Abstract
Cheetahs and certain other felids consistently ejaculate high proportions (≥60%) of malformed spermatozoa, a condition known as teratospermia, which is prevalent in humans. Even seemingly normal spermatozoa from domestic cat teratospermic ejaculates have reduced fertilizing capacity. To understand the role of sperm metabolism in this phenomenon, we conducted a comparative study in the normospermic domestic cat versus the teratospermic cat and cheetah with the general hypothesis that sperm metabolic function is impaired in males producing predominantly pleiomorphic spermatozoa. Washed ejaculates were incubated in chemically defined medium containing glucose and pyruvate. Uptake of glucose and pyruvate and production of lactate were assessed using enzyme-linked fluorescence assays. Spermatozoa from domestic cats and cheetahs exhibited similar metabolic profiles, with minimal glucose metabolism and approximately equimolar rates of pyruvate uptake and lactate production. Compared to normospermic counterparts, pyruvate and lactate metabolism were reduced in teratospermic cat and cheetah ejaculates, even when controlling for sperm motility. Rates of pyruvate and lactate (but not glucose) metabolism were correlated positively with sperm motility, acrosomal integrity, and normal morphology. Collectively, our findings reveal that pyruvate uptake and lactate production are reliable, quantitative indicators of sperm quality in these two felid species and that metabolic function is impaired in teratospermic ejaculates. Furthermore, patterns of substrate utilization are conserved between these species, including the unexpected lack of exogenous glucose metabolism. Because glycolysis is required to support sperm motility and capacitation in certain other mammals (including dogs), the activity of this pathway in felid spermatozoa is a target for future investigation.
Keywords: felid, gamete, gamete biology, glucose, lactate, pyruvate, sperm, spermatozoa, sperm metabolism, sperm motility and transport
Introduction
An interesting trait of certain felid species and genotypes is the production of unusually high proportions of sperm malformations. Species, populations, or individuals that express this condition are called teratospermic [1]. This phenomenon is especially common in species or subspecies that have low levels of gene diversity (cheetah [2–4], Florida panther [5, 6], and Asia lion [7]) and in domestic cats that have been purposefully inbred [8]. Teratospermia (defined here as the production of ≥60% structurally abnormal spermatozoa) also is common among men. A recent meta-analysis of semen characteristics by the World Health Organization revealed that >95% of men can be classified as teratospermic under this definition [9]. Our laboratory has used certain felid species and genetic lineages to better understand the impact and etiology of teratospermia. Various studies have revealed that spermatozoa from teratospermic ejaculates demonstrate delayed capacitation [10], compromised acrosomal function [10], disrupted protein tyrosine phosphorylation [11, 12], increased osmotic sensitivity [13, 14], reduced zona penetration ability [15], and increased sensitivity to cooling [16] and cryopreservation [17]. These mechanisms no doubt contribute to the reduced fertilizing ability of teratospermic ejaculates in vitro, even after processing to isolate structurally normal spermatozoa for insemination [15].
Some of these physiological impairments (e.g., tyrosine phosphorylation) could be related to a diminished capacity for energy production in malformed spermatozoa, but there is currently no knowledge of gamete metabolism in felids. Studies of mammalian sperm energy production, although conducted since the 1940s, have generally been confined to humans and fewer than 10 domesticated species [18]. Yet as Storey detailed in a recent review [18], there are considerable differences in metabolic function of male gametes, even within this small group of species. It is known that spermatozoa are capable of generating energy in the form of ATP through glycolysis and/or oxidative phosphorylation. However, the relative importance of each pathway to sperm functions, such as motility and capacitation, varies among species [18–25]. Oxidative phosphorylation is 18 times more efficient than anaerobic glycolysis and provides a significant proportion of the ATP supply in spermatozoa of most species [21]. The notable exception is the human, whose sperm appear to rely entirely on glycolysis for motility and hyperactivation [26]. Despite the efficiency of oxidative metabolism, its ability to fulfill energy demands in the distal flagellum is questionable [23, 24, 27], as mitochondria are confined to the sperm midpiece. Therefore, glycolysis may be an important supplemental source of ATP to fuel sperm motility, and glycolytic enzymes have been localized along the fibrous sheath of the flagellum in the boar, bull, rat, stallion, human, and mouse [28, 29]. Sperm production of lactate (presumably by glycolysis) is correlated positively with motility, normal morphology, acrosomal integrity, and osmotic resistance in the boar and donkey [30, 31]. One of the latter studies has suggested that these relationships are more than casual in that litter size in pigs is enhanced after artificial insemination using sperm producing high lactate concentrations [30].
In contrast to livestock species, there is a lack of information on sperm metabolism in carnivores. Gamete metabolism has been fairly well studied in the domestic dog [19, 32–39], but, to our knowledge, these pathways have not been investigated in any other carnivore species. The dog apparently is uniquely capable of sperm gluconeogenesis [19], which is surprising given that glucose synthesis requires three times more ATP than is produced by glycolysis [40]. Utilization of this pathway may explain how dog spermatozoa are able to maintain motility and achieve capacitation in a medium without glucose [19, 38]. This is especially interesting as glucose is required for capacitation in the mouse [41] and human [26], but inhibits this process in the bull [42] and guinea pig [43].
Felids are attractive models for studying gamete metabolism. The availability of multiple species within the family Felidae provides opportunities for comparative studies to understand the conservation (or diversification) of physiological processes. Furthermore, the existence of teratospermia in certain species or genetic lineages provides the opportunity to explore linkage between a complex biological phenomenon and potential causative factors. Our aim in this study was to determine the relationship between rates of glycolytic and oxidative sperm metabolism and conventional indices of cellular function (i.e., structural morphology, motility, and acrosomal integrity). The approach was unique because we took advantage of two domestic cat populations that consistently produce differing proportions of pleiomorphic spermatozoa. To increase the robustness of our findings, we conducted a cross-species comparison using the cheetah, a species that is well known to be teratospermic regardless of season or living conditions (free-range versus captive) [2–4]. Our hypotheses were that 1) metabolic rates are useful indicators of sperm quality in felids and 2) metabolic function is compromised in spermatozoa from teratospermic ejaculates compared to normospermic counterparts. We expected that elucidating the pathways of felid sperm energy production not only would provide insight into the physiological basis of teratospermia, but also might yield a reliable, quantitative indicator of ejaculate quality. The latter information has potential applied benefits. For example, identifying metabolic substrate requirements would be highly informative for enhancing the use of certain types of assisted reproductive technologies for genetically managing wild felid species [44] or domestic cat lineages studied as models of human disorders [45].
Materials and Methods
Animals
Electroejaculates were collected from adult domestic cats (ages 1.5–8 yr) that were known to consistently produce either normospermic (≥60% normal sperm per ejaculate, n = 3 males) or teratospermic (<40% normal sperm per ejaculate, n = 3 males) ejaculates. In all, 15 ejaculates were collected from normospermic males (three to eight per individual) and 19 ejaculates from teratospermic males (three to nine per individual). Males were housed individually in 2.7-m3 indoor cages at the Smithsonian Conservation Biology Institute (Front Royal, VA), maintained on a 14L:10D cycle, and provided dry, commercial cat food (Purina Cat Chow; Ralston Purina Co., St. Louis, MO) and water ad libitum.
Electroejaculates (one per male, 22 males) were collected from adult cheetahs (ages 2.5–10 yr) housed at the Cheetah Conservation Fund (CCF; Otjiwarongo, Namibia; n = 18), White Oak Conservation Center (WOCC; Yulee, FL; n = 3), or the Smithsonian's National Zoological Park (NZP; Washington, DC; n = 1). Males at CCF were wild-born and housed as described previously [46]. Males at WOCC were wild-born, housed together (in a group of three) in a 2000-m2 outdoor enclosure, and fed a commercially produced Nebraska Carnivore diet (Central Nebraska Packaging Inc., North Platte, NE). The single male at NZP was captive-born, housed on exhibit with two other males in a 1400-m2 outdoor enclosure, and fed a commercially produced carnivore diet (Carnivore Diet 10; Natural Balance Pet Foods Inc., Pacoima, CA).
