ABSTRACT
Background
Vadadustat, an inhibitor of hypoxia-inducible factor prolyl-4-hydroxylase domain dioxygenases, is an oral investigational agent in development for the treatment of anemia secondary to chronic kidney disease.
Methods
In this open-label Phase 2 trial, vadadustat was evaluated in 94 subjects receiving hemodialysis, previously maintained on epoetin alfa. Subjects were sequentially assigned to one of three vadadustat dose cohorts by starting dose: 300 mg once daily (QD), 450 mg QD or 450 mg thrice weekly (TIW). The primary endpoint was mean hemoglobin (Hb) change from pre-baseline average to midtrial (Weeks 7–8) and end-of-trial (Weeks 15–16) and was analyzed using available data (no imputation).
Results
Overall, 80, 73 and 68% of subjects in the 300 mg QD, 450 mg QD, and 450 mg TIW dose cohorts respectively, completed the study. For all dose cohorts no statistically significant mean change in Hb from pre-baseline average was observed, and mean Hb concentrations—analyzed using available data—remained stable at mid- and end-of-trial. There was one subject with an Hb excursion >13 g/dL. Overall, 83% of subjects experienced an adverse event (AE); the proportion of subjects who experienced at least one AE was similar among the three dose cohorts. The most frequently reported AEs were nausea (11.7%), diarrhea (10.6%) and vomiting (9.6%). No deaths occurred during the study. No serious AEs were attributed to vadadustat.
Conclusions
Vadadustat maintained mean Hb concentrations in subjects on hemodialysis previously receiving epoetin. These data support further investigation of vadadustat to assess its long-term safety and efficacy in subjects on hemodialysis.
Keywords: anemia, erythropoiesis, hemodialysis, hypoxia-inducible factor prolyl-4-hydroxylase inhibitor, vadadustat
INTRODUCTION
Anemia is a common complication of chronic kidney disease (CKD), the latter of which represents a major worldwide burden on public health, particularly in aging populations [1]. Anemia affects the majority of patients with advanced CKD [2] and is associated with increased cardiovascular risk, hospitalization and mortality [3].
Anemia in CKD is predominantly due to a relative deficiency in erythropoietin (EPO) production by the kidney, although concomitant functional and/or absolute iron deficiency [4] and systemic and local inflammation [5, 6] also frequently contribute to its induction and maintenance. Correction of anemia in patients with CKD has been investigated in many studies, with variable impact on prespecified outcomes [7, 8]; in most studies, higher hemoglobin (Hb) concentrations were shown to be associated with improved symptoms, as well as a reduced need for transfusion and/or hospitalization [3, 7–9].
The current standard of care for anemia secondary to CKD is the use of injectable erythropoiesis-stimulating agents (ESAs), alone, or in combination with intravenous (IV) or oral iron supplementation [10, 11]. While ESAs have been shown to be effective in treating anemia for many patients with CKD, they have some well-recognized limitations. Trials of ESAs in patients with anemia secondary to non-dialysis-dependent CKD (NDD-CKD) or dialysis-dependent CKD (DD-CKD) have demonstrated an increased risk of cardiovascular events associated with higher Hb targets [10–12]. Post hoc analyses performed by the federal Food and Drug Administration and others have shown an association between these adverse outcomes and ESA dose [13, 14]. Alternative treatments that limit EPO exposure may be useful additions to the therapeutic armamentarium for renal anemia.
In addition to reduced EPO production, iron absorption and mobilization are frequently reduced in patients with CKD [6]. Hb production depends on iron availability and is carefully orchestrated by coordinated signaling from the liver, kidney, and bone marrow. Under normal conditions, EPO induces erythroblast secretion of erythroferrone [15], a protein that suppresses hepcidin production in the liver [16]. Hepcidin acts as the master regulator of iron homeostasis [17]. Lower serum hepcidin concentrations allow for increased iron absorption and mobilization, while higher serum hepcidin concentrations block iron release from internal stores. In a large proportion of patients with CKD, hepcidin concentrations are high despite concurrent anemia; therefore, red blood cell (RBC) production remains impaired due to functional iron deficiency [18].
Several lines of evidence suggest that altitude is associated with improved outcomes in end-stage renal disease (ESRD) [19–22]. This process is thought to be mediated by the action of hypoxia-inducible factors (HIFs) [23]. HIFs are oxygen-sensitive transcription factors that play a key role in the physiologic adaptation to hypoxia by regulating the expression of multiple genes, including EPO and others involved in iron metabolism and erythropoiesis [4, 24]. HIF activity is controlled by prolyl-4-hydroxylase domain (PHD) proteins, which function as oxygen sensors [24]. Under normoxia, PHDs target the ΗIF-α subunit for degradation. Under hypoxic conditions, PHD activity is substantially decreased, which reduces HIF degradation. HIF stabilization results in increased expression of HIF target genes, including EPO [4, 24], which then stimulates RBC production and the concomitant release of erythroferrone (which reduces hepcidin production), resulting in both iron mobilization and increased Hb production [18].
Vadadustat is an oral inhibitor of HIF-PHDs, in development for the treatment of anemia in patients with CKD. In clinical trials to date, vadadustat has been shown to increase and maintain mean Hb in the target range in patients with NDD-CKD [25, 26]. In this report, we present data from a Phase 2 clinical trial evaluating vadadustat in subjects receiving hemodialysis whose anemia was previously controlled with ESA therapy.
MATERIALS AND METHODS
Study design
This Phase 2, US-based, multicenter, open-label, 16-week study examined the effect of orally administered vadadustat in subjects with renal anemia receiving maintenance hemodialysis from September 2014 to July 2015.
The trial was approved by all relevant Institutional Review Boards and conducted in compliance with International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use/Good Clinical Practice (ICH/GCP) guidelines and the Declaration of Helsinki. Subjects provided voluntary written informed consent.
Study population
The trial enrolled subjects 18–79 years of age receiving maintenance hemodialysis thrice weekly (TIW) for at least 3 months. Subjects were expected to remain on hemodialysis for the duration of the trial. For the 3 months before screening, all subjects were required to have received epoetin alfa (Epogen®) for the treatment of anemia secondary to ESRD and IV iron to maintain adequate iron stores for erythropoiesis. Subjects receiving epoetin alfa doses >24 000 U/week in the preceding 4 months were excluded from the study (further details are provided in the Supplementary Materials).
Interventions
Eligible subjects continued their ESA therapy during screening, but were required to discontinue ESA therapy prior to their baseline visit, at which time they were assigned to one of three dose cohorts irrespective of baseline characteristics or prior medical history: vadadustat 300 mg once daily (QD), 450 mg QD, or 450 mg TIW. The first dose of vadadustat was administered during the baseline visit and subsequently self-administered on an outpatient basis for 16 consecutive weeks. Dose cohorts were dosed sequentially.
Hb concentrations were measured at each study visit and used to guide dose adjustment or interruption (Supplementary data, Table S1). Vadadustat dose increase up to 600 mg per day was permitted beginning at Week 8 and through Week 12; dose decrease was allowed at any time for either intolerance or Hb level concerns. ESA administration was not allowed during the 16-week study period.
