The purpose of this study was to explore the correlations of serum hepatitis B core-related antigen (HBcrAg) with intrahepatic Hepatitis B virus (HBV) covalently closed circular DNA (cccDNA) and HBV total DNA in hepatitis B e antigen (HBeAg)-positive chronic hepatitis B (CHB) patients. Serum HBcrAg and other parameters, including HBV DNA, HBV RNA, HBeAg, hepatitis B surface antigen (HBsAg), hepatitis B core antibody (anti-HBc), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were quantitatively measured at baseline and follow-up time points.
KEYWORDS: chronic hepatitis B, covalently closed circular DNA, HBV RNA, hepatitis B core-related antigen, nucleos(t)ide analogues
ABSTRACT
The purpose of this study was to explore the correlations of serum hepatitis B core-related antigen (HBcrAg) with intrahepatic Hepatitis B virus (HBV) covalently closed circular DNA (cccDNA) and HBV total DNA in hepatitis B e antigen (HBeAg)-positive chronic hepatitis B (CHB) patients. Serum HBcrAg and other parameters, including HBV DNA, HBV RNA, HBeAg, hepatitis B surface antigen (HBsAg), hepatitis B core antibody (anti-HBc), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were quantitatively measured at baseline and follow-up time points. Intrahepatic HBV cccDNA and total DNA were quantitatively detected at baseline and 96 weeks. Grading of liver necroinflammation and staging of hepatic fibrosis were assessed at baseline and 96 weeks. Correlations between serum HBcrAg and other parameters were analyzed by Pearson’s correlation analysis. The results showed that pretreatment HBcrAg correlated significantly with HBV total DNA levels (r = 0.328, P = 0.003) in 82 CHB patients, and, after removing three outliers, with intrahepatic HBV cccDNA (r = 0.323, P = 0.004; n = 79). Serum HBcrAg correlated better with HBV cccDNA in patients with lower levels of serum HBV DNA (stratified by 7 log IU/ml of HBV DNA; r = 0.656, P = 0.003 versus r = −0.02, P = 0.866). Significant inverse correlations were found between HBcrAg and grade of liver necroinflammation (r = −0.245, P = 0.037), stage of hepatic fibrosis (r = −0.360, P = 0.002) at baseline. Serum HBcrAg presented significant correlation with intrahepatic HBV cccDNA in patients with HBeAg seroconversion at 96 weeks (r = 0.622, P = 0.006). The decrease in HBcrAg showed significant correlation with the decrease in HBV cccDNA after 96-week NA therapy (r = 0.282, P = 0.043). Serum HBcrAg also correlated significantly with other serum markers at baseline and 96 weeks of NA therapy. In conclusion, baseline HBcrAg and its decreased value were significantly correlated with the corresponding intrahepatic HBV cccDNA.
INTRODUCTION
Hepatitis B virus (HBV) infection continues to be a major public health issue worldwide. It is estimated that 292 million people in the world are HBV carriers, with approximately 86 million in China (1). Chronic hepatitis B (CHB) can progress to cirrhosis, liver failure, and hepatocellular carcinoma (HCC), which are the major causes of mortality in the world (2). Antiviral treatment has been proved to be an effective way to block and delay the progression of HBV infection and thus to decrease the incidence of liver complications. There are five oral nucleos(t)ide analogs (NAs), and two kinds of interferon alpha (IFN-α) have been approved for treating chronic hepatitis B in China (3).
Quantitation of intrahepatic HBV covalently closed circular DNA (cccDNA) is suggested to be important in evaluating antiviral therapeutic efficacy and estimating treatment endpoint (4, 5). The major obstacle for quantitation of intrahepatic HBV cccDNA in clinic is the requirement for invasive liver biopsy. Therefore, finding serum surrogate makers of intrahepatic HBV cccDNA is clinically meaningful (6). Recent studies found that serum hepatitis B core-related antigen (HBcrAg) is a potential surrogate marker for intrahepatic HBV total DNA and HBV cccDNA (7–10). Serum HBcrAg is also helpful in predicting the response to antiviral therapy and the relapse after NA discontinuation (11–14), as well as in predicting the disease progression to liver cirrhosis and the development and recurrence of HCC in CHB patients (15–17). However, no study has comprehensively investigated the correlations between HBcrAg and intrahepatic HBV cccDNA, HBV total DNA, serum HBV RNA, HBV DNA, hepatitis B e antigen (HBeAg), hepatitis B surface antigen (HBsAg), stage of hepatic fibrosis, and grade of liver necroinflammation before and after long-term NA treatment (≥96 weeks).
In this study, HBeAg-positive CHB patients who received 96-week NA optimized therapy, 69.51% (57/82) of whom were infected with genotype C of HBV, were retrospectively investigated. Intrahepatic HBV cccDNA, HBV total DNA, serum HBcrAg, HBsAg, HBeAg, hepatitis B core antibody (anti-HBc), HBV DNA, HBV RNA, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were quantitatively tested at baseline and follow-up time points. Correlations between serum HBcrAg and intrahepatic HBV cccDNA, HBV total DNA, other serum HBV markers, stage of hepatic fibrosis, and grade of liver necroinflammation before and after 96 weeks of NA treatment were statistically analyzed.
MATERIALS AND METHODS
Patients.
