Abstract
Hippo signaling is an evolutionarily conserved network that plays a central role in regulating cell proliferation and cell fate to control organ growth and regeneration. It promotes activation of the LATS kinases, which control gene expression by inhibiting the activity of the transcriptional co-activator proteins YAP and TAZ in mammals, or Yorkie in Drosophila. Diverse upstream inputs including both biochemical and biomechanical cues regulate Hippo signaling, which enable it to play a key role as a sensor of cells’ physical environment and an integrator of growth control signals. Several components of this pathway localize to cell-cell junctions, and contribute to regulation of Hippo signaling by cell polarity, cell contacts, and the cytoskeleton. Down-regulation of Hippo signaling promotes uncontrolled cell proliferation and impairs differentiation, and is associated with cancer. We review current understanding of Hippo signaling, highlighting progress in elucidating its regulatory mechanisms and biological functions.
Keywords: Hippo, YAP, Yorkie, growth, cancer
1. INTRODUCTION
The discovery of Hippo signaling originated with the identification of Drosophila tumor suppressor genes - genes that when inactivated by mutation, result in overgrowth phenotypes. Three Drosophila tumor suppressors began to be linked into a pathway in 2003 with the independent discovery by several groups of the hippo gene, and the realization that its activity was connected to the previously reported salvador and warts genes (reviewed in 95). These were soon connected to other Drosophila tumor suppressor genes, both newly discovered (eg mob as tumor suppressor (mats)) and previously identified (eg expanded, Merlin, fat, discs overgrown). These tumor suppressor genes were also shown to regulate growth largely through inhibition of the transcriptional co-activator protein Yorkie, such that by 2006 the basic outlines of a newly discovered signaling pathway could be sketched from plasma membrane to nucleus. In this initial pathway, upstream regulators, including Expanded, Merlin, and Fat, controlled the activity of a core kinase cassette, comprising the kinases Hippo and Warts, and their co-factors Salvador and Mats, and this core kinase cassette inhibited the activity of Yorkie.
Over the past 15 years, the fundamental importance of the Hippo signaling network to cell fate and organ growth has become increasingly appreciated. Concomitantly, our understanding of the complexity of this network and its interconnections with other pathways has increased tremendously, and Hippo signaling is now recognized as a major integrator of cues from cells’ physical and biochemical environment. It is also now well established that the core of the pathway is highly conserved across diverse animals species, and its contributions to mammalian oncogenesis have received considerable attention.
We overview here the organization, regulation, and functions of the Hippo signaling network, including how its mis-regulation contributes to disease. Not all Hippo signaling depends on the hippo gene, and this review will focus on processes that modulate, or depend upon, the activity of the kinase Warts, the transcription factor Yorkie, and their mammalian homologs.
2. THE HIPPO SIGNALING NETWORK
Transcriptional effectors of Hippo signaling
Hippo signaling influences cellular phenotypes by inhibiting the transcriptional co-activator protein Yorkie (Yki), or its mammalian homologs YAP1 and TAZ (henceforth collectively referred to as YAP proteins)(23; 49). YAP and TAZ have partially over-lapping activities in vivo, but they also each have unique functions (reviewed in 131). YAP proteins lack a DNA binding domain, and exert their effects through association with DNA-binding proteins. Although several DNA binding partners for YAP proteins have been identified, their main partners are the TEAD proteins, TEAD1–4 in mammals, and Scalloped (Sd) in flies. Requirements for other DNA-binding partners of YAP proteins have sometimes appeared in specific biological contexts, or control of particular downstream genes. The YAP-TEAD heterodimer has also been observed to interact with other transcription factors, including Taiman, GAGA, AP-1, and β-catenin (43; 93; 154; 155), to co-regulate downstream target genes.
Genome-wide studies assessing chromatin occupancy have revealed that YAP and TEAD can occupy proximal promoters of target genes, but more often associate with distal enhancers (35; 65; 93; 122; 154). Examination of binding partners for YAP proteins have identified multiple classes of interacting factors that can promote transcription, and through which the YAP-TEAD heterodimer mediates transcriptional activation, including components of Mediator, SWI/SNF chromatin remodeling complexes, and the Trr histone H3K4 methyl transferase complex (35; 93; 94; 105; 132). YAP proteins can also contribute to transcriptional repression (55), but in most instances they act as activators.
Although TEAD proteins are partners of YAP proteins, their loss of function phenotypes can be distinct, as has been most clearly illustrated in Drosophila. This occurs because in the absence of Yki, Sd instead associates with a transcriptional co-repressor protein, Tondu-domain-containing Growth Inhibitor (Tgi)(38; 58). In vertebrates, TEAD proteins can associate with the Tgi homolog, VGLL4. When the Hippo pathway is active, Tgi associates with Sd and actively represses “Yki” target genes. Loss of Sd thus differs from loss of Yki because it results in both loss of Yki-mediated activation, and loss of Tgi-mediated repression. VGLL4 expression is controlled by a miRNA, mir-130a, which is a target of YAP, and a similar mechanism operates in Drosophila, where the Yki-induced miRNA Bantam can repress Tgi (118). Interaction of Yki with Sd can also be competed by E2F1, which like Tgi, can suppress Yki-Sd target genes (157).
In many contexts, activation of YAP proteins is associated with increased tissue growth, and direct target genes have been identified that contribute to this, including growth promoting genes like Myc, cell cycle regulators like CycE and E2F1, and inhibitors of apoptosis like Diap1, and BIRC3 (reviewed in 131). Ligands for other signaling pathways that could contribute to regulation of tissue growth, including Wnt, Notch, EGFR, TGFβ and Jak-STAT pathways, have also been identified as targets of YAP proteins (reviewed in 40). Another class of transcriptional targets are upstream components of the Hippo pathway that negatively regulate YAP activity, including Merlin, Expanded, Kibra, AMOTL2 and LATS kinases (reviewed in 148). Genome-wide expression profiling and chromatin binding has identified thousands more candidate targets, but there are many differences amongst the sets of targets identified in studies conducted using different cell types, indicating that the much of the YAP response is tissue- or cell-type specific (131; 148).
The key role of YAP proteins in cancer has stimulated attempts to develop YAP inhibitors. Verteporfin can disrupt the interaction between YAP proteins and TEAD, and suppress expression of YAP target genes (75). However, it has high cellular toxicity, and blocking YAP/TAZ systemically using small molecules could have side effects in other tissues. An alternative approach has been developed based on the structures of YAP-TEAD and VGLL4-TEAD complexes. A polypeptide, super-TDU, was designed that blocks the YAP-TEAD interaction and suppresses tumor growth in mice (53), which could be targeted to cancer cells.
Regulation of YAP proteins
In the Hippo pathway, the activity of YAP proteins is regulated through phosphorylation by LATS family kinases (Warts in Drosophila, LATS1 and LATS2 in mammals) (95; 148). A site of LATS phosphorylation in each of the YAP proteins (Ser168 of Yki, Ser127 of human YAP1, Ser89 of human TAZ) promotes their cytoplasmic localization by creating a binding site for 14–3-3 proteins, which is a major mechanism for regulating their activity (Fig. 1A). In mammals, LATS phosphorylation also regulates YAP and TAZ by promoting their degradation through phosphorylation of YAP Ser381 or TAZ Ser311, which contributes to creation of phosphodegron motifs recognized by the SCFBeta-TRCP E3 ligase (72; 161). Another mechanism by which components of the Hippo signaling network can influence YAP family proteins is cytoplasmic sequestration. Binding to upstream regulators including Expanded, Warts, Merlin, or Angiomotins can inhibit YAP activity not only by promoting YAP phosphorylation, but also by physically excluding it from the nucleus (reviewed in 82).
Fig. 1. Regulation of YAP and the Hippo core.
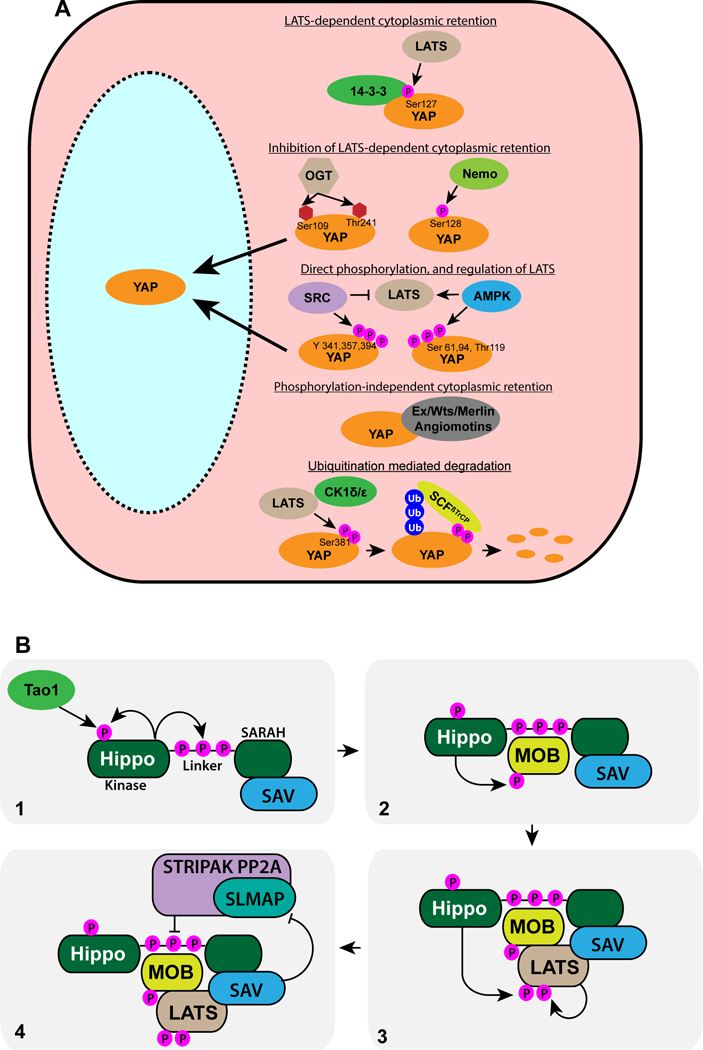
A) Several biochemical mechanisms that regulate YAP proteins, as described in the text, are shown. Amino acids modified in human YAP1 are indicated. OGT = O-N-acetylglucosaminyl transferase.