In this trial, IV iron supplementation was utilized to maintain serum ferritin concentrations between 100 and 1200 ng/mL. Oral iron was not permitted.
Trial endpoints
The primary endpoint was the mean change in Hb concentrations between the pre-baseline average (mean of the three values obtained prior to dosing at screening visit 1, screening visit 2 and the baseline visit), the mid-study average (mean of the two values obtained at Weeks 7–8 visits) and the end-of-study average (mean of the two values obtained at Weeks 15–16 visits).
Secondary endpoints included: absolute and percent change from baseline in Hb levels; reticulocyte count, reticulocyte Hb content (CHr); iron-related indices [iron, transferrin saturation (TSAT), total iron-binding capacity (TIBC), ferritin and hepcidin]; rates of ESA rescue and RBC transfusion; incidence, severity, and type of adverse events (AEs) and serious AEs (SAEs); and pre-dialysis and postdialysis plasma concentrations of vadadustat and two glucuronide metabolites.
Clinical and safety assessments (including laboratory assays and AEs) were performed at screening visit 1, screening visit 2, baseline (Day 1), during study visits/evaluations while receiving medication (Weeks 2, 4, 6, 7, 8, 10, 12, 14 and 15), at end-of-treatment (Week 16 or at early study withdrawal) and at follow-up (29 days after the last dose of study medication). Blood samples for determination of plasma levels of vadadustat and two glucuronide metabolites were drawn immediately prior to and 10 min after completion of the dialysis session at the Week 2 and 16 visits. Further details are provided in the Supplementary Materials.
Statistical analyses
The intention-to-treat (ITT) population included enrolled subjects who received at least one dose of vadadustat. The ITT population was used for analyses of demographics, baseline characteristics, pharmacokinetics, and safety parameters.
The modified ITT (mITT) population included subjects who received at least one dose of vadadustat, had a pre-baseline Hb average, and had at least one postbaseline Hb measurement. The mITT population was used for all efficacy endpoint analyses.
The per-protocol (PP) population included all subjects who had efficacy data through Week 16, were ≥80% compliant with study medication and had no major protocol deviations.
The primary efficacy analysis was based on observed data; no imputation of missing data was performed. Differences among dose cohorts were examined using a one-way analysis of variance (ANOVA) for each of the measures of change, and within-group changes from baseline were analyzed using paired t-tests.
A post hoc sensitivity analysis of the primary efficacy endpoint was performed using the mITT population and last observation carried forward (LOCF) for subjects with missing Hb data at Weeks 7–8 and/or Weeks 15–16. Within-group changes from baseline were examined with paired t-tests, and differences of mean Hb change from baseline at Weeks 7–8 and Weeks 15–16 among dose cohorts were assessed using an analysis of covariance, controlling for baseline Hb.
Descriptive statistics were used to summarize baseline characteristics and secondary endpoints. Post hoc analysis of within-group changes from baseline in iron parameters was performed using paired t-tests. All AEs recorded during the study were coded into Medical Dictionary for Regulatory Activities (MedDRA; version 16.1). Preferred Terms and System Organ Class definitions were displayed by dose cohorts. A two-sided significance level of 0.05 was used to determine statistical significance. All analyses were performed using SAS v9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Subject disposition
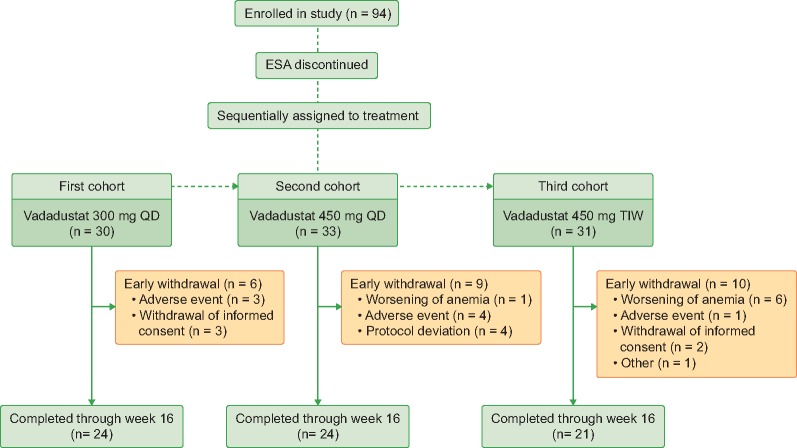
A total of 94 subjects were enrolled into the study. Twenty-four (80%), 24 (73%) and 21 (68%) subjects completed the study in the 300 mg QD, 450 mg QD, and 450 mg TIW dose cohorts, respectively (Figure 1). One subject in the 450 mg QD dose cohort and six subjects in the 450 mg TIW withdrew from the study due to worsening anemia (Figure 1). All 94 subjects met criteria for inclusion into the ITT and mITT populations, and 64 (68%) subjects comprised the PP population (Supplementary data, Table S2).
FIGURE 1.
CONSORT flow diagram of the trial design.
Baseline characteristics
Baseline characteristics were generally balanced across dose cohorts, except for a higher proportion of White/Caucasian subjects and lower average pre-baseline epoetin dose reported in the 300 mg QD dose cohort compared with the other dose cohorts (Table 1). Demographics and disease-related characteristics of the study population were reflective of the overall US population of patients on dialysis [14], with a mean ± standard deviation (SD) subject age of 58 ±11 years, median (interquartile range) dialysis vintage of 2.7 (1.4–6.1) years and 64% with diabetes mellitus.
Table 1.
