Abstract
Background:
Neuropsychological assessment can add essential information to the characterization of individuals presenting with the logopenic variant of primary progressive aphasia (lvPPA).
Objective:
This study examined the neuropsychological characteristics of lvPPA patients. We also examined differences in regional and whole brain atrophy based on neuropsychological profiles.
Methods:
We conducted a hierarchical cluster analysis on memory, executive functioning, and visuospatial neuropsychological test data for 56 individuals with lvPPA. We then compared resultant clusters to left middle temporal, inferior parietal, and superior parietal regions-of-interest using multivariate analysis of covariance. We also performed voxel-level analyses.
Results:
We identified three clusters characterized as lvPPA with no neurocognitive impairment (n = 5), lvPPA with mild neurocognitive deficits (n = 23), and lvPPA with marked cognitive deficits (n = 28). WAB-AQ was associated with left middle temporal volume. Superior parietal volumes were smaller for the lvPPA group with marked cognitive symptoms compared to the less severe groups. Voxel-level analyses showed greater atrophy in temporal, parietal, lateral occipital and frontal regions, left worse than right. Age, disease duration, gender, WAB-AQ, and PiB-PET did not account for differences between groups.
Conclusions:
LvPPA patients without other cognitive deficits in other domains were relatively uncommon while 50% of our sample exhibited pronounced neurocognitive deficits outside the language domain. Pronounced cognitive deficits in lvPPA are associated with widespread atrophy, left worse than right. Our study underscores the importance of examining neuropsychological function in addition to language in patients with lvPPA.
Keywords: primary progressive aphasia, logopenic aphasia, neuropsychological assessment, Volumetric MRI, voxel-wise analyses
Introduction
The logopenic variant of primary progressive aphasia (lvPPA) is a subtype of primary progressive aphasia (PPA) that presents with impaired spontaneous single-word retrieval, impaired sentence repetition, and phonologic paraphasias.[1, 2] Despite the logistical difficulties of using neuropsychological assessment for aphasic individuals, an increasing number of researchers have been successful using neuropsychological testing to show the presence of non-language cognitive deficits in patients who meet criteria for lvPPA. Examples include deficits in attention span [3–5], delayed verbal memory but not retention [6–8], spatial neglect [9], as well as global cognitive decline [4, 5, 8, 10–12]. One implication of this research is that in the absence of a comprehensive neuropsychological assessment, the presence or degree of cognitive impairment relative to language symptoms may go unrecognized or underappreciated in the normal course of a diagnostic work-up. Previous work demonstrates diverse cognitive profiles of patients with lvPPA [13, 14] and suggests there may also be variability of involved brain regions among lvPPA patients that is unaccounted for in the current clinical lvPPA conceptualization.
Our primary objectives were to (1) examine and describe the neuropsychological profiles of a large sample of individuals with well-characterized lvPPA; (2) examine differences in cortical volumes between lvPPA neuropsychological profiles in areas closely associated with language changes in lvPPA, namely temporal, inferior parietal, and superior parietal regions based on prior work from our group[14]; (3) examine differences in whole-brain atrophy in lvPPA based on neuropsychological profiles.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
All procedures in this study were approved by the Mayo Clinic Institutional Review Board. A total of 56 participants (50% female) with lvPPA were recruited from the Mayo Clinic, Department of Neurology, and provided written informed consent.
Subjects
All participants underwent a detailed speech and language examination, neurological evaluation, and neuropsychological testing over a span of 48–72 hours. All participants presented with primary deficits in language, with the language difficulty being the presenting complaint and dominant symptom, and the sole cause of problems in activities of daily living. Forms completed by an informant or care provider did not endorse any evidence of cognitive impairment outside of language. In addition, there was no evidence of cognitive impairment on the initial intake evaluation that suggested another diagnosis, nor were there other cognitive or behavioral changes observed during the speech/language evaluation that raised concern for another diagnosis.
All participants had video and audio recordings of their entire formal speech and language assessment, as well as general conversation. Two speech-language pathologists (JRD, EAS, or HMC) made the diagnosis of lvPPA prior to or during a consensus meeting, solely based on data from speech and language assessments without any knowledge of neurological, neuroimaging, or neuropsychological results. Table 1 provides a summary of the patient demographics.
Table 1:
Logopenic variant PPA Group Descriptive Characteristics
| lvPPA/no neurocognitive cognitive deficits (n = 5) |
lvPPA/mild neurocognitive deficits (n = 23) |
lvPPA/marked neurocognitive deficits (n = 28) |
All Participants (n=56) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Min | Max | Mean | SD | Min | Max | Mean | SD | Min | Max | Mean | SD | Min | Max | |
| Age, years | 62.0 | 10.8 | 47 | 74 | 68.4 | 7.86 | 56 | 85 | 65.4 | 8.40 | 49 | 83 | 66.5 | 8.4 | 47 | 85 |
| Education, years | 17.2 | 2.28 | 14 | 20 | 15.2 | 2.07 | 12 | 18 | 14.4 | 2.56 | 10 | 20 | 15.0 | 2.4 | 10 | 20 |
| Age at Onset, years | 59.2 | 11.8 | 42 | 72 | 64.9 | 7.04 | 52 | 80 | 62.0 | 7.89 | 47 | 78 | 63.0 | 7.9 | 42 | 80 |
| Illness Duration, years | 2.7 | 1.4 | 1.5 | 4.5 | 3.2 | 1.3 | 1.0 | 5.0 | 3.5 | 1.3 | 1 | 6 | 3.3 | 1.3 | 1 | 6 |
| PiB SUVR | 1.90 | 0.19 | 1.73 | 2.19 | 1.89 | 0.37 | 1.13 | 2.44 | 2.07 | 0.42 | 1.08 | 2.66 | 1.98 | .37 | 1.08 | 2.66 |
| WAB Aphasia Quotient | 91.1 | 5.98 | 83.0 | 98.8 | 85.6 | 5.78 | 72.6 | 95.0 | 69.9 | 16.34 | 33.5 | 89.2 | 78.2 | 14.7 | 33.5 | 98.8 |
| WAB AV Comp | 9.71 | 0.52 | 8.8 | 10 | 9.5 | .38 | 8.7 | 10 | 8.26 | 1.26 | 5.5 | 9.95 | 8.86 | 1.15 | 5.5 | 10 |
| WAV Repetition | 8.48 | 1 | 6.8 | 9.4 | 7.78 | .87 | 6.4 | 9.2 | 7.05 | 2 | 2.1 | 9.8 | 7.47 | 1.64 | 2.1 | 9.8 |
| Token Test (/22) | 15 | 5.24 | 7 | 21 | 13 | 3.85 | 6 | 19 | 6.79 | 5.01 | 0 | 17 | 9.91 | 5.69 | 0 | 21 |