We retrospectively investigated a cohort of HBeAg-positive CHB patients who received the 96-week NA (lamivudine [LAM] and adefovir dipivoxil [ADV]) optimized therapy in our study. The inclusion criteria for patients are briefly summarized as follows: 18 to 65 years old, HBsAg positive for at least 6 months, HBeAg positive and hepatitis B e antibody (anti-HBe) negative, 105 copies/ml ≤ HBV DNA ≤ 109 copies/ml, ALT ≥ 2 × upper limit of normal (ULN), and no history of antiviral therapy with NAs or interferon within the previous six months. The patients were treated with LAM (100 mg/day), and ADV (10 mg/day) was added when serum HBV DNA was >300 copies/ml at week 24 or when a virological breakthrough occurred during the 96-week treatment. After 96 weeks, the treatment was continued, but further data were not available from the CHB patients. At 96 weeks, virological response (VR; defined as HBV DNA of ≤20 IU/ml) and HBeAg seroconversion (SC) were the main endpoints. A total of 76 patients completed the follow-ups every 12 weeks from baseline to 96 weeks during NA treatment, and 82 patients at baseline and 62 patients at week 96 had liver biopsies for HBV cccDNA quantification. The study was approved by the Institutional Review Board of Peking University Health Science Center (approval number IRB00001052-11056) and conducted in accordance with the ethical standards of the Helsinki Declaration. Informed consent was obtained from each recruited patient.
Serum HBV RNA, HBV DNA, HBsAg, HBeAg, anti-HBc, and ALT measurements.
Serum HBV RNA was measured using methods reported by a previous study (18). Serum HBV DNA was quantified by the Roche Cobas TaqMan HBV test (1 IU/ml = 5.82 copies/ml; Roche Diagnostics, Mannheim, Germany). Serum HBsAg and HBeAg levels were quantified by chemiluminescent microparticle immunoassay (CMIA) using an Architect i2000SR analyzer (Abbott Diagnostics, North Chicago, IL). The HBeAg level was quantitatively detected according to the World Health Organization (WHO) HBeAg reference standard (Paul-Ehrlich-Institute units [PEIU]; Paul-Ehrlich-Institute, Germany). Anti-HBc quantification was measured using a double-sandwich immunoassay (Wantai, Beijing, China) and validated by WHO anti-HBc standards. ALT and AST levels were measured according to standard procedures in the clinical laboratories in the hospitals.
Serum HBcrAg quantification.
Serum HBcrAg was measured by the Lumipulse G HBcrAg assay, using the Lumipulse G1200 Analyzer (Fujirebio, Tokyo, Japan) with a lower detection limit of 2.0 log U/ml and a dynamic range of 3.0 log U/ml to 7.0 log U/ml. HBcrAg consists of three species of related proteins sharing an identical 149-amino-acid sequence, as follows: hepatitis B core antigen (HBcAg), HBeAg, and a 22-kDa precore protein (p22Cr). Briefly, 150 μl of pretreatment solution was added to the serum sample (150 μl), and then incubated at 60°C for 30 min. The following reaction processes were carried out in the Lumipulse G1200 analyzer. Samples with measurement values higher than the upper range of detection were diluted with HBV-negative human serum to be reanalyzed.
Intrahepatic HBV cccDNA quantification and histopathology assessment of liver tissue.
At baseline and 96 weeks after NA therapy, liver biopsies were performed, and the liver tissues were prepared for intrahepatic HBV cccDNA and HBV total DNA quantification and histopathology assessment. Intrahepatic HBV cccDNA and HBV total DNA were quantitatively measured according to the method established by our laboratory; each liver tissue sample was measured three times for cccDNA, and the mean value was used for analyses. The details of the procedures were described previously (19).
The necroinflammatory scores and fibrosis scores for liver tissue assessment were evaluated by experienced pathologists according to the guideline of prevention and treatment for chronic hepatitis B (2010 version) in China (20). Briefly, necroinflammatory scores range from 0 to 3, and higher scores indicate that the necroinflammation is more severe; fibrosis scores range from 0 to 4, and a score of 4 indicates cirrhosis.
Statistical analyses.
Continuous variables were expressed as means ± standard deviation (SD) and were compared using the Student's t test or the Mann-Whitney test. Categorical variables were expressed as counts and percentages and were compared using the chi-square test. Pearson’s correlation analysis was performed to analyze the correlations between HBcrAg levels and intrahepatic HBV cccDNA, HBV total DNA, serum HBV RNA, HBV DNA, HBsAg, HBeAg, the grade of liver necroinflammation, and the stage of hepatic fibrosis. All statistical analyses were conducted using SPSS 20.0 (SPSS, Chicago, IL). All tests were two-tailed, and a P value of <0.05 was considered statistically significant.
RESULTS
Patient characteristics at baseline and 96 weeks.
Characteristics of HBeAg-positive CHB patients at baseline and 96 weeks after NA treatment were summarized in Table 1. A total of 82 patients had undergone liver biopsy at baseline; intrahepatic HBV cccDNA and HBV total DNA, as well as serum HBcrAg and serum HBV DNA, were detectable in all 82 patients. Of the 82 patients, 8.5% (7/82) were negative (below the lower limit of detection [LoD]) for serum HBV RNA. The mean level of serum HBcrAg in 82 patients was 7.97 ± 0.96 log U/ml. At week 96, liver biopsies were conducted in 62 patients; intrahepatic HBV cccDNA and HBV total DNA, as well as serum HBcrAg, were detectable in all 62 patients, whereas in 59.7% (37/62) and 51.6% (32/62) of patients, serum HBV RNA and HBV DNA, respectively, were undetectable. The lower limits of detection of serum HBV RNA and HBV DNA were 66.67 IU/ml and 20 IU/ml, respectively, and the testing results of samples below the lower limits were recorded as 66.67 IU/ml or 20 IU/ml for serum HBV RNA and HBV DNA, respectively. After 96-week therapy, the HBcrAg level showed a significant decrease (P < 0.05), just like the parameters HBV RNA, HBV DNA, HBsAg, HBeAg, anti-HBc, ALT, AST, intrahepatic HBV cccDNA, HBV total DNA, necroinflammatory score, and fibrosis score (P < 0.05).
TABLE 1.
