Major histocompatibility complex (MHC) class II trafficking begins with the association in the endoplasmic reticulum (ER) of the class II α- and β-subunits with the invariant chain (Ii) (reviewed in Blum et al.1). The αβIi complex then travels via the trans Golgi network (TGN) or plasma membrane to the endocytic pathway. Here a combination of low pH and lysosomal proteinases causes Ii degradation, leaving CLIP (Class II-associated Invariant chain Peptide) in the MHC class II peptide binding groove. CLIP is exchanged for locally generated high-affinity peptides by the action of the non-classical MHC class II molecule, DM. Subsequent surface expression of MHC class II complexes with peptides from foreign antigens induces CD4-positive T-cell responses.
Four isoforms of Ii are present in humans.2 Two, p41 and p43, are expressed at relatively low levels and contain a protease inhibitory domain encoded by an alternatively spliced exon. The other two are p33, the shortest form, and p35, which like p43 is absent from the mouse. p35 and p43 have an extended N-terminal cytoplasmic domain, generated by alternative initiation of translation, that contains an ER retention motif consisting of three successive arginine residues. Ii assembles into trimers, and the four Ii isoforms can form mixed trimers.3 Despite the fact that p35 expression is normally only about 20% that of p33, the high probability that a trimer will contain at least one Ii subunit containing the ER retention motif results in poor release from the ER unless it associates with MHC class II molecules, which inhibit the retention motif and allow egress.
More than 20 years ago we showed that the MHC class II–Ii complex that exits the ER is a nonamer, consisting of the Ii trimer associated with three class II αβ-dimers, and suggested that formation of a complete nonamer is essential for ER export. These conclusions were based on substantial evidence, including characterization of the nonamer by chemical cross-linking, size exclusion and sedimentation velocity analysis,4 as well as pulse-chase analyses.5 The work was sufficiently convincing that the principle was incorporated into many textbook illustrations of the MHC class II trafficking pathway.
A contradictory study recently argued that only one MHC class II αβ-dimer associates with an Ii trimer and that this pentameric complex is capable of ER export.6 A hypothetical structural model suggested that the binding of one αβ-dimer ‘bends’ the putative pentamer towards the cell membrane, sterically preventing association with additional MHC class II heterodimers. This concept is clearly incompatible with our original data, and the experimental techniques used were quite different. One method involved looking for the association of epitope-tagged MHC class II subunits and non-tagged subunits with the same Ii trimer, a second asked whether different human class II isotypes (DR, DQ and DP) could be found in the same ‘mixed nonamer’. Both approaches were unsuccessful, and the model that complexes are pentameric was largely based on these negative data. Parenthetically, it was previously shown that in a transfected human B cell line, endogenous HLA-DR7 αβ-dimers and introduced mouse I-Ak αβ-dimers could associate with the same Ii trimer,7 indicating that mixed species can exist.
In this issue of Immunology and Cell Biology, Thibodeau and colleagues have creatively re-addressed this issue.8 Their work suggests that MHC class II association regulates transport from the ER by neutralizing the ER retention signals in the p35 form of Ii, allowing ER egress only when all retention motifs present in the trimer are ‘cancelled’. Either p33 trimers or p35 trimers were expressed in HEK293T cells along with DR α- and a mixture of DR β-subunits, one of which was equipped with its own cytoplasmic ER retention signal (a di-lysine motif that functions similarly to the arginine motif present in p35). In cells solely expressing p33 trimers, the DR αβ-dimer lacking an ER retention signal was expressed on the cell surface even when co-expressed with the ER-retained form. In contrast, in cells expressing p35 trimers, the ER-retained dimer inhibited surface expression of the version lacking the retention signal, indicating that association of an ER-retained DR molecule with the same p35 trimer as the ‘normal’ DR molecule reduces ER exit of the latter.
