Abstract
Objectives
Inflammatory breast carcinoma (IBC) is rare but is the most lethal type of breast cancer. Programmed death ligand 1 (PD-L1) expression in IBCs has been understudied.
Methods
In this study, tissue microarrays of 68 IBCs were immunostained with a PD-L1 antibody using an antibody clone (28-8) and detection system approved by the US Food and Drug Administration for selecting patients with non–small cell lung cancer and melanoma for anti–PD-L1 therapy.
Results
Positive PD-L1 expression was found in 25 (36.8%) of 68 samples but was not significantly associated with the clinicopathologic variables examined. Univariate analysis of overall survival (OS) revealed that worse OS was significantly associated with positive PD-L1, negative estrogen receptor, and triple-negative status. The 5-year OS rate was 36.4% for patients with PD-L1–positive IBC and 47.3% for those with PD-L1–negative IBC. In multivariate analyses, PD-L1 status remained a statistically independent predictor of OS.
Conclusions
These findings indicate that PD-L1 inhibitors could potentially improve the clinical outcome of patients with PD-L1–positive IBC.
Keywords: PD-L1, Inflammatory breast cancer, Survival, Prognosis, Breast, Immunohistochemistry
Inflammatory breast cancer (IBC) is rare but is the most aggressive type of breast cancer. Patients with IBC have characteristic clinical presentations resembling inflammatory process, including rapid onset and progression of breast swelling, redness, edema, tenderness, and warmth because of diffuse dermal lymphatic occlusion by tumor emboli.1 IBC is often associated with early metastasis and resistance to conventional therapies and poor clinical outcomes. Despite multidisciplinary approaches and multimodality treatment comprising neoadjuvant chemotherapy, radical surgery, adjuvant radiotherapy, and, if the patient is eligible, antihormonal and anti–human epidermal growth factor receptor 2 (HER2) therapy, the clinical outcomes of patients with IBC remain poor, with a 5-year overall survival (OS) rate of around 40%.1,2 Thus, it is imperative to identify innovative biomarkers related to the biologic behavior of IBC and to predict the effectiveness of novel therapeutic agents for these patients.
Programmed cell death 1 (PD-1) and its ligand, programmed death ligand 1 (PD-L1), play important roles in tumor surveillance. The interaction of PD-1 and PD-L1 leads to downregulation of the T-cell–mediated immune response to tumor cells.3-6 PD-L1 is a cell surface glycoprotein and is expressed by immune cells (such as T and B cells, macrophages, and dendritic cells), endothelial cells, and various types of cancer cells. Studies have shown that therapeutic blockade of the PD-1/PD-L1 immune checkpoint reactivates inhibited T cells, which increases antitumor immunity and promotes tumor regression. Objective, durable tumor regression with improved survival due to the blockade has been reported in patients with various advanced cancers, including melanoma, non–small cell lung cancer (NSCLC), kidney cancer, and bladder cancer.7-10 Furthermore, findings that positive PD-L1 expression predicts a higher likelihood of objective response to anti–PD-L1 agents have been reported in most studies.9,11-13
Studies of PD-L1 expression in breast cancer are relatively scant in the literature.14-29 The reported PD-L1 expression rate in breast cancer has varied substantially (1.7%-80%), likely owing to differences in testing methods, antibody clones, and scoring strategy.26,30,31 Furthermore, different studies have been contradictory regarding the prognostic effect of PD-L1 expression. Quite a few such studies have been focused on triple-negative breast cancer (TNBC) because of the aggressive natural history and the lack of targeted therapy of TNBC.20-24,26,28 Compared with other breast cancer subtypes, TNBC and basal-like breast cancer have higher PD-L1 expression rates.15-17,25,32-34
Small-scale clinical trials targeting the PD-1/PD-L1 axis in patients with recurrent/metastatic TNBC have shown encouraging results, with durable objective responses and an acceptable safety profile.35-37 In a phase Ib clinical trial in patients with advanced TNBC, Nanda et al35 reported that using an anti–PD-L1 monoclonal antibody (pembrolizumab) resulted in an overall response rate of 18.5% in heavily pretreated, advanced, PD-L1–positive TNBC. Similar findings were reported by two other studies using another type of anti–PD-L1 agent (MPDL3280A, also called atezolizumab).36,38
These promising results in initial clinical trials have inspired the evaluation of PD-L1 expression in IBC. So far, only two studies have investigated PD-L1 expression in IBC.39,40 Bertucci et al39 used DNA microarrays to evaluate PD-L1 messenger RNA (mRNA) expression in 112 pretherapeutic IBC samples and 194 non-IBC samples, and they reported that PD-L1 mRNA was upregulated in 38% of the IBCs and in 28% of the non-IBCs. Hamm et al40 used immunohistochemical (IHC) staining to stain 12 IBC tumors and reported low-intensity PD-L1 staining in three tumors and high-intensity PD-L1 staining in one tumor; also, a subset of the IBC tumors was associated with high CD8+/PD-L1+ lymphocyte infiltration.
In the present study, we retrospectively evaluated the prevalence of PD-L1 protein expression in a cohort of IBC tumors with a long follow-up duration. The PD-L1 expression was detected using IHC staining, which has been routinely used for patients with NSCLC and melanoma. We also assessed the association of PD-L1 expression of the IBCs with clinicopathologic parameters and long-term clinical outcomes.