Characteristics of HBeAg-positive CHB patients at baseline and 96 weeks after NA treatment
| Patient characteristic | Baseline (n = 82) | 96-week (n = 62) | P value |
|---|---|---|---|
| Median (range) age in yrs | 31 (18–56) | 32 (20–57) | 1.000 |
| Gender (no. of males, no. of females) | 61/21 | 46/16 | 0.979 |
| HBcrAg (log U/ml) | 7.97 ± 0.96 | 5.74 ± 1.10 | <0.001 |
| HBV RNA (log IU/ml) | 5.32 ± 1.38 | 2.49 ± 1.00 | <0.001 |
| HBV DNA (log IU/ml) | 7.48 ± 1.22 | 1.86 ± 0.92 | <0.001 |
| HBsAg (log IU/ml) | 4.05 ± 0.64 | 3.32 ± 0.90 | <0.001 |
| HBeAg (log PEIU/ml) | 2.32 ± 1.26 | 0.08 ± 1.13 | <0.001 |
| HBeAg status (no. positive/no. negative) | 82/0 | 44/18 | <0.001 |
| ALT (U/liter) | 208.87 ± 174.86 | 24.94 ± 14.47 | <0.001 |
| AST (U/liter) | 119.41 ± 107.40 | 24.07 ± 11.59 | <0.001 |
| Anti-HBc (log IU/ml) | 4.74 ± 0.46a | 3.39 ± 0.64b | <0.001 |
| HBV cccDNA level (log copies/cell) | 0.67 ± 0.74 | −0.94 ± 0.60 | <0.001 |
| HBV total DNA level (log copies/cell) | 2.86 ± 0.72 | 0.56 ± 0.75 | <0.001 |
| Necroinflammatory score of 1/2/3 (n) | 8/45/20c | 41/18/2d | <0.001 |
| Fibrosis score of 0/1/2/3/4 (n) | 2/13/27/18/13c | 3/27/16/7/8d | 0.003 |
| Infected with genotype B/C/D (n) | 23/57/2 | 19/41/2 | 0.804 |
Anti-HBc data were available from 63 patients at baseline.
Anti-HBc data were available from 59 patients at week 96.
Necroinflammatory score and fibrosis score data were available from 73 patients at baseline.
Necroinflammatory score and fibrosis score data were available from 61 patients at week 96. The lower limits of detection of serum HBV RNA and serum HBV DNA was 66.67 IU/ml and 20 IU/ml, respectively, and samples below the lower limits were recorded as 66.67 IU/ml or 20 IU/ml, respectively.
After 96-week NA treatment, 48.7% (37/76) of CHB patients obtained VR (<20 IU/ml), and 19.7% (15/76) of patients achieved HBeAg SC. Only 1.3% of CHB patients (1/76) had HBsAg loss. HBcrAg levels, as well as those of HBsAg, HBeAg, anti-HBc, and HBV DNA at baseline, had no significant differences between patients who obtained 96-week VR and those who did not. However, the level of serum HBV RNA in patients who achieved VR was significantly lower than that of those who did not achieve VR (Table S1).
Dynamic changes in serum levels of HBcrAg during 96-week treatment.
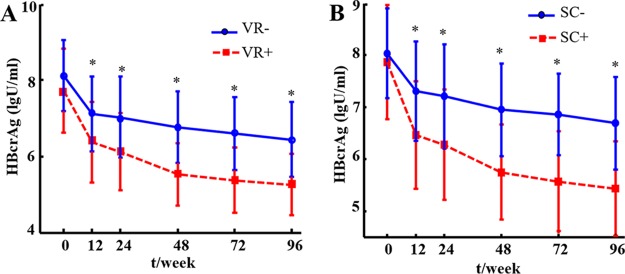
Serum HBcrAg levels decreased significantly between baseline and 96 weeks after NA therapy (from 7.97 ± 0.96 log U/ml to 5.74 ± 1.10 log U/ml). As shown in Fig. 1, at every follow-up time point except baseline (P < 0.001), significantly lower HBcrAg levels were found in patients with VR than in patients without VR. Similarly, at every follow-up time point except baseline (P < 0.05), HBcrAg levels were significantly lower in patients with HBeAg SC compared to those of patients without HBeAg SC.
FIG 1.
Dynamic changes of HBcrAg levels from baseline to 96 weeks in CHB patients who received NA therapy, stratified by virological response (A) and HBeAg seroconversion (B) at 96 weeks. *, P < 0.05; VR+, virological response (HBV DNA < 20 IU/ml); VR−, without virological response; SC+, HBeAg seroconversion; SC−, without HBeAg seroconversion.
Correlations between HBcrAg levels and intrahepatic HBV cccDNA, HBV total DNA, serum HBV RNA, HBV DNA, HBeAg, HBsAg, stage of hepatic fibrosis, and grade of liver necroinflammation at baseline and 96 weeks after NA treatment.