| Phonologic errors* | 1.2 | .45 | 1 | 2 | 1.32 | .57 | 1 | 3 | 1.41 | .8 | 0 | 3 | 1.35 | .68 | 0 | 3 |
PiB SUVR = Pittsburgh-Compound B standardized uptake value ratio, WAB = Western Aphasia Battery, AV Comp = Auditory Verbal Comprehension, Phonologic errors based on a 4-point scale (absent mild, moderate-marked, severe)
The clinical criteria used to determine a diagnosis for lvPPA were as follows: (1) presence of aphasia, (2) impaired sentence repetition and comprehension, (3) presence of anomia with evidence of relatively spared single word comprehension, (4) evidence of phonemic paraphasias, (5) slowed rate of verbal expression due to pauses for word retrieval or verbal formulation, and (6) absence of agrammatic or telegraphic verbal output. Participants were excluded if they met criteria for other variants of primary progressive aphasia (agrammatic, semantic) or primary progressive apraxia of speech, if language impairment was not the main cause of impairment of activities of daily living or if there was any evidence of a lesion that could have accounted for the clinical presentation.
Speech and language assessments included the Western Aphasia Battery (WAB), revised [15], Part A, as the primary measure of global language ability and aphasia severity, the Token Token test as a measure of auditory comprehension [16] and the Boston Naming test as a measure of confrontation naming. Repetition was determined from the repetition subtest of the WAB part 1. Table 1 also includes performance on language parameters in which some degree of heterogeneity is frequently reported in lvPPA. Phonological errors were rated on a four-point scale (absent, mild, moderate-marked, severe) during consensus review of recorded conversation, as well as spoken picture description and word and sentence repetition responses during the formal test battery. Formal interrater reliability was not assessed, but agreement about the presence/absence of aphasia and the classification of lvPPA was quickly reached (i.e., requiring little or no discussion) for the majority of subjects. Cases in which more discussion was required typically involved re-examination of data, re-review of recordings, or re-review and discussion of classification criteria before a consensus conclusion.
Neuropsychological Evaluation
A trained psychometrist administered and scored all neuropsychological tests, which was overseen by a clinical neuropsychologist (MMM). The cognitive domains assessed included (1) memory: Wechsler Memory Scale-III (WMS-III) Logical Memory I/II which assesses immediate and delayed recall of paragraph-length stories and Visual Reproduction I/II which assesses immediate and delayed recall of geometric designs,[17] and the Rey Auditory Verbal Learning Test (RAVLT)[18] which is a list learning test that includes five learning trials, an interference trial, immediate recall and delayed recall trials; (2) executive function [Trailmaking Test (TMT) [19] parts A and B which is a test of visual scanning and visuomotor tracking, divided attention, and cognitive flexibility, and Delis-Kaplan Executive Function System (D-KEFS) Sorting Test,[20] which is a conceptual task that evaluates problem-solving, verbal and nonverbal concept formation, and flexibility of thinking; and, (3) visuospatial function [Rey-Osterrieth Complex Figure Test (RCFT)[21] which is a measure of visual perception and constructional praxis and Visual Object and Space Perception (VOSP) cube and incomplete letters [22]. The VOSP cube subtest is a block counting task. The VOSP incomplete letter subtest shows a series of large alphabet letters, one to a card, which have been randomly degraded so that only 30% of the original shape remains. The subject is asked to identify the letter.
Published norms were used for the WMS-III[17], D-KEFS[20], and VOSP[22] subtests. Mayo Older American Normative Studies age-adjusted scaled scores were used for the RAVLT, Trailmaking Test, and Rey-Osterrieth Complex Figure Test[23–25]. For participants who were younger than the MOANS normative sample, the lowest age group was used to derive scaled scores. We converted age-adjusted scaled scores to z-scores for all tests.
Neuroimaging analysis
All participants underwent a 3T volumetric head MRI, which included a magnetization-prepared rapid gradient-echo (MPRAGE) sequence. MPRAGE images underwent pre-processing correction for gradient non-linearity[26] and intensity non-uniformity.[27] Freesurfer version 5.3.0 (http://surfer.nmr.mgh.harvard.edu/) was used to calculate volumes of the left middle temporal gyrus, left inferior parietal lobe, and left superior parietal lobe. Briefly, skull-stripped MPRAGE images were transformed into Talaraich space, the grey/white boundary was identified and the resultant cortical models were registered to a spherical atlas and parcellated into regions based on gyral and sulcal structure[28]. All participants also underwent Pittsburgh-Compound B (PiB) PET imaging to assess for the deposition of beta-amyloid. A global PiB standardized uptake value ratio (SUVR) was calculated for each participant as previously described.[29]
Statistical Analysis
Statistical analyses were conducted using IBM SPSS Statistics for Windows, Version 22.0 (SPSS). The variable selection approach used z-scores from all neuropsychological tests in a 2-step cluster analysis that helped determine the value of variable to the model. Variables that didn’t improve the cluster fit were removed until we had the most efficient set of variables (i.e., fewest from each domain that improved the solution.) The neuropsychological tests that were best able to produce different groups included LM-I, LM-II, RAVLT delayed recall, D-KEFS sorting, TMT-A, and RCFT copy. (WMS-III Visual Reproduction I/II and VOSP subtests did not contribute to the cluster solution.) The variables that were best able to differentiate the groups were then entered into the hierarchical cluster analysis. In addition to the approach to variable selection, we hypothesized that there would be three groups based on previous work by our group that identified three subtypes of lvPPA based on FDG-PET patterns of hypometabolism[14]. The dendrogram conceptually fit a 2–3 group solution, with one main division between lvPPA with marked neurocognitive deficits and lvPPA with mild neurocognitive deficits, and then another group somewhat closer to the lvPPA with mild neurocognitive deficits that represented a group of individuals with lvPPA with exclusively language symptoms, i.e., no neurocognitive deficits in other domains.