Semen Collection and Evaluation
A surgical plane of anesthesia was induced in domestic cats and cheetahs according to protocols determined by institutional veterinarians and similar to those previously used for semen collection in these two species [10, 15, 46]. All animal procedures were approved by NZP's Animal Care and Use Committee (ACUC) and the WOCC ACUC. Methods for semen collection and evaluation were similar to those described in previous studies [10, 15, 46]. A rectal probe of 1 cm (domestic cat) or 1.9 cm (cheetah) in diameter with three longitudinal electrodes and an electrostimulator (P.T. Electronics, Boring, OR) were used to deliver 80 stimuli (at a low voltage of 2–5 V) over a 30-min interval [47]. Ejaculates (n = 56 total) were collected in sterile, prewarmed vials as previously described [4, 47].
The volume of each ejaculate was measured using a pipette, and 3 μl of ejaculate were immediately assessed visually for sperm percentage motility and forward progressive status (FPS or speed of forward progression; scale = 0–5, with a rating of 5 equivalent to most rapid, straightforward progress [47]). A sperm motility index (SMI) was calculated using the formula (percent motility + (FPS × 20) ÷ 2 [48]). A 5-μl sample of raw semen was fixed in 0.3% glutaraldehyde in PBS (pH 7.4, 340 mOsm) for subsequent assessment of sperm morphology [13]. For each sample, 100 spermatozoa were assessed (1000× magnification) and classified as normal or having (in order of precedence) a head, acrosomal, midpiece, flagellar, or other abnormality, as previously described [47]. For all ejaculates, a second 5-μl aliquot of raw semen was fixed in 4% paraformaldehyde to evaluate acrosomal integrity (% IA). Fixed samples were processed using a modified Coomassie Blue G-250 (Fisher Biotech, Springfield, NJ) staining technique, as described earlier [49, 50], and 200 spermatozoa from each sample were evaluated (1000× magnification) and classified as having an intact or nonintact acrosome. Spermatozoa with an intact acrosome exhibited a uniform blue staining pattern overlying the acrosomal region, whereas nonintact cells had clear or patchy staining over this region [50].
Sperm Processing and Metabolic Assessments
Each ejaculate was diluted immediately with an equal volume of a chemically defined, protein-free, modified mouse tubal fluid medium (cMTF) [51] supplemented with 2% polyvinyl alcohol (PVA) [52]. The final cMTF medium (pH 7.45) contained 98.4 Mm NaCl, 4.78 mM KCl, 1.19 mM MgSO4, 1.19 mM KH2PO4, 25 mM NaHCO3, 1.71 mM CaCl2, 1 mM glucose, 1 mM Na-pyruvate, 25 mM 3-(N-morpholino) propanesulfonic acid (MOPS) buffer, and 0.02 mg/ml phenol red. All reagents were purchased from Sigma Aldrich (St. Louis, MO). The cMTF medium was prepared fresh daily from five concentrated stock solutions containing 1) NaHCO3 and phenol red, 2) CaCl2, 3) glucose and pyruvate, 4) MOPS and phenol red, and 5) all remaining reagents. All stock solutions were kept at 4°C and discarded after 2 wk (stocks 1–3) or 3 mo (stocks 4 and 5). PVA was added, and the medium was sterilized through a 0.22-μm syringe filter immediately prior to use. Osmolality of the final working medium (295–341 mOsm) was determined using a vapor pressure osmometer (Wescor, Inc., Logan, UT) and was within 10% of the physiological value of domestic cat semen (323 mOsm [53]).
Diluted ejaculates (maintained at ambient temperature, 19°–24°C) were washed by centrifugation (8 min; 300 × g for domestic cat, 200 × g for cheetah) and resuspended in fresh cMTF at a concentration of 3 × 106 motile sperm per milliliter. Sperm concentration was determined using a Nucleocounter SP-100 (Chemometec, Allerød, Denmark) [54]. Sperm samples were incubated (37°C) in microcentrifuge tubes under oil (200 μl) to prevent evaporation. Based on rates of sperm oxygen consumption in the dog and fox [55], we estimated that dissolved oxygen in cat/cheetah sperm samples would decrease by <1% after 24 h. Because sperm respiration is not limited until 90% of oxygen is depleted from the medium (starting at the atmospheric value) [56], hypoxia due to culture under oil was not of concern. A sample (130 μl) of sperm suspension was taken at 0, 1, 3, 7, and 24 h of incubation, and cells were removed by centrifugation (3 min; 1000 × g) through a CoStar Spin-X 0.22-μm nylon filter tube (Corning Incorporated, Corning, NY). The filter was removed from the tube, and the sperm-free medium was stored at −80°C until analysis. Acrosomal membrane integrity and sperm motility were assessed at 0, 1, 3, 7, and 24 h as described above and are reported as average values over each time interval to facilitate comparison with metabolic rates.
Samples of sperm-free medium were analyzed for glucose, pyruvate, and lactate concentrations using enzyme-linked fluorescence assays [51, 57]. Each assay was linked to the oxidative status of the coenzyme NADP (glucose) or NAD (pyruvate and lactate). The reduced forms of these coenzymes (NADPH and NADH) fluoresce at 445 nm when excited at 340 nm, whereas the oxidized forms do not. For the glucose assay, sperm-free medium (10 μl) was incubated (5 min, 37°C) with an enzyme cocktail (200 μl) containing 0.42 mM dithiothreitol, 3.1 mM MgSO4, 0.42 mM ATP, 1.25 mM NADP, and 0.1 U/ml hexokinase/glucose-6-phosphate dehydrogenase (HK/G6PDH) in 50 mM EPPS buffer (4-(2-hydroxyethyl)-1-piperazine propane-sulfonicacid; pH 8.0). The cocktail was stored in the dark at −80°C for up to 3 mo prior to use. The conversion of glucose to 6-phosphogluconate was carried out as shown in Equation 1. Glucose concentration determined by this assay was directly proportional to NADPH fluorescence.
| \[\begin{array}{*{20}{c}} {{\rm{glucose}} + {\rm{ATP}}\mathop \to \limits^{{\rm{HK}}} {\rm{glucose}} - 6 - {\rm{phosphate}} + {\rm{ADP}}}\\ {{\rm{glucose}} - 6 - {\rm{phosphate}} + {\rm{NAD}}{{\rm{P}}^ + }\mathop \to \limits^{{\rm{G}}6{\rm{PDH}}} 6 - {\rm{phosphoglucoate}} + {\rm{NAD}}{{\rm{P}}^ + } + {{\rm{H}}^ + }} \end{array}\] |
For the pyruvate assay, sperm-free medium was incubated with an enzyme cocktail (as above) containing 0.14 mM NADH and 0.12 U/ml lactate dehydrogenase (LDH) in 50 mM EPPS buffer (pH 8.0). The cocktail was stored in the dark at −80°C for up to 3 mo prior to use. The conversion of pyruvate to lactate was carried out as shown in Equation 2. Pyruvate concentration measured by this assay was inversely proportional to NADH fluorescence.
| \[{\rm{pyruvate}} + {\rm{NAD}}{{\rm{H}}^ + } + {{\rm{H}}^ + }\mathop \to \limits^{{\rm{LDH}}} {\rm{lactate}} + {\rm{NA}}{{\rm{D}}^ + }\] |
For the lactate assay, sperm-free medium (25 μl) was incubated (5 min, 37°C) with an enzyme cocktail (250 μl) containing 1.92 U/ml LDH, 0.2 U/ml glutamate-pyruvate transaminase (GPT), 0.42 mM NAD+, and 100 mM glutamate in 1 M glycine buffer containing 5.6 mM ethylenediaminetetraacetic acid. The buffer was stored at 4°C for up to 1 mo prior to use, and the cocktail was prepared fresh daily using NAD+ and glutamate stock solutions stored at −80°C for up to 3 mo prior to use. This assay is a nontoxic alternative to the LDH/hydrazine assay. The conversion of lactate to alanine was carried out as shown in Equation 3. Lactate concentration in this reaction was directly proportional to NADH fluorescence.
| \[\begin{array}{*{20}{c}} {{\rm{lactate}} + {\rm{NA}}{{\rm{D}}^ + }\mathop \to \limits^{{\rm{LDH}}} {\rm{pyruvate}} + {\rm{NADH}} + {{\rm{H}}^ + }}\\ {{\rm{pyruvate}} + {\rm{glutamate}}\mathop \to \limits^{{\rm{GPT}}} {\rm{alanine}} + {\rm{\alpha }} - {\rm{ketoglutarate}}} \end{array}\] |
Enzymes (LDH, #HK/G6PDH, and GPT) were purchased from Roche Applied Science (Indianapolis, IN). Fluorescence was analyzed using a Spectra Max Gemini XPS fluorescent plate reader (Molecular Devices, Sunnyvale, CA) and SoftMax Pro 5 software (Molecular Devices, Sunnyvale, CA). Metabolic rates were calculated as the change in substrate concentration over time divided by sperm concentration and are reported in nmol/106 sperm/h.