Subject demographics and baseline characteristics (ITT population)
| Vadadustat dose cohorts |
||||
|---|---|---|---|---|
| Characteristic | 300 mg QD | 450 mg QD | 450 mg TIW | All subjects |
| (n = 30) | (n = 33) | (n = 31) | (n = 94) | |
| Age (years) | 55.5 ± 12.4 | 59.4 ± 11.6 | 57.8 ± 8.3 | 57.6 ± 10.9 |
| Sex | ||||
| Male | 17 (56.7) | 18 (54.5) | 19 (61.3) | 54 (57.4) |
| Female | 13 (43.3) | 15 (45.5) | 12 (38.7) | 40 (42.6) |
| Weight (kg) | 82.7 ± 22.7 | 82.1 ± 19.7 | 84.2 ± 16.5 | 83.0 ± 19.6 |
| BMI (kg/m2) | 29.8 ± 7.7 | 29.6 ± 6.0 | 29.2 ± 5.4 | 29.5 ± 6.4 |
| Race | ||||
| Black/African American | 6 (20.0) | 9 (27.3) | 9 (29.0) | 24 (25.5) |
| White/Caucasian | 24 (80.0) | 21 (63.6) | 19 (61.3) | 64 (68.1) |
| Other | 0 (0.0) | 3 (9.1) | 3 (9.7) | 6 (6.4) |
| Etiology of CKD* | ||||
| Diabetes | 16 (53.3) | 23 (69.7) | 21 (67.7) | 60 (63.8) |
| Hypertension & large vessel disease | 15 (50.0) | 17 (51.5) | 21 (67.7) | 53 (56.4) |
| Other | 17 (56.67) | 18 (54.55) | 12 (38.71) | 47 (50.00) |
| Medical History* | ||||
| Diabetes | 18 (60.0) | 25 (75.8) | 19 (61.3) | 62 (66.0) |
| Hypertension | 28 (93.3) | 33 (100.0) | 31 (100.0) | 92 (97.9) |
| Time since initiation of dialysis (years [10th, 90th percentiles]) | 4.9 (0.9, 11.6) | 4.9 (0.7, 11.6) | 3.9 (0.5, 6.9) | 4.6 (0.8, 10.8) |
| Baseline epoetin alfa dose (U/week) | 5940 ± 4387 | 8022 ± 5208 | 8059 ± 6519 | 7370 ± 5478 |
| Blood pressure: systolic (mmHg) | 138.9 ± 18.4 | 147.8 ± 25.3 | 147.3 ± 18.2 | 144.8 ± 21.2 |
| Blood pressure: diastolic (mmHg) | 70.9 ± 11.6 | 78.3 ± 12.4 | 74.8 ± 10.7 | 74.8 ± 11.6 |
| Heart rate (beats/min) | 77.1 ± 10.0 | 80.5 ± 13.6 | 74.9 ± 10.2 | 77.6 ± 11.6 |
| Hb (g/dL) | 10.4 ± 0.7 | 10.6 ± 0.6 | 10.5 ± 0.5 | 10.5 ± 0.6 |
| Iron (µg/dL) | 70.6 ± 26.3 | 66.6 ± 23.7 | 75.4 ± 32.3 | 70.8 ± 27.5 |
| TIBC (µg/dL) | 204.5 ± 39.7 | 196.7 ± 29.4 | 188.8 ± 32.3 | 196.6 ± 34.2 |
| Ferritin (ng/mL) | 762.9 ± 470.5 | 782.0 ± 465.2 | 807.8 ± 431.0 | 784.4 ± 451.4 |
| TSAT (%) | 34.6 ± 11.6 | 33.7 ± 11.1 | 37.5 ± 13.3 | 35.2 ± 12.0 |
| Hepcidin (ng/mL) | 102.6 ± 58.9 | 119.6 ± 54.8 | 105.4 ± 46.8 | 109.5 ± 53.7 |
| C-reactive protein (mg/dL) | 1.2 ± 2.4 | 1.1 ± 1.5 | 1.6 ± 3.3 | 1.3 ± 2.4 |
| Serum bicarbonate (mEq/l) | 17.68 ± 2.88 | 19.84 ± 2.87 | 19.26 ± 2.78 | 18.96 ± 2.96 |
| CHr (pg) | 32.39 ± 1.80 | 32.12 ± 1.68 | 30.99 ± 2.37 | 31.84 ± 2.04 |
Data are presented as n (%) or mean ± SD, with the exception of vintage, reported as median (10%, 90% range)
Patients could have >1 disease etiology and >1 medical condition (medical history)
BMI, body mass index; CHr, reticulocyte Hb content; CKD, chronic kidney disease; Hb, hemoglobin; QD, once daily; SD, standard deviation; TIBC, total iron binding capacity; TIW, three times per week; TSAT, transferrin saturation.
Subject dosing
The proportion of subjects demonstrating >80% compliance with study medication by drug accountability (pill count) exceeded 90% in each of the dose cohorts. Mean ±SD prescribed doses at Weeks 14–16 in evaluable subjects who completed the study were 375 ±133 mg in the 300 mg QD dose cohort (n = 24), 488 ±167 mg in the 450 mg QD dose cohort (n = 24), and 488 ±145 mg in the 450 mg TIW dose cohort (n = 20).
Mean change in Hb, mean Hb levels and Hb excursions
Primary efficacy analysis
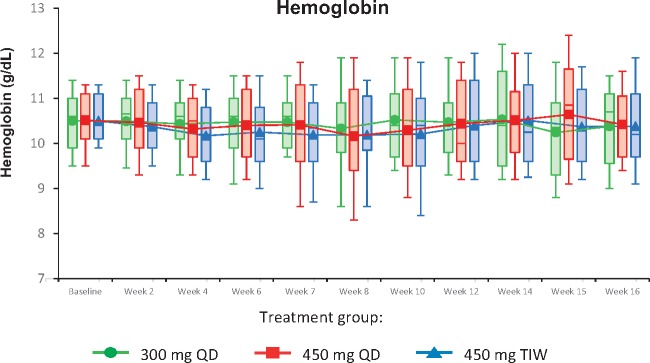
The primary efficacy analysis (using observed data from the mITT population) demonstrated no significant mean change in Hb from pre-baseline levels at Weeks 7–8 and Weeks 15–16 in the 300 mg QD, 450 mg QD or 450 mg TIW dose cohorts; in addition, no between-group differences in mean change in Hb were observed (Table 2). Mean Hb levels were maintained from pre-baseline values to Weeks 7–8 and Weeks 15–16 in each of the three dose cohorts (Table 2, Figure 2). Similar findings were observed for the per protocol analysis (Supplementary data, Table S3).
Table 2.
Mean Hb values and mean change in Hb across and between dose cohorts (mITT population)
| Mean Hb (SD), n |
Weeks 7–8 vs Pre-baseline |
Weeks 15–16 vs Pre-baseline |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Cohorts | Pre-baseline | Weeks 7–8 | Weeks 15–16 | Mean ΔHb (SD), n | 95% CI | P-valuea | Mean ΔHb (SD), n | 95% CI | P-valuea |
| 300 mg QD | 10.43 (0.65), 30 | 10.41 (0.83), 28 | 10.30 (0.97), 24 | 0.00 (0.90), 28 | −0.35, 0.35 | 0.99 | −0.03 (0.90), 24 | −0.41, 0.35 | 0.86 |
| 450 mg QD | 10.55 (0.58), 33 | 10.26 (1.17), 28 | 10.53 (1.03), 24 | −0.29 (0.97), 28 | −0.66, 0.09 | 0.13 | −0.07 (0.97), 24 | −0.48, 0.34 | 0.73 |
| 450 mg TIW | 10.52 (0.53), 31 | 10.19 (0.95), 26 | 10.37 (1.00), 21 | −0.36 (1.13), 26 | −0.81, 0.10 | 0.12 | −0.14 (1.12), 21 | −0.65, 0.37 | 0.58 |
| Weeks 7–8 |
Weeks 15–16 |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Dose cohorts, pairwise comparison | Mean difference in change in Hb (95% CI) | P-valueb | Mean difference in change in Hb (95% CI) | P-valueb | |||||
| 300 mg QD versus 450 mg QD | 0.29 (−0.25, 0.82) | 0.29 | 0.04 (−0.54, 0.61) | 0.89 | |||||
| 300 mg QD versus 450 mg TIW | 0.36 (−0.19, 0.90) | 0.19 | 0.10 (−0.49, 0.70) | 0.73 | |||||
| 450 mg QD versus 450 mg TIW | 0.07 (−0.48, 0.61) | 0.80 | 0.07 (−0.53, 0.66) | 0.82 | |||||
The analysis compared the mean Hb change relative to the baseline value to a mean change of 0.