Group differences were evaluated for age of onset, illness duration, education, Western Aphasia Battery Aphasia quotient (WAB-AQ), and PiB SUVR using multivariate analysis of variance (MANOVA).
We subsequently explored cluster group differences for volume using a one-way multivariate analysis of covariance (MANCOVA). Regions of interest were selected a priori and based on findings from a previous paper published by our group[14]. These included the left middle temporal, left inferior parietal, and left superior parietal lobes. Volumes for predetermined regions of interest, corrected by dividing by estimated total intracranial volume (eTIV), were entered as dependent variables, with age of onset, illness duration, education, WAB-AQ, and PiB SUVR entered as covariates. To assure the sample met the assumption of multivariate normality, we calculated Mahalanobis distances and chi-square values following the method described by Henson [30], and multivariate outliers were excluded from the analysis (i.e., participants with chi-square values less than .001). Box’s M calculation was run to determine if the assumption of homogeneity of variance was met.
In addition, we also performed a voxel-based analysis of grey matter volume using the well-validated and commonly used voxel-based morphometry tools in SPM12. All MRI scans were spatially normalized to the Mayo Clinic Adult Lifespan Template (MCALT) (https://www.nitrc.org/projects/mcalt/) and segmented using unified segmentation [31] with MCALT tissue priors. All grey matter images were modulated and smoothed at 8mm full-width-at-half-maximum. Two-sided t-tests were used to compare the lvPPA groups to each other, and to a healthy control cohort of 112 age and gender-matched subjects that had undergone identical imaging protocols [mean (SD) age 66.8 (8.2), 52% female] in the Mayo Clinic Study of Aging. Results were assessed corrected for multiple comparisons using the family wise error correction at p<0.05 and age and gender were included in all comparisons as covariates.
Results
Cluster Analysis
We selected the 3-cluster solution because it provided good separation of the groups and was conceptually consistent with previous work done by our group showing three patterns of FDG-PET hypometabolism with amyloid-positive lvPPA: temporal, temporoparietal, and parietal predominate. The three groups are as follows: (1) lvPPA with no neurocognitive deficits, (2) lvPPA with mild neurocognitive deficits (lvPPA-mild), and (3) lvPPA with marked neurocognitive deficits (lvPPA-marked). The dendogram for the clusteris analysis is displayed in Figure 1, and the demographics and language performance of these groups are shown in Table 1. Table 2 includes performances on neuropsychological tests used in the cluster analysis. Participants in the lvPPA group with no neurocognitive deficits had cognitive performances less than one standard deviation below the mean on neuropsychological tests. The lvPPA group with mild neurocognitive deficits had worse performance on tests more heavily mediated by language (i.e., LM-I, LM-II, and RAVLT Delayed Recall). Based on the dendrogram, the lvPPA group with no neurocognitive deficits and the lvPPA group with mild neurocognitive deficits together represent mild lvPPA. The lvPPA group with marked neurocognitive deficits showed a more severe pattern of impairment, with performances greater than two standard deviations below the mean for all neuropsychological tests, and with no difference between tasks with relatively high language demands and tasks without intensive language demands.
Figure 1:
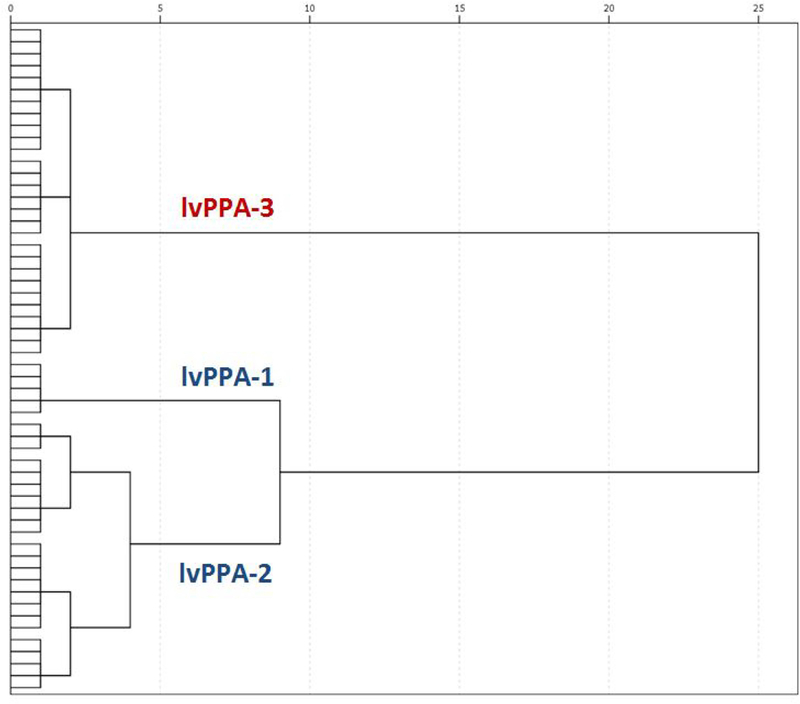
Dendrogram created by cluster analysis. The distance along the x-axis represents a measure of similarity’ between subjects, such that the closer the distance the greater the similarly between them. Cluster 1 (lvPPA-1) consisted of subjects with no neurocogmtive deficits on neuropsychological tests. Cluster 2 (lvPPA-2) showed mild neurocogmtive deficits on neuropsychological tests. Based on the dendrogram, these groups together represent milder lvPPA (i.e., lvPPA-mild). Cluster 3 (lvPPA-3) showed marked neurocogmtive deficits (i.e.. lvPP A-marked).