Statistical Analyses
Data were analyzed with Statistical Analysis Software (SAS) version 9.1 (SAS Institute, Cary, NC), and percentage data were arcsine-transformed before evaluation. Differences in ejaculate characteristics and sperm morphology among animal groups (normospermic domestic cat, teratospermic domestic cat, and cheetah) were assessed using SAS General Linear Model Procedures (GLM) [58]. To evaluate changes in SMI, % IA, and metabolic rate over time, data were analyzed using a separate GLM for each animal group [58]. Within each domestic cat group (normospermic and teratospermic), there was no interaction (P > 0.05) between individual and time, as well as no main effect of individual (P > 0.05) on SMI, % IA, or metabolism; thus, these variables were omitted from the final model. Differences in SMI, % IA, and metabolic rate among animal groups were assessed using a separate GLM for each time interval [58]. To determine if variation in sperm motility was responsible for differences in metabolic rates among animal groups, data from all individuals and time points were combined and analyzed using a GLM, with percent motile spermatozoa included as a covariate. When a significant (P < 0.05) F-statistic was measured in any GLM, differences among means were assessed using the Duncan multiple-range test. The Pearson correlation was used to evaluate the relationships between metabolic rate and sperm morphology, SMI, and % IA within and across animal groups. Results were considered significant at P < 0.05 and are reported as least-squares (LS) means ± SEM unless otherwise stated.
Results
Ejaculate and Sperm Characteristics
Semen volume and sperm concentration were similar (P > 0.05) in normospermic and teratospermic domestic cats (Table 1). Cheetah ejaculates were less concentrated (P < 0.05) than those from domestic cats, but due to larger (P < 0.05) seminal volumes, the total number of spermatozoa per ejaculate did not (P > 0.05) differ among the three animal groups (Table 1). The average SMI and percentage of structurally normal spermatozoa were similar (P > 0.05) between teratospermic domestic cats and cheetahs, both of which were less (P < 0.05) than in normospermic cats (Table 1). A bent midpiece encompassing a cytoplasmic droplet was the most prevalent deformity observed in each animal group and constituted ∼45% of all abnormalities (Table 1). This was followed by acrosomal abnormalities and proximal droplets, which were more (P < 0.05) common in the cheetah (19% and 14% of all deformities, respectively) compared to the domestic cat (∼8% and 5%, respectively). A bent flagellum encircling a cytoplasmic droplet was a less frequent (P < 0.05) deformity in the cheetah (3%) than in the domestic cat (∼12%). In both species, spermatids and midpiece bends (without a droplet) constituted <10% of all abnormalities. More than a dozen other deformities were observed rarely (≤5%) in each group but collectively comprised a significant proportion (∼15%) of total anomalies. These malformations were classified as “other” and included macro-/microcephaly, bi-/tricephaly, a misshapen head, residual cytoplasm attached to the head, a bent neck, partial or complete midpiece aplasia, a distal midpiece droplet, a misshapen midpiece, a coiled flagellum with or without a droplet, and a bi-/triflagellate spermatozoon. Images of the more prevalent deformities (i.e., constituting ≥10% of abnormalities in any group) are provided in Supplemental Figure S1 (available online at www.biolreprod.org). These depictions, along with most of the uncommon malformations listed above, also are available in earlier publications [2, 4].
Table 1.
Ejaculate characteristics of domestic cats and cheetahs (LS means ± SEM).
| Parameter | Normospermic cat | Teratospermic cat | Cheetah |
|---|---|---|---|
| No. of males | 3 | 3 | 22 |
| No. of ejaculates | 15 | 19 | 22 |
| Semen volume (ml) | 0.15 ± 0.12a | 0.17 ± 0.11a | 1.98 ± 0.10b |
| Sperm concentration (×106 cells/ml) | 345 ± 41a | 267 ± 37a | 50 ± 34b |
| Spermatozoa per ejaculate (×106) | 57 ± 21 | 48 ± 19 | 93 ± 17 |
| Sperm motility index* | 80 ± 1a | 66 ± 1b | 69 ± 1b |
| Structurally-abnormal spermatozoa (%) | 37 ± 4a | 73 ± 4b | 76 ± 3b |
| Deformity type (% of total abnormalities) | |||
| Abnormal acrosome | 8.8 ± 2.6a | 7.0 ± 2.2a | 19.3 ± 2.1b |
| Bent midpiece with cytoplasmic droplet | 49.2 ± 5.0 | 43.4 ± 4.7 | 39.8 ± 4.7 |
| Bent midpiece without droplet | 6.3 ± 1.2a | 3.4 ± 1.1a,b | 2.1 ± 1.0b |
| Normal midpiece with proximal droplet | 5.0 ± 2.2a | 5.4 ± 2.1a | 13.7 ± 1.9b |
| Bent flagellum with droplet | 12.9 ± 2.1a | 10.3 ± 1.9a | 3.0 ± 1.7b |
| Spermatid | 2.2 ± 2.1a | 9.0 ± 2.0b | 6.8 ± 1.8a,b |
| Other† | 15.7 ± 3.0 | 21.8 ± 2.8 | 15.4 ± 2.5 |
| Intact acrosomes (%) | 92 ± 2a | 84 ± 2b | 92 ± 2a |
Calculated as percent (motility + forward progressive status × 20) ÷ 2.
Macro/micro-cephaly, bi/tri-cephaly, misshapen head, residual cytoplasm attached to head, midpiece aplasia, abnormal midpiece, distal midpiece droplet, coiled flagellum with or without a droplet, biflagellate, and bent neck.
Within rows, values with different superscript letters differ (P < 0.05).
All three animal groups ejaculated high percentages of acrosome-intact spermatozoa (>80% overall; Table 1), although teratospermic cats produced a smaller proportion (P < 0.05) of cells with intact membranes compared to normospermic counterparts or cheetahs.
Sperm Motility, Acrosomal Integrity, and Metabolism over 24 h
Within each time interval, SMI and % IA were similar (P > 0.05) in teratospermic cats and cheetahs, but reduced (P < 0.05) compared to normospermic cats (Fig. 1). Sperm pyruvate uptake from 0–1 h was higher (P < 0.05) in teratospermic cats compared to cheetahs, and rates in normospermic cats were similar (P > 0.05) to both of these groups (Fig. 2A). However, after 1 h, rates were ∼70% lower (P < 0.05) in teratospermic cats and cheetahs compared to normospermic cats. Due to this inconsistency and large standard error values for the 0- to 1-h interval, rates of pyruvate uptake for this time interval were omitted from the Pearson correlations.
Fig. 1.
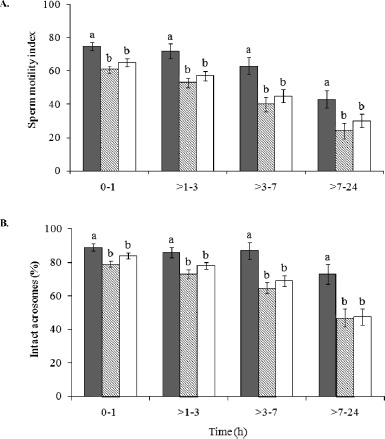
A) SMI and (B) % IA in normospermic domestic cats (solid bars), teratospermic domestic cats (lined bars), and cheetahs (open bars). Among animal groups and within each time point, bars with different lowercase letters differed (P < 0.05). Error bars represent means ± SEM.