B) Sequence of interactions and phosphorylations involved in activation of LATS proteins, as described in the text, are shown. Hippo is activated by phosphorylation within its kinase domain, mediated by Hippo or Tao1 (1). Hippo phosphorylates Ser residues in its linker region, which recruits MOB, which is then phosphorylates by Hippo (2) Phosphorylated MOB recruits LATS (together with SAV and other pathway components not shown here), and LATS is then phosphorylated by Hippo, and also autophosphorylates to generate active LATS. This complex can be deactivated by SLMAP-mediated recruitment of the STRIPAK PP2a phosphatase complex (4); this recruitment is inhibited by SAV.
Several other kinases that can phosphorylate YAP proteins have been identified. The NDR1 and NDR2 kinases are structurally related to LATS kinases, and can phosphorylate YAP on the same sites as LATS kinases (156). Nemo kinases (Drosophila Nemo, mammalian Nemo-like kinase) promote YAP protein activity through phosphorylation of Ser residues immediately adjacent to the LATS site in the 14–3-3 binding region, which inhibits LATS phosphorylation and 14–3-3 binding (46; 88). Src family kinases can phosphorylate YAP on Tyr residues and increase YAP activity, which provides a mechanism for Hippo-independent YAP regulation (61; 128). AMPK, which is regulated by cellular energy status, can phosphorylate and inhibit YAP proteins, providing another mechanism for Hippo-independent regulation, and linking YAP activity to nutritional and polarity cues (85; 138). Additional modifications that influence YAP activity have been identified, including ubiquitination, sumoylation, and glycosylation, and YAP proteins can also be regulated by autolysosomal degradation (131). Although most regulation of YAP-TEAD transcription factors occurs through YAP proteins, recent studies have revealed that direct phosphorylation of TEAD by p38 MAPK promotes cytoplasmic localization of TEAD, thereby reducing YAP-TEAD dependent transcription (68). The diversity of mechanisms for regulating YAP activity emphasize that while it is the key transcription factor of Hippo signaling, it is also regulated by many other factors.
The Hippo core - regulation of LATS kinases
LATS kinases are the key direct regulators of YAP proteins within the Hippo pathway. LATS kinases are activated by Ste20 family kinases, including Hippo, and its mammalian homologues MST1 and MST2 (collectively, Hippo kinases) (82). More recent studies identified several MAP4K-type kinases as additional LATS activators, which, depending on the context act either in parallel to Hippo kinases, or in place of them (62; 63; 83; 165). Hippo kinases are activated by phosphorylation within their activation loop, and two mechanisms for this have been identified. One involves phosphorylation of the activation loop by another family of Ste20 proteins, the TAO1 kinases (7; 102). Another involves auto-phosphorylation promoted by Hippo kinase dimerization (20; 54). Hippo kinase activity plays multiple roles in LATS activation, including activation of Hippo, promotion of MOB-Hippo binding, promotion of MOB-LATS binding, and activation of LATS.
Structural and biochemical studies have provided a more detailed understanding of how two key accessory factors long considered part of the Hippo pathway “core”, Mats and Sav, promote LATS activation (Figure 1B). MOB proteins (Mats in Drosophila, MOB1 and MOB2 in mammals) have dual roles in LATS activation, as their ability to associate with both Hippo and LATS kinases can scaffold Hippo phosphorylation of LATS, and their physical interaction with LATS induces a conformational change required for LATS activity (104; 142). MOB protein binding to Hippo kinases is promoted by autophosphorylation of a “linker” region within Hippo (16; 91). Phosphorylation of MOB proteins by Hippo kinases then promotes association of MOB proteins with LATS kinases.
Salvador was originally proposed to act as a scaffolding protein that links Hpo and Wts, but subsequent studies have identified additional roles for Sav, including recruiting Hippo to membranes (126; 147), and maintaining Hippo activity by inhibiting its dephosphorylation. The PP2A phosphatase complex, STRIPAK (Striatin-interacting phosphatase and kinase) negatively regulates Hippo (111). The STRIPAK complex was first reported to be recruited to Hippo by a subset of RASSF proteins. More recent structural and biochemical studies identified the adapter protein SLMAP as primarily responsible for recruiting the STRIPAK complex to the phosphorylated linker region of Mst1/2, and then promoting their inactivation (3; 164). Sav1 can inhibit this recruitment of SLMAP, thereby maintaining activation of Hippo kinases (3).
In addition to being regulated by Hippo and related kinases, LATS kinases can also be regulated through control of their stability. Several ubiquitin ligases that can target LATS kinases for degradation have been identified, including ITCH, CRL4, SIAH2, and NEDD4 (82). As discussed below LATS kinases are also regulated through control of their localization, and their interaction with co-factors or inhibitors.
LATS and YAP proteins are key substrates of Hippo and LATS proteins, respectively, but additional substrates have also been identified, which can contribute to cellular responses to Hippo signaling. For example, in Drosophila Warts can regulate actin polymerization through phosphorylation of the Drosophila Ena/VASP protein (76), and can influence spindle orientation through phosphorylation of Mud (22). In mammals, LATS phosphorylates Angiomotins, which contributes to YAP inhibition (1; 11; 45).
Promotion of LATS activity by junctional and apical protein complexes
Genetic studies in Drosophila identified several proteins that act upstream of Hippo and Warts, and are required for their activation. Many of these proteins localize to apical cell-cell junctions, where their ability to interact with core Hippo pathway components enables them to scaffold assembly of complexes that promote activation of Warts. Although junctional complexes play key roles in both Drosophila and mammalian Hippo signaling, there are differences amongst the specific protein complexes and how they connect to the Hippo pathway.
Expanded (Ex) is a Drosophila member of the 4.1, Ezrin, Radixin and Moesin (FERM) domain-containing proteins. Ex localizes to apical junctions through interaction with the transmembrane protein Crumbs (Crb), which also has a role in organizing apical-basal polarity. Loss of either Ex or Crb can cause overgrowth phenotypes due to increased Yki activation (125). Ex promotes Wts activation, and under conditions of Hippo pathway activation in Drosophila wing discs, activated (phosphorylated) Wts can be detected overlapping Ex at apical junctions, whereas in the absence of Ex, Wts can not localize there (126). Ex can physically associate with Hippo, Wts, Merlin, Kibra and Yki. Although Hpo can associate with Ex, Hpo localization to apical junctions depends upon Salvador (126; 147). Salvador is localized to apical junctions in Drosophila through interaction with the transmembrane Immunoglobulin-domain containing protein Echinoid (151). Ex also associates with Schip1 (Schwannomin interacting protein 1), and Schip1 associates with Tao-1 (14). Through this network of protein interactions, Ex promotes assembly of a complex of proteins that promotes Wts activation.
Another FERM domain protein, Merlin, can also bind and recruit Wts to membranes, where it plays a role similar to Ex in promoting Wts activation (147). Merlin was first identified as a tumor suppressor in humans, responsible for the inherited tumor syndrome Neurofibromatosis type 2 (NF2). Merlin is required for YAP regulation in multiple mammalian tissues (reviewed in 148). In Drosophila, Merlin overlaps Ex at apical junctions, but also exhibits a distinct apical membrane localization, to which Wts can also be recruited (124). Two mechanisms for localizing Merlin to membranes have been described. One involves the activity of the phospholipid kinase PI4KIII alpha, implying a role for phospholipids in recruiting Merlin through its FERM domain (145). The other involves the Kibra protein, which can recruit Merlin to apical membrane sites (124). The contributions of Ex and Mer to Wts activation vary amongst different Drosophila tissues. For example, in the developing wing Ex plays the more important role (39), whereas in glial cells Merlin has a major role (108). In addition to binding Wts and Kibra, Merlin can also associate with Sav (150), which links it to Hippo (108), and Kibra can bind Sav, Ex, Mer, and Pez (4; 36; 101; 150). Thus as for Ex, multiple interactions amongst Hippo pathway components occur within Merlin protein complexes, which collectively promote Wts activation.
Localization of Hippo pathway components to apical junctions and assembly of scaffolding complexes also contributes to LATS activation in mammalian cells. In vertebrates, the Crumbs homolog and tight junction protein Crb3 promotes Lats phosphorylation and YAP inhibition (127). There is no close homolog of Ex, however Merlin, Kibra, and the Pez homologue PTPN14 are conserved promoters of LATS activity in vertebrates (82; 125). A key role in promoting LATS activation is also played by a class of vertebrate-specific junctional proteins, the Angiomotins, which include Angiomotin (which has distinct p80 and P130 isoforms), Angiomotin-like 1 (AMOTL1) and Angiomotin-like 2 (AMOTL2). Angiomotins can function as scaffolding proteins that bind multiple components of the Hippo pathway, including LATS, YAP, Merlin and Kibra. Owing to their ability to bind YAP, they can modulate YAP activity by both promoting its phosphorylation and by sequestering it in the cytoplasm (10; 60; 137; 160). The Angiomotins also participate in a feed-forward regulatory loop, as their phosphorylation by LATS stabilizes them and promotes their binding to Merlin, which enhances binding of Merlin to LATS and further LATS activation (1; 46). The importance of junctional localization of Angiomotins is emphasized by observations that in mouse blastocysts their shift to apical localization correlates with loss of LATS activation (45; 60).
3. REGULATION OF HIPPO SIGNALING
Hippo signaling is affected by diverse upstream inputs, many of which relate to cells’ local physical environment. It thus functions as a network that integrates multiple factors, including both biochemical and biomechanical cues, to modulate growth and cell fate (Figure 2).
Fig. 2. Activation of core Hippo kinases.
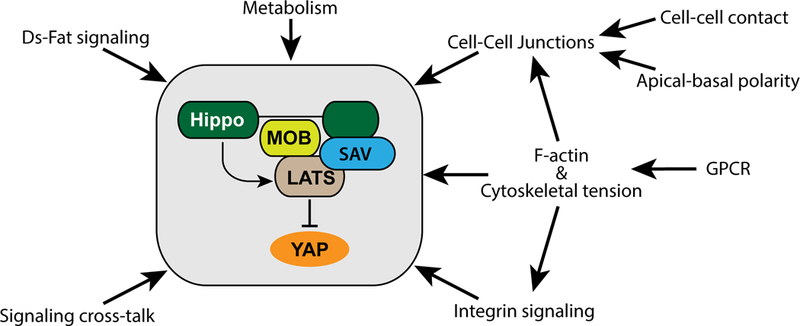
Several upstream factors that influence the activation of the core Hippo pathway kinases and YAP are shown, for details see text.