Table 2:
Logopenic variant PPA Group Neuropsychological Test Performance
| lvPPA/no neurocognitive cognitive deficits (n = 5) |
lvPPA/mild neurocognitive deficits (n = 23) |
lvPP A/marked neurocognitive deficits (n = 28) |
All Participants (n=56) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Min | Max | Mean | SD | Min | Max | Mean | SD | Min | Max | Mean | SD | Min | Max | |
| WMS-III LM-I | −0.27 | 0.83 | −1.00 | 1.00 | −2.02 | 0.58 | −3.00 | −0.67 | −2.82 | 0.32 | −3.00 | −1.67 | −2.26 | 0.88 | −3.0 | 2.0 |
| WMS-III lm-h | 0.60 | 0.86 | −0.33 | 2.00 | −1.44 | 0.54 | −2.30 | 0.00 | −2.39 | 0.54 | −3.00 | −1.00 | −1.73 | 1.03 | −3.0 | 2.0 |
| RAVLT Delayed Recall | 0.73 | 0.98 | 0.00 | 2.33 | −1.28 | 0.96 | −2.67 | 0.67 | −2.08 | 0.63 | −3.00 | −0.67 | −1.49 | 1.13 | −3.0 | 2.3 |
| TMT-A | 0.87 | 0.80 | 0.00 | 2.00 | −0.65 | 0.81 | −2.33 | 1.00 | −2.35 | 0.64 | −3.00 | −0.60 | −1.37 | 1.29 | −3.0 | 2.0 |
| D-KEFS Sorting | 0.27 | 0.60 | −0.33 | 1.00 | −0.72 | 0.61 | −2.00 | 1.00 | −2.31 | 0.69 | −3.33 | −0.67 | −1.43 | 1.13 | −3.33 | 1.0 |
| RCFT Copy | 0 87 | 0.77 | 0.00 | 2.00 | −0.77 | 1.35 | −2.67 | 2.00 | −2.34 | 0.63 | −3.00 | −1.00 | −1.41 | 1.43 | −3.0 | 2.0 |
WMS-III LM-I = Wechsler Memory Scale = Third Edition Logical Memory I, LM-II = Weclisler Memory Scale - Third Edition, Logical Memory II, RAVLT = Rey Auditory Verbal Learning Test, TMT-A = Trail Making Test Part A, D-KEFS = Delis-Kaplan Executive Function System, RCFT = Rey-Osterrieth Complex Figure Test
Multivariate Analyses
There were no multivariate outliers so all participant data were included. All five of the lvPPA participants with no neurocognitive deficits were PiB+ while 19/23 of the lvPPA participants with mild neurocognitive deficits and 24/28 of the lvPPA participants with marked neurocognitive deficits were PiB+ which is consistent with the literature showing that most individuals with lvPPA have β-amyloid (Aβ) deposition on PiB-PET [33, 34]. Because of the relatively small size of the lvPPA group with no cognitive deficits, we analyzed the data at the two-group level for multivariate analyses, combining the lvPPA with no neurocognitive deficits and lvPPA with mild neurocognitive deficits groups, referred to in subsequent analyses simply as lvPPA-mild.
lvPPA Group Differences
Multivariate tests comparing the lvPPA-mild and lvPPA-marked groups showed a significant difference for WAB-AQ, F(1, 54) = 36.34, p < .001, ηp2 = 328, with the lvPPA-marked group having lower WAB-AQ scores than the lvPPA-mild group. There were no differences in age of onset, illness duration, education, or PiB ratio between the two groups.
Brain Volume Differences
We determined that the assumption of homogeneity of variance was met, Box’s M = 5.224, p = .556, and so we used pooled variances. Levene’s Test of Equality of Error Variances showed no significant differences in variances among left middle temporal, left inferior parietal, and left superior parietal volumes. Therefore, we were able to further evaluate between-group volumetric differences in these regions.
Multivariate tests revealed significant effects for WAB-AQ, F(3, 47) = 3.019, p = .039, ηp2 = .162; PiB SUVR group differences, F(3, 47) = 4.507, p = .007, ηp2 = 223; and lvPPA subgroup classification, F(3, 47) = 4.008, p = .013, ηp2 = 204. These results indicate that in addition to group classification (i.e., lvPPA-mild vs. lvPPA-marked), WAB-AQ and PiB SUVR are independently related to cortical volumes in at least one brain region. Age of onset, illness duration, and education did not predict these brain volume differences.
At the univariate level, WAB-AQ was associated with left middle temporal lobe volume, F(1, 49) = 9.134, p = .004, ηp2 = 157, with lower WAB-AQ scores (i.e., more severe aphasia) correlating with smaller volumes. The WAB-AQ was not related to inferior or superior parietal volumes. Left superior parietal volume was significantly smaller for the lvPPA-marked group compared to the lvPPA-mild group, F(1, 49) = 12.453, p = .001, ηp2 = .203. There was also a trend toward this same pattern for left inferior parietal volume, F(1, 49) = 3.779, p = .058, ηp2 = .072. The lvPPA-mild and lvPPA-marked groups showed no significant differences in left middle temporal volume. Subgroup differences of lvPPA with no neurocognitive deficits and lvPPA with mild neurocognitive deficits separated for illustration are displayed in Figure 2. Finally, higher PiB SUVR was significantly related to smaller left superior parietal volume, F(1, 49) = 13.399, p = .001, ηp2 = 215, but was not related to left middle temporal or inferior parietal volumes.
