contain more than five alleles of rcbs with nine associated phbs, and three alleles of rcbs with five associated phbs, in the Bα and Bβ subloci (of B mating type locus), respectively [21]. The complexity of phbs and rcbs has prompted us to investigate self/non-self recognition in the pheromone interaction with its receptor. In the present study, we demonstrated that PHB peptides can activate the mating pathway in monokaryotic strains of L. edodes in a concentration-dependent manner, regardless of their B mating types (Figure 2), and even if the activation efficiency of non-self PHBs is higher than that of self PHBs (Figure (2A)). Activation of the pheromone response pathway appears to require PHB-RCB interactions from both Bα and Bβ subloci (Figures 3–5).
Both synthetic PHBs (B4 and B12) were able to induce the expression of znf2, a gene known to be controlled by HD genes from the A mating type locus [26], in the S-11 strain (B4 mating type) in a concentration-dependent manner (Figure 2(A)). Addition of synthetic PHBs induced pseudoclamps in monokaryotic mycelia (Figure 2(C)), indicating that PHBs can activate the mating pathway in monokaryons even without mating. Clamp cell formation is a hallmark of successful mating in most basidiomycetes, and is controlled by the A mating type locus-regulated clp1 gene [28,32]. Activation of A genes and A-regulated genes by mating pheromones was further demonstrated by the treatment with a mixture of self or non-self PHBs (Figure 3). priA, a gene involved in primordia formation [33], was also expressed in the PHB-treated monokaryotic strains (Figure 3). PHBs were also able to induce the expression of genes in the B mating type locus, but only upon treatment with both Bα and Bβ PHBs in the monokaryotic strains (Figures 4 and 5). Particularly, phb5 and rcb1-2 in the Bα sublocus of B4 and phb11, phb12 and rcb1-4 in the Bα sublocus of B12 were highly expressed upon treatment with a mixture of synthetic PHBs. The biological meaning of the asymmetric expression of B genes, as well as the notably high expression of the non-mating type receptor rcb3, demands further investigation.
As described above, activation of the pheromone response pathway through a compatible PHB-RCB interaction is essential at the initial stage of the mating process. In basidiomycetes, this appears to occur during the hyphal fusion between two monokaryotic mycelia, because all pheromones found in basidiomycetes are a-factor-like membrane-bound peptides. Our present study demonstrates that RCB can bind to pheromones without discriminating between self and non-self PHBs, while studies in C. cinerea and S. commune have shown specific non-self interactions [29–31]. One possible explanation of this phenomenon is that the genetically encoded pheromone is localized to the cell membrane through C-terminal farnesylation, and therefore it is not in a correct orientation to interact with the RCB in the same membrane. Provision of soluble synthetic PHBs in the present study may help overcome the orientation problem. However, if both self and non-self PHB-RCB interactions occur simultaneously in nature, how does the RCB discriminate between self and non-self PHBs? A higher binding affinity of RCB for non-self PHBs over self PHBs can be one explanation. Preferred binding of non-self PHBs to RCB may outcompete the binding of self PHBs as shown in Figure 2(A). Additionally, even if the self PHB-RCB interaction occurs and thus results in a successful mating, the dikaryon generated from incompatible partners appears to be eliminated during adaptation due to the decreased fitness. This was demonstrated by the decreased growth rate with the irregular branching and septation in the mycelia of the dikaryotic strain generated by forced mating between monokaryotic S1-11 and S1-10 strains (both of them have the same B mating type) (Figure 6).
Supplementary Material
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was carried out with the support of “Cooperation Research Program for Agriculture Science and Technology Development [Project No. PJ01368102]” Rural Development Administration, Republic of Korea. SK and MSK were supported by a scholarship from the BK 21 Plus Program, the Ministry of Education, Korea.