Regulation of Hippo signaling by the Dachsous-Fat system
The Drosophila protocadherins Fat and Dachsous (Ds) regulate both planar cell polarity (PCP) and Hippo signaling (reviewed in 52). They engage in heterophilic binding between cells, which is modulated by the Golgi-resident kinase four jointed (fj), and can act as a ligand-receptor pair for Hippo signaling. A remarkable feature of the Ds-Fat system is that it is not simply regulated by the amount or activity of Ds, but also by the steepness of the Ds and Fj gradients: a shallow gradient is associated with increased Hippo signaling, whereas a steep gradient inactivates Hippo signaling. Ds and Fj are expressed in opposing gradients in response to morphogen signaling in many developing tissues, which results in polarized localization of Ds and Fat within each cell (reviewed in 52). The key downstream effector of Fat is the atypical myosin Dachs (13; 80). Dachs localizes to apical junctions, and its membrane localization is crucial for its influence on both PCP and Hippo signaling (80; 96). Dachs localization is regulated largely through an inhibition of membrane localization by Fat (80). Recently it was shown that Fat regulates Dachs membrane association through the SH3 domain containing adapter protein, Vam/Dlish (84; 159). Earlier studies identified reduction in junctional levels of Ex and reduction in Warts levels as mechanisms by which Dachs could influence Hippo signaling (121). More recently, it was shown that Dachs can also compete with Mats for binding to Warts, and thereby suppress Warts activation (134).
Ds and Fat are conserved in vertebrates, but mutation of their mouse homologs (Dchs1 and Fat4) mainly affects PCP rather than Hippo signaling (79; 113), and Dachs is not conserved in vertebrates. However, effects on Hippo signaling have been reported in some tissues, and in cardiomyocytes it has recently been reported that interaction of the Fat4 cytoplasmic domain with AMOTL1 suppresses nuclear localization of YAP (106).
Regulation of Hippo signaling by cell-cell contact and cell polarity
Consistent with the key role of apical junctions in promoting activation of LATS proteins, cell-cell contact and cell polarity have strong effects on Hippo signaling. Components of mammalian tight junction or adherens junction complexes promote mammalian Hippo signaling. For example, loss of Crb3, E-cad, α-Cat or β-Cat can result in increased YAP activity (57; 116; 127; 133). In Drosophila, junctional localization of Crb and Ed, and consequently Ex and Sav, depend upon homophilic binding between neighboring cells, and thus require cell-cell contact (12; 151). Surprisingly however, loss of E-cad, α-Cat, or β-Cat in Drosophila wing discs decreased Yki activity (146). Nonetheless, in both mammals and flies cell contact and cell density promote Hippo signaling (2; 97; 163). Thus isolated mammalian cells typically have high levels of YAP activity, while cells cultured at high density have low levels of YAP activity. This regulation of Hippo signaling is central to a fundamental property of non-transformed cells in culture – contact inhibition of cell proliferation.
The key contribution of cell-cell junctions is also reflected in the fact that disruption of epithelial apical-basal polarity is associated with reduced Hippo signaling, and strong activation of YAP proteins. Indeed several Drosophila genes classified as neoplastic tumor suppressors, mutation of which disrupts apical-basal polarity, have been shown to induce Yki-dependent growth (reviewed in 121). Changes in cellular polarization also correlate with changes in YAP activity in mammalian cells (reviewed in 131).
Regulation of Hippo signaling by integrins and cell ECM attachment
Detachment of cultured cells from a substrate can cause cell death (anoikis) through activation of Hippo signaling (162). Integrins play a crucial role in mediating signaling from the extracellular matrix (ECM), and multiple connections between integrins and YAP activity have been identified. Integrin linked kinase (ILK) can inhibit Merlin activation by inhibiting the phosphatase MYPT1, and through activation of RAC and PAK (9; 112; 117). Integrin-mediated regulation of Hippo signaling has also been linked to Focal Adhesion Kinase (FAK)(9; 56). Integrins bound to fibronectin can stimulate FAK, which activates PI3K through Src. PDK1, which is downstream of PI3K, can then disrupt the core kinase cassette and inhibit Hippo signaling (30; 56). An alternative mechanism for FAK regulation of YAP, involving dephosphorylation of Ser397 by the protein phosphatase PP1A, has also been described (48). Src can also promote YAP activity by directly phosphorylating it, and by phosphorylating and inactivating LATS (61; 119; 128). Other downstream effectors of integrin signaling, including Jnk, Rho, and Ras, have also been linked to regulation of YAP proteins (82; 121). In multi-layered tissues like skin, integrin signaling contributes to activation of YAP in basal layers, as opposed to the inhibition of YAP in apical cell layers (27).
Biomechanical regulation of YAP activity
In addition to biochemical cues mediated through protein-protein interactions at cell-cell or cell-ECM contacts, Hippo signaling is also sensitive to mechanical stress experienced by cells. Biomechanical regulation of Hippo signaling has been revealed by the sensitivity of YAP localization to a variety of perturbations including altered cell shape, ECM stiffness, cell stretching, cell density, and shear forces (2; 5; 15; 25; 135; 163). Many of these effects depend upon the F-actin cytoskeleton, tension within the cytoskeleton, and the influence this tension has on cell-cell and cell-ECM attachments.
Cytoskeletal tension is generated by myosin, and conditions associated with high levels of actomyosin contractility are generally associated with high YAP activity, whereas conditions associated with low actomyosin contractility are associated with low YAP activity (reviewed in 125). Moreover, in most cases direct genetic or pharmacological inhibition of actomyosin contractility decreases YAP activity, whereas increasing actomyosin contractility increases YAP activity. However, actomyosin contractility has been reported in some contexts to inhibit rather than promote YAP activity, possibly because of the role of actomyosin activity in stabilizing cell-cell junctions (34; 44).
Hippo signaling can also feed back and regulate tissue mechanics and F-actin levels, both through YAP-dependent and YAP-independent mechanisms. For example, YAP is essential for mechanical force production during lung branching morphogenesis (67) and body shape in fish (103), and Warts activity influences cytoskeletal organization in Drosophila (32; 76).
Biomechanical signaling at adherens junctions
Cells experience mechanical stress through their connections to neighboring cells at adherens junctions, which are attached to the actin cytoskeleton. A mechanism by which tension at adherens junctions influences Hippo signaling was first identified in Drosophila, and involves the tension-dependent formation of a complex between Warts and a Warts inhibitor, Ajuba LIM protein (Jub)(107). The Jub protein is recruited to adherens junctions under tension, possibly through binding to a stretched conformation of α-catenin. Jub then recruits and inhibits Warts, at least in part by sequestering it away from more apical junctions where Warts activation can occur (126).
A related mechanism was recently identified in cultured mammalian cells, by establishing that LATS proteins are recruited to adherens junctions and inhibited in a tension-dependent fashion (26; 50). Two different LIM-domain containing proteins have been identified as required for this tension-dependent recruitment and inhibition of LATS: LIMD1, which is the closest mammalian homologue of Drosophila Jub, and TRIP6, which more closely related to Zyxin. TRIP6 can compete with MOB proteins for binding to LATS, which could provide a mechanism for a TRIP6-LATS complex to inhibit LATS activation (26).
These biomechanical pathways for regulation of Hippo signaling contribute to cell density-dependent regulation of Hippo signaling in both flies and mammals, including contact inhibition of cell proliferation (26; 50; 97). This occurs because as cell density increases, cytoskeletal tension decreases (50; 97), which reduces junctional recruitment and inhibition of LATS kinases.
Biomechanical signaling at focal adhesions
Several experimental regimes that alter stress on focal adhesions, including substrate stiffness, substrate stretching or compression, and increasing or decreasing cell area, influence YAP activity (2; 15; 25; 135). The mechanism by which this occurs is not well understood, but it depends upon the actin cytoskeleton, Rho activity, and cytoskeletal tension. As cytoskeletal tension can influence the size and character of focal adhesions, it could influence signaling through cell-ECM contacts, such as the integrin pathways discussed above. Mechanical stress applied to the ECM can also directly affect the nucleus through cytoskeletal connections to the nucleoskeleton (24), and has been shown to influence YAP nuclear entry by affecting nuclear pore size (28). Tension experienced at focal adhesions might also influence Hippo signaling by influencing F-actin levels, and pathways like Jnk that are sensitive to changes in F-actin.
It has been suggested that biomechanical regulation of YAP proteins is independent of Hippo signaling, because conditions that suppress F-actin formation can lead to cytoplasmic and inactive YAP even when LATS kinases are knocked down or LATS phosphorylation sites are mutated (2; 25). However, many experiments have revealed that biomechanical stresses do influence LATS activity (15; 26; 50; 107; 135; 162). Thus, while it is possible to create conditions in culture where F-actin levels and organization cannot support biomechanical regulation of YAP by LATS, the extent to which Hippo-dependent versus Hippo-independent regulation of YAP predominates in vivo remains unclear, and could vary depending upon cell types and physical conditions.
Regulation of YAP by the actin cytoskeleton and cytoskeletal tension
Both in Drosophila and in mammalian cells, F-actin levels correlate with YAP activity (2; 32; 114). The actin cytoskeleton plays a central role in regulating the mechanical properties of cells, and changes in F-actin levels could influence processes dependent upon cytoskeletal tension, as discussed above. In mammalian cells, binding of Angiomotins to F-actin releases YAP (78), which could also contribute to the influence of F-actin on YAP activity, but this could not explain the activation of Yki by elevated F-actin in Drosophila.
Several upstream inputs into Hippo signaling, including spread cell shape, cell attachment, low cell density and high matrix rigidity, are suppressed by inhibiting the key cytoskeletal regulator Rho (2; 25; 135; 162). Pharmacological activation of Rho can also promote YAP activation (162). Rho can increase both F-actin levels, and tension within the actin cytoskeleton, and so could potentially impact YAP activity in several ways, but a major part of its effect is mediated through tension at adherens junctions, as activation of YAP by Rho can be suppressed by knockdown of LIMD1 (50).
The actin cytoskeleton is connected to the membrane through the spectrin cytoskeleton. The spectrin cytoskeleton can influence Hippo signaling, but there is disagreement as to how it does so. One study suggested that spectrins are regulated by cytoskeletal tension and help transduce the effects of tension on Hippo signaling through the upstream Hippo pathway regulators Crumbs, Merlin and Kibra (33). Another reported that spectrins influence myosin phosphorylation, suggesting that they may affect Hippo signaling by influencing actomyosin contractility (19).
Metabolic status influences Hippo signaling
Integration of metabolic pathways with growth control signals is essential for proper tissue growth and homeostasis, and several connections between Hippo signaling and nutrient sensing and metabolic pathways have been identified.