The analysis was an analysis of variance (ANOVA) without multiple adjustments.
CI, confidence interval; Hb, hemoglobin; mITT, modified intent-to-treat; PP, per protocol; QD, daily dosing; SD, standard deviation; TIW, three times weekly dosing.
FIGURE 2.
Mean hemoglobin concentrations over time (mITT population). Box-and-whisker plots represent 10th, 25th, 75th and 90th percentiles. The medians are indicated by the line within the boxes, and the means are indicated by the symbol within the boxes.
Sensitivity analysis
In the mITT LOCF sensitivity analysis of mean change in Hb, no significant mean change in Hb from pre-baseline levels was observed in the 300 mg QD dose cohort. At Weeks 15–16, significant mean changes of −0.40 [95% confidence interval (CI) −0.77, −0.03] and −0.52 (95% CI −1.03, −0.02) were observed in the 450 mg QD and 450 mg TIW dose cohorts, respectively (Table 3).
Table 3.
Mean Hb values and mean change in Hb across and between dose cohorts (mITT population with LOCF imputation, sensitivity analysis)
| Mean Hba (SD), n |
Weeks 7–8 vs Pre-baseline |
Weeks 15–16 vs Pre-baseline |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Cohorts | Predose | Weeks 7–8 | Weeks 15–16 | Mean ΔHba (SD), n | 95% CI | P-valueb | Mean ΔHba (SD), n | 95% CI | P-valueb |
| 300 mg QD | 10.43 (0.65), 30 | 10.44 (0.81), 30 | 10.28 (0.91), 30 | 0.01 (0.87), 30 | −0.32, 0.33 | 0.97 | −0.15 (0.89), 30 | −0.48, 0.18 | 0.35 |
| 450 mg QD | 10.55 (0.58), 33 | 10.12 (1.17), 33 | 10.15 (1.15), 33 | −0.42 (0.99), 33 | −0.78, −0.07 | 0.02 | −0.40 (1.05), 33 | −0.77, −0.03 | 0.04 |
| 450 mg TIW | 10.52 (0.53), 31 | 10.08 (0.93), 31 | 9.99 (1.2), 31 | −0.43 (1.08), 31 | −0.83, −0.04 | 0.03 | −0.52 (1.38), 31 | −1.03, −0.02 | 0.04 |
| Weeks 7–8 |
Weeks 15–16 |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Dose cohorts, pairwise comparison | Mean difference in change in Hb, 95% CI | P-valuec | Mean difference in change in Hb, 95% CI | P-valuec | |||||
| 300 mg QD versus 450 mg QD | −0.37 (−0.85, 0.10) | 0.12 | −0.18 (−0.73, 0.36) | 0.51 | |||||
| 300 mg QD versus 450 mg TIW | −0.40 (−0.88, 0.09) | 0.11 | −0.32 (−0.88, 0.23) | 0.25 | |||||
| 450 mg QD versus 450 mg TIW | −0.02 (−0.49, 0.45) | 0.92 | −0.14 (−0.68, 0.40) | 0.61 | |||||
Subjects who received at least one dose of study medication and had one postdose Hb measurement were used in this analysis; imputation of LOCF was used to provide Hb values for subjects who did not complete the study.
The analysis compared the mean Hb change relative to the baseline value to a mean change of 0.
The analysis was an analysis of variance (ANOVA) without multiple adjustments.
CI, confidence interval; Hb, hemoglobin; LOCF, last observation carried forward; mITT, modified intent-to-treat; PP, per protocol; QD, daily dosing; SD, standard deviation; TIW, three times weekly dosing.
Additional analyses
One subject (1.1%) exhibited an Hb excursion of >13.0 g/dL. The subject, assigned to an initial dose of 300 mg QD and prescribed 150 mg QD at the time of the excursion, had an Hb of 13.1 g/dL at Week 12. Dosing was interrupted, in keeping with the protocol-specified dose adjustment algorithm. After 2 weeks, the Hb level was 11.1 g/dL, and vadadustat was restarted at 150 mg QD for the remainder of the study.
Subjects in the 450 mg TIW dose cohort who discontinued the study due to worsening anemia had a higher mean ±SD pre-baseline epoetin dose (n = 6; 14254 ±8078 U/week) compared with subjects who discontinued due to other reasons (n = 4; 8225 ±9692 U/week) or subjects who completed the study (n = 21; 6257 ±4291 U/week). The subject in the 450 mg QD dose cohort who discontinued the study due to worsening anemia had a higher pre-baseline epoetin dose (15000 U/week) than subjects who discontinued due to other reasons (n = 8; 9172 ±5695 U/week) or subjects who completed the study (n = 24; 7348 ±4981 U/week).
Secondary endpoints
Reticulocyte count and mean CHr
Absolute reticulocyte count and mean CHr (Supplementary data, Figure S1) remained stable throughout the treatment period in the three dose cohort.
Iron-related biomarkers
IV iron was administered throughout the study in 41 subjects, to maintain serum ferritin concentrations between 100 and 1200 ng/mL. The amount of IV iron administered before and during the study varied within and among dose cohort (Supplementary data, Table S4).
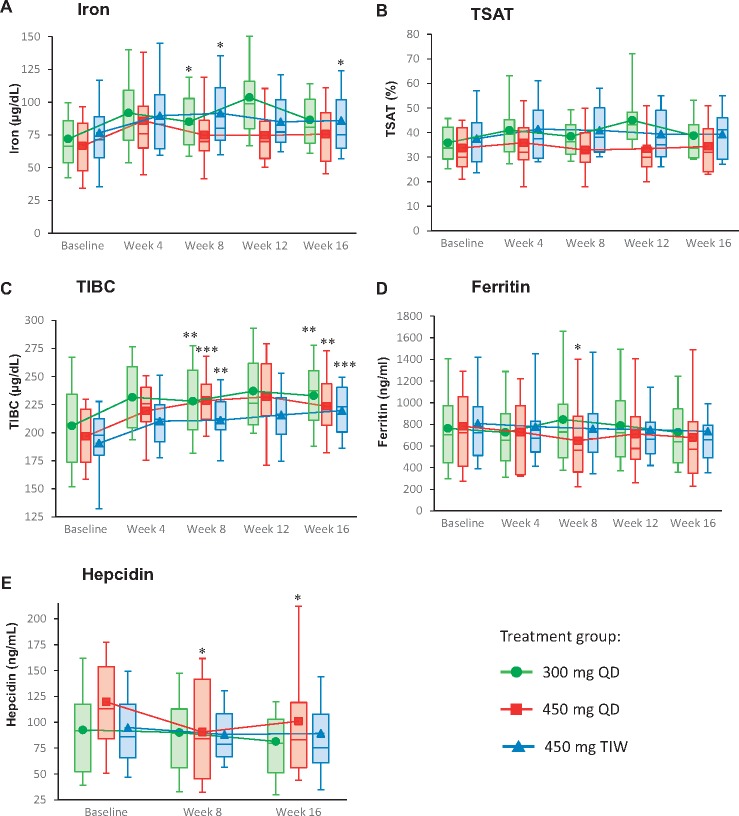
Descriptive analyses showed that vadadustat resulted in an increase in mean TIBC, iron, and TSAT (Figure 3). The mean ±SD change in TIBC at Week 16 was 27.7 ±35.27 μg/dL in the 300 mg QD cohort, 24.9 ±29.10 μg/dL in the 450 mg QD cohort, and 25.7 ±21.78 μg/dL in the 450 mg TIW cohort. The change in TIBC was similar among the two QD dose cohorts.