Fig. 2.
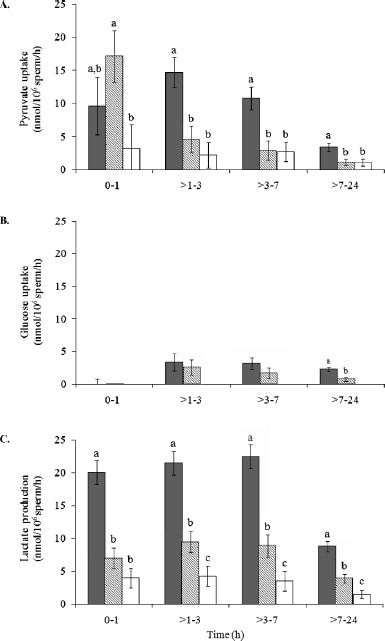
Sperm pyruvate uptake (A), glucose uptake (B), and lactate production (C) in normospermic domestic cats (solid bars), teratospermic domestic cats (lined bars), and cheetahs (open bars). Among animal groups and within each time point, bars with different lowercase letters differed (P < 0.01). Error bars represent means ± SEM.
Exogenous glucose was minimally utilized by domestic cat spermatozoa, and rates of uptake were similar (P > 0.05) overall between normospermic and teratospermic males (Fig. 2B). Extracellular glucose concentration of cheetah sperm medium samples did not change (P > 0.05) from 0–24 h, indicating no metabolism of this substrate by this species. Sperm lactate production occurred at comparatively high rates in all three groups, given the expected ratio (2:1) of lactate production to glucose uptake (Fig. 2C). Compared to normospermic cats, rates of sperm lactate production were ∼60% less (P < 0.01) in teratospermic cats and ∼80% less (P < 0.001) in cheetahs. Extreme variation in stoichiometric ratios (glucose or pyruvate uptake as a proportion of lactate production) was observed within each time interval for all three groups, with most coefficient of variation (CV) values being ≥50% (data not shown). This variation likely was related to minimal (or sometimes zero) changes in metabolic substrate concentration between consecutive time points (even in samples with high percentages of motile spermatozoa). In support of this supposition, normospermic cats demonstrated higher sperm metabolic rates and, overall, less variation in stoichiometric ratios (CV = 12%–392%) compared to the other two groups. To eliminate as much assay “noise” as possible, stoichiometric ratios were recalculated based on the total change in substrate concentration from 0 to 24 h of incubation. Using this method, most (60%–70%) lactate production was attributed to the uptake and reduction of pyruvate, whereas a lesser portion (0%–40%) was credited to the metabolism of exogenous glucose metabolism (Table 2).
Table 2.
Substrate uptake/lactate production ratios in domestic cat and cheetah spermatozoa.
| Parameter | Normospermic cat | Teratospermic cat | Cheetah* |
|---|---|---|---|
| No. of males | 3 | 3 | 22 |
| No. of ejaculates | 15 | 19 | 22 |
| Glucose | 0.2 ± 0.4 | 0.1 ± 0.4 | ND |
| Pyruvate | 0.6 ± 0.2 | 0.7 ± 0.2 | 0.7 ± 0.1 |
ND, not detected.
Relationship Between Metabolic Rates and Sperm Quality
When data from all ejaculates (n = 56) were combined, rates of pyruvate uptake were positively correlated (P < 0.001) to SMI (r = 0.44) and % IA (r = 0.43; Table 3). Because rates of lactate production were correlated positively (P < 0.001) to normal sperm morphology across all ejaculates, as well as with SMI and % IA (r = 0.42–0.50; Table 3), this metric was found to be a more accurate indicator of overall cellular quality than pyruvate uptake. Furthermore, rates of lactate production were correlated positively (P < 0.05) to SMI and % IA within each group (r = 0.34–0.67). Rates of glucose uptake were not (P > 0.05) correlated with SMI or % IA in any group.
Table 3.
Correlation coefficient (r) values for metabolic rate versus sperm quality in domestic cats and cheetahs.*
| Parameter | Overall | Normospermic cat | Teratospermic cat | Cheetah |
|---|---|---|---|---|
| No. of males | 28 | 3 | 3 | 22 |
| No. of ejaculates | 56 | 15 | 19 | 22 |
| Pyruvate uptake (nmol/106sperm per hour) | ||||
| Sperm motility index | 0.44b | 0.44a | 0.50b | NS |
| Intact acrosomes (%) | 0.43b | 0.41a | 0.52b | NS |
| Structurally-normal spermatozoa (% ) | NS | NS | NS | NS |
| Lactate production (nmol/106sperm per hour) | ||||
| Sperm motility index | 0.45b | 0.37a | 0.50b | 0.67b |
| Intact acrosomes (%) | 0.42b | 0.34a | 0.51b | 0.62b |
| Structurally-normal spermatozoa (% ) | 0.50b | NS | NS | NS |
NS, not significant (P > 0.05).
P < 0.05
P < 0.001.
To determine if decreased metabolic rates in spermatozoa of teratospermic cats and cheetahs were an artifact of reduced motility in these cells, data were reanalyzed with sperm motility (% motile and SMI) included as covariates in the GLM. Results were consistent with the previous analysis: sperm pyruvate uptake and subsequent lactate production were decreased (P < 0.05) in teratospermic cats and cheetahs compared to normospermic cats, thereby indicating a direct relationship to the teratospermic condition.
Given that rates of lactate production were correlated positively to normal sperm morphology across all felid ejaculates, we were curious about the metabolic function of cheetah ejaculates containing relatively high proportions (∼40%) of structurally normal spermatozoa. Specifically, do these high-quality cheetah ejaculates demonstrate normal metabolic function when compared to domestic cat counterparts? Conversely, is metabolic function more severely compromised in cheetah ejaculates with very low (∼5%) proportions of structurally normal spermatozoa? To address these questions, cheetah ejaculates from the existing dataset were ranked in order of increasing percentages of structurally normal sperm (% N), and those with the lowest and highest percentage values were selected for comparison of lactate production (n = 5 per group; Fig. 3). The mean % N in each ejaculate group was 7% ± 3% (range 5%–10%) and 42% ± 3% (range 35%–58%), respectively. Domestic cat ejaculates (n = 9 total, 4 males) having % N values within the 35%–58% range (mean 44% ± 2%) were selected from the existing dataset as a control for normal rates of lactate production. Despite the large difference in % N between the two cheetah groups, rates of sperm lactate production were similar (P > 0.05) and were reduced (P < 0.05) compared to the domestic cat control group (Fig. 3). Pyruvate and glucose metabolism was not reassessed, because rates of uptake were not correlated with any sperm quality index in the cheetah (Table 3).
Fig. 3.
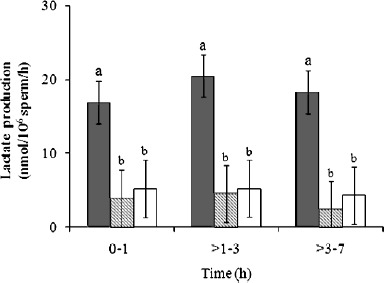
Sperm lactate production in cheetah ejaculates selected for either normal (lined bars) or abnormal (open bars) morphology, compared to a domestic cat control group (closed bars). Among animal groups and within each time point, bars with different lowercase letters differed (P < 0.05). Error bars represent means ± SEM.
Discussion
This was the first study of sperm metabolism in any felid species, and we made four significant discoveries. First, we determined that felid spermatozoa (at least from the two species studied here) did not rely on exogenous glucose as a source of energy. Rather, based on the observed ratios of substrate uptake/production, it appeared likely that these cells generated ATP from the catabolism of one or more unidentified endogenous sources. Secondly, certain cellular mechanisms related to sperm energy production were conserved between the domestic cat and cheetah, indicated by similar patterns of substrate metabolism between the species. Third, metabolic function was impaired in spermatozoa from teratospermic ejaculates, as revealed by relatively low rates of pyruvate uptake and lactate production in males producing high proportions of pleiomorphisms. This observation was consistent with previous reports that linked teratospermia to disruptions in multiple components of sperm function, including several energy-dependent processes [10, 11, 15, 59]. Finally, rates of lactate production were correlated positively to multiple measures of sperm function in both the domestic cat and cheetah. Therefore, this substrate may prove to be a valuable indicator of ejaculate quality.