The insulin-IGF signaling (IIS) pathway plays a central role in growth and metabolism, and YAP proteins are activated by IIS signaling and necessary for insulin-induced growth (123). This occurs through PDK1, with additional contributions from AKT. Energy stress induced by culturing cells in glucose free conditions activates the energy sensor AMPK, which decreases YAP/TAZ activity both by directly phosphorylating YAP, and also by phosphorylating AMOTL1 to promote LATS activation (21; 85; 138). Glucose levels also influence O-GlcNAc transferases, which attach GlcNAc to Ser or Thr residues of cellular proteins. Under high glucose conditions Yap can undergo O-GlcNAcylation at Ser109 and Thr241, which decreases YAP phosphorylation by LATS (100; 158). The key glycolytic enzyme, Phospho-Fructo Kinase (PFK) can bind TEAD directly and promote YAP-TEAD activity (29). The TSC-Tor pathway, which is responsive to nutrient levels including amino acids, and regulates multiple downstream effectors to modulate growth and metabolism, also influences YAP and TAZ levels and activity. In a mouse cancer model, mutation of TSC was observed to lead to an mTOR and autophagy-dependent up-regulation of YAP and TAZ (66). In Drosophila, inhibition of TOR was observed to inhibit nuclear Yki activity through an unknown mechanism (99).
The mevalonate synthesis pathway converts acetyl-coA into lipid precursors, including compounds involved in protein prenylation. This pathway promotes YAP/TAZ activity through the Rho GTPases, which require prenylation for their membrane localization and activity (120; 141). Thus, inhibition of this pathway by drugs including statins can inhibit YAP/TAZ activity.
Hippo signaling also feeds back and influences cellular metabolism.
YAP induces multiple genes involved in hexosamine biosynthesis pathways including OGT, Nudt9 and SLC5A3, increases glucose uptake, and promotes global O-GlcNAcylation (158). Many cancer cells alter their metabolism, including increased aerobic glycolysis (the Warburg effect) and glucose uptake. Aerobic glycolysis regulates YAP and in turn YAP promotes glucose uptake which could potentiate tumor growth (29; 158). Activation of Yki/YAP also promotes TOR signaling through miRNA-mediated inhibition of PTEN, and controlling the expression of the high-affinity leucine transporter (41; 130).
Regulation of Hippo signaling by cross-talk with other pathways
Integration of growth and cell fate decisions also occurs through cross-talk of other signaling pathways with Hippo signaling, and by now most major signaling pathways have been found to regulate or cross-talk with Hippo signaling. For example, multiple G protein coupled receptor (GPCR) signaling pathways can regulate YAP through their influence on Rho activity (149). Several points of cross-talk with Wnt signaling have been identified, including interactions of YAP with Dsh and with APC, and co-regulation of downstream genes by YAP and β-catenin (40; 131). Interactions between YAP proteins and SMADs provide a point of cross-talk between Hippo and BMP pathways, as does regulation of Fat signaling by Dpp signaling (52; 131). Multiple mechanisms through which EGFR and related pathways can influence YAP activity have been identified, including both Hippo pathway-dependent and independent effects (30; 47; 109). JNK signaling can also have multiple, even opposing effects on YAP activity (reviewed in 51). YAP and Hedgehog signaling interact in multiple tissues, including intestine, skin, and nervous system (40; 131).
4. ROLES FOR HIPPO SIGNALING IN DEVELOPMENT, PHYSIOLOGY AND DISEASE
Hippo signaling is closely identified with the control of organ growth, but an ever-increasing number of additional biological roles for Hippo signaling have been described (Figure 3). The diverse roles of YAP proteins are also reflected in their contributions to disease, and the therapeutic potential for modulation of YAP activity.
Fig 3. Cellular consequences of YAP activity.
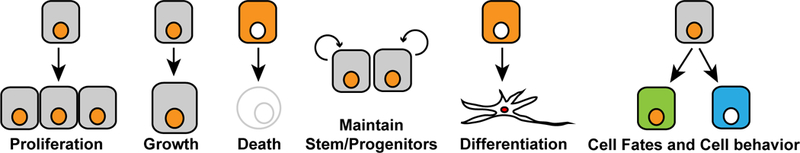
As described in the text, YAP activity has diverse consequences for cells during normal development and physiology. It often increases tissue growth by promoting cellular growth and cell proliferation, and inhibiting apoptosis. It also helps to maintain stem and progenitor cells in many tissues, while suppressing differentiation, but in other contexts can influence cell fate decisions or modify cellular phenotypes.
Organ Size Control
Mutations in Hippo pathway components can cause overgrowth or undergrowth not only in Drosophila, but also in some mammalian tissues (reviewed in 131; 148). It has been less clear however, to what extent physiological modulation of Hippo signaling normally occurs during development, how it is normally controlled, and what it contributes to the normal regulation of organ size. The role of Hippo signaling in cell density-dependent control of cell proliferation suggests that it could contribute to organ size control by acting as a physical sensor of cell density (2; 97; 163). However, other observations suggest that Hippo signaling does not have a universal role in organ size control. For example, sd mutant clones, which presumably are insensitive to Hippo signaling, nonetheless appear to grow essentially normally within the developing Drosophila notum and eye (74; 143). Activation of YAP promotes overgrowth in some vertebrate organs, like the liver (23), but not in others, like the kidney (110).
In addition to promoting growth cell autonomously, YAP proteins also have non-autonomous effects on growth that contribute to the influence of Hippo signaling on organ size. For example, as noted above, ligands for other signaling pathways that promote growth, such as Wnts and EGFR ligands, are transcriptional targets of YAP proteins. Final organ size depends not only on the rate of growth, but also the duration of growth, which is typically controlled by hormonal signals. In Drosophila, the main hormone controlling developmental transitions is 20-hydroxy-ecdysone (ecdysone). Hippo signaling intersects with ecdysone signaling at several levels. It modulates basal ecdysone production as well as coupling Insulin signaling with ecdysone production (87). Yki also controls the expression of dilp8, which plays an important role in coordinated growth of different organs by modulating ecdysone production (8), and cooperates with the ecdysone pathway transcription factor Taiman to control downstream gene expression (155).
Controlling cell fate decisions and cell behavior during organogenesis and homeostasis
A wide variety of cell fate decisions depend on YAP proteins. These include the earliest cell fate decision during mouse embryogenesis, the subdivision of the blastocyst into the trophectoderm (TE) and the inner cell mass (ICM)(92). YAP is active in outer blastocyst cells, which become TE, whereas Hippo signaling in inner cells suppresses YAP, and these cells form the ICM. Regulation of Hippo signaling in the blastocyst has been linked to differences in cell-cell contacts, cell polarity, and cytoskeletal tension between inner and outer cells (reviewed in 115). For example, AMOT is localized to cell-cell junctions of inner cells, where it promotes LATS activity, but is localized to the apical membrane in outer cells, where LATS is less active (45). There is also evidence that cell stretching contributes to YAP activation in TE cells (77).
Many other cell fate decisions are also influenced by YAP proteins. For example, YAP directs the differentiation path of mesenchymal stem cells (MSC) (25). At least in culture, differentiation of MSC can be controlled by the stiffness of the ECM upon which they are cultured, with a soft substrate directing cells towards neural lineages or adipocytes, a stiff substrate towards osteogenic fates, and an intermediate stiffness towards myogenic fates. This differentiation of MSC depends upon, and can be recapitulated by, regulation of YAP activity (25).
As an example of YAP-mediated regulation of cell behaviors, we note the crucial roles of YAP proteins in the vasculature. Endothelial-specific deletion of YAP and TAZ leads to impaired vascularization and embryonic lethality (140). Vascular endothelial growth factor (VEGF) is a key driver of angiogenesis and YAP proteins function as downstream effectors of VEGF-VEGFR2 signaling (139). VEGF activates YAP through its effect on the cytoskeleton, and activated YAP induces a transcriptional program that regulates the dynamics of the cytoskeleton and promotes multiple aspects of angiogenesis including, cell migration, junctional assembly, endothelial cell proliferation and metabolism (31; 139). YAP proteins are also regulated within endothelial cells by shear stresses induced by fluid flow (90), and YAP activation by ECM stiffness has been linked to pulmonary hypertension (6). Moreover, disturbed vascular flow can trigger inappropriately elevated levels of YAP proteins, which contribute to atherosclerosis (136). This has led to the suggestion that part of the effect of the widely prescribed statins, which are generally thought to reduce cardiovascular disease by decreasing cholesterol levels, might actually be due to their ability to indirectly inhibit Rho, and consequently YAP activation.
Unexpectedly, Hippo signaling also participates in immune responses. In Drosophila, the Hippo pathway contributes to regulation of innate immunity. Activated Yki induces the transcription of the IκB homolog, Cactus, in the Fat body, which suppresses the transcription of the antimicrobial peptides (71). Consequently, flies with activated Yki are more susceptible to Gram-positive bacteria. Gram-positive bacteria also activate Hippo-Yki signaling through the Toll-Myd88-Pelle pathway, where Pelle phosphorylates and degrades the CKa subunit of the STRIPAK PP2A complex. In mouse models, the Hippo pathway can suppress anti-tumor activity (89). Loss of Lats1/2 in the tumor cells leads to tumor regression that is dependent on the adaptive immune response. Mechanistically, tumor cells lacking LATS1/2 produced nucleic-acid rich extracellular vesicles, which induced a type-1 interferon response. Thus, Lats1/2 play an important role in modulating tumor immunogenicity, and this influence of LATS on tumor immunity will complicate efforts to suppress tumor growth by activating the Hippo pathway.
Promoting Stem and progenitor cells
A common theme that has emerged from examination of requirements for YAP proteins in many tissues is that they often play key roles in promoting stem or progenitor cell fates. In Drosophila, Yki promotes maintenance of undifferentiated stem/progenitor cells in the nervous system and intestine (reviewed in 121). In mammals, YAP proteins have been linked to stem/progenitor cell fates in a wide range of tissues, including skin, nervous system, muscle, liver, lung, teeth, mammary gland, and pancreas (reviewed in 86). YAP proteins also suppress differentiation and promote survival of human ES cells (65), and transient activation of YAP can convert differentiated cells into stem cells (98). Moreover, the physical architecture of tissues can play a role in regulating differentiation through Hippo signaling. For example, in skin, YAP is active in basal progenitor cells but inactive in most differentiating suprabasal cells, due both to the loss of contact with basement membrane, and the acquisition of an apical membrane domain that contains proteins that promote LATS activation (27).