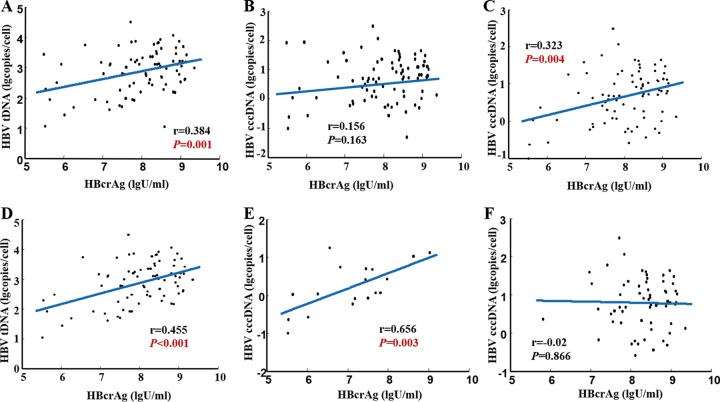
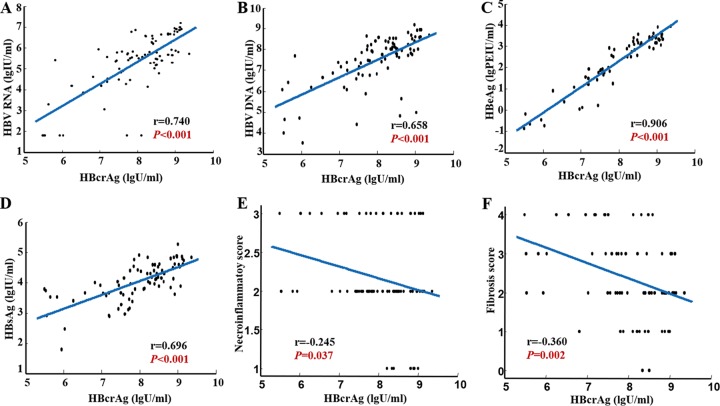
At baseline, serum HBcrAg showed a significant correlation with intrahepatic HBV total DNA level (r = 0.384, P = 0.001; Fig. 2A) but not with intrahepatic HBV cccDNA level (r = 0.156, P = 0.163; Fig. 2B) in 82 HBeAg-positive CHB patients. There were three samples with outliers for which we retested the serum HBcrAg; the testing results of these three samples were 6.3 log U/ml, 5.7 log U/ml, and 8.5 log U/ml, respectively. There was no significant difference compared with previous testing results (5.9 log U/ml, 5.5 log U/ml, and 8.6 log U/ml). If we remove these three outliers, significant correlations appeared between serum HBcrAg and intrahepatic HBV cccDNA levels (r = 0.323, P = 0.004; Fig. 2C) (n = 79) and between serum HBcrAg and intrahepatic HBV total DNA levels (r = 0.455, P < 0.001; Fig. 2D). Moreover, we found that serum HBcrAg correlated better with intrahepatic HBV cccDNA level in patients with lower levels of serum HBV DNA than in those with higher levels of HBV DNA (stratified by 7 log IU/ml of HBV DNA, r = 0.656, P = 0.003 versus r = −0.02, P = 0.866) (Fig. 2E and F). There were 61 (77.2%) patients with baseline serum HBV DNA higher than 7 log IU/ml and 18 (22.8%) patients with serum HBV DNA lower than 7 log IU/ml. Serum HBcrAg correlated significantly with serum HBV RNA (r = 0.740, P < 0.001), HBV DNA (r = 0.658, P < 0.001), HBeAg (r = 0.906, P < 0.001), and HBsAg (r = 0.696, P < 0.001) (Fig. 3A to D). Significant inverse correlations were found between HBcrAg value and grade of liver necroinflammation (r = −0.245, P = 0.037; Fig. 3E), stage of hepatic fibrosis (r = −0.360, P = 0.002; Fig. 3F). As shown in Table 2, the correlation between grade of liver necroinflammation and stage of hepatic fibrosis and intrahepatic HBV cccDNA, HBV total DNA, HBV RNA, HBV DNA, HBeAg, HBsAg, ALT, and AST were statistically analyzed. Furthermore, the correlation of intrahepatic HBV cccDNA and HBV total DNA with serum parameters were analyzed. We found that intrahepatic HBV cccDNA and HBV total DNA had more significant correlations with HBV DNA than with other parameters (Table S2).
FIG 2.
Correlation between HBcrAg and HBV total DNA (A), intrahepatic HBV cccDNA (B), intrahepatic HBV cccDNA (after removing three outliers) (C), HBV total DNA (after removing three outliers) (D), intrahepatic HBV cccDNA (with HBV DNA of <7 log U/ml) (E), intrahepatic HBV cccDNA (with HBV DNA of ≥7 log U/ml) (F). cccDNA, covalently closed circular DNA; HBV total DNA, hepatitis B virus total DNA; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; HBcrAg, hepatitis B core-related antigen.
FIG 3.
Correlation between HBcrAg and serum HBV RNA (A), HBV DNA (B), HBeAg (C), HBsAg (D), the grade of liver necroinflammation (E), and stage of hepatic fibrosis (F) in HBeAg-positive CHB patients at baseline. HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; HBcrAg, hepatitis B core-related antigen.
TABLE 2.
Correlations between the grade of liver necroinflammation and stage of hepatic fibrosis and the measured parameters at baseline
| Factors | Grade of liver necroinflammation |
Stage of hepatic fibrosis |
||
|---|---|---|---|---|
| r | P | r | P | |
| HBV cccDNA | −0.042 | 0.725 | 0.008 | 0.948 |
| HBV total DNA | −0.113 | 0.340 | −0.098 | 0.409 |
| HBcrAg | −0.245 | 0.037 | −0.360 | 0.002 |
| HBV RNA | −0.155 | 0.192 | −0.144 | 0.225 |
| HBV DNA | −0.343 | 0.003 | −0.286 | 0.014 |
| HBeAg | −0.337 | 0.004 | 0.405 | <0.001 |
| HBsAg | −0.362 | 0.002 | −0.319 | 0.006 |
| ALT | 0.246 | 0.036 | 0.017 | 0.886 |
| AST | 0.363 | 0.002 | 0.089 | 0.455 |
At 96 weeks after NA treatment, no correlations were found between serum HBcrAg and intrahepatic HBV cccDNA (r = 0.108, P = 0.403) or between serum HBcrAg and intrahepatic HBV total DNA (r = −0.051, P = 0.694), as shown in Fig. S1A and B. Besides, there were no correlations between serum HBcrAg and intrahepatic HBV cccDNA in patients who achieved or did not achieve VR at 96 weeks after NA treatment (r = 0.307, P = 0.087; r = 0.228, P = 0.226, respectively). However, serum HBcrAg presented a significant correlation with intrahepatic HBV cccDNA in patients with HBeAg SC, other than in patients without HBeAg SC at 96 weeks after NA treatment (r = 0.622, P = 0.006; r = −0.095, P = 0.540, respectively) (Fig. S1C and D). Serum HBcrAg correlated significantly with serum HBV RNA (r = 0.679, P < 0.001), HBV DNA (r = 0.512, P < 0.001), HBeAg (r = 0.868, P < 0.001), and HBsAg (r = 0.452, P < 0.001) at 96 weeks (Fig. S1E to H). The samples in which HBV DNA and HBV RNA were undetectable were included in the correlation analysis. If we removed the samples in which HBV DNA was undetectable, there was no significant correlation between serum HBcrAg and HBV DNA (r = 0.334, P = 0.071). If we removed the samples in which HBV RNA was undetectable, there was also a significant correlation between serum HBcrAg and HBV RNA (r = 0.600, P = 0.002). Correlations between grade of liver necroinflammation and stage of hepatic fibrosis and intrahepatic HBV cccDNA, HBV total DNA, HBcrAg, HBV RNA, HBV DNA, HBeAg, HBsAg, ALT, and AST at 96 weeks were statistically analyzed, as shown in Table S3. Only intrahepatic HBV total DNA correlated with liver necroinflammation and fibrosis scores (P ≤ 0.05). Moreover, after 96-week NA therapy, we observed that intrahepatic HBV cccDNA was associated with HBsAg (r = 0.39, P = 0.002), and that there were no correlations between intrahepatic HBV markers and other serum markers (Table S4).