Materials and Methods
Patients
This study included patients with primary IBC who were treated at The University of Texas MD Anderson Cancer Center from September 1994 through August 2004 with available tumor tissue for analysis and clinical follow-up information. Diagnosis; preoperative and postoperative treatments; biomarker studies encompassing estrogen receptor (ER), progesterone receptor (PR), and HER2 status; and tissue microarray (TMA) construction have been previously reported by our group for these patients.41 Three cores (each 1.0 mm in dimension) for each tumor were used for this study. TMA was built up using post-neoadjuvant resected residual tumors because many pretreated core needle biopsy samples (mostly obtained at local hospitals) were not available. Patients with pathologic complete response were not included. A total of 68 patients and their tumor tissue were analyzed in this study. This study was approved by the institutional review board.
PD-L1 Expression
Immunohistochemical staining for PD-L1 was performed on 4-µm-thick paraffin sections of TMA slides. Each tumor had three cores obtained from different areas of the tumor. Antigen retrieval was conducted by steaming the slides in 10 mmol/L citrate buffer (pH 6.0) for 25 minutes. The sections were incubated with the primary monoclonal rabbit anti–PD-L1 antibody, clone 28-8 (pharmDX; Dako, Carpinteria, CA) with Autostainer Link 48 (Dako), followed by the EnVision FLEX visualization system (Dako) per the manufacturer’s instructions. The combination of this PD-L1 clone and detection system was approved by US Food and Drug Administration (FDA) for selecting patients with NSCLC and melanoma for anti–PD-L1 therapy (ie, nivolumab).42 The tissues were then counterstained with Mayer’s hematoxylin solution and evaluated under a light-field microscope.
The staining results were evaluated with known positive and negative tissue controls. Percentage of membranous staining in viable invasive tumor cells was enumerated and recorded for each case. Interpretation of IHC staining was performed independently by three pathologists (J.H., J.Z., and Y.G.). Discrepancies among the three pathologists were resolved by discussion at a multihead microscope until a consensus was reached. Positive PD-L1 expression was defined as when more than 1% of viable invasive tumor cells showed partial or complete membranous staining at any intensity, according to previous studies.9,12,34 Staining intensity for positive cases was scored as weak, moderate, or strong. The correlations of PD-L1 expression with clinicopathologic parameters and survival data were evaluated.
Statistical Analyses
Fisher exact test was used to evaluate associations between PD-L1 expression and clinicopathologic variables. OS was calculated from the date of initial pathologic diagnosis of the primary tumor to the date of death from any cause or the last follow-up date. Of note, OS and disease-specific survival were highly correlated (data not shown); therefore, only OS data are presented. OS was estimated by the Kaplan-Meier method, and distributions were compared using the log-rank test. Cox proportional hazards regression was used to assess the association between OS and PD-L1 expression and clinicopathologic factors.
Univariate Cox proportional hazards and Firth penalized (for covariates with zero deaths) regression models were used to test the associations between several potential prognostic factors and OS. From these models, hazard ratios (HRs) for each potential prognostic factor and corresponding 95% confidence intervals (CIs) were estimated. All potential prognostic factors with P values of less than .10 in the univariate analysis were then included in multivariate Cox models. All analyses were two-sided, and P values of .05 or less were considered statistically significant. Statistical analyses were carried out using R version 3.3.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
The clinicopathologic characteristics of the patients studied are summarized in Table 1. Of the 68 patients with IBC included in this study, 52 (76.5%) were white, 12 (17.6%) were Hispanic, and four (5.9%) were of other races/ethnicities. Patient age at the time of the initial diagnosis ranged from 23 to 75 years (median, 48 years). Fifty-two patients had stage IIIb disease, 10 had stage IIIc disease, and six had stage IV disease. Sixty patients received chemotherapy, and 21 patients received hormonal treatment.
Table 1.
Associations Between PD-L1 (Clone 28-8) and Clinicopathologic Variables
| Variable | PD-L1 Negative, No. (%) | PD-L1 Positive, No. (%) | P Value |
|---|---|---|---|
| Age | 1.000 | ||
| <45 years | 15 (34.9) | 8 (32.0) | |
| ≥45 years | 28 (65.1) | 17 (68.0) | |
| Race/ethnicity | 1.000 | ||
| Hispanic | 8 (18.6) | 4 (16.0) | |
| Other | 3 (7.0) | 1 (4.0) | |
| White | 32 (74.4) | 20 (80.0) | |
| Lymph node status | 1.000 | ||
| Negative | 4 (9.8) | 2 (8.3) | |
| Positive | 37 (90.2) | 22 (91.7) | |
| Histologic type | .549 | ||
| Ductal | 37 (86.0) | 24 (96.0) | |
| Lobular | 4 (9.3) | 1 (4.0) | |
| Other | 2 (4.7) | 0 (0.0) | |
| Lymphovascular invasion | 1.000 | ||
| No | 6 (14.6) | 3 (13.0) | |
| Yes | 35 (85.4) | 20 (87.0) | |
| Tumor grade | 1.000 | ||
| High | 34 (79.1) | 20 (80.0) | |
| Intermediate | 7 (16.3) | 4 (16.0) | |
| Low | 2 (4.7) | 1 (4.0) | |
| Estrogen receptor status | 1.000 | ||
| Negative | 27 (64.3) | 16 (64.0) | |
| Positive | 15 (35.7) | 9 (36.0) | |
| Progesterone receptor status | .787 | ||
| Negative | 28 (66.7) | 18 (72.0) | |
| Positive | 14 (33.3) | 7 (28.0) | |
| HER2 status | .453 | ||
| Negative | 22 (52.4) | 15 (62.5) | |
| Positive | 20 (47.6) | 9 (37.5) | |
| Triple-negative status | .276 | ||
| Yes | 10 (24.4) | 9 (37.5) | |
| No | 31 (75.6) | 15 (62.5) | |
HER2, human epidermal growth factor receptor 2; PD-L1, programmed death ligand 1.