The lack of glucose uptake by domestic cat and cheetah spermatozoa is unexpected, given that glycolysis is required to support sperm motility [21, 26, 34] and capacitation [19, 26, 60, 61] in the human and domestic dog. This finding perhaps could result from low hexokinase activity in felids compared to other species or, in the case of the cheetah, the complete absence of this enzyme. Reduced hexokinase activity would limit NADPH production, a key component of the glutathione-mediated defense system that protects cellular membranes against lipid peroxidation damage [62]. This is an intriguing possibility, as spermatozoa from teratospermic felids (including the domestic cat and cheetah) are unusually susceptible to membrane damage [13, 16, 46]. However, it also remains possible that felid spermatozoa possess fully functional hexokinase but metabolize glucose at modest rates relative to oxidative substrates. We currently are using the glycolytic inhibitor α-chlorohydrin to more thoroughly understand the role of glycolysis in felid sperm cellular function.
Given the observed lack of glucose uptake by cat and cheetah spermatozoa, we were surprised to discover that these cells consistently produced lactate, which is an end-product of glycolysis [63]. Under our experimental conditions, we consider there to be three possible sources of lactate: 1) endogenous glycogen, 2) endogenous phospholipids, and/or 3) imported pyruvate. These potential mechanisms of lactate production are not mutually exclusive and may also contribute, to varying degrees, in the generation of NADH and ATP (Fig. 4). In each case, lactate formation could occur in cytosol or mitochondria, as sperm-specific lactate dehydrogenase has been found in these compartments [64–67]. Lactate and NADH production also could occur in separate cellular locations, since reducing equivalents can be transferred between the cytosol and mitochondria by the malate-aspartate shuttle (present in spermatozoa of several species [68–70]).
Fig. 4.
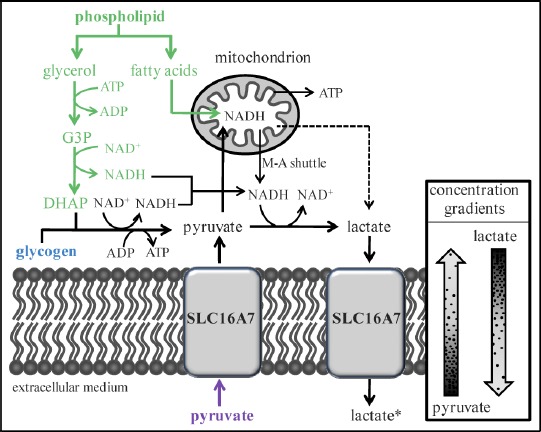
Theoretical model showing three possible mechanisms of lactate production by domestic cat and cheetah spermatozoa, with NADH, lactate, and ATP generated from the metabolism of endogenous glycogen (blue), phospholipid (green), and/or extracellular pyruvate (purple). Common metabolic products or intermediates are illustrated in black. Dashed line indicates the possibility of intramitochondrial lactate formation. For clarity, all enzymes and certain metabolic products or intermediates are omitted from the figure. The absence of lactate in the starting medium is noted by an asterisk (*). SLC16A7, monocarboxylic acid transporter 2 (formerly MCT2); G3P, glycerol-3-phosphate; DHAP, dihydroxyacetone phosphate; M-A shuttle, malate-aspartate shuttle.
Glycogen is known to be metabolized by spermatozoa of the domestic dog, another carnivore [36]. Intracellular glycogen breakdown would yield ATP, and cytosolic NAD+ would be regenerated by lactate production (Fig. 4). However, phospholipid is considered the primary endogenous substrate for most mammalian spermatozoa [21] and could provide greater amounts of cellular ATP. Phospholipid hydrolysis would yield glycerol and fatty acids (Fig. 4). Glycerol would enter the glycolytic pathway via conversion to dihydroxyacetone phosphate and would be metabolized to produce lactate, NADH, and ATP [71]. Mitochondrial oxidation of fatty acids would provide substantial amounts of NADH, which could contribute to ATP and/or lactate production (Fig. 4).
Because we used a protein-free, chemically defined medium, the only possible exogenous source of lactate in our study was the uptake and reduction of extracellular pyruvate. Although pyruvate reduction would require an NADH source and would not generate ATP [63], these molecules could be provided by endogenous substrate metabolism (as described above). Pyruvate uptake likely would occur via the facultative transporter SLC16A7 (monocarboxylic acid transporter 2, previously known as MCT2), the primary monocarboxylate transporter in mature spermatozoa of species studied to date [72–74]. Given the starting composition of our cMTF medium (1 mM pyruvate, 0 mM lactate) and the kinetic properties of SLC16A7 and LDH [75–77], rapid pyruvate uptake and reduction theoretically should occur independently of sperm energy production. Examination of stoichiometric ratios revealed that 60%–70% of produced lactate could have been explained on the basis of pyruvate uptake and reduction (in contrast to only 0%–40% generated from exogenous glucose catabolism). These are important observations given that lactate production (measured by enzyme-linked fluorescence) has been used as an indicator of glycolytic metabolism for other species [25, 30, 31]. Ongoing research in our laboratory supports this mechanism of lactate production in felid spermatozoa. Initial findings determined that sperm lactate production was approximately four times greater in the presence of exogenous pyruvate compared to equimolar amounts of exogenous glucose. Furthermore, in the absence of both substrates in the culture medium, no lactate was produced. Collectively, these observations suggested that mammalian spermatozoa may produce lactate independently of glycolysis, implying that the functional importance of this pathway could have been misinterpreted in earlier studies. More detailed studies are in progress in our laboratory using chemical inhibitors of oxidative and glycolytic metabolism to identify the primary energy substrates for felid spermatozoa.
Although the source of lactate was unclear, rates of production provided a consistent indicator of sperm motility and acrosomal function in both felid species. Intriguingly, lactate production also was correlated to proportions of structurally normal spermatozoa among domestic cat (normospermic and teratospermic combined), but not cheetah, ejaculates. This finding may be related to previous reports that even structurally intact cells from teratospermic ejaculates can be functionally compromised. Specifically, these spermatozoa may demonstrate increased osmotic sensitivity, delayed acrosome reaction, or reduced zona penetration ability [10, 11, 15]. Therefore, rates of lactate production may provide an accurate indicator of ejaculate quality in both species, and may reveal disrupted cellular physiology in apparently normal cheetah spermatozoa. However, lactate production should be validated against a more direct measure of sperm fertilizing ability such as the zona pellucida assay, particularly given its unclear relationship to ATP generation. Because spermatozoa must be capable of multiple energy-dependent processes to achieve successful fertilization, a zona penetration assay could provide a more robust test of the functional relevance of lactate production than any single measure of sperm function (e.g., motility). Understanding the source and biological significance of lactate produced by felid spermatozoa could yield a quantitative, field-friendly indicator of ejaculate quality, which would significantly facilitate developing and refining reproductive technologies for improving felid management and conservation.
Because so little is known about gamete metabolism in felids, this taxon is an excellent target for more detailed investigation in this area of study. The existence of >30 species in the family Felidae (including several with the teratospermic phenotype [78]) also provides important opportunities to determine the etiology and evolution of certain physiological attributes influencing reproductive success. Indeed, direct comparisons across species are fundamental to identifying differences in the mechanisms involving energy production in mammalian gametes, particularly given the ambiguities of published findings relating to sperm metabolism. For example, Carey et al. [79] have reported that mouse spermatozoa metabolize endogenous oxidative substrates to remain motile for >4 h in vitro in the absence of supplemental energy sources. Yet Mukai and Okuno [80] have found that mouse spermatozoa become nonmotile within 30 min in a substrate-free medium and have presented evidence for an obligate role of glycolysis in supporting cellular motility. Contradictory findings also have been reported regarding the need for exogenous glucose to achieve capacitation in dog spermatozoa [61, 81]. Such equivocations often can be avoided and more confidence generated by comparatively evaluating two taxonomically related species using a standardized experimental protocol. In the present study, we found similar patterns of substrate uptake/production in the domestic cat and cheetah, suggesting that spermatozoa of both species relied on the same energy source(s). Relatively low rates of substrate uptake/production in cheetah ejaculates revealed that sperm metabolic efficiency was compromised in this species. More importantly, comparison with the teratospermic cat model then allowed us to link this finding to ejaculate phenotype. Still, we uncovered a subtle but key physiological difference between these species. Specifically, in contrast to the domestic cat, even apparently “high-quality” cheetah ejaculates (i.e., containing higher proportions of structurally normal spermatozoa) demonstrated compromised metabolic function. Thus, while the collective results confirmed that the teratospermic cat was an excellent model for expanding knowledge about gamete physiology in wild felids, findings revealed important functional differences between these two related species.