Correlations between the decreases in HBcrAg and in intrahepatic HBV cccDNA, HBV total DNA, serum HBV RNA, HBV DNA, HBeAg, HBsAg, grade of liver necroinflammation, and stage of hepatic fibrosis after 96 weeks of NA treatment.
A total of 52 patients had liver biopsy samples both before and after 96 weeks of NA treatment, and we obtained paired (baseline and week 96) intrahepatic HBV cccDNA data. Of the 52 patients, serum HBcrAg, HBV RNA, HBV DNA, HBsAg, HBeAg, intrahepatic HBV cccDNA, and HBV total DNA decreased by 2.26 ± 1.35 log U/ml, 2.82 ± 1.47 log IU/ml, 5.45 ± 1.49 log IU/ml, 0.74 ± 0.88 log IU/ml, 2.29 ± 1.50 log PEIU/ml, 1.60 ± 0.87 log copies/cell, and 2.24 ± 1.03 log copies/cell, respectively, after 96 weeks of NA treatment. As shown in Fig. S2, the decreased HBcrAg value presented significant correlations with the decreases in intrahepatic HBV cccDNA (r = 0.282, P = 0.043), serum HBV RNA (r = 0.765, P < 0.001), HBV DNA (r = 0.545, P < 0.001), HBeAg (r = 0.877, P < 0.001), and HBsAg (r = 0.459, P = 0.001) values, but not with that of HBV total DNA (r = 0.260, P = 0.063). As shown in Table S5, there were no significant correlations between the improvement of necroinflammation and fibrosis and the decreases in intrahepatic HBV cccDNA, HBV total DNA, serum HBcrAg, HBV RNA, HBV DNA, HBsAg, HBeAg, ALT, and AST.
DISCUSSION
Previous studies have shown a strong correlation between HBcrAg and intrahepatic HBV cccDNA (7, 8, 21). Wong et al. (8) found that HBcrAg was significantly associated with intrahepatic HBV cccDNA (r = 0.70, P < 0.0001) and intrahepatic HBV total DNA (r = 0.67, P < 0.0001) in 305 CHB patients (HBeAg positive, n = 133; HBeAg negative, n = 172) at baseline. Chen et al. (22) also reported that the correlation between serum HBcrAg and intrahepatic HBV cccDNA was strong (r = 0.790, P < 0.001) in 52 CHB patients in the immune clearance phase of CHB. In our study, the significant correlation between intrahepatic HBV cccDNA level and serum HBcrAg existed, although the correlation coefficient was not as high as previously reported. In Chen et al.’s study, 66.10% (39/59) of patients were infected with genotype B (22), while 69.51% (57/82) of the patients in our study were infected with genotype C. It has been reported that the genotype may influence the correlations between HBV parameters (23, 24). Additionally, the quantification method of intrahepatic HBV cccDNA and the distribution of intrahepatic HBV cccDNA and HBV DNA values were different, which might be related to the inconsistent results between the study by Chen et al. (22) and ours. Compared with the study of Wong et al. (8), in which the mean level of serum HBV DNA was 4.0 log IU/ml (95% confidence interval [CI], 1.3 to 14 log IU/ml), in our study, the mean value of serum HBV DNA was higher, reaching up to 7.48 log IU/ml (95% CI, 4.05 to 8.96 log IU/ml). Therefore, we stratified the data by HBV DNA levels. The results showed that serum HBcrAg correlated significantly with intrahepatic HBV cccDNA levels in patients with serum HBV DNA levels lower than 7 log IU/ml (r = 0.656, P = 0.003). Wong et al. (8) performed correlation analysis in the 130 samples from patients with undetectable serum HBV DNA at the first year after NA treatment, among which HBcrAg was detectable in 101 (78%) patients, and HBcrAg correlated positively with intrahepatic HBV cccDNA (r = 0.42, P < 0.0001). However, the correlation of HBcrAg and intrahepatic HBV cccDNA in patients with detectable HBV DNA was not included in the analysis. In our study, there was no significant correlation between serum HBcrAg and intrahepatic HBV cccDNA in 32 patients achieved VR at 96 weeks after NA treatment (r = 0.307, P = 0.087). The discrepancy of the results of two studies may be due to the differences in the case number or the treatment duration. In addition, we observed that serum HBcrAg presented a significant correlation with intrahepatic HBV cccDNA in patients with HBeAg SC at 96 weeks after NA treatment, while no correlation was found in patients without HBeAg SC (Fig. S1C and D). To our knowledge, this is the first report on the correlation between HBcrAg and intrahepatic HBV cccDNA stratified by HBeAg status after long-term NA treatment, which suggests that there is still much work to be done to study serum HBcrAg.