Figure 2:
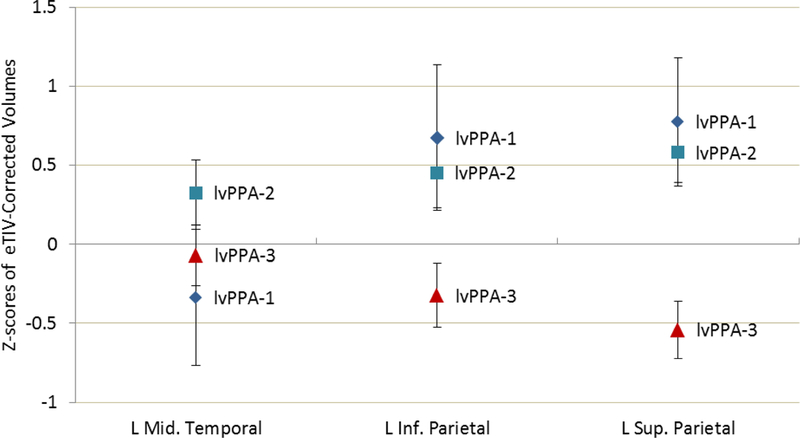
Z-scores of estimated total intracranial volume-corrected volumes for each lvPPA cluster in the left middle temporal, left inferior parietal, and left superior parietal regions of interest for each lvPPA group identified by cluster analysis. lvPPA-1 = no neurocogmtive deficits, lvPPA-2 = mild neurocogmtive deficits. lvPPA-3 = marked neurocogmtive deficits
Voxel-level MRI
Due to the comparatively small size of the lvPPA group with no cognitive deficits, we also combined this group with the lvPPA group with mild neurocognitive deficits (referred to as lvPPA-mild) for the voxel-level analyses. Relative to controls the lvPPA-mild group showed grey matter loss predominantly in the left temporal lobe. There were also small regions of grey matter loss in the left inferior parietal cortex, left inferior frontal region, and right temporal lobe (Figure 3). The lvPPA-marked group showed striking grey matter loss, left greater than right, in temporoparietal regions with extension posteriorly to the occipital lobes, cingulate gyrus and precuneus bilaterally, and frontal regions, left greater than right. When comparing the lvPPA-mild and lv-PPA marked directly to each other, the lv-PPA marked group had small regions of more grey matter loss in the superior parietal lobe as expected, but also in the inferior parietal lobe, lateral occipital lobe, and superior frontal gyri. There were no areas in which the lvPPA-mild group had more grey matter loss than the lvPPA-marked group.
Figure 3:
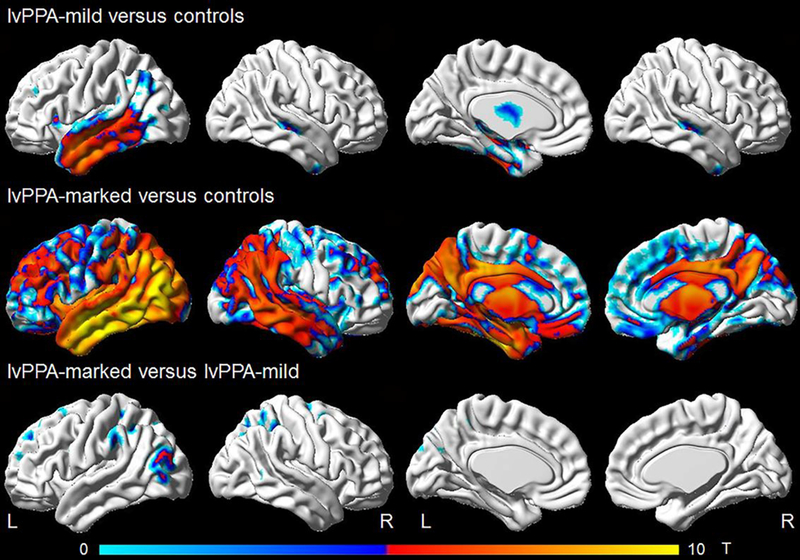
VBM maps comparing the lvPPA-mild and lvPP A-marked to 112 matched controls (3:1) and to each other (shown using FWE correction at p<_05.)
Discussion
The main findings of this study are: (1) lvPPA presents with a range of neuropsychological deficits. (2) lvPPA without concomitant neurocognitive deficits in other domains is relatively uncommon in our sample. (3) The severity of neuropsychological deficits correlates with left superior parietal volume. This is consistent with our previous work showing that FDG-PET hypometabolism in the left superior parietal lobe is associated with deficits in executive function and behavioral dyscontrol in individuals with lvPPA[14]. In addition, the lvPPA-mild group had grey matter loss mainly in the left temporal lobe whereas the lvPPA-marked group showed striking grey matter loss in the left temporo-parietal regions with extension posteriorly to the occipital lobes, cingulate, precuneus, and frontal regions. (4) The severity of aphasia is correlated with left middle temporal volume. (5) The range of neuropsychological deficits is not directly associated with age at onset, disease duration, education, or PiB SUVR.
Our findings show compelling evidence for at least two coherent neuropsychological profiles in this lvPPA sample, with one division showing relatively circumscribed language deficits or relatively mild neurocognitive deficits in other domains (i.e., lvPPA-mild) while the lvPPA-marked group showed more severe, multi-domain decline co-occurring with the logopenic presentation. From the mild cognitive profiles, the subset of participants who showed no significant non-language cognitive deficits represented about 9% of our sample, suggesting that lvPPA without concomitant cognitive deficits is a relatively uncommon presentation in our clinic.
Other studies have shown that lvPPA has varying degrees of neuropsychological impairment and that assessing neuropsychological function in individuals presenting with a primary language complaint can help distinguish lvPPA from healthy controls [4, 5, 32, 33], other types of primary progressive aphasia (e.g., agrammatic aphasia, semantic dementia) [4, 6, 33–35], and MCI and Alzheimer’s dementia with typical amnestic presentations [4, 7, 8]. One possible reason that visual memory measures did not contribute to the cluster solution in our study is that level of performance on visual memory measures may not contribute significant variance to distinguishing the groups [6, 7]. None of these studies, however, used performance on neuropsychological measures (rather than performance on language measures) to identify subgroups or characterized the heterogeneity of cognitive deficits in a well-characterized group of individuals who meet strict clinical criteria for lvPPA.