Lymph node involvement was found in 59 (90.8%) of 65 patients with available data. Histologically, 61 (89.7%) of 68 tumors were ductal, 54 (79.4%) of 68 were high tumor grade, and 55 (85.9%) of 64 showed lymphovascular invasion. ER status was positive in 24 (35.8%) of 67 tumors, PR status was positive in 21 (31.3%) of 67, HER2 status was positive in 29 (43.9%) of 66, and triple-negative status was found in 19 (29.2%) of 65.
One patient had the same date recorded for diagnosis and last follow-up and hence was excluded from survival analysis. Of the remaining 67 patients, the median follow-up time was 3.75 years (range, 0.29-17.54 years). The median OS time was 3.78 years (95% CI, 2.45-10.17 years). The 5-year OS rate was 43.4% and the 10-year OS rate was 36.4%. Forty-four deaths had occurred among these patients by the time of analysis.
Correlation of PD-L1 Expression With Clinicopathologic Parameters and Outcomes
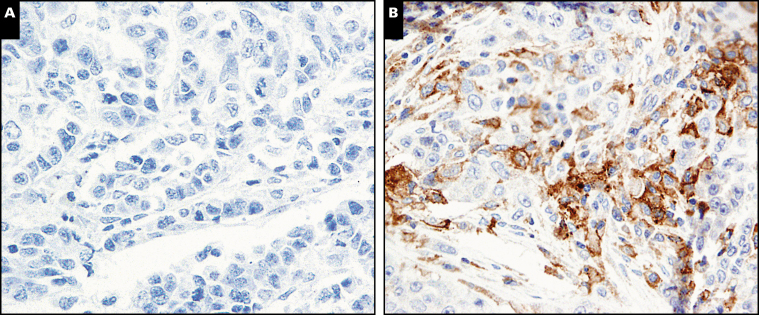
Positive PD-L1 expression in tumor cells was found in 25 (36.8%) of 68 IBC tumors, with a generally low-level staining intensity and heterogeneous distribution Image 1. Only four of the 25 positive cases showed strong intensity, and the rest showed low to intermediate intensity. Heterogeneous staining was observed within individual cores of TMA in some cases and among cores in other cases, with only five of the positive cases showing staining in more than 50% of tumor cells. PD-L1 expression was not statistically significantly associated with any of the clinicopathologic variables, including histologic type; tumor grade; lymphovascular invasion; lymph node status; ER, PR, and HER2 status; and triple-negative status (Table 1).
Image 1.
An inflammatory breast cancer (IBC) sample with negative programmed death ligand 1 (PD-L1) expression (A) and another IBC sample showing PD-L1 expression in approximately 40% of tumor cells (B) (×400).
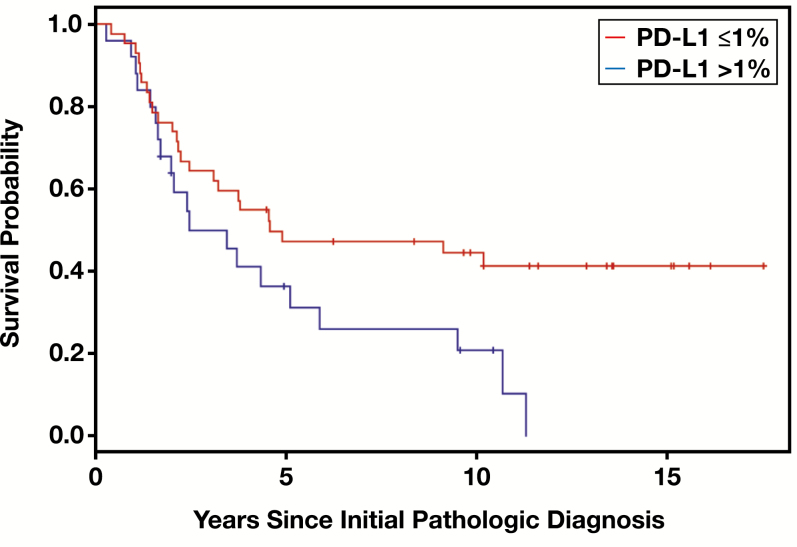
In univariate analysis, worse OS was significantly associated with positive PD-L1 expression (P = .040), negative ER status (P = .008), and triple-negative status (P = .048) Table 2. The 5-year OS rate was 36.4% for patients with PD-L1–positive tumors and 47.3% for those with PD-L1–negative tumors Figure 1. In multivariate analyses, PD-L1 status remained a statistically independent predictor of OS in a Cox model including race/ethnicity and ER status (HR, 1.90; 95% CI, 1.03-3.50; P = .042); similar yet slightly attenuated results were observed in a separate Cox model including race/ethnicity and triple-negative status (HR, 1.76; 95% CI, 0.95-3.25; P = .078). Of note, because of their strong association, ER and triple-negative status could not both be included in a significant multivariate model.
Table 2.