These authors also asked whether different class II isotypes could interact with the same Ii trimer. When DQ αβ-dimers were introduced into cells along with ER-retained DR αβ-dimers, DQ expression on the surface was reduced in the presence of p35 trimers but not in the presence of p33 trimers. Curiously, some DQ was surface expressed in the presence of p35 but these molecules did not contain the Ii-derived CLIP peptide, suggesting that this subset was transported to the surface in an Ii-independent manner. Thus, its presence at the cell surface was irrelevant to the conclusion that ER-retained DR molecules could restrain DQ surface expression in the presence of p35 trimers. Again, the interpretation is that DR and DQ molecules can ‘share’ an Ii trimer.
Overall, the data are compatible with the following model (schematized in Figure 1). If Ii trimers only contain p33 (or potentially the analogous p41 alternatively spliced form), a pentameric structure consisting of an Ii trimer and an MHC class II heterodimer can leave the ER. If the trimers are exclusively composed of p35 (or p43), only MHC class II–Ii nonamers can escape. One can extrapolate and suggest that pentamers can also leave the ER if the trimer contains only one p35 or p43 molecule, whereas heptamers (containing two class II αβ-dimers) can leave the ER if the Ii trimer has two of them. Thibodeau and colleagues suggest that the first MHC class II heterodimer to bind may neutralize an ER retention signal or not, depending on which of the three Ii molecules it associates with in the mixed trimer. This could explain the results of our original studies, which indicated that nonamer formation was essentially complete even though the amount of p33 significantly exceeded that of p35. At a minimum, it seems likely that MHC class II–Ii complex stoichiometry varies depending on the ratio of p33/p35 expressed. It would, of course, be very informative to examine the stoichiometry of the complex in mouse cells, in which p35 and p43 do not exist.
Figure 1.
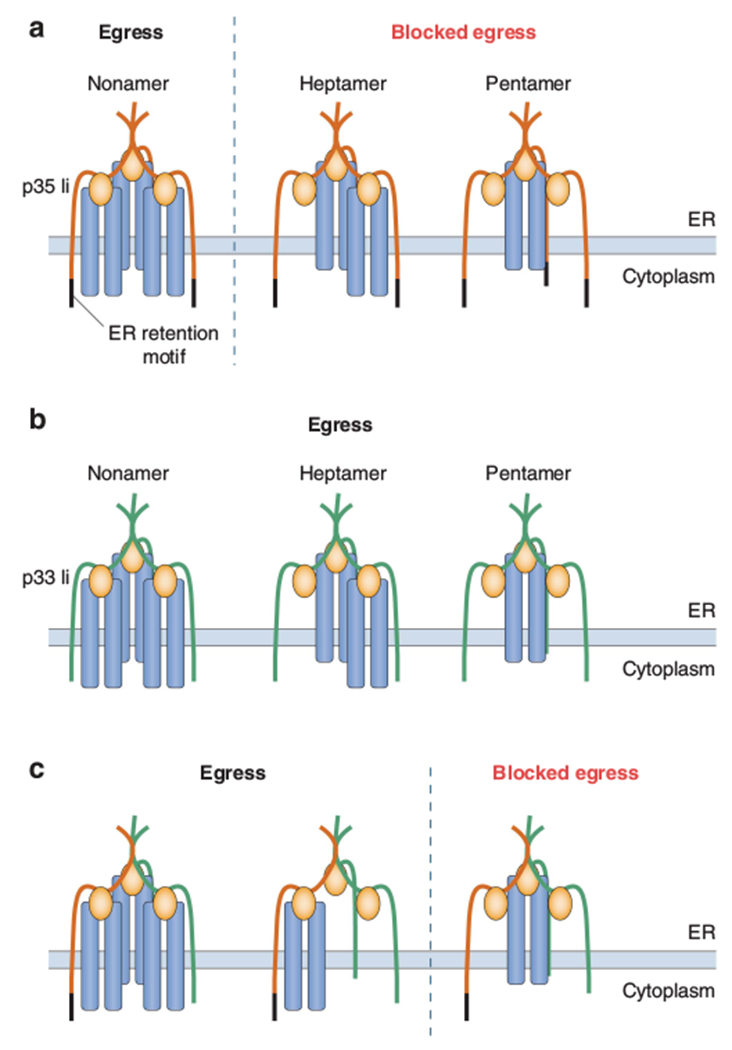
Model for the regulation of MHC class II release from the ER by the composition of the Ii trimer. (a) Trimers containing three p35 forms (orange), shown with the N-terminal-extended cytoplasmic domain containing an ER retention signal (black), remain in the ER unless they associate with three MHC class II αβ-dimers (blue). If only one or two αβ-dimers associate, the ‘unblocked’ ER retention sequence(s) remain functional and transport of the complex from the ER is prevented. (b) Trimers containing one, two or three αβ-dimers can leave the ER if all three Ii species are the p33 form (green) that lacks the ER retention signal. (c) A mixed Ii, trimer that contains only one p35 molecule may leave the ER with a single αβ-dimer if the latter associates with p35 and not one of the two p33 molecules also present in the trimer.