Region-of-interest analyses showed that for all participants the severity of aphasia, as measured by WAB-AQ, was related to left middle temporal lobe volume loss. The lvPPA-mild and lvPPA-marked groups showed no systematic differences in temporal volume loss, despite the lvPPA-marked group having worse aphasia scores, which suggests the worse non-language cognitive difficulties may have contributed to worse WAB-AQ performances. Additionally, the lvPPA-marked group showed significantly lower superior parietal volumes compared to the lvPPA-mild group. Amyloid-beta burden, as measured by PiB SUVR, was associated with lower cortical volume in the left parietal regions, particularly the left superior cortex.
Voxel-level analyses showed that for the lvPPA-mild group, atrophy was primarily in the left temporal, left inferior parietal lobe, and left inferior frontal gyrus whereas the lvPPA-marked group showed widespread atrophy encompassing temporoparietal cortices extending to lateral occipital cortex as well as frontal regions, left worse than right. This is striking given the comparable age at onset and disease duration between the groups and points to the importance of assessing other domains of cognition in addition to language in order to capture the extent of disease in individuals presenting with a primary language complaint. We also previously found that occipital PiB was associated with widespread cognitive impairment in lvPPA, and these individuals had evidence of slightly more occipital hypometabolism on FDG-PET[36].
These findings, taken together, show that left middle temporal lobe volume loss is associated with aphasia severity for all three lvPPA groups identified in our study, while aphasia severity in the lvPPA-marked group is also associated with more generalized atrophy. The additional cognitive deficits may account for the lvPPA-marked group’s lower WAB-AQ scores without corresponding left middle temporal volume loss. The difference, then, among the lvPPA groups is the degree to which brain regions beyond those normally associated with language dysfunction are affected, and may reflect different rates of disease progression.
Our findings that all participants presented with language as the sole cause of difficulties with daily living is consistent with a previous report that also examined the neuropsychological profiles of patients with lvPPA [8] and underscores the importance of thoroughly examining neuropsychological function when patients present with a primary language – as opposed to memory – complaint. Previous studies report that the majority of individuals with lvPPA are on the Alzheimer’s Disease continuum because they have elevated levels of amyloid [35–39]. Our findings are consistent this, given that 86% of our participants were PiB positive and support the current conceptualization of lvPPA representing an atypical presentation of Alzheimer’s Disease.
In terms of classification accuracy, the lvPPA-mild group may represent the more traditional conceptualization of lvPPA as a distinct, relatively focal aphasic syndrome. The classification of the lvPPA-marked group is more complicated because, while these individuals present with a primary language concern and therefore meet criteria for lvPPA, their more severe neurocognitive deficits reflect a more widespread neurodegenerative process. This raises the question of whether this group indeed would better be classified as another condition (e.g., aphasic dementia[40]). We argue that some variation of lvPPA remains the best classification for several reasons. First, because the primary presenting concern and cause for impairments in daily life is aphasia, the constellation of these individuals’ essential clinical features is best captured by the lvPPA classification. Secondly, our evaluation of lvPPA was made blinded to neuropsychological assessment results, and instead used the less stringent but acceptable sources of information to evaluate cognitive impairment, such as self- and collateral reports; using this standard from the consensus criteria, individuals met full criteria for lvPPA. Thirdly, there are little actuarial data that specify what constitutes non-language cognitive impairment and to what degree a non-language symptom is impaired compared to language symptoms, which makes systematic judgments of inclusion and exclusion challenging. Finally, classification as another neurodegenerative condition would obscure the unique presentation and disease course of these individuals. We propose that these individuals would be more precisely characterized as having logopenic dementia, or a more severe spectrum of lvPPA.
This study demonstrates that neuropsychological assessment can add essential information to the evaluation of PPA. The PPA criteria stipulate that the presenting concern of the patient must be predominant language difficulty with no prominent difficulty in episodic memory, verbal memory, or visuospatial function; any non-language cognitive impairment (i.e., cognitive impairment not directly related to PPA) must not exceed the severity of the primary language impairment; and that non-language cognitive impairments cannot be substantially impairing to adaptive functioning. The consensus criteria permit a patient’s history, self- or collateral-report, brief cognitive screening, or comprehensive neuropsychological testing to satisfy these criteria. However, the criteria do not operationalize how relative severity of cognitive impairment should be assessed, but instead raise concerns about the validity of language-dependent neuropsychological assessment for patients with aphasia.[2] The present results are consistent with other studies showing that despite the barriers related to language impairment, aphasia in itself does not preclude the acquisition of meaningful clinical data [4–8, 11, 12, 14]. Our results are also supported by cortical volume findings which correlate with our neuropsychologically defined groups. For the subset of lvPPA participants with exclusive language symptoms, neuropsychological performances were fully within the normal range.
One of the primary implications of these findings is that when lvPPA is diagnosed according to the consensus criteria, but in the absence of a comprehensive neuropsychological evaluation, practitioners may underestimate the presence and severity of co-occurring neurocognitive deficits. Patients may have a similar presenting concern of language change, but potentially underappreciated cognitive deficits may result in suboptimal treatment planning and access to coping resources that would otherwise be helpful for patients and their caregivers. We propose that the gold-standard for evaluating patients with possible lvPPA should include formal neuropsychological assessment. This study demonstrates that, in addition to comprehensive speech and language assessment, comprehensive neuropsychological evaluations are an important tool that can add unique clinical information about clinical profiles and that correlate meaningfully with relevant structural changes.
Limitations and Future Directions
The lack of longitudinal data for these individuals limits our understanding of how these cognitive difficulties have evolved over time. We statistically accounted for differences in age at onset, illness duration, level of education and amount of amyloid deposition, but additional research will be needed to evaluate the clinical trajectories of these groups. Additionally, for the lvPPA-marked group we are unable to determine if language indeed remains more impaired than non-language deficits because of the degree of impairment on all measures. Future studies correlating neuropsychological and speech and language data with other biomarkers (e.g., tau PET) may further elucidate these relationships.