FIGURE 3.
Mean iron (A), TSAT (B), TIBC (C), ferritin (D) and hepcidin (E) levels over time (mITT population). Box-and-whisker plots represent 10th, 25th, 75th and 90th percentiles. The medians are indicated by the line within the boxes, and the means are indicated by the symbol within the boxes. Statistical significance values are only provided for measurements at Weeks 8 and 16, relative to the baseline values. *P<0.05; **P<0.01; ***P<0.001.
Mean serum hepcidin and ferritin concentration values decreased in the two QD dose cohorts and suggested a dose-dependent response (Figure 3). At Week 16, the mean ±SD change from baseline in serum ferritin was −56.7 ±317.1 ng/mL in the 300 mg QD cohort and −115.4 ±276.6 ng/mL in the 450 mg QD cohort. In the 450 mg TIW cohort, a decrease of 39.0 ±485.0 ng/mL was observed. The mean ±SD change from baseline in serum hepcidin at Week 16 was −15.1 ±46.5 ng/mL in the 300 mg QD cohort, −21.7 ±45.4 ng/mL in the 450 mg QD cohort, and −4.9 ±50.4 ng/dL in the 450 mg TIW cohort.
In post hoc analyses, statistically significant differences from baseline in iron parameters were observed as shown in Supplementary data, Table S5.
ESA administration and RBC transfusion
Although blood transfusions and ESA administration were not permitted during the 16-week treatment period, 12/94 (13%) subjects received one or more doses of ESA during the study (300 mg QD cohort, n = 4; 450 mg QD cohort, n = 6; and 450 mg TIW cohort, n = 2). ESA was administered inadvertently in 10 subjects. In the other two subjects, ESA was prescribed during hospitalization for an SAE (heart failure, worsening hyperkalemia) assessed by study investigators as unrelated to vadadustat. There were no Hb excursions ≥13.0 g/dL during coadministration of vadadustat and ESA. RBC transfusions were administered to 2/94 (2.1%) subjects during the study period. Each received a single blood transfusion while hospitalized for an SAE [arteriovenous (AV) fistula thrombosis, pneumonia] assessed as unrelated to vadadustat.
Plasma levels of vadadustat and its metabolites
Although a formal dose proportionality analysis was not conducted, visual inspection of the data showed that plasma concentrations of vadadustat and the metabolites acyl- and O-glucuronide increased in an apparent dose-dependent manner. Repeated dosing of vadadustat over the 16 weeks of treatment did not appear to result in accumulation of vadadustat or its metabolites (Supplementary data, Figure S2). Based on a single sample taken at Weeks 2 and 16 predialysis and at 10 min postdialysis, plasma concentrations of vadadustat were similar. Plasma concentrations of the acyl- and O-glucuronide metabolites were lower following the dialysis treatment.
Safety
Overall, 78/94 (83%) subjects experienced at least one AE; the percentage of subjects who experienced one or more AE was similar among the three dose cohorts, with no apparent dose effect on the frequency of AEs (Table 4). The most frequently reported AEs were nausea (11/94 subjects, 11.7%), diarrhea (10/94, 10.6%) and vomiting (9/94, 9.6%). Among these, 22/94 (23.4%) subjects had at least one AE reported by investigators as related to vadadustat therapy; the most common drug-related AEs were nausea (7/94, 7.4%) and vomiting (4/94, 4.3%). SAEs occurred in 13 (13.8%) subjects. Pneumonia (3/94, 3.2%), AV fistula thrombosis (2/94, 2.1%) and acute myocardial infarction (MI) (2/94, 2.1%) were the only SAEs that were reported in more than one subject. One subject with a history of coronary artery bypass graft and left internal mammary artery stenting was diagnosed with a non-ST elevation MI based on an incidental elevated troponin level in the absence of symptoms of myocardial ischemia. The other subject had a history of type 2 diabetes, hypertension and hypercholesterolemia, and was diagnosed with acute MI and advanced three-vessel coronary artery disease on study Day 27—10 days after treatment with vadadustat had been discontinued due to a major protocol deviation. None of the SAEs was considered by the trial investigators to be related to vadadustat therapy. There were no deaths reported during the study period.
Table 4.
AEs (ITT population)
| Category | Vadadustat dose cohorts |
|||
|---|---|---|---|---|
| 300 mg QD | 450 mg QD | 450 mg TIW | All subjects | |
| (n = 30) | (n = 33) | (n = 31) | (n = 94) | |
| Number of AEs | 110 | 95 | 89 | 294 |
| Subjects with ≥1 AE, n (%) | 26 (86.7) | 26 (78.8) | 26 (83.9) | 78 (83.0) |
| AEs reported in ≥5% of subjects, n (%) | ||||
| Nausea | 4 (13.3) | 3 (9.1) | 4 (12.9) | 11 (11.7) |
| Diarrhea | 4 (13.3) | 4 (12.1) | 2 (6.5) | 10 (10.6) |
| Vomiting | 3 (10.0) | 3 (9.1) | 3 (9.7) | 9 (9.6) |
| Headache | 3 (10.0) | 2 (6.1) | 3 (9.7) | 8 (8.5) |
| Dizziness | 2 (6.7) | 3 (9.1) | 2 (6.5) | 7 (7.4) |
| Abdominal pain | 2 (6.7) | 4 (12.1) | 0 | 6 (6.4) |
| Muscle spasms | 3 (10.0) | 2 (6.1) | 1 (3.2) | 6 (6.4) |
| AV fistula thrombosis | 3 (10.0) | 1 (3.0) | 1 (3.2) | 5 (5.3) |
| Back pain | 1 (3.3) | 1 (3.0) | 3 (9.7) | 5 (5.3) |
| Subjects with ≥1 SAEa, n (%) | 2 (6.7) | 6 (18.2) | 5 (16.1) | 13 (13.8) |
| SAEs reported in ≥1 subject, n (%) | ||||
| Pneumonia | 1 (3.3) | 1 (3.0) | 1 (3.2) | 3 (3.2) |
| AV fistula thrombosis | 1 (3.3) | 0 | 1 (3.2) | 2 (2.1) |
| Acute MI | 1 (3.3) | 1 (3.0) | 0 | 2 (2.1) |
| Subjects with ≥1 drug-related AEb, n (%) | 10 (33.3) | 6 (18.2) | 6 (19.4) | 22 (23.4) |
| Subjects with AE leading to study withdrawal, n (%) | 3 (10.0) | 4 (12.1) | 1 (3.2) | 8 (8.5) |
| Subjects with ≥1 drug-related SAEb, n (%) | 0 | 0 | 0 | 0 |
| Deaths, n (%) | 0 | 0 | 0 | 0 |
A total of 22 SAEs were reported in 13/94 (13.8%). Pneumonia (n = 3, 3.2%), AV fistula thrombosis (n = 2, 2.1%), and acute MI (n = 2, 2.1%) were the only SAEs that were reported in >1 of the 94 subjects. No strokes or transient ischemic attacks were reported.