In conclusion, study results underscored the importance of species diversity in fundamental reproductive phenomena, as has been emphasized recently in the contexts of biological conservation and human health [82]. We predict that understanding the unique mechanisms of energy production in felid spermatozoa will facilitate increased efficiency of assisted reproductive technologies that have been used for producing offspring in wild felids [83, 84]. These technologies also are important for propagating at least eight domestic cat models of human genetic disease (W.F. Swanson, personal communication) [85]. For example, such information would be important for optimizing medium composition to maximize sperm survival for artificial insemination, in vitro culture (including for in vitro fertilization), and cryopreservation. Furthermore, we observed that metabolic efficiency reflected other sperm quality metrics across both individuals and species, thus providing the first objective, field-friendly indicator of gamete function in felids. Such findings also offer new insights for improving reproduction in small populations of endangered species or rare biomedical genotypes. Indeed, a basic understanding of the reproductive uniqueness of a previously unstudied species has been critical to recovery or development of self-sustaining populations [82]. The opportunity to conduct these fundamental studies is one of the invaluable contributions of ex situ (hedge) felid populations to the conservation of Earth's biodiversity [86].
Supplementary Material
Acknowledgments
The authors thank Drs. JoGayle Howard, Budhan Pukazhenthi, Bernard Rees, Copper Aitken-Palmer, and Rebecca Spindler for their helpful advice. We also thank Drs. Luis Padilla, Katharine Hope, Carlos Sanchez, and Katey Pelican for veterinary support and Jenny Santiestevan and Marianne de Jonge for technical assistance.
References
- 1. Pukazhenthi BS, Wildt DE, Howard JG. The phenomenon and significance of teratospermia in felids. J Reprod Fertil Suppl 2001; 57:423–433. [PubMed] [Google Scholar]
- 2. Wildt DE, Bush M, Howard JG, O'Brien SJ, Meltzer D, van Dyk A, Ebedes H, Brand DJ. Unique seminal quality in the South African cheetah and a comparative evaluation in the domestic cat. Biol Reprod 1983; 29:1019–1025. [DOI] [PubMed] [Google Scholar]
- 3. Wildt DE, Brown JL, Bush M, Barone MA, Cooper KA, Grisham J, Howard JG. Reproductive status of cheetahs (Acinonyx jubatus) in North American zoos: the benefits of physiological surveys for strategic planning. Zoo Biol 1993; 12:45–80. [Google Scholar]
- 4. Crosier AE, Marker L, Howard JG, Pukazhenthi BS, Henghali JN, Wildt DE. Ejaculate traits in the Namibian cheetah (Acinonyx jubatus): influence of age, season and captivity. Reprod Fertil Dev 2007; 19:370–382. [DOI] [PubMed] [Google Scholar]
- 5. Barone MA, Roelke ME, Howard J, Brown JL, Anderson AE, Wildt DE. Reproductive characteristics of male Florida panthers: comparative studies from Florida, Texas, Colorado, Latin America and North American Zoos. J Mammal 1994; 75:150–162. [Google Scholar]
- 6. Roelke ME, Martenson JS, O'Brien SJ. The consequences of demographic reduction and genetic depletion in the endangered Florida panther. Curr Biol 1993; 3:340–350. [DOI] [PubMed] [Google Scholar]
- 7. Wildt DE, Bush M, Goodrowe KL, Packer C, Pusey AE, Brown JL, Joslin P, O'Brien SJ. Reproductive and genetic consequences of founding isolated lion populations. Nature 1987; 329:328–331. [Google Scholar]
- 8. Neubauer K, Jewgenow K, Blottner S, Wildt DE, Pukazhenthi BS. Quantity rather than quality in teratospermic males: a histomorphometric and flow cytometric evaluation of spermatogenesis in the domestic cat. Biol Reprod 2004; 71:1524–1571. [DOI] [PubMed] [Google Scholar]
- 9. Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HWG, Behre HM, Haugen TB, Kruger T, Wang C, Mbizvo MT, Vogelsong KM. World Health Organization reference values for human semen characteristics. Hum Reprod Update 2010; 16:231–245. [DOI] [PubMed] [Google Scholar]
- 10. Long JA, Wildt DE, Wolfe BA, Crister JK, DeRossi RV, Howard JG. Sperm capacitation and the acrosome reaction are compromised in teratospermic domestic cats. Biol Reprod 1996; 54:638–646. [DOI] [PubMed] [Google Scholar]
- 11. Pukazhenthi BS, Wildt DE, Ottinger MA, Howard JG. Compromised sperm protein phosphorylation after capacitation, swim-up and zona pellucida exposure in teratospermic domestic cats. J Androl 1996; 17:409–419. [PubMed] [Google Scholar]
- 12. Pukazhenthi BS, Long JA, Wildt DE, Ottinger MA, Armstrong DL, Howard JG. Regulation of sperm function by protein tyrosine phosphorylation in diverse wild felid species. J Androl 1998; 19:675–685. [PubMed] [Google Scholar]
- 13. Pukazhenthi BS, Noiles E, Pelican K, Donoghue AM, Wildt DE, Howard JG. Osmotic effects on feline spermatozoa from normospermic versus teratospermic donors. Cryobiology 2000; 40:139–150. [DOI] [PubMed] [Google Scholar]
- 14. Pukazhenthi BS, Spindler RE, Wildt DE, Bush LM, Howard JG. Osmotic properties of spermatozoa from felids producing different proportions of pleiomorphisms: influence of adding and removing cryoprotectant. Cryobiology 2002; 44:288–300. [DOI] [PubMed] [Google Scholar]
- 15. Howard JG, Donoghue AM, Johnston LA, Wildt DE. Zona pellucida filtration of structurally abnormal spermatozoa and reduced fertilization in teratospermic cats. Biol Reprod 1993; 49:131–139. [DOI] [PubMed] [Google Scholar]
- 16. Pukazhenthi B, Pelican K, Wildt D, Howard JG. Sensitivity of domestic cat (Felis catus) sperm from normospermic versus teratospermic donors to cold-induced acrosome damage. Biol Reprod 1999; 61:135–141. [DOI] [PubMed] [Google Scholar]
- 17. Pukazhenthi B, Santymire R, Crosier A, Howard JG, Wildt DE. Challenges in cryopreserving endangered mammal spermatozoa: morphology and the value of acrosomal integrity as markers of cryo-survival. Roldan ERS, Gomendio M. Spermatology, vol. 65. Nottingham, UK: Nottingham University Press; 2008:433–444. [PubMed] [Google Scholar]
- 18. Storey B. Mammalian sperm metabolism: oxygen and sugar, friend and foe. Int J Dev Biol 2008; 52:427–437. [DOI] [PubMed] [Google Scholar]
- 19. Albarracín JL, Fernández-Novell JM, Ballester J, Rauch MC, Quintero-Moreno A, Peña A, Mogas T, Rigau T, Yañez A, Guinovart JJ, Slebe JC, Concha II et al. Gluconeogenesis-linked glycogen metabolism is important in the achievement of in vitro capacitation of dog spermatozoa in a medium without glucose. Biol Reprod 2004; 71:1437–1445. [DOI] [PubMed] [Google Scholar]
- 20. Marín S, Chiang K, Bassilian S, Lee WNP, Boros L, Fernandáz-Novell JM, Centelles JJ, Medrano A, Rodriguíz-Gil JE, Cascante M. Metabolic strategy of boar spermatozoa revealed by a metabolomic characterization. FEBS Lett 2003; 554:342–346. [DOI] [PubMed] [Google Scholar]