Conclusion
We found distinct neurocognitive profiles in a sample of well characterized individuals with lvPPA that differed in terms of degree of cognitive deficits beyond language, which we characterized as a milder, more traditional lvPPA group (lvPPA-mild) and a more severe, “logopenic dementia” group (lvPPA-marked). Our volumetric analyses demonstrate a strong association with left superior and inferior parietal volume losses in lvPPA-marked (i.e., logopenic dementia) that is reflective of a more widespread disease process.
Acknowledgements
This study was funded by R01 DC010367 (PI: KAJ) and R01 AG050603 (PI: JLW) from the National Institutes of Health. We would like to acknowledge Dr. Ronald Petersen (PI) of the Mayo Clinic Study of Aging which provided imaging data from normal controls for the VBM group comparisons.
Footnotes
Author Disclosures
Tyler Owens – Reports no disclosures
Mary Machulda – NIH funding: R01-DC12519, U01-AG06786, R01-AG50603, P50-AG16574, AG49810
Joseph Duffy – NIH funding: R01-DC12519, R21-NS94684
Edythe Strand – Reports no disclosures
Heather Clark – NIH funding: R01-DC12519, R01-NS89757, R21-NS94684
Sarah Boland – R01-DC12519, R01-NS89757, R21-NS94684, R01-AG50603
Jennifer Whitwell – NIH funding: R01-DC12519, R01-NS89757, R21-NS94684, R01-AG50603
Val Lowe - consults for Bayer Schering Pharma, Piramal Life Sciences and Merck Research and receives research support from GE Healthcare, Siemens Molecular Imaging, AVID Radiopharmaceuticals and the NIH (NIA, NCI).
Clifford R. Jack – serves as a consultant for Eli Lily and receives research funding from the NIH (R01-AG011378, R01-AG041851, R01-AG037551, U01-HL096917, U01-AG032438, U01-AG024904) and the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Foundation.
Keith Josephs – NIH funding: R01-AG037491, R01-DC12519, R01-NS89757, R21-NS094684, and R01-AG50603.
References
- [1].Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Ogar JM, Rohrer JD, Black S, Boeve BF, Manes F, Dronkers NF, Vandenberghe R, Rascovsky K, Patterson K, Miller BL, Knopman DS, Hodges JR, Mesulam MM, Grossman M (2011) Classification of primary progressive aphasia and its variants. Neurology 76, 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mesulam M-M (2003) Primary Progressive Aphasia — A Language-Based Dementia. New England Journal of Medicine 349, 1535–1542. [DOI] [PubMed] [Google Scholar]
- [3].Foxe DG, Irish M, Hodges JR, Piguet O (2013) Verbal and Visuospatial Span in Logopenic Progressive Aphasia and Alzheimer’s Disease. Journal of the International Neuropsychological Society 19, 247–253. [DOI] [PubMed] [Google Scholar]
- [4].Rohrer JD, Ridgway GR, Crutch SJ, Hailstone J, Goll JC, Clarkson MJ, Mead S, Beck J, Mummery C, Ourselin S, Warrington EK, Rossor MN, Warren JD (2010) Progressive logopenic/phonological aphasia: Erosion of the language network. NeuroImage 49, 984–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Leyton CE, Hodges JR, McLean CA, Kril JJ, Piguet O, Ballard KJ (2015) Is the logopenic-variant of primary progressive aphasia a unitary disorder? Cortex 67, 122–133. [DOI] [PubMed] [Google Scholar]
- [6].Butts AM, Machulda MM, Duffy JR, Strand EA, Whitwell JL, Josephs KA (2015) Neuropsychological Profiles Differ among the Three Variants of Primary Progressive Aphasia. Journal of the International Neuropsychological Society 21, 429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Weintraub S, Rogalski E, Shaw E, Sawlani S, Rademaker A, Wieneke C, Mesulam MM (2013) Verbal and nonverbal memory in primary progressive aphasia: The Three Words-Three Shapes Test. Behavioural Neurology 26, 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Magnin E, Chopard G, Ferreira S, Sylvestre G, Dariel E, Ryff I, Mertz C, Lamidieu C, Hidalgo J, Tio G, Haffen S, Galmiche J, Moulin T, Vandel P, Rumbach L (2013) Initial neuropsychological profile of a series of 20 patients with logopenic variant of primary progressive aphasia. Journal of Alzheimer’s Disease 36, 799–808. [DOI] [PubMed] [Google Scholar]
- [9].Zilli EM, Heilman KM (2016) Spatial neglect in a patient with logopenic progressive aphasia. Neurocase 22, 30–39. [DOI] [PubMed] [Google Scholar]
- [10].Louwersheimer E, Keulen MA, Steenwijk MD, Wattjes MP, Jiskoot LC, Vrenken H, Teunissen CE, van Berckel BNM, van der Flier WM, Scheltens P, van Swieten JC, Pijnenburg YAL (2016) Heterogeneous Language Profiles in Patients with Primary Progressive Aphasia due to Alzheimer’s Disease. Journal of Alzheimer’s Disease 51, 581–590. [DOI] [PubMed] [Google Scholar]
- [11].Teichmann M, Kas A, Boutet C, Ferrieux S, Nogues M, Samri D, Rogan C, Dormont D, Dubois B, Migliaccio R (2013) Deciphering logopenic primary progressive aphasia: a clinical, imaging and biomarker investigation. Brain 136, 3474–3488. [DOI] [PubMed] [Google Scholar]
- [12].Leyton CE, Hsieh S, Mioshi E, Hodges JR (2013) Cognitive decline in logopenic aphasia: More than losing words. Neurology. [DOI] [PubMed] [Google Scholar]
- [13].Machulda MM, Whitwell JL, Duffy JR, Strand EA, Dean PM, Senjem ML, Jack CR Jr, Josephs KA (2013) Identification of an atypical variant of logopenic progressive aphasia. Brain and Language 127, 139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Krishnan K, Machulda MM, Whitwell JL, Butts AM, Duffy JR, Strand EA, Senjem ML, Spychalla AJ, Jack CR Jr, Lowe VJ, Josephs KA (2017) Varying degress of temporoparietal hypometabolism on FDG-PET reveal amyloid-positive aphasia is not a homogeneous clinical entity. Journal of Alzheimer’s Disease 55, 1019–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kertesz A (2007) Psych Corp., San Antonio, TX.