As assessed by the investigator as possibly or probably related to vadadustat.
Over the course of the trial, no meaningful changes from baseline in mean systolic and diastolic blood pressures, pulse rate, and respiratory rate were noted across the three dose cohorts. Hypertension was reported as an AE in 4/94 subjects (Supplementary data, Table S6) and was reported by the investigators as related to vadadustat in 3/4 subjects. All four subjects had a prior history of hypertension and showed wide variation in their systolic blood pressure readings between the screening and baseline visits, as well as during the treatment period. Hypotension was reported as an AE in 4/94 subjects; none of these events was considered by the study investigators to be associated with vadadustat treatment.
There were no trends in vital signs, electrocardiographic parameters or safety laboratory values, and the number of subjects with recorded abnormal laboratory values were similar in each dose cohort. Serum bicarbonate (Supplementary data, Figure S3) levels remained stable throughout the treatment period in the three dose cohorts. No trend was observed for mean plasma vascular endothelial growth factor (VEGF) and serum cholesterol or triglyceride measurements (Supplementary data, Table S7).
DISCUSSION
Vadadustat belongs to a new class of potential treatment options that stabilize HIF by reversibly inhibiting HIF-PHD dioxygenases—hydroxylating enzymes that target the oxygen-sensitive HIF-α subunit for rapid proteasomal degradation in the presence of oxygen. In two Phase 2 trials of subjects with NDD-CKD, vadadustat increased reticulocyte production and raised or maintained mean Hb concentrations with limited excursions above the target range [25, 26]. In addition, in subjects with NDD-CKD, vadadustat treatment was associated with favorable changes in iron-related biomarkers, namely, increased serum TIBC and decreased ferritin and hepcidin levels [25, 26].
This 16-week, open-label Phase 2 trial evaluated the effect of vadadustat on Hb concentrations and iron indices in subjects receiving hemodialysis who had been on prior epoetin alpha therapy, who have similar baseline characteristics to the US population of patients on dialysis [14]. Vadadustat starting doses of 300 mg QD, 450 mg QD, and 450 mg TIW maintained mean Hb concentrations, from pre-baseline values to Weeks 7–8 and 15–16 in the prespecified primary efficacy analysis using observed data. In the sensitivity analysis using LOCF to account for early discontinuations, mean Hb levels remained stable in the 300 mg QD dose cohort and modest declines were observed in the 450 mg QD and 450 mg TIW dose cohorts. Three percent and 19% of subjects in the 450 mg QD and 450 mg TIW dose cohorts, respectively, discontinued due to worsening of anemia; analysis of baseline characteristics revealed a higher pre-baseline epoetin alfa dose in these subjects. Additionally, in this study, dose titration of vadadustat was not permitted until Week 8. These findings warrant further assessment in future studies.
The erythropoietic effects of vadadustat in subjects receiving hemodialysis were consistent with the known actions of HIF, which include increased renal and hepatic EPO production, a reduction in serum hepcidin concentration, increased intestinal iron uptake, and enhanced release of stored iron from enterocytes, hepatocytes and macrophages [4, 27]. It is worth noting that the effect on iron homeostasis is one of the key potential differences between vadadustat and ESAs. Unlike ESAs, which indirectly affect iron mobilization through hepcidin modulation [28, 29], HIF-PHD inhibitors are predicted to have direct effects on iron metabolism through regulation of several genes involved in iron transport and absorption independent of EPO stimulation [30, 31].
Patients on hemodialysis have greater iron requirements than patients without renal impairment, due to a combination of increased systemic inflammation and sustained blood loss with recurrent dialysis and phlebotomy [32]. IV iron is administered concurrently with ESA in order to ensure that patients have adequate iron resources to keep up with the demands of a strong erythropoietic signal produced by ESAs [31, 33], but patients generally do not experience increased intestinal iron absorption after treatment [30, 31, 34–36]. In contrast, studies with other HIF-PHD inhibitors [37–39] or at high altitude (hypobaric hypoxia) [19, 22] in patients receiving hemodialysis have indicated an additional increase in iron utilization, presumably due to improved intestinal iron absorption and mobilization [40]. In the current study, the apparent iron mobilization seen with vadadustat may be explained by less stringent study eligibility criteria regarding iron saturation (a wide ferritin target range and unrestricted TSAT values), but our observations with respect to iron parameters are also consistent with drug class findings of improved iron mobilization. Further study is warranted in this patient subpopulation with respect to iron supplementation requirements and utilization in the context of new therapeutic approaches.
Based upon limited sampling, plasma concentrations of vadadustat appeared unaffected by hemodialysis. In a previous study, when vadadustat was given 2 h prior to and 4 h after hemodialysis, dialysis had no significant impact on either the maximal drug concentration in serum (Cmax) or extent (AUCinf) of vadadustat or its phenolic glucuronide, whereas the plasma concentration of its acyl glucuronide was lower following dialysis (this metabolite represented <1% of vadadustat plasma exposure) [41].
Patients with ESRD are an older, fragile population, characterized by a high prevalence of cardiovascular and other comorbidities [42–45]. In this study of subjects with ESRD, AEs were primarily mild or moderate in severity, and there was no discernable trend in the type or frequency of events across the three dose cohorts. Eight of the 94 (9%) subjects discontinued treatment because of AEs, which were primarily gastrointestinal in nature. None of these eight AEs was reported in more than one subject who discontinued. No deaths were reported, and none of the observed SAEs was reported by the investigators as related to vadadustat. With respect to cardiovascular events, two MIs were reported in this study and were not considered related to vadadustat.
Our study was limited by the small sample size, treatment duration, open-label design, and the lack of a control group. Iron administration was not standardized and the ferritin target range was wide; as a result, firm conclusions regarding the impact of vadadustat on iron metabolism in the subjects with ESRD will require additional study. The trial excluded patients receiving epoetin doses >24 000 U/week. Furthermore, because HIF transcription factors are involved in regulating multiple signaling and metabolic pathways, systemic HIF-PHD inhibition has the potential to have effects beyond increasing Hb, some of which have been described in genetic animal models [46–48]. For example, HIF promotes angiogenesis through upregulation of VEGF [49]. Though no discernible effect on VEGF was noted with the use of vadadustat in CKD subjects at the tested doses, concerns remain regarding the oncogenic potential of HIF-activating compounds [50, 51] and their use in patients with proliferative retinopathy [52, 53]. HIF has also been directly implicated in tumor cell growth and metastasis, as well as the pathogenesis of diabetes [54] and pulmonary hypertension [51], raising additional cardiovascular safety concerns. Conversely, HIF activation has been shown to have cytoprotective [51] and beneficial metabolic and anti-inflammatory effects [51, 55, 56]. Although some of these non-erythropoietic actions are dependent on cell type, the degree and duration of HIF activation, and these effects may only be detectable in genetic animal models, careful and comprehensive safety evaluations are needed in patients on long-term therapy with HIF-PHD inhibitors. The long-term safety profile of vadadustat in subjects with anemia of NDD-CKD and DD-CKD is currently being investigated in several large Phase 3 clinical trials. Comparative studies among different HIF-PHD inhibitors will be needed to assess potential drug differences.