Univariate Cox Analysis of Prognostic Factors for Overall Survival in Breast Cancers
| Prognostic Factor | No. | HR (95% CI) | P Valuea |
|---|---|---|---|
| Age | 67 | .780 | |
| <45 years | 23 | Reference | |
| ≥45 years | 44 | 1.10 (0.57-2.10) | |
| Race/ethnicity | 67 | .064 | |
| Hispanic | 12 | Reference | |
| Other | 4 | 0.12 (0.00-0.99) | |
| White | 51 | 1.10 (0.53-2.62) | |
| Lymph node status | 64 | .558 | |
| Negative | 6 | Reference | |
| Positive | 58 | 0.72 (0.26-2.04) | |
| Histologic type | 67 | .728 | |
| Ductal | 61 | Reference | |
| Lobular | 4 | 0.60 (0.14-2.48) | |
| Others | 2 | 0.78 (0.11-5.71) | |
| Lymphovascular invasion | 63 | .166 | |
| No | 9 | Reference | |
| Yes | 54 | 1.95 (0.69-5.46) | |
| Tumor grade | 67 | .158 | |
| High | 54 | Reference | |
| Intermediate | 10 | 0.41 (0.15-1.16) | |
| Low | 3 | 0.68 (0.16-2.81) | |
| Estrogen receptor status | 66 | .008 | |
| Negative | 43 | Reference | |
| Positive | 23 | 0.41 (0.21-0.83) | |
| Progesterone receptor status | 66 | .226 | |
| Negative | 46 | Reference | |
| Positive | 20 | 0.66 (0.33-1.32) | |
| HER2 status | 65 | .276 | |
| Negative | 36 | Reference | |
| Positive | 29 | 0.71 (0.39-1.32) | |
| Triple-negative status | 64 | .048 | |
| Yes | 19 | Reference | |
| No | 45 | 0.51 (0.27-0.97) | |
| PD-L1 staining | 67 | .040 | |
| ≤1% | 42 | Reference | |
| >1% | 25 | 1.90 (1.04-3.47) |
CI, confidence interval; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; PD-L1, programmed death ligand 1.
a P values are based on the likelihood ratio test.
Figure 1.
Kaplan-Meier plots of overall survival for patients with inflammatory breast cancer by programmed death ligand 1 (PD-L1) status. The P value from the log-rank test was .040. Positive PD-L1 expression was associated with worse overall survival.
Discussion
Recent clinical trials have demonstrated that anti–PD-L1 therapy leads to an objective, substantive, and durable response in patients with various advanced malignancies, and positive PD-L1 expression predicts better response to this therapy in most studies. At the moment, IHC staining for PD-L1 is the best predictive biomarker to identify patients who are most likely to respond to anti–PD-L1 therapy. For patients with IBC, obtaining information of PD-L1 expression in their tumors and its clinicopathologic implication is the first step before considering potential application of anti–PD-L1 therapy. In this study, we determined PD-L1 expression in a cohort of IBC tumors using an antibody clone and IHC detection system that has been approved by the FDA for NSCLC.
Our study showed that PD-L1 was expressed in 36.8% of IBC tumors and that worse OS was significantly associated with positive PD-L1 expression, negative ER status, and triple-negative status. We also demonstrated that positive PD-L1 expression was associated with unfavorable OS in univariate and multivariate analyses. However, we did not find statistically significant associations between PD-L1 expression and any of the clinicopathologic variables examined. The PD-L1 expression rate in our study is similar to what has been reported by two IBC study groups. Bertucci et al39 used DNA microarray to evaluate PD-L1 mRNA and observed PD-L1 overexpression in 38% of IBC samples and 28% of non-IBC samples. In addition, PD-L1 mRNA overexpression in IBC was reportedly associated with ER-negative status, basal and HER2-enriched aggressive subtypes, and better pathologic response to chemotherapy but was not associated with metastasis-free survival and overall specific survivals. Hamm et al40 reported that four (33.3%) of 12 IBC tumors expressed PD-L1 and, as in our findings, most positive cases had low staining intensity by IHC.
The evaluation of PD-L1 expression is challenging owing to the lack of a standardized and reproducible staining method and interpretation protocol. To date, most studies addressing PD-L1 expression in breast cancer have used IHC staining on formalin-fixed, paraffin-embedded tissue with variability in antibody clones, scoring methods, and cutoffs to define positive expression and reported prevalence varying considerably, from 1.7% to 80% Table 3.14-16,18-21,23-28 The lack of consensus standards in PD-L1 detection makes the reliability of these study results a bit skeptical.
Table 3.