The role of YAP proteins in maintaining progenitor cells is also reflected in their contributions to organoid culture (98). Advances in controlling developmental programs in vitro have made it possible to create and culture multicellular organoids created by reprogramming stem cells that resemble mammalian organs. In self-renewing intestinal organoids, YAP is active in the growing crypt-like regions, but cytoplasmic in differentiated regions (37). Moreover, by controlling the local stiffness of the matrices that intestinal stem cells and their derivatives are grown in, it is possible to promote either self-renewal or differentiation through effects on YAP.
Regeneration and Repair
YAP proteins play key roles in wound repair and regeneration. This reflects both their roles in promoting progenitor cell fates and organ growth, and their regulation by tissue integrity, which is disrupted in damaged tissues. Indeed activation of YAP proteins occurs both in response to changes in cell-cell contact, cell density, and cell stretching that can accompany wounding, and also in response to pathways that are activated in association with tissue damage, infection, or inflammation. The role of YAP proteins in repair makes them a potential therapeutic target for stimulating healing.
In Drosophila, Yki has been implicated in regenerative growth after tissue damage in developing imaginal discs, as well as in the adult intestine, which, like the mammalian intestine, is continually replenished by proliferation of intestinal stem cells. A key factor promoting Yki activation in response to wounding or infection of discs or intestine is Jnk pathway activation, which acts through a Jub-dependent process to inhibit Wts activity (reviewed in 121).
YAP proteins play key roles in regeneration of multiple mammalian tissues, including intestine, skin, mammary gland, and lungs, and increased YAP/TAZ activity can facilitate wound healing, tissue repair and regeneration (reviewed in 131; 148). It is particularly notable that key roles for YAP proteins in regeneration have been identified even in tissues where YAP does not appear to contribute significantly to homeostasis of adult tissues, such as the adult intestine. Remarkably, increased YAP activity can even facilitate regeneration in the adult mouse heart, which normally has very little ability to regenerate. YAP promotes cardiomyocyte proliferation during embryonic development, and newborn mice can regenerate cardiac tissue after injury, but lose this capability after birth as cardiomyocytes exit the cell cycle. However, genetically inducing YAP activity by expression of activated-YAP, or by mutation of upstream Hippo pathway genes like Sav, can promote cardiomyocyte proliferation, and aid in repair of hearts damaged by heart attack (18; 42; 70; 144). This stimulation of regenerative capacity in adult mouse hearts suggests that increasing YAP activity could be an important therapeutic approach for human heart attack patients.
Fibrotic Diseases
YAP proteins have also been linked to other clinically important diseases, including fibrotic diseases, such as liver cirrhosis and pulmonary fibrosis, which are associated with abnormal and excessive ECM deposition by activated fibroblasts, leading to tissue stiffening and cellular dysfunction. YAP proteins are activated in fibrotic diseases, consistent with the stiffening of the ECM, and also contribute to a feedback loop that worsens the disease by promoting expression of genes that contribute to fibrosis (73; 81).
Cancer
Early indications of a link between defective Hippo signaling and cancer included the overgrowth phenotypes of Drosophila Hippo pathway mutants, observations of elevated tumorigenesis in LATS mutant mice, and linkage of the human tumor suppressor NF2 to Hippo signaling (95). Subsequent observations have strengthened and elaborated ties between YAP activity and cancer. Immunohistological analysis shows that a significant fraction of human solid tumors in a wide variety of cancers, including lung, colon, breast, ovarian, pancreatic, liver, skin, brain and other cancers, are associated with elevated levels and/or increased nuclear localization of YAP proteins, and analysis of many mouse cancer models has established the functional relevance of elevated YAP activity to tumorigenesis (reviewed in 153). Moreover, elevated levels of YAP proteins correlate with poor prognosis in multiple human cancers.
Although YAP is activated in many tumors, mutations in core upstream components of the Hippo pathway are infrequently identified, and in most cases, the basis for YAP activation in particular cancers has not been defined. However, it seems likely to stem from a combination of cross-talk with other oncogenic pathways, such as EGFR-RAS, JNK, WNT, SRC, GPCR or PI3K signaling, together with changes in the tumor microenvironment such as inflammation and increased ECM stiffness, which as described above promote YAP activity. Indeed, increased tissue stiffness is not only an established activator of YAP, both also a classic hallmark of tumorigenesis.
YAP activity promotes oncogenesis not only through its well known role in promoting cell proliferation and growth, but also through several other activities, including promoting cancer stem cell behavior, altering metabolism, promoting chemo-resistance, and promoting metastasis (reviewed in 153). In part, these likely reflect normal contributions of YAP proteins to tissue repair and regeneration hijacked by cancer, such as suppressed differentiation of progenitor cells. In addition, rapidly proliferating cells modify their metabolic pathways to meet the increased demand for biosynthetic building blocks, and as noted above YAP promotes metabolic adaptations that support rapid proliferation and aerobic glycolysis. Similarly, in zebrafish, expressing active YAP in the liver leads to overgrowth and increased Glutamine synthesis due to enhanced expression of the Glutamine synthetase, which in turn supports nucleotide synthesis (17). Tumors can contain heterogenous mixtures of cells, a subset of which, referred to as cancer stem cells, have an ability to establish new tumors. Cancer stem cells have been observed to have elevated activity of YAP proteins. Elevated YAP activity has also been correlated with resistance to chemotherapy (59; 69; 129), which may in part stem from YAPs role in suppressing apoptosis. YAP proteins, particularly TAZ, have also been linked to metastasis, perhaps reflecting influences on cytoskeletal organization, as well as suppression of anoikis. The key roles of YAP proteins in cancer have stimulated attempts to develop YAP inhibitors, such as disrupters of YAP-TEAD binding, as discussed above. Alternatively, drugs that affect upstream inputs could be used to indirectly inhibit YAP (reviewed in 152).
5. CONCLUSIONS and FUTURE PERSPECTIVES
Extensive research over the past 15 years has provided tremendous insight into the regulation and role of Hippo signaling in development, physiology and disease. Despite the advances in our understanding of the molecular mechanisms by which myriad factors impinge on Hippo signaling, and the role of this pathway in physiological and disease conditions, critical questions remain unanswered. The actin cytoskeleton is a focal point for regulation of the pathway, but we still have only a limited understanding of the mechanisms by which the cytoskeleton, and tension within the cytoskeleton, regulate YAP in vivo. More generally, the physiological signals that act in vivo to control normal organ size and physiology through Hippo signaling are not well understood. There are also still gaps in our understanding of the cellular and molecular mechanisms that control Hippo signaling, including how the activity of Hippo and related kinases is normally controlled, and where LATS kinases are activated within mammalian cells. A greater understanding of mechanisms of Hippo signaling and its physiological roles will also help in realizing the potential of therapeutics to modulate YAP protein activity, enhancing regeneration and repair, or suppressing oncogenesis.
SUMMARY POINTS.
-
-
Hippo signaling is mediated by negative regulation of YAP transcription factors.
-
-
Hippo signaling is effected through assembly, often at cell-cell junctions, of complexes of proteins that promote activation of Hippo and Lats family kinases.
-
-
YAP transcription factors respond to multiple upstream inputs, both Hippo-dependent and Hippo-independent, which provide information on both the physical and biochemical environment.
-
-
Hippo signaling plays diverse essential roles during development in promoting growth, maintaining progenitor cells, and modulating cell fates.
-
-
Promotion of YAP activity may promote wound healing and regeneration, and is a candidate for therapy to improve cardiac function after heart attack.
-
-
Dysregulation of YAP activity occurs in many cancers, and is also implicated in other major diseases including atherosclerosis and fibrotic diseases.
ACKNOWLEDGEMENTS
The authors’ research is supported by NIH grants R01 GM78620 (KDI) R01 GM121537 (KDI) and K99 HD092553 (JRM). We apologize to authors whose work could not be cited due to space and reference constraints.