In summary, in this open-label Phase 2 trial investigating QD (300 and 450 mg) and TIW dosing (450 mg), subjects receiving hemodialysis who remained on vadadustat treatment maintained stable mean Hb concentrations after being converted from epoetin alfa. Given its effects on erythropoiesis and preliminary safety profile, vadadustat has the potential to provide an alternative to conventional ESA therapy for the treatment of anemia secondary to CKD.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Lucid Partners Ltd and Qing Zuraw for editorial assistance, Wenli Luo for statistical analysis help, and Ana Bozas for project management assistance with this work. The authors also thank all patients and their families, as well as the Study Monitoring Team (SMT), study investigators, research nurses, trial coordinators and other site staff who participated in this trial. In particular, the authors thank Steven Fishbane and Robert Shalwitz for study design and SMT participation, Krishna Polu for SMT participation, Karen Annis for assistance with trial execution, monitoring support and data analysis, and Charlotte Hartmann for study design, execution, monitoring support and assistance with data analysis and interpretation of trial results.
FUNDING
Funding for this research was provided by Akebia Therapeutics, Inc.
AUTHORS’ CONTRIBUTIONS
All authors contributed to the final analysis and interpretation of data, and had full access to the study data and associated analyses. All authors participated in the review process and editing of the manuscript for important intellectual content, approve of the final version to be published, and have full confidence in the accuracy and integrity of the work.
CONFLICT OF INTEREST STATEMENT
P.E.P. is supported by honoraria and lecture fees from Akebia Therapeutics. E.M.d.G., Z.K., A.S. and B.J.M. (former employee) are employed by Akebia Therapeutics and own stock/stock options. G.A.B., G.M.C., V.H.H. and P.A.M. are scientific advisors and have received honoraria from Akebia Therapeutics. V.H.H. is supported by the Krick-Brooks Chair in Nephrology.
REFERENCES
- 1. Levey AS, Atkins R, Coresh J. et al. Chronic kidney disease as a global public health problem: approaches and initiatives - a position statement from Kidney Disease Improving Global Outcomes. Kidney Int 2007; 72: 247–259 [DOI] [PubMed] [Google Scholar]
- 2. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 150. [DOI] [PubMed] [Google Scholar]
- 3. Schmidt RJ, Dalton CL.. Treating anemia of chronic kidney disease in the primary care setting: cardiovascular outcomes and management recommendations. Osteopath Med Prim Care 2007; 1: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Koury MJ, Haase VH.. Anaemia in kidney disease: harnessing hypoxia responses for therapy. Nat Rev Nephrol 2015; 11: 394–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yilmaz MI, Solak Y, Covic A. et al. Renal anemia of inflammation: the name is self-explanatory. Blood Purif 2011; 32: 220–225 [DOI] [PubMed] [Google Scholar]
- 6. de Francisco AL, Stenvinkel P, Vaulont S.. Inflammation and its impact on anaemia in chronic kidney disease: from haemoglobin variability to hyporesponsiveness. NDT plus 2009; 2: i18–i26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pisoni RL, Bragg-Gresham JL, Young EW. et al. Anemia management and outcomes from 12 countries in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2004; 44: 94–111 [DOI] [PubMed] [Google Scholar]
- 8. Palmer SC, Saglimbene V, Mavridis D. et al. Erythropoiesis-stimulating agents for anaemia in adults with chronic kidney disease: a network meta-analysis. Cochrane Database Syst Rev 2014; CD010590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Palazzuoli A, Quatrini I, Calabro A. et al. Anemia correction by erythropoietin reduces BNP levels, hospitalization rate, and NYHA class in patients with cardio-renal anemia syndrome. Clin Exp Med 2011; 11: 43–48 [DOI] [PubMed] [Google Scholar]
- 10. Pfeffer MA, Burdmann EA, Chen CY. et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009; 361: 2019–2032 [DOI] [PubMed] [Google Scholar]
- 11. Singh AK, Szczech L, Tang KL. et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006; 355: 2085–2098 [DOI] [PubMed] [Google Scholar]
- 12. Besarab A, Bolton WK, Browne JK. et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 1998; 339: 584–590 [DOI] [PubMed] [Google Scholar]
- 13. McCullough PA, Barnhart HX, Inrig JK. et al. Cardiovascular toxicity of epoetin-alfa in patients with chronic kidney disease. Am J Nephrol 2013; 37: 549–558 [DOI] [PubMed] [Google Scholar]
- 14. Unger EF, Thompson AM, Blank MJ. et al. Erythropoiesis-stimulating agents–time for a reevaluation. N Engl J Med 2010; 362: 189–192 [DOI] [PubMed] [Google Scholar]
- 15. Koury MJ. Erythroferrone: a missing link in iron regulation. The Hematologist 2015; 12; http://www.hematology.org/Thehematologist/Years-Best/3599.aspx (18 October 2017, date last accessed) [Google Scholar]
- 16. Kim A, Nemeth E.. New insights into iron regulation and erythropoiesis. Curr Opin Hematol 2015; 22: 199–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ganz T, Nemeth E.. Hepcidin and iron homeostasis. Biochim Biophys Acta 2012; 1823: 1434–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ganz T, Nemeth E.. Iron balance and the role of hepcidin in chronic kidney disease. Semin Nephrol 2016; 36: 87–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brookhart MA, Schneeweiss S, Avorn J. et al. The effect of altitude on dosing and response to erythropoietin in ESRD. J Am Soc Nephrol 2008; 19: 1389–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blumberg A, Keller H, Marti HR.. Effect of altitude on erythropoiesis and oxygen affinity in anaemic patients on maintenance dialysis. Eur J Clin Invest 1973; 3: 93–97 [DOI] [PubMed] [Google Scholar]
- 21. Winkelmayer WC, Liu J, Brookhart MA.. Altitude and all-cause mortality in incident dialysis patients. JAMA 2009; 301: 508–512 [DOI] [PubMed] [Google Scholar]
- 22. Sibbel S, Maroni BJ, Brunelli SM.. The effect of altitude on erythropoiesis-stimulating agent dose, hemoglobin level, and mortality in hemodialysis patients. J Nephrol 2017; 30: 821–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Windsor JS, Rodway GW.. Heights and haematology: the story of haemoglobin at altitude. Postgrad Med J 2007; 83: 148–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kaelin WG Jr, Ratcliffe PJ.. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell 2008; 30: 393–402 [DOI] [PubMed] [Google Scholar]