What does this mean functionally? Ii facilitates the assembly of MHC class II molecules, and its ER retention ensures that αβ-dimers generally do not leave home without it. Ii trimer formation improves ER retention, at least in the human system, because each trimer is likely to contain an ER retention signal. The cytoplasmic domain of all the Ii forms also contains an essential di-leucine motif that targets MHC class II–Ii complexes to the endocytic pathway, and the presence of three such targeting signals in an MHC class II–Ii complex may improve its association with clathrin-associated adaptor proteins, enhancing delivery to appropriate antigen-processing compartments. Furthermore, MHC class II–Ii complexes containing p35 follow a different intracellular trafficking pathway to antigen-processing compartments than those that do not,9 potentially affecting the repertoire of bound antigenic peptides. Although these issues have faded into the rear-view mirror for most immunologists, Thibodeau and colleagues are to be commended for continuing to shine light upon this intriguing problem.
Contributor Information
Peter Cresswell, Department of Immunobiology and Howard Hughes Medical Institute, Yale University Medical School, New Haven, CT, USA.
Paul A Roche, Experimental Immunology Branch, National Cancer Institute, NIH, Bethesda, MD, USA.
References
- 1.Blum JS, Wearsch PA, Cresswell P. Pathways of antigen processing. Ann Rev Immunol 2013; 31: 443–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cresswell P. Invariant chain structure and MHC class II function. Cell 1996; 84: 505–507. [DOI] [PubMed] [Google Scholar]
- 3.Marks MS, Blum JS, Cresswell P. Invariant chain trimers are sequestered in the rough endoplasmic reticulum in the absence of association with HLA class II antigens. J Cell Biol 1990; 111: 839–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roche PA, Marks MS, Cresswell P. Formation of a nine-subunit complex by HLA class II glycoproteins and the invariant chain. Nature 1991; 354: 392–394. [DOI] [PubMed] [Google Scholar]
- 5.Lamb CA, Cresswell P. Assembly and transport properties of invariant chain trimers and HLA-DR-invariant chain complexes. J Immunol 1992; 148: 3478–3482. [PubMed] [Google Scholar]
- 6.Koch N, Zacharias M, Konig A, Temme S, Neumann J, Springer S. Stoichiometry of HLA class II-invariant chain oligomers. PLoS ONE 2011; 6: e17257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riberdy JM, Cresswell P. The antigen-processing mutant T2 suggests a role for MHC-linked genes in class II antigen presentation. J Immunol 1992; 148: 2586–2590. [PubMed] [Google Scholar]
- 8.Cloutier M, Gauthier C, Fortin J-S, Thibodeau J. The invariant chain p35 isoform promotes formation of nonameric complexes with MHC II molecules. Immunol Cell Biol 2014; 92: 553–556. [DOI] [PubMed] [Google Scholar]
- 9.Warmerdam PA, Long EO, Roche PA. Isoforms of the invariant chain regulate transport of MHC class II molecules to antigen processing compartments. J Cell Biol 1996; 133: 281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]