Our study also found that the decreased value of HBcrAg presented a significant correlation with the decrease of intrahepatic HBV cccDNA in 52 patients (Fig. S2A). Similarly, a previous study by Wong et al. (8) also proved that HBcrAg levels decreased in a similar manner as that of intrahepatic HBV cccDNA in patients treated with NAs. Another study included 43 patients who had been on continuous (median of follow-up duration, 10 years; range, 5 to 12 years) NA therapy, the results of which showed that a positive correlation was observed between the logarithmic reduction of HBcrAg and intrahepatic HBV cccDNA (r = 0.419, P = 0.005) (25), which was also similar to those of our study. Although there was no significant correlation between decreases of serum HBcrAg and intrahepatic HBV total DNA (r = 0.260, P = 0.063), the P value was close to 0.05, which indicated that the decreased value of serum HBcrAg may correlated with the decline of intrahepatic HBV total DNA as well.
As a new viral marker, serum HBV RNA might play a role for CHB patients during NA therapy. Synthesized from the intrahepatic HBV cccDNA, HBV RNA can serve as the template for viral replication via reverse transcription using the HBV polymerase, as well as for protein synthesis. Studies show that serum HBV RNA is confirmed to be pregenomic RNA (pgRNA) present in virus-like particles (18). HBV pgRNA virions were produced from encapsidated particles in which the pgRNA was reverse transcribed partially or not at all (26). Our study first analyzed the correlation between serum HBcrAg and HBV RNA, two relatively new viral markers. The results demonstrated that serum HBcrAg correlated strongly with HBV RNA at baseline, as well as at 96 weeks (Fig. 3A and Fig. S1E). In addition, the decreased value of HBcrAg also presented a strong correlation with the decrease in serum HBV RNA in CHB patients after 96-week therapy (Fig. S2C).
Serum HBV DNA is a widely used viral marker, has a vital role in disease monitoring and predicting (27), and had a significant association with HBcrAg (r = 0.69, P < 0.0001) (Fig. S2D) (8). However, after receiving antiviral therapy, patients often have undetectable serum HBV DNA, which makes the correlation analysis not feasible. A study recruited 222 Chinese CHB patients on continuous entecavir treatment; after 7 years of therapy, the rate of undetectable HBV DNA was 98.7%, whereas only 32.0% of patients (47/222) had undetectable HBcrAg levels (28). In our study, after 96 weeks of NA treatment, 48.7% (37/76) of CHB patients achieved undetectable HBV DNA levels, while serum HBcrAg could be detected in all patients. The divergence between the serum HBcrAg and HBV DNA can be explained by the action of NAs on reverse transcription and subsequent prevention of HBV DNA replication, whereas HBcrAg production remains unaffected.
In our study, the correlations between serum HBcrAg and HBeAg were stronger at both baseline and 96 weeks (Fig. 3C and Fig. S1G), and the decreased value of HBcrAg also presented a strong correlation with the decreased value of HBeAg (Fig. S2E). One study also found that HBcrAg levels correlated strongly with those of HBeAg (r = 0.9, P < 0.001) at baseline in HBeAg-positive CHB patients (11), which was similar to results of our study. The explanation for the strong correlation between HBcrAg and HBeAg is that HBeAg is a part of HBcrAg, which consists of three components (HBcAg, HBeAg, and P22cr). However, serum HBcrAg correlated moderately with HBsAg at either baseline or 96 weeks (Fig. 3D and Fig. S1H), and the decrease in HBcrAg was also moderately correlated with the decrease in HBsAg (Fig. S2F). A previous study revealed that there was also a weak correlation between HBcrAg and HBsAg (r = 0.45, P < 0.0001) (8). The possible reason for the weak correlation may be the presence of abundant circulating empty HBsAg particles. The possibility of HBsAg synthesized from integrated HBV DNA may also weaken the correlation.
Our study revealed that there were significant inverse correlations between the serum HBcrAg value and grade of liver necroinflammation (r = −0.245, P < 0.037; Fig. 3E), stage of hepatic fibrosis (r = −0.36, P < 0.002; Fig. 3F) at baseline. Zhang et al. (29) also proved that serum HBcrAg had significant negative correlations with the liver necroinflammation score (r = −0.318, P < 0.001) and hepatic fibrosis score (r = −0.386, P < 0.01) in HBeAg-positive patients, which were similar to those in our study. As shown in Table S3, the results indicated that the grade of liver necroinflammation and stage of hepatic fibrosis were not significantly correlated with intrahepatic HBV cccDNA, serum HBcrAg, HBV RNA, HBV DNA, HBsAg, HBeAg, ALT, or AST at 96 weeks, but were correlated with intrahepatic HBV total DNA. Table S5 also presents results that there were no significant correlations between the improvement of necroinflammation and fibrosis and the declines in intrahepatic HBV cccDNA, HBV total DNA, serum HBcrAg, HBV RNA, HBV DNA, HBsAg, HBeAg, ALT, and AST at 96 weeks. The possible reason may be that the improvement of necroinflammation and fibrosis is an outcome of much longer treatment. However, only data from the 96-week follow-up were available for our study.
In conclusion, significant correlations between serum HBcrAg and intrahepatic HBV total DNA and cccDNA (HBV DNA level, <7 log IU/ml) were observed among the CHB patients at baseline, and the same correlation between the decreased values of serum HBcrAg and intrahepatic HBV cccDNA after 96-week therapy was also found, which suggested that HBcrAg is a potentially reliable surrogate serum marker for intrahepatic HBV cccDNA. Serum HBcrAg also correlated significantly with the grade of liver necroinflammation, stage of hepatic fibrosis at baseline, and other serum markers at baseline and 96 weeks.
Supplementary Material
ACKNOWLEDGMENTS
We declare no conflicts of interest.