LITERATURE CITED
- 1.Adler JJ, Johnson DE, Heller BL, Bringman LR, Ranahan WP, et al. 2013. Serum deprivation inhibits the transcriptional co-activator YAP and cell growth via phosphorylation of the 130-kDa isoform of Angiomotin by the LATS1/2 protein kinases. Proceedings of the National Academy of Sciences of the United States of America 110:17368–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, et al. 2013. A Mechanical Checkpoint Controls Multicellular Growth through YAP/TAZ Regulation by Actin-Processing Factors. Cell 154:1047–59 [DOI] [PubMed] [Google Scholar]
- 3.Bae SJ, Ni L, Osinski A, Tomchick DR, Brautigam CA, Luo X. 2017. SAV1 promotes Hippo kinase activation through antagonizing the PP2A phosphatase STRIPAK. Elife 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baumgartner R, Pörnbacher I, Buser N, Hafen E, Stocker H. 2010. The WW domain protein Kibra acts upstream of Hippo in Drosophila. Dev Cell 18:309–16 [DOI] [PubMed] [Google Scholar]
- 5.Benham-Pyle BW, Pruitt BL, Nelson WJ. 2015. Cell adhesion. Mechanical strain induces E-cadherin-dependent Yap1 and β-catenin activation to drive cell cycle entry. Science 348:1024–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertero T, Oldham WM, Cottrill KA, Pisano S, Vanderpool RR, et al. 2016. Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. The Journal of Clinical Investigation 126:3313–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boggiano JC, Vanderzalm PJ, Fehon RG. 2011. Tao-1 Phosphorylates Hippo/MST Kinases to Regulate the Hippo-Salvador-Warts Tumor Suppressor Pathway. Developmental Cell 21:888–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boone E, Colombani J, Andersen DS, Léopold P. 2016. The Hippo signalling pathway coordinates organ growth and limits developmental variability by controlling dilp8 expression. Nature communications 7:13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakraborty S, Njah K, Pobbati AV, Lim YB, Raju A, et al. 2017. Agrin as a Mechanotransduction Signal Regulating YAP through the Hippo Pathway. Cell reports 18:2464–79 [DOI] [PubMed] [Google Scholar]
- 10.Chan SW, Lim CJ, Chong YF, Pobbati AV, Huang C, Hong W. 2011. Hippo pathway-independent restriction of TAZ and YAP by angiomotin. The Journal of biological chemistry 286:7018–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan SW, Lim CJ, Guo F, Tan I, Leung T, Hong W. 2013. Actin-binding and cell proliferation activities of angiomotin family members are regulated by Hippo pathway-mediated phosphorylation. Journal of Biological Chemistry 288:37296–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen CL, Gajewski KM, Hamaratoglu F, Bossuyt W, Sansores-Garcia L, et al. 2010. The apical-basal cell polarity determinant Crumbs regulates Hippo signaling in Drosophila. Proc Natl Acad Sci U S A 107:15810–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho E, Feng Y, Rauskolb C, Maitra S, Fehon R, Irvine KD. 2006. Delineation of a Fat tumor suppressor pathway. Nat Genet 38:1142–50 [DOI] [PubMed] [Google Scholar]
- 14.Chung H-L, Augustine GJ, Choi K-W. 2016. Drosophila Schip1 Links Expanded and Tao-1 to Regulate Hippo Signaling. Developmental Cell 36:511–24 [DOI] [PubMed] [Google Scholar]
- 15.Codelia VA, Sun G, Irvine KD. 2014. Regulation of YAP by Mechanical Strain through Jnk and Hippo Signaling. Current biology 24:2012–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Couzens AL, Xiong S, Knight JDR, Mao DY, Guettler S, et al. 2017. MOB1 Mediated Phospho-recognition in the Core Mammalian Hippo Pathway. Molecular & cellular proteomics : MCP 16:1098–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox AG, Hwang KL, Brown KK, Evason K, Beltz S, et al. 2016. Yap reprograms glutamine metabolism to increase nucleotide biosynthesis and enable liver growth. Nat Cell Biol 18:886–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Del Re DP, Yang Y, Nakano N, Cho J, Zhai P, et al. 2013. Yes-associated Protein Isoform 1 (Yap1) Promotes Cardiomyocyte Survival and Growth to Protect against Myocardial Ischemic Injury. The Journal of biological chemistry 288:3977–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng H, Wang W, Yu J, Zheng Y, Qing Y, Pan D. 2015. Spectrin regulates Hippo signaling by modulating cortical actomyosin activity. eLife 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng Y, Matsui Y, Zhang Y, Lai Z-C. 2013. Hippo activation through homodimerization and membrane association for growth inhibition and organ size control. Developmental Biology 375:152–9 [DOI] [PubMed] [Google Scholar]
- 21.DeRan M, Yang J, Shen CH, Peters EC, Fitamant J, et al. 2014. Energy stress regulates hippo-YAP signaling involving AMPK-mediated regulation of angiomotin-like 1 protein. Cell Rep 9:495–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dewey EB, Sanchez D, Johnston CA. 2015. Warts phosphorylates mud to promote pins-mediated mitotic spindle orientation in Drosophila, independent of Yorkie. Curr Biol 25:2751–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong J, Feldmann G, Huang J, Wu S, Zhang N, et al. 2007. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 130:1120–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Driscoll TP, Cosgrove BD, Heo S-J, Shurden ZE, Mauck RL. 2015. Cytoskeletal to Nuclear Strain Transfer Regulates YAP Signaling in Mesenchymal Stem Cells. Biophysical journal 108:2783–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, et al. 2011. Role of YAP/TAZ in mechanotransduction. Nature 474:179–83 [DOI] [PubMed] [Google Scholar]
- 26.Dutta S, Mana-Capelli S, Paramasivam M, Dasgupta I, Cirka H, et al. 2017. TRIP6 inhibits Hippo signaling in response to tension at adherens junctions. EMBO Rep [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elbediwy A, Vincent-Mistiaen ZI, Spencer-Dene B, Stone RK, Boeing S, et al. 2016. Integrin signalling regulates YAP/TAZ to control skin homeostasis. Development (Cambridge, England) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elosegui-Artola A, Andreu I, Beedle AEM, Lezamiz A, Uroz M, et al. 2017. Force Triggers YAP Nuclear Entry by Regulating Transport across Nuclear Pores. Cell 171:1397–410.e14 [DOI] [PubMed] [Google Scholar]
- 29.Enzo E, Santinon G, Pocaterra A, Aragona M, Bresolin S, et al. 2015. Aerobic glycolysis tunes YAP/TAZ transcriptional activity. EMBO J 34:1349–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan R, Kim N-G, Gumbiner BM. 2013. Regulation of Hippo pathway by mitogenic growth factors via phosphoinositide 3-kinase and phosphoinositide-dependent kinase-1. Proceedings of the National Academy of Sciences of the United States of America 110:2569–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feng X, Liu P, Zhou X, Li M-T, Li F-L, et al. 2016. Thromboxane A2 Activates YAP/TAZ Protein to Induce Vascular Smooth Muscle Cell Proliferation and Migration. The Journal of biological chemistry 291:18947–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernández BG, Gaspar P, Brás-Pereira C, Jezowska B, Rebelo SR, Janody F. 2011. Actin-Capping Protein and the Hippo pathway regulate F-actin and tissue growth in Drosophila. Development 138:2337–46 [DOI] [PubMed] [Google Scholar]
- 33.Fletcher GC, Elbediwy A, Khanal I, Ribeiro PS, Tapon N, Thompson BJ. 2015. The Spectrin cytoskeleton regulates the Hippo signalling pathway. The EMBO Journal 34:940–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Furukawa KT, Yamashita K, Sakurai N, Ohno S. 2017. The Epithelial Circumferential Actin Belt Regulates YAP/TAZ through Nucleocytoplasmic Shuttling of Merlin. Cell reports 20:1435–47 [DOI] [PubMed] [Google Scholar]
- 35.Galli GG, Carrara M, Yuan WC, de Laat W, Calogero RA, Camargo FD. 2015. YAP drives growth by controlling transcriptional pause release from dynamic enhancers. Molecular Cell 60:328–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Genevet A, Wehr MC, Brain R, Thompson BJ, Tapon N. 2010. Kibra is a regulator of the Salvador/Warts/Hippo signalling network. Dev Cell 18:300–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gjorevski N, Sachs N, Manfrin A, Giger S, Bragina ME, et al. 2016. Designer matrices for intestinal stem cell and organoid culture. Nature 539:560–4 [DOI] [PubMed] [Google Scholar]
- 38.Guo T, Lu Y, Li P, Yin MX, Lv D, et al. 2013. A novel partner of Scalloped regulates Hippo signaling via antagonizing Scalloped-Yorkie activity. Cell Res 23:1201–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamaratoglu F, Willecke M, Kango-Singh M, Nolo R, Hyun E, et al. 2006. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat Cell Biol 8:27–36 [DOI] [PubMed] [Google Scholar]
- 40.Hansen CG, Moroishi T, Guan K-L. 2015. YAP and TAZ: a nexus for Hippo signaling and beyond. Trends in cell biology 25:499–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hansen CG, Ng YL, Lam WL, Plouffe SW, Guan KL. 2015. The Hippo pathway effectors YAP and TAZ promote cell growth by modulating amino acid signaling to mTORC1. Cell Res 25:1299–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heallen T, Morikawa Y, Leach J, Tao G, Willerson JT, et al. 2013. Hippo signaling impedes adult heart regeneration. Development (Cambridge, England) 140:4683–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, et al. 2011. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science 332:458–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirata H, Samsonov M, Sokabe M. 2017. Actomyosin contractility provokes contact inhibition in E-cadherin-ligated keratinocytes. Scientific reports 7:46326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirate Y, Hirahara S, Inoue K-i, Suzuki A, Alarcon VB, et al. 2013. Polarity-dependent distribution of angiomotin localizes Hippo signaling in preimplantation embryos. Current biology 23:1181–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hong AW, Meng Z, Yuan HX, Plouffe SW, Moon S, et al. 2017. Osmotic stress-induced phosphorylation by NLK at Ser128 activates YAP. EMBO Rep 18:72–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hong X, Nguyen HT, Chen Q, Zhang R, Hagman Z, et al. 2014. Opposing activities of the Ras and Hippo pathways converge on regulation of YAP protein turnover. The EMBO Journal 33:2447–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu JK, Du W, Shelton SJ, Oldham MC, DiPersio CM, Klein OD. 2017. An FAK-YAP-mTOR Signaling Axis Regulates Stem Cell-Based Tissue Renewal in Mice. Cell stem cell 21:91–106 e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang J, Wu S, Barrera J, Matthews K, Pan D. 2005. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell 122:421–34 [DOI] [PubMed] [Google Scholar]
- 50.Ibar C, Kirichenko E, Keepers B, Enners E, Irvine KD. 2018. Tension-dependent regulation of mammalian Hippo signaling through LIMD1. J Cell Science:in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Irvine KD. 2012. Integration of intercellular signaling through the Hippo pathway. Seminars in Cell & Developmental Biology 23:812–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Irvine KD, Harvey KF. 2015. Control of organ growth by patterning and hippo signaling in Drosophila. Cold Spring Harbor perspectives in biology 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiao S, Wang H, Shi Z, Dong A, Zhang W, et al. 2014. A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer cell 25:166–80 [DOI] [PubMed] [Google Scholar]
- 54.Jin Y, Dong L, Lu Y, Wu W, Hao Q, et al. 2012. Dimerization and cytoplasmic localization regulate Hippo kinase signaling activity in organ size control. Journal of Biological Chemistry 287:5784–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim M, Kim T, Johnson RL, Lim DS. 2015. Transcriptional co-repressor function of the hippo pathway transducers YAP and TAZ. Cell Rep 11:270–82 [DOI] [PubMed] [Google Scholar]