References
- 1. Raudaskoski M, Kothe E. Basidiomycete mating type genes and pheromone signaling. Eukaryot Cell. 2010;9:847–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kües U. From two to many: multiple mating types in Basidiomycetes. Fungal Biol Rev. 2015;29:126–166. [Google Scholar]
- 3. Park H-O, Bi E. Central roles of small GTPases in the development of cell polarity in yeast and beyond. Microbiol Mol Biol Rev. 2007;71:48–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bardwell L, Cook JG, Inouye CJ, et al. . Signal propagation and regulation in the mating pheromone response pathway of the yeast Saccharomyces cerevisiae . Dev Biol. 1994;166:363–379. [DOI] [PubMed] [Google Scholar]
- 5. Tam A, Nouvet FJ, Fujimura-Kamada K, et al. . Dual roles for Ste24p in yeast a-factor maturation: NH2-terminal proteolysis and COOH-terminal CAAX processing. J Cell Biol. 1998;142:635–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tam A, Schmidt WK, Michaelis S. The multispanning membrane protein Ste24p catalyzes CAAX proteolysis and NH2-terminal processing of the yeast a-factor precursor. J Biol Chem. 2001;276:46798–46806. [DOI] [PubMed] [Google Scholar]
- 7. Michaelis S, Barrowman J. Biogenesis of the Saccharomyces cerevisiae pheromone a-factor, from yeast mating to human disease. Microbiol Mol Biol Rev. 2012;76:626–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hrycyna CA, Clarke S. Farnesyl cysteine C-terminal methyltransferase activity is dependent upon the STE14 gene product in Saccharomyces cerevisiae . Mol Cell Biol. 1990;10:5071–5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. He B, Chen P, Chen S-Y, et al. . RAM2, an essential gene of yeast, and RAM1 encode the two polypeptide components of the farnesyltransferase that prenylates a-factor and Ras proteins. Proc Natl Acad Sci USA. 1991;88:11373–11377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Caldwell GA, Naider F, Becker JM. Fungal lipopeptide mating pheromones: a model system for the study of protein prenylation. Microbiol Rev. 1995;59:406–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Casselton LA, Olesnicky NS. Molecular genetics of mating recognition in basidiomycete fungi. Microbiol Mol Biol Rev. 1998;62:55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Raper JR, Baxter MG, Ellingboe AH. The genetic structure of the incompatibility factors of Schizophyllum Commune: the A-factor. Proc Natl Acad Sci USA. 1960;46:833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Riquelme M, Challen MP, Casselton LA, et al. . The origin of multiple B mating specificities in Coprinus cinereus . Genetics. 2005;170:1105–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fowler TJ, DeSimone SM, Mitton MF, et al. . Multiple sex pheromones and receptors of a mushroom-producing fungus elicit mating in yeast. Mol Biol Cell. 1999;10:2559–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fowler TJ, Mitton MF, Vaillancourt LJ, et al. . Changes in mate recognition through alterations of pheromones and receptors in the multisexual mushroom fungus Schizophyllum commune . Genetics. 2001;158:1491–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kües U. Life history and developmental processes in the basidiomycete Coprinus cinereus . Microbiol Mol Biol Rev. 2000;64:316–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Olesnicky NS, Brown AJ, Honda Y, et al. . Self-compatible B mutants in Coprinus with altered pheromone-receptor specificities. Genetics. 2000;156:1025–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Szabo Z, Tönnis M, Kessler H, et al. . Structure-function analysis of lipopeptide pheromones from the plant pathogen Ustilago maydis . Mol Genet Genom. 2002;268:362–370. [DOI] [PubMed] [Google Scholar]
- 19. Ha BS, Kim SI, Ro HS. Isolation and characterization of monokaryotic strains of Lentinula edodes showing higher fruiting rate and better fruiting body production. Mycobiology. 2015;43:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ha B, Kim S, Kim M, et al. . Diversity of A mating type in Lentinula edodes and mating type preference in the cultivated strains. J Microbiol. 2018;56:416–425. [DOI] [PubMed] [Google Scholar]
- 21. Ha B, Moon YJ, Song Y, et al. . Molecular analysis of B mating type diversity in Lentinula edodes . Sci Hortic. 2019;243:55–63. [Google Scholar]
- 22. Wu L, van Peer A, Song W, et al. . Cloning of the Lentinula edodes B mating-type locus and identification of the genetic structure controlling B mating. Gene. 2013;531:270–278. [DOI] [PubMed] [Google Scholar]
- 23. Kosted PJ, Gerhardt SA, Anderson CM, et al. . Structural requirements for activity of the pheromones of Ustilago hordei . Fungal Genet Biol. 2000;29:107–117. [DOI] [PubMed] [Google Scholar]
- 24. Diaz-Rodriguez V, Distefano MD. a-Factor: a chemical biology tool for the study of protein prenylation. Curr Top Pept Protein Res. 2017;18:133–151. [PMC free article] [PubMed] [Google Scholar]
- 25. Olesnicky NS, Brown AJ, Dowell SJ, et al. . A constitutively active G-protein-coupled receptor causes mating self-compatibility in the mushroom Coprinus . EMBO J. 1999;18:2756–2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heimel K, Scherer M, Vranes M, et al. . The transcription factor Rbf1 is the master regulator for b-mating type controlled pathogenic development in Ustilago maydis . PLoS Pathog. 2010;6:e1001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mead ME, Hull CM. Transcriptional control of sexual development in Cryptococcus neoformans . J Microbiol. 2016;54:339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Inada K, Morimoto Y, Arima T, et al. . The clp1 gene of the mushroom Coprinus cinereus is essential for A-regulated sexual development. Genetics. 2001;157:133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. O’Shea SF, Chaure PT, Halsall JR, et al. . A large pheromone and receptor gene complex determines multiple B mating type specificities in Coprinus cinereus . Genetics. 1998;148:1081–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Halsall JR, Milner MJ, Casselton LA. Three subfamilies of pheromone and receptor genes generate multiple B mating specificities in the mushroom Coprinus cinereus . Genetics. 2000;154:1115–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brown AJ, Casselton LA. Mating in mushrooms: increasing the chances but prolonging the affair. Trends Genet. 2001;17:393–400. [DOI] [PubMed] [Google Scholar]
- 32. Yi R, Mukaiyama H, Tachikawa T, et al. . A-mating type gene expression can drive clamp formation in the bipolar mushroom Pholiota microspore (Pholiota nameko). Eukaryot Cell. 2010;9:1109–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kajiwara S, Yamaoka K, Hori K, et al. . Isolation and sequence of a developmentally regulated putative novel gene, priA, from the basidiomycete Lentinus edodes . Gene. 1992;114:173–178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.