The study was supported by a Major Science and Technology Special Project of China Thirteenth 5-year plan (no. 2018ZX10732401-003-015).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.01303-18.
REFERENCES
- 1.Polaris Observatory Collaborators. 2018. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol 3:383–403. doi: 10.1016/S2468-1253(18)30056-6. [DOI] [PubMed] [Google Scholar]
- 2.Trépo C, Chan HL, Lok A. 2014. Hepatitis B virus infection. Lancet 384:2053–2063. doi: 10.1016/S0140-6736(14)60220-8. [DOI] [PubMed] [Google Scholar]
- 3.Zheng X, Wang J, Yang D. 2015. Antiviral therapy for chronic hepatitis B in China. Med Microbiol Immunol 204:115–120. doi: 10.1007/s00430-014-0380-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nassal M. 2015. HBV cccDNA: viral persistence reservoir and key obstacle for a cure of chronic hepatitis B. Gut 64:1972–1984. doi: 10.1136/gutjnl-2015-309809. [DOI] [PubMed] [Google Scholar]
- 5.Bourne EJ, Dienstag JL, Lopez VA, Sander TJ, Longlet JM, Hall JG, Kwiatkowski RW, Wright T, Lai CL, Condreay LD. 2007. Quantitative analysis of HBV cccDNA from clinical specimens: correlation with clinical and virological response during antiviral therapy. J Viral Hepat 14:55–63. doi: 10.1111/j.1365-2893.2006.00775.x. [DOI] [PubMed] [Google Scholar]
- 6.Sung JJ, Wong ML, Bowden S, Liew CT, Hui AY, Wong VW, Leung NW, Locarnini S, Chan HL. 2005. Intrahepatic hepatitis B virus covalently closed circular DNA can be a predictor of sustained response to therapy. Gastroenterology 128:1890–1897. doi: 10.1053/j.gastro.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Honda M, Shirasaki T, Terashima T, Kawaguchi K, Nakamura M, Oishi N, Wang X, Shimakami T, Okada H, Arai K, Yamashita T, Sakai Y, Yamashita T, Mizukoshi E, Kaneko S. 2016. Hepatitis B virus (HBV) core-related antigen during nucleos(t)ide analog therapy is related to intra-hepatic HBV replication and development of hepatocellular carcinoma. J Infect Dis 213:1096–1106. doi: 10.1093/infdis/jiv572. [DOI] [PubMed] [Google Scholar]
- 8.Wong DK, Seto WK, Cheung KS, Chong CK, Huang FY, Fung J, Lai CL, Yuen MF. 2017. Hepatitis B virus core–related antigen as a surrogate marker for covalently closed circular DNA. Liver Int 37:995–1001. doi: 10.1111/liv.13346. [DOI] [PubMed] [Google Scholar]
- 9.Matsuzaki T, Tatsuki I, Otani M, Akiyama M, Ozawa E, Miuma S, Miyaaki H, Taura N, Hayashi T, Okudaira S, Takatsuki M, Isomoto H, Takeshima F, Eguchi S, Nakao K. 2013. Significance of hepatitis B virus core-related antigen and covalently closed circular DNA levels as markers of hepatitis B virus re-infection after liver transplantation. J Gastroen Hepatol 28:1217–1222. doi: 10.1111/jgh.12182. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki F, Miyakoshi H, Kobayashi M, Kumada H. 2009. Correlation between serum hepatitis B virus core-related antigen and intrahepatic covalently closed circular DNA in chronic hepatitis B patients. J Med Virol 81:27–33. doi: 10.1002/jmv.21339. [DOI] [PubMed] [Google Scholar]
- 11.van Campenhout MJ, Brouwer WP, van Oord GW, Xie Q, Zhang Q, Zhang N, Guo S, Tabak F, Streinu-Cercel A, Wang J, Pas SD, Sonneveld MJ, de Knegt RJ, Boonstra A, Hansen BE, Janssen HL. 2016. Hepatitis B core-related antigen levels are associated with response to entecavir and peginterferon add-on therapy in hepatitis B e antigen-positive chronic hepatitis B patients. Clin Microbiol Infect 22:571.e5–579. doi: 10.1016/j.cmi.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto A, Tanaka E, Minami M, Okanoue T, Yatsuhashi H, Nagaoka S, Suzuki F, Kobayashi M, Chayama K, Imamura M, Yotsuyanagi H, Nakaoka S, Maki N, Kawata S, Kumada H, Iino S, Kiyosawa K. 2007. Low serum level of hepatitis B core-related antigen indicates unlikely reactivation of hepatitis after cessation of lamivudine therapy. Hepatol Res 37:661–666. doi: 10.1111/j.1872-034X.2007.00094.x. [DOI] [PubMed] [Google Scholar]
- 13.Seto WK, Wong DK, Chan TS, Hwang YY, Fung J, Liu KS, Gill H, Lam YF, Cheung KS, Lie AK, Lai CL, Kwong YL, Yuen MF. 2016. Association of hepatitis B core-related antigen with hepatitis B virus reactivation in occult viral carriers undergoing high-risk immunosuppressive therapy. Am J Gastroenterol 111:1788–1795. doi: 10.1038/ajg.2016.436. [DOI] [PubMed] [Google Scholar]
- 14.Jung KS, Park JY, Chon YE, Kim HS, Kang W, Kim BK, Kim SU, Kim DY, Han KH, Ahn SH. 2016. Clinical outcomes and predictors for relapse after cessation of oral antiviral treatment in chronic hepatitis B patients. J Gastroenterol 51:830–839. doi: 10.1007/s00535-015-1153-1. [DOI] [PubMed] [Google Scholar]
- 15.Tada T, Kumada T, Toyoda H, Kobayashi N, Akita T, Tanaka J. 2018. Hepatitis B virus core-related antigen levels predict progression to liver cirrhosis in hepatitis B carriers. J Gastroenterol Hepatol 33:918–925. doi: 10.1111/jgh.13989. [DOI] [PubMed] [Google Scholar]