- 56.Kim N-G, Gumbiner BM. 2015. Adhesion to fibronectin regulates Hippo signaling via the FAK-Src-PI3K pathway. The Journal of Cell Biology 210:503–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim NG, Koh E, Chen X, Gumbiner BM. 2011. E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proceedings of the National Academy of Sciences of the United States of America 108:11930–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koontz LM, Liu-Chittenden Y, Yin F, Zheng Y, Yu J, et al. 2013. The Hippo effector Yorkie controls normal tissue growth by antagonizing scalloped-mediated default repression. Dev Cell 25:388–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lai D, Ho KC, Hao Y, Yang X. 2011. Taxol resistance in breast cancer cells is mediated by the hippo pathway component TAZ and its downstream transcriptional targets Cyr61 and CTGF. Cancer research 71:2728–38 [DOI] [PubMed] [Google Scholar]
- 60.Leung CY, Zernicka-Goetz M. 2013. Angiomotin prevents pluripotent lineage differentiation in mouse embryos via Hippo pathway-dependent and -independent mechanisms. Nature communications 4:2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li P, Silvis MR, Honaker Y, Lien W-H, Arron ST, Vasioukhin V. 2016. αE-catenin inhibits a Src-YAP1 oncogenic module that couples tyrosine kinases and the effector of Hippo signaling pathway. Genes & Development 30:798–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li Q, Li S, Mana-Capelli S, Roth Flach RJ, Danai LV, et al. 2014. The conserved misshapen-warts-Yorkie pathway acts in enteroblasts to regulate intestinal stem cells in Drosophila. Developmental Cell 31:291–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li S, Cho YS, Yue T, Ip YT, Jiang J. 2015. Overlapping functions of the MAP4K family kinases Hppy and Msn in Hippo signaling. Cell Discovery 1:15038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li Y, Zhou H, Li F, Chan SW, Lin Z, et al. 2015. Angiomotin binding-induced activation of Merlin/NF2 in the Hippo pathway. Cell research 25:801–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lian I, Kim J, Okazawa H, Zhao J, Zhao B, et al. 2010. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev 24:1106–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liang N, Zhang C, Dill P, Panasyuk G, Pion D, et al. 2014. Regulation of YAP by mTOR and autophagy reveals a therapeutic target of tuberous sclerosis complex. J Exp Med 211:2249–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lin C, Yao E, Zhang K, Jiang X, Croll S, et al. 2017. YAP is essential for mechanical force production and epithelial cell proliferation during lung branching morphogenesis. eLife 6:e21130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lin KC, Moroishi T, Meng Z, Jeong HS, Plouffe SW, et al. 2017. Regulation of Hippo pathway transcription factor TEAD by p38 MAPK-induced cytoplasmic translocation. Nat Cell Biol 19:996–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lin L, Sabnis AJ, Chan E, Olivas V, Cade L, et al. 2015. The Hippo effector YAP promotes resistance to RAF- and MEK-targeted cancer therapies. Nature Genetics 47:250–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin Z, von Gise A, Zhou P, Gu F, Ma Q, et al. 2014. Cardiac-Specific YAP Activation Improves Cardiac Function and Survival in an Experimental Murine MI ModelNovelty and Significance. Circulation Research 115:354–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu B, Zheng Y, Yin F, Yu J, Silverman N, Pan D. 2016. Toll Receptor-Mediated Hippo Signaling Controls Innate Immunity in Drosophila. Cell 164:406–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu C-Y, Zha Z-Y, Zhou X, Zhang H, Huang W, et al. 2010. The hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCF{beta}-TrCP E3 ligase. The Journal of biological chemistry 285:37159–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu F, Lagares D, Choi KM, Stopfer L, Marinkovic A, et al. 2015. Translational Research in Acute Lung Injury and Pulmonary Fibrosis: Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. American Journal of Physiology - Lung Cellular and Molecular Physiology 308:L344–L57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu X, Grammont M, Irvine KD. 2000. Roles for scalloped and vestigial in regulating cell affinity and interactions between the wing blade and the wing hinge. Dev Biol 228:287–303 [DOI] [PubMed] [Google Scholar]
- 75.Liu-Chittenden Y, Huang B, Shim JS, Chen Q, Lee SJ, et al. 2012. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev 26:1300–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lucas EP, Khanal I, Gaspar P, Fletcher GC, Polesello C, et al. 2013. The Hippo pathway polarizes the actin cytoskeleton during collective migration of Drosophila border cells. J Cell Biol 201:875–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maître J-L, Turlier H, Illukkumbura R, Eismann B, Niwayama R, et al. 2016. Asymmetric division of contractile domains couples cell positioning and fate specification. Nature 536:344–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mana-Capelli S, Paramasivam M, Dutta S, McCollum D. 2014. Angiomotins link F-actin architecture to Hippo pathway signaling. Molecular biology of the cell 25:1676–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mao Y, Mulvaney J, Zakaria S, Yu T, Morgan KM, et al. 2011. Characterization of a Dchs1 mutant mouse reveals requirements for Dchs1-Fat4 signaling during mammalian development. Development 138:947–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mao Y, Rauskolb C, Cho E, Hu WL, Hayter H, et al. 2006. Dachs: an unconventional myosin that functions downstream of Fat to regulate growth, affinity and gene expression in Drosophila. Development 133:2539–51 [DOI] [PubMed] [Google Scholar]
- 81.Martin K, Pritchett J, Llewellyn J, Mullan AF, Athwal VS, et al. 2016. PAK proteins and YAP-1 signalling downstream of integrin beta-1 in myofibroblasts promote liver fibrosis. Nature communications 7:12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Meng Z, Moroishi T, Guan K-L. 2016. Mechanisms of Hippo pathway regulation. Genes & Development 30:1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meng Z, Moroishi T, Mottier-Pavie V, Plouffe SW, Hansen CG, et al. 2015. MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2 in the Hippo pathway. Nature communications 6:8357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Misra JR, Irvine KD. 2016. Vamana Couples Fat Signaling to the Hippo Pathway. Developmental Cell 39:254–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mo J-S, Meng Z, Kim YC, Park HW, Hansen CG, et al. 2015. Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway. Nature Cell Biology 17:500–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mo J-S, Park HW, Guan K-L. 2014. The Hippo signaling pathway in stem cell biology and cancer. EMBO reports 15:642–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moeller ME, Nagy S, Gerlach SU, Soegaard KC, Danielsen ET, et al. 2017. Warts Signaling Controls Organ and Body Growth through Regulation of Ecdysone. Curr Biol 27:1652–9 e4 [DOI] [PubMed] [Google Scholar]
- 88.Moon S, Kim W, Kim S, Kim Y, Song Y, et al. 2017. Phosphorylation by NLK inhibits YAP-14–3-3-interactions and induces its nuclear localization. EMBO Rep 18:61–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moroishi T, Hayashi T, Pan WW, Fujita Y, Holt MV, et al. 2016. The Hippo Pathway Kinases LATS1/2 Suppress Cancer Immunity. Cell 167:1525–39 e17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nakajima H, Yamamoto K, Agarwala S, Terai K, Fukui H, et al. 2017. Flow-Dependent Endothelial YAP Regulation Contributes to Vessel Maintenance. Developmental Cell 40:523–36.e6 [DOI] [PubMed] [Google Scholar]
- 91.Ni L, Zheng Y, Hara M, Pan D, Luo X. 2015. Structural basis for Mob1-dependent activation of the core Mst-Lats kinase cascade in Hippo signaling. Genes & Development 29:1416–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nishioka N, Inoue K, Adachi K, Kiyonari H, Ota M, et al. 2009. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev Cell 16:398–410 [DOI] [PubMed] [Google Scholar]
- 93.Oh H, Slattery M, Ma L, Crofts A, White KP, et al. 2013. Genome-wide Association of Yorkie with Chromatin and Chromatin-Remodeling Complexes. Cell Reports 3:309–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oh H, Slattery M, Ma L, White KP, Mann RS, Irvine KD. 2014. Yorkie promotes transcription by recruiting a histone methyltransferase complex. Cell Reports 8:449–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pan D 2010. The hippo signaling pathway in development and cancer. Dev Cell 19:491–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pan G, Feng Y, Ambegaonkar AA, Sun G, Huff M, et al. 2013. Signal transduction by the Fat cytoplasmic domain. Development 140:831–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pan Y, Heemskerk I, Ibar C, Shraiman BI, Irvine KD. 2016. Differential growth triggers mechanical feedback that elevates Hippo signaling. Proceedings of the National Academy of Sciences of the United States of America 113:E6974–E83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Panciera T, Azzolin L, Fujimura A, Di Biagio D, Frasson C, et al. 2016. Induction of Expandable Tissue-Specific Stem/Progenitor Cells through Transient Expression of YAP/TAZ. Cell stem cell 19:725–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Parker J, Struhl G. 2015. Scaling the Drosophila Wing: TOR-Dependent Target Gene Access by the Hippo Pathway Transducer Yorkie. PLoS biology 13:e1002274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Peng C, Zhu Y, Zhang W, Liao Q, Chen Y, et al. 2017. Regulation of the Hippo-YAP Pathway by Glucose Sensor O-GlcNAcylation. Mol Cell 68:591–604 e5 [DOI] [PubMed] [Google Scholar]
- 101.Poernbacher I, Baumgartner R, Marada SK, Edwards K, Stocker H. 2012. Drosophila Pez acts in Hippo signaling to restrict intestinal stem cell proliferation. Curr Biol 22:389–96 [DOI] [PubMed] [Google Scholar]
- 102.Poon CLC, Lin JI, Zhang X, Harvey KF. 2011. The Sterile 20-like Kinase Tao-1 Controls Tissue Growth by Regulating the Salvador-Warts-Hippo Pathway. Developmental Cell 21:896–906 [DOI] [PubMed] [Google Scholar]
- 103.Porazinski S, Wang H, Asaoka Y, Behrndt M, Miyamoto T, et al. 2015. YAP is essential for tissue tension to ensure vertebrate 3D body shape. Nature 521:217–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Praskova M, Xia F, Avruch J. 2008. MOBKL1A/MOBKL1B phosphorylation by MST1 and MST2 inhibits cell proliferation. Curr Biol 18:311–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Qing Y, Yin F, Wang W, Zheng Y, Guo P, et al. 2014. The Hippo effector Yorkie activates transcription by interacting with a histone methyltransferase complex through Ncoa6. eLife:e02564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ragni CV, Diguet N, Le Garrec JF, Novotova M, Resende TP, et al. 2017. Amotl1 mediates sequestration of the Hippo effector Yap1 downstream of Fat4 to restrict heart growth. Nat Commun 8:14582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rauskolb C, Sun S, Sun G, Pan Y, Irvine KD. 2014. Cytoskeletal tension inhibits Hippo signaling through an Ajuba-Warts complex. Cell 158:143–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Reddy BV, Irvine KD. 2011. Regulation of Drosophila glial cell proliferation by Merlin-Hippo signaling. Development 138:5201–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Reddy BV, Irvine KD. 2013. Regulation of Hippo signaling by EGFR-MAPK signaling through Ajuba family proteins. Dev Cell 24:459–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Reginensi A, Scott RP, Gregorieff A, Bagherie-Lachidan M, Chung C, et al. 2013. Yap- and Cdc42-Dependent Nephrogenesis and Morphogenesis during Mouse Kidney Development. PLoS genetics 9:e1003380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ribeiro PS, Josue F, Wepf A, Wehr MC, Rinner O, et al. 2010. Combined functional genomic and proteomic approaches identify a PP2A complex as a negative regulator of Hippo signaling. Mol Cell 39:521–34 [DOI] [PubMed] [Google Scholar]