Summarized Key Studies Showing PD-L1 Expression in Breast Carcinoma Cells Assessed Using Immunohistochemistry Staining and Its Clinicopathologic Relevance
| First Author (Year) | No. of Cases | Antibody Clone | Cutoff for PD-L1 Positivity (Cell Compartment Evaluated) | PD-L1–Positive Rate | Clinicopathologic Variables Significantly Associated With Positive PD-L1 Expression | Survival Variables Associated With PD-L1 Positivity |
|---|---|---|---|---|---|---|
| Ghebeh (2006)27 | 44 breast cancer samples (frozen section) | MIH1 | Any positive cells (cell membrane and/or cytoplasm) | 34.0% | ER–, PR– | NA |
| Mittendorf (2014)28 | 120 TNBCs (TMA) | 5H1 | >5% (cell membrane) | 19.0% | NA | NA |
| Muenst (2014)14 | 650 breast cancer samples (TMA) | Rabbit polyclonal antibody | H-score ≥100 (cell membrane and/or cytoplasm) | 23.4% | Higher grade and Ki-67 index, larger size, positive nodal status, ER– | Shorter OS in univariate and multivariate analysis |
| Qin (2015)16 | 870 breast cancer samples (TMA) | Rabbit polyclonal antibody | >5% (cell membrane and/or cytoplasm) | 21.7% (55.9% in TNBC) | Larger size, higher grade, LVI, ER–, PR– | Shorter DFS, OS |
| Ali (2015)15 | 3,916 breast cancer samples (TMA) | Rabbit polyclonal antibody | >1% (not specified) | 1.7% | Basal-like subtype | Better DFS (P = .08) in ER– tumors |
| Guo (2016)23 | 183 TNBCs (TMA) | SP142 | Not specified (cell membrane and/or cytoplasm) | 8.7% | Higher grade | Not associated with survival |
| Cimino-Mathews (2016)21 | 45 breast cancer samples (whole sections) | 5H1 | ≥5% (cell membrane) | 21.0% | Higher grade | Less likely to have distant metastasis |
| Beckers (2016)20 | 161 TNBCs (TMA) | E1L3N | ≥1% and ≥5% (cell membrane) | 64.0% and 60.0%, respectively | Not associated with any variables | Not associated with survival |
| ≥1% and ≥5% (cell cytoplasm) | 80.0% and 77.8%, respectively | Not associated with any variables | Lower risk of cancer- specific death | |||
| Bae (2016)18 | 465 breast cancer samples (TMA) | E1L3N | H-score ≥100 (cell membrane and/or cytoplasm) | 13.5% | Higher grade and Ki-67 index, negative nodal status, ER–, PR–, HER2+ | Longer DFS and OS in univariate analysis |
| Li (2016)24 | 136 TNBCs (whole sections) | E1L3N | Any positive cells (cell membrane) | 21.0% | NA | Not associated with DFS |
| Baptista (2016)19 | 192 breast cancer samples (TMA) | Rabbit polyclonal antibody | Allred score >0 (cell membrane and/or cytoplasm) | 56.6% | Larger size, positive nodal status, ER–, distant recurrence | Better OS |
| Li (2016)25 | 501 breast cancer samples (whole sections) | ab58810 | H-score ≥100 (cell membrane and/or cytoplasm) | 46.1% | Higher grade, aggressive intrinsic subtype, ER–, PR– | Shorter OS |
| Mori (2017)43 | 248 TNBCs (whole sections) | E1L3N | ≥1% (cell membrane) | 41.5% | Higher grade, and Ki-67 index | Not associated with prognosis |
DFS, disease-free survival; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; LVI, lymphovascular invasion; NA, not available; OS, overall survival; PD-L1, programmed death ligand 1; PR, progesterone receptor; TMA, tissue microarray; TNBC, triple-negative breast cancer.
In addition to the inconsistent expression rate of PD-L1 by IHC found in breast cancer, the prognostic value of PD-L1 as to whether PD-L1 expression is a favorable or adverse prognostic variable in breast cancer has been contradictory in the literature. In fact, similar conflicting results are seen not only in breast cancer research but also in studies of other cancer types. Given the immunosuppressive role of PD-L1 in mediating an immune evasion mechanism, PD-L1 expression may plausibly be associated with a poor prognosis. PD-L1 has been shown to be an unfavorable predictor in NSCLC,44,45 melanoma,46-48 renal cell carcinoma,49-51 hepatocellular carcinoma,52 pancreatic carcinoma,53 esophageal carcinoma,54 gastric carcinoma,55 colorectal carcinoma,56 and ovarian carcinoma.57 A meta-analysis of 3,107 patients with various solid tumors reported a similar association.58 However, the opposite findings are not uncommon, and the association of PD-L1 expressions in tumor cells with better outcomes has been reported in NSCLC,59 melanoma,12 Merkel cell carcinoma,60 and colorectal cancer.61
In breast cancer, the frequency of PD-L1 expression (as measured by IHC) in tumor cells and the association of this expression with clinicopathologic variables and prognostic effect in 13 key studies are summarized in Table 3. Consistent with our findings, several studies showed an adverse prognostic effect of PD-L1 expression (Table 3).14,16,25,32 Muenst et al14 found that PD-L1 expression was significantly associated with high tumor grade and Ki-67 index, large tumor size, positive nodal status, and negative ER status, and positive PD-L1 was an independent negative prognostic factor for OS. Similar findings were reported in two other studies by Li et al25 and Qin et al.16 Soliman et al32 also examined 61 breast cancer specimens using IHC and found an association between PD-L1 protein expression and positive lymph node status. These findings suggest that PD-L1 expression in tumor cells may facilitate activation of the PD-1/PD-L1 pathway and allow the tumor cells to evade antitumor immune surveillance and consequently proliferate and spread more rapidly. Notably, most of these studies reported a significant association between positive PD-L1 expression in tumor cells and unfavorable clinicopathologic features (Table 3),14-16,18,19,21,23,25,27 suggesting that PD-L1 may be a high-risk factor in patients with breast cancer. Interestingly, despite its association with poor clinicopathologic features, including distant recurrence in one study,19 PD-L1 expression in tumor cells was paradoxically associated with improved survival in some of these studies (Table 3).15,18,19,21 The biologic mechanism of such dilemma is not yet understood.