- 25. Martin ER, Smith MT, Maroni BJ. et al. Clinical trial of vadadustat in patients with anemia secondary to Stage 3 or 4 chronic kidney disease. Am J Nephrol 2017; 45: 380–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pergola PE, Spinowitz BS, Hartman CS. et al. Vadadustat, a novel oral HIF stabilizer, provides effective anemia treatment in nondialysis-dependent chronic kidney disease. Kidney Int 2016; 90: 1115–1122 [DOI] [PubMed] [Google Scholar]
- 27. Haase VH. Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev 2013; 27: 41–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gammella E, Diaz V, Recalcati S. et al. Erythropoietin’s inhibiting impact on hepcidin expression occurs indirectly. Am J Physiol Regul Integr Comp Physiol 2015; 308: R330–R335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ashby DR, Gale DP, Busbridge M. et al. Plasma hepcidin levels are elevated but responsive to erythropoietin therapy in renal disease. Kidney Int 2009; 75: 976–981 [DOI] [PubMed] [Google Scholar]
- 30. Reddy GC, Devaki R, Rao P.. Iron indices in patients with functional anemia in chronic kidney disease. EJIFCC 2013; 24: 129–136 [PMC free article] [PubMed] [Google Scholar]
- 31. Fishbane S, Frei GL, Maesaka J.. Reduction in recombinant human erythropoietin doses by the use of chronic intravenous iron supplementation. Am J Kidney Dis 1995; 26: 41–46 [DOI] [PubMed] [Google Scholar]
- 32. Besarab A, Coyne DW.. Iron supplementation to treat anemia in patients with chronic kidney disease. Nat Rev Nephrol 2010; 6: 699–710 [DOI] [PubMed] [Google Scholar]
- 33. Macdougall IC, Tucker B, Thompson J. et al. A randomized controlled study of iron supplementation in patients treated with erythropoietin. Kidney Int 1996; 50: 1694–1699 [DOI] [PubMed] [Google Scholar]
- 34. Honda H, Kobayashi Y, Onuma S. et al. Associations among erythroferrone and biomarkers of erythropoiesis and iron metabolism, and treatment with long-term erythropoiesis-stimulating agents in patients on hemodialysis. PLoS One 2016; 11: e0151601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kuji T, Toya Y, Fujikawa T. et al. Acceleration of iron utilization after intravenous iron administration during activated erythropoiesis in hemodialysis patients: a randomized study. Ther Apher Dial 2015; 19: 131–137 [DOI] [PubMed] [Google Scholar]
- 36. Onuma S, Honda H, Kobayashi Y. et al. Effects of long-term erythropoiesis-stimulating agents on iron metabolism in patients on hemodialysis. Ther Apher Dial 2015; 19: 582–589 [DOI] [PubMed] [Google Scholar]
- 37. Provenzano R, Besarab A, Wright S. et al. Roxadustat (FG-4592) versus epoetin alfa for anemia in patients receiving maintenance hemodialysis: a Phase 2, randomized, 6- to 19-Week, open-label, active-comparator, dose-ranging, safety and exploratory efficacy study. Am J Kidney Dis 2016; 67: 912–924 [DOI] [PubMed] [Google Scholar]
- 38. Chen N, Qian J, Chen J. et al. Phase 2 studies of oral hypoxia-inducible factor prolyl hydroxylase inhibitor FG-4592 for treatment of anemia in China. Nephrol Dial Transplant 2017; 32: 1373–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Holdstock L, Meadowcroft AM, Maier R. et al. Four-week studies of oral hypoxia-inducible factor-prolyl hydroxylase inhibitor GSK1278863 for treatment of anemia. J Am Soc Nephrol 2016; 27: 1234–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Besarab A, Szczech L, Wish JB.. What are the considerations in balancing benefits and risks in iron treatment?: the benefits of intravenous iron. Semin Dial 2017; 30: 20–22 [DOI] [PubMed] [Google Scholar]
- 41. Buch A, Maroni BJ, Hartman CS.. Dose exposure relationship of vadadustat is independent of the level of renal function. J Am Soc Nephrol 2015; 26: #SA–PO537 [Google Scholar]
- 42. Van Buren PN, Lewis JB, Dwyer JP. et al. The phosphate binder ferric citrate and mineral metabolism and inflammatory markers in maintenance dialysis patients: results from prespecified analyses of a randomized clinical trial. Am J Kidney Dis 2015; 66: 479–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Besarab A, Zeig SN, Martin ER. et al. An open-label, sequential, dose-finding study of peginesatide for the maintenance treatment of anemia in chronic hemodialysis patients. BMC Nephrol 2012; 13: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Covic A, Mircescu G.. The safety and efficacy of intravenous ferric carboxymaltose in anaemic patients undergoing haemodialysis: a multi-centre, open-label, clinical study. Nephrol Dial Transplant 2010; 25: 2722–2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fishbane S, Schiller B, Locatelli F. et al. Peginesatide in patients with anemia undergoing hemodialysis. N Engl J Med 2013; 368: 307–319 [DOI] [PubMed] [Google Scholar]
- 46. Semenza GL. Regulation of metabolism by hypoxia-inducible factor 1. Cold Spring Harb Symp Quant Biol 2011; 76: 347–353 [DOI] [PubMed] [Google Scholar]
- 47. Haase VH. Renal cancer: oxygen meets metabolism. Exp Cell Res 2012; 318: 1057–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Girgis CM, Cheng K, Scott CH. et al. Novel links between HIFs, type 2 diabetes, and metabolic syndrome. Trends Endocrinol Metab 2012; 23: 372–380 [DOI] [PubMed] [Google Scholar]
- 49. Manalo DJ, Rowan A, Lavoie T. et al. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood 2005; 105: 659–669 [DOI] [PubMed] [Google Scholar]
- 50. Bonomini M, Del Vecchio L, Sirolli V. et al. New treatment approaches for the anemia of CKD. Am J Kidney Dis 2016; 67: 133–142 [DOI] [PubMed] [Google Scholar]
- 51. Maxwell PH, Eckardt KU.. HIF prolyl hydroxylase inhibitors for the treatment of renal anaemia and beyond. Nat Rev Nephrol 2016; 12: 157–168 [DOI] [PubMed] [Google Scholar]
- 52. Abcouwer SF. Angiogenic factors and cytokines in diabetic retinopathy. J Clin Cell Immunol 2013; Suppl 1: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Campochiaro PA. Molecular pathogenesis of retinal and choroidal vascular diseases. Prog Retin Eye Res 2015; 49: 67–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ichiki T, Sunagawa K.. Novel roles of hypoxia response system in glucose metabolism and obesity. Trends Cardiovasc Med 2014; 24: 197–201 [DOI] [PubMed] [Google Scholar]
- 55. Taylor CT, Doherty G, Fallon PG. et al. Hypoxia-dependent regulation of inflammatory pathways in immune cells. J Clin Invest 2016; 126: 3716–3724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cummins EP, Keogh CE, Crean D. et al. The role of HIF in immunity and inflammation. Mol Aspects Med 2016; 47–48: 24–34 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.