- 112.Sabra H, Brunner M, Mandati V, Wehrle-Haller B, Lallemand D, et al. 2017. β1 integrin-dependent Rac/group I PAK signaling mediates YAP activation of Yes-associated protein 1 (YAP1) via NF2/merlin. Journal of Biological Chemistry 292:19179–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Saburi S, Hester I, Fischer E, Pontoglio M, Eremina V, et al. 2008. Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat Genet 40:1010–5 [DOI] [PubMed] [Google Scholar]
- 114.Sansores-Garcia L, Bossuyt W, Wada K-I, Yonemura S, Tao C, et al. 2011. Modulating F-actin organization induces organ growth by affecting the Hippo pathway. The EMBO Journal 30:2325–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sasaki H 2015. Position- and polarity-dependent Hippo signaling regulates cell fates in preimplantation mouse embryos. Seminars in cell & developmental biology 47–48:80–7 [DOI] [PubMed] [Google Scholar]
- 116.Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, et al. 2011. Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell 144:782–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Serrano I, McDonald PC, Lock F, Muller WJ, Dedhar S. 2013. Inactivation of the Hippo tumour suppressor pathway by integrin-linked kinase. Nature communications 4:2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shen S, Guo X, Yan H, Lu Y, Ji X, et al. 2015. A miR-130a-YAP positive feedback loop promotes organ size and tumorigenesis. Cell Res 25:997–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Si Y, Ji X, Cao X, Dai X, Xu L, et al. 2017. Src Inhibits the Hippo Tumor Suppressor Pathway through Tyrosine Phosphorylation of Lats1. Cancer Res 77:4868–80 [DOI] [PubMed] [Google Scholar]
- 120.Sorrentino G, Ruggeri N, Specchia V, Cordenonsi M, Mano M, et al. 2014. Metabolic control of YAP and TAZ by the mevalonate pathway. Nat Cell Biol 16:357–66 [DOI] [PubMed] [Google Scholar]
- 121.Staley BK, Irvine KD. 2012. Hippo signaling in Drosophila: recent advances and insights. Developmental Dynamics 241:3–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Stein C, Bardet AF, Roma G, Bergling S, Clay I, et al. 2015. YAP1 Exerts Its Transcriptional Control via TEAD-Mediated Activation of Enhancers. PLoS genetics 11:e1005465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Straßburger K, Tiebe M, Pinna F, Breuhahn K, Teleman AA. 2012. Insulin/IGF signaling drives cell proliferation in part via Yorkie/YAP. Dev Biol 367:187–96 [DOI] [PubMed] [Google Scholar]
- 124.Su T, Ludwig MZ, Xu J, Fehon RG. 2017. Kibra and Merlin Activate the Hippo Pathway Spatially Distinct from and Independent of Expanded. Developmental Cell 40:478–90.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sun S, Irvine KD. 2016. Cellular Organization and Cytoskeletal Regulation of the Hippo Signaling Network. Trends in cell biology 26:694–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sun S, Reddy BVVG, Irvine KD. 2015. Localization of Hippo Signaling complexes and Warts activation in vivo. Nature Communications 6:8402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Szymaniak AD, Mahoney JE, Cardoso WV, Varelas X. 2015. Crumbs3-Mediated Polarity Directs Airway Epithelial Cell Fate through the Hippo Pathway Effector Yap. Developmental Cell 34:283–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Taniguchi K, Wu L-W, Grivennikov SI, de Jong PR, Lian I, et al. 2015. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature 519:57–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Touil Y, Igoudjil W, Corvaisier M, Dessein A-F, Vandomme J, et al. 2014. Colon Cancer Cells Escape 5FU Chemotherapy-Induced Cell Death by Entering Stemness and Quiescence Associated with the c-Yes/YAP Axis. Clinical cancer research : an official journal of the American Association for Cancer Research 20:837–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tumaneng K, Schlegelmilch K, Russell RC, Yimlamai D, Basnet H, et al. 2012. YAP mediates crosstalk between the Hippo and PI(3)K-TOR pathways by suppressing PTEN via miR-29. Nat Cell Biol 14:1322–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Varelas X 2014. The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development (Cambridge, England) 141:1614–26 [DOI] [PubMed] [Google Scholar]
- 132.Varelas X, Sakuma R, Samavarchi-Tehrani P, Peerani R, Rao BM, et al. 2008. TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nat Cell Biol 10:837–48 [DOI] [PubMed] [Google Scholar]
- 133.Varelas X, Samavarchi-Tehrani P, Narimatsu M, Weiss A, Cockburn K, et al. 2010. The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-beta-SMAD pathway. Developmental cell 19:831–44 [DOI] [PubMed] [Google Scholar]
- 134.Vrabioiu AM, Struhl G. 2015. Fat/Dachsous Signaling Promotes Drosophila Wing Growth by Regulating the Conformational State of the NDR Kinase Warts. Developmental Cell 35:737–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wada K-I, Itoga K, Okano T, Yonemura S, Sasaki H. 2011. Hippo pathway regulation by cell morphology and stress fibers. Development 138:3907–14 [DOI] [PubMed] [Google Scholar]
- 136.Wang KC, Yeh YT, Nguyen P, Limqueco E, Lopez J, et al. 2016. Flow-dependent YAP/TAZ activities regulate endothelial phenotypes and atherosclerosis. Proc Natl Acad Sci U S A 113:11525–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang W, Huang J, Chen J. 2011. Angiomotin-like proteins associate with and negatively regulate YAP1. Journal of Biological Chemistry 286:4364–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang W, Xiao Z-D, Li X, Aziz KE, Gan B, et al. 2015. AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nature Cell Biology 17:490–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang X, Freire Valls A, Schermann G, Shen Y, Moya IM, et al. 2017. YAP/TAZ Orchestrate VEGF Signaling during Developmental Angiogenesis. Dev Cell 42:462–78 e7 [DOI] [PubMed] [Google Scholar]
- 140.Wang Y, Hu G, Liu F, Wang X, Wu M, et al. 2014. Deletion of Yes-Associated Protein (YAP) Specifically in Cardiac and Vascular Smooth Muscle Cells Reveals a Crucial Role for YAP in Mouse Cardiovascular Development. Circulation Research 114:957–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang Z, Wu Y, Wang H, Zhang Y, Mei L, et al. 2014. Interplay of mevalonate and Hippo pathways regulates RHAMM transcription via YAP to modulate breast cancer cell motility. Proc Natl Acad Sci U S A 111:E89–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wei X, Shimizu T, Lai ZC. 2007. Mob as tumor suppressor is activated by Hippo kinase for growth inhibition in Drosophila. Embo J 26:1772–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wu S, Liu Y, Zheng Y, Dong J, Pan D. 2008. The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev Cell 14:388–98 [DOI] [PubMed] [Google Scholar]
- 144.Xin M, Kim Y, Sutherland LB, Murakami M, Qi X, et al. 2013. Hippo pathway effector Yap promotes cardiac regeneration. Proceedings of the National Academy of Sciences of the United States of America 110:13839–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yan Y, Denef N, Tang C, Schupbach T. 2011. Drosophila PI4KIIIalpha is required in follicle cells for oocyte polarization and Hippo signaling. Development 138:1697–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yang C-C, Graves HK, Moya IM, Tao C, Hamaratoglu F, et al. 2015. Differential regulation of the Hippo pathway by adherens junctions and apical-basal cell polarity modules. Proceedings of the National Academy of Sciences of the United States of America 112:1785–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yin F, Yu J, Zheng Y, Chen Q, Zhang N, Pan D. 2013. Spatial organization of Hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2. Cell 154:1342–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yu F-X, Zhao B, Guan K-L. 2015. Hippo Pathway in Organ Size Control, Tissue Homeostasis, and Cancer. Cell 163:811–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, et al. 2012. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 150:780–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yu J, Zheng Y, Dong J, Klusza S, Deng W-M, Pan D. 2010. Kibra functions as a tumor suppressor protein that regulates Hippo signaling in conjunction with Merlin and Expanded. Dev Cell 18:288–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yue T, Tian A, Jiang J. 2012. The Cell Adhesion Molecule Echinoid Functions as a Tumor Suppressor and Upstream Regulator of the Hippo Signaling Pathway. Developmental cell 22:255–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zanconato F, Battilana G, Cordenonsi M, Piccolo S. 2016. YAP/TAZ as therapeutic targets in cancer. Current opinion in pharmacology 29:26–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zanconato F, Cordenonsi M, Piccolo S. 2016. YAP/TAZ at the Roots of Cancer. Cancer cell 29:783–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zanconato F, Forcato M, Battilana G, Azzolin L, Quaranta E, et al. 2015. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat Cell Biol 17:1218–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang C, Robinson BS, Xu W, Yang L, Yao B, et al. 2015. The Ecdysone Receptor Coactivator Taiman Links Yorkie to Transcriptional Control of Germline Stem Cell Factors in Somatic Tissue. Developmental Cell 34:168–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang L, Tang F, Terracciano L, Hynx D, Kohler R, et al. 2015. NDR Functions as a Physiological YAP1 Kinase in the Intestinal Epithelium. Current biology : CB 25:296–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhang P, Pei C, Wang X, Xiang J, Sun BF, et al. 2017. A Balance of Yki/Sd Activator and E2F1/Sd Repressor Complexes Controls Cell Survival and Affects Organ Size. Dev Cell 43:603–17 e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang X, Qiao Y, Wu Q, Chen Y, Zou S, et al. 2017. The essential role of YAP O-GlcNAcylation in high-glucose-stimulated liver tumorigenesis. Nat Commun 8:15280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhang Y, Wang X, Matakatsu H, Fehon R, Blair SS. 2016. The novel SH3 domain protein Dlish/CG10933 mediates fat signaling in Drosophila by binding and regulating Dachs. Elife 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhao B, Li L, Lu Q, Wang LH, Liu C-Y, et al. 2011. Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes & Development 25:51–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. 2010. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP). Genes Dev 24:72–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhao B, Li L, Wang L, Wang C-Y, Yu J, Guan K-L. 2012. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes & Development 26:54–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhao B, Wei X, Li W, Udan RS, Yang Q, et al. 2007. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev 21:2747–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zheng Y, Liu B, Wang L, Lei H, Pulgar Prieto KD, Pan D. 2017. Homeostatic Control of Hpo/MST Kinase Activity through Autophosphorylation-Dependent Recruitment of the STRIPAK PP2A Phosphatase Complex. Cell Rep 21:3612–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zheng Y, Wang W, Liu B, Deng H, Uster E, Pan D. 2015. Identification of Happyhour/MAP4K as Alternative Hpo/Mst-like Kinases in the Hippo Kinase Cascade. Developmental Cell 34:642–55 [DOI] [PMC free article] [PubMed] [Google Scholar]