Beckers et al20 examined PD-L1 expression in 161 TNBC samples and reported that cytoplasmic PD-L1 staining, but not membranous staining, was significantly associated with a lower risk of cancer-specific death. Li et al24 reported a significant association between disease-free survival and positive PD-L1 expression in stromal cells but not in tumor cells. A recent study reported that PD-L1 expression by tumor cells was not associated with recurrence-free survival or OS; however, the combination of PD-L1 expression and decreased tumor-infiltrating lymphocytes was associated with a poor prognosis in 248 TNBC samples.43
The variation in the prevalence and clinical implications of PD-L1 expression could be attributed, at least in part, to differences in cohort size, sample type (eg, tissue microarray vs whole section or frozen tissue vs formalin-fixed, paraffin-embedded tissue), IHC methods, antibody clones, scoring methods (eg, H-score vs percentage of positive cells), cutoff values, and composition of cancer subtypes in the study population (eg, basal or triple-negative type vs all types).26,30,31
Because of the concerns regarding the reliability of IHC staining for PD-L1, two previous studies used mRNA-based tests.17,29 Schalper et al,29 using in situ mRNA hybridization, reported PD-L1 mRNA expression in 60% of 636 breast tumors in TMAs, and positive expression was significantly associated with longer recurrence-free survival. Sabatier et al17 reported that PD-L1 gene expression based on gene microarrays was upregulated in 20% of all clinical samples and 38% of basal cancers. The high expression was associated with larger tumor size, higher grade, negative ER and PR status, positive HER2 and Ki-67 status, and basal and HER2-enriched subtypes. However, upon survival analysis, PD-L1 upregulation was not associated with survival in the whole population but was associated with better metastasis-free and overall specific survivals in basal cancers.
Owing to its technical feasibility, IHC remains the most commonly used method to detect PD-L1 expression. In this study with IBC samples, we adopted the FDA-approved IHC technique, antibody clone, and scoring strategy that are currently used for NSCLC and melanoma with the hope that the result would be more reliable. Our study is important because, to our knowledge, it is the first large study of PD-L1 expression in IBC (as measured by IHC) with a long duration of clinical follow-up information. This study demonstrated PD-L1 expression in more than one-third of IBCs examined, indicating that these tumors are potential candidates for innovative PD-L1–targeting agents, especially for patients whose tumor progressed on conventional therapies. With the encouraging results for anti–PD-L1 monoclonal antibodies in the ongoing clinical trials of breast cancer,35,36,38,62 it is reasonable to believe that PD-L1 inhibitors could be implemented in patients with IBC in the future.
Our study has several limitations. First, all the patients with IBC required neoadjuvant chemotherapy, and a subset of pretreated core needle biopsy samples that were obtained at local hospitals was unavailable for the PD-L1 study. Thus, post-neoadjuvant resected tumors that were obtained at our institution were used. Second, because of rarity of IBC tumors, the study series comprised a modest sample size of patients with IBC, although this cohort remained the largest series compared with those reported in the literature. Third, PD-L1 IHC expression was assessed on TMA, as in many other such studies (Table 2),14-16,18-20,23,28 and small tumor pieces in TMA may lead to false-negative results due to intratumoral heterogeneity of PD-L1 expression.34,63 However, given the cutoff of PD-L1 positivity being 1%, we believe that possibility of false-negative results should be quite low. On the other hand, to minimize this potential drawback, we used multiple cores from each tumor. Last, TMA cores contain largely tumor cells with minimal or no stromal component and thus are not suitable for evaluating PD-L1 expression in the tumor microenvironment. The expression of PD-L1 in the tumor microenvironment, especially in tumor-infiltrating lymphocytes, was reportedly associated with clinical outcomes and might be of predictive value to immune checkpoint inhibitors.14,20,23,24,26 Using whole-tissue sections would overcome the shortcomings associated with TMA.
In a few studies, clinical responses to PD-L1 blockade therapy were observed in patients with PD-L1–negative tumors,12,64 indicating that clinical benefit from inhibition of PD-L1 may extend beyond the PD-L1–positive tumor population. Currently, however, positive PD-L1 expression seems to be the best predictor of response to anti–PD-L1 therapy.
In conclusion, our study provides information of PD-L1 expression in IBC tumors and its prognostic relevance. Further studies, preferably prospective, consisting of larger cohorts with whole-tissue sections, are required to further delineate the biologic significance of PD-L1 in IBC tumor cells and stromal components, as well as their prognostic implication and predictive value in relation to anti–PD-L1 therapies.
Acknowledgment:
We thank Sarah Bronson in the Department of Scientific Publications at The University of Texas MD Anderson Cancer Center for editing this manuscript.