- 21. Ford WCL, Rees JM. The bioenergetics of mammalian sperm motility. Gagnon C. Controls of Sperm Motility: Biological and Clinical Aspects. Boca Raton, FL: CRC Press; 1990:175–202. [Google Scholar]
- 22. Nascimento JM, Shi LZ, Tam J, Chandsawangbhuwana C, Durrant B, Botvinick EL, Berns MW. Comparison of glycolysis and oxidative phosphorylation as energy sources for mammalian sperm motility, using the combination of fluorescence imaging, laser tweezers and real-time automated tracking and trapping. J Cell Physiol 2008; 217:745–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ford WCL. Glycolysis and sperm motility: does a spoonful of sugar help the flagellum go round? Hum Reprod Update 2006; 12:269–274. [DOI] [PubMed] [Google Scholar]
- 24. Ruiz-Pesini E, Díez-Sánchez C, López-Pérez MJ, Enríquez JA. The role of the mitochondrion in sperm function: is there a place for oxidative phosphorylation or is this a purely glycolytic process? Curr Top Dev Biol 2007; 77:3–19. [DOI] [PubMed] [Google Scholar]
- 25. Garrett LJA, Revell SG, Leese HJ. Adenosine triphosphate production by bovine spermatozoa and its relationship to semen fertilizing ability. J Androl 2008; 29:449–458. [DOI] [PubMed] [Google Scholar]
- 26. Williams AC, Ford WC. The role of glucose in supporting motility and capacitation in human spermatozoa. J Androl 2001; 22:680–695. [PubMed] [Google Scholar]
- 27. Turner R. Tales from the tail: what do we really know about sperm motility? J Androl 2003; 24:790–803. [DOI] [PubMed] [Google Scholar]
- 28. Westhoff D, Kamp G. Glyceraldehyde 3-phosphate dehydrogenase is bound to the fibrous sheath of mammalian spermatozoa. J Cell Sci 1997; 110:1821–1829. [DOI] [PubMed] [Google Scholar]
- 29. Travis AJ, Foster JA, Rosenbaum NA, Visconti PE, Gerton GL, Kopf GS, Moss SB. Targeting of a germ cell-specific type 1 hexokinase lacking a porin-binding domain to the mitochondria as well as to the head and fibrous sheath of murine spermatozoa. Mol Cell Biol 1998; 9:263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rigau T, Piedrafita J, Reverter A, Canal M, Rodríguez-Gil JE. The rate of L-lactate production: a feasible parameter for the fresh diluted boar semen quality analysis. Anim Reprod Sci 1996; 43:161–172. [Google Scholar]
- 31. Miró J, Lobo V, Quintero-Moreno A, Medrano A, Peňa A, Rigau T. Sperm motility patterns and metabolism in Catalonian donkey semen. Theriogenology 2005; 63:1706–1716. [DOI] [PubMed] [Google Scholar]
- 32. Songsasen N, Spindler RE, Wildt DE. Requirement for, and patterns of, pyruvate and glutamine metabolism in the domestic dog oocyte in vitro. Mol Reprod Dev 2007; 74:870–877. [DOI] [PubMed] [Google Scholar]
- 33. Palomo MJ, Fernández-Novell JP, Peña A, Guinovart JJ, Rigau T, Rodríguez-Gil JE. Glucose- and fructose-induced dog-sperm glycogen synthesis shows specific changes in the location of the sperm glycogen deposition. Mol Reprod Dev 2003; 64:349–359. [DOI] [PubMed] [Google Scholar]
- 34. Rigau T, Farré M, Ballester J, Mogas T, Peña A, Rodríguez-Gil JE. Effects of glucose and fructose on motility patterns of dog spermatozoa from fresh ejaculates. Theriogenology 2001; 56:801–815. [DOI] [PubMed] [Google Scholar]
- 35. Rigau T, Rivera M, Palomo MJ, Fernández-Novell JM, Mogas T, Ballester J, Peña A, Otaegui PJ, Guinovart JJ, Rodríguez-Gil JE. Differential effects of glucose and fructose on hexose metabolism in dog spermatozoa. Reproduction 2002; 123:579–591. [PubMed] [Google Scholar]
- 36. Ballester J, Fernández-Novell JP, Rutllant J, García-Rocha M, Palomo MJ, Mogas T, Peña A, Rigau T, Guinovart JJ, Rodríguez-Gil JE. Evidence for a functional glycogen metabolism in mature mammalian spermatozoa. Mol Reprod Dev 2000; 56:207–219. [DOI] [PubMed] [Google Scholar]
- 37. Fernández-Novell JM, Ballester J, Medrano A, Otaegui PJ, Rigau T, Guinovart JJ, Rodríguez-Gil JE. The presence of a high-Km hexokinase activity in dog, but not in boar sperm. FEBS Lett 2004; 570:211–216. [DOI] [PubMed] [Google Scholar]
- 38. Albarracín JL, Mogas T, Palomo MJ, Peña A, Rigau T, Rodríguez-Gil JE. In vitro capacitation and acrosome reaction of dog spermatozoa can be feasibly attained in a defined medium without glucose. Reprod Domest Anim 2004; 39:129–135. [DOI] [PubMed] [Google Scholar]
- 39. Bucci D, Isani G, Spinaci M, Tamanini C, Mari G, Zambelli D, Galeati G. Comparative immunolocalization of GLUTs 1, 2, 3 and 5 in boar, stallion and dog spermatozoa. Reprod Domest Anim 2010; 45:315–322. [DOI] [PubMed] [Google Scholar]
- 40. Pilkis SJ, Granner DK. Molecular physiology of the regulation of hepatic gluconeogenesis and glycolysis. Annu Rev Physiol 1992; 54:885–909. [DOI] [PubMed] [Google Scholar]
- 41. Hoppe PC. Glucose requirement for mouse sperm capacitation in vitro. Biol Reprod 1976; 15:39–45. [DOI] [PubMed] [Google Scholar]
- 42. Parrish JJ, Susko-Parrish J, First NL. Capacitation of bovine sperm by heparin: inhibitory role of glucose and role of intracellular pH. Biol Reprod 1989; 38:683–689. [DOI] [PubMed] [Google Scholar]
- 43. Rogers BJ, Yanagimachi R. Retardation of guinea pig sperm acrosome reaction by glucose: the possible importance of pyruvate and lactate metabolism in capacitation and the acrosome reaction. Biol Reprod 1975; 13:568–575. [DOI] [PubMed] [Google Scholar]
- 44. Wildt DE. Assisted reproduction for managing and conserving threatened felids. Int Zoo Yearb 1997; 35:164–172. [Google Scholar]
- 45. O'Brien SJ, Johnson W, Driscoll C, Pontius J, Pecon-Slattery J, Menotti-Raymond M. State of cat genomics. Trends Genet 2008; 24:268–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Crosier A, Henghali J, Howard JG, Pukazhenthi BS, Terrell KA, Marker LL, Wildt D. Improved quality of cryopreserved cheetah (Acinonyx jubatus) spermatozoa after centrifugation through Accudenz. J Androl 2009; 30:298–308. [DOI] [PubMed] [Google Scholar]
- 47. Howard JG. Semen collection and analysis in carnivores. Fowler ME. Zoo and Wild Animal Medicine: Current Therapy III. Philadelphia: WB Saunders; 1993:390–399. [Google Scholar]
- 48. Howard JG, Brown JL, Bush M, Wildt DE. Teratospermic and normospermic domestic cats: ejaculate traits, pituitary-gonadal hormones and improvement of spermatozoal motility and morphology after swim-up processing. J Androl 1990; 11:204–215. [PubMed] [Google Scholar]
- 49. Larson JL, Miller DJ. Simple histochemical stain for acrosomes on sperm from several species. Mol Reprod Dev 1999; 52:445–449. [DOI] [PubMed] [Google Scholar]
- 50. Crosier AE, Pukazhenthi BS, Henghali JN, Howard JG, Dickman AJ, Marker L, Wildt DE. Cryopreservation of spermatozoa from wild-born Namibian cheetahs (Acinonyx jubatus) and influence of glycerol on cryosurvival. Cryobiology 2006; 52:169–181. [DOI] [PubMed] [Google Scholar]