- 16.Hosaka T, Suzuki F, Kobayashi M, Hirakawa M, Kawamura Y, Yatsuji H, Sezaki H, Akuta N, Suzuki Y, Saitoh S, Arase Y, Ikeda K, Kobayashi M, Kumada H. 2010. HBcrAg is a predictor of post-treatment recurrence of hepatocellular carcinoma during antiviral therapy. Liver Int 30:1461–1470. doi: 10.1111/j.1478-3231.2010.02344.x. [DOI] [PubMed] [Google Scholar]
- 17.Tada T, Kumada T, Toyoda H, Kiriyama S, Tanikawa M, Hisanaga Y, Kanamori A, Kitabatake S, Yama T, Tanaka J. 2016. HBcrAg predicts hepatocellular carcinoma development: an analysis using time-dependent receiver operating characteristics. J Hepatol 65:48–56. doi: 10.1016/j.jhep.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 18.Huang H, Wang J, Li W, Chen R, Chen X, Zhang F, Xu D, Lu F. 2018. Serum HBV DNA plus RNA shows superiority in reflecting the activity of intrahepatic cccDNA in treatment-naïve HBV-infected individuals. J Clin Virol 99-100:71–78. doi: 10.1016/j.jcv.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 19.Wang M, Qiu N, Lu S, Xiu D, Yu J, Wang XT, Lu F, Li T, Liu X, Zhuang H. 2013. Serum hepatitis B surface antigen is correlated with intrahepatic total HBV DNA and cccDNA in treatment-naïve patients with chronic hepatitis B but not in patients with HBV related hepatocellular carcinoma. J Med Virol 85:219–227. doi: 10.1002/jmv.23461. [DOI] [PubMed] [Google Scholar]
- 20.Chinese Society of Hepatology and Chinese Society of Infectious Diseases, Chinese Medical Association. 2010. The guideline of prevention and treatment for chronic hepatitis B (2010 version). Guoji Liuxingbingxue Chuanranbingxue Zazhi 38:1–12. (In Chinese.) doi: 10.3760/cma.j.issn.1673-4149.2011.01.001. [DOI] [Google Scholar]
- 21.Wong DK, Tanaka Y, Lai CL, Mizokami M, Fung J, Yuen MF. 2007. Hepatitis B virus core-related antigens as markers for monitoring chronic hepatitis B infection. J Clin Microbiol 45:3942–3947. doi: 10.1128/JCM.00366-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen EQ, Feng S, Wang ML, Liang LB, Zhou LY, Du LY, Yan LB, Tao CM, Tang H. 2017. Serum hepatitis B core-related antigen is a satisfactory surrogate marker of intrahepatic covalently closed circular DNA in chronic hepatitis B. Sci Rep 7:173. doi: 10.1038/s41598-017-00111-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maasoumy B, Wiegand SB, Jaroszewicz J, Bremer B, Lehmann P, Deterding K, Taranta A, Manns MP, Wedemeyer H, Glebe D, Cornberg M. 2015. Hepatitis B core-related antigen (HBcrAg) levels in the natural history of hepatitis B virus infection in a large European cohort predominantly infected with genotypes A and D. Clin Microbiol Infect 21:606.e1–610. doi: 10.1016/j.cmi.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 24.van Campenhout MJH, van Bömmel F, Pfefferkorn M, Fischer J, Deichsel D, Boonstra A, van Vuuren AJ, Berg T, Hansen BE, Janssen HLA. 2018. Host and viral factors associated with serum hepatitis B virus RNA levels among patients in need for treatment. Hepatology 68:839–847. doi: 10.1002/hep.29872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong DK, Kopaniszen M, Seto WK, Fung J, Hung I, Young JLP, Yuen JCH, Ngai VWS, Yuen MF, Lai CL. 2015. Reduction of hepatitis B core related antigen by long term nucleoside nucleotide analogue therapy and its correlation with intrahepatic HBV DNA reduction. Hepatol Int 9:202.25788188 [Google Scholar]
- 26.Wang J, Shen T, Huang X, Kumar GR, Chen X, Zeng Z, Zhang R, Chen R, Li T, Zhang T, Yuan Q, Li PC, Huang Q, Colonno R, Jia J, Hou J, McCrae MA, Gao Z, Ren H, Xia N, Zhuang H, Lu F. 2016. Serum hepatitis B virus RNA is encapsidated pregenome RNA that may be associated with persistence of viral infection and rebound. J Hepatol 65:700–710. doi: 10.1016/j.jhep.2016.05.029. [DOI] [PubMed] [Google Scholar]
- 27.Zacharakis G, Koskinas J, Kotsiou S, Tzara F, Vafeiadis N, Papoutselis M, Maltezos E, Sivridis E, Papoutselis K. 2008. The role of serial measurement of serum HBV DNA levels in patients with chronic HBeAg (−) hepatitis B infection: association with liver disease progression. A prospective cohort study. J Hepatol 49:884–891. doi: 10.1016/j.jhep.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 28.Lam YF, Seto WK, Wong D, Cheung KS, Fung J, Mak LY, Yuen J, Chong CK, Lai CL, Yuen MF. 2017. Seven-year treatment outcome of entecavir in a real-world cohort: effects on clinical parameters, HBsAg and HBcrAg levels. Clin Transl Gastroenterol 8:e125. doi: 10.1038/ctg.2017.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang ZQ, Lu W, Wang YB, Weng QC, Zhang ZY, Yang ZQ, Feng YL. 2016. Measurement of the hepatitis B core-related antigen is valuable for predicting the pathological status of liver tissues in chronic hepatitis B patients. J Virol Methods 235:92–98. doi: 10.1016/j.jviromet.2016.05.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.