References
- 1. Hance KW, Anderson WF, Devesa SS et al. . Trends in inflammatory breast carcinoma incidence and survival: the surveillance, epidemiology, and end results program at the national cancer institute. J Natl Cancer Inst. 2005;97:966-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cristofanilli M, Valero V, Buzdar AU et al. . Inflammatory breast cancer (IBC) and patterns of recurrence: understanding the biology of a unique disease. Cancer. 2007;110:1436-1444. [DOI] [PubMed] [Google Scholar]
- 3. Blank C, Gajewski TF, Mackensen A. Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother. 2005;54:307-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Iwai Y, Ishida M, Tanaka Y et al. . Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99:12293-12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reiss KA, Forde PM, Brahmer JR. Harnessing the power of the immune system via blockade of PD-1 and PD-L1: a promising new anticancer strategy. Immunotherapy. 2014;6:459-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lipson EJ, Forde PM, Hammers HJ et al. . Antagonists of PD-1 and PD-L1 in cancer treatment. Semin Oncol. 2015;42:587-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. 2015;14:847-856. [DOI] [PubMed] [Google Scholar]
- 8. Powles T, Eder JP, Fine GD et al. . MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558-562. [DOI] [PubMed] [Google Scholar]
- 9. Topalian SL, Hodi FS, Brahmer JR et al. . Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brahmer JR, Tykodi SS, Chow LQ et al. . Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Herbst RS, Soria JC, Kowanetz M et al. . Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taube JM, Klein A, Brahmer JR et al. . Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064-5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tumeh PC, Harview CL, Yearley JH et al. . PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Muenst S, Schaerli AR, Gao F et al. . Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2014;146:15-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ali HR, Glont SE, Blows FM et al. . PD-L1 protein expression in breast cancer is rare, enriched in basal-like tumours and associated with infiltrating lymphocytes. Ann Oncol. 2015;26:1488-1493. [DOI] [PubMed] [Google Scholar]
- 16. Qin T, Zeng YD, Qin G et al. . High PD-L1 expression was associated with poor prognosis in 870 Chinese patients with breast cancer. Oncotarget. 2015;6:33972-33981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sabatier R, Finetti P, Mamessier E et al. . Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget. 2015;6:5449-5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bae SB, Cho HD, Oh MH et al. . Expression of programmed death receptor ligand 1 with high tumor-infiltrating lymphocytes is associated with better prognosis in breast cancer. J Breast Cancer. 2016;19:242-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baptista MZ, Sarian LO, Derchain SF et al. . Prognostic significance of PD-L1 and PD-L2 in breast cancer. Hum Pathol. 2016;47:78-84. [DOI] [PubMed] [Google Scholar]
- 20. Beckers RK, Selinger CI, Vilain R et al. . Programmed death ligand 1 expression in triple-negative breast cancer is associated with tumour-infiltrating lymphocytes and improved outcome. Histopathology. 2016;69:25-34. [DOI] [PubMed] [Google Scholar]
- 21. Cimino-Mathews A, Thompson E, Taube JM et al. . PD-L1 (B7-H1) expression and the immune tumor microenvironment in primary and metastatic breast carcinomas. Hum Pathol. 2016;47:52-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. García-Teijido P, Cabal ML, Fernández IP et al. . Tumor-infiltrating lymphocytes in triple negative breast cancer: the future of immune targeting. Clin Med Insights Oncol. 2016;10:31-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guo L, Li W, Zhu X et al. . PD-L1 expression and CD274 gene alteration in triple-negative breast cancer: implication for prognostic biomarker. Springerplus. 2016;5:805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li X, Wetherilt CS, Krishnamurti U et al. . Stromal PD-L1 expression is associated with better disease-free survival in triple-negative breast cancer. Am J Clin Pathol. 2016;146:496-502. [DOI] [PubMed] [Google Scholar]
- 25. Li Z, Dong P, Ren M et al. . PD-L1 expression is associated with tumor FOXP3(+) regulatory T-cell infiltration of breast cancer and poor prognosis of patient. J Cancer. 2016;7:784-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sun WY, Lee YK, Koo JS. Expression of PD-L1 in triple-negative breast cancer based on different immunohistochemical antibodies. J Transl Med. 2016;14:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ghebeh H, Mohammed S, Al-Omair A et al. . The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia. 2006;8:190-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mittendorf EA, Philips AV, Meric-Bernstam F et al. . PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2:361-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schalper KA, Velcheti V, Carvajal D et al. . In situ tumor PD-L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas. Clin Cancer Res. 2014;20:2773-2782. [DOI] [PubMed] [Google Scholar]
- 30. Rimm D, Schalper K, Pusztai L. Unvalidated antibodies and misleading results. Breast Cancer Res Treat. 2014;147:457-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Scognamiglio G, De Chiara A, Di Bonito M et al. . Variability in immunohistochemical detection of programmed death ligand 1 (PD-L1) in cancer tissue types. Int J Mol Sci. 2016;17:E790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Soliman H, Khalil F, Antonia S. PD-L1 expression is increased in a subset of basal type breast cancer cells. PLoS One. 2014;9:e88557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gatalica Z, Snyder C, Maney T et al. . Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type. Cancer Epidemiol Biomarkers Prev. 2014;23:2965-2970. [DOI] [PubMed] [Google Scholar]
- 34. Dill EA, Gru AA, Atkins KA et al. . PD-L1 expression and intratumoral heterogeneity across breast cancer subtypes and stages: an assessment of 245 primary and 40 metastatic tumors. Am J Surg Pathol. 2017;41:334-342. [DOI] [PubMed] [Google Scholar]