- 51. Gardner DK, Leese HJ. Concentrations of nutrients in mouse oviduct fluid and their effects on embryo development and metabolism in vitro. J Reprod Fertil 1990; 88:361–368. [DOI] [PubMed] [Google Scholar]
- 52. Bavister BD. Substitution of a synthetic polymer for protein in a mammalian gamete culture system. J Exp Zool 1981; 217:45–51. [DOI] [PubMed] [Google Scholar]
- 53. Johnston SD, Osborne CA, Lipowitz AJ. Characterization of seminal plasma, prostatic fluid and bulbourethral gland secretions in the domestic cat. In:Proceedings of the 11th International Congress on Animal Reproduction and Artifical Insemination,Dublin,1988; 4:560. [Google Scholar]
- 54. Shah D, Naciri M, Clee P, Al-Rubeai M. NucleoCounter: an efficient technique for the determination of cell number and viability in animal cell culture processes. Cytotechnology 2006; 51:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bishop DW. Oxygen consumption of fox sperm. Biol Bull 1942; 83:353–362. [Google Scholar]
- 56. Christen R, Schackmann RW, Shapiro BM. Metabolism of sea urchin sperm. J Biol Chem 1983; 258:5392–5399. [PubMed] [Google Scholar]
- 57. Passonneau JV, Lowry OH. Enzymatic Analysis: A Practical Guide. Totowa, NJ: Humana Press; 1993. [Google Scholar]
- 58. Statistical Analysis System. SAS/STAT User's Guide: Statistics. Version 9.1. Cary, NC: Statistical Analysis System Institute; 2002. [Google Scholar]
- 59. Penfold LM, Jost L, Evenson DP, Wildt DE. Normospermic versus teratospermic domestic cat sperm chromatin integrity evaluated by flow cytometry and intracytoplasmic injection. Biol Reprod 2003; 69:1730–1735. [DOI] [PubMed] [Google Scholar]
- 60. Mahadevan MM, Miller MM, Moutos DM. Absence of glucose decreases human fertilization and sperm movement characteristics in vitro. Hum Reprod 1997; 12:119–123. [DOI] [PubMed] [Google Scholar]
- 61. Mahi CA, Yanagimachi R. Capacitation, acrosome reaction and egg penetration by canine spermatozoa in a simple defined medium. Gamete Res 1978; 1:101–109. [Google Scholar]
- 62. Voet D, Voet JG. Other pathways of carbohydrate metabolism. Harris D, Fitzgerald P. Biochemistry, 3rd ed. Hoboken, NJ: John Wiley & Sons; 2004:843–870. [Google Scholar]
- 63. Voet D, Voet JG. Glycolysis. Harris D, Fitzgerald P. Biochemistry, 3rd ed. Hoboken, NJ: John Wiley & Sons; 2004:581–624. [Google Scholar]
- 64. Storey BT, Kayne FJ. Energy metabolism of spermatozoa VI. Direct intramitochondrial lactate oxidation by rabbit sperm mitochondria. Biol Reprod 1977; 16:549–556. [PubMed] [Google Scholar]
- 65. Gallina FG, Deburgos NMG, Burgos C, Coronel CE, Blanco A. The lactate/pyruvate shuttle in spermatozoa: operation in vitro. Arch Biochem Biophys 1994; 308:515–519. [DOI] [PubMed] [Google Scholar]
- 66. Wang SX, Luo AM, Liang ZG, Song JF, Wang HA, Chen YX. Preparation and characterization of monoclonal antibodies against sperm-specific lactate dehydrogenase C4. J Androl 1990; 11:319–324. [PubMed] [Google Scholar]
- 67. Burgos C, Maldonado C, Gerez de Burgos NM, Aoki A, Blanco A. Intracellular localization of the testicular and sperm-specific lactate dehydrogenase isozyme C4 in mice. Biol Reprod 1995; 53:84–92. [DOI] [PubMed] [Google Scholar]
- 68. Blanco A. On the functional significance of LDH X. Johns Hopkins Med J 1980; 146:231–235. [PubMed] [Google Scholar]
- 69. Calvin J, Tubbs PK. Mitochondrial transport processes and oxidation of NADH by hypotonically-treated boar spermatozoa. Eur J Biochem 1978; 89:315–320. [DOI] [PubMed] [Google Scholar]
- 70. Voet D, Voet JG. Electron transport and oxidative phosphorylation. Harris D, Fitzgerald P. Biochemistry, 3rd ed. Hoboken, NJ: John Wiley & Sons; 2004:797–842. [Google Scholar]
- 71. Voet D, Voet JG. Lipid metabolism. Harris D, Fitzgerald P. Biochemistry, 3rd ed. Hoboken, NJ: John Wiley & Sons; 2004:909–984. [Google Scholar]
- 72. Merezhinskaya N, Fishbein WN. Monocarboxylate transporters: past, present and future. Histol Histopathol 2009; 24:243–264. [DOI] [PubMed] [Google Scholar]
- 73. Garcia CK, Brown MS, Pathak RK, Goldstein JL. CDNA cloning of MCT2, a second monocarboxylate transporter expressed in different cells than MCT1. J Biol Chem 1995; 270:1843–1849. [DOI] [PubMed] [Google Scholar]
- 74. Halestrap AP, Price NT. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem J 1999; 343:281–299. [PMC free article] [PubMed] [Google Scholar]
- 75. Brooks GA. Intra- and extra-cellular lactate shuttles. Med Sci Sports Exerc 2000; 32:790–799. [DOI] [PubMed] [Google Scholar]
- 76. Brooks GA. Mammalian fuel utilization during sustained exercise. Comp Biochem Physiol B Biochem Mol Biol 1998; 120:89–107. [DOI] [PubMed] [Google Scholar]
- 77. Brooks GA, Dubouchaud H, Brown M, Sicurello JP, Butz CE. Role of mitochondrial lactate dehydrogenase and lactate oxidation in the intracellular lactate shuttle. Proc Natl Acad Sci U S A 1999; 96:1129–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pukazhenthi BS, Neubauer K, Jewgenow K, Howard JG, Wildt DE. The impact and potential etiology of teratospermia in the domestic cat and its wild relatives. Theriogenology 2006; 66:112–121. [DOI] [PubMed] [Google Scholar]
- 79. Carey JE, Oldsclarke P, Storey BT. Oxidative metabolism of spermatozoa from inbred and random bred mice. J Exp Zool 1981; 216:285–292. [DOI] [PubMed] [Google Scholar]
- 80. Mukai C, Okuno M. Glycolysis plays a major role for adenosine triphosphate supplementation in mouse sperm flagellar movement. Biol Reprod 2004; 71:540–547. [DOI] [PubMed] [Google Scholar]
- 81. Rota A, Peña AI, Linde-Forsberg C, Rodríguez-Martínez H. In vitro capacitation of fresh, chilled and frozen-thawed dog spermatozoa assessed by the chlorotetracycline assay and changes in motility patterns. Anim Reprod Sci 1999; 57:199–215. [DOI] [PubMed] [Google Scholar]
- 82. Wildt DE, Comizzoli P, Pukazhenthi B, Songsasen N. Lessons from biodiversity—the value of nontraditional species to advance reproductive science, conservation and human health. Mol Reprod Dev 2010; 77:397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Howard JG, Wildt DE. Approaches and efficacy of artificial insemination in felids and mustelids. Theriogenology 2009; 71:130–148. [DOI] [PubMed] [Google Scholar]
- 84. Swanson WF. Application of assisted reproduction for population management in felids: the potential and reality for conservation of small cats. Theriogenology 2006; 66:49–58. [DOI] [PubMed] [Google Scholar]
- 85. Swanson WF. Research in nondomestic species: experiences in reproductive physiology research for conservation of endangered felids. ILAR J 2003; 44:307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wildt D, Swanson W, Brown J, Sliwa A, Vargas A. Felids ex situ: managed programmes, research and species recovery. Macdonald DW, Loveridge AJ. Biology and Conservation of Wild Felids. New York: Oxford University Press; 2010:217–235. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