- [16].De Renzi E, Vignolo L (1962) The Token Test: a sensitive test to detect receptive disturbances in aphasies. Brain 85, 665–768. [DOI] [PubMed] [Google Scholar]
- [17].Weschler D (1997) Weschler memory scale-III, Psychological Corporation, New York. [Google Scholar]
- [18].Rey A (1964) L’examen clinique en psychologie [The clinical psychological examination]. Paris: Presses Universitaires de France. [Google Scholar]
- [19].Reitan RM (1958) Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and Motor Skills 8, 271–276. [Google Scholar]
- [20].Delis D, Kaplan E, Kramer J (2001) Delis-Kaplan Executive Function System (D-KEFS) Examiner’s Manual, The Psychological Corporation, San Antonio, TX. [Google Scholar]
- [21].Osterrieth PA (1944) Le test de copie d’une figure complexe; contribution à l’étude de la perception et de la mémoire. [Test of copying a complex figure; contribution to the study of perception and memory.]. Archives de Psychologie 30, 206–356. [Google Scholar]
- [22].Warrington E, James M (1991) Thames Valley Test Company, Bury St. Edmunds, Suffolk, England.
- [23].Machulda MM, Ivnik RJ, Smith GE, Ferman TJ, Boeve BF, Knopman D, Petersen RC, Tangalos EG (2007) Mayo’s Older Americans Normative Studies: Visual Form Discrimination and copy trial of the Rey–Osterrieth Complex Figure. Journal of Clinical and Experimental Neuropsychology 29, 377–384. [DOI] [PubMed] [Google Scholar]
- [24].Ivnik RJ, Malec JF, Smith GE, Tangalos EG, Petersen RC (1996) Neuropsychological tests’ norms above age 55: COWAT, BNT, MAE token, WRAT-R reading, AMNART, STROOP, TMT, and JLO. The Clinical Neuropsychologist 10, 262–278. [Google Scholar]
- [25].Ivnik RJ, Malec JF, Smith GE, Tangalos EG, Petersen RC, Kokmen E, Kurland LT (1992) Mayo’s older americans normative studies: Updated AVLT norms for ages 56 to 97. Clinical Neuropsychologist 6, 83–104. [Google Scholar]
- [26].Sled JG, Zijdenbos AP, Evans AC (1998) A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Transactions on Medical Imaging 17, 87–97. [DOI] [PubMed] [Google Scholar]
- [27].Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, Kennedy D, Schmitt F, Brown G, MacFall J, Fischl B, Dale A (2006) Reliability in multi-site structural MRI studies: Effects of gradient non-linearity correction on phantom and human data. NeuroImage 30, 436–443. [DOI] [PubMed] [Google Scholar]
- [28].Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 31, 968–980. [DOI] [PubMed] [Google Scholar]
- [29].Jack JCR, Lowe VJ, Senjem ML, Weigand SD, Kemp BJ, Shiung MM, Knopman DS, Boeve BF, Klunk WE, Mathis CA, Petersen RC (2008) 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain 131, 665–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Henson RK (1999) Multivariate normality: What is it and how is it assessed. Advances in social science methodology 5, 193–211. [Google Scholar]
- [31].Ashburner J, Friston KJ (2005) Unified segmentation. NeuroImage 26, 839–851. [DOI] [PubMed] [Google Scholar]
- [32].Leyton CE, Hodges JR (2013) Towards a Clearer Definition of Logopenic Progressive Aphasia. Current Neurology and Neuroscience Reports 13, 396. [DOI] [PubMed] [Google Scholar]
- [33].Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, Johnson JK, Weiner MW, Miller BL (2004) Cognition and anatomy in three variants of primary progressive aphasia. Annals of Neurology 55, 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Amici S, Ogar J, Brambati S, Miller B, Neuhaus J, Dronkers N, BGorno-Tempini M (2007) Performance in specific language tasks correlates with regional volume changes in progressive aphasia. Cognitive and Behavioral Neurology 20, 203–211. [DOI] [PubMed] [Google Scholar]
- [35].Rabinovici GD, Jagust WJ, Furst AJ, Ogar JM, Racine CA, Mormino EC, O’Neil JP, Lal RA, Dronkers NF, Miller BL, Gorno-Tempini ML (2008) Aβ amyloid and glucose metabolism in three variants of primary progressive aphasia. Annals of Neurology 64, 388–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Whitwell JL, Lowe VJ, Duffy JR, Strand EA, Machulda MM, Kantarci K, Wille SM, Senjem ML, Murphy MC, Gunter JL, Jack CR, Josephs KA (2013) Elevated occipital β-amyloid deposition is associated with widespread cognitive impairment in logopenic progressive aphasia. Journal of Neurology, Neurosurgery & Psychiatry 84, 1357–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Leyton CE, Villemagne VL, Savage S, Pike KE, Ballard KJ, Piguet O, Burrell JR, Rowe CC, Hodges JR (2011) Subtypes of progressive aphasia: application of the international consensus criteria and validation using β-amyloid imaging. Brain 134, 3030–3043. [DOI] [PubMed] [Google Scholar]
- [38].Rohrer JD, Rossor MN, Warren JD (2012) Alzheimer’s pathology in primary progressive aphasia. Neurobiology of Aging 33, 744–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Warren JD, Fletcher PD, Golden HL (2012) The paradox of syndromic diversity in Alzheimer disease. Nature Reviews Neurology 8, 451. [DOI] [PubMed] [Google Scholar]
- [40].Josephs KA, Whitwell JL, Duffy JR, Vanvoorst WA, Strand EA, Hu WT, Boeve BF, Graff-Radford NR, Parisi JE, Knopman DS, Dickson DW, Jack CR, Petersen RC (2008) Progressive aphasia secondary to Alzheimer disease vs FTLD pathology. Neurology 70, 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]