- 35. Nanda R, Chow LQ, Dees EC et al. . Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J Clin Oncol. 2016;34:2460-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Emens LA, Braiteh FS, Cassier P et al. . Inhibition of PD-L1 by MPDL3280A leads to clinical activity in patients with metastatic triple-negative breast cancer (TNBC) [abstract]. In: Proceedings of the 106th Annual Meeting of the American Association for Cancer Research; 2015 Apr 18-22; Philadelphia, PA Philadelphia (PA): AACR. Cancer Res; 2015;75(suppl):Abstract 2859. [Google Scholar]
- 37. Bertucci F, Finetti P, Birnbaum D et al. . The PD1/PDl1 axis, a promising therapeutic target in aggressive breast cancers. Oncoimmunology. 2016;5:e1085148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Adams S, Diamond J, Hamilton E et al. . Safety and clinical activity of atezolizumab (anti-PDL1) in combination with nab-paclitaxel in patients with metastatic triple-negative breast cancer. In: Proceedings of the 38th Annual CTRC-AACR San Antonio Breast Cancer Symposium: December 8-12, 2015; San Antonio, TXCancer Res. 2016;76(suppl):abstract OT1-01-06. [Google Scholar]
- 39. Bertucci F, Finetti P, Colpaert C et al. . PDl1 expression in inflammatory breast cancer is frequent and predicts for the pathological response to chemotherapy. Oncotarget. 2015;6:13506-13519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hamm CA, Moran D, Rao K et al. . Genomic and immunological tumor profiling identifies targetable pathways and extensive CD8+/PDl1+ immune infiltration in inflammatory breast cancer tumors. Mol Cancer Ther. 2016;15:1746-1756. [DOI] [PubMed] [Google Scholar]
- 41. Gong Y, Huo L, Liu P et al. . Polycomb group protein EZH2 is frequently expressed in inflammatory breast cancer and is predictive of worse clinical outcome. Cancer. 2011;117:5476-5484. [DOI] [PubMed] [Google Scholar]
- 42. Sholl LM, Aisner DL, Allen TC et al. ; Members of Pulmonary Pathology Society Programmed death ligand-1 immunohistochemistry—a new challenge for pathologists: a perspective from members of the Pulmonary Pathology Society. Arch Pathol Lab Med. 2016;140:341-344. [DOI] [PubMed] [Google Scholar]
- 43. Mori H, Kubo M, Yamaguchi R et al. . The combination of PD-L1 expression and decreased tumor-infiltrating lymphocytes is associated with a poor prognosis in triple-negative breast cancer. Oncotarget. 2017;8:15584-15592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Azuma K, Ota K, Kawahara A et al. . Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol. 2014;25:1935-1940. [DOI] [PubMed] [Google Scholar]
- 45. Mu CY, Huang JA, Chen Y et al. . High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol. 2011;28:682-688. [DOI] [PubMed] [Google Scholar]
- 46. Hino R, Kabashima K, Kato Y et al. . Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer. 2010;116:1757-1766. [DOI] [PubMed] [Google Scholar]
- 47. Massi D, Brusa D, Merelli B et al. . PD-L1 marks a subset of melanomas with a shorter overall survival and distinct genetic and morphological characteristics. Ann Oncol. 2014;25:2433-2442. [DOI] [PubMed] [Google Scholar]
- 48. Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013;19:5300-5309. [DOI] [PubMed] [Google Scholar]
- 49. Thompson RH, Dong H, Kwon ED. Implications of B7-H1 expression in clear cell carcinoma of the kidney for prognostication and therapy. Clin Cancer Res. 2007;13:709s-715s. [DOI] [PubMed] [Google Scholar]
- 50. Thompson RH, Gillett MD, Cheville JC et al. . Costimulatory B7-H1 in renal cell carcinoma patients: indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A. 2004;101:17174-17179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shin SJ, Jeon YK, Kim PJ et al. . Clinicopathologic analysis of PD-L1 and PD-L2 expression in renal cell carcinoma: association with oncogenic proteins status. Ann Surg Oncol. 2016;23:694-702. [DOI] [PubMed] [Google Scholar]
- 52. Gao Q, Wang XY, Qiu SJ et al. . Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15:971-979. [DOI] [PubMed] [Google Scholar]
- 53. Nomi T, Sho M, Akahori T et al. . Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13:2151-2157. [DOI] [PubMed] [Google Scholar]
- 54. Ohigashi Y, Sho M, Yamada Y et al. . Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2005;11:2947-2953. [DOI] [PubMed] [Google Scholar]
- 55. Eto S, Yoshikawa K, Nishi M et al. . Programmed cell death protein 1 expression is an independent prognostic factor in gastric cancer after curative resection. Gastric Cancer. 2016;19:466-471. [DOI] [PubMed] [Google Scholar]
- 56. Song M, Chen D, Lu B et al. . PTEN loss increases PD-L1 protein expression and affects the correlation between PD-L1 expression and clinical parameters in colorectal cancer. PLoS One. 2013;8:e65821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hamanishi J, Mandai M, Iwasaki M et al. . Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:3360-3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wu P, Wu D, Li L et al. . PD-L1 and survival in solid tumors: a meta-analysis. PLoS One. 2015;10:e0131403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Velcheti V, Schalper KA, Carvajal DE et al. . Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest. 2014;94:107-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lipson EJ, Vincent JG, Loyo M et al. . PD-L1 expression in the Merkel cell carcinoma microenvironment: association with inflammation, Merkel cell polyomavirus and overall survival. Cancer Immunol Res. 2013;1:54-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Droeser RA, Hirt C, Viehl CT et al. . Clinical impact of programmed cell death ligand 1 expression in colorectal cancer. Eur J Cancer. 2013;49:2233-2242. [DOI] [PubMed] [Google Scholar]
- 62. Hartkopf AD, Taran FA, Wallwiener M et al. . PD-1 and PD-L1 immune checkpoint blockade to treat breast cancer. Breast Care (Basel). 2016;11:385-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Botti G, Scognamiglio G, Cantile M. PD-L1 immunohistochemical detection in tumor cells and tumor microenvironment: main considerations on the use of tissue micro arrays. Int J Mol Sci. 2016;17:E1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56-61. [DOI] [PubMed] [Google Scholar]